Hydrogenation of Levulinic Acid over Nickel Catalysts Supported on Aluminum Oxide to Prepare γ-Valerolactone
Abstract
:1. Introduction
2. Results and Discussion
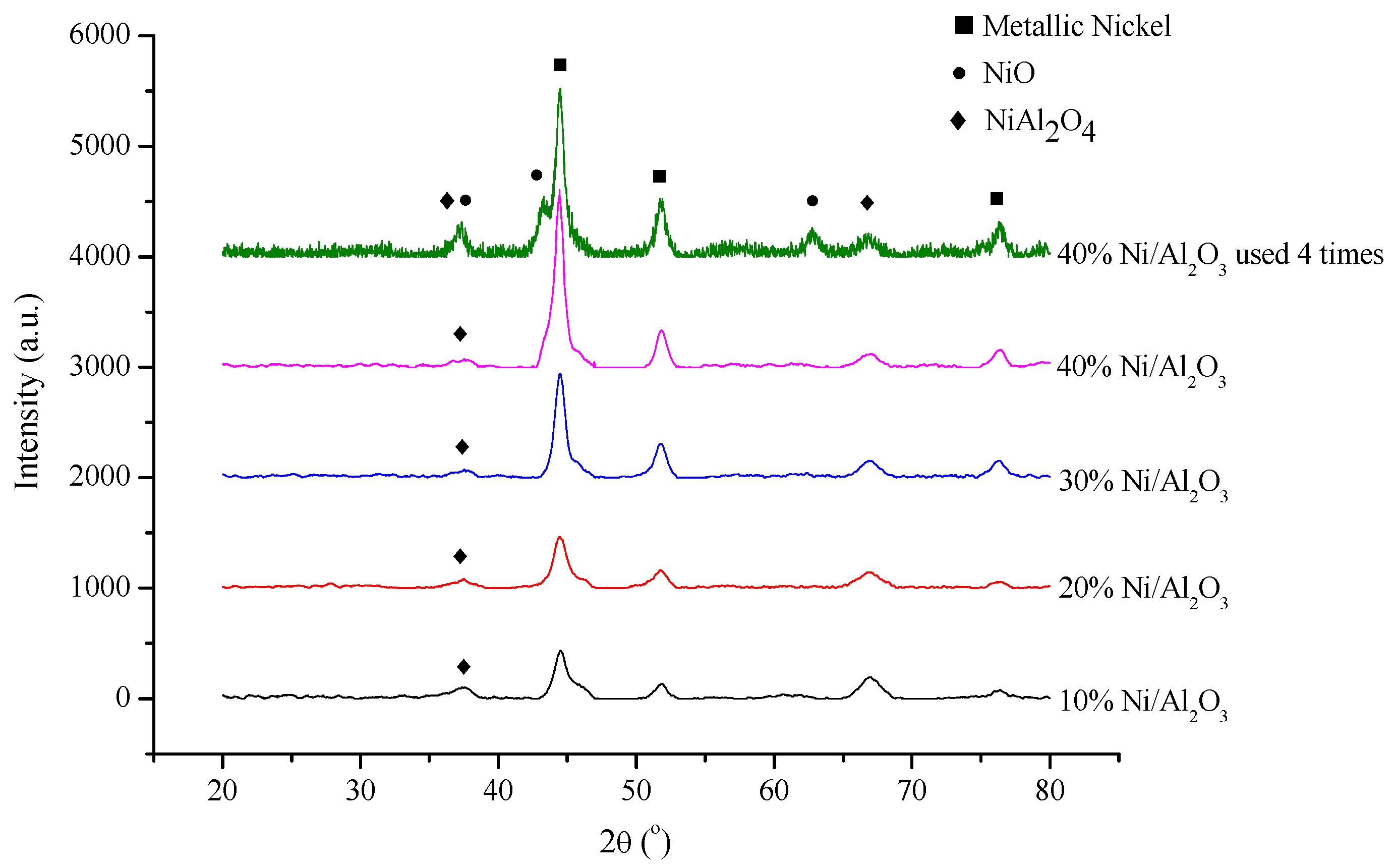
2.1. Catalyst Characterization
| Catalyst | BET Surface Area (m2/g) | Pore Volume a (cm³/g) | Pore Size b (nm) | Average Crystalline Size of Metallic Ni c (nm) |
|---|---|---|---|---|
| 10% Ni/Al2O3 | 181.3 | 0.483 | 9.80 | 8 |
| 20% Ni/Al2O3 | 168.8 | 0.426 | 8.52 | 14.7 |
| 30% Ni/Al2O3 | 145.7 | 0.377 | 8.86 | 17.7 |
| 40% Ni/Al2O3 | 133.7 | 0.327 | 8.32 | 19.3 |
| 40% Ni/Al2O3 (used 4 times) | 133.1 | 0.275 | 7.50 | 19.1 |
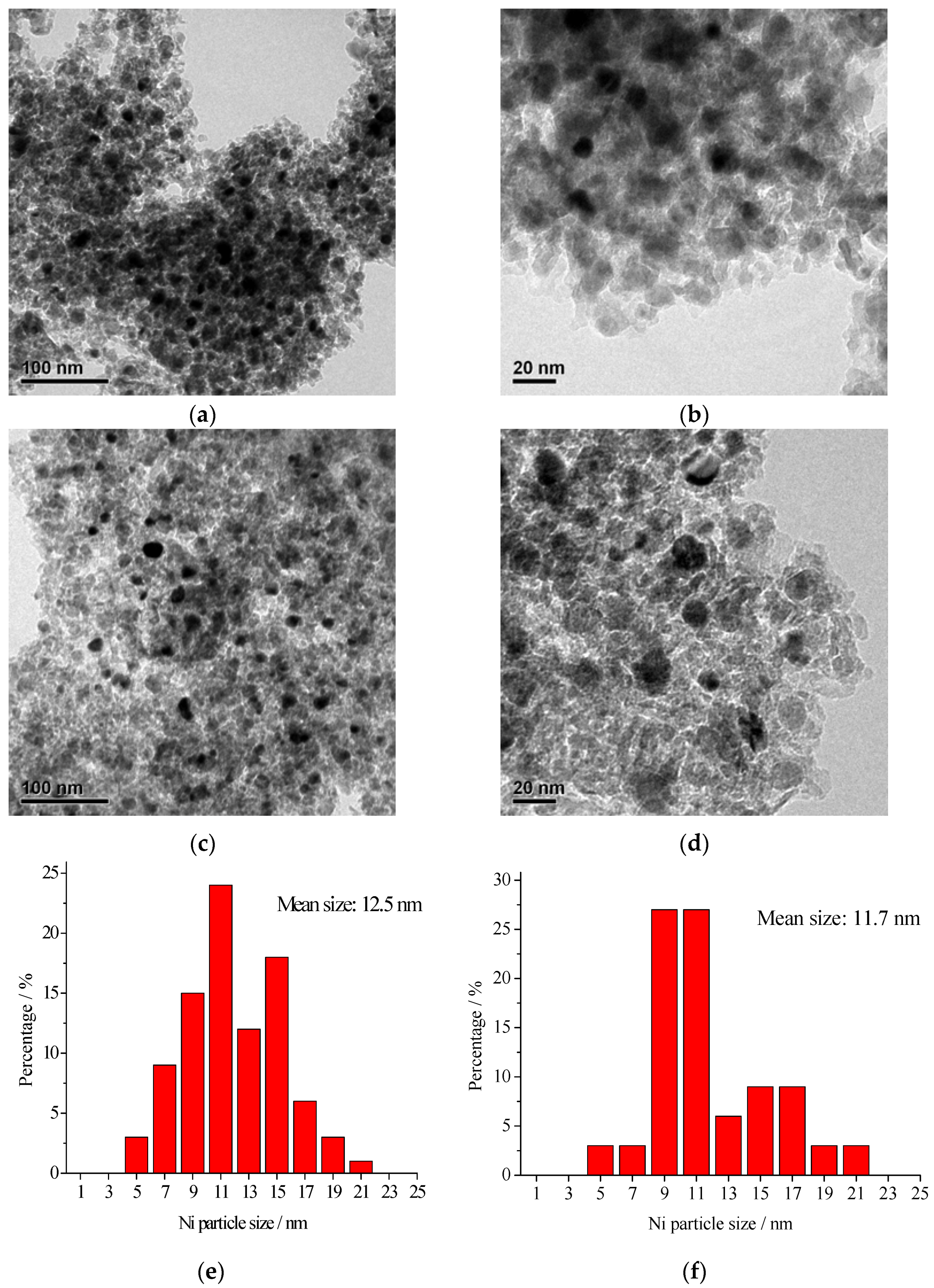
2.2. Catalytic Activity and Stability
| Catalyst | Solvent | T/°C | H2 P/MPa | Time/h | Conversion/% | Yield/% | Selectivity/% |
|---|---|---|---|---|---|---|---|
| 10%Ni/Al2O3 | water | 200 | 3 | 3 | 28.8 | 26.5 | 92.2 |
| 20%Ni/Al2O3 | water | 200 | 3 | 3 | 94.0 | 88.0 | 93.6 |
| 30%Ni/Al2O3 | water | 200 | 3 | 3 | 96.2 | 86.9 | 90.3 |
| 40%Ni/Al2O3 | water | 200 | 3 | 3 | 98.2 | 93.4 | 95.1 |
| 10%Ni/Al2O3 | dioxane | 180 | 3 | 2 | 68.6 | 66.2 | 96.5 |
| 20%Ni/Al2O3 | dioxane | 180 | 3 | 2 | 96.0 | 94.8 | 98.8 |
| 30%Ni/Al2O3 | dioxane | 180 | 3 | 2 | 100.0 | 98.3 | 98.3 |
| 40%Ni/Al2O3 | dioxane | 180 | 3 | 2 | 100.0 | 99.2 | 99.2 |
| 10%Ni/Al2O3 | dioxane | 180 | 3 | 1 | 40.7 | 39.7 | 97.5 |
| 20%Ni/Al2O3 | dioxane | 180 | 3 | 1 | 57.3 | 55.2 | 96.3 |
| 30%Ni/Al2O3 | dioxane | 180 | 3 | 1 | 77.2 | 74.8 | 96.9 |
| 40%Ni/Al2O3 | dioxane | 180 | 3 | 1 | 82.2 | 80.7 | 98.2 |

2.3. Effects of Temperature, Hydrogen Pressure, and Substrate/Catalyst Ratio
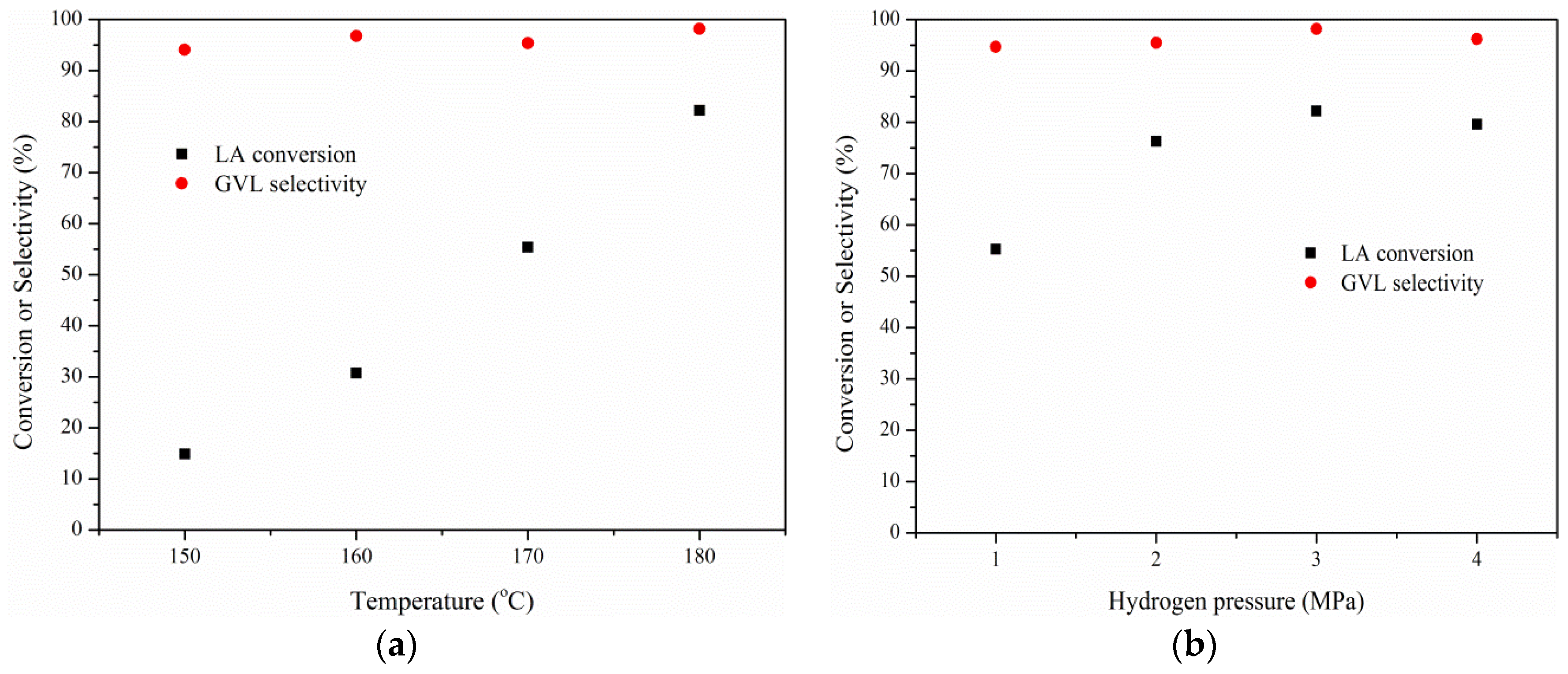
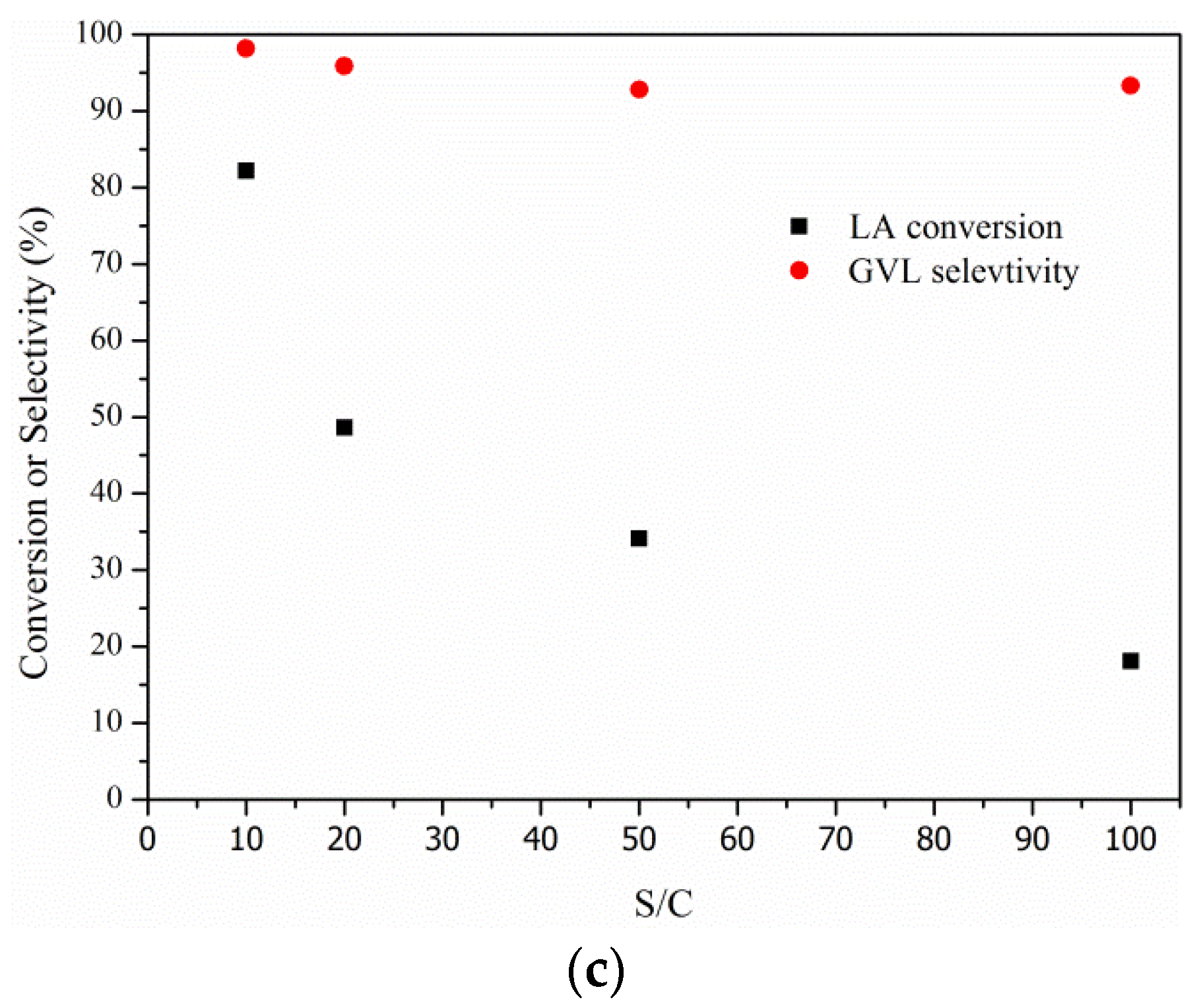
3. Experimental Section
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Experimental Procedure
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry catalysts and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Ruiz, J.C.; West, R.M.; Dumesic, J.A. Catalytic conversion of renewable biomass resources to fuels and chemicals. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 79–100. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Jing, Q.; Lü, X. Kinetics of non-catalyzed decomposition of glucose in high-temperature liquid Water. Chin. J. Chem. Eng. 2008, 16, 890–894. [Google Scholar] [CrossRef]
- Yang, F.; Fu, J.; Mo, J.; Lu, X. Synergy of Lewis and Brønsted acids on catalytic hydrothermal decomposition of hexose to levulinic acid. Energy Fuels 2013, 27, 6973–6978. [Google Scholar] [CrossRef]
- Bui, L.; Luo, H.; Gunther, W.R.; Román-Leshkov, Y. Domino reaction catalyzed by zeolites with Bronsted and Lewis acid sites for the production of gamma-valerolactone from furfural. Angew. Chem. Int. Ed. 2013, 52, 8022–8025. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone—A sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Bozell, J.J. Connecting biomass and petroleum processing with a chemical bridge. Science 2010, 329, 522–523. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated catalytic conversion of γ-valerolactone to liquid alkenes for transportation fuels. Science 2010, 327, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.; Filonenko, G.A.; van Putten, R.; Hensen, E.J.; Pidko, E.A. Heterogeneous and homogeneous catalysis for the hydrogenation of carboxylic acid derivatives: History, advances and future directions. Chem. Soc. Rev. 2015, 44, 3808–3833. [Google Scholar] [CrossRef] [PubMed]
- Liguori, F.; Moreno-Marrodan, C.; Barbaro, P. Environmentally friendly synthesis of γ-valerolactone by direct catalytic conversion of renewable sources. ACS Catal. 2015, 5, 1882–1894. [Google Scholar] [CrossRef]
- Upare, P.P.; Lee, J.; Hwang, D.W.; Halligudi, S.B.; Hwang, Y.K.; Chang, J. Selective hydrogenation of levulinic acid to γ-valerolactone over carbon-supported noble metal catalysts. J. Ind. Eng. Chem. 2011, 17, 287–292. [Google Scholar] [CrossRef]
- Manzer, L.E. Catalytic synthesis of α-methylene-γ-valerolactone: A biomass-derived acrylic monomer. Appl. Catal. A 2004, 272, 249–256. [Google Scholar] [CrossRef]
- Yan, Z.; Lin, L.; Liu, S. Synthesis of gamma-valerolactone by hydrogenation of biomass-derived levulinic acid over RU/C catalyst. Energy Fuels 2009, 23, 3853–3858. [Google Scholar] [CrossRef]
- Al-Shaal, M.G.; Wright, W.R.H.; Palkovits, R. Exploring the ruthenium catalysed synthesis of γ-valerolactone in alcohols and utilisation of mild solvent-free reaction conditions. Green Chem. 2012, 14, 1260–1263. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; Antonetti, C.; De Luise, V.; Martinelli, M. A sustainable process for the production of γ-valerolactone by hydrogenation of biomass-derived levulinic acid. Green Chem. 2012, 14, 688–694. [Google Scholar] [CrossRef]
- Ortiz-Cervantes, C.; García, J.J. Hydrogenation of levulinic acid to γ-valerolactone using ruthenium nanoparticles. Inorg. Chim. Acta 2013, 397, 124–128. [Google Scholar] [CrossRef]
- Du, X.; Liu, Y.; Wang, J.; Cao, Y.; Fan, K. Catalytic conversion of biomass-derived levulinic acid into γ-valerolactone using iridium nanoparticles supported on carbon nanotubes. Chin. J. Catal. 2013, 34, 993–1001. [Google Scholar] [CrossRef]
- Luo, W.; Sankar, M.; Beale, A.M.; He, Q.; Kiely, C.J.; Bruijnincx, P.C.A.; Weckhuysen, B.M. High performing and stable supported nano-alloys for the catalytic hydrogenation of levulinic acid to γ-valerolactone. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Hengne, A.M.; Rode, C.V. Cu-ZrO2 nanocomposite catalyst for selective hydrogenation of levulinic acid and its ester to γ-valerolactone. Green Chem. 2012, 14, 1064–1072. [Google Scholar] [CrossRef]
- Yuan, J.; Li, S.; Yu, L.; Liu, Y.; Cao, Y.; He, H.; Fan, K. Copper-based catalysts for the efficient conversion of carbohydrate biomass into γ-valerolactone in the absence of externally added hydrogen. Energy Environ. Sci. 2013, 6, 3308–3313. [Google Scholar] [CrossRef]
- Upare, P.P.; Lee, J.; Hwang, Y.K.; Hwang, D.W.; Lee, J.; Halligudi, S.B.; Hwang, J.; Chang, J. Direct hydrocyclization of biomass-derived levulinic acid to 2-methyltetrahydrofuran over nanocomposite copper/silica catalysts. ChemSusChem 2011, 4, 1749–1752. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Chen, A. Efficient hydrogenation of biomass-derived furfural and levulinic acid on the facilely synthesized noble-metal-free Cu-Cr catalyst. Energy 2013, 58, 357–363. [Google Scholar] [CrossRef]
- Yan, K.; Chen, A. Selective hydrogenation of furfural and levulinic acid to biofuels on the ecofriendly Cu-Fe catalyst. Fuel 2014, 115, 101–108. [Google Scholar] [CrossRef]
- Obregón, I.; Corro, E.; Izquierdo, U.; Requies, J.; Arias, P.L. Levulinic acid hydrogenolysis on Al2O3-based Ni-Cu bimetallic catalysts. Chin. J. Catal. 2014, 35, 656–662. [Google Scholar] [CrossRef]
- Shimizu, K.; Kanno, S.; Kon, K. Hydrogenation of levulinic acid to γ-valerolactone by Ni and MoOx co-loaded carbon catalysts. Green Chem. 2014, 16, 3899–3903. [Google Scholar] [CrossRef]
- Hengst, K.; Schubert, M.; Carvalho, H.W.P.; Lu, C.; Kleist, W.; Grunwaldt, J. Synthesis of γ-valerolactone by hydrogenation of levulinic acid over supported nickel catalysts. Appl. Catal. A 2015, 502, 18–26. [Google Scholar] [CrossRef]
- Mohan, V.; Venkateshwarlu, V.; Pramod, C.V.; Raju, B.D.; Rao, K.S.R. Vapour phase hydrocyclisation of levulinic acid to γ-valerolactone over supported in catalysts. Catal. Sci. Technol. 2014, 4, 1253–1259. [Google Scholar] [CrossRef]
- Lv, J.; Rong, Z.; Wang, Y.; Xiu, J.; Wang, Y.; Qu, J. Highly efficient conversion of biomass-derived levulinic acid into γ-valerolactone over Ni/MgO catalyst. RSC Adv. 2015, 5, 72037–72045. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Solvents; ChemTec. Publishing: New York, NY, USA, 2001; pp. 754–757. [Google Scholar]
- Crozier, T.E.; Yamamoto, S. Solubility of hydrogen in water, sea water, and sodium chloride solutions. J. Chem. Eng. Data 1974, 19, 242–244. [Google Scholar] [CrossRef]
- Brunner, E. Solubility of hydrogen in 10 organic solvents at 298.15, 323.15, and 373.15 K. J. Chem. Eng. Data 1985, 30, 269–273. [Google Scholar] [CrossRef]
- Rothaemel, M.; Hanssen, K.F.; Blekkan, E.A.; Schanke, D.; Holmen, A. The effect of water on cobalt Fischer-Tropsch catalysts studied by steady-state isotopic transient kinetic analysis (SSITKA). Catal. Today 1997, 38, 79–84. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, J.; Sheng, D.; Lu, X. Hydrogenation of Levulinic Acid over Nickel Catalysts Supported on Aluminum Oxide to Prepare γ-Valerolactone. Catalysts 2016, 6, 6. https://doi.org/10.3390/catal6010006
Fu J, Sheng D, Lu X. Hydrogenation of Levulinic Acid over Nickel Catalysts Supported on Aluminum Oxide to Prepare γ-Valerolactone. Catalysts. 2016; 6(1):6. https://doi.org/10.3390/catal6010006
Chicago/Turabian StyleFu, Jie, Dong Sheng, and Xiuyang Lu. 2016. "Hydrogenation of Levulinic Acid over Nickel Catalysts Supported on Aluminum Oxide to Prepare γ-Valerolactone" Catalysts 6, no. 1: 6. https://doi.org/10.3390/catal6010006
APA StyleFu, J., Sheng, D., & Lu, X. (2016). Hydrogenation of Levulinic Acid over Nickel Catalysts Supported on Aluminum Oxide to Prepare γ-Valerolactone. Catalysts, 6(1), 6. https://doi.org/10.3390/catal6010006






