Abstract
A series of metal cation modified layered-double hydroxides (LDHs) and mixed oxides were prepared and used to be the selective oxidation of toluene with O2. The results revealed that the modified LDHs exhibited much higher catalytic performance than their parent LDH and the modified mixed oxides. Moreover, the metal cations were also found to play important roles in the catalytic performance and stabilities of modified catalysts. Under the optimal reaction conditions, the highest toluene conversion reached 8.7% with 97.5% of the selectivity to benzyldehyde; moreover, the catalytic performance remained after nine catalytic runs. In addition, the reaction probably involved a free-radical mechanism.
1. Introduction
The selective oxidation of alkanes is described as ”the Holy Grail of organic chemistry”, and is accepted as a very challenging problem. Toluene, as the simplest member of alkyl aromatics, can be oxidized into benzyl alcohol, benzyldehyde, benzoic acid, benzyl benzoate, etc. These oxidation products were wildly used in dyes, solvents, perfumery, plasticizer, preservatives, flame retardant and other fields [1,2,3,4]. Recently, with the increasing of demand for chlorine-free benzyldehyde in perfumery and pharmaceutical industries, the traditional toluene chlorination hydrolysis had been unable to meet needs [3,4]. Thus, the selective oxidation of toluene side chain attracted much attention in academia and industry. However, due to the inertia of toluene to oxidation reaction and the presence of side reactions such as deep oxidation and ring-hydroxylation, the conversion of toluene and the selectivity to object product were often low, and the atomic economy was poor. Therefore, in recent years, much effort has been paid to explore the high efficient oxidation process for toluene.
Among all the processes of selective oxidation of toluene, the liquid phase oxidation process with O2, especially in the absence of organic solvent, was eye-catching due to the ”green” and mild reaction conditions as well as high selectivity to object product, and various catalytic systems have been reported [5,6,7,8,9,10,11,12]. Xu et al. used CuFe/γ-Al2O3 to the selective oxidation of toluene with O2, and the 7.4% of toluene conversion and 46.5% of selectivity to benzyldehy were obtained [5]. Mac Leod et al. reported that Mn (salen) could catalytically oxidize toluene at room temperature with acetonitrile as slolvent, the benzyldehyde selectivity could reach 98%, but the toluene conversion was only 5% [6]. Kesavan et al. found that Au-Pd/C showed effective catalytic activity to the selective oxidation of toluene under solvent-free conditions. The conversion of toluene reached 94.4%, but the main product was benzyl benzoate, and the selectivity to benzyldehyde was less than 15% [7]. Thus, there was still an urgent need to develop effective catalysts for selective oxidation of toluene to benzyldehyde.
The solid Mg-Al hydrotalcites (HT) and their calcined products of mixed oxides were often considered as promising catalysts or catalyst supports owing to their perfectly adjustable surface basicity, along with high surface area and thermal stability. Some cation and anion modified Mg-Al hydrotalcites or mixed oxides had been prepared for aromatization, condensation, cyanoethylation, cyclohexene dehydration, alcohol oxidation, etc. [13,14,15,16,17]. However, to the best of our knowledge, there are few reports on the design of cation modified Mg-Al hydrotalcites and mixed oxides for the selective oxidation of toluene. Here, we reported the efficient solvent-free selective oxidation of toluene with O2 catalyzed by nine kinds of transition metal cation, seven kinds of rare-earth metal catoin and two kinds of noble metal cation modified layered-double hydroxides (LDHs) as well as their corresponding mixed oxides. The roles of the metal cations in the catalytic performance and stabilities of modified LDHs and their mixed oxides were discussed in detail.
2. Results and Discussion
2.1. Characterization of Catalysts
The 18 kinds of metal modified LDH obtained and 18 kinds of metal modified mixed oxides were characterized by X-ray diffraction (XRD), elemental analysis, N2 sorption and thermogravimetric analysis (TGA) techniques. Considering the similarity of characterization chart, Ti3+/Mg3Al-LDH and Ti/Mg3(Al)O were selected as the representative catalysts for metal cation modified hydrotalcites and mixed oxides, respectively.
XRD patterns of Ti3+/Mg3Al-HT showed typical layered material containing sharp and intense lines at low 2 theta values and less intense asymmetric lines at higher 2 theta angular values (see Figure 1). Three sharp and asymmetrical peaks of (003), (006) and (110) planes appeared, suggesting the formation of a highly crystalline material [17]. For Ti/Mg3(Al)O, the planes of 003 and 006 in hydrotalcites disappeared, while two peaks at about 43° and 63° due to the MgAl mixed oxides and two peaks at about 25° and 36° related to TiO2 were detected. This indicated that the hydrotalcite-like structures were destructed and new phases of mixed oxides appeared during calcination.
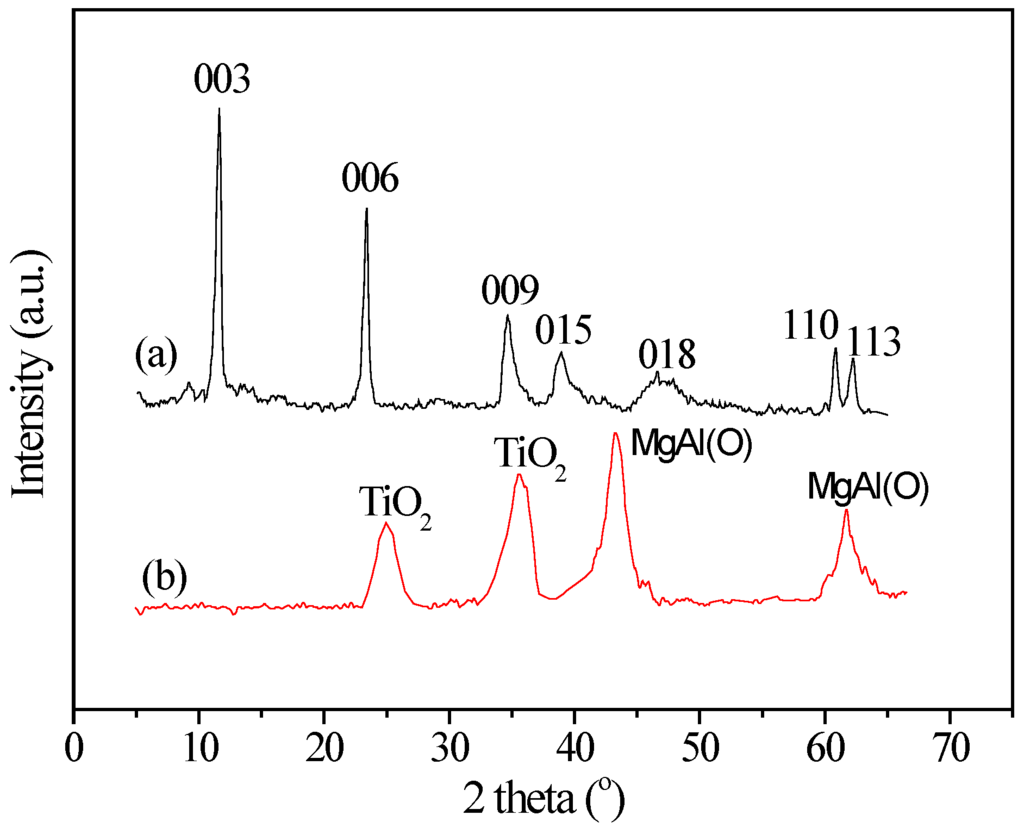
Figure 1.
XRD patterns of (a) Ti3+/Mg3Al-LDH; (b) Ti/Mg3(Al)O.
Chemical analysis of metal cation modified LDHs showed almost identical molar ratios of Mg, Al and the third metal (see Table 1 and Table 2). This indicated that the introduction of metal cations did not change the elemental composition of their parent LDH. Simultaneously, no significant change in the molar ratio of Mg, Al and the third metal was found before and after the calcination, suggesting that no loss took place during calcination.
TGA curves of Ti3+/Mg3Al-LDH were typically illustrated in Figure 2. Two major weight losses appeared at about 100 °C and 400 °C, respectively. The former could be attributed to the elimination of physically adsorbed and interlayer water, while the latter was related to the dehydroxylation of the layers. This indicated that Ti/Mg3(Al)O could be successfully prepared by the complete decomposition of the Ti3+/Mg3Al-LDH at 450 °C.

Table 1.
Catalytic performance of metal cation modified layered-double hydroxides (LDHs) in toluene oxidation a.
| Entry | Catalysts | Metal Cation Radius (Å) | Mg/Al/M Molar Ratio | SBET (m2/g) | Toluene Con. (mol %) | Sel. (mol %) | ||
|---|---|---|---|---|---|---|---|---|
| Benzyl Alcohol | Benzyldehyde | Benzoic Acid | ||||||
| 1 | blank | --- e | --- | --- | 0 | 0 | 0 | 0 |
| 2 | Mg3Al-LDH | --- | --- | 92 | 1.1 | 4.2 | 93.6 | 2.2 |
| 3 | Ti3+/Mg3Al-LDH | 0.76 | 2.93/1.0/0.017 | 87 | 8.7 | 2.5 | 97.5 | 0 |
| 4 | Ti3+/Mg3Al-LDH b | ditto | ditto | ditto | 1.2 | 3.2 | 87.5 | 9.3 |
| 5 | Ti3+/Mg3Al-LDH c | ditto | ditto | ditto | 1.6 | 5.6 | 83.0 | 11.4 |
| 6 | Ti3+/Mg3Al-LDH d | ditto | ditto | ditto | 0.7 | 10.2 | 76.3 | 13.5 |
| 7 | Cr3+/Mg3Al-LDH | 0.69 | 2.93/1.0/0.018 | 85 | 6.8 | 3.8 | 95.2 | 1.0 |
| 8 | Mn2+/Mg3Al-LDH | 0.80 | 2.93/1.0/0.015 | 87 | 2.8 | 6.1 | 91.8 | 2.1 |
| 9 | Fe2+/Mg3Al-LDH | 0.76 | 2.93/1.0/0.017 | 86 | 1.6 | 5.3 | 93.5 | 1.2 |
| 10 | Fe3+/Mg3Al-LDH | 0.64 | 2.93/1.0/0.018 | 86 | 5.2 | 6.5 | 92.0 | 1.5 |
| 11 | Co2+/Mg3Al-LDH | 0.74 | 2.93/1.0/0.016 | 83 | 2.8 | 4.3 | 93.3 | 1.4 |
| 12 | Ni2+/Mg3Al-LDH | 0.72 | 2.93/1.0/0.016 | 85 | 3.9 | 7.8 | 91.4 | 0.8 |
| 13 | Cu2+/Mg3Al-LDH | 0.69 | 2.93/1.0/0.018 | 87 | 3.2 | 8.5 | 91.5 | 0 |
| 14 | Zn2+/Mg3Al-LDH | 0.74 | 2.93/1.0/0.017 | 84 | 2.3 | 5.4 | 92.4 | 2.2 |
| 15 | La3+/Mg3Al-LDH | 1.06 | 2.93/1.0/0.016 | 86 | 6.5 | 3.5 | 95.2 | 1.3 |
| 16 | Nd3+/Mg3Al-LDH | 0.99 | 2.93/1.0/0.017 | 86 | 5.2 | 5.3 | 92.7 | 2.0 |
| 17 | Sm3+/Mg3Al-LDH | 0.96 | 2.93/1.0/0.017 | 85 | 4.7 | 5.7 | 92.5 | 1.8 |
| 18 | Gd3+/Mg3Al-LDH | 0.94 | 2.93/1.0/0.015 | 82 | 3.8 | 5.2 | 94.0 | 0.8 |
| 19 | Dy3+/Mg3Al-LDH | 0.91 | 2.93/1.0/0.016 | 87 | 4.0 | 5.1 | 93.5 | 1.4 |
| 20 | Ho3+/Mg3Al-LDH | 0.89 | 2.93/1.0/0.018 | 86 | 5.0 | 4.9 | 94.2 | 0.9 |
| 21 | Yb3+/Mg3Al-LDH | 0.86 | 2.93/1.0/0.017 | 88 | 4.4 | 5.3 | 93.6 | 1.1 |
| 22 | Au+/Mg3Al-LDH | 1.37 | 2.93/1.0/0.018 | 86 | 9.2 | 3.2 | 96.5 | 0.3 |
| 23 | Au+/Pd2+/Mg3Al-LDH | 1.37/0.86 | 2.93/1.0/0.018 | 86 | 9.5 | 4.0 | 95.0 | 1.0 |
a Reaction conditions: catalyst 0.18 g; toluene 0.1 mol; Oxygen pressure1 MPa, temperature 150 °C; time 8 h; b Adding pyrocatechol (0.02 mol); c adding resorcinol (0.02 mol); d adding hydroquinone (0.02 mol); e “---” means no detection.

Table 2.
Catalytic performance of metal modified mixed oxides in toluene oxidation a.
| Entry | Catalysts | Mg/Al/M Molar Ratio | SBET (m2/g1) | Toluene Con. (mol %) | Sel. (mol %) | ||
|---|---|---|---|---|---|---|---|
| Benzyl Alcohol | Benzyldehyde | Benzoic Acid | |||||
| 1 | Mg3(Al)O | ---b | 210 | 0.5 | 10.0 | 87.5 | 2.5 |
| 2 | Ti/Mg3(Al)O | 2.93/1.0/0.016 | 205 | 3.5 | 11.1 | 86.2 | 2.7 |
| 3 | Cr/Mg3(Al)O | 2.93/1.0/0.016 | 207 | 3.7 | 11.5 | 85.3 | 3.2 |
| 4 | Mn/Mg3(Al)O | 2.93/1.0/0.015 | 204 | 2.0 | 12.5 | 82.7 | 4.7 |
| 5 | Fe/Mg3(Al)O | 2.93/1.0/0.016 | 205 | 0.8 | 11.2 | 84.0 | 4.8 |
| 6 | Fe/Mg3(Al)O | 2.93/1.0/0.015 | 205 | 2.9 | 13.2 | 80.8 | 6.0 |
| 7 | Co/Mg3(Al)O | 2.93/1.0/0.017 | 207 | 1.7 | 12.0 | 82.5 | 5.5 |
| 8 | Ni/Mg3(Al)O | 2.93/1.0/0.015 | 206 | 1.0 | 10.6 | 87.5 | 1.9 |
| 9 | Cu/Mg3(Al)O | 2.93/1.0/0.016 | 206 | 1.3 | 9.2 | 86.2 | 4.6 |
| 10 | Zn/Mg3(Al)O | 2.93/1.0/0.017 | 205 | 0.9 | 11.5 | 85.5 | 3.0 |
| 11 | La/Mg3(Al)O | 2.93/1.0/0.017 | 204 | 3.2 | 12.8 | 83.5 | 3.7 |
| 12 | Nd/Mg3(Al)O | 2.93/1.0/0.016 | 206 | 2.5 | 11.8 | 82.0 | 6.2 |
| 13 | Sm/Mg3(Al)O | 2.93/1.0/0.015 | 205 | 2.7 | 9.6 | 84.4 | 5.8 |
| 14 | Gd/Mg3(Al)O | 2.93/1.0/0.016 | 205 | 2.1 | 9.5 | 86.0 | 4.5 |
| 15 | Dy/Mg3(Al)O | 2.93/1.0/0.017 | 206 | 2.0 | 8.9 | 88.2 | 2.9 |
| 16 | Ho/Mg3(Al)O | 2.93/1.0/0.016 | 206 | 2.2 | 10.2 | 87.5 | 2.3 |
| 17 | Yb/Mg3(Al)O | 2.93/1.0/0.016 | 204 | 2.9 | 8.9 | 88.0 | 3.1 |
| 18 | Au/Mg3(Al)O | 2.93/1.0/0.017 | 203 | 5.5 | 9.8 | 86.7 | 3.5 |
| 19 | Au/Pd/Mg3(Al)O | 2.93/1.0/0.016 | 205 | 5.0 | 10.3 | 85.5 | 4.2 |
a Reaction conditions: catalyst 0.18 g; toluene 0.1 mol; Oxygen pressure1 MPa, temperature 150 °C; time 8 h; b “---” means no detection.
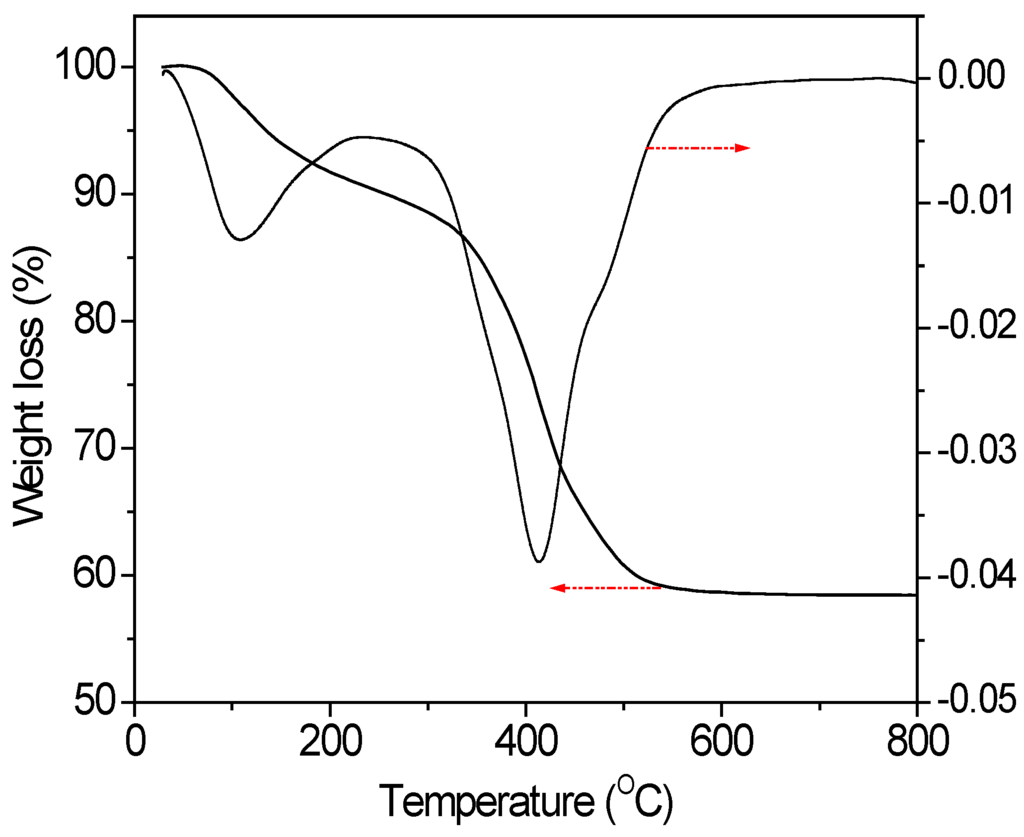
Figure 2.
Thermogravimetric weight loss curve and derivative plots of Ti3+/Mg3Al-LDH.
The N2 adsorption-desorption isotherms of Ti3+/Mg3Al-LDH and Ti/Mg3(Al)O both displayed type IV isotherms with clear hysteresis loops associated with capillary condensation (see Figure 3). This indicated that the mesoporous structures formed, probably because of particle aggregation [18]. Moreover, compared to Ti3+/Mg3Al-LDH, Ti/Mg3(Al)O showed a much higher surface area (see Table 1 and Table 2), which might be associated with the evolving of a large amount of CO2 from the Ti3+/Mg3Al-LDH during calcination.
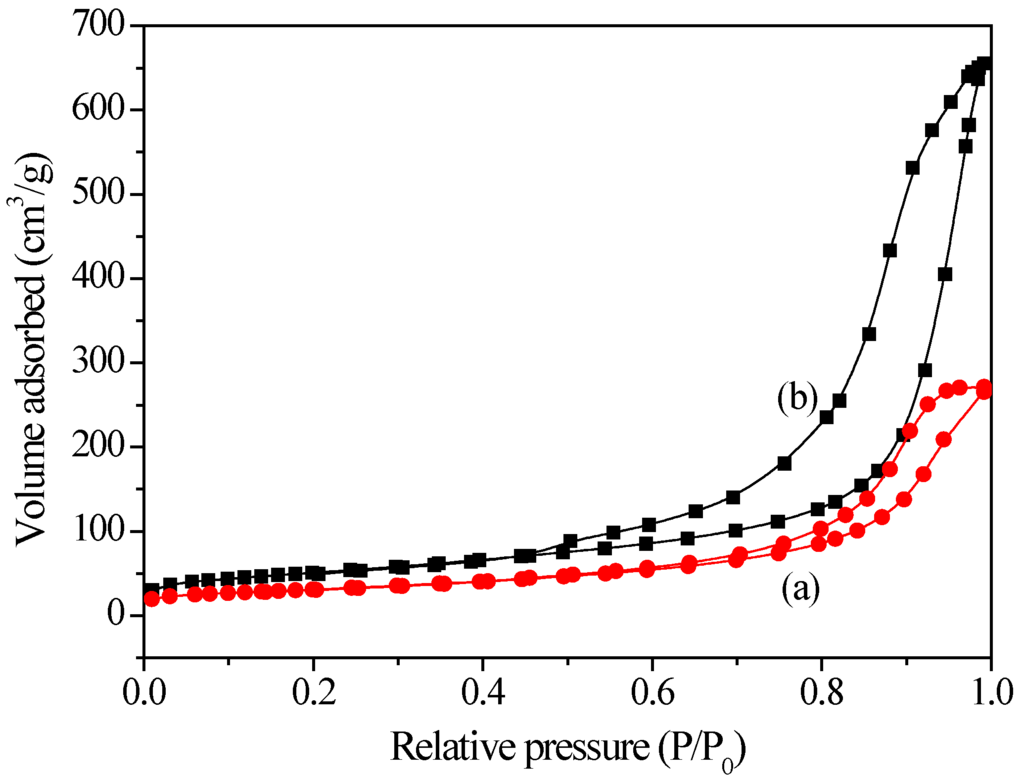
Figure 3.
N2 adsorption-desorption isotherms of (a) Ti3+/Mg3Al-LDH; (b) Ti/Mg3(Al)O.
2.2. Catalytic Performance of Metal Cation Modified LDHs
Without any organic solvent, phase transfer catalyst or additive, the obtained catalysts were used in the selective oxidation of toluene. Under the present reaction conditions, only three products—benzyl alcohol, benzyldehyde and benzoic acid—were detected.
Overall, compared to the parent Mg3Al-LDH, all metal cation modified LDHs showed enhanced catalytic performance, indicating that it was feasible to tune the catalytic performance of LDHs by introducing metal cations. Among the nine kinds of transition metal cation modified LDHs, trivalent cation modified LDHs exhibited much higher catalytic performance than divalent cation modified ones (see Entries 3–14 in Table 1). On the LDH laminates with trivalent metal cations, more surface –OH were decomposed to OH− to balance the excessive positive charge [19,20]. This led to the enhanced surface basicity and could afford the higher catalytic performance of trivalent cation modified LDHs. For the divalent metal modified catalysts, when the metal cations were Ni2+ or Cu2+ with the similar ionic radius to Mg2+, the obtained catalysts showed relatively high catalytic performance in the selective oxidation of toluene. This could be attributed to the substitution of Mg2+ in hydrotalcite lattice by Ni2+ or Cu2+ in the process of catalyst preparation, which induced the increased disorder on hydrotalcite laminates and made more catalytic activity “exposure”. In the present reaction conditions, Ti3+/Mg3Al-LDH exhibited best catalytic performance, probably because the introduction of Ti3+ with larger ionic radius to LDHs promoted the decomposition of surface –OH to OH− due to its relatively weak binding capacity.
Moreover, seven kinds of rare-earth metal cation modified LDHs were more active in the selective oxidation of toluene than most of the transition metal cation modified LDHs except Ti3+/Mg3Al-LDH, which could be related to their larger cation radius. Among them, La3+/Mg3Al-LDH exhibited the highest toluene conversion of 6.5%, with the selectivity of 95.2% to benzyldehyde.
For comparison, two kinds of noble metal modified LDHs were also prepared for the toluene oxidation. Au+/Pd2+/Mg3Al-LDH exhibited much higher catalytic performance than Au+/Mg3Al-LDH, which could be attributed to the synergistic catalytic effect of Pd2+ and Au+, similar phenomena had also been reported previously [7].
2.3. Catalytic Performance of Metal Modified Mixed Oxides
Compared to LDHs, the same metal modified mixed oxides showed lower catalytic performance. During calcination, as described in the TGA experiment, the dehydroxylation of layer –OH on hydrotalcite laminates took place, so the amount of surface B basic sites reduced. This indicated that B basic sites derived from the surface –OH played an important role in the catalytic performance of modified LDHs. In addition, compared to their parent Mg3(Al)O, the metal modified mixed oxides showed slightly higher catalytic performance. Among all modified mixed oxides, noble metal modified mixed oxides exhibited highest catalytic performance, simultaneously, inexpensive Ti3+, Cr3+ and La3+ modified mixed oxides were also effective in toluene oxidation. Moreover, the trivalent metal modified mixed oxides also exhibited much higher catalytic performance than divalent metal modified ones due to the relatively more surface B basic sites.
2.4. Stabilities of Catalysts
On the basis of the above experiments, the following three metal cation modified LDHs, which exhibited high catalytic performance in toluene oxidation, were selected as the representative catalysts to investigate their stabilities. After the first catalytic run, the catalysts were separated from the reaction system, washed several times with water to remove any physisorbed molecules, and then dried overnight. The obtained used catalysts were further used in another nine catalytic runs. The results in Figure 4 revealed that the three catalysts exhibited high stabilities in the present reaction conditions. Particularly, Ti3+/Mg3Al-LDH was still effective until being reused nine times. Chemical analysis revealed that the recovered Ti catalyst after nine times held almost the same elemental composition (Mg/Al/M molar ratio = 2.93/1.0/0.016) as the fresh catalyst (Mg/Al/M molar ratio = 2.93/1.0/0.017), indicating that metal leaching was negligible.
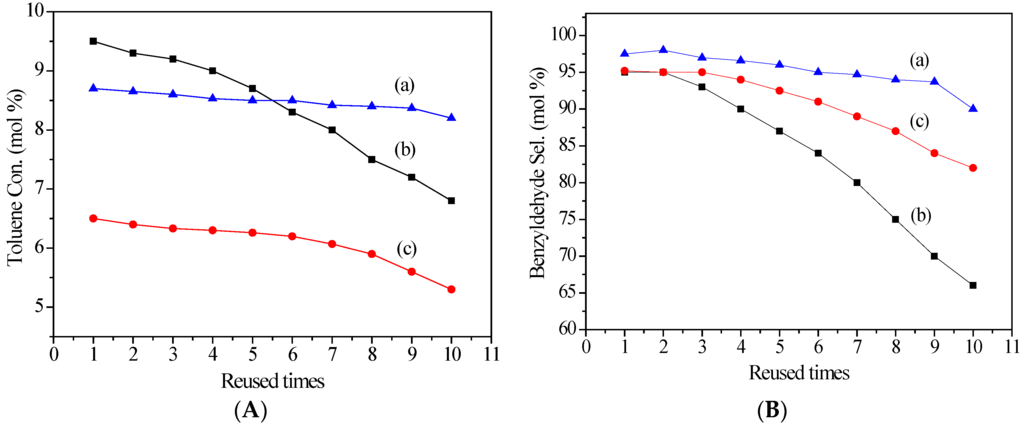
Figure 4.
Reusability of (a) Ti3+/Mg3Al-LDH; (b) Au+/Pd2+/Mg3Al-LDH; (c) La3+/Mg3Al-LDH of (A) Effect of reused times on the toluene conversion; (B) Eeffect of reused time on the benzyldehyde selectivity. Reaction conditions: catalyst 0.18 g; toluene 0.1 mol; Oxygen pressure1 MPa, temperature 150 °C; time 8 h.
2.5. Effect of Reaction Conditions on the Catalytic Performance of Catalyst
The effect of reaction conditions on the catalytic performance of catalysts was also investigated used Ti3+/Mg3Al-LDH as the representative catalyst. The reaction temperature was first investigated, and the results were shown in Figure 5. When the reaction was performed at less than 70 °C, no products were detected. With the increasing of temperature from 70 °C to 220 °C, the conversion of toluene increased continuously. This could be attributed to the increased contact possibility between reactant molecules and surface active sites of catalyst with the increased reaction temperature in multi phase catalytic system. Simultaneously, the selectivity to main product (benzyldehyde) was found to increase firstly and then decrease, while the selectivity to over oxidation product (benzoic acid) increased continuously. This indicated that a much too high reaction temperature was more conducive to over oxidation of benzyldehyde. The optimum reaction temperature was 150 °C. The effect of reaction time was illustrated in Figure 6. It was found that the toluene oxidation reacted fast in the present catalytic system, the reaction almost reached equilibrium for 8 h, and the further increase in reaction time was more favorable to the over oxidation of the obtained benzyldehyde (see Figure 6).
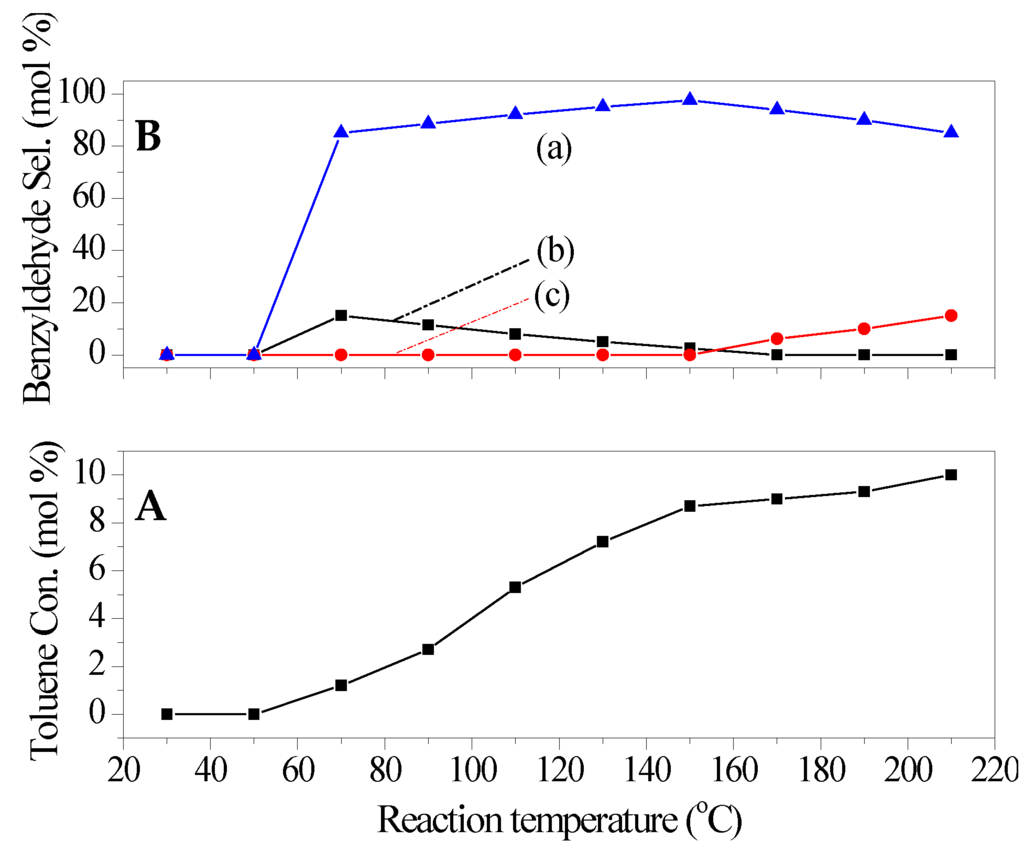
Figure 5.
Effect of reaction temperature on the catalytic performance of catalyst (a) benzyldehyde; (b) benzyl alcohol; (c) benzoic acid. (A) Toluene conversion (B) Benzyldehyde selectivity. Reaction conditions: catalyst 0.18 g, toluene 0.1 mol, oxygen pressure 1 MPa, time 8 h.
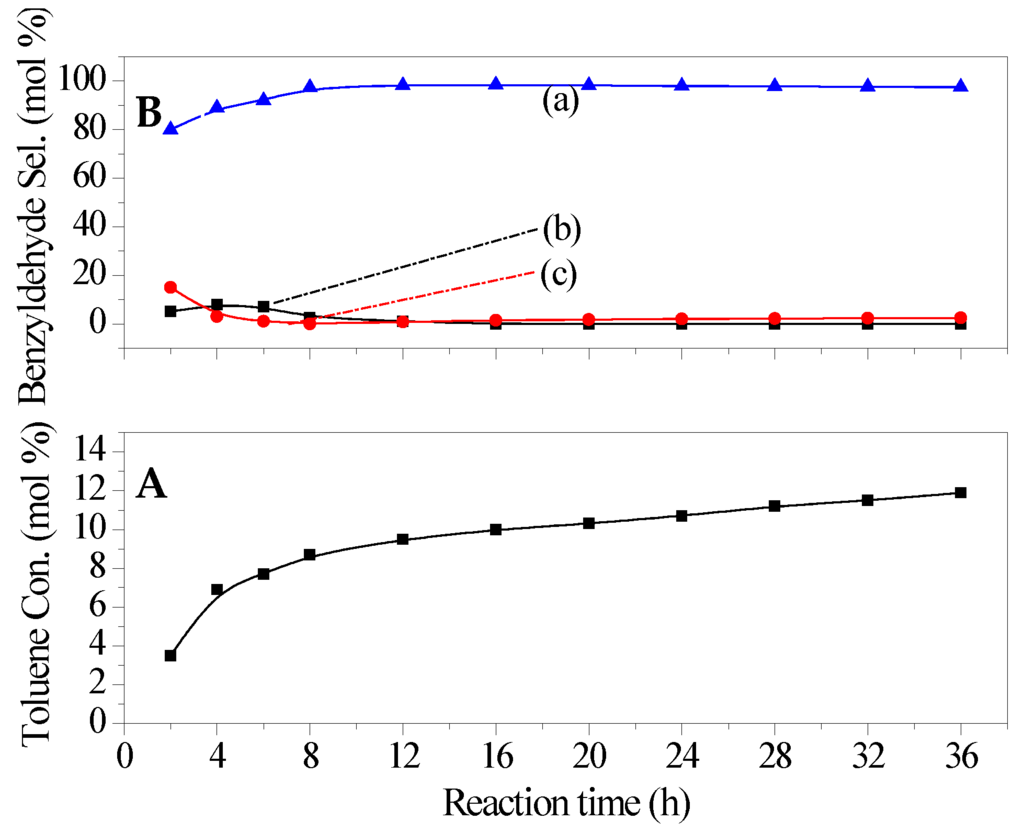
Figure 6.
Effect of reaction time on the catalytic performance of catalyst (a) benzyldehyde; (b) benzyl alcohol; (c) benzoic acid. (A) Toluene conversion (B) Benzyldehyde selectivity. Reaction conditions: catalyst 0.18 g, toluene 0.1 mol, Oxygen pressure 1 MPa, temperature 150 °C.
The dosage of O2 was also an important factor in the catalytic performance of the catalyst (see Figure 7). With the increase in the dosage of oxidant, toluene conversion and benzyldehyde selectivity both increased at the beginning stage, and then reached the maximum value at O2 pressure of 1 MPa. The further improvement in the O2 pressure led to a significant decrease in benzyldehyde selectivity, along with a slight increased toluene conversion. Meanwhile, the increased amount of benzoic acid were detected by GC. This indicated that the O2 pressure of 1 MPa was optimum in the present catalytic system.
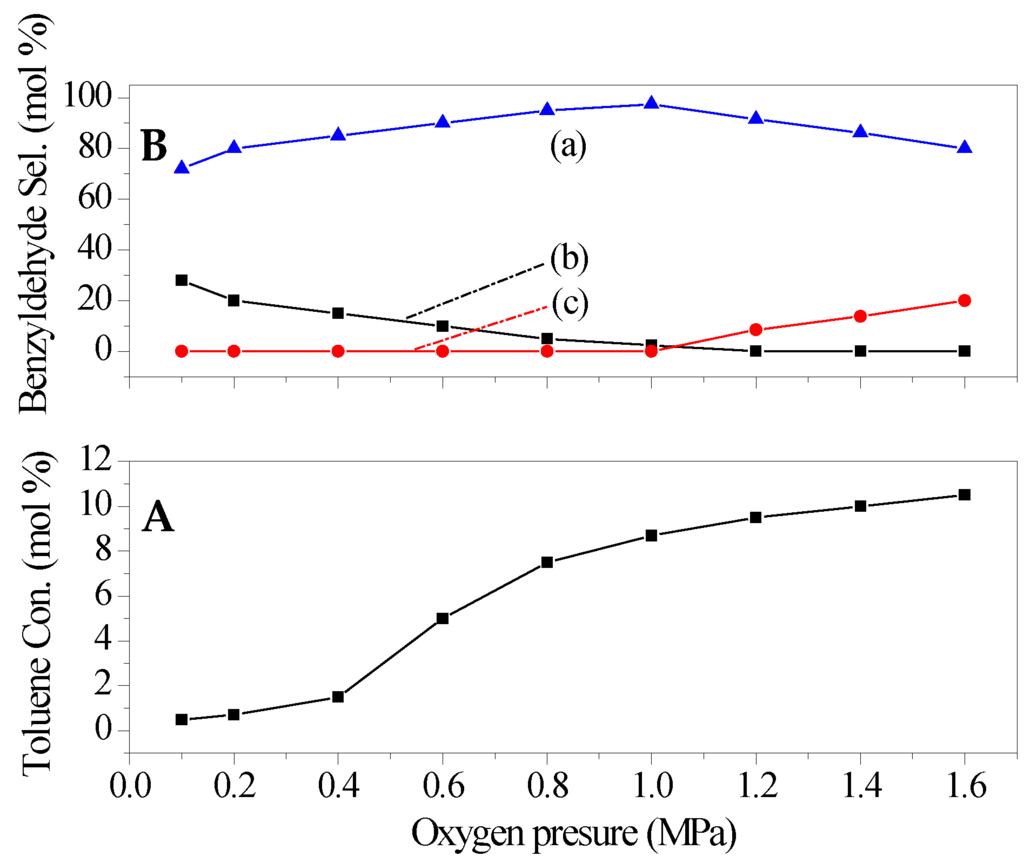
Figure 7.
Effect of O2 pressure on the catalytic performance of catalyst (a) benzyldehyde; (b) benzyl alcohol; (c) benzoic acid. (A) Toluene conversion (B) Benzyldehyde selectivity. Reaction conditions: catalyst 0.18 g, toluene 0.1 mol, temperature 150 °C, time 8 h.
The amount of catalyst was further investigated, and the results were shown in Figure 8. The toluene conversion was found to increase with the increased amount of catalyst, but the increasing trends became gradually slow. There was a turning point for the selectivity to benzyldehyde with the increasing of catalyst dosage. This could be related to the increased diffusion limitation originated from the increased amount of catalyst. Thus, the excess catalyst had an adverse effect on the selective oxidation of toluene. Through the above experiments, the best toluene conversion of 8.7% with 97.5% of the selectivity to benzyldehyde was achieved when the reaction was run for 8 h at 150 °C with O2 presure of 1 MPa.
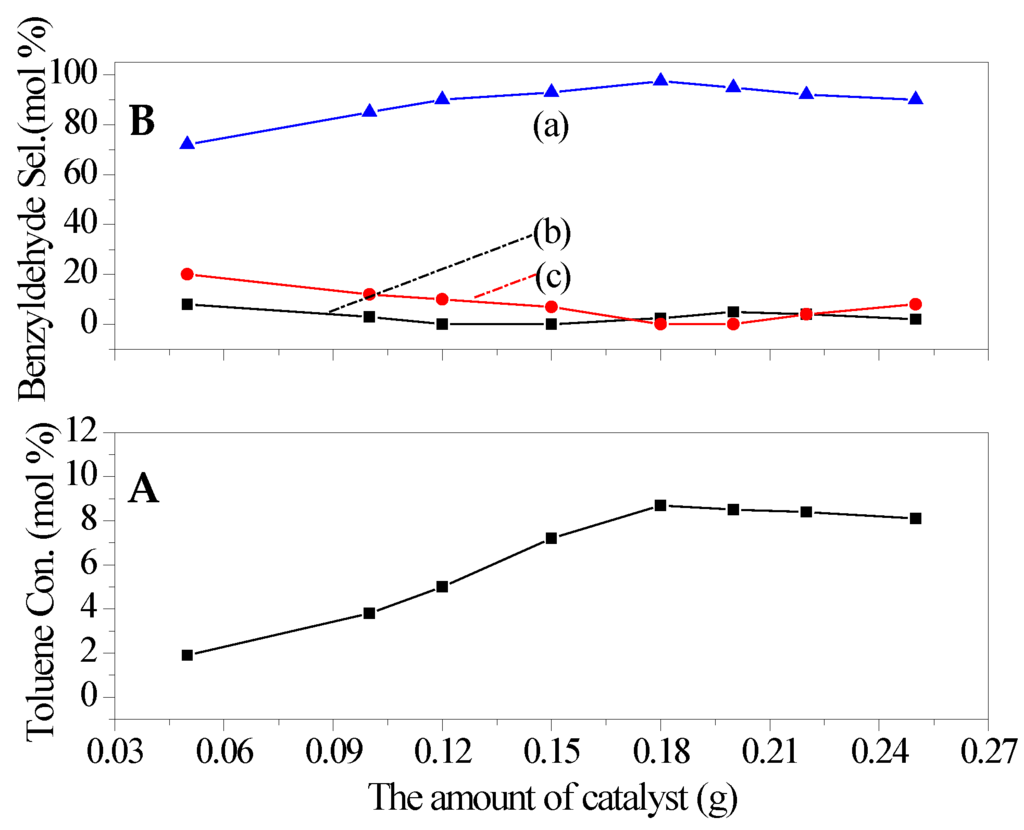
Figure 8.
Effect of catalyst dosage on the catalytic performance of catalyst (a) benzyldehyde; (b) benzyl alcohol; (c) benzoic acid. (A) Toluene conversion (B) Benzyldehyde selectivity. Reaction conditions: toluene 0.1 mol, Oxygen pressure1 MPa, temperature 150 °C, time 8 h.
2.6. Reaction Mechanism
The mechanistic probe for the selective oxidation of toluene with O2 was attracting continuous interests, and the prevailing accepted one was the free-radical mechanism. In the present investigation, in the presence of metal cation modified LDH, the inhibition experiments of radicals were designed by the use of three conventional radical scavengers (pyrocatechol, resorcinol and hydroquinone). A significant lowering of the toluene conversion was observed (see Table 1), further confirming the involvement of free-radical mechanism. Based on the above experiments and previous research on toluene oxidation [1,4], a proposed reaction mechanism was shown in Scheme 1. Namely, toluene was firstly converted into benzyl radical over catalyst. Then, an electron was transferred from benzyl radical to the catalyst, which led to the benzyl cation, and, simultaneously, the catalyst was regenerated to the original form. The benzyl cation was further transformed to benzyl alcohol. The formation of benzyldehyde was originated from the further combination of the benzyl radical with O2. According to the proposed mechanism, the pathway of forming benzaldehyde and benzyl alcohol was independent. This could afford the increasing selectivity to both benzaldehyde and benzyl alcohol before 6 h (see Figure 6).
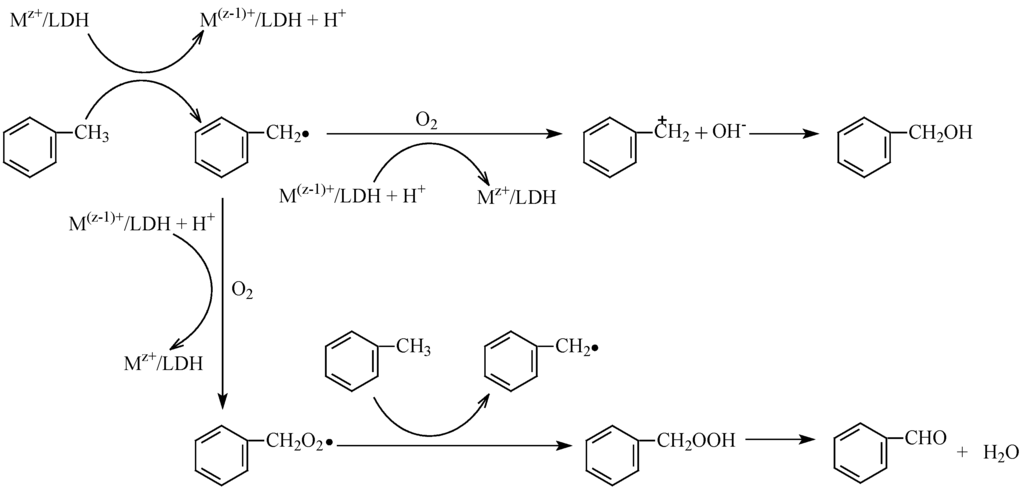
Scheme 1.
Possible reaction mechanism of the selective oxidation of toluene.
3. Experimental Section
3.1. Catalyst Preparation
Hydrotalcites containing CO32− (Mg3Al-LDH) was firstly prepared by a co-precipitation method and hydrothermal treatment as described in our previous report [21]. Then, the obtained Mg3Al-HT was modified with a series of metal cations. Typically, 1.0 g Mg3Al-HT was added to a 100 mL aqueous solution contained 0.0005 mol of metal chloride MClx·nH2O (M = Ti3+, Cr3+, Mn2+, Fe2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, La3+, Nd3+, Sm3+, Gd3+, Dy3+, Ho3+, Yb3+, Au+, Au+/Pd2+) with vigorous stirring at 25 °C for 1 h. The resulting slurry was filtered, and the residue was washed with deionized water until natural. The solid sample was further dried at 100 °C in air for approximately 12 h. In this way, a series of metal cation modified hydrotalcites was prepared, and was denoted as Mz+/Mg3Al-LDH. Furthermore, the resulting hydrotalcites were calcined in an oven at 450 °C for 8 h under a flowing stream of pure nitrogen. Then, the a series of metal modified Mg-Al mixed oxides (M/Mgx(Al)O) were also obtained.
3.2. Characterization
Powder X-ray diffraction experiments of samples were carried out on a Rigaku Miniflex diffractometer (Rigak, Tokyo, Japan), equipped with an automatic slit and with Cu Kα radiation at 50 kV and 30 mA, the data was collected at a scanning speed of 2° per minute. The specific surface area and average pore diameter were measured by N2 adsorption-desorption method using a Micromeritics Tristar 3000 (Micromeritics, Norcross, GA, USA). The samples were outgassed at 80 °C and 10−4 Pa overnight and then the adsorption-desorption isotherms were conducted by passing nitrogen into the samples, which were kept under liquid nitrogen temperature, specific surface area was calculated by BET. TGA was carried out on a Setaram TGA-92 thermal analyzer (Setaram, Lyon, France) connected to a PC via a TAC7/DX thermal controller (Setaram, Kansas, MO, USA). The samples were heated from 30 °C to 800 °C at 10 °C/min under nitrogen.
3.3. Catalytic Test
The oxidation of toluene was carried out in a 100 mL Teflon-lined and magnetically stirred stainless steel autoclave. In a typical experiment, a certain amount of catalyst and 0.1 mol toluene was stirred for about 10 min, and then O2 was introduced. The autoclave was heated to the required temperature within 5 min. After the reaction run for the desired time, the products were filtered out of the catalyst and then were analyzed using a gas chromatograph with a capillary 30 m HP-5 column and an FID detector (Agilent, Santa Clara, CA, USA).
4. Conclusions
The metal cation modified LDHs and mixed oxides, especially the modified LDHs, were promising catalysts for the selective oxidation of toluene to benzyldehyde with O2. Owing to the introduction of metal cations with excessive positive charge and larger ionic radius, more surface –OH on the LDH laminates were decomposed to OH−, which led to more B basic sites and could afford their high catalytic performance. The highest toluene conversion reached 8.7% with 97.5% of the selectivity to benzyldehyde, when the reaction was run for 8 h at 150 °C with O2 pressure of 1 MPa. Moreover, the catalysts could be reused nine times. We believe this work will be helpful for the design of high effective heterogeneous catalysts for toluene oxidation.
Acknowledgments
The authors acknowledge the financial support from the National Natural Science Foundation of China (21003073, 21203093), the Natural Science Foundation of Jiangsu Province (BK20141388), the Qing Lan Project of Jiangsu Province, the Academic Talents Training Project of Nanjing Institute of Technology, and the College students practice innovation training program of Jiangsu Province.
Author Contributions
W.G.D. conceived and designed the experiments; W.X.L. analyzed the data and wrote the paper; L.H. and L.Q.B. performed the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jin, C.; Zhang, L.; Su, W.K. Direct benzylic oxidation with sodim hypochlorite using a new efficient catalytic system: TEMPO/Co(OAc)2. Synlett 2011, 2011, 1435–1438. [Google Scholar]
- Mandal, S.; Bando, K.K.; Santra, C.; Maity, S.; James, O.O.; Mehta, D.; Chowdhury, B. Sm-CeO2 supported gold nanoparticle catalyst for benzyl alcohol oxidation using molecular O2. Appl. Catal. A 2013, 452, 94–104. [Google Scholar] [CrossRef]
- Xue, M.; Yu, J.; Chen, H.; Shen, J. Surface Acidic and Redox Properties of V-Ag-O/TiO2 Catalysts for the Selective Oxidation of Toluene to Benzaldehyde. Catal. Lett. 2009, 128, 373–378. [Google Scholar] [CrossRef]
- Ma, B.C.; Zhang, Z.X.; Song, W.F.; Xue, X.L.; Yu, U.Z.; Zhao, Z.S.; Ding, Y. Solvent-free selective oxidation of C-H bonds of toluene and substituted toluene to aldehydes by vanadium-substituted polyoxometalate catalyst. J. Mol. Catal. A 2013, 369, 152–158. [Google Scholar] [CrossRef]
- Wang, F.; Xu, J.; Li, X.Q.; Gao, J.; Zhou, L.P.; Ohnishi, R. Liquid phase oxidation of toluene to benzaldehyde with molecular oxygen over Copper-based heterogeneous catalysts. Adv. Synth. Catal. 2005, 347, 1987–1992. [Google Scholar] [CrossRef]
- Mac Leod, T.C.O.; Kirillova, M.V.; Pombeiro, A.J.L.; Schiavon, M.A.; Assis, M.D. Mild oxidation of alkanes and toluene by tert-butyl hydroperoxide catalyzed by an homogeneous and immobilized Mn(salen) complex. Appl. Catal. A 2010, 372, 191–198. [Google Scholar] [CrossRef]
- Kesavan, L.; Tiruvalam, R.; Rahim, M.H.A.; Saiman, M.I.; Enache, D.I.; Jenkins, R.L.; Dimitratos, N.; Lopez-Sanchez, J.A.; Taylor, S.H.; Knight, D.W.; et al. Solvent-free oxidation of primary carbon-hydrogen bonds in toluene using Au-Pd alloy nanoparticles. Science 2011, 331, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Luo, J.; Deng, C.C.; Guo, Y.A.; Zhao, S.K.; Zhou, H.; Wei, S. Catalytic oxidation of toluene with molecular oxygen over manganese tetraphenylporphyrin supported on chitosan. Appl. Catal. A 2008, 338, 83–86. [Google Scholar] [CrossRef]
- Guo, C.C.; Liu, Q.; Wang, X.T.; Hu, H.Y. Selective liquid phase oxidation of toluene with air. Appl. Catal. A 2005, 282, 55–59. [Google Scholar] [CrossRef]
- Lv, J.G.; Shen, Y.; Peng, L.M.; Guo, X.F.; Ding, W.P. Exclusively selective oxidation of toluene to benzaldehyde on ceria nanocubes by molecular oxygen. Chem. Commun. 2010, 46, 5909–5911. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.W.; Chen, H.; Zhang, H.L.; Auroux, A.; Shen, J.Y. Preparation and characterization of V-Ag-O catalysts for the selective oxidation of toluene. Appl. Catal. A 2010, 379, 7–14. [Google Scholar] [CrossRef]
- Fu, B.S.; Zhu, X.W.; Xiao, G.M. Solvent-free selective aerobic oxidation of toluene by ultra fine nano-palladium catalyst. Appl. Catal. A 2012, 415, 47–52. [Google Scholar] [CrossRef]
- Davis, R.J.; Derouane, E.G. A non-porous supported-platinum catalyst for aromatization of n-hexane. Nature 1991, 349, 313–315. [Google Scholar] [CrossRef]
- Nikolopoulos, A.A.; Jang, B.W.L.; Spivey, J.J. Acetone condensation and selective hydrogenation to MIBK on Pd and Pt hydrotalcite-derived Mg Al mixed oxide catalysts. Appl. Catal. A 2005, 296, 128–136. [Google Scholar] [CrossRef]
- Bîjega, R.; Pavel, O.D.; Costentin, G.; Che, M.; Angelescu, E. Rare-earth elements modified hydrotalcites and corresponding mesoporous mixed oxides as basic solid catalysts. Appl. Catal. A 2005, 288, 185–193. [Google Scholar] [CrossRef]
- Dussault, L.; Dupin, J.C.; Martinez, H.; Dumitriu, E.; Auroux, A.; Guimon, C. Influence of the metal nature (Ni, Cu, Mg) on the surface acid–base properties of mixed oxides elaborated from LDH. Surf. Interface Anal. 2006, 38, 234–237. [Google Scholar] [CrossRef]
- Wang, X.L.; Wu, G.D.; Wang, F.; Ding, K.Q.; Zhang, F.; Liu, X.F.; Xue, Y.B. Base-free selective oxidation of glycerol with 3% H2O2 catalyzed by sulphonato-salen-chromium (III) intercalated LDH. Catal. Commum. 2012, 28, 73–76. [Google Scholar] [CrossRef]
- Zhou, C.H.; Beltramini, J.N.; Lin, C.X.; Xu, Z.P.; Lu, G.Q.; Tanksale, A. Selective oxidation of biorenewable glycerol with molecular oxygen over Cu-containing layered double hydroxide-based catalysts. Catal. Sci Technol. 2011, 1, 111–122. [Google Scholar] [CrossRef]
- Motokura, K.; Nishimura, D.; Mori, K.; Mizugaki, T.; Ebitani, K.; Kaneda, K. A ruthenium-grafted hydrotalcite as a multifunctional catalyst for direct α-alkylation of nitriles with primary alcohols. J. Am. Chem. Soc. 2004, 126, 5662–5663. [Google Scholar] [CrossRef] [PubMed]
- Motokura, K.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Multifunctional catalysis of a ruthenium-grafted hydrotalcite: On pot synthesis of quinolines from 2-aminobenzyl alcohol and various carbonyl compounds via aerobic oxidation and aldol reaction. Tetrahedron Lett. 2004, 45, 6029–6032. [Google Scholar] [CrossRef]
- Wu, G.D.; Wang, X.L.; Chen, B.; Li, J.P.; Zhao, N.; Wei, W.; Sun, Y.H. Fluorine-modified mesoporous Mg-Al mixed oxides: Mild and stable base catalysts for o-methylation of phenol with dimethyl carbonate. Appl. Catal. A 2007, 329, 106–111. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).