Simple Preparation of Pd Core Nanoparticles for Pd Core/Pt Shell Catalyst and Evaluation of Activity and Durability for Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Properties of Pd/CB and Pt Monolayer-Modified Pd/CB
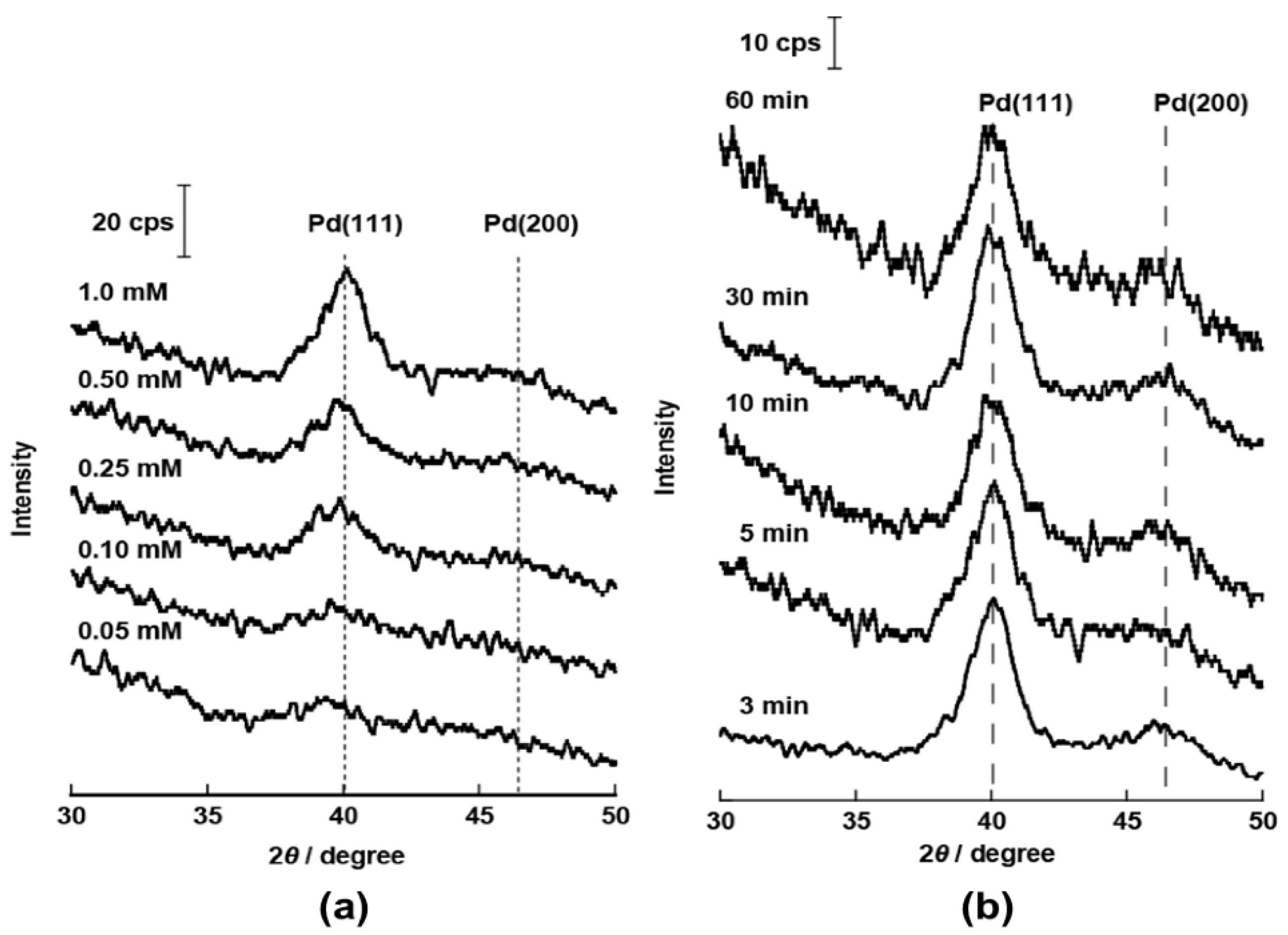
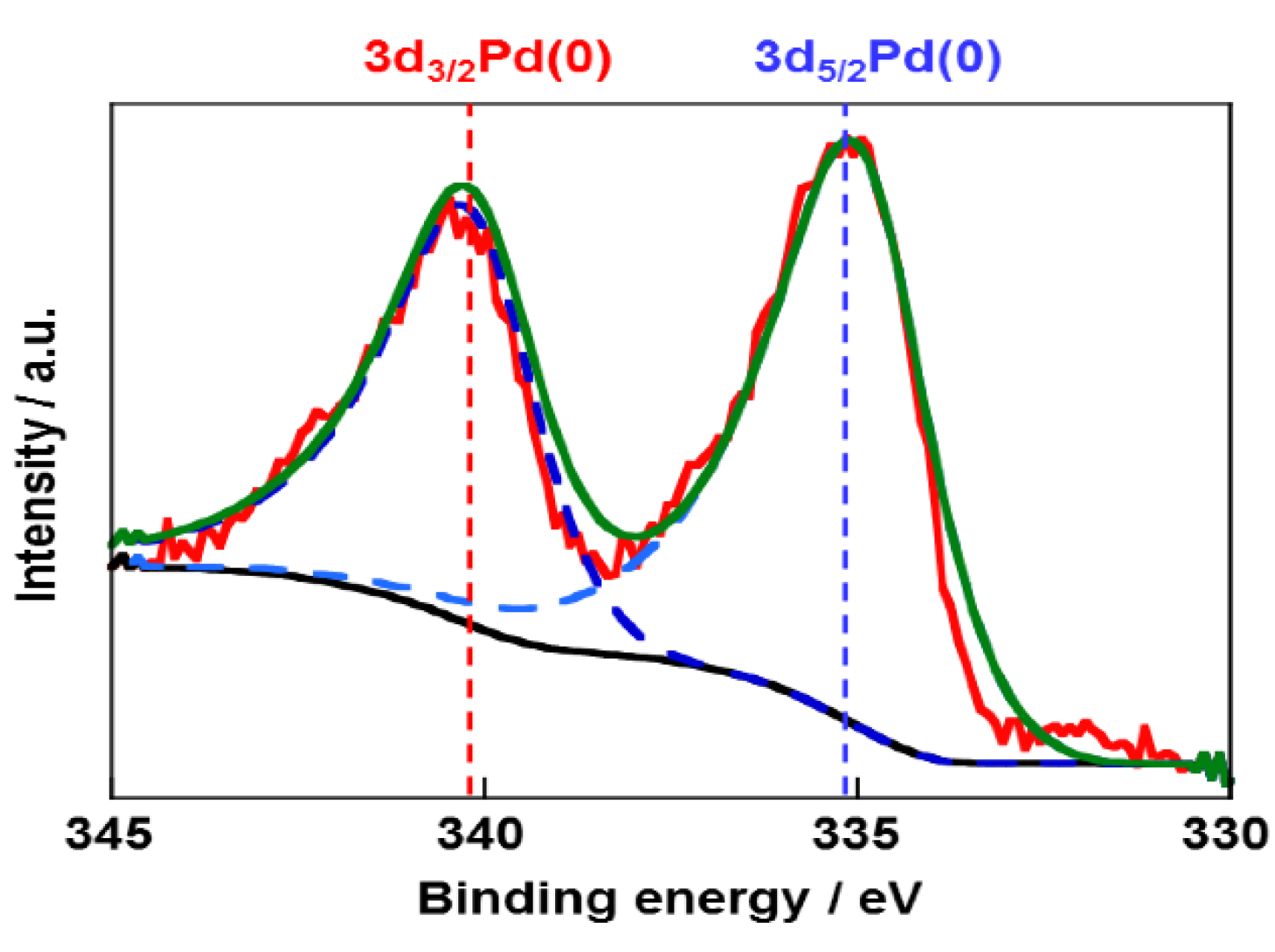
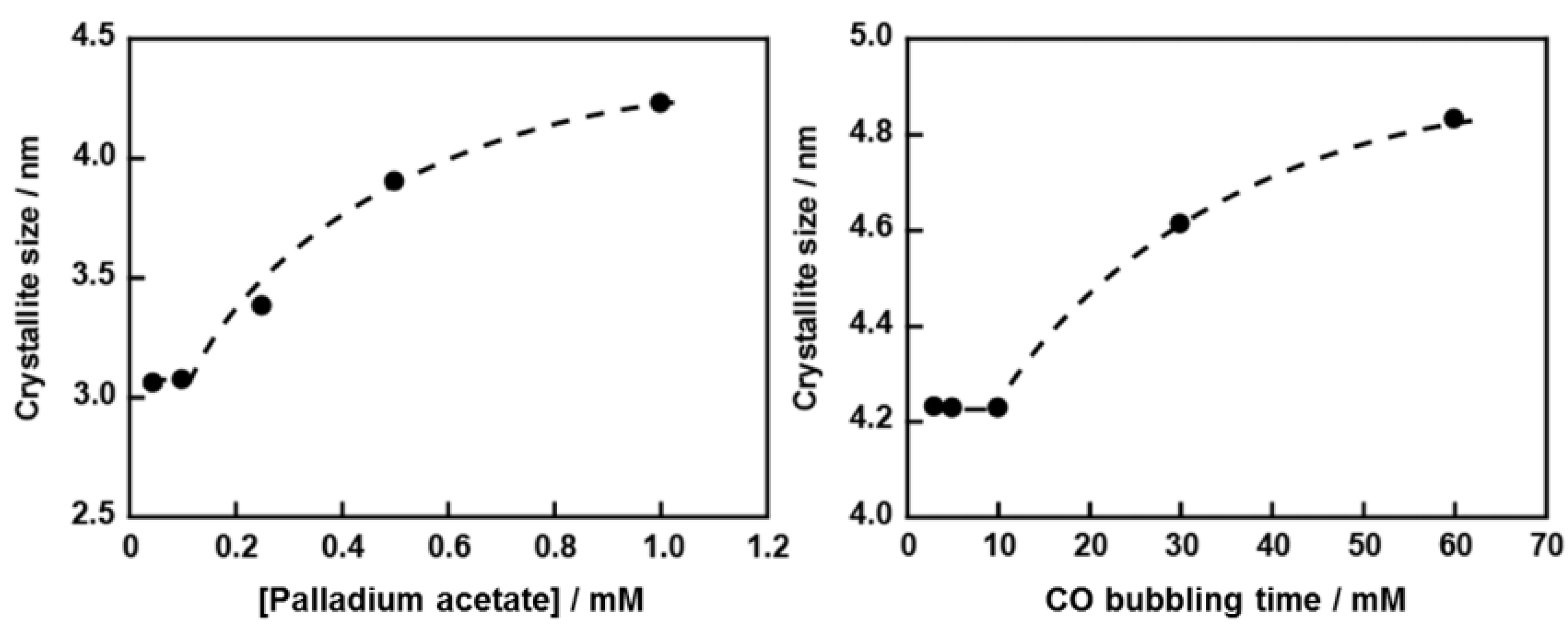
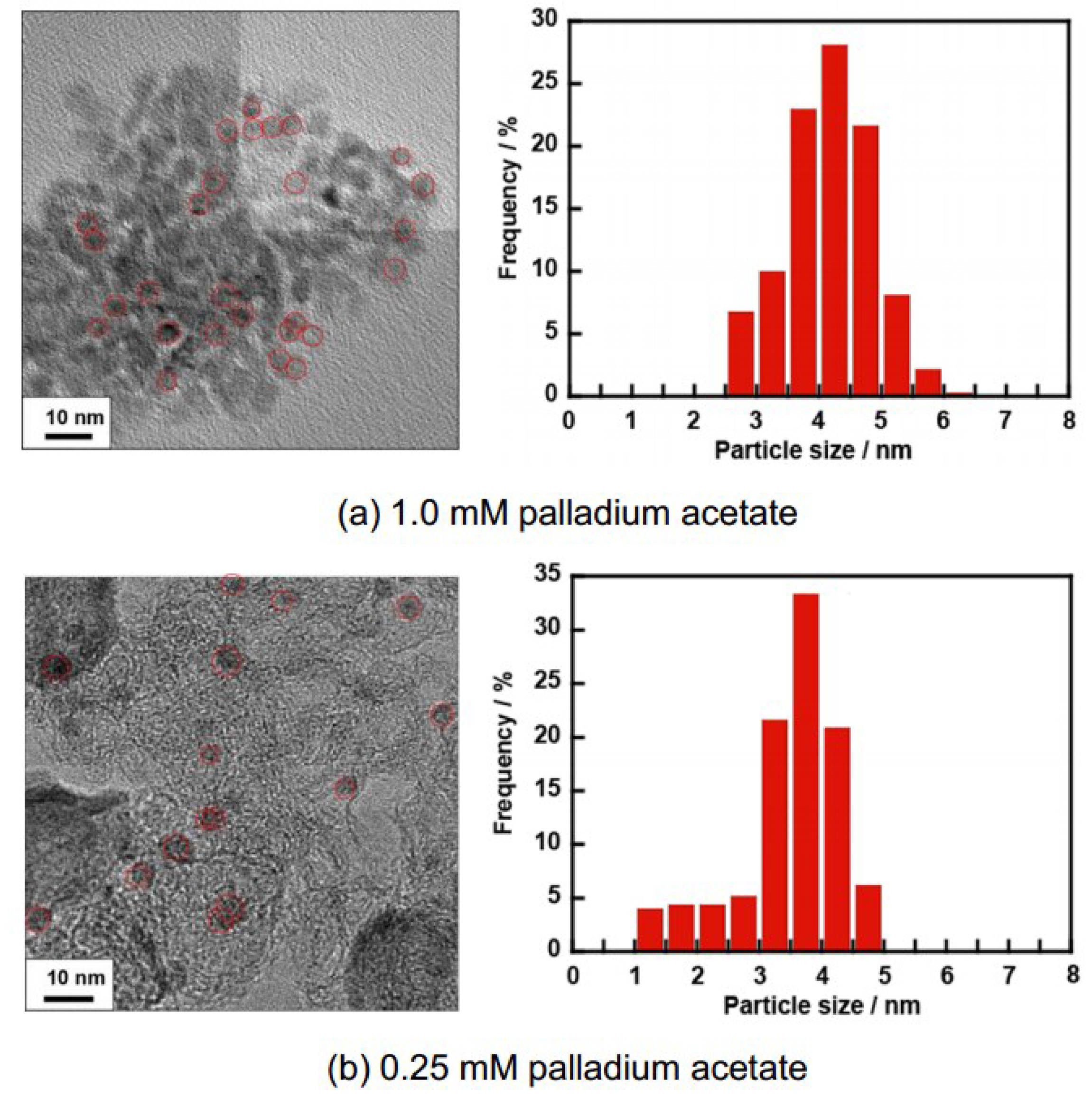
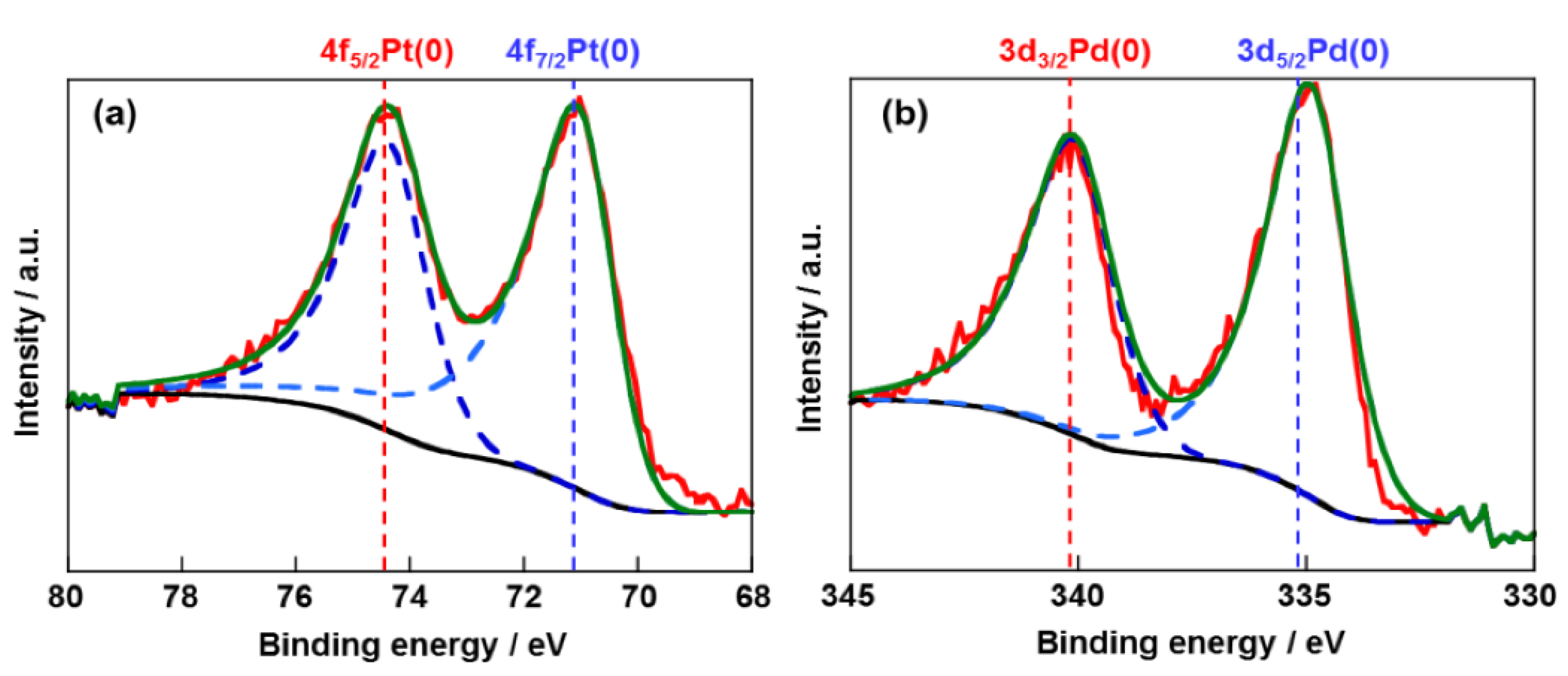
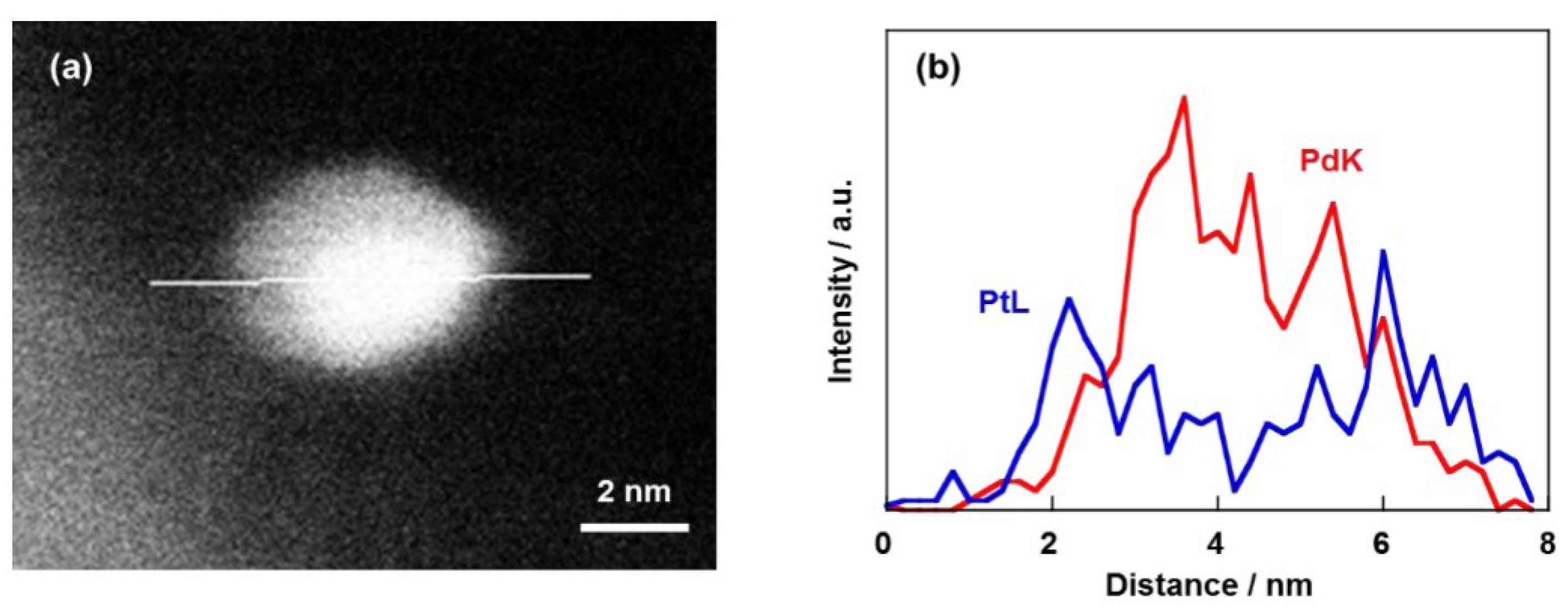
2.2. Electrochemical Properties of Pt/Pd/CB Electrodes
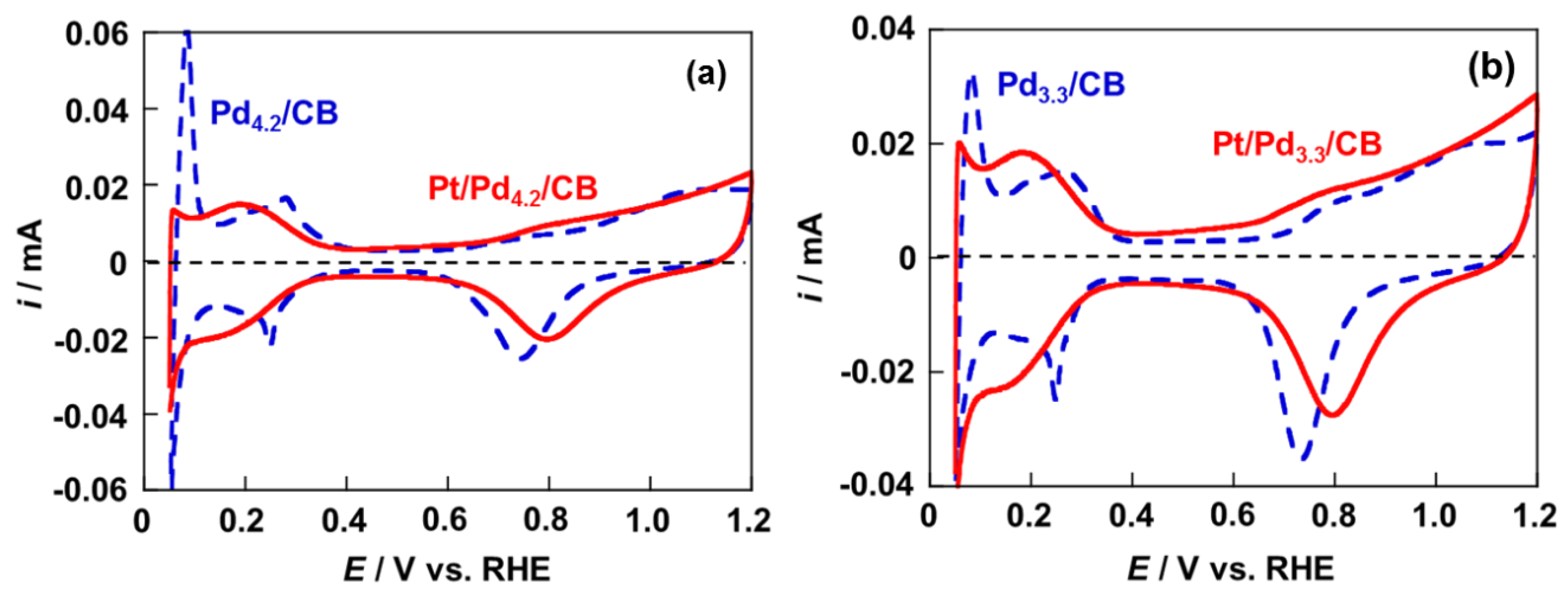
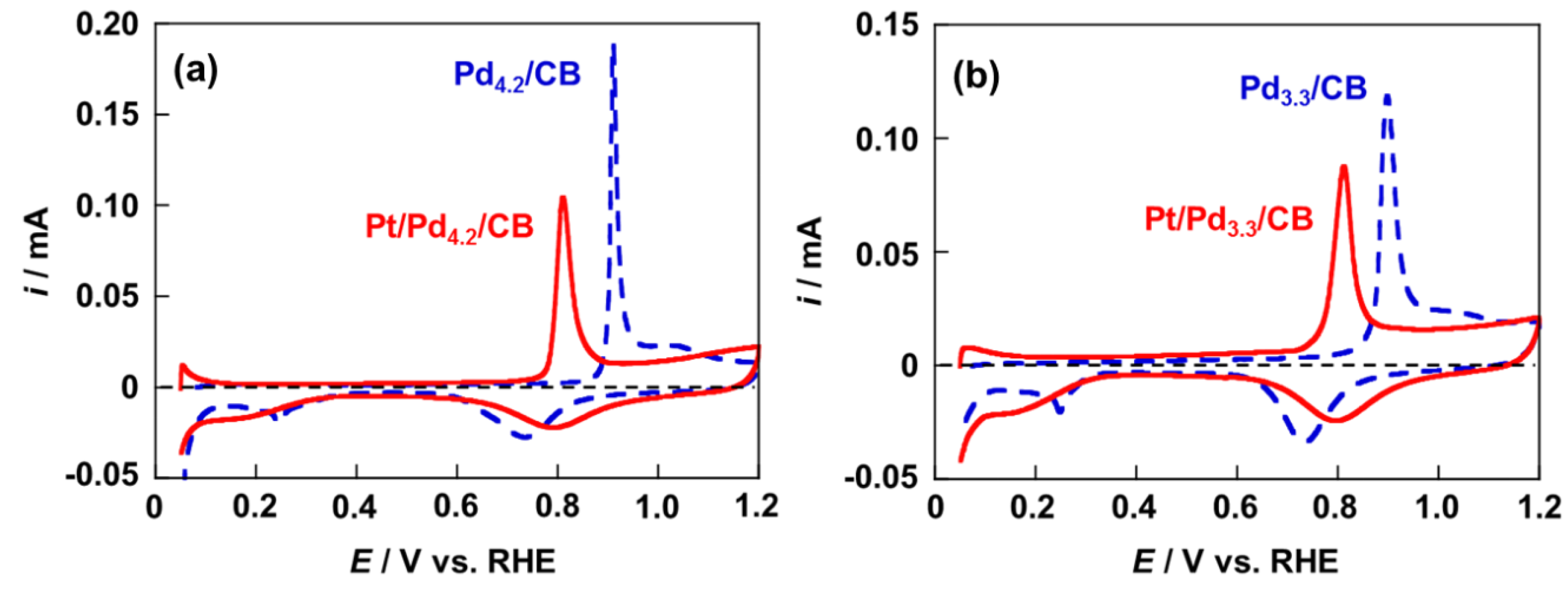
2.3. Activity and Durability for ORR of Pt/Pd4.2/CB and Pt/Pd3.3/CB Electrodes
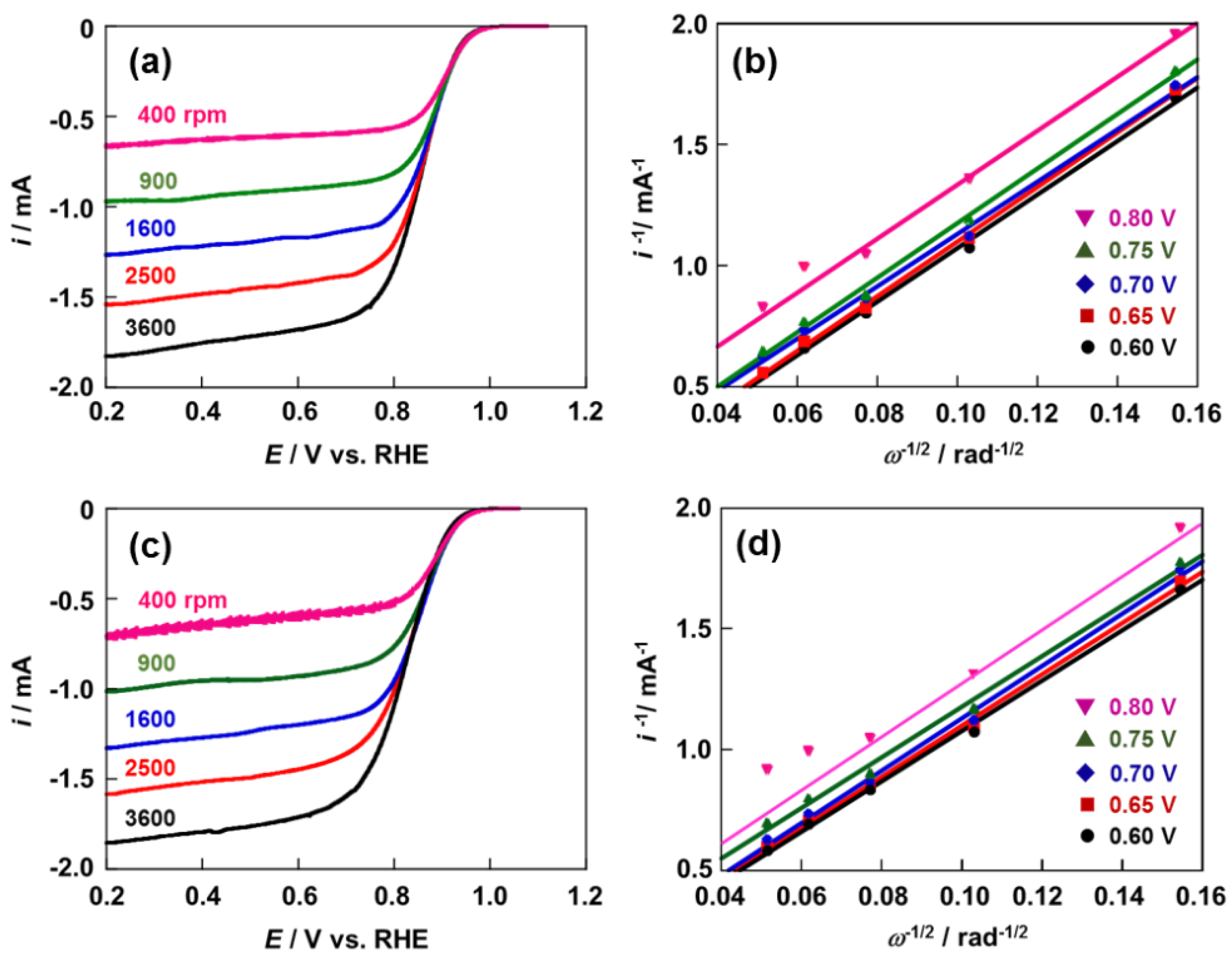
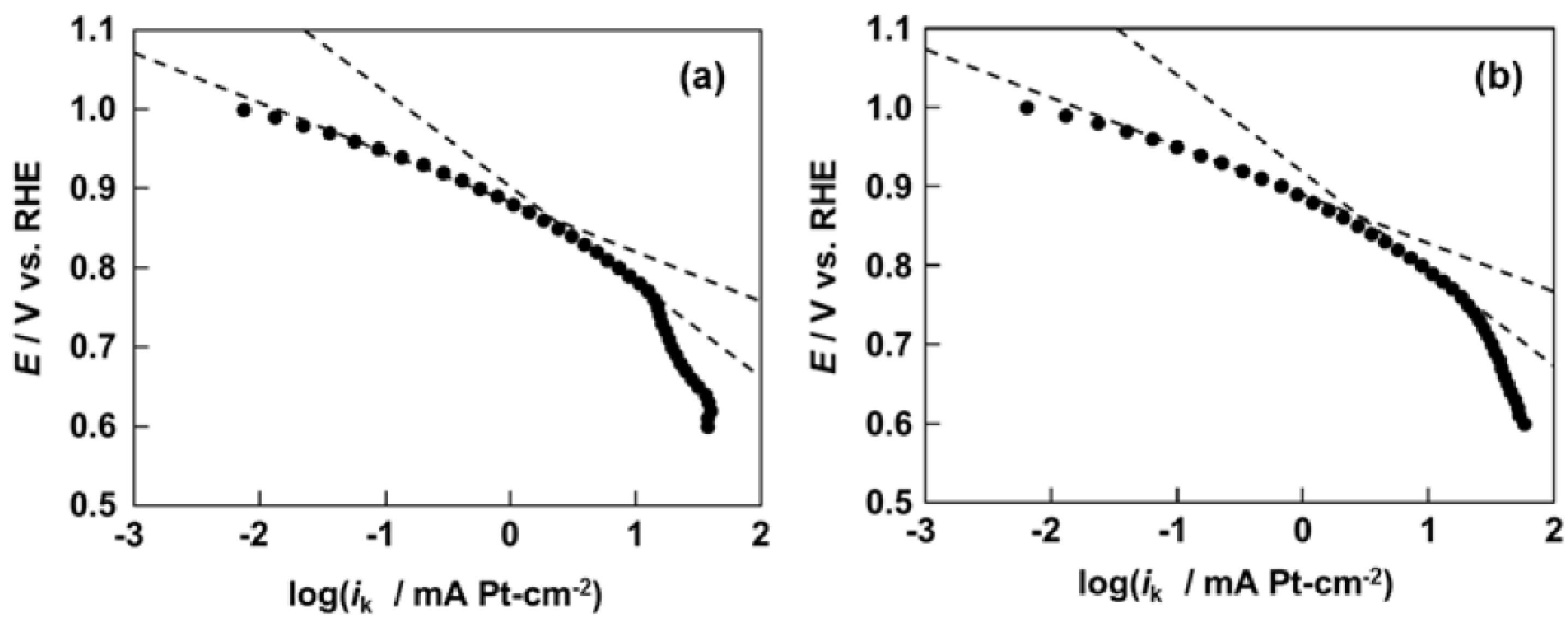
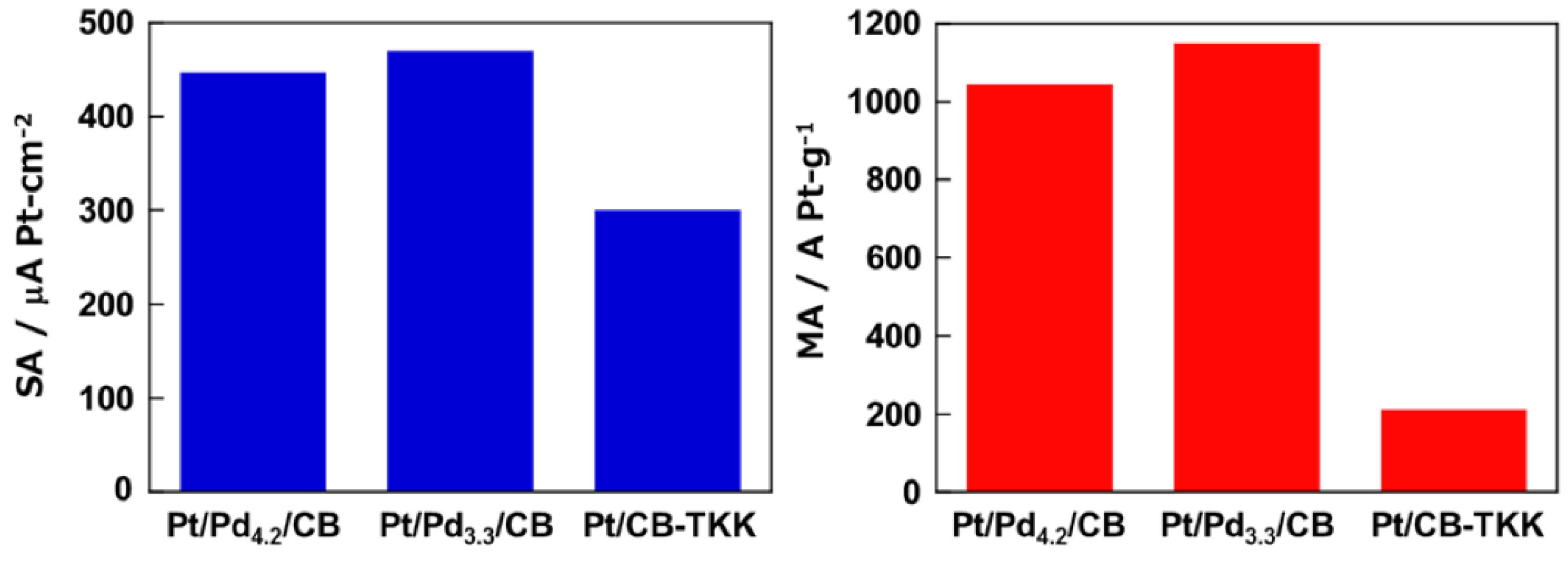
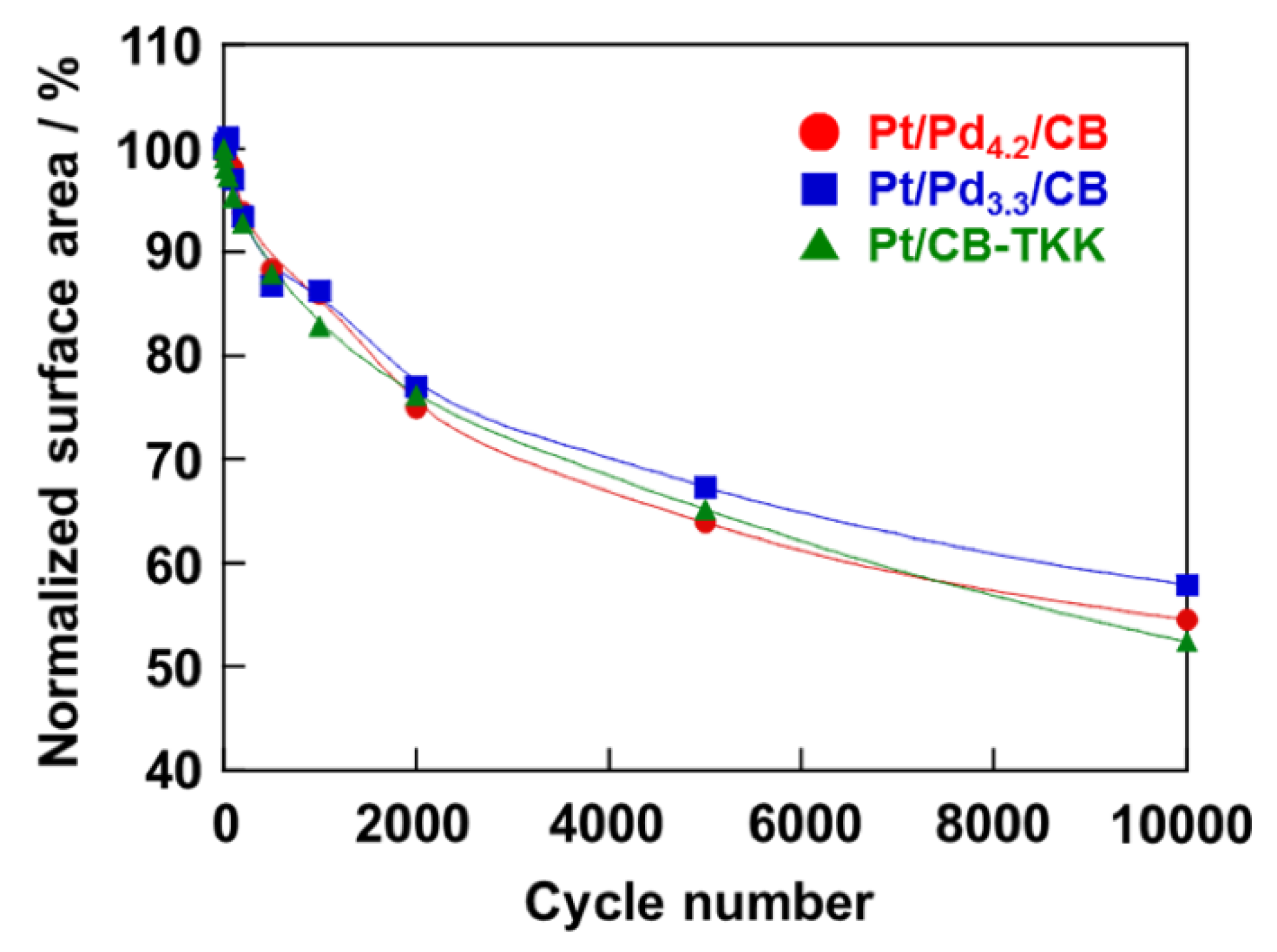
3. Experimental Section
3.1. Preparation and Characterization of Pd/CB
3.2. Modification of Pd Core Nanoparticles Loaded on CB with Pt Shell
3.3. Electrochemical Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Toda, T.; Igarashi, H.; Uchida, H.; Watanabe, M. Enhancement of the electroreduction of oxygen on Pt alloys with Fe, Ni, and Co. J. Electrochem. Soc. 1999, 146, 3750–3756. [Google Scholar] [CrossRef]
- Mukerjee, S.; Srinivasan, S.; Soriaga, M.P.; McBreen, J. Role of Structural and electronic properties of Pt and Pt alloys on electrocatalysis of oxygen reduction: An in situ XANES and EXAFS investigation. J. Electrochem. Soc. 1995, 142, 1409–1422. [Google Scholar] [CrossRef]
- Greeley, J.; Stephens, I.E.L.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Norskov, J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Paulus, U.A.; Wokaun, A.; Scherer, G.G.; Schmidt, T.J.; Stamenkovic, V.; Radmilovic, V.; Markovic, N.M.; Ross, P.N. Oxygen reduction on carbon-supported Pt-Ni and Pt-Co alloy catalysts. J. Phys. Chem. B 2002, 106, 4181–4191. [Google Scholar] [CrossRef]
- Paffett, M.T.; Berry, J.G.; Gottesfeld, S. Oxygen reduction at Pt0.65Cr0.35, Pt0.2Cr0.8 and roughened platinum. J. Electrochem. Soc. 1998, 135, 1431–1436. [Google Scholar] [CrossRef]
- Smith, M.C.; Gilbert, J.A.; Mawdsley, J.R.; Seifert, S.; Myers, D.J. In situ small-angle X-ray scattering observation of Pt catalyst particle growth during potential cycling. J. Am. Chem. Soc. 2008, 130, 8112–8113. [Google Scholar] [CrossRef] [PubMed]
- Salgado, J.R.C.; Antolini, E.; Gonzalez, E.R. Structure and activity of carbon-supported Pt-Co electrocatalysts for oxygen reduction. J. Phys. Chem. B 2004, 108, 17767–17774. [Google Scholar] [CrossRef]
- Yano, H.; Kataoka, M.; Yamashita, H.; Uchida, H.; Watanabe, M. Oxygen reduction activity of carbon-supported Pt-M (M = V, Ni, Cr, Co, and Fe) alloys prepared by nanocapsule method. Langmuir 2007, 23, 6438–6445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mo, Y.; Vukmirovic, M.B.; Klie, R.; Sasaki, K.; Adzic, R.R. Platinum monolayer electrocatalysts for O2 reduction: Pt monolayer on Pd(111) and on carbon-supported Pd nanoparticles. J. Phys. Chem. B 2004, 108, 10955–10964. [Google Scholar] [CrossRef]
- Vukmirovic, M.B.; Zhang, J.; Sasaki, K.; Nilekar, A.U.; Uribe, F.; Mavrikakis, M.; Adzic, R.R. Platinum monolayer electrocatalysts for oxygen reduction. Electrochim. Acta 2007, 52, 2257–2263. [Google Scholar] [CrossRef]
- Inaba, M.; Ito, H.; Tuji, H.; Wada, T.; Banno, M.; Yamada, H.; Saito, M.; Tasaka, A. Effect of core size on activity and durability of Pt core-shell catalysts for PEFCs. ECS Trans. 2010, 33, 231–238. [Google Scholar]
- Li, Y.; El-Sayed, M.A. The effect of stabilizers on the catalytic activity and stability of Pd colloidal nanoparticles in the suzuki reactions in aqueous solution. J. Phys. Chem. B 2001, 105, 8938–8943. [Google Scholar] [CrossRef]
- Shao, M.; Odell, J.; Humbert, M.; Yu, T.; Xia, Y. Electrocatalysis on shape-controlled palladium nanocrystals: Oxygen reduction reaction and formic acid oxidation. J. Phys. Chem. C 2013, 117, 4172–4180. [Google Scholar] [CrossRef]
- Vidal-Iglesias, F.J.; Aran-Ais, R.M.; Solla-Gullon, J.; Garnier, E.; Herrero, E.; Aldaz, A.; Feliu, J.M. Shape-dependent electrocatalysis: Formic acid electrooxidation on cubic Pd nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 10258–10265. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, V.; Sun, S. Oleylamine-mediated synthesis of Pd nanoparticles for catalytic formic acid oxidation. J. Am. Chem. Soc. 2009, 131, 4588–4589. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Zhu, Q.; Chen, R. Controlled synthesis of Pd/Pt core shell nanoparticles using area-selective atomic layer deposition. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, E.; Taguchi, A.; Hayashi, K.; Inoue, H. Electrocatalytic activity for oxygen reduction reaction of Pt nanoparticle catalysts with narrow size distribution prepared from [Pt3(CO)3(μ-CO)3]n2− (n = 3–8) complexes. J. Electroanal. Chem. 2011, 663, 84–89. [Google Scholar] [CrossRef]
- Higuchi, E.; Hayashi, K.; Chiku, M.; Inoue, H. Simple preparation of Au nanoparticles and their application to Au core/Pt shell catalysts for oxygen reduction reaction. Electrocatalysis 2012, 3, 274–283. [Google Scholar] [CrossRef]
- Erdogan, H.; Metin, O.; Ozkar, S. In situ-generated PVP-stabilized palladium(0) nanocluster catalyst in hydrogen generation from the methanolysis of ammonia-borane. Phys. Chem. Chem. Phys. 2009, 11, 10519–10525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qiu, C.; Ma, H.; Liu, X. Facile fabrication and unexpected electrocatalytic activity of palladium thin films with hierarchical architectures. J. Phys. Chem. C 2008, 112, 13970–13975. [Google Scholar] [CrossRef]
- Gabrielli, C.; Grand, P.P.; Lasia, A.; Perrot, H. Investigation of hydrogen adsorption and absorption in palladium thin films: II. Cyclic voltammetry. J. Electrochem. Soc. 2004, 151, A1937–A1942. [Google Scholar] [CrossRef]
- Damjanovic, A.; Genshaw, M.A. Dependence of the kinetics of O2 dissolution at Pt on the conditions for adsorption of reaction intermediates. Electrochim. Acta 1970, 15, 1281–1283. [Google Scholar] [CrossRef]
- Markovic, N.M.; Ross, P.N. Electrocatalysis as well-defined surfaces: Kinetics of oxygen reduction and hydrogen oxidation/evolution on Pt(hkl) electrodes; mechanism of methanol electro-oxidation. In Interfacial Electrochemistry, Theory, Experiment, and Applications; Wieckowski, A., Ed.; Marcel Dekker: New York, NY, USA, 1999; pp. 821–841. [Google Scholar]
- Hammer, B.; Nørskov, J.K. Theoretical Surface Science and Catalysis—Calculations and Concepts. Adv. Catal. 2000, 45, 71–129. [Google Scholar]
- Wang, J.X.; Inada, H.; Wu, L.; Zhu, Y.; Choi, Y.; Liu, P.; Zhou, W.−P.; Adzic, R.R. Oxygen reduction on well-defined core-shell nanocatalysts: Particle size, facet, and Pt shell thickness effects. J. Am. Chem. Soc. 2009, 131, 17298–17302. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Orikasa, Y.; Takesue, Y.; Inoue, H.; Nakamura, M.; Minato, T.; Hoshi, N.; Uchimoto, Y. Quantitating the lattice strain dependence of monolayer Pt shell activity toward oxygen reduction. J. Am. Chem. Soc. 2013, 135, 5938–5941. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, H.; Sakai, R.; Kuwahara, T.; Chiku, M.; Higuchi, E. Simple Preparation of Pd Core Nanoparticles for Pd Core/Pt Shell Catalyst and Evaluation of Activity and Durability for Oxygen Reduction Reaction. Catalysts 2015, 5, 1375-1387. https://doi.org/10.3390/catal5031375
Inoue H, Sakai R, Kuwahara T, Chiku M, Higuchi E. Simple Preparation of Pd Core Nanoparticles for Pd Core/Pt Shell Catalyst and Evaluation of Activity and Durability for Oxygen Reduction Reaction. Catalysts. 2015; 5(3):1375-1387. https://doi.org/10.3390/catal5031375
Chicago/Turabian StyleInoue, Hiroshi, Ryotaro Sakai, Taiki Kuwahara, Masanobu Chiku, and Eiji Higuchi. 2015. "Simple Preparation of Pd Core Nanoparticles for Pd Core/Pt Shell Catalyst and Evaluation of Activity and Durability for Oxygen Reduction Reaction" Catalysts 5, no. 3: 1375-1387. https://doi.org/10.3390/catal5031375
APA StyleInoue, H., Sakai, R., Kuwahara, T., Chiku, M., & Higuchi, E. (2015). Simple Preparation of Pd Core Nanoparticles for Pd Core/Pt Shell Catalyst and Evaluation of Activity and Durability for Oxygen Reduction Reaction. Catalysts, 5(3), 1375-1387. https://doi.org/10.3390/catal5031375




