Synthesis and Electrocatalytic Performance of Multi-Component Nanoporous PtRuCuW Alloy for Direct Methanol Fuel Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microstructural Characterization of np-PtRuCuW
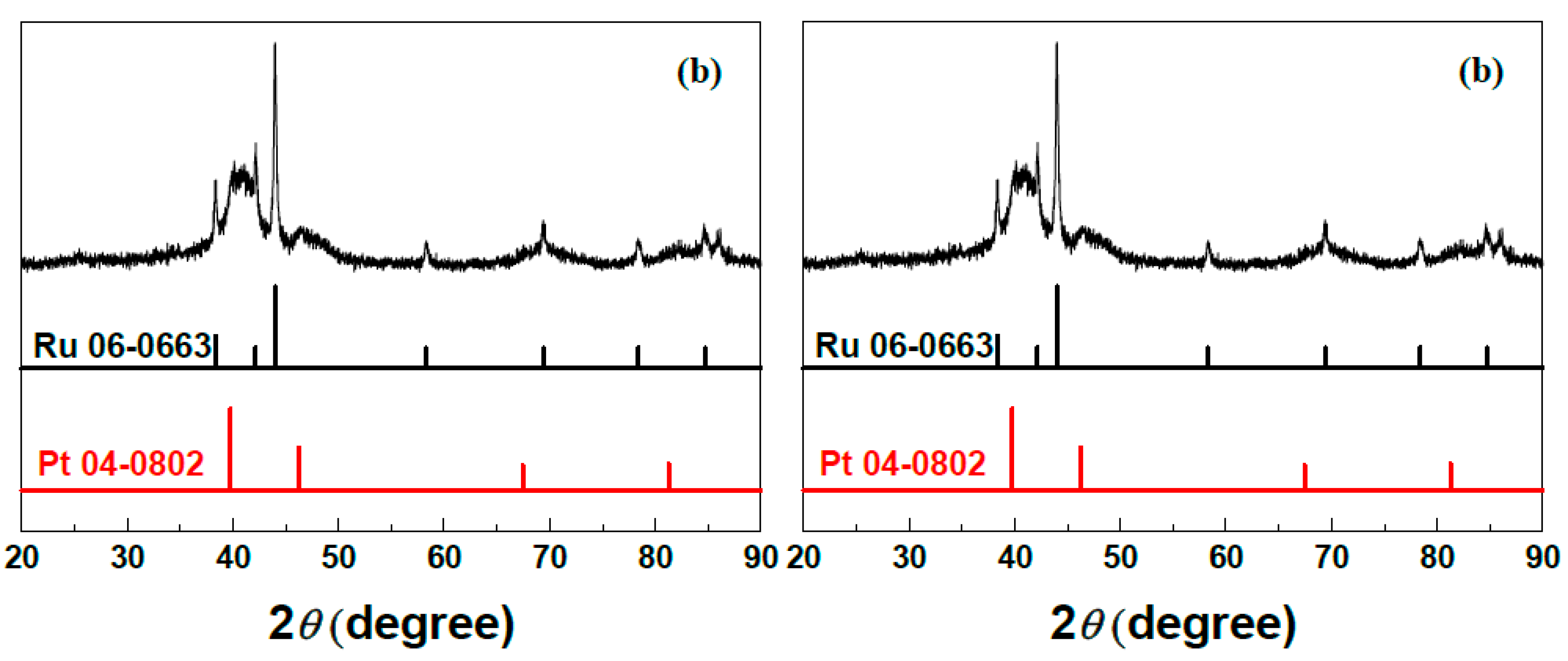
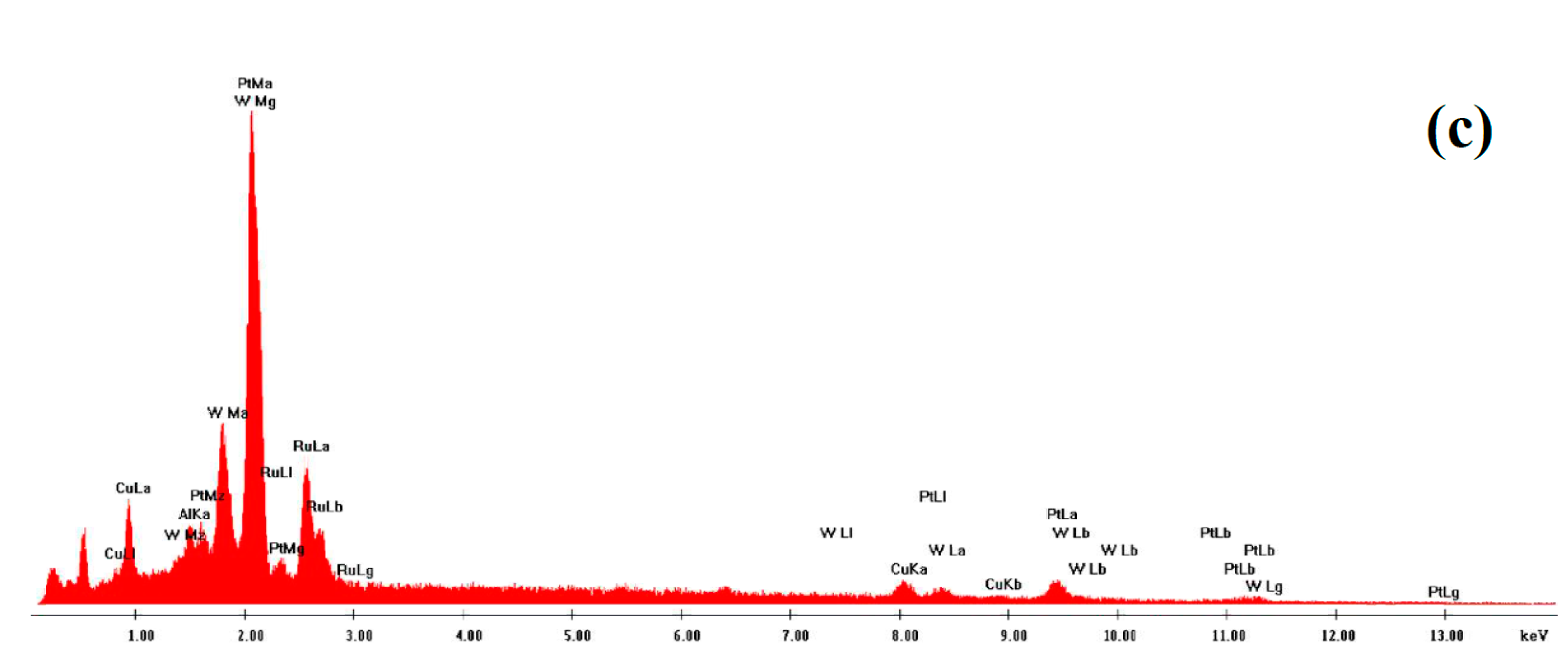
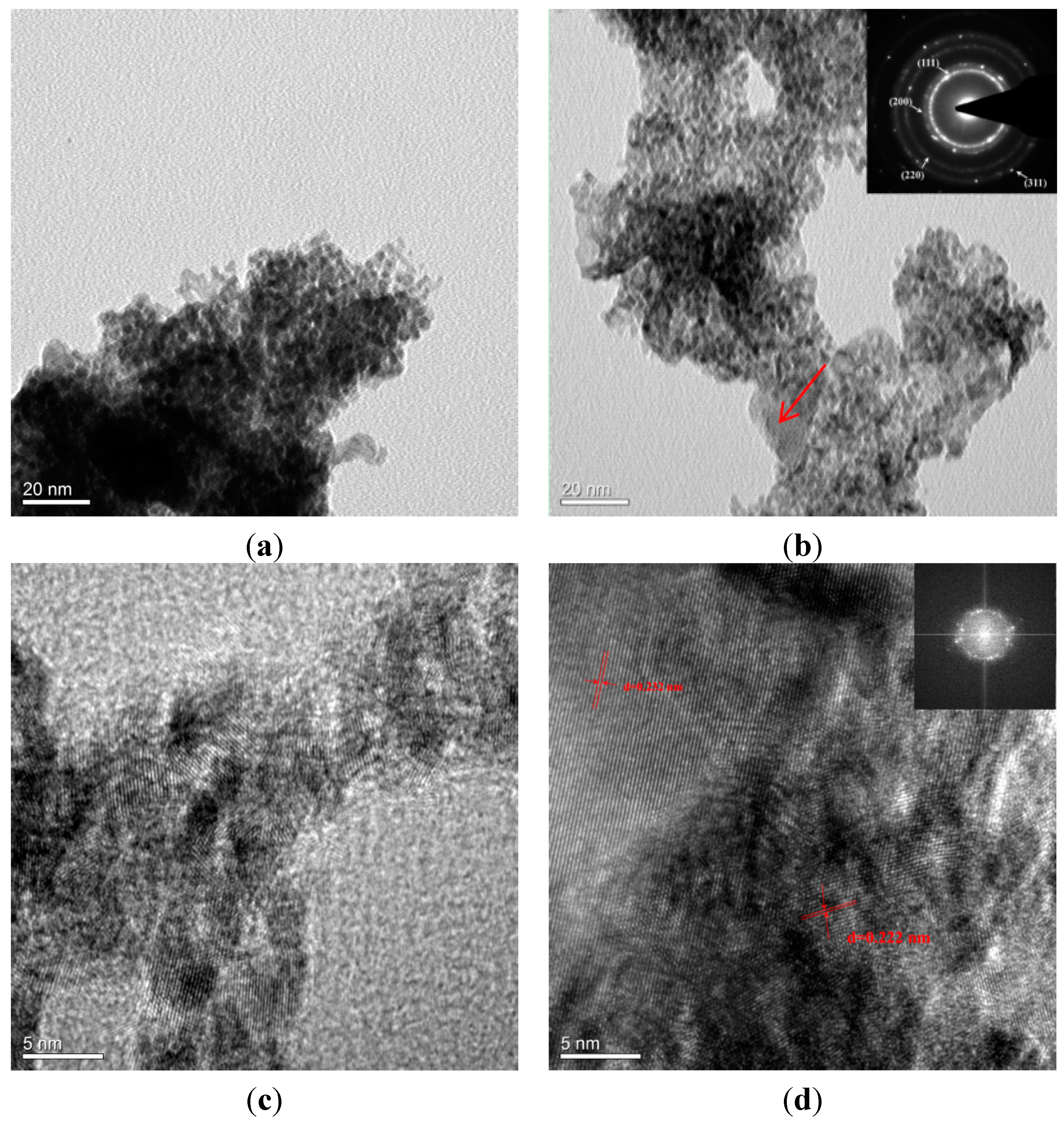
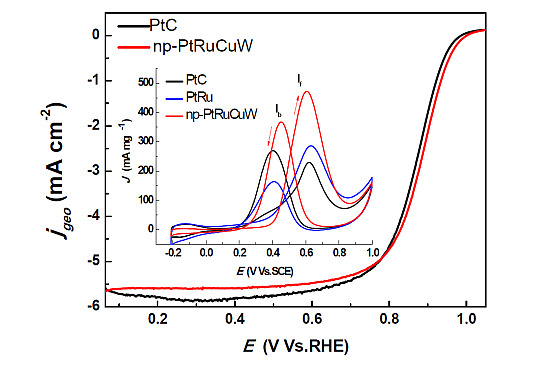
2.2. Catalytic Activity of np-PtRuCuW at Anode
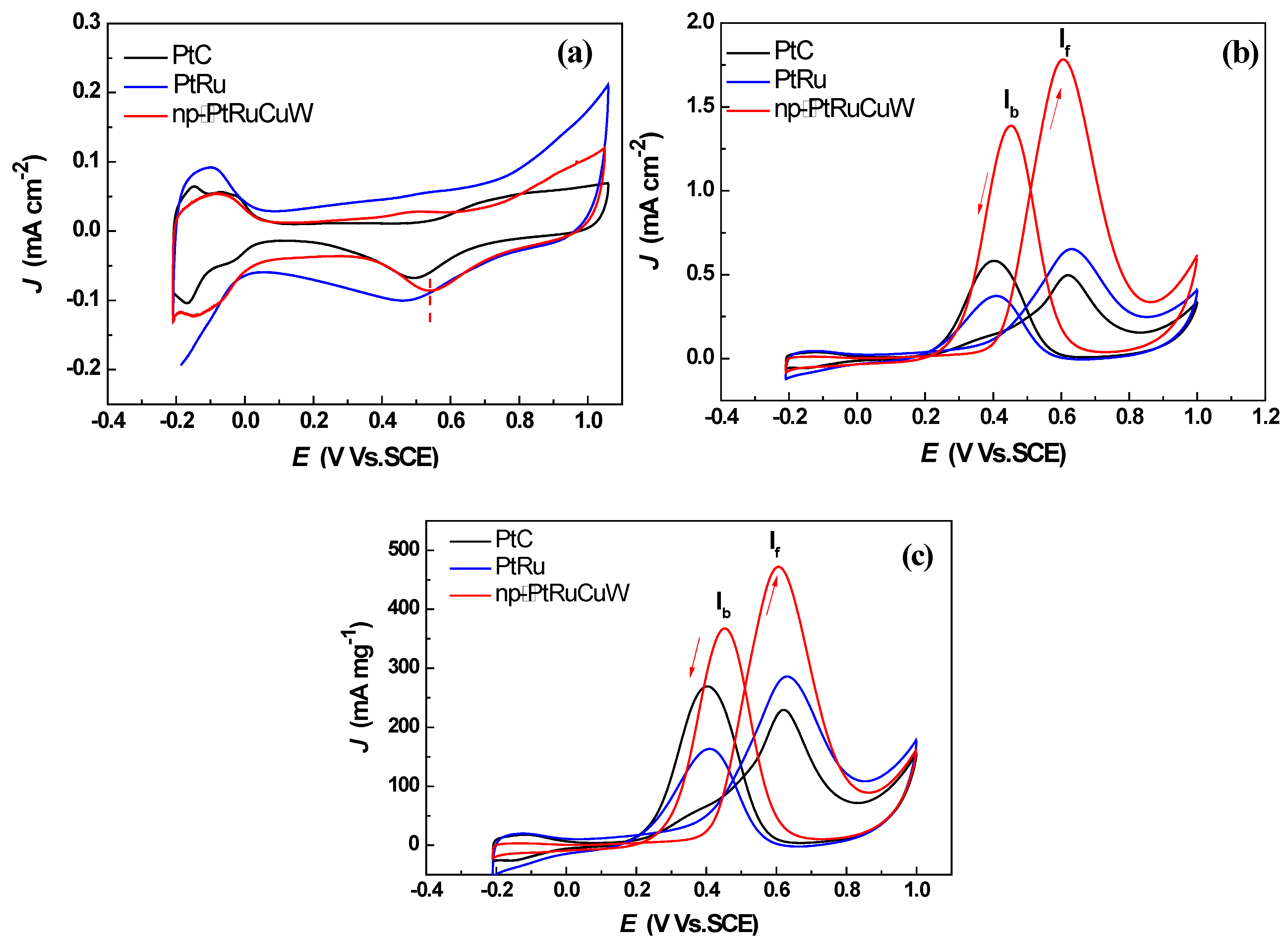
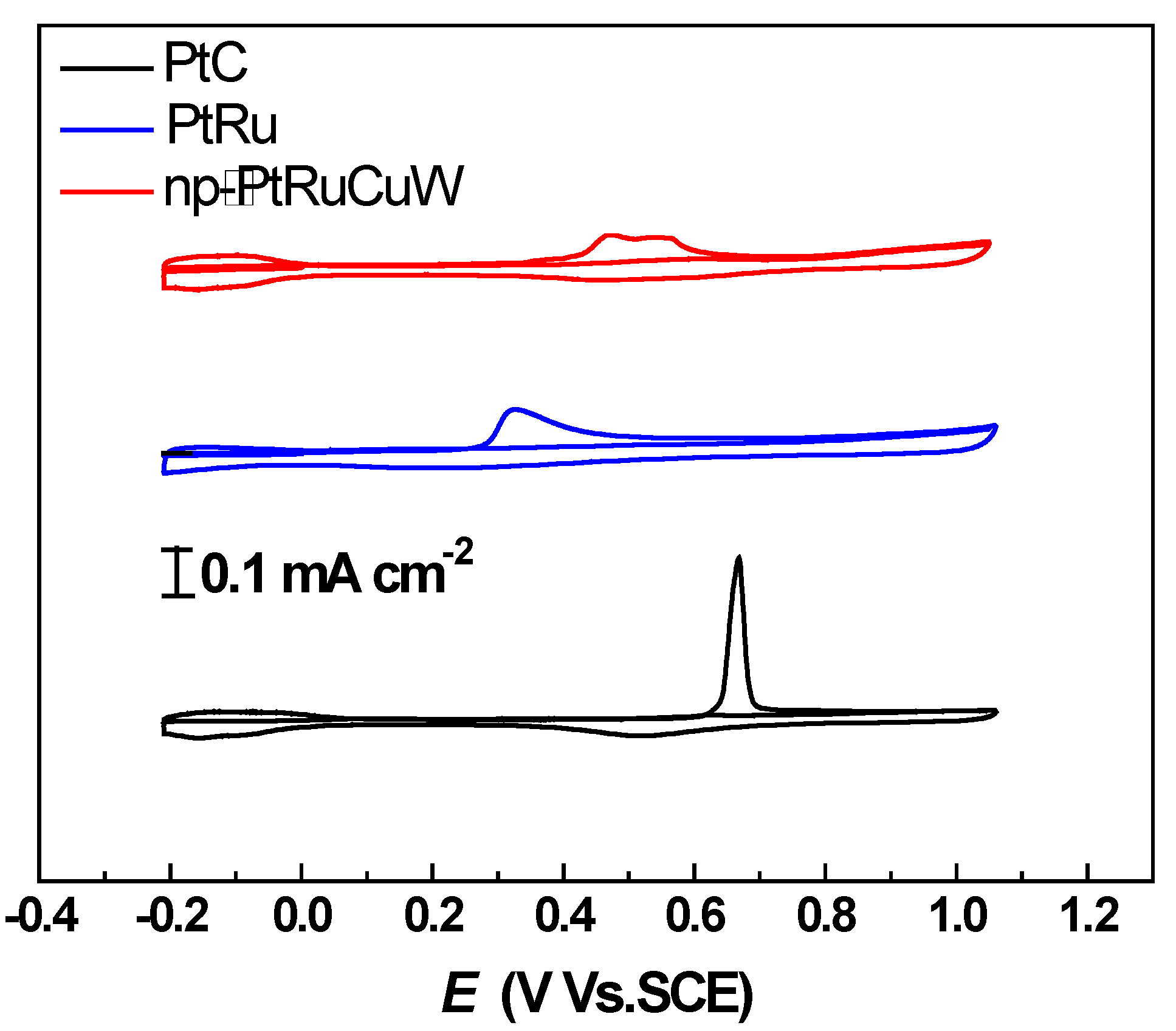
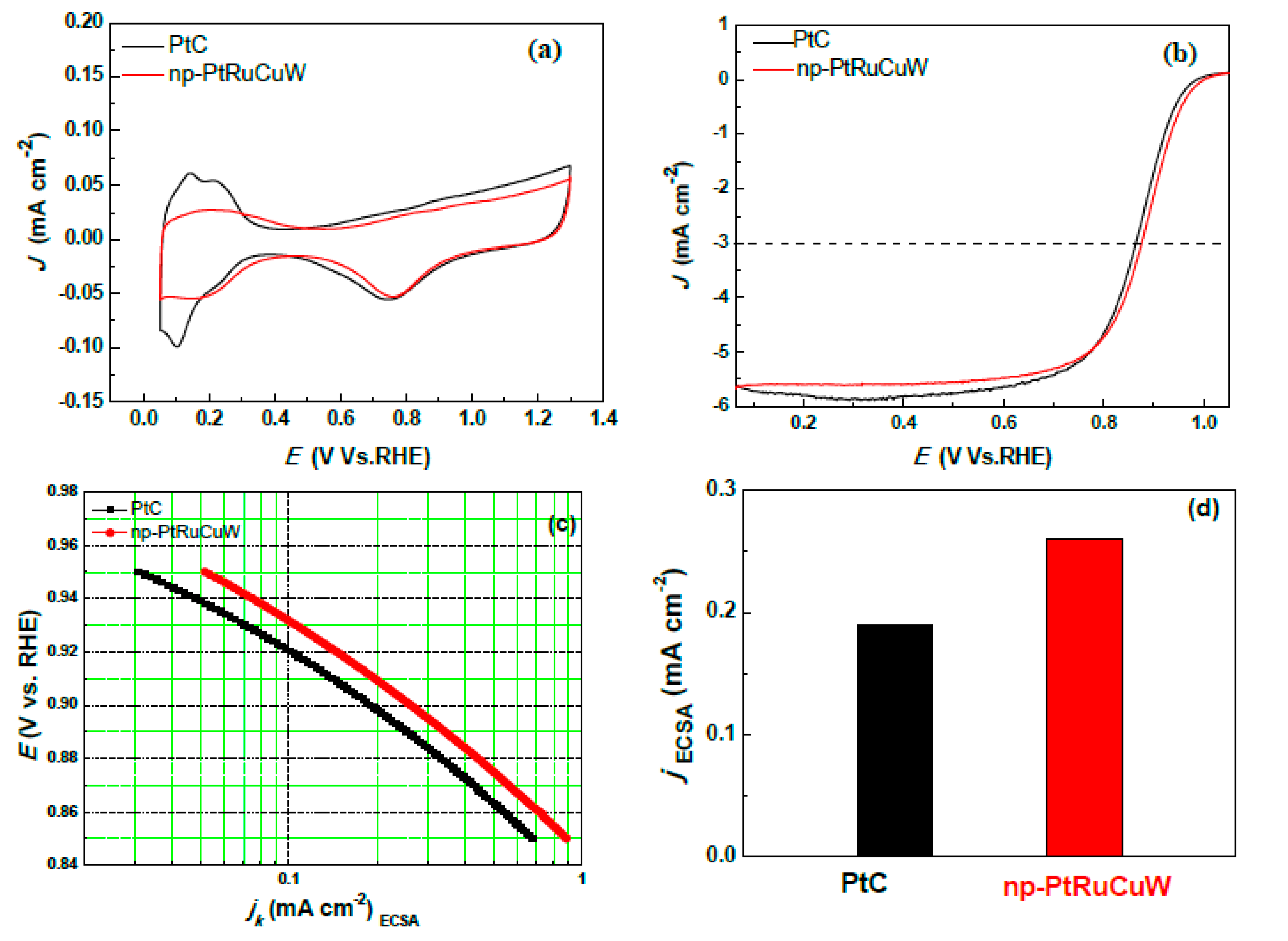
2.3. Catalytic Activity of np-PtRuCuW towards ORR
3. Experimental Section
3.1. Synthesis and Characterizations of np-PtRuCuW
3.2. Electrochemical Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kamarudin, S.K.; Achmad, F.; Daud, W.R.W. Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices. Int. J. Hyd. Energy 2009, 34, 6902–6916. [Google Scholar] [CrossRef]
- Zhang, G.; Xia, B.Y.; Wang, X. Strongly Coupled NiCo2O4-Rgo Hybrid Nanosheets as a Methanol-Tolerant Electrocatalyst for the Oxygen Reduction Reaction. Adv. Mater. 2014, 26, 2408–2412. [Google Scholar] [CrossRef] [PubMed]
- Reddington, E.; Sapienza, A.; Gurau, B.; Viswanathan, R.; Sarangapani, S.; Smotkin, E.S.; Mallouk, T.E. Combinatorial electrochemistry: A highly parallel, optical screening method for discovery of better electrocatalysts. Science 1998, 280, 1735–1737. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Luo, L.; Zhu, L.; Yu, L.; Sheng, L.; An, K.; Ando, Y.; Zhao, X. Pt–Fe catalyst nanoparticles supported on single-wall carbon nanotubes: Direct synthesis and electrochemical performance for methanol oxidation. J. Power Sour. 2013, 241, 274–280. [Google Scholar] [CrossRef]
- Wang, C.; Markovic, N.M.; Stamenkovic, V.R. Advanced platinum alloy electrocatalysts for the oxygen reduction reaction. ACS Catal. 2012, 2, 891–898. [Google Scholar] [CrossRef]
- Ahmadi, R.; Amini, M.; Bennett, J. Pt–Co alloy nanoparticles synthesized on sulfur-modified carbon nanotubes as electrocatalysts for methanol electrooxidation reaction. J. Catal. 2012, 292, 81–89. [Google Scholar] [CrossRef]
- Xu, C.; Hou, J.; Pang, X.; Li, X.; Zhu, M.; Tang, B. Nanoporous PtCo and PtNi alloy ribbons for methanol electrooxidation. Int. J. Hyd. Energy 2012, 37, 10489–10498. [Google Scholar] [CrossRef]
- Choi, S.-I.; Xie, S.; Shao, M.; Odell, J.H.; Lu, N.; Peng, H.-C.; Protsailo, L.; Guerrero, S.; Park, J.; Xia, X. Synthesis and characterization of 9 nm Pt–Ni octahedra with a record high activity of 3.3 A/mgPt for the oxygen reduction reaction. Nano Lett. 2013, 13, 3420–3425. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Gan, L.; Heggen, M.; Rudi, S.; Strasser, P. Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis. Nat. Mater. 2013, 12, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, D.; Dong, D.; Wang, H.; Webley, P.A. One-step fabrication of ordered Pt–Cu alloy nanotube arrays for ethanol electrooxidation. Mater. Lett. 2010, 64, 1169–1172. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, G.; Geng, D.; Chen, Y.; Banis, M.N.; Li, R.; Cai, M.; Sun, X. Direct growth of single-crystal Pt nanowires on Sn@CNT nanocable: 3D electrodes for highly active electrocatalysts. Chem. A Eur. J. 2010, 16, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Guo, J.; Liu, J.; Zou, Z.; Akins, D.L.; Yang, H. Highly alloyed PtRu black electrocatalysts for methanol oxidation prepared using magnesia nanoparticles as sacrificial templates. J. Power Sources. 2014, 248, 356–362. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, T.; Prabhuram, J.; Chen, R.; Wong, C. Preparation and characterization of a PtRu/C nanocatalyst for direct methanol fuel cells. Electrochim. Acta 2005, 51, 754–763. [Google Scholar] [CrossRef]
- Lipkowski, J.; Ross, P.N. Electrocatalysis; John Wiley & Sons: New York, NY, USA, 1998; Volume 3, pp. 70–200. [Google Scholar]
- Kua, J.; Goddard, W.A. Oxidation of methanol on 2nd and 3rd row group viii transition metals (Pt, Ir, Os, Pd, Rh, and Ru): Application to direct methanol fuel cells. J. Am. Chem. Soc. 1999, 121, 10928–10941. [Google Scholar] [CrossRef]
- Tong, Y.; Rice, C.; Wieckowski, A.; Oldfield, E. A detailed NMR-based model for CO on Pt catalysts in an electrochemical environment: Shifts, relaxation, back-bonding, and the Fermi-Level local density of states. J. Am. Chem. Soc. 2000, 122, 1123–1129. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, Z.; Song, S.; Li, W.; Sun, G.; Tsiakaras, P.; Xin, Q. Pt based anode catalysts for direct ethanol fuel cells. Appl. Catal. B 2003, 46, 273–285. [Google Scholar] [CrossRef]
- Liu, H.; Song, C.; Zhang, L.; Zhang, J.; Wang, H.; Wilkinson, D.P. A review of anode catalysis in the direct methanol fuel cell. J. Power Sour. 2006, 155, 95–110. [Google Scholar] [CrossRef]
- Liu, Z.; Lee, J.Y.; Han, M.; Chen, W.; Gan, L.M. Synthesis and characterization of PtRu/C catalysts from microemulsions and emulsions. J. Mater. Chem. 2002, 12, 2453–2458. [Google Scholar] [CrossRef]
- Almeida, T.; Palma, L.; Leonello, P.; Morais, C.; Kokoh, K.; de Andrade, A. An optimization study of PtSn/C catalysts applied to direct ethanol fuel cell: Effect of the preparation method on the electrocatalytic activity of the catalysts. J. Power Sour. 2012, 215, 53–62. [Google Scholar] [CrossRef]
- Liu, L.; Huang, Z.; Wang, D.; Scholz, R.; Pippel, E. The fabrication of nanoporous Pt-based multimetallic alloy nanowires and their improved electrochemical durability. Nanotechnology 2011, 22, 105604. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Waje, M.; Li, W.; Yan, Y. Supportless Pt and PtPd nanotubes as electrocatalysts for oxygen-reduction reactions. Angew. Chem. Int. Ed. 2007, 46, 4060–4063. [Google Scholar] [CrossRef] [PubMed]
- You, T.; Niwa, O.; Tomita, M.; Hirono, S. Characterization of platinum nanoparticle-embedded carbon film electrode and its detection of hydrogen peroxide. Anal. Chem. 2003, 75, 2080–2085. [Google Scholar] [CrossRef] [PubMed]
- Weissmüller, J.; Viswanath, R.; Kramer, D.; Zimmer, P.; Würschum, R.; Gleiter, H. Charge-induced reversible strain in a metal. Science 2003, 300, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Geng, H.; Wang, X.; Zhao, C.; Ji, H.; Zhang, C.; Xu, J.; Zhang, Z. Novel nanocrystalline PdNi alloy catalyst for methanol and ethanol electro-oxidation in alkaline media. J. Power Sour. 2011, 196, 5823–5828. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Y.; Hao, Q.; Duan, H. Nanoporous PdNi alloys as highly active and methanol-tolerant electrocatalysts towards oxygen reduction reaction. J. Mater. Chem. A 2013, 1, 13542–13548. [Google Scholar] [CrossRef]
- Jeon, M.K.; Cooper, J.S.; McGinn, P.J. Methanol electro-oxidation by a ternary Pt–Ru–Cu catalyst identified by a combinatorial approach. J. Power Sour. 2008, 185, 913–916. [Google Scholar] [CrossRef]
- Naidoo, Q.-L.; Naidoo, S.; Petrik, L.; Nechaev, A.; Ndungu, P.; Vaivars, G. Synthesis highly active platinum tri-metallic electrocatalysts using “one-step” organometallic chemical vapour deposition technique for methanol oxidation process. IOP Conf. Ser. Mater. Sci. Eng. 2012. [Google Scholar] [CrossRef]
- Kang, D.K.; Noh, C.S.; Park, S.T.; Sohn, J.M.; Kim, S.K.; Park, Y.-K. The effect of PtRuW ternary electrocatalysts on methanol oxidation reaction in direct methanol fuel cells. Korean J. Chem. Eng. 2010, 27, 802–806. [Google Scholar] [CrossRef]
- Jeon, M.K.; Lee, K.R.; Woo, S.I. Ternary Pt45Ru45M10/C (M = Mn, Mo and W) catalysts for methanol and ethanol electro-oxidation. Korean J. Chem. Eng. 2009, 26, 1028–1033. [Google Scholar] [CrossRef]
- Aricò, A.S.; Antonucci, P.L.; Modica, E.; Baglio, V.; Kim, H.; Antonucci, V. Effect of PtRu alloy composition on high-temperature methanol electro-oxidation. Electrochim. Acta 2002, 47, 3723–3732. [Google Scholar] [CrossRef]
- Ammam, M.; Easton, E.B. Quaternary PtMnCuX/C (X = Fe, Co, Ni, and Sn) and PtMnMoX/C (X = Fe, Co, Ni, Cu and Sn) alloys catalysts: Synthesis, characterization and activity towards ethanol electrooxidation. J. Power Sour. 2012, 215, 188–198. [Google Scholar] [CrossRef]
- Qian, L.; Chen, M. Ultrafine nanoporous gold by low-temperature dealloying and kinetics of nanopore formation. Appl. Phys. Lett. 2007, 91, 083105. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, Y.; Sun, J.; Jin, C.; Zhang, Z. Highly active nanoporous Pt-based alloy as anode and cathode catalyst for direct methanol fuel cells. J. Power Sour. 2014, 267, 212–218. [Google Scholar] [CrossRef]
- Pozio, A.; de Francesco, M.; Cemmi, A.; Cardellini, F.; Giorgi, L. Comparison of high surface Pt/C catalysts by cyclic voltammetry. J. Power Sour. 2002, 105, 13–19. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Ko, A.-R.; Han, S.-B.; Kim, H.-S.; Park, K.-W. Synthesis of octahedral Pt–Pd alloy nanoparticles for improved catalytic activity and stability in methanol electrooxidation. Phys. Chem. Chem. Phys. 2011, 13, 5569–5572. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Cui, C.H.; Zhao, S.; Yao, H.B.; Gao, M.R.; Fan, F.J.; Yu, S.H. Mixed-PtPd-shell PtPdCu nanoparticle nanotubes templated from copper nanowires as efficient and highly durable electrocatalysts. Adv. Energy Mater. 2012, 2, 1182–1187. [Google Scholar] [CrossRef]
- He, C.; Liang, Y.; Fu, R.; Wu, D.; Song, S.; Cai, R. Nanopores array of ordered mesoporous carbons determine Pt’s activity towards alcohol electrooxidation. J. Mater. Chem. 2011, 21, 16357–16364. [Google Scholar] [CrossRef]
- Mancharan, R.; Goodenough, J.B. Methanol oxidation in acid on ordered NiTi. J. Mater. Chem. 1992, 2, 875–887. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Wang, R.; Wang, K.; Zhang, Y.; Tian, F.; Ding, Y. Nanotubular mesoporous bimetallic nanostructures with enhanced electrocatalytic performance. Adv. Mater. 2009, 21, 2165–2169. [Google Scholar] [CrossRef]
- Maillard, F.; Schreier, S.; Hanzlik, M.; Savinova, E.R.; Weinkauf, S.; Stimming, U. Influence of particle agglomeration on the catalytic activity of carbon-supported Pt nanoparticles in Co monolayer oxidation. Phys. Chem. Chem. Phys. 2005, 7, 385–393. [Google Scholar] [CrossRef]
- Ley, K.L.; Liu, R.; Pu, C.; Fan, Q.; Leyarovska, N.; Segre, C.; Smotkin, E. Methanol oxidation on single-phase Pt-Ru-Os ternary alloys. J. Electrochem. Soc. 1997, 144, 1543–1548. [Google Scholar] [CrossRef]
- Lei, H.-W.; Suh, S.; Gurau, B.; Workie, B.; Liu, R.; Smotkin, E.S. Deuterium isotope analysis of methanol oxidation on mixed metal anode catalysts. Electrochim. Acta 2002, 47, 2913–2919. [Google Scholar] [CrossRef]
- Zhang, X.; Choi, I.; Qu, D.; Wang, L.; Lee, C.-W.J. Coverage-dependent electro-catalytic activity of Pt sub-monolayer/Au bi-metallic catalyst toward methanol oxidation. Int. J. Hyd. Energy 2013, 38, 5665–5670. [Google Scholar] [CrossRef]
- Hodnik, N.; Jeyabharathi, C.; Meier, J.C.; Kostka, A.; Phani, K.L.; Rečnik, A.; Bele, M.; Hočevar, S.; Gaberšček, M.; Mayrhofer, K.J. Effect of ordering of PtCu3 nanoparticle structure on the activity and stability for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2014, 16, 13610–13615. [Google Scholar] [CrossRef] [PubMed]
- Stephens, I.E.L.; Bondarenko, A.S.; Grønbjerg, U.; Rossmeisl, J.; Chorkendorff, I. Understanding the electrocatalysis of oxygen reduction on platinum and its alloys. Energy Environ. Sci. 2012, 5, 6744–6762. [Google Scholar] [CrossRef]
- Hu, Y.; Jensen, J.O.; Zhang, W.; Cleemann, L.N.; Xing, W.; Bjerrum, N.J.; Li, Q. Hollow spheres of iron carbide nanoparticles encased in graphitic layers as oxygen reduction catalysts. Angew. Chem. Int. Ed. 2014, 53, 3675–3679. [Google Scholar] [CrossRef] [PubMed]
- Kongkanand, A.; Kuwabata, S.; Girishkumar, G.; Kamat, P. Single-wall carbon nanotubes supported platinum nanoparticles with improved electrocatalytic activity for oxygen reduction reaction. Langmuir 2006, 22, 2392–2396. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, S.; Sun, S. Tuning nanoparticle catalysis for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2013, 52, 8526–8544. [Google Scholar] [CrossRef] [PubMed]
- Toda, T.; Igarashi, H.; Uchida, H.; Watanabe, M. Enhancement of the electroreduction of oxygen on Pt alloys with Fe, Ni, and Co. J. Electrochem. Soc. 1999, 146, 3750–3756. [Google Scholar] [CrossRef]
- Toda, T.; Igarashi, H.; Watanabe, M. Role of electronic property of Pt and Pt alloys on electrocatalytic reduction of oxygen. J. Electrochem. Soc. 1998, 145, 4185–4188. [Google Scholar] [CrossRef]
- Zhang, J.; Vukmirovic, M.B.; Xu, Y.; Mavrikakis, M.; Adzic, R.R. Controlling the catalytic activity of platinum-monolayer electrocatalysts for oxygen reduction with different substrates. Angew. Chem. Int. Ed. 2005, 44, 2132–2135. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Si, C.; Gao, Y.; Frenzel, J.; Sun, J.; Eggeler, G.; Zhang, Z. Multi-component nanoporous platinum-ruthenium-copper-osmium-iridium alloy with enhanced electrocatalytic activity towards methanol oxidation and oxygen reduction. J. Power Sour. 2015, 273, 324–332. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wang, H.; Wang, Y.; Bai, Q.; Gao, Y.; Zhang, Z. Synthesis and Electrocatalytic Performance of Multi-Component Nanoporous PtRuCuW Alloy for Direct Methanol Fuel Cells. Catalysts 2015, 5, 1003-1015. https://doi.org/10.3390/catal5031003
Chen X, Wang H, Wang Y, Bai Q, Gao Y, Zhang Z. Synthesis and Electrocatalytic Performance of Multi-Component Nanoporous PtRuCuW Alloy for Direct Methanol Fuel Cells. Catalysts. 2015; 5(3):1003-1015. https://doi.org/10.3390/catal5031003
Chicago/Turabian StyleChen, Xiaoting, Hao Wang, Ying Wang, Qingguo Bai, Yulai Gao, and Zhonghua Zhang. 2015. "Synthesis and Electrocatalytic Performance of Multi-Component Nanoporous PtRuCuW Alloy for Direct Methanol Fuel Cells" Catalysts 5, no. 3: 1003-1015. https://doi.org/10.3390/catal5031003
APA StyleChen, X., Wang, H., Wang, Y., Bai, Q., Gao, Y., & Zhang, Z. (2015). Synthesis and Electrocatalytic Performance of Multi-Component Nanoporous PtRuCuW Alloy for Direct Methanol Fuel Cells. Catalysts, 5(3), 1003-1015. https://doi.org/10.3390/catal5031003