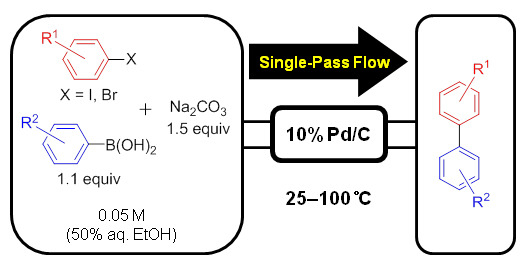
Palladium on Carbon-Catalyzed Suzuki-Miyaura Coupling Reaction Using an Efficient and Continuous Flow System
Abstract
:1. Introduction
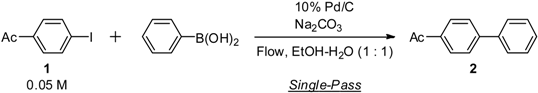
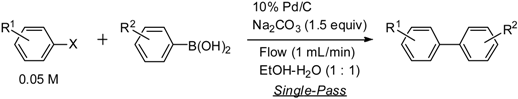
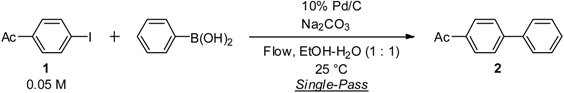
2. Results and Discussion
| Entry | Flow rate (mL/min) | Temperature (°C) | Na2CO3 (equiv) | 1H NMR ratio |
|---|---|---|---|---|
| 1:2 | ||||
| 1 | 1 | 25 | 1.5 | 0:100 (100) a |
| 2 | 2 | 25 | 1.5 | 21:79 |
| 3 | 2 | 100 | 1.5 | 0:100 |
| 4 | 3 | 25 | 1.5 | 32:68 |
| 5 | 3 | 100 | 1.5 | 6:94 |
| 6 | 1 | 25 | 1.2 | 56:44 |
| 7 | 1 | 25 | 1.0 | 100:0 |
| Entry | X | R1 | R2 | Temperature (°C) | Yield (%) a |
|---|---|---|---|---|---|
| 1 [9] | I | 4-Ac | H | 25 | 100 |
| 2 [16] | I | 4-CO2Et | H | 25 | 92 |
| 3 [18] | I | 2-CO2Et | H | 75 | 88 |
| 4 [9] | I | 4-Me | H | 75 | 91 |
| 5 [16] | I | 4-OMe | H | 75 | 81 |
| 6 [19] | I | 4-Ac | 3-Ac | 25 | 99 |
| 7 [18] | I | 4-CO2Et | 4-Ac | 25 | 91 |
| 8 [9] | I | 4-OMe | 4-Ac | 75 | 97 |
| 9 [9] | I | 4-Ac | 4-OMe | 50 | 89 |
| 10 [18] | I | 4-CO2Et | 4-OMe | 75 | 97 |
| 11 [20] | I | 4-Me | 4-OMe | 75 | 100 |
| 12 [21] | I | 3-Me | 4-OMe | 75 | 98 |
| 13 [21] | I | 2-Me | 4-OMe | 75 | 98 |
| 14 [22] | I | 4-OMe | 2,4-di-OMe | 100 | 78 |
| 15 [9] | Br | 4-Ac | H | 50 | 90 |
| 16 [16] | Br | 4-CO2Et | H | 100 | 78 |
| 17 [9] | Br | 4-Ac | 4-OMe | 50 | 97 |
| 18 [18] | Br | 4-CO2Et | 4-OMe | 75 | 100 |
| Entry | Layer | 1H NMR ratio | Pd leaching |
|---|---|---|---|
| 1:2 | |||
| 1 | Organic | 0:100 (99) a | <1 ppm |
| 2 | Aquous | <1 ppm |
3. Experimental Section
3.1. General Procedure
3.2. Typical Procedure for the Palladium on Carbon-Catalyzed Cross-Coupling Reaction under Flow Conditions
3.3. Leaching Test of Palladium (Table 3)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Bulger, P.G.; Sarlah, D. Palladium-Catalyzed Cross-Coupling Reaction in Total Synthesis. Angew. Chem. Int. Ed. 2005, 44, 4442–4489. [Google Scholar] [CrossRef]
- Kotha, S.; Lahiri, K.; Kashinath, D. Recent Applications of the Suzuki-Miyaura Cross-Coupling Reaction in Organic Synthesis. Tetrahedron 2002, 58, 9633–9695. [Google Scholar] [CrossRef]
- Luis, S.V.; Garcia-Verdugo, E. Chemical Reactions and Processes under Flow Conditions; RSC Publishing: Cambridge, UK, 2010. [Google Scholar]
- Frost, C.G.; Mutton, L. Heterogeneous Catalytic Synthesis Using Microreactor Technology. Green Chem. 2010, 12, 1687–1703. [Google Scholar] [CrossRef]
- Noël, T.; Buchwald, S.L. Cross-coupling in Flow. Chem. Soc. Rev. 2011, 40, 5010–5029. [Google Scholar] [CrossRef]
- Gemoets, H.P.L.; Hessel, V.; Noël, T. Recent example for the flow reaction using homogeneous catalyst. Org. Lett. 2014, 16, 5800–5803. [Google Scholar] [CrossRef]
- Hattori, T.; Tsubone, A.; Sawama, Y.; Monguchi, Y.; Sajiki, H. Systematic Evaluation of the Palladium-Catalyzed Hydrogenation under Flow Condition. Tetrahedron 2014, 70, 4790–4798. [Google Scholar] [CrossRef]
- Mennecke, K.; Kirschning, A. Polyionic Polymers-Heterogeneous Media for Metal Nanoparticles as Catalyst in Suzuki-Miyaura and Heck-Mizoroki Reactions under Flow Conditions. Beilstein. J. Org. Chem. 2009, 5. [Google Scholar] [CrossRef]
- De Muñouz, J.M.; Alcázar, J.; de la Hoz, A.; Díaz-Ortiz, A. Cross-Coupling in Flow Using Supported Catalysts: Mild, Clean, Efficient and Sustainable Suzuki-Miyaura Coupling in a Single Pass. Adv. Synth. Catal. 2012, 354, 3456–3460. [Google Scholar] [CrossRef]
- Mateos, C.; Rincón, J.A.; Martin-Hidalgo, B.; Villanueva, J. Green and Scalable Procedure for Extremely Fast Ligandless Suzuki-Miyaura Cross-Coupling Reactions in Aqueous IPA Using Solid-Supported Pd in Continuous Flow. Tetrahedron Lett. 2014, 55, 3701–3705. [Google Scholar] [CrossRef]
- Glasnov, T.N.; Kappe, C.O. Toward a Continuous-Flow Synthesis of Boscalid. Adv. Synth. Catal. 2010, 352, 3089–3097. [Google Scholar] [CrossRef]
- Nӧel, T.; Musacchio, A.J. Suzuki-Miyaura Cross-Coupling of Heteroaryl Halides and Arylboronic Acids in Continuous Flow. Org. Lett. 2011, 13, 5180–5183. [Google Scholar] [CrossRef] [PubMed]
- Shu, W.; Pellegatti, L.; Oberli, M.A.; Buchwald, S.L. Continuous-Flow Synthesis of Biaryls Enabled by Multistep Solid-Handling in a Lithiation/Borylation/Suzuki-Miyaura Cross-Coupling. Angew. Chem. Int. Ed. 2011, 50, 10665–10669. [Google Scholar] [CrossRef]
- Christakakou, M.; Shӧn, M.; Schnürch, M.; Mihovilovic, M.D. Arylation of Pyridines via Suzuki-Miyaura Cross-Coupling and Pyridine-Directed C-H Activation Using a Continuous-Flow Approach. Synlett 2013, 24, 2411–2418. [Google Scholar] [CrossRef]
- Maegawa, T.; Kitamura, Y.; Satoko, S.; Udzu, T.; Sakurai, A.; Tanaka, A.; Kobayashi, Y.; Endo, K.; Bora, U.; Kurita, T.; et al. Heterogeneous Pd/C-Catalyzed Ligand-Free, Room-Temperature Suzuki-Miyaura Coupling Reactions in Aqueous Media. Chem. Eur. J. 2007, 13, 5937–5943. [Google Scholar] [CrossRef] [PubMed]
- Cantillo, D.; Kappe, C.O. Immobilized Transition Metals as Catalysts for Cross-Couplings in Continuous Flow—A Critical Assessment of the Reaction Mechanism and Metal Leaching. ChemCatChem 2014, 6, 3286–3305. [Google Scholar]
- Baghbanzadeh, M.; Pilger, C.; Kappe, C.O. Rapid Nickel-Catalyzed Suzuki-Miyaura Cross-Coupling of Aryl Carbamates and Sulfamates Utilizing Microwave-Heating. J. Org. Chem. 2011, 76, 1507–1510. [Google Scholar] [CrossRef] [PubMed]
- Gauchot, V.; Kroutil, W.; Schmitzer, A.R. Highly Recyclable Chemo-/Biocatalyzed Cascade Reactions with Ionic Liquids: One-Pot Synthesis of Chiral Biaryl Alcohols. Chem. Eur. J. 2010, 16, 6748–6751. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Sakurai, A.; Udzu, T.; Maegawa, T.; Tanaka, A.; Monguchi, Y.; Sajiki, H. Heterogeneous Pd/C-Catalyzed Ligand-Free Suzuki-Miyaura Coupling Reaction Using Aryl Boronic Esters. Tetrahedron 2007, 63, 10596–10602. [Google Scholar] [CrossRef]
- Li, X.-J.; Zhang, J.-L.; Geng, Y.; Jin, Z. Nickel-Catalyzed Suzuki-Miyaura Coupling of Heteroarylethers with Arylboronic Acid. J. Org. Chem. 2013, 78, 5078–5084. [Google Scholar] [CrossRef] [PubMed]
- Subhas, M.S.; Racharlawar, S.S.; Sridhar, B.; Kennady, P.K.; Likhar, P.R.; Kantam, M.L.; Bhargava, S.K. New Cyclopalladated Benzothiophenes: A Catalyst Precursor for the Suzuki Coupling of Deactivated Aryl Chlorides. Org. Biomol. Chem. 2010, 8, 3001–3006. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hattori, T.; Tsubone, A.; Sawama, Y.; Monguchi, Y.; Sajiki, H. Palladium on Carbon-Catalyzed Suzuki-Miyaura Coupling Reaction Using an Efficient and Continuous Flow System. Catalysts 2015, 5, 18-25. https://doi.org/10.3390/catal5010018
Hattori T, Tsubone A, Sawama Y, Monguchi Y, Sajiki H. Palladium on Carbon-Catalyzed Suzuki-Miyaura Coupling Reaction Using an Efficient and Continuous Flow System. Catalysts. 2015; 5(1):18-25. https://doi.org/10.3390/catal5010018
Chicago/Turabian StyleHattori, Tomohiro, Aya Tsubone, Yoshinari Sawama, Yasunari Monguchi, and Hironao Sajiki. 2015. "Palladium on Carbon-Catalyzed Suzuki-Miyaura Coupling Reaction Using an Efficient and Continuous Flow System" Catalysts 5, no. 1: 18-25. https://doi.org/10.3390/catal5010018
APA StyleHattori, T., Tsubone, A., Sawama, Y., Monguchi, Y., & Sajiki, H. (2015). Palladium on Carbon-Catalyzed Suzuki-Miyaura Coupling Reaction Using an Efficient and Continuous Flow System. Catalysts, 5(1), 18-25. https://doi.org/10.3390/catal5010018