Application of Fischer–Tropsch Synthesis in Biomass to Liquid Conversion
Abstract
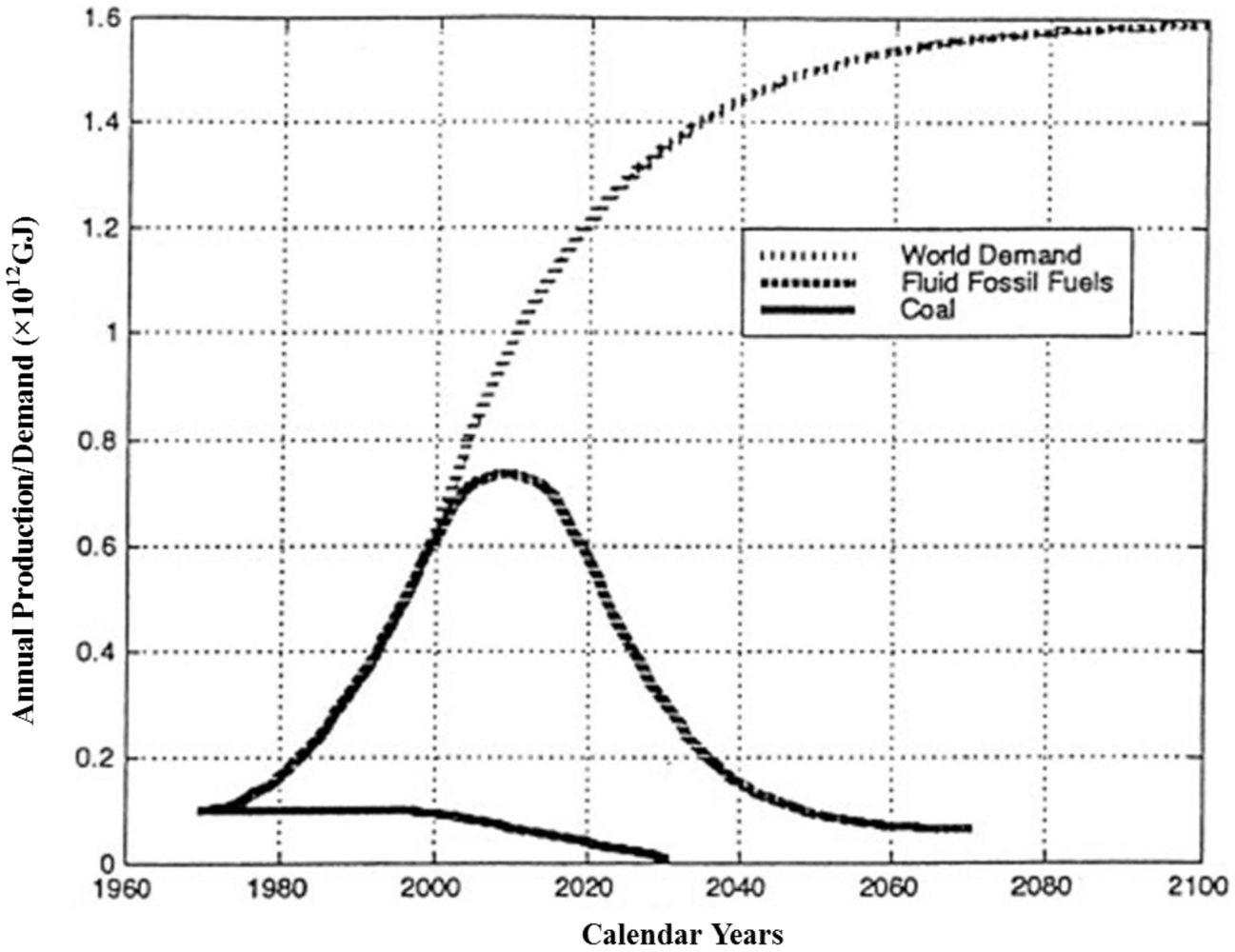
:1. Introduction
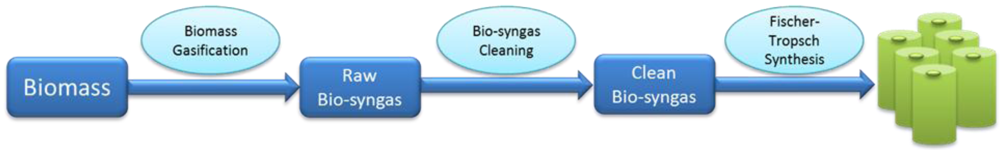
2. Process Analysis
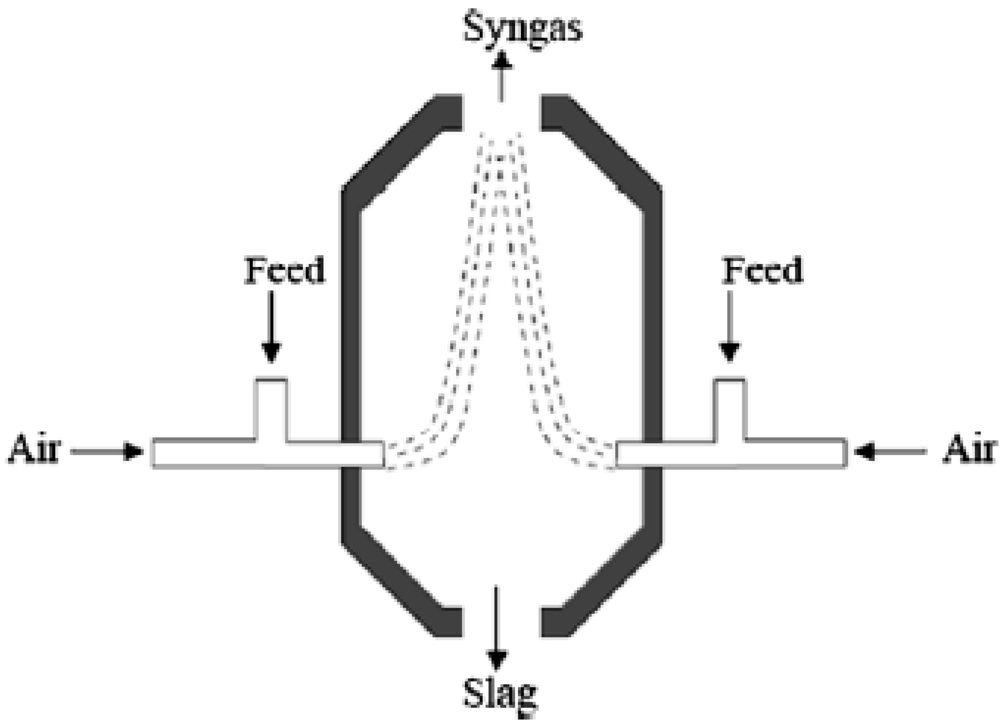
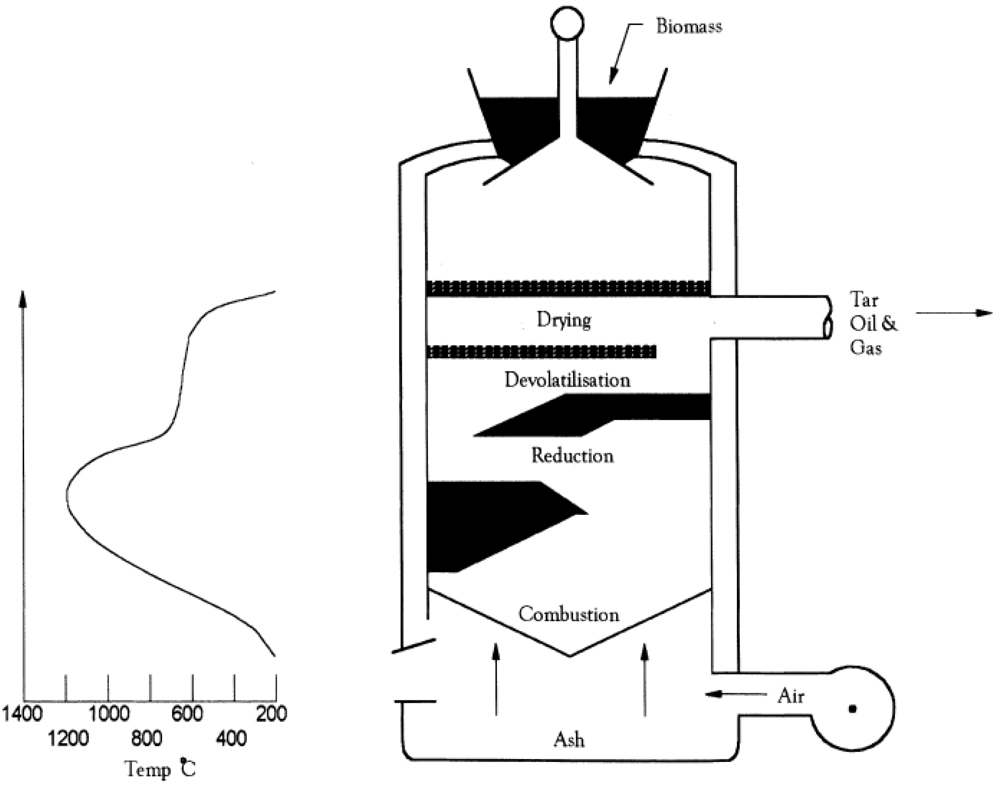
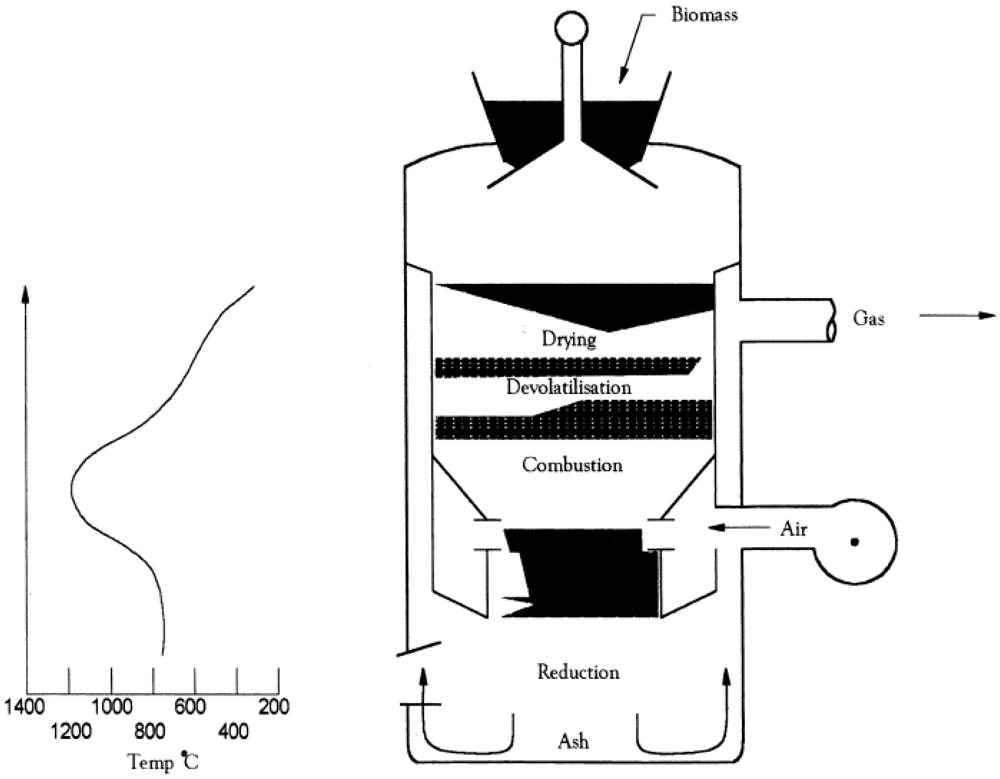
2.1. Biomass Gasification
| Biomass | Ultimate analysis (wt%) | HHV a (MJ/kg) | Density (kg/m3) | x | y | z | Percentage conversion of carbon | |||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | |||||||
| Bagasse | 43.8 | 5.8 | 0.4 | 47.1 | 16.29 | 111 | 3.65 | 5.8 | 2.94 | 81 |
| Coconut coir | 47.6 | 5.7 | 0.2 | 45.6 | 14.67 | 151 | 3.97 | 5.7 | 2.85 | 72 |
| Coconut Shell | 50.2 | 5.7 | 0 | 43.4 | 20.5 | 661 | 4.18 | 5.7 | 2.71 | 65 |
| Coir pith | 44 | 4.7 | 0.7 | 43.4 | 18.07 | 94 | 3.67 | 4.7 | 2.71 | 74 |
| Corn Cob | 47.6 | 5 | 0 | 44.6 | 15.65 | 188 | 3.97 | 5 | 2.79 | 70 |
| Corn stalks | 41.9 | 5.3 | 0 | 46 | 16.54 | 129 | 3.49 | 5.3 | 2.88 | 82.3 |
| Cotton gin waste | 42.7 | 6 | 0.1 | 49.5 | 17.48 | 109 | 3.56 | 6 | 3.1 | 87 |
| Ground nut shell | 48.3 | 5.7 | 0.8 | 39.4 | 18.65 | 299 | 4.03 | 5.7 | 2.46 | 61.2 |
| Millet husk | 42.7 | 6 | 0.1 | 33 | 17.48 | 201 | 3.56 | 6 | 2.06 | 58 |
| Rice husk | 38.9 | 5.1 | 0.6 | 32 | 15.29 | 617 | 3.24 | 5.1 | 2 | 62 |
| Rice straw | 36.9 | 5 | 0.4 | 37.9 | 16.78 | 259 | 3.08 | 5 | 2.37 | 82.4 |
| Subabul wood | 48.2 | 5.9 | 0 | 45.1 | 19.78 | 259 | 4.02 | 5.9 | 2.82 | 70.2 |
| Wheat straw | 47.5 | 5.4 | 0.1 | 35.8 | 17.99 | 222 | 3.96 | 5.4 | 2.24 | 56.5 |
| AVERAGE | 44.6 | 5.5 | 0.3 | 41.8 | 17.32 | 253.84 | 3.72 | 5.49 | 2.61 | 70.89 |
| Advantages | Disadvantages |
|---|---|
| Fixed/moving bed, updraft | |
| Simple, inexpensive process Exit gas temperature about 250 °C Operates satisfactorily under pressure High carbon conversion efficiency Low dust levels in gas High thermal efficiency | Large tar production Potential channeling Potential bridging Small feed size Potential clinkering |
| Fixed/moving bed, downdraft | |
| Simple process Only traces of tar in gas product | Minimum feed size Limited ash content allowable in feed Limits to scale up capacity Potential for bridging and clinkering |
| Fluidized bed | |
| Flexible feed rate and composition High ash fuels acceptable Able to pressurize High CH4 in gas product High volumetric capacity Easy temperature control | Operating temperature limited by ash clinkering High gas product temperature High tar and fines content in gas Possibility of high C content in fly ash |
| Circulating fluidized bed | |
| Flexible process Up to 850 °C operating temperature | Corrosion and attrition problems Poor operational control using biomass |
| Double fluidized bed | |
| Oxygen not required High CH4 due to low bed Temperature Temperature limit in the oxidizer | More tar due to lower bed temperature Difficult to operate under pressure |
| Entrained bed | |
| Very low in tar and CO2 Flexible to feedstock Exit gas temperature | Low in CH4 Extreme feedstock size reduction required Complex operational control Carbon loss with ash Ash slagging |
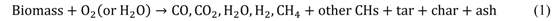
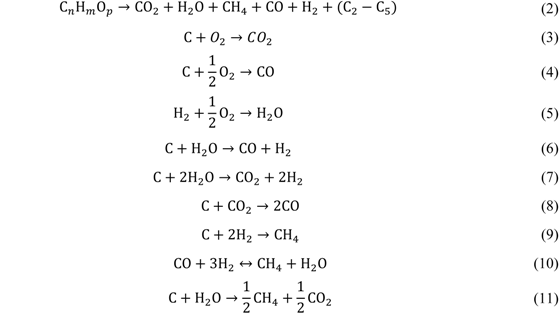
| Component | Wood gas (air) | Charcoal gas (air) | Bio-syngas (nitrogen free) |
|---|---|---|---|
| N2 | 50–60 | 55–65 | 0 |
| CO | 14–25 | 28–32 | 28–36 |
| CO2 | 9–15 | 1–3 | 22–32 |
| H2 | 10–20 | 4–10 | 21–30 |
| CH4 | 2–6 | 0–2 | 8–11 |
| C2H4 | n/a | n/a | 2–4 |
| BTX | n/a | n/a | 0.84–0.96 |
| C2H5 | n/a | n/a | 0.16–0.22 |
| Tar | n/a | n/a | 0.15–0.24 |
| Others | n/a | n/a | <0.021 |
2.2. Bio-Syngas Cleaning
| Impurity | Specification |
|---|---|
| H2S + COS + CS2 | <1 ppmv a |
| NH3 + HCN | <1 ppmv |
| HCl + HBr + HF | <10 ppbv b |
| Alkali metals (Na + K) | <10 ppbv |
| Particles (soot, ash) | “almost removed” |
| Organic components (tar) | below dew point |
| Hetero-organic components (S, N, O) | <1 ppmv |
2.2.1. Organic Impurities Removal
2.2.2. Inorganic and Other Impurities Removal
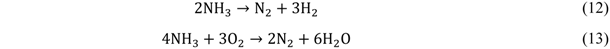
2.3. Fischer–Tropsch Synthesis
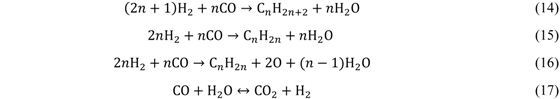
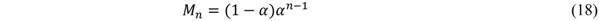
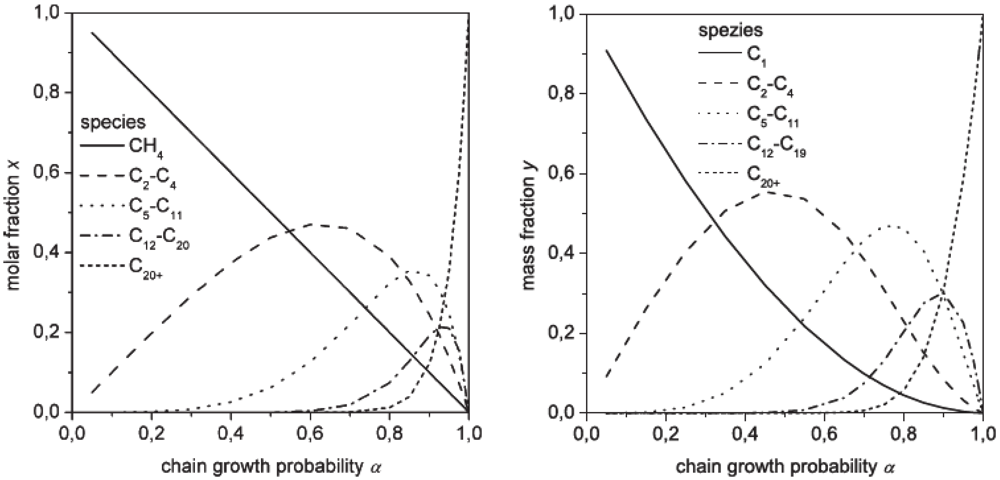
| Feature | Fixed bed | Fluid bed (circulating) | Slurry |
|---|---|---|---|
| Temperature control | Poor | Good | Good |
| Heat exchanger surface | 240 m2 per 1000 m3 feed | 15–30 m2 per 2000 m3 feed | 50 m2 per 1000 m3 feed |
| Max. reactor diameter | <0.08 m | Large | Large |
| CH4 formation | Low | High | As fixed bed or lower |
| Flexibility | Intermediate | Little | High |
| Product | Full range | Low mol. Weight | Full range |
| Space-time yield (C2+) | >1000 kg/m3 day | 4000–12000 kg/m3 day | 1000 kg/m3 day |
| Catalyst affectivity | Lowest | Highest | Intermediate |
| Back-mixing | Little | Intermediate | Large |
| Minimum H2/CO feed | As slurry or higher | Highest | Lowest |
| Construction | Simplest |
3. Increasing Carbon Utilization
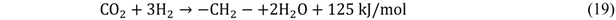
4. Enhancing Catalyst Activity
5. Selectivity Maximization
6. Catalyst Deactivation
6.1. Carbon Deposition Related Deactivation
6.2. Sintering (Aging)
| Variable | Effect |
|---|---|
| Temperature | Sintering rates are exponentially dependent on T; Eact varies from 30 to 150 KJ/mol; Eact decreases with increasing metal loading; it increases in the following order with atmosphere: NO, O2, H2, N2 |
| Atmosphere | Sintering rates are much higher for noble metals in O2 than in H2 and higher for noble and base metals in H2 relative to N2; sintering rate decreases for supported Pt in atmospheres in the following order: NO, O2, H2, N2 |
| Metal | Observed order of decreasing thermal stability in H2 is Ru > Ir ≈ Rh > Pt; thermal stability in O2 is a function of (1) volatility of metal oxide and (2) strength of metal oxide-support interaction |
| Support | Metal-support interactions are weak (bond strengths of 5–15 KJ/mol); with a few exceptions, thermal stability for a given metal decreases with support in the following order Al2O3 > SiO2 > carbon |
| Promoters | Some additives decrease atom mobility, e.g., C, O, CaO, BaO, CeO2, GeO2; others increase atom mobility, e.g., Pb, Bi, Cl, F, or S; oxides of Ba, Ca, or Sr are “trapping agents” that decrease sintering rate |
| Pore size | Sintering rates are lower for porous vs. non-porous supports; they decrease as crystallite diameters approach those of the pores |
6.3. Poisoning
7. Conclusion and Outlook
Acknowledgments
Disclaimer
References
- Kaygusuz, K. Energy for sustainable development: A case of developing countries. Renew. Sustain. Energy Rev. 2012, 16, 1116–1126. [Google Scholar] [CrossRef]
- Veziroğlu, T.N.; Şahi'n, S. 21st Century’s energy: Hydrogen energy system. Energy Convers. Manag. 2008, 49, 1820–1831. [Google Scholar] [CrossRef]
- Davis, S.J.; Caldeira, K. Consumption-based accounting of CO2 emissions. Proc. Natl. Acad. Sci. USA 2010, 107, 5687–5692. [Google Scholar]
- Street, J.; Yu, F. Production of high-value products including gasoline hydrocarbons from thermochemical conversion of syngas. Biofuels 2011, 2, 677–691. [Google Scholar] [CrossRef]
- Le Quere, C.; Raupach, M.R.; Canadell, J.G.; Marland, G. Trends in the sources and sinks of carbon dioxide. Nat. Geosci. 2009, 2, 831–836. [Google Scholar] [CrossRef]
- Tilman, D.; Socolow, R.; Foley, J.A.; Hill, J.; Larson, E.; Lynd, L.; Pacala, S.; Reilly, J.; Searchinger, T.; Somerville, C.; Williams, R. Beneficial biofuels—the food, energy, andenvironment trilemma. Science 2009, 325, 270–271. [Google Scholar]
- Yang, J.H.; Kim, H.J.; Chun, D.H.; Lee, H.T.; Hong, J.C.; Jung, H.; Yang, J.I. Mass transfer limitations on fixed-bed reactor for Fischer–Tropsch synthesis. Fuel Process. Technol. 2010, 91, 285–289. [Google Scholar] [CrossRef]
- Demirbas, A. Global biodiesel strategies. Energy Educ. Sci. Technol. 2006, 17, 27–63. [Google Scholar]
- Tijmensen, M.J.A.; Faaij, A.P.C.; Hamelinck, C.N.; van Hardeveld, M.R.M. Exploration of the possibilities for production of Fischer Tropsch liquids and power via biomass gasification. Biomass Bioenergy 2002, 23, 129–152. [Google Scholar] [CrossRef]
- Klass, D.L. Biomass for Renewable Energy, Fuels and Chemicals; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Beenackers, A.A.C.M.; Swaaij, W.P.M. Thermochemical Processing of Biomass; Butterworth: London, UK, 1984. [Google Scholar]
- Wei, L.; Thomasson, J.A.; Bricka, R.M.; Sui, R.; Wooten, J.R.; Columbus, E.P. Syngas quality evaluation for biomass gasification with a downdraft gasifier. Trans. ASABE 2009, 52, 21–37. [Google Scholar]
- Dry, M.E. The Fischer–Tropsch process: 1950–2000. Catal. Today 2002, 71, 227–241. [Google Scholar]
- den Breejen, J.P.; Radstake, P.B.; Bezemer, G.L.; Bitter, J.H.; Frøseth, V.; Holmen, A.; de Jong, K.P. On the origin of the cobalt particle size effects in Fischer–Tropsch catalysis. J. Am. Chem. Soc. 2009, 131, 7197–7203. [Google Scholar]
- Davis, B.H. Fischer–Tropsch synthesis: Reaction mechanisms for iron catalysts. Catal. Today 2009, 141, 25–33. [Google Scholar]
- den Breejen, J.P.; Sietsma, J.R.A.; Friedrich, H.; Bitter, J.H.; de Jong, K.P. Design of supported cobalt catalysts with maximum activity for the Fischer–Tropsch synthesis. J. Catal. 2010, 270, 146–152. [Google Scholar]
- Khodakov, A.Y. Fischer–Tropsch synthesis: Relations between structure of cobalt catalysts and their catalytic performance. Catal. Today 2009, 144, 251–257. [Google Scholar] [CrossRef]
- Yan, Z.; Bukur, D.B.; Goodman, D.W. Silica-supported rhodium-cobalt catalysts for Fischer–Tropsch synthesis. Catal. Today 2011, 160, 39–43. [Google Scholar]
- Lualdi, M.; Lögdberg, S.; Regali, F.; Boutonnet, M.; Järås, S. Investigation of mixtures of a Co-based catalyst and a Cu-based catalyst for the Fischer–Tropsch synthesis with bio-syngas: The importance of indigenous water. Top. Catal. 2011, 54, 977–985. [Google Scholar]
- Mogorosi, R.P.; Fischer, N.; Claeys, M.; van Steen, E. Strong-metal-support interaction by molecular design: Fe-silicate interactions in Fischer–Tropsch catalysts. J. Catal. 2012, 289, 140–150. [Google Scholar]
- Hamelinck, C.N.; Faaij, A.P.C.; den Uil, H.; Boerrigter, H. Production of FT transportation fuels from biomass: Technical options, process analysis and optimisation, and development potential. Energy 2004, 29, 1743–1771. [Google Scholar] [CrossRef]
- Raveendran, K.; Ganesh, A.; Khilar, K.C. Influence of mineral matter on biomass pyrolysis characteristics. Fuel 1995, 74, 1812–1822. [Google Scholar] [CrossRef]
- Kirubakaran, V.; Sivaramakrishnan, V.; Nalini, R.; Sekar, T.; Premalatha, M.; Subramanian, P. A review on gasification of biomass. Renew. Sustain. Energy Rev. 2009, 13, 179–186. [Google Scholar] [CrossRef]
- Faaij, A.; van den Broek, R.; van Engelenburg, B.; Lysen, E. Global availability of biomass for energy and possibilities and constraints for large scale international trade. In Proceedings of the Fifth International Conference on Greenhouse Gas Control Technologies; Williams, D.J., Ed.; CSIRO Publishing: Collingwood, Australia, 2001; pp. 1145–1151. [Google Scholar]
- Faaij, A.; van Ree, R.; Meuleman, B. Long Term Perspectives of Biomass Integrated Gasification with Combined Cycle Technology: Costs and Efficiency and a Comparison with Combustion; EWAB Report 9840; The Netherlands Agency for Energy and the Environmen (NOVEM): Utrecht, The Netherlands, 1998. [Google Scholar]
- McKendry, P. Energy production from biomass (part 3): Gasification technologies. Bioresour. Technol. 2002, 83, 55–63. [Google Scholar] [CrossRef]
- van Ree, R.; Oudhuis, A.; Faaij, A.; Curvers, A. Modelling of a Biomass Integrated Gasifier/Combined Cycle (BIG/CC) System with the Flowsheet Simulation Programme ASPEN+; ECN Report ECN-C–95-041; Energy research Centre of the Netherlands (ECN): Petten, The Netherlands, 1995. [Google Scholar]
- Bergman, P.C.A.; Boersma, A.R.; Zwart, R.W.R.; Kiel, J.H.A. Torrefaction for Biomass Co-Firing in Existing Coal-Fired Power Stations “BIOCOAL”; ECN Report ECN-C–05-013; Energy research Centre of the Netherlands (ECN): Petten, The Netherlands, 2005. [Google Scholar]
- Yaman, S. Pyrolysis of biomass to produce fuels and chemical feedstock. Energy Convers. Manag. 2004, 45, 651–671. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Evans, G.D. An Assessment of Thermochemical Conversion Systems for Processing Biomass and Refuse; ETSU Report ETSU/B/T–1/00207/REP; Energy Technology Support Unit (ETSU): Harwell, UK, 1993. [Google Scholar]
- Koppejan, J.; Meulman, P.D.M. The Market for Fuel Pellet Produced from Biomass and Waste in the Netherlands; EWAB Report 1; The Netherlands Agency for Energy and the Environmen (NOVEM): Utrecht, The Netherlands, 2001. [Google Scholar]
- Uslu, A.; Faaij, A.P.C.; Bergman, P.C.A. Pre-treatment technologies, and their effect on international bioenergy supply chain logistics. Techno-economic evaluation of torrefaction, fast pyrolysis and pelletisation. Energy 2008, 33, 1206–1223. [Google Scholar] [CrossRef]
- Prins, M.J. Thermodynamic analysis of biomass gasification and torrefaction. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands.
- Kumar, A.; Jones, D.; Hanna, M. Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Marsh, R.; Hewlett, S.; Griffiths, T.; Williams, K. Advanced thermal treatment for solid waste—a wastemanager’s guide. In Proceedings of the 22nd International Conference on Solid Waste Technology and Management, Philadelphia, PA, USA, 18–21 March 2007.
- Zhang, L.; Xu, C.; Champagne, P. Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Rampling, T.; Gill, P. Fundamental Research on the Thermal Treatment of Wastes and Biomass: Literature Review of Part Research on Thermal Treatment of Biomass and Waste; ETSU Report ETSU B/T1/00208/Rep/1; Energy Technology Support Unit (ETSU): Harwell, UK, 1993. [Google Scholar]
- Balat, M. New biofuel production technologies. Energy Educ. Sci. Technol. 2009, 22, 147–161. [Google Scholar]
- Rajvanshi, A.K. Biomass gasification. In Alternative Energy in Agriculture; Goswami, D.Y., Ed.; CRC Press: Boca Raton, FL, USA, 1986; Volume 2, pp. 83–102, Chapter 4. [Google Scholar]
- Cao, Y.; Wang, Y.; Riley, J.T.; Pan, W.P. A novel biomass air gasification process for producing tar-free higher heating value fuel gas. Fuel Process. Technol. 2006, 87, 343–353. [Google Scholar] [CrossRef]
- Balat, M. Mechanisms of thermochemical biomass conversion processes. Part 2: Reactions of gasification. Energy Sources A 2008, 30, 636–648. [Google Scholar] [CrossRef]
- Basu, P. Combustion and Gasification in Fluidized Beds; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Stassen, H.E.M.; Knoef, H.A.M. Small Scale Gasification Systems; Biomass Technology Group, University of Twente: Enschede, The Netherlands, 1993. [Google Scholar]
- Demirbas, A. Progress and recent trends in biofuels. Prog. Energy Combust. Sci. 2007, 33, 1–18. [Google Scholar] [CrossRef]
- Demirbas, A. Converting biomass derived synthetic gas to fuels via Fisher–Tropsch synthesis. Energy Sources A 2007, 29, 1507–1512. [Google Scholar] [CrossRef]
- Demirbas, A. Hydrogen production from carbonaceous solid wastes by steam reforming. Energy Sources A 2008, 30, 924–931. [Google Scholar] [CrossRef]
- Hanaoka, T.; Inoue, S.; Uno, S.; Ogi, T.; Minowa, T. Effect of woody biomass components on air-steam gasification. Biomass Bioenergy 2005, 28, 69–76. [Google Scholar] [CrossRef]
- Barneto, A.G.; Carmona, J.A.; Gálvez, A.; Conesa, J. Effects of the compositing and the heating rate on biomass gasification. Energy Fuels 2009, 23, 951–957. [Google Scholar] [CrossRef]
- Yamazaki, T.; Kozu, H.; Yamagata, S.; Murao, N.; Ohta, S.; Shiya, S.; Ohba, T. Effect of superficial velocity on tar from downdraft gasification of biomass. Energy Fuels 2005, 19, 1186–1191. [Google Scholar] [CrossRef]
- Lv, P.M.; Xiong, Z.H.; Chang, J.; Wu, C.Z.; Chen, Y.; Zhu, J.X. An experimental study on biomass air-steam gasification in a fluidized bed. Bioresour. Technol. 2004, 95, 95–101. [Google Scholar] [CrossRef]
- Lucas, C.; Szewczyk, D.; Blasiak, W.; Mochida, S. High-temperature air and steam gasification of densified biofuels. Biomass Bioenergy 2004, 27, 563–575. [Google Scholar] [CrossRef]
- Boerrigter, H.; Calis, H.P.; Slort, D.J. Cleaning for Integrated Biomass Gasification (BG) and Fischer–Tropsch (FT) Systems; ECN Report ECN-C–04-056; Energy research Centre of the Netherlands (ECN): Petten, The Netherlands, 2004. [Google Scholar]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G. A review of the primary measures for tar elimination in biomass gasification processes. Biomass Bioenergy 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Milne, T.A.; Evans, R.J.; Abatzoglou, N. Biomass Gasifier “Tars”: Their Nature, Formation and Conversion; NREL Report NREL/TP-570-25357; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 1998. [Google Scholar]
- Warnecke, R. Gasification of biomass: comparison of fixed bed and fluidized bed gasifier. Biomass Bioenergy 2000, 18, 489–497. [Google Scholar] [CrossRef]
- Boerrigter, H.; den Uil, H.; Calis, H. Green Diesel from Biomass via Fischer–Tropsch Synthesis: New Insights in Gas Cleaning and Process Design. In Pyrolysis and Gasification of Biomass and Waste; Bridgwater, A.V., Ed.; CPL Press: Newbury, UK, 2003; pp. 385–394. [Google Scholar]
- Li, Y.; Wang, T. 100t/a-Scale demonstration of direct dimethyl ether synthesis from corncob-derived syngas. Renew. Energy 2010, 35, 583–587. [Google Scholar] [CrossRef]
- Badwal, S.; Ciacchi, F.; Zelizko, V.; Giampietro, K. Oxygen removal and level control with zirconia-Yttria membrane cells. Ionics 2003, 9, 315–320. [Google Scholar]
- Tamaru, K. A “new” general mechanism of ammonia synthesis and decomposition on transition metals. Acc. Chem. Res. 1988, 21, 88–94. [Google Scholar] [CrossRef]
- Salker, A.V.; Weisweiler, W. Catalytic behaviour of metal based ZSM-5 catalysts for NOx reduction with NH3 in dry and humid conditions. Appl. Catal. A 2000, 203, 221–229. [Google Scholar] [CrossRef]
- Yung, M.M.; Jablonski, W.S.; Magrini-Bair, K.A. Review of catalytic conditioning of biomass-derived syngas. Energy Fuels 2009, 23, 1874–1887. [Google Scholar] [CrossRef]
- Fischer, F.; Tropsch, H. The preparation of synthetic oil mixtures (synthol) from carbon monoxide and hydrogen. Brennst. Chem. 1923, 4, 276–285. [Google Scholar]
- Patzlaff, J.; Liu, Y.; Graffmann, C.; Gaube, J. Studies on product distributions of iron and cobalt catalyzed Fischer–Tropsch synthesis. Appl. Catal. A 1999, 186, 109–119. [Google Scholar] [CrossRef]
- Guettel, R.; Kunz, U.; Turek, T. Reactors for Fischer–Tropsch Synthesis. Chem. Eng. Technol. 2008, 31, 746–754. [Google Scholar] [CrossRef]
- Tavakoli, A.; Sohrabi, M.; Kargari, A. Application of Anderson–Schulz–Flory (ASF) equation in the product distribution of slurry phase FT synthesis with nanosized iron catalysts. Chem. Eng. J. 2008, 136, 358–363. [Google Scholar] [CrossRef]
- Dry, M.E. Present and future applications of the Fischer–Tropsch process. Appl. Catal. A 2004, 276, 1–3. [Google Scholar] [CrossRef]
- Sie, S.T.; Krishna, R. Fundamentals and selection of advanced Fischer–Tropsch reactors. Appl. Catal. A 1999, 186, 55–70. [Google Scholar] [CrossRef]
- Davis, B.H. Fischer–Tropsch synthesis: Overview of reactor development and future potentialities. Top. Catal. 2005, 32, 143–168. [Google Scholar]
- Mark, E.D. Practical and theoretical aspects of the catalytic Fischer–Tropsch process. Appl. Catal. A 1996, 138, 319–344. [Google Scholar] [CrossRef]
- Knochen, J.; Güttel, R.; Knobloch, C.; Turek, T. Fischer–Tropsch synthesis in milli-structured fixed-bed reactors: Experimental study and scale-up considerations. Chem. Eng. Process. 2010, 49, 958–964. [Google Scholar] [CrossRef]
- Guettel, R.; Turek, T. Comparison of different reactor types for low temperature Fischer–Tropsch synthesis: A simulation study. Chem. Eng. Sci. 2009, 64, 955–964. [Google Scholar] [CrossRef]
- Jager, B.; Espinoza, R. Advances in low temperature Fischer–Tropsch synthesis. Catal. Today 1995, 23, 17–28. [Google Scholar] [CrossRef]
- Myrstad, R.; Eri, S.; Pfeifer, P.; Rytter, E.; Holmen, A. Fischer–Tropsch synthesis in a microstructured reactor. Catal. Today 2009, 147, S301–S304. [Google Scholar]
- Dry, M.E. High quality diesel via the Fischer–Tropsch process—a review. J. Chem. Technol. Biotechnol. 2002, 77, 43–50. [Google Scholar] [CrossRef]
- Unruh, D.; Rohde, M.; Schaub, G. Improving carbon utilization in biomass conversion to synthetic hydrocarbons via Fischer–Tropsch synthesis. Stud. Surf. Sci. Catal. 2004, 153, 91–96. [Google Scholar] [CrossRef]
- James, O.O.; Mesubi, A.M.; Ako, T.C.; Maity, S. Increasing carbon utilization in Fischer–Tropschsynthesis using H2-deficient or CO2-rich syngas feeds. Fuel Process. Technol. 2010, 91, 136–144. [Google Scholar] [CrossRef]
- Yao, Y.; Hildebrandt, D.; Glasser, D. Fischer–Tropsch Synthesis Using H2/CO/CO2 Syngas Mixtures over a Cobalt Catalyst. Ind. Eng. Chem. Res. 2010, 49, 11061–11066. [Google Scholar]
- Robert, D.W.; Hardy, D.R.; Williams, F.W.; Willaue, H.D. Catalytic CO2 hydrogenation to feedstock chemicals for jet fuel synthesis using multi-walled carbon nanotubes as support. In Advances in CO2 Conversion and Utilization; Hu, Y.H., Ed.; American Chemical Society: Washington, DC, USA, 2010; pp. 125–139, ACS Symposium Series 1056. [Google Scholar]
- Visconti, C.G.; Lietti, L.; Tronconi, E.; Forzatti, P.; Zennaro, R.; Finocchio, E. Fischer–Tropsch synthesis on a Co/Al2O3 catalyst with CO2 containing syngas. Appl. Catal. A 2009, 355, 61–68. [Google Scholar] [CrossRef]
- Riedel, T.; Claeys, M.; Schulz, H.; Schaub, G.; Nam, S.S.; Jun, K.W.; Choi, M.J.; Kishan, G.; Lee, K.W. Comparative study of FTS with H2/CO and H2/CO2 syngas using Fe and Co catalysts. Appl. Catal. A 1999, 186, 201–213. [Google Scholar] [CrossRef]
- Jun, K.W.; Roh, H.S.; Kim, K.S.; Ryu, J.S.; Lee, K.W. Catalytic investigation for Fischer–Tropsch synthesis from bio-mass derived syngas. Appl. Catal. A 2004, 259, 221–226. [Google Scholar] [CrossRef]
- Ando, H.; Xu, Q.; Fujiwara, M.; Matsumura, Y.; Tanaka, M.; Souma, Y. Hydrocarbon synthesis from CO2 over Fe–Cu catalysts. Catal. Today 1998, 45, 229–234. [Google Scholar] [CrossRef]
- Tominaga, H.; Nagai, M. Density functional study of carbon dioxide hydrogenation on molybdenum carbide and metal. Appl. Catal. A 2005, 282, 5–13. [Google Scholar] [CrossRef]
- Riedel, T.; Claeys, M.; Schulz, H.; Schaub, G.; Nam, S.S.; Jun, K.W.; Choi, M.J.; Kishan, G.; Lee, K.W. Comparative study of Fischer–Tropsch synthesis with H2/CO and H2/CO2 syngas using Fe- and Co-based catalysts. Appl. Catal. A 1999, 186, 201–213. [Google Scholar] [CrossRef]
- Zhang, Y.; Jacobs, G.; Sparks, D.E.; Dry, M.E.; Davis, B.H. CO and CO2 hydrogenation study on supported cobalt Fischer–Tropsch synthesis catalysts. Catal. Today 2002, 71, 411–418. [Google Scholar]
- Dorner, R.W.; Hardy, D.R.; Williams, F.W.; Davis, B.H.; Willauer, H.D. Influence of gas feed composition and pressure on the catalytic conversion of CO2 to Hydrocarbons using a traditional cobalt-based Fischer–Tropschcatalyst. Energy Fuels 2009, 23, 4190–4195. [Google Scholar]
- Pour, A.N.; Shahri, S.M.K.; Bozorgzadeh, H.R.; Zamani, Y.; Tavasoli, A.; Marvast, M.A. Effect of Mg, La and Ca promoters on the structure and catalytic behavior of iron-based catalysts in Fischer–Tropsch synthesis. Appl. Catal. A 2008, 348, 201–208. [Google Scholar] [CrossRef]
- Pour, A.N.; Shahri, S.M.K. Promoter effect on the CO2–H2O formation during Fischer–Tropsch synthesis on iron-based catalysts. J. Nat. Gas Chem. 2010, 19, 193–197. [Google Scholar] [CrossRef]
- Gaube, J.; Klein, H.F. The promoter effect of alkali in Fischer–Tropsch iron and cobalt catalysts. Appl. Catal. A 2008, 350, 126–132. [Google Scholar] [CrossRef]
- Escalona, N.; Medina, C.; García, R.; Reyes, P. Fischer–Tropsch reaction from a mixture similar to biosyngas. Influence of promoters on surface and catalytic properties of Co/SiO2 catalysts. Catal. Today 2009, 143, 76–79. [Google Scholar]
- Zhang, H.; Ma, H.; Zhang, H.; Ying, W.; Fang, D. Effects of Zr and K promoters on precipitated iron-based catalysts for Fischer–Tropschsynthesis. Catal. Lett. 2012, 142, 131–137. [Google Scholar]
- Ma, W.; Kugler, E.L.; Dadyburjor, D.B. Promotional Effect of copper on activity and selectivity to Hydrocarbons and Oxygenates for Fischer–TropschSynthesis over potassium-promoted iron catalysts supported on activated carbon. Energy Fuels 2011, 25, 1931–1938. [Google Scholar] [CrossRef]
- Dinse, A.; Aigner, M.; Ulbrich, M.; Johnson, G.R.; Bell, A.T. Effects of Mn promotion on the activity and selectivity of Co/SiO2 for Fischer–Tropsch synthesis. J. Catal. 2012, 288, 104–114. [Google Scholar]
- Hans, S. Short history and present trends of Fischer–Tropsch synthesis. Appl. Catal. A 1999, 186, 3–12. [Google Scholar] [CrossRef]
- Yang, Y.; Xiang, H.W.; Xu, Y.; Bai, L.; Li, Y. Effect of potassium promoter on precipitated iron-manganese catalyst for Fischer–Tropsch synthesis. Appl. Catal. A 2004, 266, 181–194. [Google Scholar] [CrossRef]
- Forzatti, P.; Lietti, L. Catalyst deactivation. Catal. Today 1999, 52, 165–181. [Google Scholar]
- Lohitharn, N.; Goodwin, J.G., Jr. Effect of K promotion of Fe and FeMn Fischer–Tropsch synthesis catalysts: Analysis at the site level using SSITKA. J. Catal. 2008, 260, 7–16. [Google Scholar]
- Zhang, C.H.; Yang, Y.; Tao, Z.; Zhang, C.; Xiang, H.; Li, Y. Study of an iron-manganese Fischer–Tropsch synthesis catalyst promoted with copper. J. Catal. 2006, 237, 405–415. [Google Scholar]
- Lohitharn, N.; Goodwin, J.G., Jr.; Lotero, E. Fe-based Fischer–Tropsch synthesis catalysts containing carbide-forming transition metal promoters. J. Catal. 2008, 255, 104–113. [Google Scholar]
- Xu, D.; Li, W.; Duan, H.; Ge, Q.; Xu, H. Effect of Pt, Ru and Pd Promoters on the Performance of Co/gamma-Al2O3Catalysts for Fischer–Tropsch Synthesis. Chin. J. Catal. 2005, 26, 780–784. [Google Scholar]
- Enrique, I. Design, synthesis, and use of cobalt-based Fischer–Tropsch synthesis catalysts. Appl. Catal. A 1997, 161, 59–78. [Google Scholar] [CrossRef]
- Bertole, C.J.; Mims, C.A.; Kiss, G. Support and rhenium effects on the intrinsic site activity and methane selectivity of cobalt Fischer–Tropsch catalysts. J. Catal. 2004, 221, 191–203. [Google Scholar]
- Withers, H.P., Jr.; Eliezer, K.F.; Mitchell, J.W. Slurry-phase Fischer–Tropsch synthesisand kinetic studies over supported cobalt carbonyl derived catalysts. Ind. Eng. Chem. Res. 1990, 29, 1807–1814. [Google Scholar]
- All, S.; Chen, B.; Goodwin, J.G. Zr Promotion of Co/SiO2 for Fischer–Tropsch Synthesis. J. Catal. 1995, 157, 35–41. [Google Scholar]
- Feller, A.; Claeys, M.; van Steen, E.J. Cobalt Cluster Effects in Zirconium Promoted Co/SiO2 Fischer–Tropsch Catalysts. J. Catal. 1999, 185, 120–130. [Google Scholar]
- Pham, H.N.; Nowicki, L.; Xu, J.; Datye, A.K.; Bukur, D.B.; Bartholomew, C. Attrition Resistance of Supports for Iron Fischer–Tropsch Catalysts. Ind. Eng. Chem. Res. 2003, 42, 4001–4008. [Google Scholar] [CrossRef]
- Soled, S.L.; Iglesia, E.; Fiato, R.A.; Baumgartner, J.E.; Vroman, H.; Miseo, S. Control of Metal Dispersion and Structure by Changes in the Solid-State Chemistry of Supported Cobalt Fischer–Tropsch Catalysts. Top. Catal. 2003, 26, 101–109. [Google Scholar]
- Bartholomew, C.H.; Reuel, R.C. Cobalt-support interactions: Their effects on adsorption and carbon monoxide hydrogenation activity and selectivity properties. Ind. Eng. Chem. Prod. Res. Dev. 1985, 24, 56–61. [Google Scholar] [CrossRef]
- Jacobs, G.; Das, T.K.; Zhang, Y.; Li, J.; Racoillet, G.; Davis, B.H. Fischer–Tropsch synthesis: Support, loading, and promoter effects on the reducibility of cobalt catalysts. App. Catal. A 2002, 233, 263–281. [Google Scholar] [CrossRef]
- Bertole, C.J.; Mims, C.A.; Kiss, G. Support and rhenium effects on the intrinsic site activity and methane selectivity of cobalt Fischer–Tropsch catalysts. J. Catal. 2004, 221, 191–203. [Google Scholar]
- Storsæter, S.; Tøtdal, B.; Walmsley, J.C.; Tanem, B.S.; Holmen, A. Characterization of alumina-, silica-, and titania-supported cobalt Fischer–Tropsch catalysts. J. Catal. 2005, 236, 139–152. [Google Scholar]
- Oh, J.H.; Bae, J.; Park, S.J.; Khanna, P.; Jun, K.W. Slurry-Phase Fischer–Tropsch Synthesis Using Co/γ-Al2O3, Co/SiO2 and Co/TiO2: Effect of Support on Catalyst Aggregation. Catal. Lett. 2009, 130, 403–409. [Google Scholar] [CrossRef]
- Enache, D.I.; Roy-Auberger, M.; Revel, R. Differences in the characteristics and catalytic properties of cobalt-based Fischer–Tropsch catalysts supported on zirconia and alumina. Appl. Catal. A 2004, 268, 51–60. [Google Scholar] [CrossRef]
- Chang, C.D.; Lang, W.H.; Silvestri, A.J.; Smith, R.L. Conversion of synthesis gas to hydrocarbons mixtures. US Patent 4,096,163,20, 20 June 1978. [Google Scholar]
- Marschner, F.; Moeller, F.W. Methanol Synthesis. In Applied Industrial Catalysis; Leach, B.E., Ed.; Academic Press: New York, NY, USA, 1983; Volume 2, pp. 215–243. [Google Scholar]
- Chang, C.D.; Lang, W.H.; Bell, W.K. Dual-Functional Coupling of Methanol Synthesis and Hydrocarbons Synthesis from Methanol. Chem. Energy Prod. Symp. Proc. 1980, 1, 127–132. [Google Scholar]
- Ereña, J.; Arandes, J.M.; Bilbao, J.; Aguayo, A.T.; de Lasa, H.I. Study of physical mixtures of Cr2O3–ZnO and ZSM-5 catalysts for the transformation of syngas into liquid hydrocarbons. Ind. Eng. Chem. Res. 1998, 37, 1211–1219. [Google Scholar] [CrossRef]
- Simard, F.; Sedran, U.A.; Sepúlveda, J.; Fígoli, N.S.; de Lasa, H.I. ZnO–Cr2O3 + ZSM-5 catalyst with very low Zn/Cr ratio for the transformation of synthesis gas to hydrocarbons. Appl. Catal. A 1995, 125, 81–98. [Google Scholar] [CrossRef]
- Liu, S.; Gujar, A.C.; Thomas, P.; Toghiani, H.; White, M.G. Synthesis of gasoline-range hydrocarbons over Mo/HZSM-5 catalysts. Appl. Catal. A 2009, 357, 18–25. [Google Scholar] [CrossRef]
- Street, J.; Yu, F.; Wooten, J.; Columbus, E.; White, M.G.; Warnock, J. Gasoline-range hydrocarbon production using biomass derived synthesis gas over Mo/H+ZSM-5. Fuel 2012, 96, 239–249. [Google Scholar]
- Udaya, V.; Rao, S.; Gormley, R.J. Bifunctional catalysis in syngas conversions. Catal. Today 1990, 6, 207. [Google Scholar]
- Dry, M.E. The Fischer–Tropsch Synthesis. In Catalysis: Science and Technology; Anderson, J.R., Boudart, M., Eds.; Springer: Berlin, Germany, 1981; Volume 1, Chapter 4. [Google Scholar]
- Guczi, L.; Kiricsi, I. Zeolite supported mono- and bimetallic systems: Structure and performance as CO hydrogenation catalysts. Appl. Catal. A 1999, 186, 375–394. [Google Scholar] [CrossRef]
- Wang, P.; Kang, J.; Zhang, Q.; Wang, Y. Lithium ion-exchanged zeolite faujasite as support of iron catalyst for Fischer–Tropsch synthesis. Catal. Lett. 2007, 114, 178–184. [Google Scholar] [CrossRef]
- Ravishankar, R.; Li, M.M.; Borgna, A. Novel utilization of MCM-22 molecular sieves as supports of cobalt catalysts in the Fischer–Tropsch synthesis. Catal. Today 2005, 106, 149–153. [Google Scholar]
- Bessell, S. Investigation of bifunctional zeolite supported cobalt Fischer–Tropsch catalysts. Appl. Catal. A 1995, 126, 235–244. [Google Scholar] [CrossRef]
- Lv, P.; Yuan, Z. Bio-syngas production from biomass catalytic gasification. Energy Convers. Manag. 2007, 48, 1132–1139. [Google Scholar] [CrossRef]
- Albright, L.F.; Baker, R.T.K. Coke Formation on Metal Surfaces; ACS Symposium Series 202; American Chemical Society: Washington, DC, USA, 1982. [Google Scholar]
- Menon, P.G. Coke on catalysts-harmful, harmless, invisible and beneficial type. J. Mol. Catal. 1990, 59, 207–220. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R.; Sehested, J. Hydrogen and synthesis gas by steam- and CO2 reforming. Adv. Catal. 2002, 47, 65–139. [Google Scholar] [CrossRef]
- Bengaard, H.S.; Norskov, J.K.; Sehested, J.; Clausen, B.S.; Nielsen, L.P.; Molenbroek, A.M.; Rostrup-Nielsen, J.R.J. Steam reforming and graphite formation on Ni catalysts. J. Catal. 2002, 209, 365–384. [Google Scholar]
- Besenbacher, F.; Chorkendorff, I.; Clausen, B.S.; Hammer, B.; Molenbroek, A.M.; Norskov, J.K.; Stensgaard, I. Design of a surface alloy catalyst for steam reforming. Science 1998, 279, 1913–1915. [Google Scholar]
- Trimm, D.L. Coke formation and minimisation during steam reforming reactions. Catal. Today 1997, 37, 233–238. [Google Scholar]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Li, J.; Coville, N.J. Effect of boron on the sulfur poisoning of Co/TiO2 Fischer–Tropsch catalysts. Appl. Catal. A 2001, 208, 177–184. [Google Scholar]
- Bartholomew, C.H.; Bowman, R.M. Sulfur poisoning of cobalt and iron Fischer–Tropsch catalysts. Appl. Catal. 1985, 15, 59–67. [Google Scholar] [CrossRef]
- Magrini-Bair, K.A.; Czernik, S.; French, R.; Parent, Y.O.; Chornet, E.; Dayton, D.C.; Feik, C.; Bain, R. Fluidizable reforming catalyst development for conditioningbiomass-derived syngas. Appl. Catal. A 2007, 318, 199–206. [Google Scholar] [CrossRef]
- Srinakruang, J.; Sato, K.; Vitidsant, T.; Fujimoto, K. Highly efficient sulfur and coking resistance catalysts for tar gasification with steam. Fuel 2006, 85, 2419–2426. [Google Scholar] [CrossRef]
- Bain, R.L.; Dayton, D.C.; Carpenter, D.L.; Czernik, S.R.; Feik, C.J.; French, R.J.; Magrini-Bair, K.A.; Phillips, S.D. Evaluation of catalyst deactivation during catalytic steam reforming of biomass-derived syngas. Ind. Eng. Chem. Res. 2005, 44, 7945–7956. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hu, J.; Yu, F.; Lu, Y. Application of Fischer–Tropsch Synthesis in Biomass to Liquid Conversion. Catalysts 2012, 2, 303-326. https://doi.org/10.3390/catal2020303
Hu J, Yu F, Lu Y. Application of Fischer–Tropsch Synthesis in Biomass to Liquid Conversion. Catalysts. 2012; 2(2):303-326. https://doi.org/10.3390/catal2020303
Chicago/Turabian StyleHu, Jin, Fei Yu, and Yongwu Lu. 2012. "Application of Fischer–Tropsch Synthesis in Biomass to Liquid Conversion" Catalysts 2, no. 2: 303-326. https://doi.org/10.3390/catal2020303
APA StyleHu, J., Yu, F., & Lu, Y. (2012). Application of Fischer–Tropsch Synthesis in Biomass to Liquid Conversion. Catalysts, 2(2), 303-326. https://doi.org/10.3390/catal2020303