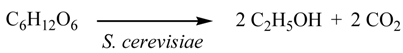
Abstract
Ethanol and pentose were produced from lignocellulosic napiergrass by the simultaneous saccharification and fermentation process (SSF) using hydrolytic enzyme and S. Cerevisiae. After the ethanol was removed, the pentose solution was subjected to photocatalytic hydrogen evolution with Pt-loaded TiO2 under UV-irradiation. This process converted 100 g of napiergrass into 12.3 g of ethanol and 1.76 g of hydrogen whose total combustion energy of (∆H) was 615 kJ. This was close to the ∆H (639 kJ) of the pentose (13.6 g) and hexose (27.4 g) obtained by the cellulose-saccharification of 100 g of napiergrass.
1. Introduction
Bio-fuel has been receiving a great amount of interest from the standpoint of utilizing renewable resources [1]. However, commercially available bio-ethanol has been prepared from the starch of maize, sugarcane, and sugar sorghum, which are in competition with food sources for human consumption [2]. Therefore, we are interested in herbaceous lignocellulosic napiergrass (Pennisetum purpureum Schumach) which is a kind of pasture used in stock farms and therefore, is not in competition with food sources [3]. Recently we have reported the production of bio-ethanol from napiergrass through an enzymatic saccharification and a fermentation with yeast (Saccharomyces cerevisiae) [4]. In this process, 8.8 g of ethanol was produced from 100 g of the leaf part of napiergrass which contained 44 g of cellulosic components. Thus the ethanol yield was low because of the high content of hemicellulose composed by pentose which cannot be fermented into ethanol by yeast. Therefore, the transformation of pentose into bio-fuels is an unavoidable process in the lignocellulosic biomass conversion. Photocatalytic hydrogen production, using hexose and pentose acting as a sacrificial reagent, is a prospective candidate to transform pentose into bio-fuels [5,6,7].
It is well-known that the photocatalytic hydrogen-evolution from H2O by a Pt-loaded TiO2 (Pt/TiO2) is initiated by the charge-separation on TiO2 under photoexcitation [8]. The electron reduced water to generate H2 on the Pt-loaded on TiO2 (Equation 1). Although the oxidation pathway of sacrificial saccharides (1) by the hole (h+) is still unclear, Fu et al. have postulated a mechanism by which the h+ oxidizes directly with the alcohol moiety of glucose [7]. We have proposed that the h+ oxidize the HO− to produce HO radical which oxidize 1 through the hydrogen abstraction from the α-carbon of the alcoholic and formyl groups [5]. Formally the reaction of 1 with four equivalents of HO radical, eliminated one mole of CO2 and three moles of H2O (Equation 2). At the same time, it is expected that 2 equivalents of H2 was evolved by the reduction of water with 4-electrons.
Here, we examined the hydrogen evolution using 1 obtained from napiergrass through the combination of biological treatment (Equations 3 and 4) and the subsequent photocatalytic reaction with the Pt/TiO2 catalyst.
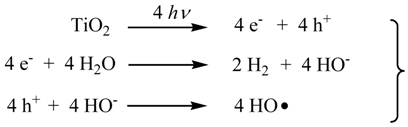
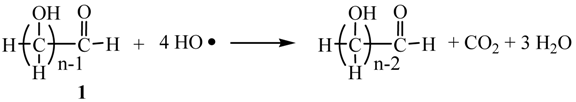
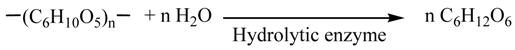
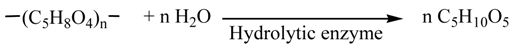
2. Results and Discussion
2.1. Alkali-Treatment of Napiergrass
The stem part of a dwarf type of napiergrass was dried and powdered by a blender until the powder passed through a sieve with 150 μm of mesh. The powdered napiergrass (5.0 g) was treated with a 1% aqueous solution of NaOH at 95 °C for 1 h in order to remove colored materials such as lignin and chlorophyll, which disturbed the light-absorption of the photocatalyst. The holocellulose (a mixture of cellulose and hemi-cellulose) was isolated as a pale yellow precipitate by centrifugation. The colored materials were dissolved in the aqueous NaOH solution. Lignin was collected as dark brown precipitate by centrifugation of the supernatant NaOH solution which was neutralized to pH 5.0 by a dilute HCl solution. As for the results, 2.70 g (54.0%) of holocellulose and 0.61 g (12.2%) of lignin were obtained from 5.0 g of powdered napiergrass. The residue (1.69 g, 33.8%) contained ash components and others.
2.2. Biological Treatment of Holocellulose
The holocellulose was turned into the reducing saccharides (1) by three methods shown in Figure 1. In Method A, the holocellulose (2.5 g) was turned into pentose and hexose by hydrolytic enzyme (Equations 3 and 4). The SA was performed using a hydrolytic enzyme (250 mg, Acremozyme, cellulase from acremonium, Kyowa Kasei) [4] in an acetate buffer solution (60 mL, pH 5.0) for 48 h under vigorous shaking at 45 °C. The solution was subjected to centrifugation to produce the supernatant solutions (1a) containing both pentose (0.63 g) and hexose (1.27 g). HPLC analysis showed that the pentose and hexose were mainly xylose and glucose, respectively. Holocellulose was recovered in 0.49 g, which was 80.4% of the conversion of saccharification.
After the Method A, the 1a was subjected to the fermentation in an acetate buffer solution (180 mL, pH 5.0) containing the 1a (12.49 g) and the suspension solution (3.6 mL) of S. cerevisiae at 35 °C (Method B). The reaction was monitored by the CO2-evolution (Equation 5). After the CO2-evolution was stopped for 107 h, ethanol (3.2 g) was formed. Pentose (3.6 g) was remained without a reaction. After the ethanol was removed from the reaction mixture by evaporation under reduced pressure, an aqueous pentose solution (1b) was obtained and subjected to the following photocatalytic reaction.
In Method C, the holocellulose was converted into ethanol and pentose by the simultaneous saccharification and fermentation process (SSF) according to Equations 3, 4, and 5. SSF was performed in an acetate buffer solution (180 mL, pH 5.0) containing holocellulose (16.2 g), Acremozyme (3.0 g), and the suspension solution (3.6 mL) of S. cerevisiae. The CO2-evolution was stopped for 89 h. The SSF process produced ethanol (3.69 g) and pentose (3.85 g). The ethanol was removed by evaporation of the solution to produce an aqueous pentose solution (1c). Table 1 summarizes the products yields based on 100 g of napiergrass.
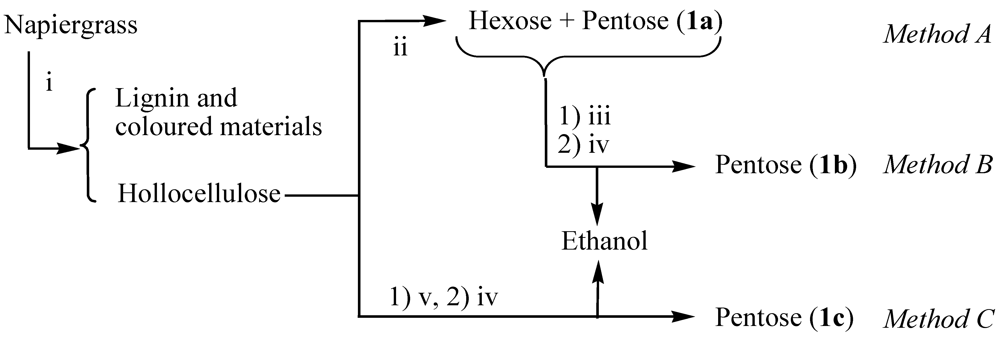
Figure 1.
Conversion from napiergrass to the saccharides (1). Operation: (i) the alkali treatment to remove lignin and others, (ii) saccharification with cellulase (SE) for 48 h, (iii) fermentation with Saccharomyces cerevisiae (FE) for 107 h, (iv) distillation under reduced pressure to isolate ethanol, (v) simultaneous saccharification and fermentation (SSF) of holocellolose using Acremozyme and S. cerevisiae for 89 h.
Figure 1.
Conversion from napiergrass to the saccharides (1). Operation: (i) the alkali treatment to remove lignin and others, (ii) saccharification with cellulase (SE) for 48 h, (iii) fermentation with Saccharomyces cerevisiae (FE) for 107 h, (iv) distillation under reduced pressure to isolate ethanol, (v) simultaneous saccharification and fermentation (SSF) of holocellolose using Acremozyme and S. cerevisiae for 89 h.

Table 1.
The yields of products obtained by the biological and photocatalytic treatments of the holocellulose occurring in napiergrass a.
| Biological treatment | PC-treatment d | ∆H/kJ g | ||
|---|---|---|---|---|
| Method b | Product (yields/g) c | [N] e (yield/g) f | ||
| A (SA) | Pentose (13.6) → | H2 [8.7] (4.23) | 603 | |
| Hexose (27.4) → | ||||
| B (SA/FE) | Pentose (12.0) | Pentose (12.1) → | H2 [9.7] (1.66) | 555 |
| Hexose (27.2) → | Hexose (0.9) → | |||
| EtOH (10.7) | ||||
| C (SSF) | Pentose (12.8) → | H2 [10.2] (1.74) | 615 | |
| Ethanol (12.3) | ||||
a Holocellolose (54.0 g), lignin (12.2 g), and others (33.8 g) was obtained by alkali-treatment of napiergrass (100 g). b SA = the saccarification with Acremozyme for 48 h, FE = the fermentation with S. cerevisiae for 107 h, SSF = the simultaneous saccharification and fermentation with Acremozyme and S. cerevisiae for 89 h. c Product yield based on the 54.0 g of holocellulose produced 100 g of napiergrass. d The photocatalytic reaction (PC) with Pt/TiO2 of saccharide solution. In Methods B and C, ethanol was removed before PC reaction. e The limiting hydrogen mole (N) obtained from the intercept of Figure 2. f The amount of H2 = 2 N (WP/150 + WH/180) where WP and WH denoted the weight of pentose and hexose. g Total combustion energy (∆H/kJ mol−1) of H2 and ethanol where ∆H = ethanol (1367) and hydrogen (285). The ∆H of the mixture of hexose (27.2 g) and pentose (12.0 g) was calculated to be 639 kJ where the ∆H of glucose was 2803 and ∆H of pentose was calculated to be 2336 kJ mol−1 by 2803 × 5/6. Data were referred from reference [9].
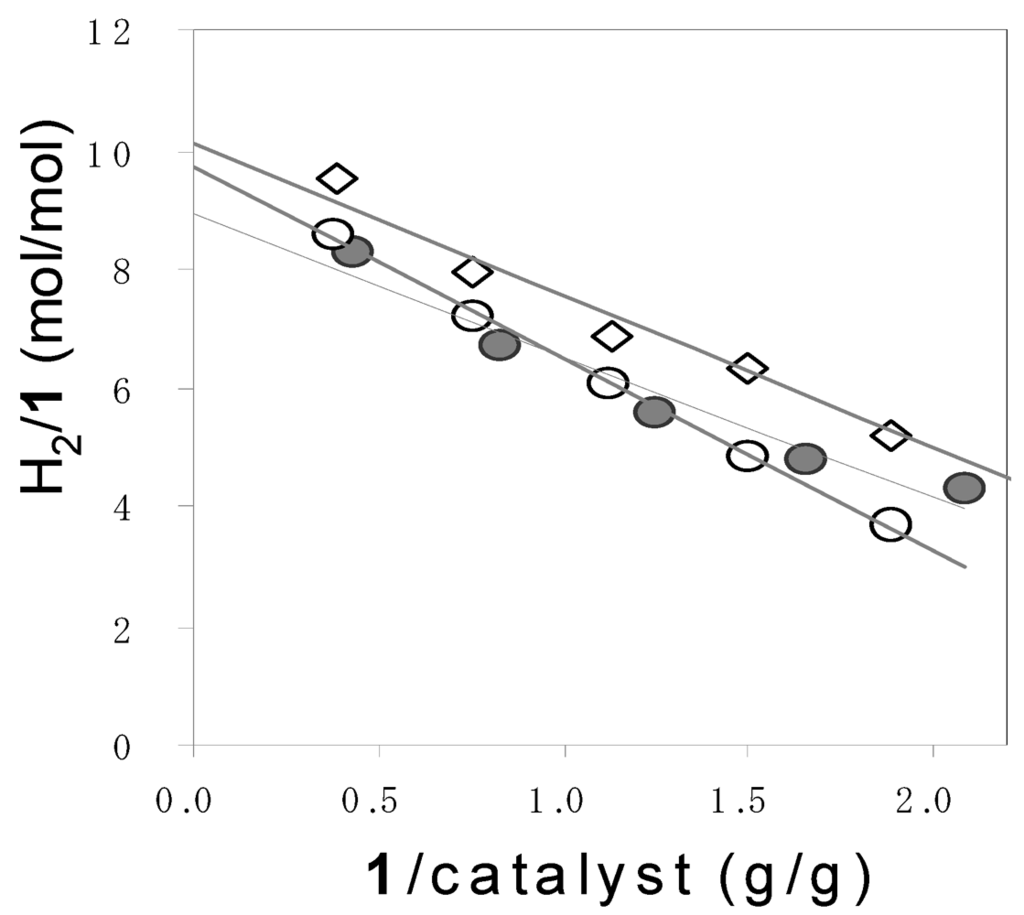
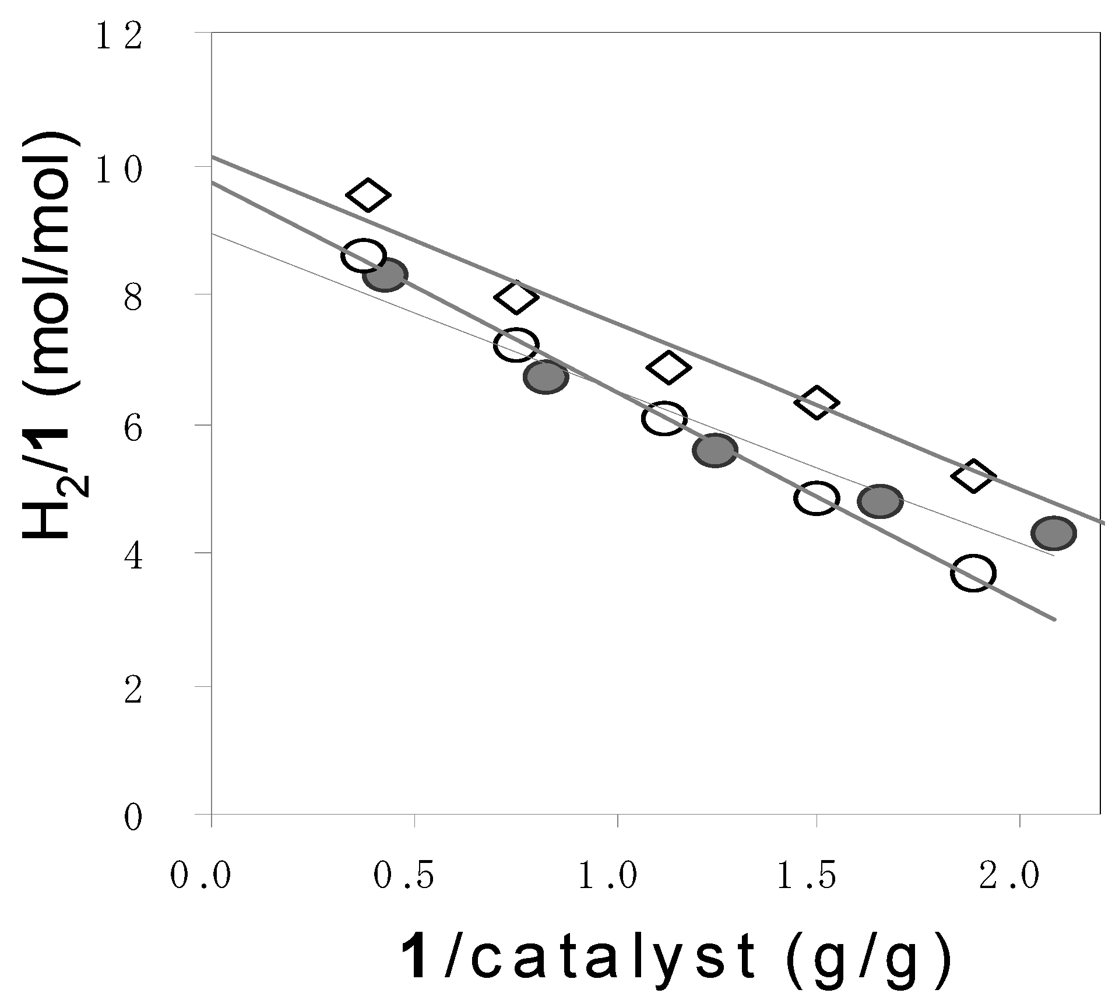
Figure 2.
Dependence of H2/1 on 1 used in photocatalytic hydrogen evolution from 1a (●), 1b (◯), and 1c (◇). Reaction conditions: catalyst = 100 mg, water = 150 mL. Intercept (N) = 8.7 (1a), 9.7 (1b), and 10.2 (1c).
Figure 2.
Dependence of H2/1 on 1 used in photocatalytic hydrogen evolution from 1a (●), 1b (◯), and 1c (◇). Reaction conditions: catalyst = 100 mg, water = 150 mL. Intercept (N) = 8.7 (1a), 9.7 (1b), and 10.2 (1c).
2.3. Photocatalytic Hydrogen Evolution (PC) Using the Saccharide (1) as Sacrificial Agent
The Pt/TiO2 (100 mg) was suspended in an aqueous solution (150 mL) containing 1 (0–249 mg) and the oxygen was purged by bubbling with N2. The suspended solution was irradiated by a high-pressure mercury lamp under vigorous stirring with a magnetic stirrer. The band gap of anatase-type of TiO2 is known to be 3.20 eV which is corresponding to 385 nm. Therefore, the TiO2 can be excited by 366 nm-emission from high-pressure mercury lamp [10]. The evolved gas was collected by mess-cylinder to measure the total volume of the evolved gas. The irradiation was performed until the gas evolution ceased. The quantitative analysis of hydrogen, oxygen, nitrogen, and carbon dioxide were performed by GLC. The results are shown in Table 2. Figure 3 shows the plots of the evolved H2, CO2, and O2 volumes against the amounts of 1 used. The evolved H2 and CO2 volumes increased gradually with the increase of 1. When smaller amounts of 1 were used, the volume ratio of H2 to CO2 (H2/CO2) exceeded over 2.0 which was the stoichiometric value of Equations 6 and 7, because of the dissolution of CO2 into aqueous reaction solution (Table 2). However, with the use of higher amounts of 1, the H2/CO2 became close to 2.0.
Moreover, it was confirmed that the H2 evolution from water was small (2 mL) in the absence of the sacrificial reagent. At the same time, 18 mL of O2 was evolved. Therefore, the volume ratio of H2 became O2 (H2/O2) in the photocatalytic reaction over stoichiometric value (2.0) [11]. Also, in the presence of sacrificial agent, a considerable amount of O2 was evolved. At the present time, the evolution mechanism of O2 is still under investigation. Other gases such as CH4 and CO [12] were not observed in the evolved gas.
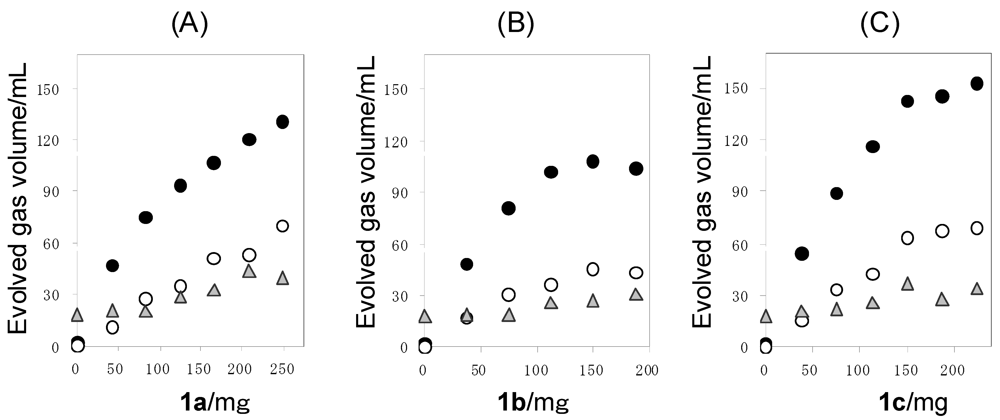
Figure 3.
Plots of the volumes of H2 (●), CO2 (○), and O2 (▲) against the amounts of 1 used in the photoreaction of 1a (A), 1b (B), and 1c (C) with the Pt/TiO2. Reaction conditions: catalyst = 100 mg, water = 150 mL.
Figure 3.
Plots of the volumes of H2 (●), CO2 (○), and O2 (▲) against the amounts of 1 used in the photoreaction of 1a (A), 1b (B), and 1c (C) with the Pt/TiO2. Reaction conditions: catalyst = 100 mg, water = 150 mL.
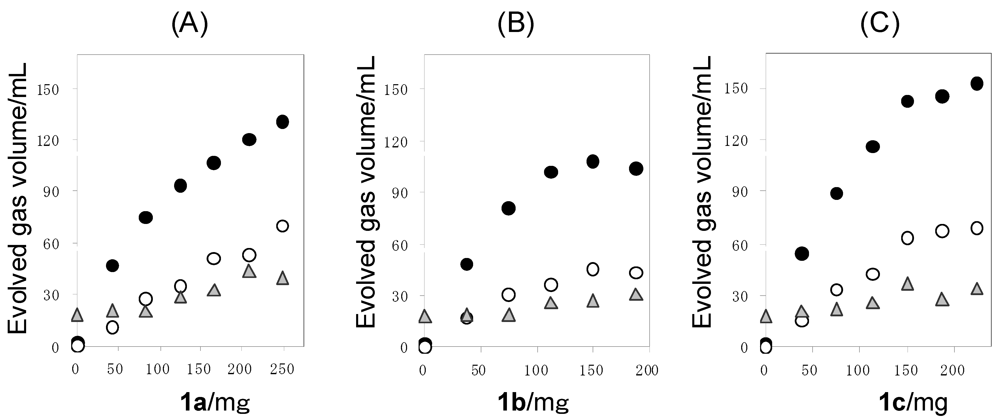

Table 2.
Photocatalytic H2 evolution (PC) using the saccharides (1a–1c) as sacrificial agent a.
| 1/mg b | T/h c | Gas volume/mL | Molar ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total d | H2 | CO2 | O2 | N2e | H2/1 | H2/CO2 | |||
| 0 | 5 | 13 | 2 | 0 | 18 | −7 | - | - | |
| 1a | 42 | 10 | 72 | 47 | 11 | 21 | −7 | 8.4 | 4.3 |
| 1a | 83 | 21 | 110 | 75 | 27 | 20 | −12 | 6.7 | 2.8 |
| 1a | 125 | 30 | 158 | 94 | 35 | 29 | 0 | 5.6 | 2.7 |
| 1a | 166 | 49 | 184 | 107 | 51 | 33 | −7 | 4.8 | 2.1 |
| 1a | 208 | 53 | 215 | 120 | 53 | 44 | −2 | 4.3 | 2.3 |
| 1a | 249 | 74 | 240 | 131 | 70 | 40 | −1 | 3.9 | 1.9 |
| 1b | 38 | 17 | 80 | 48 | 17 | 19 | −23 | 8.6 | 2.9 |
| 1b | 75 | 25 | 117 | 81 | 31 | 19 | −13 | 7.2 | 2.6 |
| 1b | 113 | 31 | 157 | 102 | 36 | 26 | −7 | 6.1 | 2.8 |
| 1b | 150 | 45 | 160 | 108 | 45 | 27 | −20 | 4.8 | 2.4 |
| 1b | 188 | 53 | 161 | 104 | 43 | 31 | −17 | 3.7 | 2.4 |
| 1c | 38 | 14 | 78 | 54 | 15 | 21 | −12 | 9.6 | 3.6 |
| 1c | 75 | 26 | 134 | 89 | 33 | 22 | −10 | 8.0 | 2.7 |
| 1c | 113 | 29 | 174 | 116 | 42 | 26 | −10 | 6.9 | 2.8 |
| 1c | 150 | 40 | 225 | 142 | 63 | 20 | 0 | 6.3 | 2.3 |
| 1c | 188 | 53 | 225 | 145 | 67 | 28 | −15 | 5.2 | 2.2 |
| 1c | 225 | 43 | 230 | 153 | 69 | 34 | −26 | 4.6 | 2.2 |
a Irradiation was performed for an aqueous solution (150 mL) containing 1 and Pt/TiO2 (the Pt content was 2 wt%, 100 mg); b The saccharides (1a, 1b, and 1c) were obtained from Methods A, B, and C, respectively; c Irradiation time (T) to reach the maximum volume of hydrogen; d The total gas volume collected over water by mess-cylinder; e The amounts of N2 was calculated by the subtraction of dead space volume from the measured amounts of N2.
According to Equations 6 and 7, the 10 and 12 equivalents of H2 were theoretically obtained from 1 mole of pentose and hexose, respectively. However, the molar ratio of the evolved H2 to 1 (H2/1) did not reach the theoretical values. Moreover, the H2/1 depended on the amount of 1 used. Therefore, the H2/1 values were plotted against the weight ratio of 1 to catalyst (1/catalyst), as shown in Figure 2. As the 1/catalyst values decreased, the H2/1 values increased. The intercept of the plots represents the limiting H2/1 values (N) at an infinite amount of a catalyst. The N values were nearly equaled to the theoretical values. Judging from these results, it is suggested that the deactivation of the catalyst occurred at the high turnover.
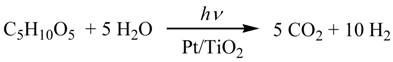
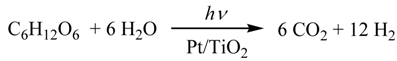
The amounts of the hydrogen obtained from the photocatalytic reaction of 1 were estimated by multiplying N by the moles of 1: The amount of H2 in g = 2 N (WP/150 + WH/180) where WP and WH denoted the weight of pentose and hexose. The results are shown in Table 1. Thus, the amounts of H2 were determined to be 4.23, 1.66, and 1.74 g from 1a, 1b, and 1c which were derived from 100 g of napiergrass, respectively.
2.4. The Combustion Energy of the Products
The three processes were compared from the standpoint of the combustion energy (∆H/kJ mol−1) of the bio-fuel produced by biological treatment and PC reaction, as shown in Table 1. The Method A process and PC produced 4.23 g of H2. The total ∆H was calculated to be 603 kJ using the ∆H of H2 (285 kJ mol−1) and ethanol (1,367 kJ mol−1) [9]. The Method B and PC processes produced ethanol (10.7 g) and H2 (1.66 g) whose total ∆H was 555 kJ. Also the Method C and PC process gave ethanol (12.3 g) and H2 (1.74 g) whose total ∆H was 615 kJ. Thus, the combination of the SSF process (Method C) with the PC process was most effective process. This ∆H was close to the 639 kJ which was ∆H of 27.4 g of hexose and 13.6 g of pentose which were formed from 100 g of napiergrass.
3. Experimental Section
3.1. Preparation of the Photocatalyst
Anatase-type of TiO2 (ST-01) was purchased from Ishihara Sangyo, Japan. According to the literature [13], an aqueous solution (50 mL) containing TiO2 (1.0 g), K2PtCl6 (10–100 mg), and 2-propanol (0.38 mL) was irradiated by high-pressure mercury lamp for 24 h with stirring to give the Pt-loaded TiO2 catalyst (Pt/TiO2). The optimized Pt-content on TiO2 was determined to be 2.0 wt% by the comparison of the amounts of hydrogen-evolution by the Pt-doped TiO2 (100 mg) under irradiation by high-pressure mercury lamp for 6 h using glucose (100 mg) as sacrificial reagent. Thus the Pt/TiO2 (2 wt% of Pt) was used throughout the present investigation.
3.2. Analysis
The amount of the saccharides (1) formed by the enzymatic saccharification (SA) process was analyzed by the modified Somogyi-Nelson method assuming the composition of 1 as C6H12O6 [14]. Also the amounts of hexose and pentose were analyzed by a Shimadzu LC-20AD high-performance liquid chromatography system using anion exchange column (Shodex Asahipak NH2P-50 4E). Ethanol concentrations were determined by a Shimadzu GC-2014 gas chromatograph using a glass column of 5% Thermon 1000 on Sunpak-A (Shimadzu) with 2-propanol as an internal standard. Hydrogen, carbon dioxide, and nitrogen were analyzed on a Shimadzu GC-8A equipped with TCD detector at temperature raised from 40 to 180 °C using a stainless column (3 mmΦ, 6 m) packed with a SHINCARBON ST (Shimadzu).
3.3. Alkali-Treatment of Napiergrass
A dwarf type of napiergrass (Pennisetum purpureum Schumach; dwarf variety of late-heading type) [3] was cultivated in the Sumiyoshi Ranch, Faculty of Agriculture, University of Miyazaki. The powdered stem part of the napiergrass (30 g) was treated with a 1% aqueous solution of NaOH (400 mL) at 95 °C for 1 h. Holocellulose was isolated as pale yellow precipitate by centrifugation. The neutralization of the supernatant solution to pH 5.0 by a dilute HCl solution gave a dark brown precipitate of lignin which was collected by centrifugation.
3.4. Saccharification of Hollocellulose with Enzyme (SA)
The holocellulose (2.5 g) was dispersed in an acetate buffer solution (60 mL, pH 5.0) and a hydrolytic enzyme (Acremozyme, Kyowa Kasei, 250 mg) was added to the sterile solution. The saccharification was performed by under vigorous shaking at 45 °C for 48 h [4]. The solution was subjected to centrifugation at 12,000 rpm to produce the supernatant solutions of 1 which were used in the following process.
3.5. Fermentation of the Saccharide (1) with S. cerevisiae
Saccharomyces cerevisiae NBRC 2044 was cultured at 30 °C for 24 h in a basal medium (initial pH 5.5) consisting of glucose (20 g L–1), bactotryptone (1.0 g L–1, Difco), yeast extract (1 g L–1), NaHPO4 (1 g L–1), and MaSO4 (3 g L–1) [4]. After incubating for 24 h, the cell suspension solution of S. cerevisiae was obtained. The suspension solution (3.6 mL) of S. cerevisiae was added to a solution of 1 (180 mL). The fermentation (FE) was performed at 35 °C with stirring using a magnetic stirrer until the evolution of CO2 was stopped.
The simultaneous saccharification and fermentation process (SSF) was performed as follows. The holocellulose (16.2 g) was dispersed in an acetate buffer solution (180 mL, pH 5.0) and was sterilized in autoclave at 120 °C. Acremozyme (3.0 g) and the cell suspension (3.6 mL) of S. cerevisiae were added to the suspension. The suspension was stirred vigorously with a magnetic stirrer at 35 °C until the evolution of CO2 was stopped.
3.6. Photocatalytic Reaction
The catalyst (usually 100 mg) and the aqueous solution of 1 (usually 5.0 mL) were introduced to a reaction vessel. The volume of the reaction solution was adjusted to 150 mL with water (usually 145 mL). A high-pressure mercury lamp (100 W, UVL-100HA, Riko, Japan) was inserted into the reaction vessel, which was attached to a mess-cylinder to correct the evolved gas and set in a water bath to keep it at a constant temperature (usually 20 °C) (Figure 4). After the oxygen was purged by nitrogen gas, irradiation was performed with vigorous stirring using magnetic stirrer. The evolved gas was collected by a mess-cylinder to measure the total volume of the evolved gas (Figure 4(A)). The quantitative analysis of hydrogen, nitrogen, and carbon dioxide were performed by GLC using the peak area (A) measured by the GLC-analysis, the sample gas volume (V3, 500 μL), and the slopes of the calibration curves (f) which were determined to be 3380, 334, 388 and 377 for H2, N2, O2, and CO2, respectively (Figure 4(B)). According to Equation (8) where V1 was the total volume of evolved gas (mL) corrected by mess-cylinder, V2 was the volume (mL) of the dead space of the vessel before reaction (usually 230 mL), the amounts of for H2, N2, O2, and CO2 were obtained
The amounts of gas (mL) = A (V1 + V2)/(f ×V3) (8)
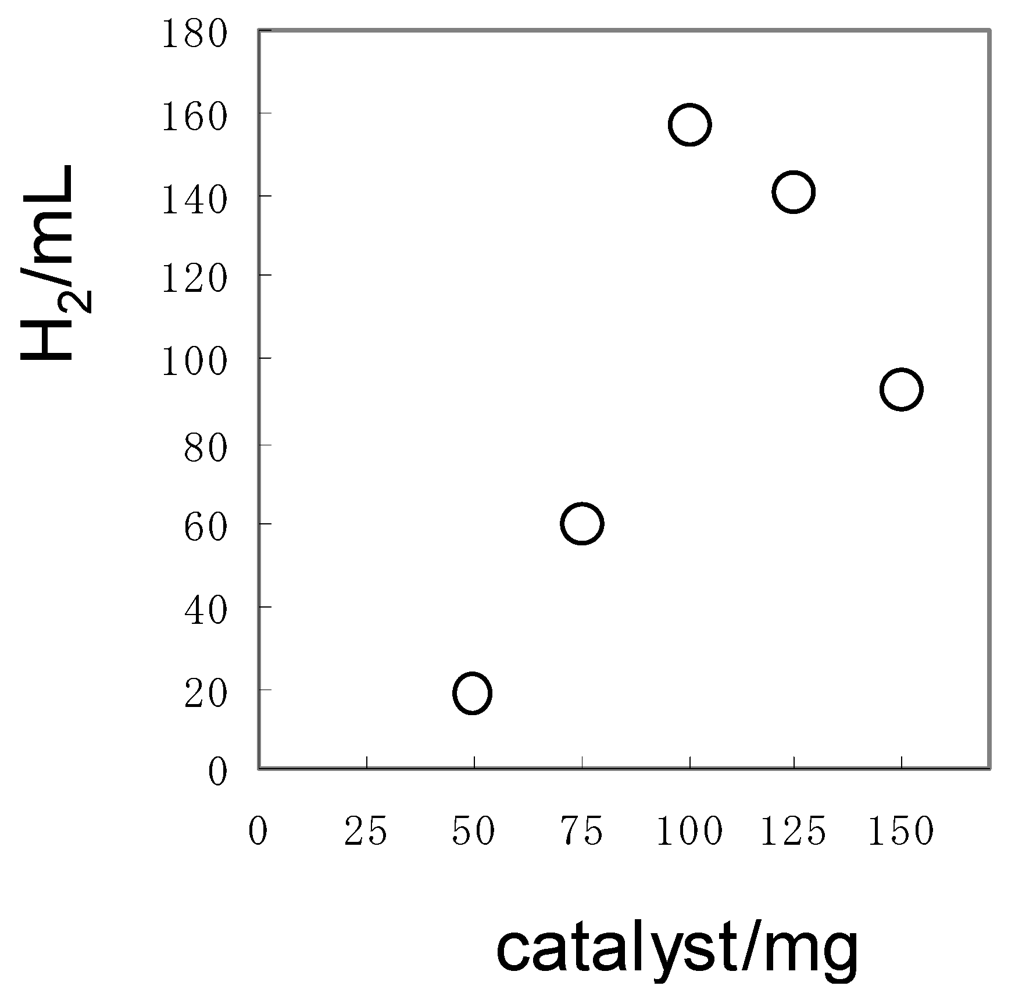
In order to efficiently irradiate the Pt/TiO2 suspended in solution, the amount of the Pt/TiO2 was optimized using 150 mg of 1a. When 100 mg (1.25 mmol) of the Pt/TiO2 (2.0 wt% of Pt content) was used for an aqueous solution (150 mL), the largest amounts of H2 evolved, as shown in Figure 5.
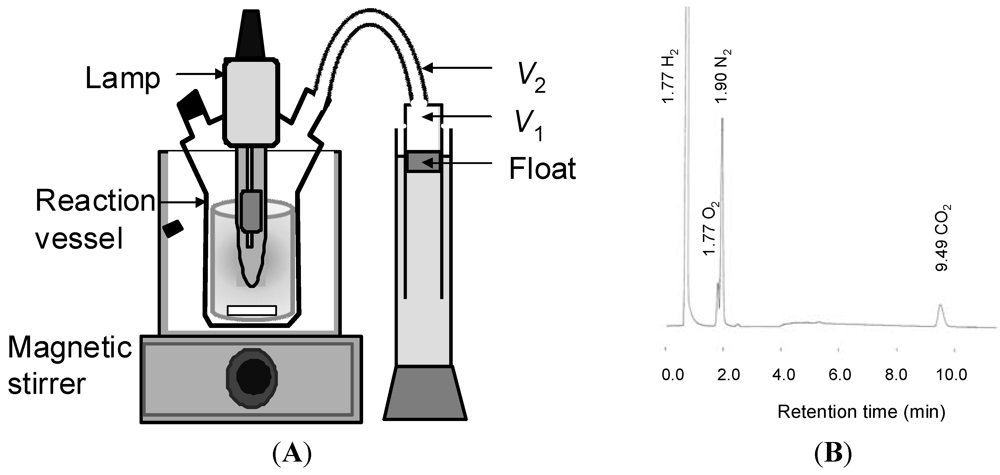
Figure 4.
(A) Outlines of photoreaction apparatus and (B) GLC chart of the evolved gas from the photocatalytic reaction of Pt/TiO2.
Figure 4.
(A) Outlines of photoreaction apparatus and (B) GLC chart of the evolved gas from the photocatalytic reaction of Pt/TiO2.
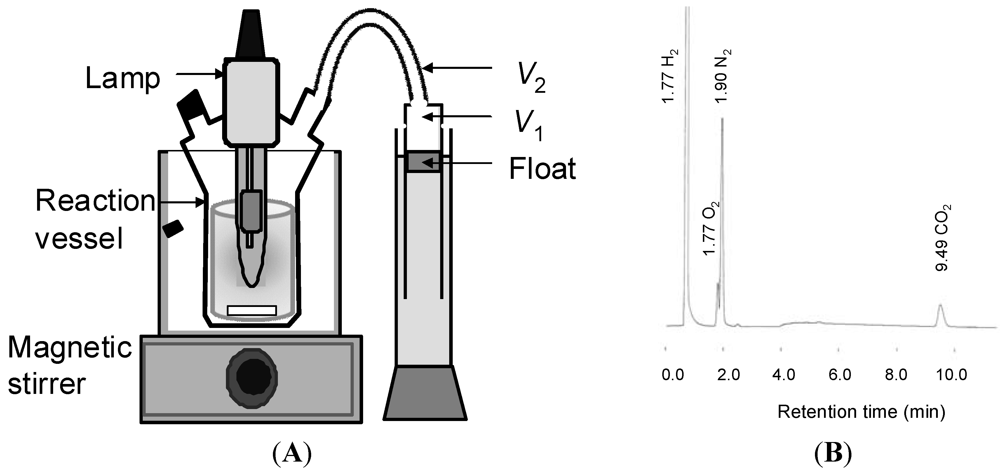
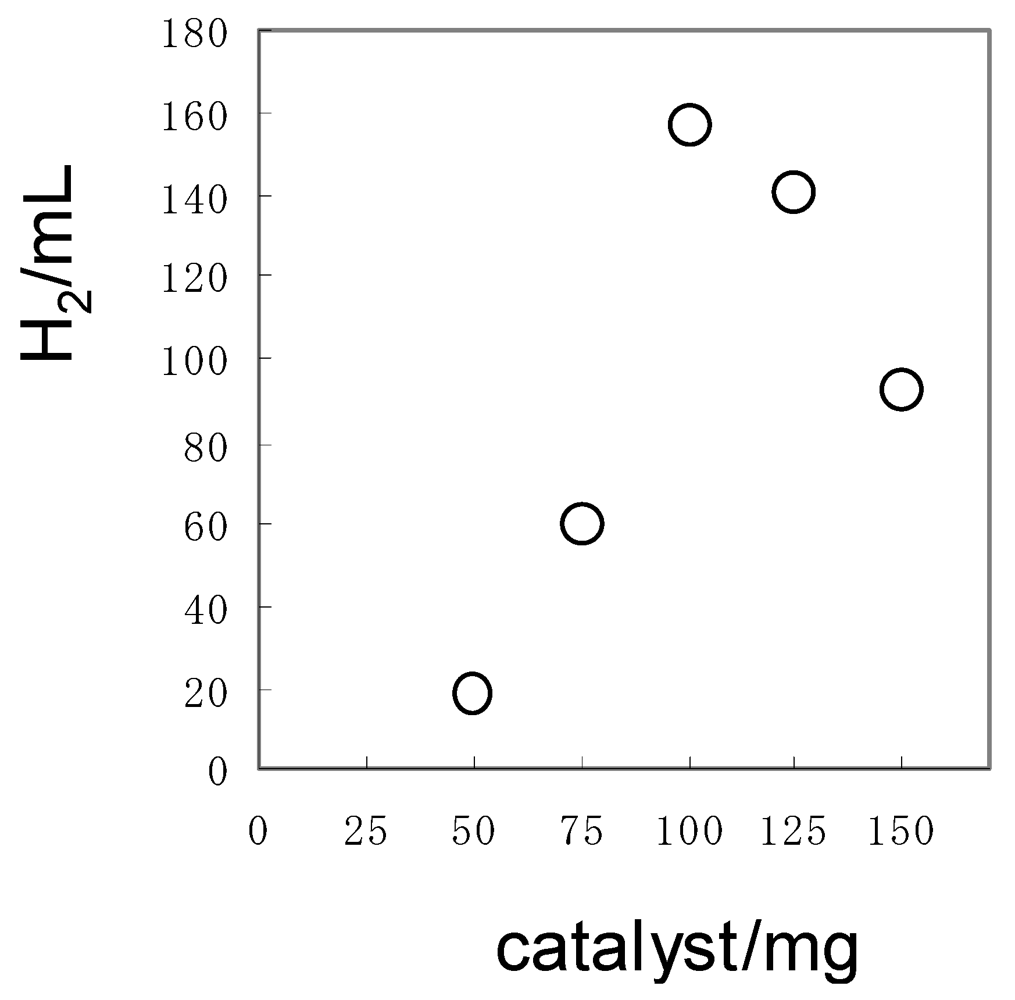
Figure 5.
Dependence of H2 volume on the weight of Pt/TiO2 in the photocatalytic hydrogen evolution using 1a. The irradiation was performed until the H2-evolution stopped. Reaction conditions: 1a = 150 mg, water = 150 mL.
Figure 5.
Dependence of H2 volume on the weight of Pt/TiO2 in the photocatalytic hydrogen evolution using 1a. The irradiation was performed until the H2-evolution stopped. Reaction conditions: 1a = 150 mg, water = 150 mL.
4. Conclusions
The photocatalytic hydrogen production with Pt/TiO2 has been widely developed using methanol [15], ethanol [16], glycerol [17], pentose [4], glucose [7], carboxylic acids such as glycolic acid [18] and acetic acid [19]. Recently we have compared the N values among the variety of saccharides and the related compounds to evaluate their sacrificial ability [4]. We have found the N values of alcoholic substances such as glycerol and arabitol were nearly equaled to the theoretical amounts (0.5m + 2n − k) for CnHmOk (Equation 9). On the other hand, the N values of glucose, xylose and the carboxylic compounds did not reach the theoretical amounts, showing that the sacrificial ability of saccharides was inferior to those of the alcohols. However, the photocatalytic hydrogen production from pentose is an important step in the transformation from lignocellulose to bio-fuel. Moreover, there are no reports on the photocatalytic H2 evolution combined with the biological saccharification of biomass, so far.

The present study showed that saccharides derived from napiergrass could operate effectively as sacrificial agent with the same activity as pentose [5] and glucose [7]. The SSF process poses an advantage in the pentose-production from the standpoints of simplicity of the manufacturing process, shortening of the reaction time, and yield of ethanol. The formed bio-fuel, ethanol and hydrogen, has almost the same combustion energy (∆H) as saccharide occurring in lignocellulosic napiergrass. If the UV light in sunlight is used as the light source for catalytic reaction, it will provide a useful method to produce H2 from pentose.
References
- Ward, O.P.; Singh, A. Bioethanol technology: Development and perspectives. Adv. Appl. Microbiol. 2002, 51, 53–80. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Recent trends in global production and utilization of bio-ethanol fuel. Appl. Energy 2009, 86, 2273–2282. [Google Scholar] [CrossRef]
- Ishii, Y.; Mukhtar, M.; Idota, S.; Fukuyama, K. Rotational grazing system for beef cows on dwarf napiergrass pasture oversown with Italian ryegrass for 2 years after establishment. Grassl. Sci. 2005, 51, 209–220. [Google Scholar]
- Yasuda, M.; Miura, A.; Yuki, R.; Nakamura, Y.; Shiragami, T.; Ishii, Y.; Yokoi, H. The effect of TiO2-photocatalytic pretreatment on the biological production of ethanol from lignocelluloses. J. Photochem. Photobiol. A 2011, 220, 195–199. [Google Scholar] [CrossRef]
- Shiragami, T.; Tomo, T.; Tsumagari, H.; Yuki, R.; Yamashita, T.; Yasuda, M. Pentose acting as a sacrificial multi-electron source in photocatalytic hydrogen evolution from water by Pt-doped TiO2. Chem. Lett. 2012, in press.. [Google Scholar]
- Kawai, T.; Sakata, T. Conversion of carbohydrate into hydrogen fuel by a photocatalytic process. Nature 1980, 286, 474–476. [Google Scholar] [CrossRef]
- Fu, X.; Long, J.; Wang, X.; Leung, Y.; Ding, Z.; Wu, L.; Zhang, Z.; Li, Z.; Fu, X. Photocatalytic reforming of biomass: A systematic study of hydrogen evolution from glucose solution. Int. J. Hydrog. Energy 2008, 33, 6484–6491. [Google Scholar]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Gslinska, A.; Walendziewski, J. Photocatalytic water-splitting over Pt-TiO2 in the presence of sacrificial Reagents. J. Energy Fuels 2005, 19, 1143–1147. [Google Scholar] [CrossRef]
- Kitano, M.; Tsujimaru, K.; Anpo, M. Development of water in the separate evolution of hydrogen and oxygen using visible-responsive TiO2 thin film photocatalysts: Effect of the work function of the substrates on the yield of the reaction. Appl. Catal. A 2006, 314, 179–183. [Google Scholar] [CrossRef]
- Bahruji, H.; Bowker, M.; Davies, P.R.; Pedrono, F. New insights into the mechanism of photocatalytic reforming on Pd/TiO2. Appl. Catal. B 2011, 107, 205–209. [Google Scholar] [CrossRef]
- Atkins, P.W. Physical Chemistry, 5th ed; Oxford University Press: Oxford, UK, 1994; pp. 922–926. [Google Scholar]
- Kennedy, J.C., III; Datye, A.K. Photochemical heterogeneous oxidation of ethanol over Pt/TiO2. J. Catal. 1998, 179, 375–389. [Google Scholar]
- Kim, Y.-K.; Sakano, Y. Analyses of reducing sugars on a thin-layer chromatographic plate with modified Somogyi and Nelson reagents, and with copper bicinchoninate. Biosci. Biotechnol. Biochem. 1996, 60, 594–597. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Aguirre, M.H.; Selli, E. Hydrogen production by photocatalytic stream reforming of methanol on noble metal-modified TiO2. J. Catal. 2010, 273, 182–190. [Google Scholar]
- Yang, Y.Z.; Chang, C.-H.; Idriss, H. Photo-catalytic production of hydrogen from ethanol over M/TiO2 catalysts (M = Pd, Pt, or Rh). Appl. Catal. 2006, 67, 217–222. [Google Scholar] [CrossRef]
- Daskalaki, V.D.; Kondarides, D.I. Efficient production of hydrogen by photo-induced reforming of glycerol at ambient conditions. Catal. Today 2009, 144, 75–80. [Google Scholar]
- Ho, C.-H.; Shieh, C.-Y.; Tseng, C.-L.; Chen, Y.-K.; Lin, J.-L. Decomposition pathways of glycolic acid on titanium dioxide. J. Catal. 2009, 261, 150–157. [Google Scholar]
- Zheng, X.-J.; Wei, L.-F.; Zhang, Z.-H.; Jiang, Q.-J.; Wei, Y.-J.; Xie, B.; Wei, M.-B. Research on photocatalytic H2 production from acetic acid solution by Pt/TiO2 nanoparticles under UV irradiation. Int. J. Hydrog. Energy 2009, 24, 9033–9041. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
