The Synthesis of Bis(α-aryl-methylphosphonoyl)amines by the Microwave-Assisted Catalyst-Free Tandem Kabachnik–Fields Reaction
Abstract
1. Introduction
2. Results and Discussion
2.1. Kabachnik–Fields Reaction of the α-Amino-Benzylphosphonates
2.2. NMR Identification of the Products
2.3. Bis(α-aryl-methylphosphonoyl)-amines 2a–d
2.4. (α-Aryl-methylphosphonoyl)-(α-phenyl-methylphosphonoyl)-amines 3b–d
3. Materials and Methods
3.1. General Information
3.2. Syntheses
3.2.1. General Method for the Preparation of Bis(α-aryl-methylphosphonoyl)-amines (2a–d)
3.2.2. General Method for the Preparation of Bis(α-aryl-methylphosphonoyl)-(α-phenyl-methylphosphonoyl)-amines (3b–d)
3.3. Alternative Procedure for the Synthesis of Compounds 3b–d
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dormán, G.; Szalai, Z.; Keglevich, G. Cytotoxic activity of distinct families of phosphonic acid derivatives—A chemocentric approach to assess their molecular action. ChemMedChem 2024, 19, e202400370. [Google Scholar] [CrossRef] [PubMed]
- Mucha, A.; Kafarski, P.; Berlicki, L. Remarkable potential of the α-aminophosphonate/phosphinate structural motif in medicinal chemistry. J. Med. Chem. 2011, 54, 5955–5980. [Google Scholar] [CrossRef]
- Kang, S.-U.; Shi, Z.-D.; Worthy, K.M.; Bindu, L.K.; Dharmawardana, P.G.; Choyke, S.J.; Bottaro, D.P.; Fisher, R.J.; Burke, T.R. Examination of phosphoryl-mimicking functionalities within a macrocyclic Grb2 SH2 domain-binding platform. J. Med. Chem. 2005, 48, 3945–3948. [Google Scholar] [CrossRef][Green Version]
- Robbins, B.L.; Srinivas, R.V.; Kim, C.; Bischofberger, N.; Fridland, A. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), Bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob. Agents Chemother. 1998, 42, 612–617. [Google Scholar] [CrossRef]
- Sienczyk, M.; Oleksyszyn, J. Irreversible inhibition of serine proteases-design and in vivo activity of diaryl α-aminophosphonate derivatives. Curr. Med. Chem. 2009, 16, 1673–1687. [Google Scholar] [CrossRef] [PubMed]
- Kafarski, P.; Lejczak, B. Aminophosphonic acids of potential medical importance. Curr. Med. Chem. Anticancer. Agents 2001, 1, 301–312. [Google Scholar] [CrossRef]
- Varga, P.R.; Dinnyési, E.; Tóth, S.; Szakács, G.; Keglevich, G. Optimized synthesis and cytotoxic activity of α-aminophosphonates against a multidrug resistant uterine sarcoma cell line. Lett. Drug Des. Discov. 2023, 20, 365–371. [Google Scholar] [CrossRef]
- Keglevich, G.; Bálint, E. The Kabachnik–Fields reaction; mechanism and synthetic use. Molecules 2012, 17, 12821–12835. [Google Scholar] [CrossRef]
- Varga, P.R.; Keglevich, G. The last decade of optically active α-aminophosphonates. Molecules 2023, 28, 6150. [Google Scholar] [CrossRef]
- Kukhar, V.P.; Hudson, H.R. Aminophosphonic and Aminophosphinic Acids: Chemistry and Biological Activity; John Wiley & Sons: Chichester, UK, 2000. [Google Scholar]
- Naydenova, E.D.; Todorov, P.T.; Troev, K.D. Recent synthesis of aminophosphonic acids as potential biological importance. Amino Acids 2009, 38, 23–30. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.M.; Ali, T.E.; Abdel-Kariem, S.M. Methods for synthesis of N-heterocyclyl/heteroaryl-α-aminophosphonates and α-(azaheterocyclyl)phosphonates. Arkivoc 2016, 2016, 183–211. [Google Scholar] [CrossRef]
- Shastri, R.A. Review on the synthesis of α-aminophosphonate derivatives. Chem. Sci. Trans. 2019, 8, 359–367. [Google Scholar] [CrossRef]
- Cherkasov, R.A.; Garifzyanov, A.R.; Koshkin, S.A. Synthesis of optically active α-aminophosphine oxides and enantioselective membrane transport of acids with their participation. Russ. J. Gen. Chem. 2011, 81, 773–774. [Google Scholar] [CrossRef]
- Vagapova, L.I.; Burilov, A.R.; Voronina, J.K.; Syakaev, V.V.; Sharafutdinova, D.R.; Amirova, L.R.; Pudovik, M.A.; Garifzyanov, A.R.; Sinyashin, O.G. Phosphorylated aminoacetal in the synthesis of new acyclic, cyclic, and heterocyclic polyphenol structures. Heteroat. Chem. 2014, 25, 178–185. [Google Scholar] [CrossRef]
- Hellal, A.; Chafaa, S.; Chafai, N.; Touafri, L. Synthesis, antibacterial screening and DFT studies of series of α-amino-phosphonates derivatives from aminophenols. J. Mol. Struct. 2017, 1134, 217–225. [Google Scholar] [CrossRef]
- Ordonez, M.; Tibhe, G.; Bedolla-Medrano, M.; Cativiela, C. Phenylboronic acid as efficient and eco-friendly catalyst for the one-pot, three-component synthesis of α-aminophosphonates under solvent-free conditions. Synlett 2012, 23, 1931–1936. [Google Scholar] [CrossRef]
- Ordóñez, M.; Bedolla-Medrano, M.; Hernández-Fernández, E. Phenylphosphonic acid as efficient and recyclable catalyst in the synthesis of α-aminophosphonates under solvent-free conditions. Synlett 2014, 25, 1145–1149. [Google Scholar] [CrossRef]
- Sun, P.; Hu, Z.; Huang, Z. Gallium triiodide catalyzed organic reaction: A convenient synthesis of α-amino phosphonates. Synth. Commun. 2004, 34, 4293–4299. [Google Scholar] [CrossRef]
- Xu, F.; Luo, Y.; Deng, M.; Shen, Q. One-pot synthesis of α-amino phosphonates using samarium diiodide as a catalyst precursor. Eur. J. Org. Chem. 2003, 35, 4728–4730. [Google Scholar] [CrossRef]
- Ranu, B.C.; Hajra, A.; Jana, U. General procedure for the synthesis of α-amino phosphonates from aldehydes and ketones using indium(III) chloride as a catalyst. Org. Lett. 1999, 1, 1141–1143. [Google Scholar] [CrossRef]
- Lee, S.-G.; Park, J.H.; Kang, J.; Lee, J.K. Lanthanide triflate-catalyzed three component synthesis of α-aminophosphonates in ionic liquids. A catalyst reactivity and reusability study. Chem. Commun. 2001, 47, 1698–1699. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-G.; Lee, J.K.; Song, C.E.; Kim, D.-C. Microwave-assisted Kabachnik-Fields reaction in ionic liquid. Bull. Korean Chem. Soc. 2002, 23, 667–668. [Google Scholar] [CrossRef]
- Matveeva, E.D.; Podrugina, T.A.; Tishkovskaya, E.V.; Tomilova, L.G.; Zefirov, N.S. A novel catalytic three-component synthesis (Kabachnick-Fields reaction) of α-aminophosphonates from ketones. Synlett 2003, 2003, 2321–2324. [Google Scholar] [CrossRef]
- Bhagat, S.; Chakraborti, A.K. An extremely efficient three-component reaction of aldehydes/ketones, amines, and phosphites (Kabachnik–Fields Reaction) for the synthesis of α-aminophosphonates catalyzed by magnesium perchlorate. J. Org. Chem. 2007, 72, 1263–1270. [Google Scholar] [CrossRef]
- Wu, J.; Sun, W.; Xia, H.-G.; Sun, X. A facile and highly efficient route to α-amino phosphonates via three-component reactions catalyzed by Mg(ClO4)2 or molecular iodine. Org. Biomol. Chem. 2006, 4, 1663–1666. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Iranpoor, N.; Sobhani, S. Metal triflate-catalyzed one-pot synthesis of α-aminophosphonates from carbonyl compounds in the absence of solvent. Synthesis 2004, 2004, 2692–2696. [Google Scholar] [CrossRef]
- Ghosh, R.; Maiti, S.; Chakraborty, A.; Maiti, D.K. In(OTf)3 catalysed simple one-pot synthesis of α-amino phosphonates. J. Mol. Catal. A 2004, 210, 53–57. [Google Scholar] [CrossRef]
- Bhattacharya, A.K.; Kaur, T. An efficient one-pot synthesis of α-amino phosphonates catalyzed by bismuth nitrate pentahydrate. Synlett 2007, 38, 745–748. [Google Scholar] [CrossRef]
- Zhan, Z.-P.; Li, J.-P. Bismuth(III) chloride–catalyzed three-component coupling: Synthesis of α-amino phosphonates. Synth. Commun. 2005, 35, 2501–2508. [Google Scholar] [CrossRef]
- Wu, J.; Sun, W.; Wang, W.-Z.; Xiu, H.-G. A highly efficient catalyst FeCl3 in the synthesis of α-amino phosphonates via three-component reactions. Chin. J. Chem. 2006, 24, 1054–1057. [Google Scholar] [CrossRef]
- Xu, F.; Luo, Y.; Wu, J.; Shen, Q.; Chen, H. Facile one-pot synthesis of α-amino phosphonates using lanthanide chloride as catalyst. Heteroat. Chem. 2006, 17, 389–392. [Google Scholar] [CrossRef]
- Ravinder, K.; Vijender Reddy, A.; Krishnaiah, P.; Venkataramana, G.; Niranjan Reddy, V.L.; Venkateswarlu, Y. CAN Catalyzed one-pot synthesis of α-amino phosphonates from carbonyl compounds. Synth. Commun. 2004, 34, 1677–1683. [Google Scholar] [CrossRef]
- Keglevich, G.; Szekrényi, A. Eco-friendly accomplishment of the extended Kabachnik–Fields reaction; a solvent- and catalyst-free microwave-assisted synthesis of α-aminophosphonates and α-aminophosphine oxides. Lett. Org. Chem. 2008, 5, 616–622. [Google Scholar] [CrossRef]
- Kafarski, P.; Gorniak, M.G.V.; Andrasiak, I. Kabachnik-Fields reaction under green conditions—A critical overview. Curr. Green Chem. 2015, 2, 218–222. [Google Scholar] [CrossRef]
- Varga, P.R.; Karaghiosoff, K.; Sári, É.V.; Simon, A.; Hegedűs, L.; Drahos, L.; Keglevich, G. New N-acyl-, as well as N-phosphonoylmethyl-and N-phoshinoylmethyl-α-amino-benzylphosphonates by acylation and a tandem Kabachnik–Fields protocol. Org. Biomol. Chem. 2023, 21, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Bircher, M.; Karaghiosoff, K.; Czugler, M.; Takács, A.; Kőhidai, L.; Drahos, L.; Keglevich, G. New cytotoxic derivatives by the phosphorylation and phosphinoylation of diethyl α-amino-α-aryl-methylphosphonates. Chem. Eur. J. 2025, 31, e202500370. [Google Scholar] [CrossRef]
- Bajusz, B.; Nagy, D.; Tóth, R.; Szalai, Z.; Gömöry, Á.; Takács, A.; Kőhidai, L.; Keglevich, G. Synthesis of alkyl α-amino-benzylphosphinates by the aza-Pudovik reaction; the preparation of the butyl phenyl-H-phosphinate starting P-reagent. Molecules 2025, 30, 339. [Google Scholar] [CrossRef] [PubMed]
- Varga, P.R.; Oláhné Szabó, R.; Dormán, G.; Bősze, S.; Keglevich, G. Cytotoxyc activity of α-aminophosphonic derivatives coming from the tandem Kabachnik–Fields reaction and acylation. Pharmaceuticals 2023, 16, 506. [Google Scholar] [CrossRef]
- Li, Y.-J.; Ye, M.-Y.; Huang, R.-Z.; Yao, G.-Y.; Pan, Y.-M.; Liao, Z.-X.; Wang, H.-S. Coumarin-containing aminophosphonates bridged with chiral side chain: Synthesis and influence of chirality on cytotoxicity and DNA binding. Med. Chem. Res. 2014, 23, 3144–3156. [Google Scholar] [CrossRef]
- Li, Y.-J.; Wang, C.-Y.; Ye, M.-Y.; Yao, G.-Y.; Wang, H.-S. Novel coumarin-containing aminophosphonatesas antitumor agent: Synthesis, cytotoxicity, DNA-binding and apoptosis evaluation. Molecules 2015, 20, 14791–14809. [Google Scholar] [CrossRef]
- Schmidpeter, A.; Zirzow, K.-H. Dilithium-diphenyltetraseleno-hypodiphosphonat, demonstration eines AA’X spinsystems. Phosphorus Sulfur Silicon Relat. Elem. 1988, 36, 15–21. [Google Scholar] [CrossRef]
- Radeglia, A. On the NMR spectrum of the X part of an AA’X spin system. J. Prakt. Chem. 1989, 331, 863–866. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, M.; Chen, M.; Su, J.; Du, J.; Song, Q. β-Ketophosphonates formation via deesterification or deamidation of cinnamyl/alkynyl carboxylates or amides with H-phosphonates. RSC Adv. 2015, 5, 103977. [Google Scholar] [CrossRef]
- Bagan, A.; Lopez-Ruiz, A.; Abas, S.; Molins, E.; Perez, B.; Muneta-Arrate, I.; Callado, L.F.; Escolano, C. Synthesis of diversely substituted diethyl (pyrrolidin-2-Yl)Phosphonates. Molecules 2025, 30, 2078. [Google Scholar] [CrossRef] [PubMed]
- Karaghiosoff, K. Phosphorus-31 NMR. In Encyclopedia of Nuclear Magnetic Resonance; Grant, D.M., Harris, R.K., Eds.; Wiley: Chichester, UK, 1996; Volume 6, pp. 3612–3619. [Google Scholar]
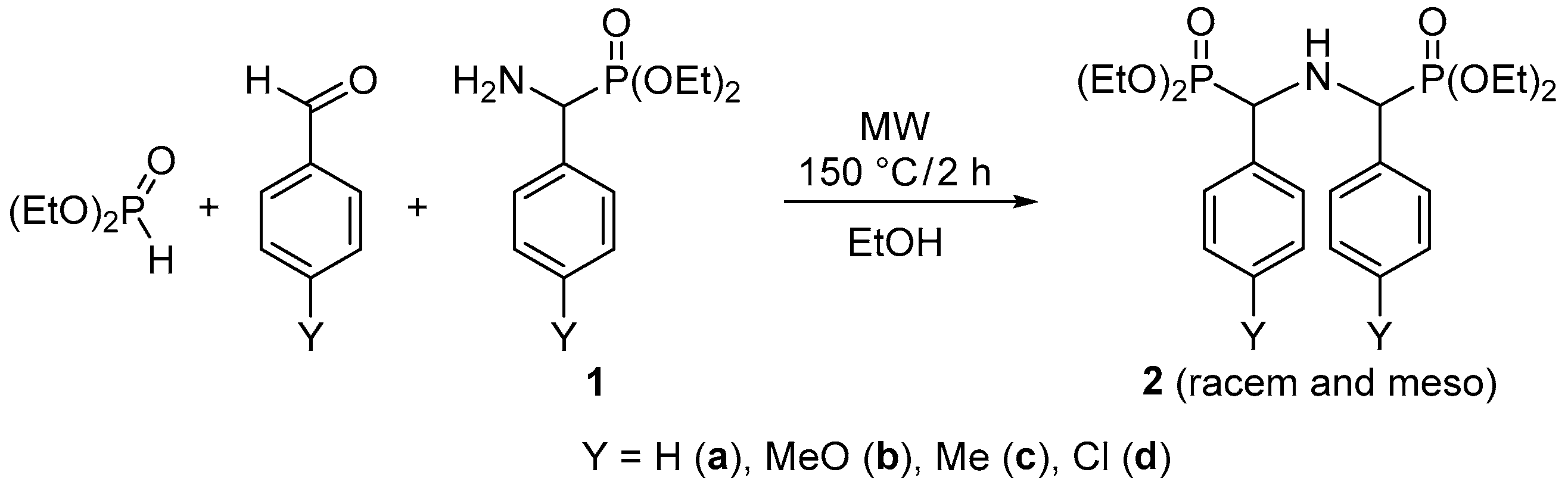

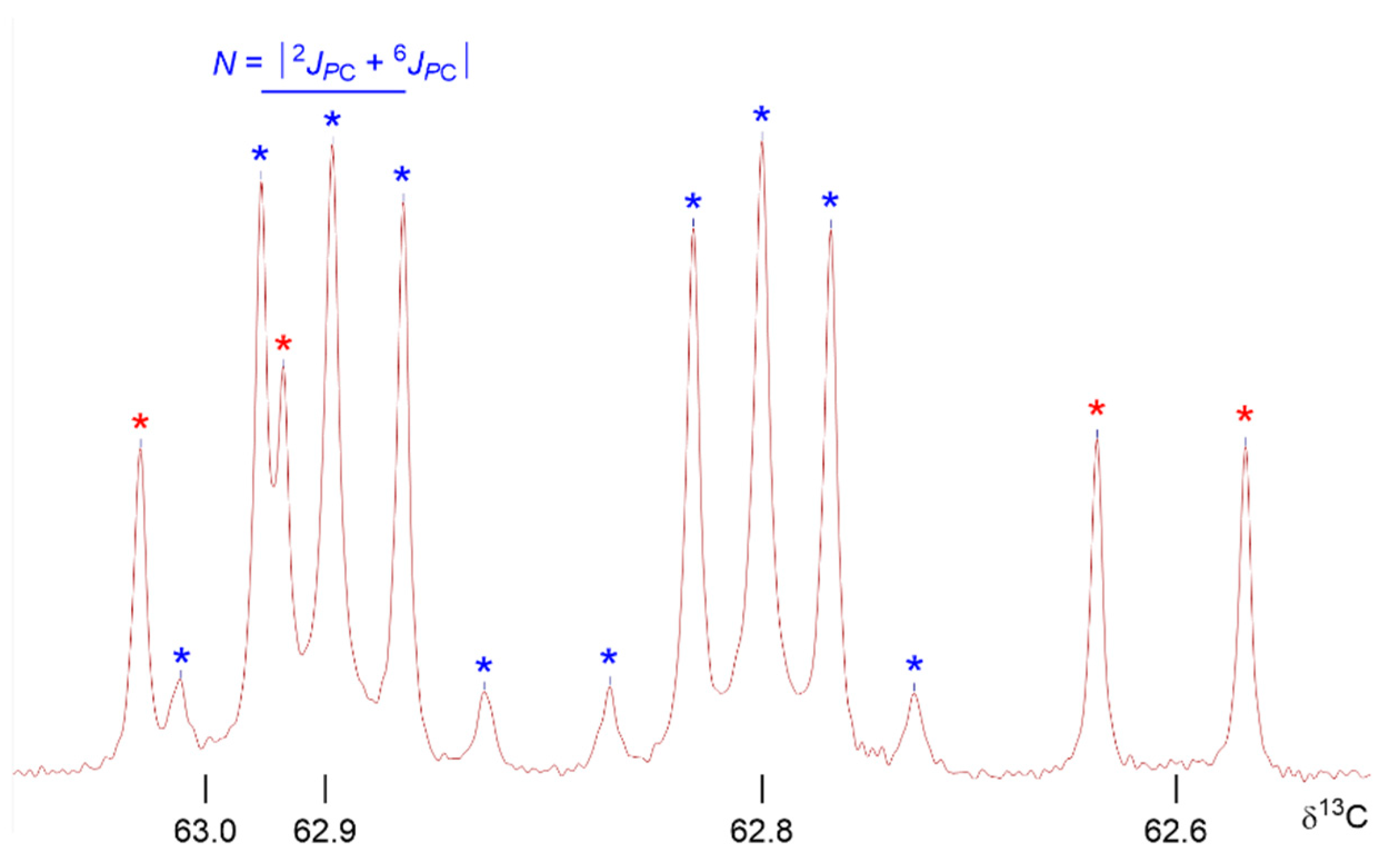

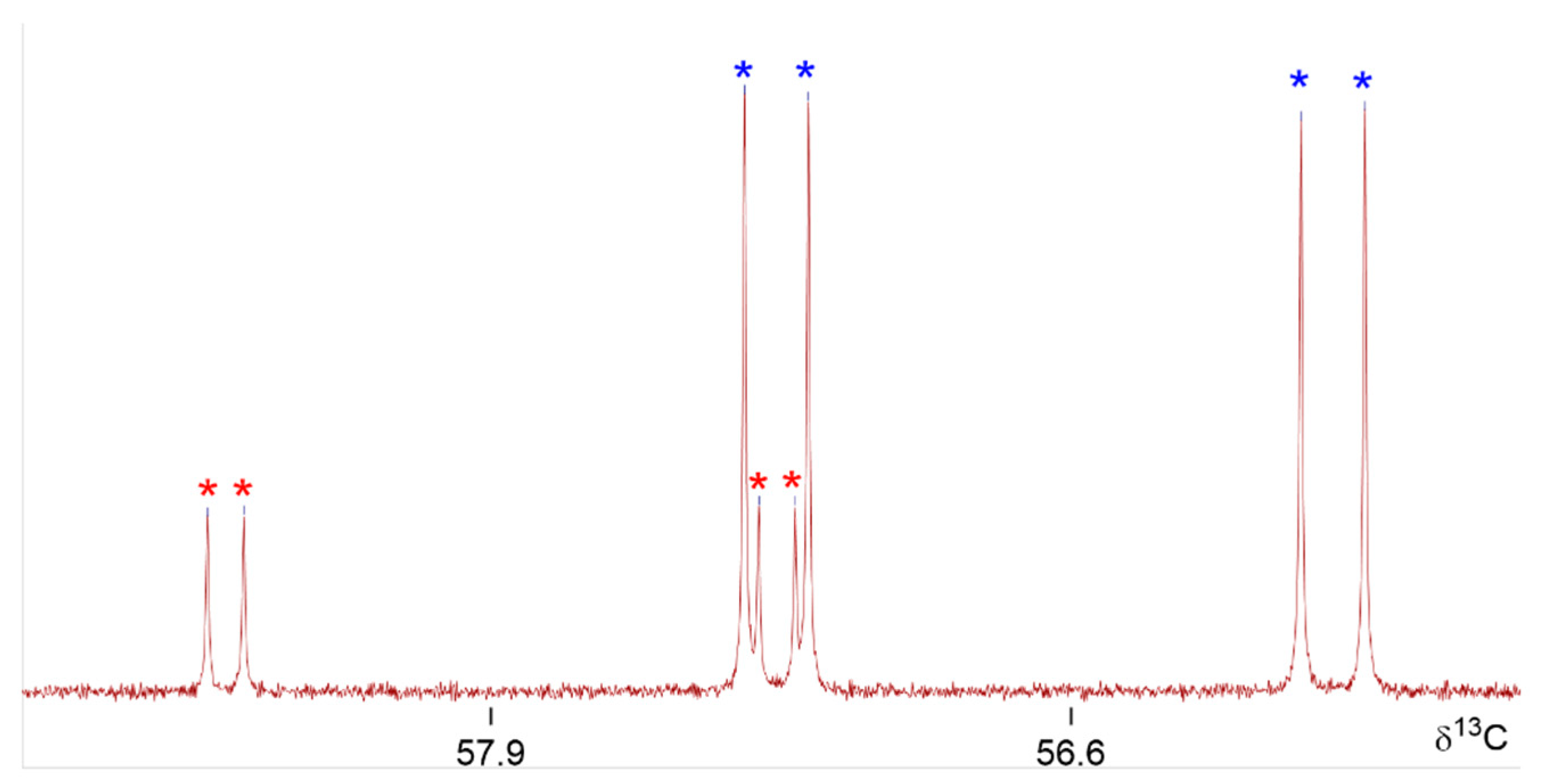



| Y = MeO | 3b1 | 3b2 | 2b1 | 2b2 | 2a1 | 2a2 | |
| composition (%) | 74 | 21 | ~5 | <1 | |||
| δP (CDCl3) | 22.76 and 22.98 (J = 7.0 Hz) | 23.02 and 23.25 | |||||
| [M+H] (m/z) | 500 | 530 | 470 | ||||
| Y = Me | 3c1 | 3c2 | 2c1 | 2c2 | 2a1 | 2a2 | |
| composition (%) | 65 | 26 | <1 | 5 * | 4 * | ||
| δP (CDCl3) | 22.76 and 22.91 (J = 7.0 Hz) | 23.06 and 23.19 | 22.67 | 22.99 | |||
| [M+H] (m/z) | 484 | 500 | 470 | ||||
| Y = Cl | 3d1 | 3d2 | 2d1 | 2d2 | 2a1 | 2a2 | |
| composition (%) | 59 | 23 | 3 * | 1 * | 9 * | 5 * | |
| δP (CDCl3) | 22.08 and 22.52 (J = 6.0 Hz) | 22.44 and 22.84 | 21.89 | 22.26 | 22.72 | 23.05 | |
| [M+H] (m/z) | 504 | 538 | 470 |
| 2a | 2b | 2c | 2d | ||||||
|---|---|---|---|---|---|---|---|---|---|
| δ31P | 22.8 (55%) | 23.1 (45%) | 23.3 (80%) | 23.5 (20%) | 23.1 (86%) | 23.4 (14%) | 22.0 (73%) | 22.3 (27%) | |
| δ1H | PCH | 3.82 | 4.30 | 3.69 | 4.16 | 3.71 | 4.20 | 3.68 | 4.16 |
| 2JPH (Hz) | 21.8 | 16.8 | 21.3 | 16.3 | 22.0 | 16.4 | 22.2 | 17.4 | |
| δ13C | PCH | 57.6 | 58.8 | 56.6 | 57.9 | 57.1 | 58.2 | 57.0 | 58.2 |
| 1JPC (Hz) | 155.0 | 153.4 | 156.7 | 155.3 | 155.6 | 154.1 | 155.8 | 152.7 | |
| 3JPC (Hz) | 17.8 | 10.3 | 18.0 | 10.2 | 18.0 | 10.1 | 17.6 | 10.2 | |
| 3b | 3c | 3d | |||||
|---|---|---|---|---|---|---|---|
| amount of isomer (%) | 86.9 | 13.3 | 82.0 | 18.0 | 71 | 29 | |
| δ31P | 22.9 (Pa) | 23.2 | 22.9 (Pa) | 23.2 (Pa) | 22.6 (Pa) | 22.9 (Pa) | |
| 22.8 (Pb) | 23.4 | 23.0 (Pb) | 23.3 (Pb) | 22.1 (Pb) | 22.5 (Pb) | ||
| 4JPP (Hz) | 6.7 | 0.8 | 6.8 | 1.0 | 6.6 | 0.6 | |
| δ1H | PaCH | 3.83 | 4.30 | 3.84 | 4.33 | 3.76 | 4.23 |
| 2JPH (Hz) | 20.2 | 17.1 | 22.0 | 16.9 | 20.4 | 16.8 | |
| δ1H | PbCH | 3.77 | 4.26 | 3.79 | 4.27 | 3.61 | 4.30 |
| 2JPH (Hz) | 18.9 | 16.1 | 22.0 | 16.3 | 19.0 | 17.7 | |
| δ13C | PaCH | 57.4 | 58.6 | 57.4 | 58.6 | 57.6 | 58.8 |
| 1JPC (Hz) | 155.3 | 153.7 | 155.1 | 153.3 | 155.1 | 153.0 | |
| 3JPC (Hz) | 17.7 | 10.6 | 17.8 | 10.0 | 18.3 | 10.5 | |
| δ13C | PbCH | 56.8 | 58.0 | 57.2 | 58.3 | 56.9 | 58.2 |
| 1JPC (Hz) | 156.8 | 155.2 | 155.6 | 154.2 | 154.3 | 153.3 | |
| 3JPC (Hz) | 17.8 | 10.0 | 17.8 | 10.3 | 18.6 | 10.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Bajusz, B.; Karaghiosoff, K.; Drahos, L.; Gömöry, Á.; Keglevich, G. The Synthesis of Bis(α-aryl-methylphosphonoyl)amines by the Microwave-Assisted Catalyst-Free Tandem Kabachnik–Fields Reaction. Catalysts 2026, 16, 148. https://doi.org/10.3390/catal16020148
Bajusz B, Karaghiosoff K, Drahos L, Gömöry Á, Keglevich G. The Synthesis of Bis(α-aryl-methylphosphonoyl)amines by the Microwave-Assisted Catalyst-Free Tandem Kabachnik–Fields Reaction. Catalysts. 2026; 16(2):148. https://doi.org/10.3390/catal16020148
Chicago/Turabian StyleBajusz, Bence, Konstantin Karaghiosoff, László Drahos, Ágnes Gömöry, and György Keglevich. 2026. "The Synthesis of Bis(α-aryl-methylphosphonoyl)amines by the Microwave-Assisted Catalyst-Free Tandem Kabachnik–Fields Reaction" Catalysts 16, no. 2: 148. https://doi.org/10.3390/catal16020148
APA StyleBajusz, B., Karaghiosoff, K., Drahos, L., Gömöry, Á., & Keglevich, G. (2026). The Synthesis of Bis(α-aryl-methylphosphonoyl)amines by the Microwave-Assisted Catalyst-Free Tandem Kabachnik–Fields Reaction. Catalysts, 16(2), 148. https://doi.org/10.3390/catal16020148









