Production of Methanol by CO2 Hydrogenation Using a Membrane Reactor
Abstract
1. Introduction
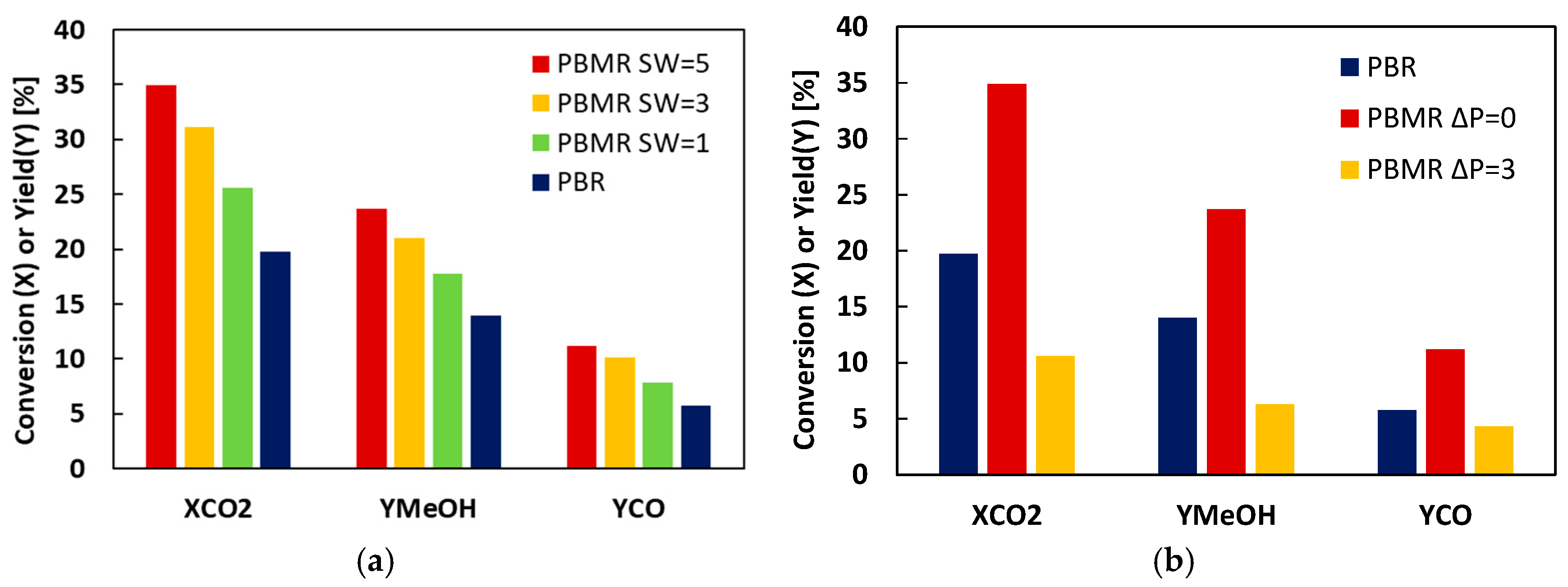
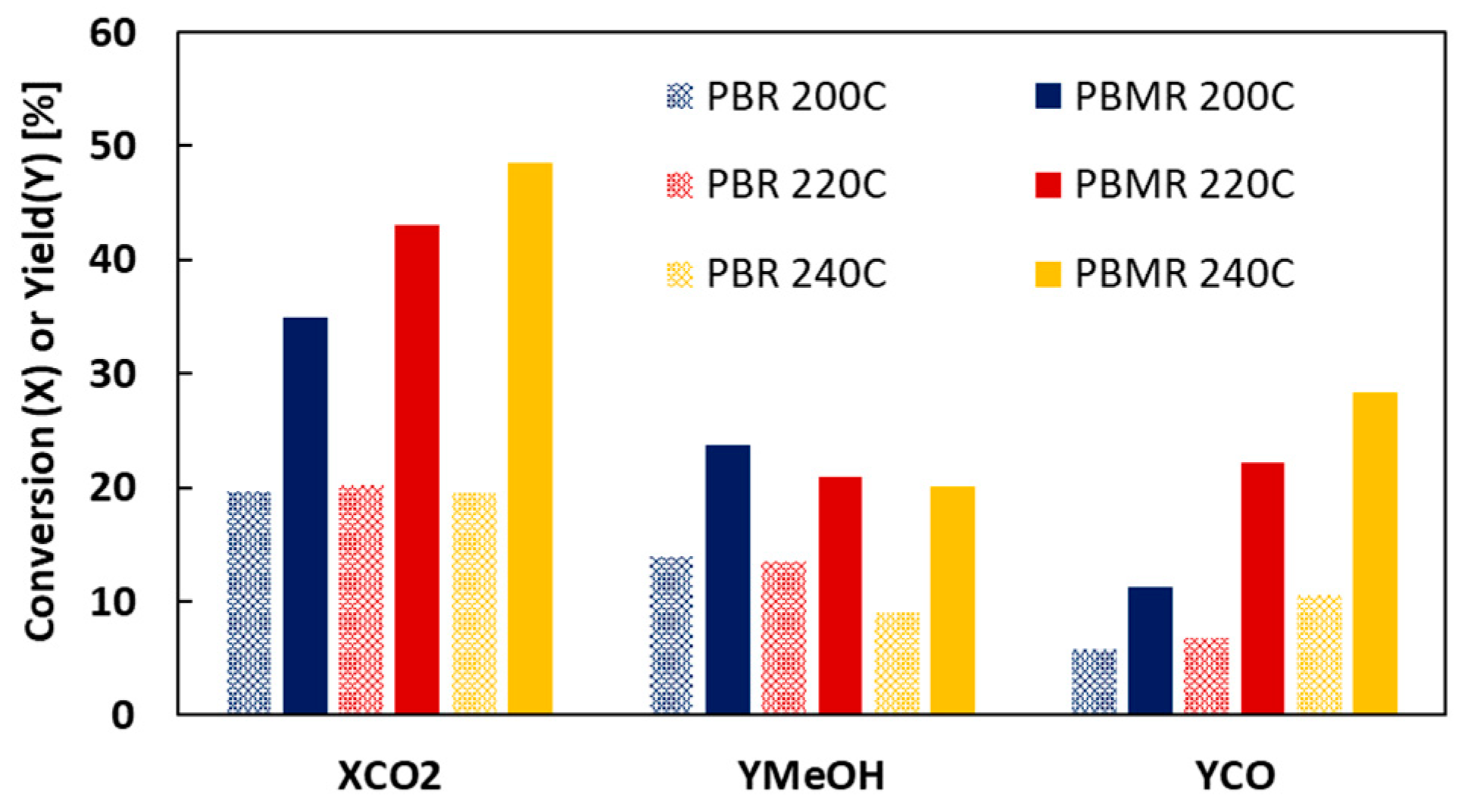
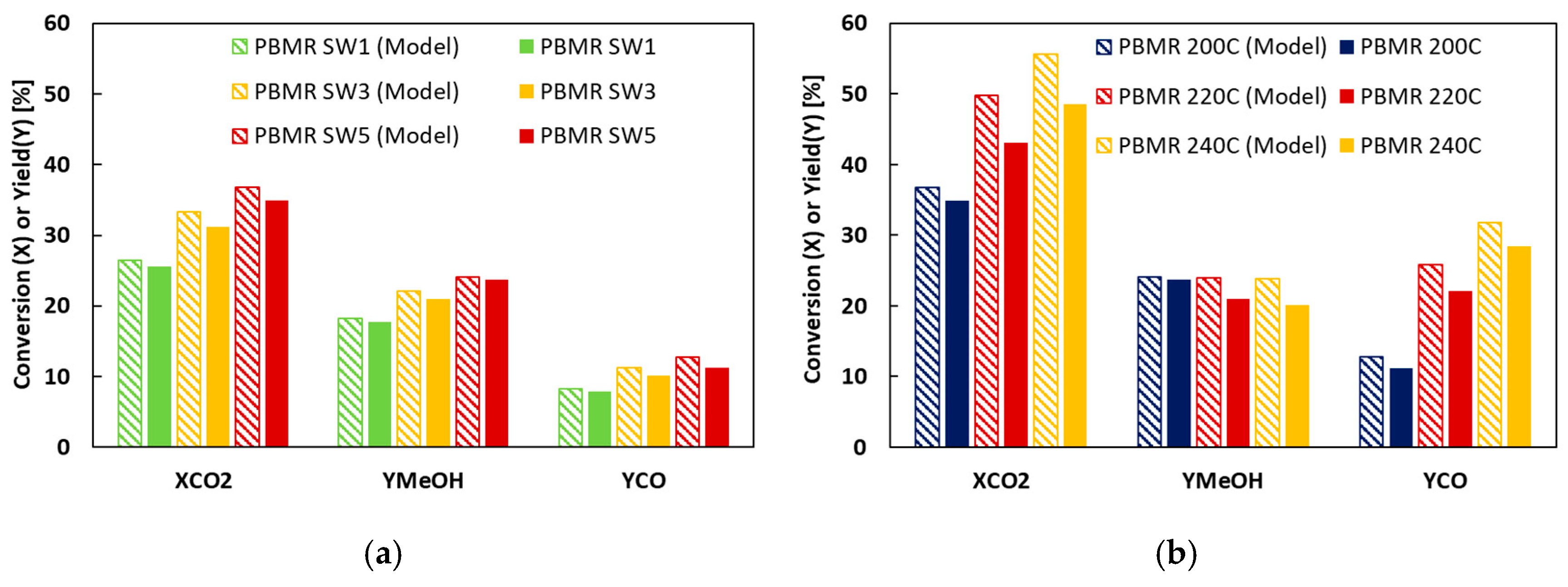
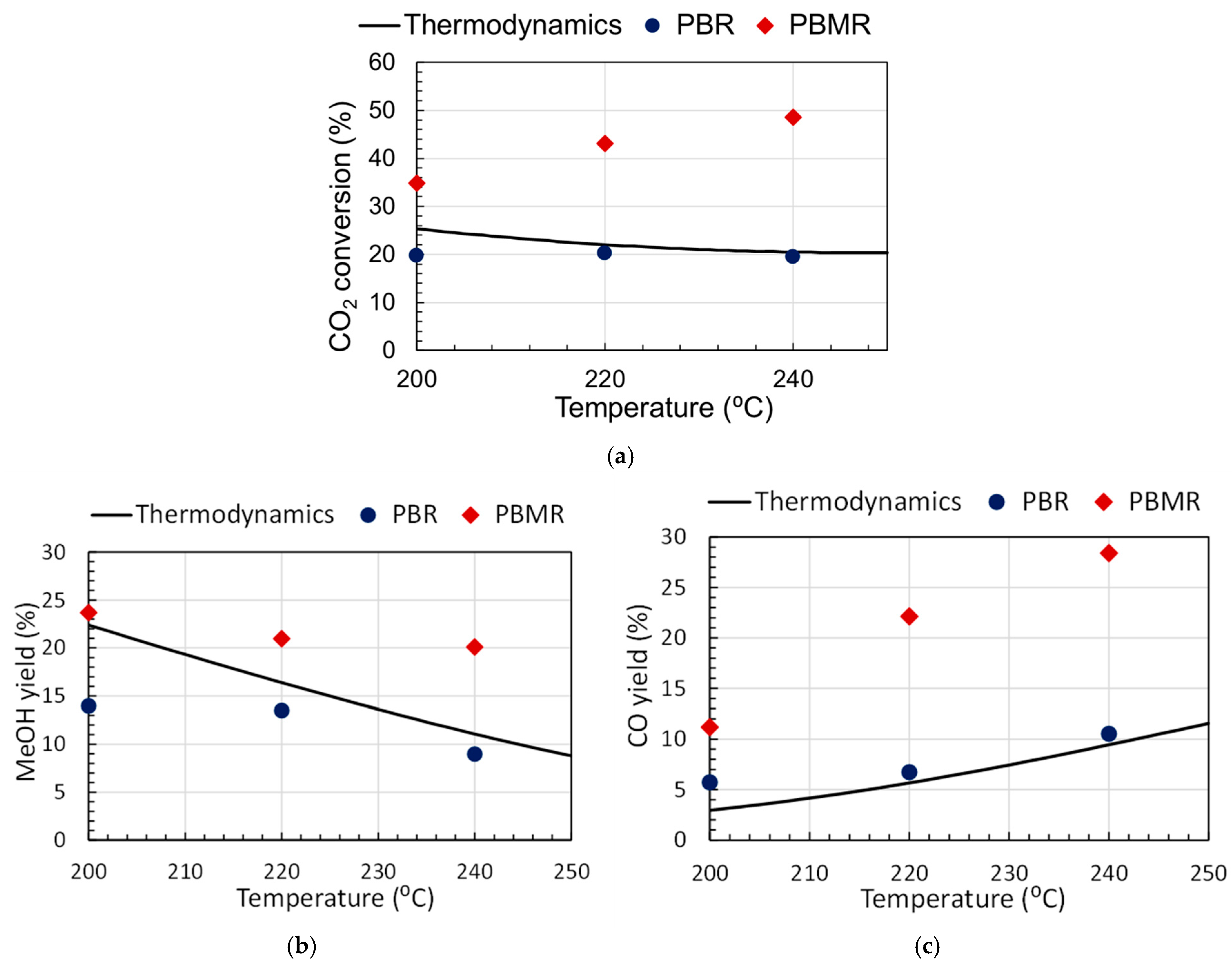
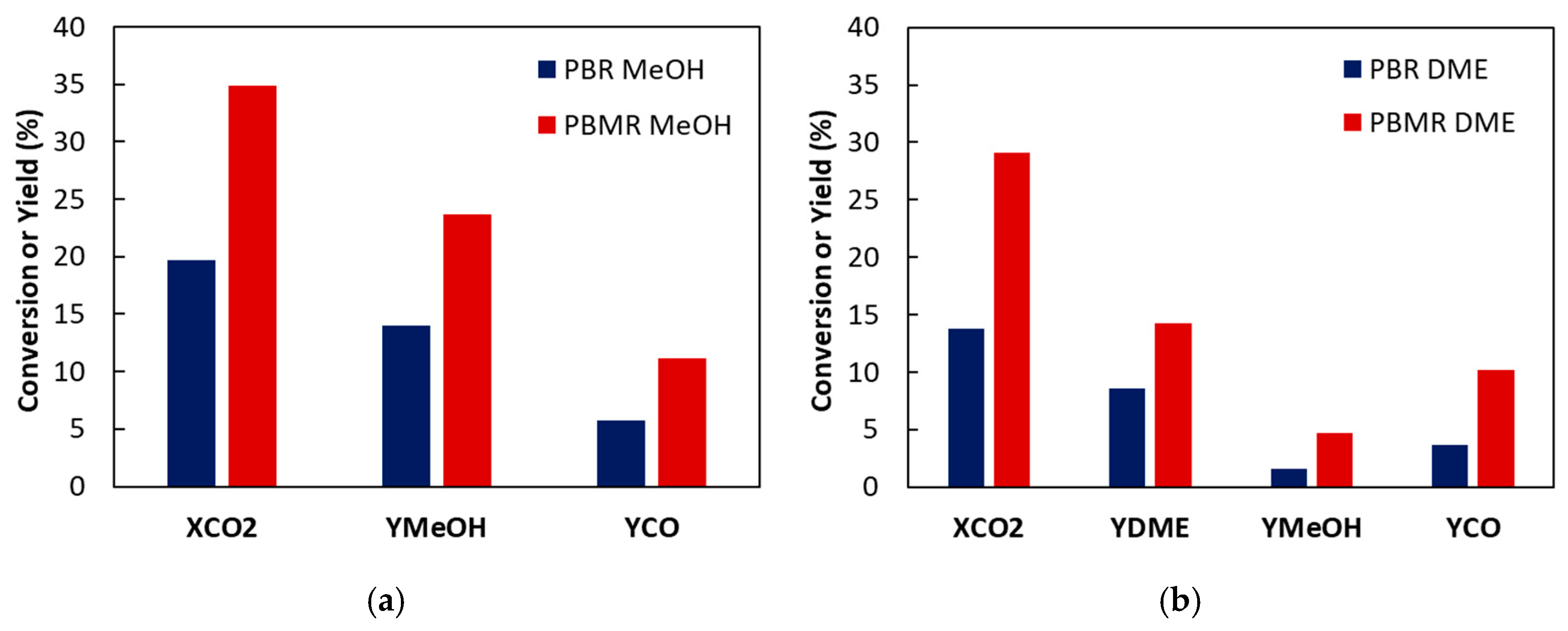
2. Results and Discussion
| CO2 transmembrane flow | ||
| Reactant loss | ||
| Reactant cofeeding | ||
3. Experimental Methods
3.1. Al-CMSM Preparation
Permeation Results
3.2. Methanol Synthesis
3.2.1. Catalyst
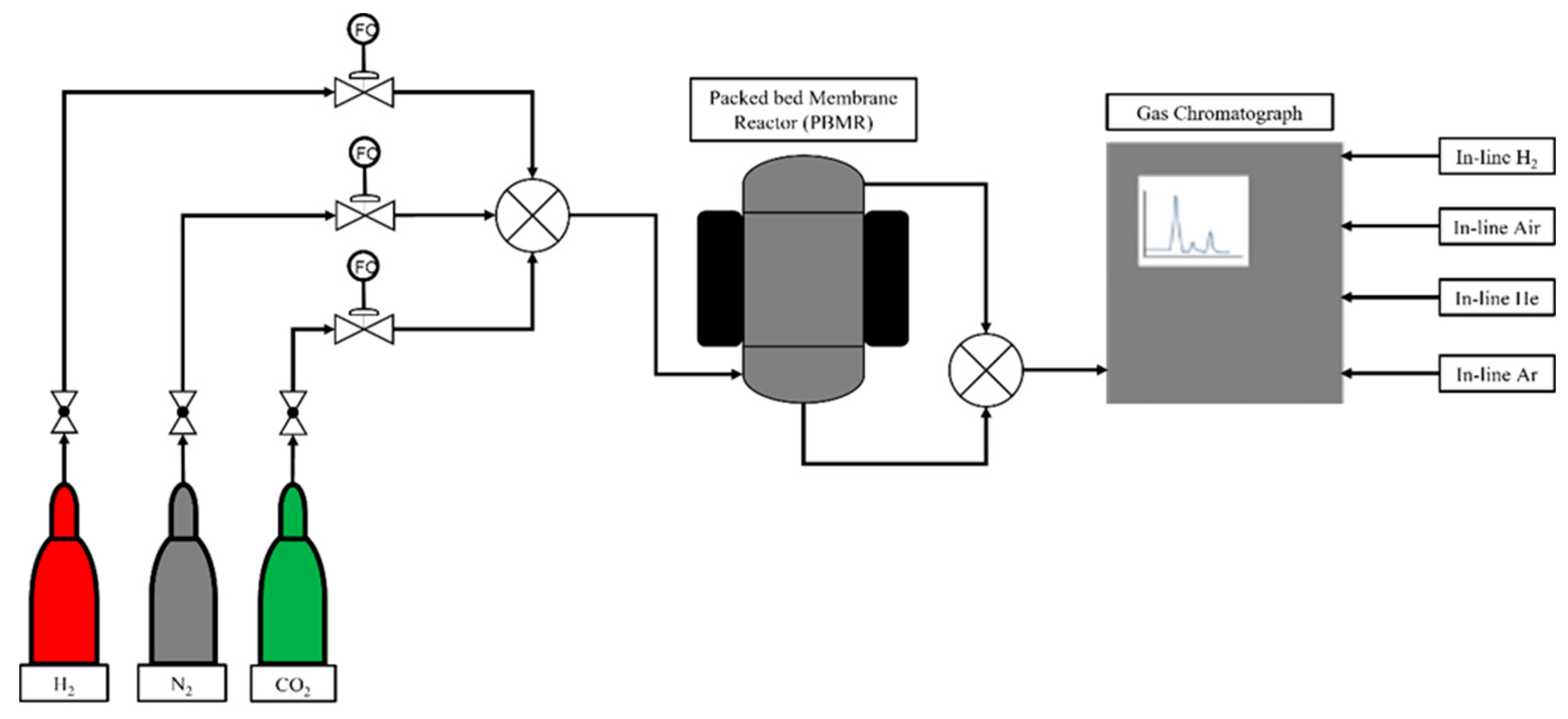
3.2.2. Packed-Bed Membrane Reactor (PBMR)
3.2.3. Packed-Bed Reactor (PBR)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Parameters | Conversion CO2 [%] | Yield MeOH [%] | Yield CO [%] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sweep/Reacting Gases SW [-] | Pressure Difference ΔP [bar] | Temperature [°C] | PBR | PBMR | PBR | PBMR | PBR | PBMR | |
| MR1 | 1 | 0 | 200 | 19.7 | 5.6 | 14.0 | 17.7 | 5.7 | 7.9 |
| MR6 | 3 | 0 | 200 | 19.7 | 31.1 | 14.0 | 21.0 | 5.7 | 10.1 |
| MR2 | 5 | 0 | 200 | 19.7 | 34.9 | 14.0 | 23.7 | 5.7 | 11.2 |
| MR5 | 5 | 3 | 200 | 19.7 | 10.6 | 14.0 | 6.3 | 5.7 | 4.3 |
| MR3 | 5 | 0 | 220 | 20.2 | 43.1 | 13.5 | 21 | 6.7 | 22.1 |
| MR4 | 5 | 0 | 240 | 19.5 | 48.5 | 9.0 | 20.1 | 10.6 | 28.4 |
| Temperature [°C] | CO2 Conversion [%] | MeOH Yield [%] | CO Yield [%] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PBR | PBMR | Therm | PBR | PBMR | Therm | PBR | PBMR | Therm | |
| 200 | 19.7 | 34.9 | 25.4 | 14.0 | 23.7 | 22.4 | 5.7 | 11.2 | 3.0 |
| 220 | 20.2 | 43.1 | 22.1 | 13.5 | 21.0 | 16.4 | 6.7 | 22.1 | 5.7 |
| 240 | 19.5 | 48.5 | 20.5 | 9.0 | 20.1 | 11.1 | 10.6 | 28.4 | 9.4 |
| Temperature [°C] | CO2 Conversion [%] | MeOH Yield [%] | CO Yield [%] | DME Yield [%] | ||||
|---|---|---|---|---|---|---|---|---|
| PBR | PBMR | PBR | PBMR | PBR | PBMR | PBR | PBMR | |
| 200 | 13.7 | 29.1 | 1.6 | 4.7 | 3.6 | 10.2 | 8.6 | 14.3 |
Appendix B. Model Equations and Assumptions
- Steady state conditions.
- 1D ideal plug flow (i.e., axial and radial dispersion is neglected by considering L/dp ≥ 50 and D/dp ≥ 25, respectively).
- Kinetic control regime (i.e., the solid and gas phases are described as a single pseudo-homogeneous phase, due to the absence of mass transfer limitations).
- Negligible pressure drops in the permeation side.
- Kinetics by Lu et al. [23] valid for conventional and membrane reactors.
- Inert membrane material under reaction conditions.
| Kinetic Parameter | Value |
|---|---|
References
- European parlament and the Council of the European Union. Amending Regulation (EU) 2019/631 as regards strengthening the CO2 emission performance standards for new passenger cars and new light commercial vehicles in line with the Union’s increased climate ambition. Off. J. Eur. Union 2023, L 110, 5–20. [Google Scholar]
- Wang, W.; Zeng, C.; Tsubaki, N. Recent advancements and perspectives of the CO2 hydrogenation reaction. Green Carbon 2023, 1, 133–145. [Google Scholar] [CrossRef]
- Shi, K.; Guan, B.; Zhuang, Z.; Chen, J.; Chen, Y.; Ma, Z.; Zhu, C.; Hu, X.; Zhao, S.; Dang, H.; et al. Perspectives and Outlook of E-fuels: Production, Cost Effectiveness, and Applications. Energy Fuels 2024, 38, 7665–7692. [Google Scholar] [CrossRef]
- Richard, S.; Olivier, P.; Jegoux, M.; Makhloufi, C.; Gallucci, F. Membrane reactors technologies for e-fuel production & processing: A review. Int. J. Hydrogen Energy 2025, 112, 446–467. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, J.; Wu, C.; Fu, J.; Liu, J.; Duan, X. The application prospect and challenge of the alternative methanol fuel in the internal combustion engine. Sci. Total Environ. 2024, 913, 169708. [Google Scholar] [CrossRef]
- Dieterich, V.; Buttler, A.; Hanel, A.; Spliethoff, H.; Fendt, S. Power-to-liquid via synthesis of methanol, DME or Fischer–Tropsch-fuels: A review. Energy Environ. Sci. 2020, 13, 3207–3252. [Google Scholar] [CrossRef]
- Ebrahimzadeh Sarvestani, M.; Norouzi, O.; Di Maria, F.; Dutta, A. From catalyst development to reactor Design: A comprehensive review of methanol synthesis techniques. Energy Convers. Manag. 2024, 302, 118070. [Google Scholar] [CrossRef]
- Ma, J.; Sun, N.; Zhang, X.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. A short review of catalysis for CO2 conversion. Catal. Today 2009, 148, 221–231. [Google Scholar] [CrossRef]
- Etim, U.J.; Song, Y.; Zhong, Z. Improving the Cu/ZnO-Based Catalysts for Carbon Dioxide Hydrogenation to Methanol, and the Use of Methanol As a Renewable Energy Storage Media. Front. Earth Sci. 2020, 8, 1–26. [Google Scholar] [CrossRef]
- Portha, J.F.; Parkhomenko, K.; Kobl, K.; Roger, A.C.; Arab, S.; Commenge, J.M.; Falk, L. Kinetics of Methanol Synthesis from Carbon Dioxide Hydrogenation over Copper-Zinc Oxide Catalysts. Ind. Eng. Chem. Res. 2017, 56, 13133–13145. [Google Scholar] [CrossRef]
- Li, D.; Xu, F.; Tang, X.; Dai, S.; Pu, T.; Liu, X.; Tian, P.; Xuan, F.; Xu, Z.; Wachs, I.E.; et al. Induced activation of the commercial Cu/ZnO/Al2O3 catalyst for the steam reforming of methanol. Nat. Catal. 2022, 5, 99–108. [Google Scholar] [CrossRef]
- Gallucci, F.; Basile, A. A theoretical analysis of methanol synthesis from CO2 and H2 in a ceramic membrane reactor. Int. J. Hydrogen Energy 2007, 32, 5050–5058. [Google Scholar] [CrossRef]
- Mohammed, M.G.; Hashim, N.A.; Daud, W.M.A.W.; Hartley, U.W.; Aroua, M.K.; Wohlrab, S. Overview of the latest progress and prospects in the catalytic hydrogenation of carbon dioxide (CO2) to methanol in membrane reactors. Int. J. Hydrogen Energy 2024, 77, 936–957. [Google Scholar] [CrossRef]
- Gallucci, F.; Paturzo, L.; Basile, A. An experimental study of CO2 hydrogenation into methanol involving a zeolite membrane reactor. Chem. Eng. Process. 2004, 43, 1029–1036. [Google Scholar] [CrossRef]
- Gorbe, J.; Lasobras, J.; Francés, E.; Herguido, J.; Menéndez, M.; Kumakiri, I.; Kita, H. Preliminary study on the feasibility of using a zeolite A membrane in a membrane reactor for methanol production. Sep. Purif. Technol. 2018, 200, 164–168. [Google Scholar] [CrossRef]
- Maréchal, F.; Heyen, G.; Kalitventzeff, B. Energy savings in methanol synthesis: Use of heat integration techniques and simulation tools. Comput. Chem. Eng. 1997, 21, S511–S516. [Google Scholar]
- Prodinger, S.; Derewinski, M.A. Recent Progress to Understand and Improve Zeolite Stability in the Aqueous Medium. Pet. Chem. 2020, 60, 420–436. [Google Scholar] [CrossRef]
- Neves, T.M.; Pollo, L.D.; Marcilio, N.R.; Tessaro, I.C. Insights into the development of carbon molecular sieve membranes from polymer blends for gas separation: A review. Gas Sci. Eng. 2024, 131, 205472. [Google Scholar] [CrossRef]
- Ismail, A.F.; Goh, P.S.; Sanip, S.M.; Aziz, M. Transport and separation properties of carbon nanotube-mixed matrix membrane. Sep. Purif. Technol. 2009, 70, 12–26. [Google Scholar] [CrossRef]
- Llosa Tanco, M.A.; Pacheco Tanaka, D.A.; Rodrigues, S.C.; Texeira, M.; Mendes, A. Composite-alumina-carbon molecular sieve membranes prepared from novolac resin and boehmite. Part I: Preparation, characterization and gas permeation studies. Int. J. Hydrogen Energy 2015, 40, 5653–5663. [Google Scholar] [CrossRef]
- Poto, S.; Endepoel, J.G.H.; Llosa-Tanco, M.A.; Pacheco-Tanaka, D.A.; Gallucci, F.; Neira d’Angelo, M.F. Vapor/gas separation through carbon molecular sieve membranes: Experimental and theoretical investigation. Int. J. Hydrogen Energy 2022, 47, 11385–11401. [Google Scholar] [CrossRef]
- Poto, S.; Llosa Tanco, M.A.; Pacheco Tanaka, D.A.; Neira d′Angelo, M.F.; Gallucci, F. Experimental investigation of a packed bed membrane reactor for the direct conversion of CO2 to dimethyl ether. J. CO2 Util. 2023, 72, 102513. [Google Scholar] [CrossRef]
- Lu, W.; Teng, L.; Xiao, W. Simulation and experiment study of dimethyl ether synthesis from syngas in a fluidized-bed reactor. Chem. Eng. Sci. 2004, 59, 5455–5464. [Google Scholar] [CrossRef]
- Giacinti Baschetti, M.; De Angelis, M.G. Vapour Permeation Modelling; Elsevier Ltd.: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Bussche KMVanden Froment, G.F. A Steady-State Kinetic Model for Methanol Synthesis and the Water Gas Shift Reaction on a Commercial Cu/ZnO/Al2O3 Catalyst. J. Catal. 1996, 10, 1–10. [Google Scholar] [CrossRef]
- Zhang, H.T.; Cao, F.H.; Liu, D.H.; Fang, D.Y. Thermodynamic analysis for synthesis of dimethyl ether and methanol from synthesis gas. J. East China Univ. Sci. Technol. 2001, 27, 198–201. [Google Scholar]
- Taler, D.; Taler, J. Simple heat transfer correlations for turbulent tube flow. E3S Web Conf. 2017, 13, 1–7. [Google Scholar] [CrossRef]
- Backer, J.J. Heat Transfer in Packed Beds. Ind. Eng. Chem. 1965, 57, 43–51. [Google Scholar] [CrossRef]
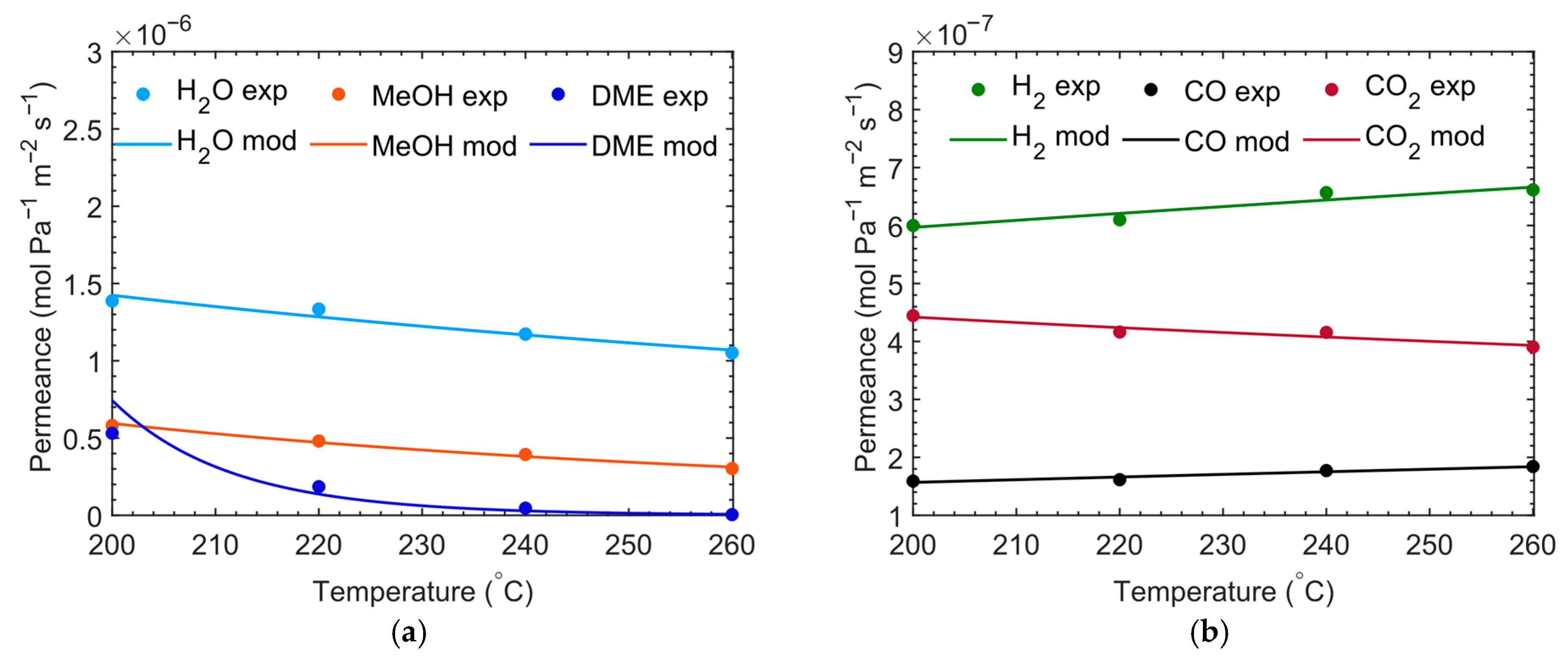
| Membrane Property | CMSM Tested |
|---|---|
| (mol·Pa−1·m−2·s−1) | 1.39 × 10−6 |
| (-) | 2.06 |
| (-) | 2.37 |
| (-) | 3.99 |
| (-) | 2.39 |
| (-) | 2.61 |
| Sweep Gas/Reacting Gas SW [-] | Pressure Difference ΔP [bar] | Temperature T [°C] | |
|---|---|---|---|
| MR1 | 1 | 0 | 200 |
| MR2 | 5 | 0 | 200 |
| MR3 | 5 | 0 | 220 |
| MR4 | 5 | 0 | 240 |
| MR5 | 5 | 3 | 200 |
| MR6 | 3 | 0 | 200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Gallucci, F.; Poto, S.; Tanco, M.A.L.; Pacheco Tanaka, D.A. Production of Methanol by CO2 Hydrogenation Using a Membrane Reactor. Catalysts 2026, 16, 53. https://doi.org/10.3390/catal16010053
Gallucci F, Poto S, Tanco MAL, Pacheco Tanaka DA. Production of Methanol by CO2 Hydrogenation Using a Membrane Reactor. Catalysts. 2026; 16(1):53. https://doi.org/10.3390/catal16010053
Chicago/Turabian StyleGallucci, Fausto, Serena Poto, Margot Anabell Llosa Tanco, and David Alfredo Pacheco Tanaka. 2026. "Production of Methanol by CO2 Hydrogenation Using a Membrane Reactor" Catalysts 16, no. 1: 53. https://doi.org/10.3390/catal16010053
APA StyleGallucci, F., Poto, S., Tanco, M. A. L., & Pacheco Tanaka, D. A. (2026). Production of Methanol by CO2 Hydrogenation Using a Membrane Reactor. Catalysts, 16(1), 53. https://doi.org/10.3390/catal16010053












