Activity and Stability Enhancement of Carbonic Anhydrase Entrapped Within Biomimetic Silica by Methyl-Substituted Silanes
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Recombinant SazCA
2.2. Effect of SazCA Concentration on Entrapment
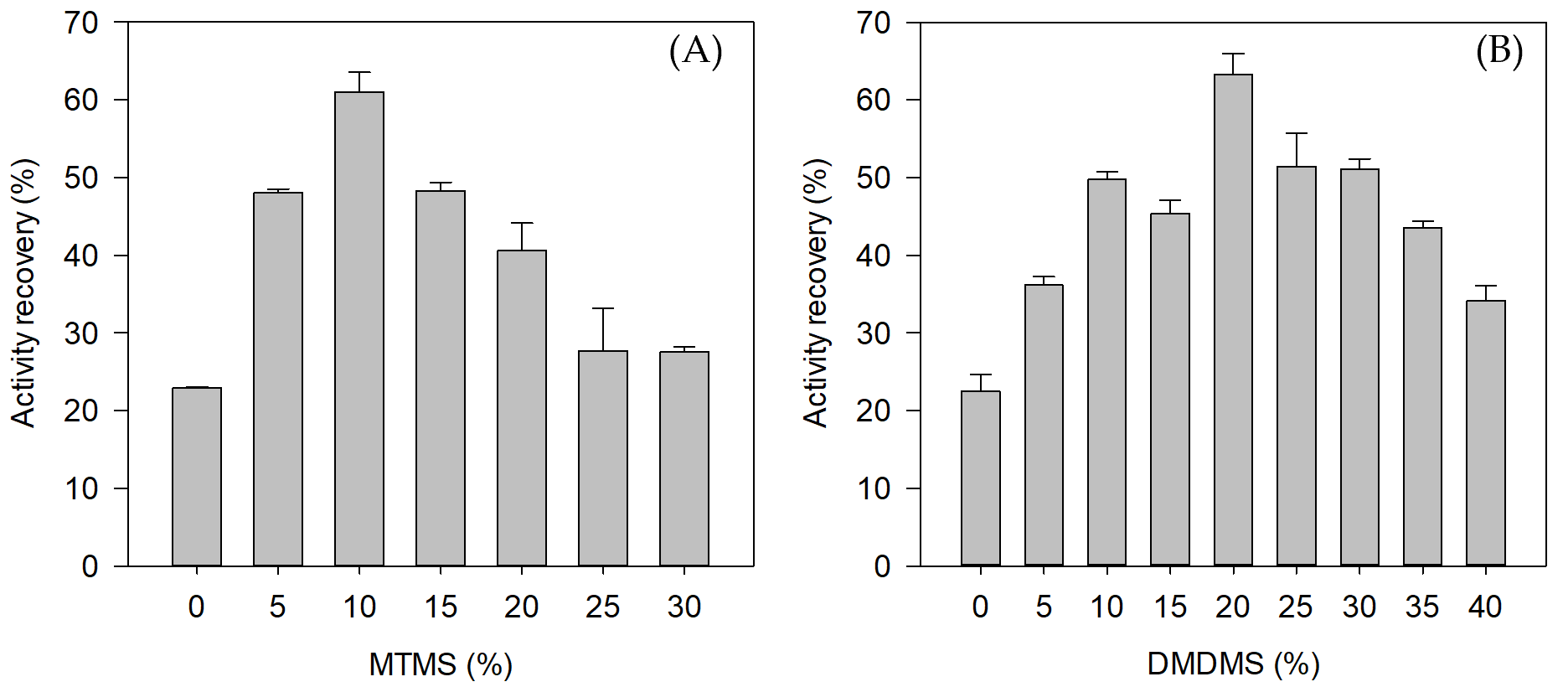
2.3. Effect of MTMS and DMDMS on Entrapped SazCA
2.4. Thermal Stability
2.5. pH Stability
2.6. Storage Stability
2.7. Reusability by Hydratase Activity
2.8. Carbon Sequestration
3. Materials and Methods
3.1. Plasmid Construction and Propagation
3.2. Expression and Purification of SazCA
3.3. Activity Assay
3.4. Entrapment of SazCA
- Immobilization efficiency = (amount of entrapped SazCA/amount of added SazCA) × 100%.
- Activity recovery = (specific activity of entrapped SazCA/specific activity of free SazCA) × 100%.
3.5. Thermal, pH, and Storage Stability
3.6. Determination of Reusability by Hydratase Activity
3.7. Determination of Reusability by Carbon Sequestration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CA | Carbonic anhydrase |
| SazCA | CA from Sulfurihydrogenibium azorense |
| MTMS | Methyltrimethoxysilane |
| DMDMS | Dimethyldimethoxysilane |
| TMOS | Tetramethoxysilane |
References
- Bose, H.; Satyanarayana, T. Microbial carbonic anhydrases in biomimetic carbon sequestration for mitigating global warming: Prospects and perspectives. Front. Microbiol. 2017, 8, 1615. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar]
- Liu, E.; Lu, X.; Wang, D. A systematic review of carbon capture, utilization and storage: Status, progress and challenges. Energies 2023, 16, 2865. [Google Scholar] [CrossRef]
- Capasso, C.; Supuran, C.T. An overview of the selectivity and efficiency of the bacterial carbonic anhydrase inhibitors. Curr. Med. Chem. 2015, 22, 2130–2139. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Maciel, A.; Christakopoulos, P.; Rova, U.; Antonopoulou, I. Carbonic anhydrase to boost CO2 sequestration: Improving carbon capture utilization and storage (CCUS). Chemosphere 2022, 299, 134419. [Google Scholar] [CrossRef]
- Ali, J.; Faridi, S.; Sardar, M. Carbonic anhydrase as a tool to mitigate global warming. Environ. Sci. Pollut. Res. 2023, 30, 83093–83112. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.L.; Maberly, S.C.; Gontero, B. Insights on the functions and ecophysiological relevance of the diverse carbonic anhydrases in microalgae. Int. J. Mol. Sci. 2020, 21, 2922. [Google Scholar] [CrossRef]
- De Simone, G.; Monti, S.M.; Alterio, V.; Buonanno, M.; De Luca, V.; Rossi, M.; Carginale, V.; Supuran, C.T.; Capasso, C.; Di Fiore, A. Crystal structure of the most catalytically effective carbonic anhydrase enzyme known, SazCA from the thermophilic bacterium Sulfurihydrogenibium azorense. Bioorg. Med. Chem. Lett. 2015, 25, 2002–2006. [Google Scholar]
- Luca, V.D.; Vullo, D.; Scozzafava, A.; Carginale, V.; Rossi, M.; Supuran, C.T.; Capasso, C. An α-carbonic anhydrase from the thermophilic bacterium Sulphurihydrogenibium azorense is the fastest enzyme known for the CO2 hydration reaction. Bioorg. Med. Chem. 2013, 21, 1465–1469. [Google Scholar]
- Kumari, M.; Lee, J.; Lee, D.W.; Hwang, I. High-level production in a plant system of a thermostable carbonic anhydrase and its immobilization on microcrystalline cellulose beads for CO2 capture. Plant. Cell Rep. 2020, 39, 1317–1329. [Google Scholar]
- Hou, J.; Li, X.; Kaczmarek, M.B.; Chen, P.; Li, K.; Jin, P.; Liang, Y.; Daroch, M. Accelerated CO2 hydration with thermostable Sulfurihydrogenibium azorense carbonic anhydrase-chitin binding domain fusion protein immobilised on chitin support. Int. J. Mol. Sci. 2019, 20, 1494. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, K.; Yuan, H.; Zhou, R.; Mao, L.; Zhang, R.; Zhang, G. An antifouling epoxy coated metal surface containing silica-immobilized carbonic anhydrase supraparticles for CO2 capture through microalgae. Int. J. Biol. Macromol. 2024, 269, 132075. [Google Scholar] [CrossRef]
- Sumper, M.; Kröger, N. Silica formation in diatoms: The function of long chain polyamines and silaffins. J. Mater. Chem. 2004, 14, 2059–2065. [Google Scholar] [CrossRef]
- Kröger, N.; Deutzmann, R.; Sumper, M. Polycationic peptides from diatom biosilica that direct silica nanosphere formation. Science 1999, 286, 1129–1132. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.A.; Pack, S.P. Biomimetic and bioinspired silicifications: Recent advances for biomaterial design and applications. Acta Biomater. 2021, 120, 38–56. [Google Scholar] [CrossRef]
- Fu, W.-D.; Hsieh, C.-J.; Hu, C.-J.; Yu, C.-Y. Entrapment of carbonic anhydrase from Sulfurihydrogenibium azorense with polyallylamine-mediated biomimetic silica. Bioresour. Technol. Rep. 2022, 20, 101217. [Google Scholar] [CrossRef]
- Reetz, M.T.; Zonta, A.; Simpelkamp, J. Efficient heterogeneous biocatalysts by entrapment of lipases in hydrophobic sol–Gel materials. Angew. Chem. Int. Ed. Engl. 1995, 34, 301–303. [Google Scholar] [CrossRef]
- Weiser, D.; Boros, Z.; Hornyánszky, G.; Tóth, A.; Poppe, L. Disubstituted dialkoxysilane precursors in binary and ternary sol–Gel systems for lipase immobilization. Process Biochem. 2012, 47, 428–434. [Google Scholar] [CrossRef]
- Luckarift, H.R.; Spain, J.C.; Naik, R.R.; Stone, M.O. Enzyme immobilization in a biomimetic silica support. Nat. Biotech. 2004, 22, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Jo, B.H.; Seo, J.H.; Yang, Y.J.; Baek, K.; Choi, Y.S.; Pack, S.P.; Oh, S.H.; Cha, H.J. Bioinspired silica nanocomposite with autoencapsulated carbonic anhydrase as a robust biocatalyst for CO2 sequestration. ACS Catal. 2014, 4, 4332–4340. [Google Scholar] [CrossRef]
- Kuan, I.C.; Chuang, C.-A.; Lee, S.-L.; Yu, C.-Y. Alkyl-substituted methoxysilanes enhance the activity and stability of D-amino acid oxidase encapsulated in biomimetic silica. Biotechnol. Lett. 2012, 34, 1493–1498. [Google Scholar] [CrossRef]
- Cao, X.; Yang, J.; Shu, L.; Yu, B.; Yan, Y. Improving esterification activity of Burkholderia cepacia lipase encapsulated in silica by bioimprinting with substrate analogues. Process Biochem. 2009, 44, 177–182. [Google Scholar]
- Micalella, C.; Caglio, R.; Mozzarelli, A.; Valetti, F.; Pessione, E.; Giunta, C.; Bruno, S. Ormosil gels doped with engineered catechol 1,2 dioxygenases for chlorocatechol bioremediation. Biotechnol. Appl. Biochem. 2014, 61, 297–303. [Google Scholar] [CrossRef]
- Corîci, L.N.; Frissen, A.E.; van Zoelen, D.-J.; Eggen, I.F.; Peter, F.; Davidescu, C.M.; Boeriu, C.G. Sol–Gel immobilization of alcalase from Bacillus licheniformis for application in the synthesis of C-terminal peptide amides. J. Mol. Catal. B Enzym. 2011, 73, 90–97. [Google Scholar] [CrossRef]
- Xu, L.; Ke, C.; Huang, Y.; Yan, Y. Immobilized Aspergillus niger lipase with SiO2 nanoparticles in sol-gel materials. Catalysts 2016, 6, 149. [Google Scholar] [CrossRef]
- Biró, E.; Budugan, D.; Todea, A.; Péter, F.; Klébert, S.; Feczkó, T. Recyclable solid-phase biocatalyst with improved stability by sol–gel entrapment of β-D-galactosidase. J. Mol. Catal. B Enzym. 2016, 123, 81–90. [Google Scholar]
- Lopez-Gallego, F.; Betancor, L.; Hidalgo, A.; Dellamora-Ortiz, G.; Mateo, C.; Fernández-Lafuente, R.; Guisán, J.M. Stabilization of different alcohol oxidases via immobilization and post immobilization techniques. Enzym. Microb. Technol. 2007, 40, 278–284. [Google Scholar] [CrossRef]
- Ungurean, M.; Paul, C.; Peter, F. Cellulase immobilized by sol–Gel entrapment for efficient hydrolysis of cellulose. Bioprocess Biosyst. Eng. 2013, 36, 1327–1338. [Google Scholar]
- Forsyth, C.; Yip, T.W.; Patwardhan, S.V. CO2 sequestration by enzyme immobilized onto bioinspired silica. Chem. Commun. 2013, 49, 3191–3193. [Google Scholar]
- Capasso, C.; De Luca, V.; Carginale, V.; Cannio, R.; Rossi, M. Biochemical properties of a novel and highly thermostable bacterial alpha-carbonic anhydrase from Sulfurihydrogenibium yellowstonense YO3AOP1. J. Enzyme Inhib. Med. Chem. 2012, 27, 892–897. [Google Scholar]







| Without SazCA | With SazCA | |||
|---|---|---|---|---|
| Precursor Used 1 | Specific Surface Area (m2/g) | Pore Size (Å) | Specific Surface Area (m2/g) | Pore Size (Å) |
| 100% TMOS | 25 | 71 | 21 | 57 |
| 10% MTMS and 90% TMOS | 25 | 76 | 20 | 96 |
| 20% DMDMS and 80% TMOS | 21 | 117 | 24 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
How, S.-C.; Kong, X.-S.; Hu, C.-J.; Yu, C.-Y. Activity and Stability Enhancement of Carbonic Anhydrase Entrapped Within Biomimetic Silica by Methyl-Substituted Silanes. Catalysts 2025, 15, 907. https://doi.org/10.3390/catal15090907
How S-C, Kong X-S, Hu C-J, Yu C-Y. Activity and Stability Enhancement of Carbonic Anhydrase Entrapped Within Biomimetic Silica by Methyl-Substituted Silanes. Catalysts. 2025; 15(9):907. https://doi.org/10.3390/catal15090907
Chicago/Turabian StyleHow, Su-Chun, Xen-Shuan Kong, Chia-Jung Hu, and Chi-Yang Yu. 2025. "Activity and Stability Enhancement of Carbonic Anhydrase Entrapped Within Biomimetic Silica by Methyl-Substituted Silanes" Catalysts 15, no. 9: 907. https://doi.org/10.3390/catal15090907
APA StyleHow, S.-C., Kong, X.-S., Hu, C.-J., & Yu, C.-Y. (2025). Activity and Stability Enhancement of Carbonic Anhydrase Entrapped Within Biomimetic Silica by Methyl-Substituted Silanes. Catalysts, 15(9), 907. https://doi.org/10.3390/catal15090907







