Characterization of a Novel Thermostable and Alkaliphilic β-Mannanase for Gel-Breaking in Guar Gum Fracturing Fluids
Abstract
1. Introduction
2. Results and Discussion
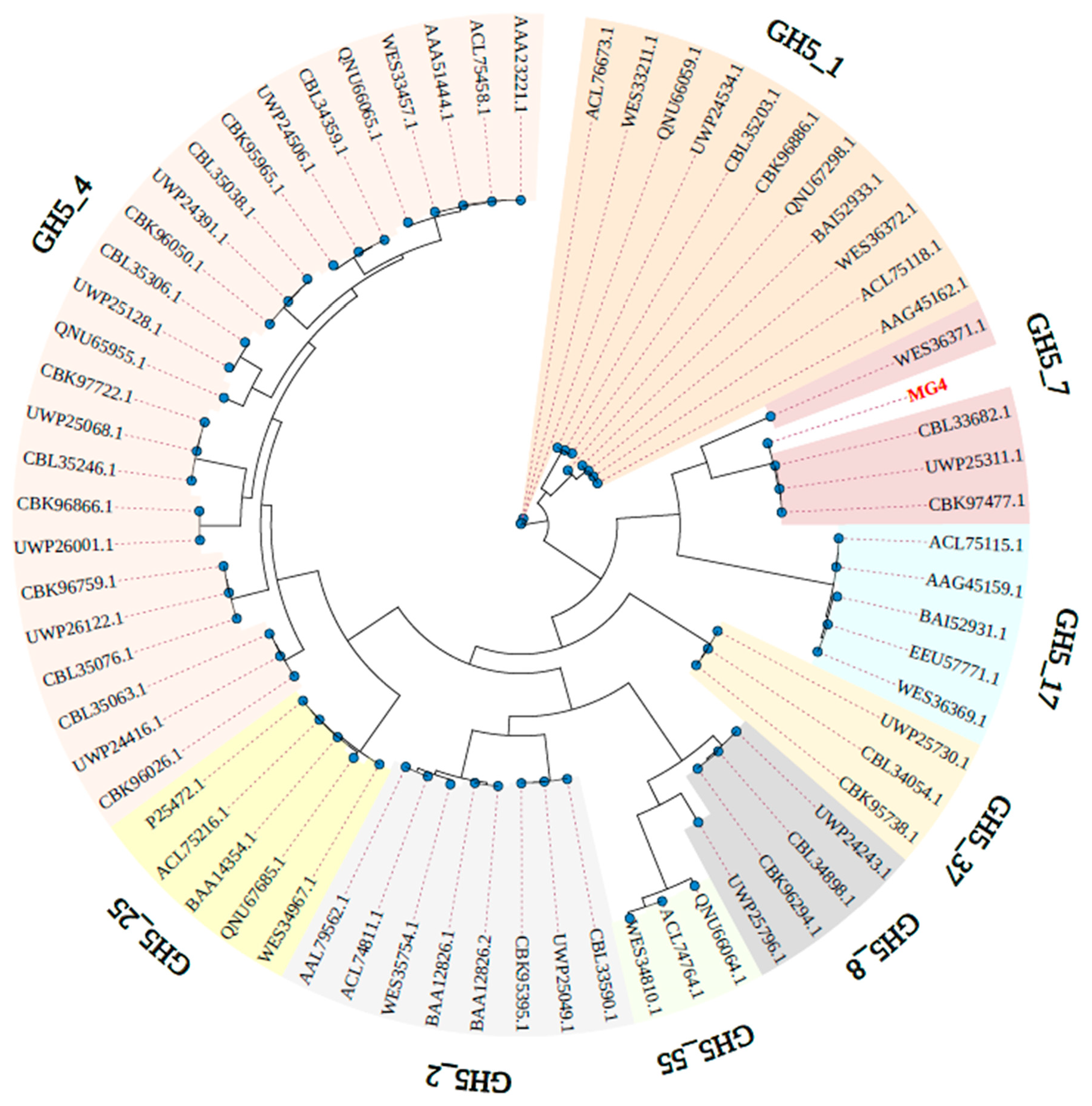
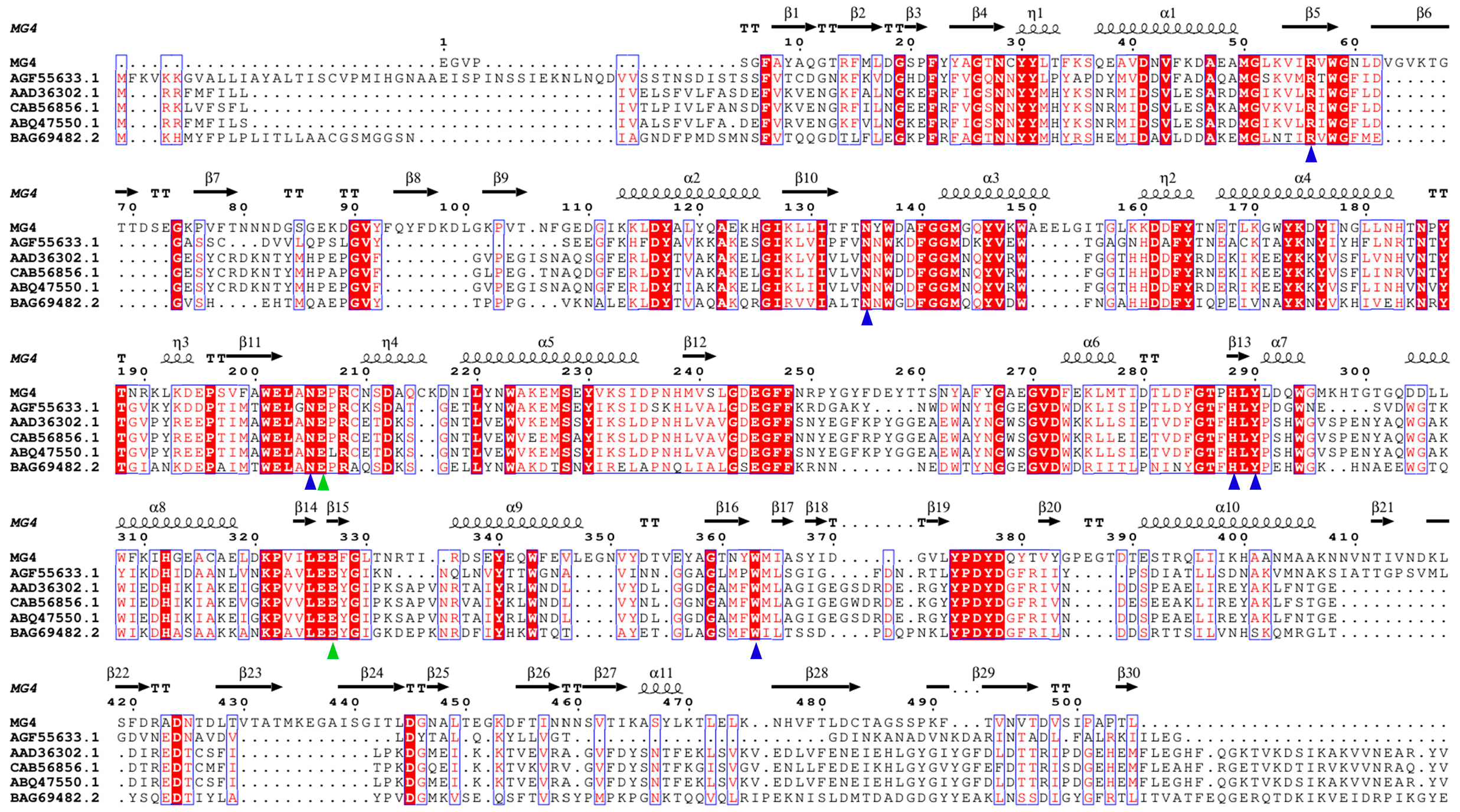
2.1. Identification and Sequence Analysis of MG4
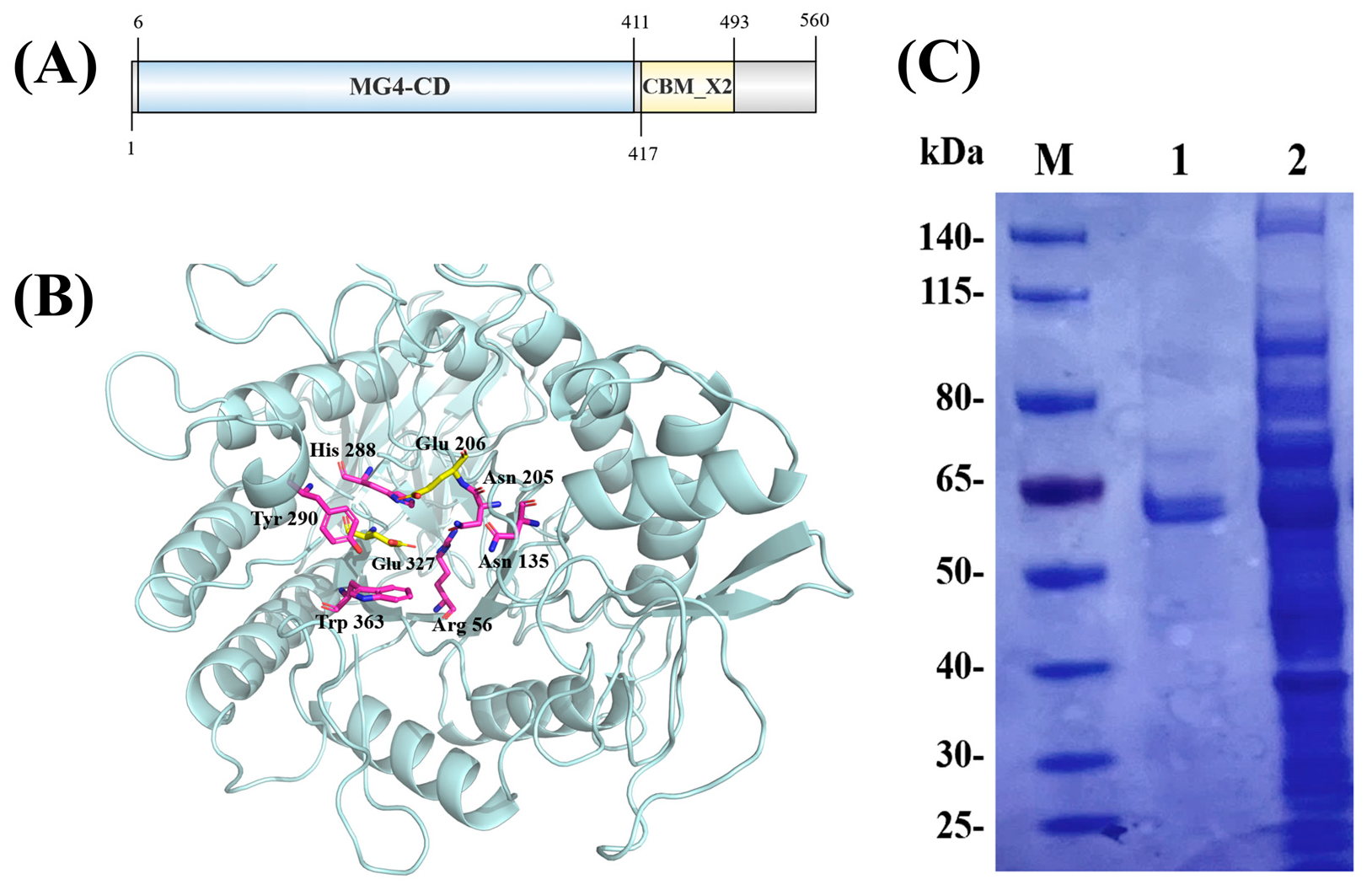
2.2. Functional and Structural Analysis of MG4
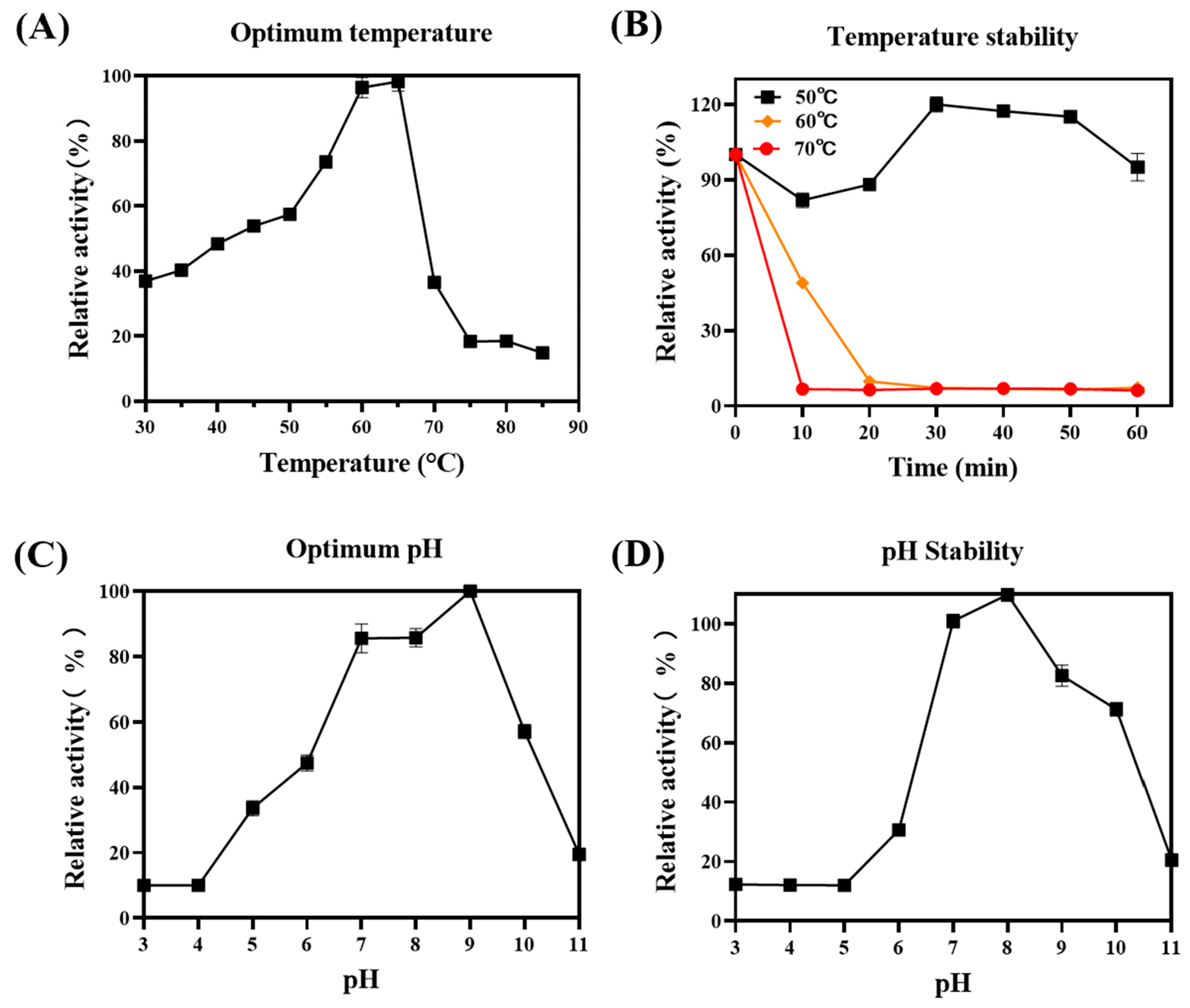
2.3. SDS-PAGE Analysis and Enzyme Activity Assay
2.4. Effect of Metal Ions and EDTA on Enzyme Activity
2.5. Substrate Specificity and Enzyme Kinetic
2.6. Hydrolysis Product Analysis
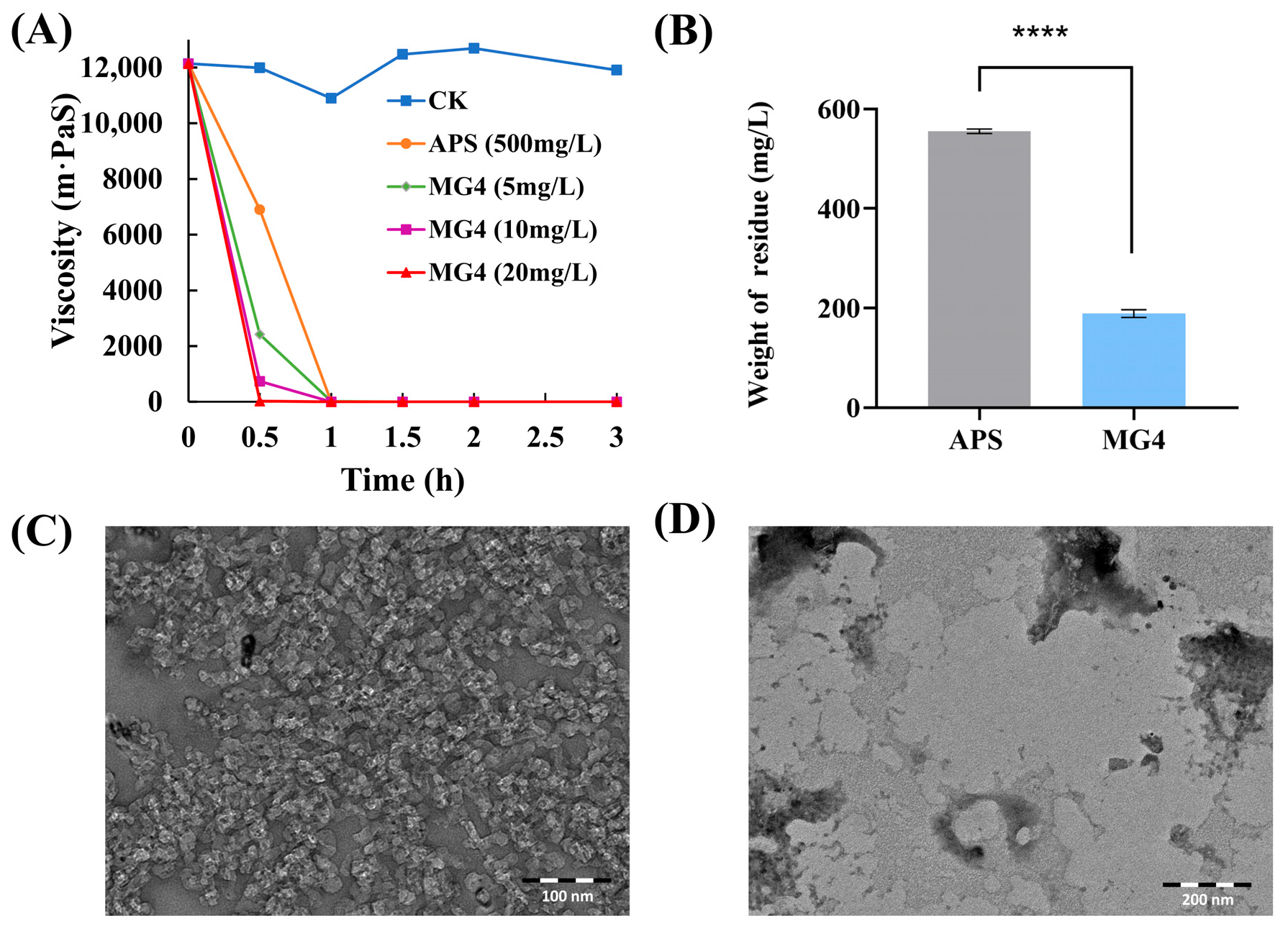
2.7. Gel Breaking of Guar Gum Fracturing Fluid
3. Materials and Methods
3.1. Strains and Reagents
3.2. Synthesis and Expression of Recombinant MG4
3.3. Sequence Analysis
3.4. Structural Analysis and Molecular Docking
3.5. Expression and Purification of MG4
3.6. Enzyme Activity Assay
3.7. Effect of Metal Ions and EDTA on Enzyme Activity
3.8. Substrate Specificity and Enzyme Kinetic Parameters
3.9. Hydrolysis Products
3.10. Guar Gum Fracturing Fluid Breaking
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MOS | Manno-oligosaccharides |
| GEM | Genomes of Earth’s microbiomes |
| LBG | Locust bean gum |
| GG | Guar gum |
| KGM | Konjac glucomannan |
| INM | Ivory nut mannan |
| DNS | 3,5-dinitrosalicylic acid |
| XG | Xanthan gum |
| CMC | Carboxymethyl cellulose |
| TLC | Thin-layer chromatography |
| APS | Ammonium persulfate |
| TEM | Transmission electron microscopy |
| GH | Glycoside hydrolase |
| CBM | Carbohydrate binding module |
| CD | Catalytic domain |
| TIM | Triose phosphate isomerase |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| DP | Degrees of polymerization |
References
- Wang, P.; Pei, X.; Zhou, W.; Zhao, Y.; Gu, P.; Li, Y.; Gao, J. Research and Application Progress of Microbial β-Mannanases: A Mini-Review. World J. Microbiol. Biotechnol. 2024, 40, 169. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Puri, N.; Sharma, P.; Gupta, N. Mannanases: Microbial Sources, Production, Properties and Potential Biotechnological Applications. Appl. Microbiol. Biotechnol. 2012, 93, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Han, J.; Zhou, Y.; Zhang, H.; Xu, X.; He, B.; Liu, M.; Wang, J.; Wang, Q. A Rumen-Derived Bifunctional Glucanase/Mannanase Uncanonically Releases Oligosaccharides with a High Degree of Polymerization Preferentially from Branched Substrates. Carbohydr. Polym. 2024, 330, 121828. [Google Scholar] [CrossRef]
- Nadaroglu, H.; Adiguzel, G.; Adiguzel, A.; Sonmez, Z. A Thermostable-Endo-β-(1,4)-Mannanase from Pediococcus Acidilactici (M17): Purification, Characterization and Its Application in Fruit Juice Clarification. Eur. Food Res. Technol. 2017, 243, 193–201. [Google Scholar] [CrossRef]
- David, A.; Singh Chauhan, P.; Kumar, A.; Angural, S.; Kumar, D.; Puri, N.; Gupta, N. Coproduction of Protease and Mannanase from Bacillus Nealsonii PN-11 in Solid State Fermentation and Their Combined Application as Detergent Additives. Int. J. Biol. Macromol. 2018, 108, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.T.; Deng, Z.; Sokale, A.; Frederick, B.; Kim, S.W. Nutritional and Functional Roles of β-Mannanase on Intestinal Health and Growth of Newly Weaned Pigs Fed Two Different Types of Feeds. J. Anim. Sci. 2024, 102, skae206. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, X.; Wang, Y.; Na, J.; Ping, W.; Ge, J. Purification, Biochemical and Secondary Structural Characterisation of β-Mannanase from Lactobacillus Casei HDS-01 and Juice Clarification Potential. Int. J. Biol. Macromol. 2020, 154, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, G.; Khatri, M.; Kaur, A.; Arya, S.K. Thermo and Alkali Stable β-Mannanase: Characterization and Application for Removal of Food (Mannans Based) Stain. Int. J. Biol. Macromol. 2019, 134, 536–546. [Google Scholar] [CrossRef]
- Kumar Suryawanshi, R.; Kango, N. Production of Mannooligosaccharides from Various Mannans and Evaluation of Their Prebiotic Potential. Food Chem. 2021, 334, 127428. [Google Scholar] [CrossRef]
- Meng, Y.; Zhao, F.; Jin, X.; Feng, Y.; Sun, G.; Lin, J.; Jia, B.; Li, P. Performance Evaluation of Enzyme Breaker for Fracturing Applications under Simulated Reservoir Conditions. Molecules 2021, 26, 3133. [Google Scholar] [CrossRef]
- Wang, T.; Ye, J. Rheological and Fracturing Characteristics of a Cationic Guar Gum. Int. J. Biol. Macromol. 2023, 224, 196–206. [Google Scholar] [CrossRef]
- Hasan, A.M.A.; Abdel-Raouf, M.E. Applications of Guar Gum and Its Derivatives in Petroleum Industry: A Review. Egypt. J. Pet. 2018, 27, 1043–1050. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Wang, C.; Zhao, F.; Lei, S.; Yi, H.; Guo, J. Influence of Nanomaterial Morphology of Guar-Gum Fracturing Fluid, Physical and Mechanical Properties. Carbohydr. Polym. 2020, 234, 115915. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, G.; Ding, R.; Zheng, D.; Yang, Z.; Sun, Z.; Zhou, F.; Wang, D. Research on Water Blocking and Residue Damage Mechanism of Fracturing Fluid in Yongjin Tight Reservoirs. Phys. Fluids 2024, 36, 042018. [Google Scholar] [CrossRef]
- Liang, T.; Zhou, F.; Lu, J.; DiCarlo, D.; Nguyen, Q. Evaluation of Wettability Alteration and IFT Reduction on Mitigating Water Blocking for Low-Permeability Oil-Wet Rocks after Hydraulic Fracturing. Fuel 2017, 209, 650–660. [Google Scholar] [CrossRef]
- Ma, X.; Song, P.; Liu, L.; Da, Q.; Lei, G.; Yao, C.; Shor, L.M. Low-Temperature pH-Regulable Gel-Breaking of Galactomannan-Based Fracturing Fluids by the Mannanase from Bacillus Aerius. Int. Biodeterior. Biodegrad. 2021, 160, 105226. [Google Scholar] [CrossRef]
- Murthy, R.V.V.R.; Chavali, M. A Novel Hydraulic Fracturing Gel Realization for Unconventional Reservoirs. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 37. [Google Scholar] [CrossRef]
- Reddy, T.T.; Tammishetti, S. Free Radical Degradation of Guar Gum. Polym. Degrad. Stab. 2004, 86, 455–459. [Google Scholar] [CrossRef]
- Trabelsi, S.; Kakadjian, S. Comparative Study between Guar and Carboxymethylcellulose Used as Gelling Systems in Hydraulic Fracturing Application. In Proceedings of the SPE Production and Operations Symposium, Oklahoma City, OK, USA, 23–26 March 2013; p. SPE-164486-MS. [Google Scholar]
- Barati, R.; Liang, J. A Review of Fracturing Fluid Systems Used for Hydraulic Fracturing of Oil and Gas Wells. J. Appl. Polym. Sci. 2014, 131, app.40735. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Z.; Da, Q.; Cheng, M.; Yao, C.; Lei, G. Application of Guar Gum Degrading Bacteria in Microbial Remediation of Guar-Based Fracturing Fluid Damage. Energy Fuels 2017, 31, 7894–7903. [Google Scholar] [CrossRef]
- Chen, X.; Sun, P.; Li, L.; Zhou, X.; Han, C.; Ma, S.; Cai, Y.; Zhang, W.; Li, Y.; Cao, Z. Sustainable Enhancing Oil Recovery in Different Reservoirs via Reservoir Adaptability and Multifunction of Bacillus velezensis. Fuel 2025, 388, 134488. [Google Scholar] [CrossRef]
- Abdelrahim, M.A.; Ghosh, D.B.; Belhaj, D.H.; Ghosh, D. High-Temperature Stable Specific Enzyme for Guar Polymer Based Fracturing Fluid Degradation. In Proceedings of the SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition, Virtual, 12–14 October 2021; p. D012S032R062. [Google Scholar]
- Naeem, M.; Khalil, A.B.; Tariq, Z.; Mahmoud, M. A Review of Advanced Molecular Engineering Approaches to Enhance the Thermostability of Enzyme Breakers: From Prospective of Upstream Oil and Gas Industry. Int. J. Mol. Sci. 2022, 23, 1597. [Google Scholar] [CrossRef]
- Erkan, S.B.; Ozcan, A.; Yilmazer, C.; Gurler, H.N.; Karahalil, E.; Germec, M.; Yatmaz, E.; Kucukcetin, A.; Turhan, I. The Effects of Mannanase Activity on Viscosity in Different Gums. J. Food Process. Preserv. 2021, 45, e14820. [Google Scholar] [CrossRef]
- Lv, L.; Lin, J.; Feng, Y.; Wang, W.; Li, S. Coated Recombinant Escherichia Coli for Delayed Release of β-Mannanase in the Water-Based Fracturing Fluid. Process Biochem. 2021, 107, 121–128. [Google Scholar] [CrossRef]
- Glasner, M.E. Finding Enzymes in the Gut Metagenome. Science 2017, 355, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Nayfach, S.; Roux, S.; Seshadri, R.; Udwary, D.; Varghese, N.; Schulz, F.; Wu, D.; Paez-Espino, D.; Chen, I.-M.; Huntemann, M.; et al. A Genomic Catalog of Earth’s Microbiomes. Nat. Biotechnol. 2021, 39, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Aspeborg, H.; Coutinho, P.M.; Wang, Y.; Brumer, H.; Henrissat, B. Evolution, Substrate Specificity and Subfamily Classification of Glycoside Hydrolase Family 5 (GH5). BMC Evol. Biol. 2012, 12, 186. [Google Scholar] [CrossRef]
- Parker, K.N.; Chhabra, S.R.; Lam, D.; Callen, W.; Duffaud, G.D.; Snead, M.A.; Short, J.M.; Mathur, E.J.; Kelly, R.M. Galactomannanases Man2 and Man5 from Thermotoga Species: Growth Physiology on Galactomannans, Gene Sequence Analysis, and Biochemical Properties of Recombinant Enzymes. Biotechnol. Bioeng. 2001, 75, 322–333. [Google Scholar] [CrossRef]
- Santos, C.R.; Squina, F.M.; Navarro, A.M.; Ruller, R.; Prade, R.; Murakami, M.T. Cloning, Expression, Purification, Crystallization and Preliminary X-Ray Diffraction Studies of the Catalytic Domain of a Hyperthermostable Endo-1,4-β-D-Mannanase from Thermotoga petrophila RKU-1. Acta Crystallogr. Sect. F 2010, 66, 1078–1081. [Google Scholar] [CrossRef]
- Tanaka, M.; Umemoto, Y.; Okamura, H.; Nakano, D.; Tamaru, Y.; Araki, T. Cloning and Characterization of a β-1,4-Mannanase 5C Possessing a Family 27 Carbohydrate-Binding Module from a Marine Bacterium, Vibrio Sp. Strain MA-138. Biosci. Biotechnol. Biochem. 2009, 73, 109–116. [Google Scholar] [CrossRef][Green Version]
- Kosugi, A.; Amano, Y.; Murashima, K.; Doi, R.H. Hydrophilic Domains of Scaffolding Protein CbpA Promote Glycosyl Hydrolase Activity and Localization of Cellulosomes to the Cell Surface of Clostridium cellulovorans. J. Bacteriol. 2004, 186, 6351–6359. [Google Scholar] [CrossRef]
- Chanal, A.; Mingardon, F.; Bauzan, M.; Tardif, C.; Fierobe, H.-P. Scaffoldin Modules Serving as “Cargo” Domains to Promote the Secretion of Heterologous Cellulosomal Cellulases by Clostridium Acetobutylicum. Appl. Environ. Microbiol. 2011, 77, 6277–6280. [Google Scholar] [CrossRef]
- Pasari, N.; Adlakha, N.; Gupta, M.; Bashir, Z.; Rajacharya, G.H.; Verma, G.; Munde, M.; Bhatnagar, R.; Yazdani, S.S. Impact of Module-X2 and Carbohydrate Binding Module-3 on the Catalytic Activity of Associated Glycoside Hydrolases towards Plant Biomass. Sci. Rep. 2017, 7, 3700. [Google Scholar] [CrossRef]
- Tao, X.; Liu, J.; Kempher, M.L.; Xu, T.; Zhou, J. In Vivo Functional Characterization of Hydrophilic X2 Modules in the Cellulosomal Scaffolding Protein. Front. Microbiol. 2022, 13, 861549. [Google Scholar] [CrossRef] [PubMed]
- St John, F.J.; González, J.M.; Pozharski, E. Consolidation of Glycosyl Hydrolase Family 30: A Dual Domain 4/7 Hydrolase Family Consisting of Two Structurally Distinct Groups. FEBS Lett. 2010, 584, 4435–4441. [Google Scholar] [CrossRef]
- Wang, C.-H.; Lu, L.-H.; Huang, C.; He, B.-F.; Huang, R.-B. Simultaneously Improved Thermostability and Hydrolytic Pattern of Alpha-Amylase by Engineering Central Beta Strands of TIM Barrel. Appl. Biochem. Biotechnol. 2020, 192, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Höcker, B.; Jürgens, C.; Wilmanns, M.; Sterner, R. Stability, Catalytic Versatility and Evolution of the (Βα)8-Barrel Fold. Curr. Opin. Biotechnol. 2001, 12, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Li, C.-X.; Pan, J.; Ni, Y.; Zhang, X.-Y.; Xu, J.-H. Performance of a New Thermostable Mannanase in Breaking Guar-Based Fracturing Fluids at High Temperatures with Little Premature Degradation. Appl. Biochem. Biotechnol. 2014, 172, 1215–1226. [Google Scholar] [CrossRef]
- Sadaqat, B.; Sha, C.; Rupani, P.F.; Wang, H.; Zuo, W.; Shao, W. Man/Cel5B, a Bifunctional Enzyme Having the Highest Mannanase Activity in the Hyperthermic Environment. Front. Bioeng. Biotechnol. 2021, 9, 637649. [Google Scholar] [CrossRef]
- Rahmani, N.; Kashiwagi, N.; Lee, J.; Niimi-Nakamura, S.; Matsumoto, H.; Kahar, P.; Lisdiyanti, P.; Yopi; Prasetya, B.; Ogino, C.; et al. Mannan Endo-1,4-β-Mannosidase from Kitasatospora sp. Isolated in Indonesia and Its Potential for Production of Mannooligosaccharides from Mannan Polymers. AMB Express 2017, 7, 100. [Google Scholar] [CrossRef]
- Li, H. An Alternative Amino Acid Leaching of Base Metals from Waste Printed Circuit Boards Using Alkaline Glutamate Solutions: A Comparative Study with Glycine. Sep. Purif. Technol. 2025, 356, 129953. [Google Scholar] [CrossRef]
- Gu, X.; Lu, H.; Chen, W.; Meng, X. Characterization of a Novel Thermophilic Mannanase and Synergistic Hydrolysis of Galactomannan Combined with Swollenin. Catalysts 2021, 11, 254. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, G.; Zhang, G.; He, Q.; Wei, Z.; Zheng, W.; Qian, T.; Wu, Q. Effect of Mixed Chelators of EDTA, GLDA, and Citric Acid on Bioavailability of Residual Heavy Metals in Soils and Soil Properties. Chemosphere 2018, 209, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, G.C.; Cho, S.S.; Choi, Y.H.; Choi, Y.S.; Jee, J.-P.; Seong, C.N.; Yoo, J.C. An Extremely Alkaline Mannanase from Streptomyces sp. CS428 Hydrolyzes Galactomannan Producing Series of Mannooligosaccharides. World J. Microbiol. Biotechnol. 2016, 32, 84. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.Y.; Pradeep, G.C.; Kim, S.W.; Park, D.H.; Choi, Y.H.; Suh, J.W.; Yoo, J.C. A Novel Low-Molecular Weight Alkaline Mannanase from Streptomyces Tendae. Biotechnol. Bioprocess Eng. 2015, 20, 453–461. [Google Scholar] [CrossRef]
- O’Connell, A. The Structure and Dynamics of Locust Bean Gum in Aqueous Solution. Food Hydrocoll. 2023, 138, 108446. [Google Scholar] [CrossRef]
- Zhu, F. Modifications of Konjac Glucomannan for Diverse Applications. Food Chem. 2018, 256, 419–426. [Google Scholar] [CrossRef]
- Suzuki, K.; Michikawa, M.; Sato, H.; Yuki, M.; Kamino, K.; Ogasawara, W.; Fushinobu, S.; Kaneko, S. Purification, Cloning, Functional Expression, Structure, and Characterization of a Thermostable β-Mannanase from Talaromyces trachyspermus B168 and Its Efficiency in Production of Mannooligosaccharides from Coffee Wastes. J. Appl. Glycosci. 2018, 65, 13–21. [Google Scholar] [CrossRef]
- Mirzaei, M.; Movahhed, S.; Asadollahzadeh, M.J.; Ahmadi Chenarbon, H. Effect of Carboxymethylcellulose and Locust Bean Gums on Some of Physicochemical, Mechanical, and Textural Properties of Extruded Rice. J. Texture Stud. 2021, 52, 91–100. [Google Scholar] [CrossRef]
- Moreira, L.R.S.; Filho, E.X.F. An Overview of Mannan Structure and Mannan-Degrading Enzyme Systems. Appl. Microbiol. Biotechnol. 2008, 79, 165–178. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Wiemann, M.; Stålbrand, H. β-Mannanase BoMan26B from Bacteroides Ovatus Produces Mannan-Oligosaccharides with Prebiotic Potential from Galactomannan and Softwood β-Mannans. LWT 2021, 151, 112215. [Google Scholar] [CrossRef]
- Ratnakomala, S.; Kahar, P.; Kashiwagi, N.; Lee, J.; Kudou, M.; Matsumoto, H.; Apriliana, P.; Yopi, Y.; Prasetya, B.; Ogino, C.; et al. Manno-Oligosaccharide Production from Biomass Hydrolysis by Using Endo-1,4-β-Mannanase (ManNj6-379) from Nonomuraea Jabiensis ID06-379. Processes 2022, 10, 269. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Liao, J.; Yan, B.; Lai, C.; Zeng, K.; Yang, Y.; Zhu, Y.; Yin, B.; Huang, C. The State-of-the-Art Preparation, Purification and Biological Activities of Mannan Oligosaccharides. Ind. Crops Prod. 2025, 225, 120594. [Google Scholar] [CrossRef]
- Wang, J.; Ke, S.; Strappe, P.; Ning, M.; Zhou, Z. Structurally Orientated Rheological and Gut Microbiota Fermentation Property of Mannans Polysaccharides and Oligosaccharides. Foods 2023, 12, 4002. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, T.; Huang, C.; Lai, C.; Ling, Z.; Zhou, Y.; Yong, Q. Incomplete Degradation Products of Galactomannan from Sesbania cannabina Modulated the Cecal Microbial Community of Laying Hens. J. Anim. Sci. 2022, 100, skac087. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.-Y.; Lin, Y.-J.; Saburi, W.; Vieths, S.; Scheurer, S.; Schülke, S.; Toda, M. β-(1→4)-Mannobiose Acts as an Immunostimulatory Molecule in Murine Dendritic Cells by Binding the TLR4/MD-2 Complex. Cells 2021, 10, 1774. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N. Exploring the Potential of Mannan Oligosaccharides in Enhancing Animal Growth, Immunity, and Overall Health: A Review. Carbohydr. Polym. Technol. Appl. 2025, 9, 100603. [Google Scholar] [CrossRef]
- Jana, U.K.; Suryawanshi, R.K.; Prajapati, B.P.; Kango, N. Prebiotic Mannooligosaccharides: Synthesis, Characterization and Bioactive Properties. Food Chem. 2021, 342, 128328. [Google Scholar] [CrossRef]
- Pason, P.; Tachaapaikoon, C.; Suyama, W.; Waeonukul, R.; Shao, R.; Wongwattanakul, M.; Limpaiboon, T.; Chonanant, C.; Ngernyuang, N. Anticancer and Anti-Angiogenic Activities of Mannooligosaccharides Extracted from Coconut Meal on Colorectal Carcinoma Cells in Vitro. Toxicol. Rep. 2024, 12, 82–90. [Google Scholar] [CrossRef]
- Zheng, J.; Li, H.; Zhang, X.; Jiang, M.; Luo, C.; Lu, Z.; Xu, Z.; Shi, J. Prebiotic Mannan-Oligosaccharides Augment the Hypoglycemic Effects of Metformin in Correlation with Modulating Gut Microbiota. J. Agric. Food Chem. 2018, 66, 5821–5831. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Wang, S.; Li, H.; Lu, Z.; Shi, J.; Xu, Z. Mannan-Oligosaccharide Modulates the Obesity and Gut Microbiota in High-Fat Diet-Fed Mice. Food Funct. 2018, 9, 3916–3929. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Majtorp, L.; Birgersson, S.; Wiemann, M.; Sreenivas, K.; Verbrugghe, P.; Van Aken, O.; Van Niel, E.; Stålbrand, H. Cross-Feeding and Enzymatic Catabolism for Mannan-Oligosaccharide Utilization by the Butyrate-Producing Gut Bacterium Roseburia Hominis A2-183. Microorganisms 2022, 10, 2496. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, S.; Wang, Q.; Feng, Y.; Shuai, Y. Improving the Fracturing Fluid Loss Control for Multistage Fracturing by the Precise Gel Breaking Time Design. J. Nat. Gas Sci. Eng. 2015, 25, 367–370. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, M.; Liu, J.; Zhang, Y.; Gao, M.; Song, X.; Sun, N.; Li, L.; Wu, Y.; Dai, C. Study on Formation Process and Reservoir Damage Mechanism of Blockages Caused by Polyacrylamide Fracturing Fluid in Production Wells. Fuel 2024, 358, 130154. [Google Scholar] [CrossRef]
- Huang, Q.; Li, J.; Liu, S.; Wang, G. Experimental Study on the Adverse Effect of Gel Fracturing Fluid on Gas Sorption Behavior for Illinois Coal. Int. J. Coal Sci. Technol. 2021, 8, 1250–1261. [Google Scholar] [CrossRef]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial Use of Immobilized Enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Li, S.; Zheng, H.; Wei, Z.; Liu, D.; Raza, W.; Shen, Q.; Xu, Y. A New Acidophilic Thermostable Endo-1,4-β-Mannanase from Penicillium Oxalicum GZ-2: Cloning, Characterization and Functional Expression in Pichia Pastoris. BMC Biotechnol. 2014, 14, 90. [Google Scholar] [CrossRef]
- Liberato, M.V.; Silveira, R.L.; Prates, É.T.; De Araujo, E.A.; Pellegrini, V.O.A.; Camilo, C.M.; Kadowaki, M.A.; Neto, M.D.O.; Popov, A.; Skaf, M.S.; et al. Molecular Characterization of a Family 5 Glycoside Hydrolase Suggests an Induced-Fit Enzymatic Mechanism. Sci. Rep. 2016, 6, 23473. [Google Scholar] [CrossRef]
- Sanjaya, R.E.; Putri, K.D.A.; Kurniati, A.; Rohman, A.; Puspaningsih, N.N.T. In Silico Characterization of the GH5-Cellulase Family from Uncultured Microorganisms: Physicochemical and Structural Studies. J. Genet. Eng. Biotechnol. 2021, 19, 143. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Jiang, Z.; Wei, Y.; Li, D.; Li, L.; Chai, P.; Kusakabe, I. High-Level Production, Purification and Characterization of a Thermostable β-Mannanase from the Newly Isolated Bacillus Subtilis WY34. Carbohydr. Polym. 2006, 66, 88–96. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- SYT 7627-2021; Technical Requirements of Water-Based Fracturing Fluid. Petroleum Industry Press: Beijing, China, 2021.
- Ma, Y.-X.; Du, Y.-R.; Zou, C.-H.; Lai, J.; Ma, L.-Y.; Guo, J.-C. A High-Temperature-Resistant and Metallic-Crosslinker-Free Fracturing Fluid Based on Guar Gum/Montmorillonite Nanocomposite. J. Nat. Gas Sci. Eng. 2022, 105, 104712. [Google Scholar] [CrossRef]

| Metal Ion | Relative Activity (%) * |
|---|---|
| None | 100.00 |
| Cu2+ | 6.3 ± 0.7 |
| Zn2+ | 10.6 ± 0.3 |
| Fe3+ | 26.3 ± 0.04 |
| EDTA | 53.5 ± 1.9 |
| Ni2+ | 75.8 ± 1.1 |
| K+ | 114.2 ± 1.6 |
| Mn2+ | 121.1 ± 0.8 |
| Fe2+ | 122.6 ± 1.3 |
| Na+ | 130.4 ± 0.6 |
| Ba2+ | 132.1 ± 1.4 |
| Mg2+ | 143.2 ± 2.1 |
| Ca2+ | 200.4 ± 4.6 |
| Substrate | Specific Activity (U/mg) 1 |
|---|---|
| LBG | 25.77 ± 0.68 |
| KGM | 27.27 ± 0.76 |
| GG | 8.89 ± 0.85 |
| INM | ND 2 |
| XG | ND 2 |
| CMC | ND 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, W.; Lv, T.; Wang, S.; Wang, W.; Wang, Z.; Chen, S.; Tian, Y.; Yun, Y.; Li, G.; Ma, T. Characterization of a Novel Thermostable and Alkaliphilic β-Mannanase for Gel-Breaking in Guar Gum Fracturing Fluids. Catalysts 2025, 15, 905. https://doi.org/10.3390/catal15090905
Tian W, Lv T, Wang S, Wang W, Wang Z, Chen S, Tian Y, Yun Y, Li G, Ma T. Characterization of a Novel Thermostable and Alkaliphilic β-Mannanase for Gel-Breaking in Guar Gum Fracturing Fluids. Catalysts. 2025; 15(9):905. https://doi.org/10.3390/catal15090905
Chicago/Turabian StyleTian, Wenzhuo, Tianhua Lv, Shaojing Wang, Weilong Wang, Zhiwei Wang, Shuai Chen, Yutong Tian, Yuan Yun, Guoqiang Li, and Ting Ma. 2025. "Characterization of a Novel Thermostable and Alkaliphilic β-Mannanase for Gel-Breaking in Guar Gum Fracturing Fluids" Catalysts 15, no. 9: 905. https://doi.org/10.3390/catal15090905
APA StyleTian, W., Lv, T., Wang, S., Wang, W., Wang, Z., Chen, S., Tian, Y., Yun, Y., Li, G., & Ma, T. (2025). Characterization of a Novel Thermostable and Alkaliphilic β-Mannanase for Gel-Breaking in Guar Gum Fracturing Fluids. Catalysts, 15(9), 905. https://doi.org/10.3390/catal15090905







