1. Introduction
With the ever-increasing global demand for energy, coupled with the finite reserves of fossil fuels and their tendency to cause environmental pollution, the search for clean and renewable energy sources has become paramount. Among these, hydrogen energy stands out as a quintessential clean energy source, attracting significant attention. Hydrogen evolution reaction (HER) via water electrolysis offers advantages such as process simplicity, environmental friendliness, and recyclability [
1,
2]. The water splitting reaction comprises two half-reactions: The hydrogen evolution reaction (HER) at the cathode and the oxygen evolution reaction (OER) at the anode. Both reactions require highly efficient catalysts to achieve high current densities at low overpotentials [
3,
4]. However, in the water electrolysis process, OER, due to its sluggish kinetics, constitutes the rate-limiting step and typically necessitates substantial amounts of precious metal catalysts to reach the required current density (
) [
5]. Consequently, researchers have focused on exploring earth-abundant 3d transition metal compounds to develop low-cost, long-lasting, and highly active OER catalysts, such as perovskite oxides [
6], metal phosphates [
7,
8], selenide composites [
9,
10], nickel borate composites [
11,
12], among others.
Among these 3d transition metal catalysts, transition metal compounds (e.g., oxides [
13,
14,
15], phosphides [
7,
16], selenides [
17,
18]) are regarded as promising alternatives to precious metal OER catalysts, owing to their high catalytic activity, excellent stability, unique redox characteristics, and low cost [
19,
20,
21]. In particular, metal selenides have garnered considerable interest in the OER field due to their tunable electronic structure, corrosion resistance, and favorable intrinsic activity [
22,
23]. For instance, Nath’s group reported Ni
3Se
2 synthesized via electrodeposition, requiring only
overpotential to achieve
in
KOH [
23]. Cai et al. demonstrated high stability for dendrite-like nanostructured NiSe
2 synthesized via a co-reduction method, showing an overpotential decay of only
after
cycles [
24]. Chen et al. grew NiSe
2 on nickel foam in a tube furnace at
and used it as a highly efficient HER catalyst [
25]. Furthermore, studies indicate that incorporating a third element or other external functional materials into binary alloy catalysts often significantly enhances their electrocatalytic performance. For example, research has found that even trace amounts of iron (
) doping can dramatically boost the OER activity of
and
based catalysts [
26,
27]. Boettcher and colleagues, while investigating the electrocatalytic water oxidation performance of solution-cast metal oxide films in alkaline medium, affirmed the enhancement effect of iron [
28]. Subsequently, the same research group reported the effects of iron—either accidentally or intentionally incorporated into Ni(OH)
2/NiOOH electrodes—during the OER in
KOH. They also observed that the OER activity of Ni(OH)
2 peaked when the iron content reached an atomic percentage between
and
[
29,
30]. As for iron doping in nickel selenides, it was first reported by Du et al., who synthesized dendritic nanostructured Ni
0.5Fe
0.5Se
2 and urchin-like Ni
1.12Fe
0.45Se
2 catalysts, both exhibiting outstanding OER activity and durability in alkaline media [
31,
32]. Later, Chang et al. synthesized Fe-doped nickel selenide on carbon nanotubes, which significantly enhanced their electrocatalytic oxygen evolution activity, requiring only an overpotential of
to achieve a current density of
[
33]. Deng et al. fabricated an iron-doped nickel selenide (NF/NiSe/Ni
3Se
2-Fe) catalyst on nickel foam via electrodeposition. This catalyst exhibited overpotentials of
for OER at a current density of
[
18].
It is evident that many studies have demonstrated that iron doping in transition metal compounds can significantly enhance the oxygen evolution reaction (OER) activity of nickel-based catalysts. For transition metal oxides, numerous systematic studies have reported the effects of iron doping on their morphology, composition, and catalytic performance, and the optimal iron doping levels have been comprehensively discussed. However, research on iron doping in transition metal selenides, particularly nickel selenides, remains insufficient. In particular, the influence of low-concentration iron doping on OER activity and the specific mechanisms underlying the activity enhancement have yet to be fully elucidated.
This study evaluates the performance of two NiFeSe catalysts with low iron doping levels for the oxygen evolution reaction (OER). The results demonstrate that Ni0.9Fe0.1Se2 exhibits the best OER catalytic activity among all tested catalysts. Its iron doping level is lower than those reported for Ni0.5Fe0.5Se2 and Ni1.12Fe0.45Se2 in the literature, yet it achieves an overpotential of only 231 mV at a current density of , with a Tafel slope of .
2. Results and Discussion
This study successfully prepared NiSe
2, FeSe
2, and iron-doped nickel-iron selenide (Ni
1−xFe
xSe
2) nanomaterials using an efficient and controllable wet-chemical method. The key advantage of this approach lies in its ability to achieve molecular-level uniform dispersion and complete dissolution of reaction precursors in an organic solvent system under relatively low temperature and ambient pressure conditions, thereby providing an ideal environment for the nucleation and growth of nanocrystals with uniform morphology and controllable composition. Specifically, a mixed solution of oleylamine (OAm) and tetralin was selected as the reaction medium. Three reaction systems were designed to synthesize the target products by adjusting the precursor combinations: (1) In the single-nickel-source system, Ni(acac)
2 and selenium powder (Se) were used as reactants to generate pure-phase NiSe
2. (2) In the single-iron-source system, iron sulfate (Fe
2(SO
4)
3) and selenium powder (Se) served as reactants to produce pure-phase FeSe
2. (3) In the nickel-iron mixed-source system, Ni(acac)
2, iron sulfate (Fe(SO
4)
3), and selenium powder (Se) were jointly used as reactants. In this system, both nickel and iron ions participated in the reaction in the solution to form Ni
1−xFe
xSe
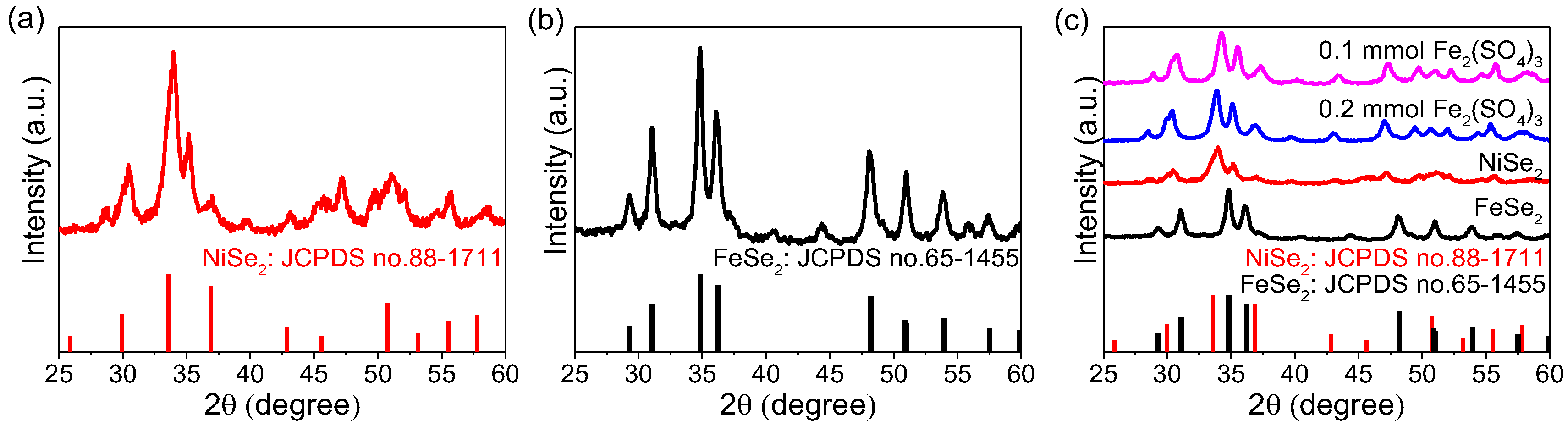
2. The XRD patterns of the as-synthesized products are shown in
Figure 1.
When the solute was Ni(acac)
2 and selenium powder, the diffraction peaks exhibited the characteristic pattern of the cubic NiSe
2 phase (pyrite-type structure). The diffraction peaks located at
values of
,
,
,
,
,
,
, and
correspond to the (
), (
), (
), (
), (
), (
), (
), and (
) crystal planes of cubic NiSe
2 (JCPDS card No.88-1711), respectively. When the solute was Fe
2(SO
4)
3 and selenium powder, the XRD pattern of the sample displayed the typical FeSe
2 crystalline phase. Comparison with the standard JCPDS card No.65-1455 revealed that the diffraction peaks at
values of
,
,
,
,
,
,
,
,
, and
correspond to the (
), (
), (
), (
), (
), (
), (
), (
), (
), and (
) planes of FeSe
2, respectively.
Figure 1c compares the XRD patterns of samples prepared with different amounts of Fe
2(SO
4)
3 with those of NiSe
2 and FeSe
2. The results indicate that the diffraction peak positions of samples prepared with
and
Fe
2(SO
4)
3, respectively, are similar. Furthermore, these diffraction peaks correspond simultaneously to the characteristic peaks of FeSe
2 (JCPDS No.65-1455) and NiSe
2 (JCPDS No.88-1711). This demonstrates that the final product is the Ni
xFe
1−xSe
2, signifying the coexistence of NiSe
2 and FeSe
2 phases. This finding is consistent with previous reports [
32]. Furthermore, no detectable impurity peaks are observed in the pattern, suggesting the relatively high purity of the synthesized sample.
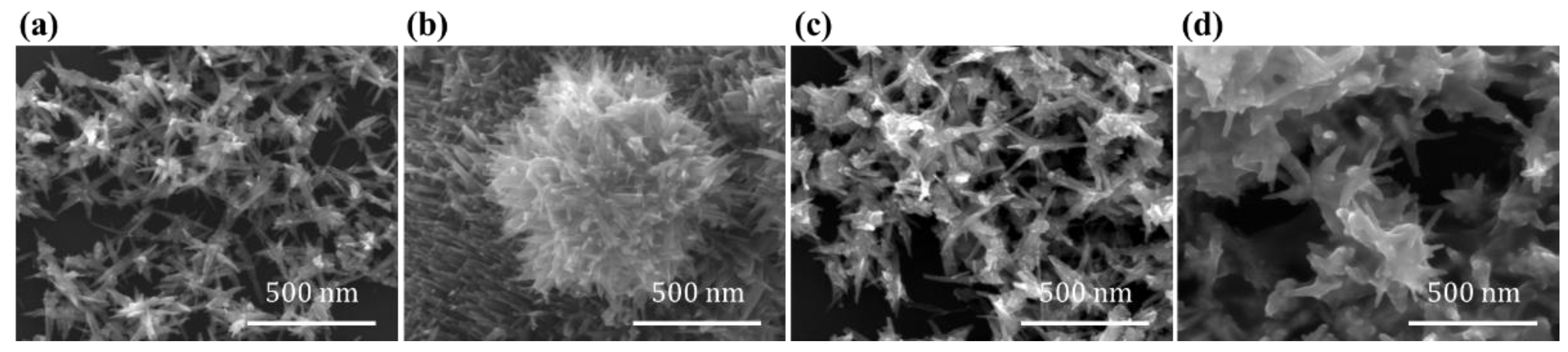
The morphology of the samples was characterized by SEM, as shown in
Figure 2. The images reveal that undoped NiSe
2 exhibits a nanodendritic structure, while FeSe
2 displays a nanourchin-like morphology, which is consistent with the structures reported by Du et al. Upon iron doping, a coating layer gradually forms on the surface of NiSe
2, resulting in the disruption of its original dendritic architecture. Although iron doping was achieved by adjusting the addition level of Fe
2(SO
4)
3, the final composition of the samples required verification through characterization. For this purpose, elemental distribution was analyzed using the Energy Dispersive X-ray Spectroscopy (EDS) detector equipped on the SEM, obtaining quantitative compositional information. The EDS spectra of the samples obtained with the two different addition amounts are shown in
Figure S1. Based on the EDS analysis, the atomic ratios of the various elements in the samples under different ferric sulfate doping levels were determined. When the amount of Fe
2(SO
4)
3 added was
, the atomic percentages of Fe, Ni, and Se were
,
, and
, respectively, corresponding to a chemical formula of Ni
0.9Fe
0.1Se
2. When the addition amount was
, the atomic percentages of the three elements were
,
, and
, respectively, corresponding to a chemical formula of Ni
0.83Fe
0.17Se
2. It should be noted that due to the introduction of Fe
2(SO
4)
3 during the preparation process, sulfate residues remained in the samples, resulting in signals of O and S in the EDS spectra. Furthermore, the atomic percentage of Se was significantly more than twice the sum of Fe and Ni, which is attributed to the addition of excess Se powder during the synthesis process. Additionally, from the SEM-EDS mapping results shown in
Figure S2, it can be observed that the elements Fe, Ni, and Se are uniformly distributed in the sample.
To further investigate the effects of different Fe
2(SO
4)
3 doping amounts on the products, we performed XPS characterization on the products, with the results shown in
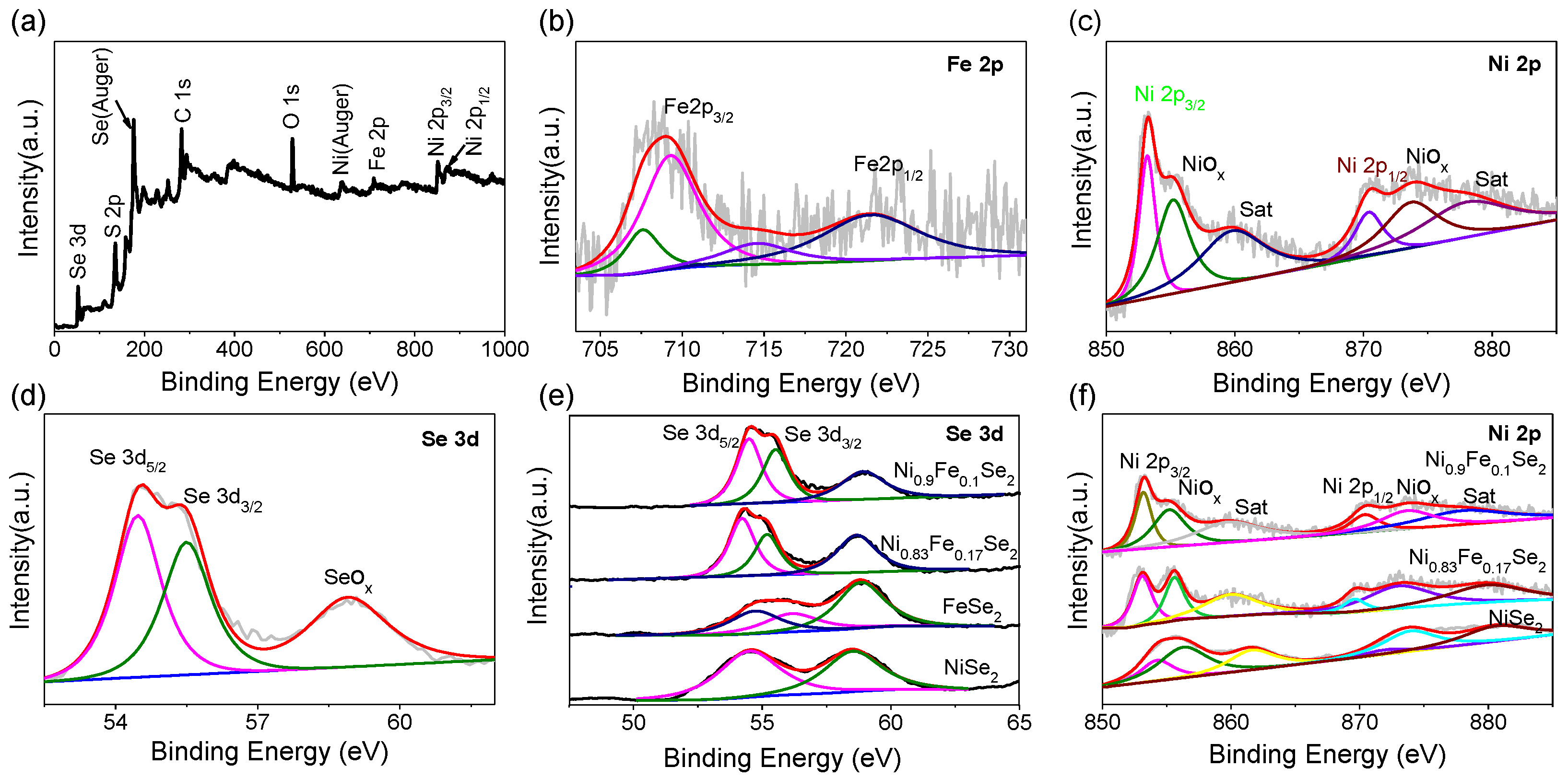
Figure 3.
Figure 3a displays the XPS survey spectrum of the Ni
0.9Fe
0.1Se
2 sample obtained by doping with
Fe
2(SO
4)
3.
Figure 3b–d present the corresponding high-resolution spectra of Fe2p, Ni2p, and Se3d for this sample, respectively.
Figure 3e,f show the comparative spectra of Se3d and Ni2p for the four samples: Ni
0.9Fe
0.1Se
2, Ni
0.83Fe
0.17Se
2, FeSe
2, and NiSe
2. All spectra were calibrated using the C1s peak at the binding energy of
.
From the XPS survey spectrum in
Figure 3a, characteristic peaks can be observed at binding energies of 854 eV (Ni 2p), 709 eV (Fe 2p), and 52 eV (Se 3d). Analysis of the Fe2p spectrum in
Figure 3b reveals that the Fe2p
3/2 peak can be deconvoluted into three peaks located at
,
, and
, attributed to the metallic iron, the iron component in Ni
xFe
1−xSe
2, and its satellite peak, respectively [
31,
32]. Analysis of the Ni2p spectrum in
Figure 3c indicates that for the Ni
0.9Fe
0.1Se
2 sample, the peaks at
and
correspond to the Ni2p
3/2 and Ni2p
1/2 main peaks, respectively, while the peaks at
and
are assigned to nickel oxide species and the Ni satellite peak [
31,
32]. Analysis of the Se3d spectrum in
Figure 3d shows peaks at
,
, and
, corresponding to Se3d
5/2, Se3d
3/2, and selenium oxide (SeO
x), respectively [
31,
32]. The results in
Figure 3a–d collectively confirm the successful incorporation of iron. Furthermore, comparative analysis of the Se3d and Ni2p spectra (
Figure 3e,f) shows that the Se3d
5/2 peak position (
) in Ni
0.9Fe
0.1Se
2 and Ni
0.83Fe
0.17Se
2 is lower than that in pure NiSe
2 (
). Similarly, the Ni2p
3/2 peak position (
) in Ni
0.9Fe
0.1Se
2 and Ni
0.83Fe
0.17Se
2 is lower than that in pure NiSe
2 (
). In summary, compared to NiSe
2, both Ni and Se binding energies in the doped samples (Ni
0.9Fe
0.1Se
2, Ni
0.83Fe
0.17Se
2) exhibit a shift towards lower binding energy [
31,
32]. This result aligns with the findings reported by Du et al., indicating the introduction of strong electronic interactions between Fe, Ni, and Se atoms after Fe doping [
31,
32].
The electrochemical performance of all materials was characterized in the
KOH electrolyte using the standard three-electrode system, with relevant data presented in
Figure 4. The catalysts were uniformly coated onto a
glassy carbon electrode (GCE), with a total catalyst loading of approximately
. The working electrode potential (vs. Ag/AgCl) was converted to the reversible hydrogen electrode (RHE) scale using the formula:
. During electrochemical measurements, the working electrode was rotated at
to remove generated oxygen bubbles. Polarization curves for the synthesized samples were recorded at a low scan rate of
to minimize capacitive current effects.
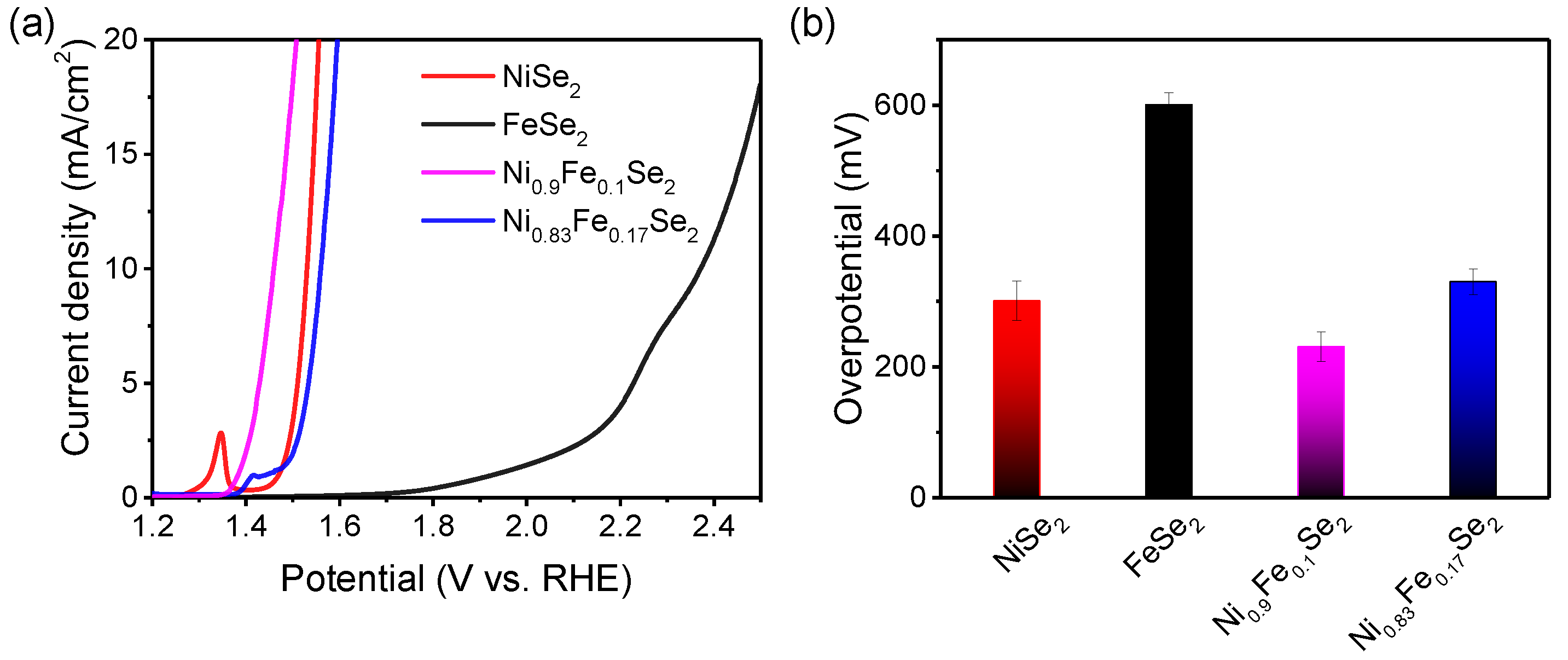
As shown in
Figure 4a, all samples exhibited significant catalytic activity for the oxygen evolution reaction (OER). The oxidation peak observed for NiSe
2 at approximately
before the OER onset potential is attributed to the Ni(OH)
2/NiOOH transformation, consistent with literature reports [
31,
34]. The overpotentials required for the four samples to achieve a current density of
were statistically analyzed, and the results are shown in
Figure 4b. It can be seen that FeSe
2 demonstrated the lowest catalytic activity, requiring a high overpotential of
to achieve a current density of
. When doped with
of Fe
2(SO
4)
3, the resulting Ni
0.9Fe
0.1Se
2 showed significantly enhanced OER performance. However, higher Fe doping levels were not beneficial; increasing the doping amount to
resulted in decreased OER performance. Among the four catalyst samples, Ni
0.9Fe
0.1Se
2 exhibited the highest catalytic activity, requiring an overpotential of only
at a current density of
. In comparison, the overpotentials for NiSe
2 and Ni
0.83Fe
0.17Se
2 were
and
, respectively. These results indicate that the Fe/Ni ratio during the reaction not only influences the morphology and composition of the samples but also significantly modulates their OER performance. Notably, Ni
0.9Fe
0.1Se
2 prepared with a low doping amount (
) exhibited optimal performance, demonstrating superior characteristics compared to various selenides and transition metal compound catalysts reported in the literature [
23,
33,
35,
36,
37], as shown in
Table S1. Furthermore, compared to the higher-cost NiSe
2 and the poorly performing FeSe
2 in terms of catalytic activity, the doping of a small amount of Fe reduces the cost while enhancing the catalytic activity of the material, resulting in excellent cost-effectiveness (see
Table S2). Regarding nickel-based oxygen evolution catalysts, the introduction of iron (Fe)—whether accidental or intentional—has been widely demonstrated to significantly enhance the catalytic activity for the oxygen evolution reaction (OER). The proposed mechanisms for this iron-enhanced performance primarily include the following [
27]: First, Fe
3+ ions can increase the electrical conductivity of the catalytic film, thereby improving OER performance; second, Fe
3+ can acquire sufficient charge transfer capability from conductive substrates (such as nickel or cobalt oxyhydroxides) and conductive supports (such as redox-activated gold), leading to superior electrocatalytic performance; third, Fe
3+ sites are regarded as “fast” active centers in OER kinetics due to their near-optimal adsorption energy for OER intermediates; fourth, the incorporation of Fe
3+ can suppress metal oxidation steps and promote reduction steps related to oxygen evolution, thereby optimizing the OER process. Nevertheless, a unified and definitive theory regarding the enhancement mechanism of iron has not yet been established. In this study, XPS results indicate that compared to undoped NiSe
2, the binding energies of Ni and Se in the iron-doped samples (Ni
0.9Fe
0.1Se
2 and Ni
0.83Fe
0.17Se
2) shift negatively. This observation suggests that the introduction of Fe induces significant reconstruction of the local electronic structure of NiSe
2, specifically manifested as electron transfer from Fe to Ni and Se regions, increasing the electron cloud density of Ni and Se atoms. The enhanced catalytic activity of Ni
0.9Fe
0.1Se
2 is likely attributed to this modulation of the electronic structure, which optimizes the adsorption strength of nickel active sites toward key oxygen intermediates (such as *O, *OH, and *OOH) [
18,
27], bringing it closer to the optimal adsorption energy required for OER. The optimized adsorption energy of intermediates effectively reduces the energy barriers of various steps in the OER pathway, particularly that of the rate-determining step (RDS), thereby significantly lowering the reaction overpotential.
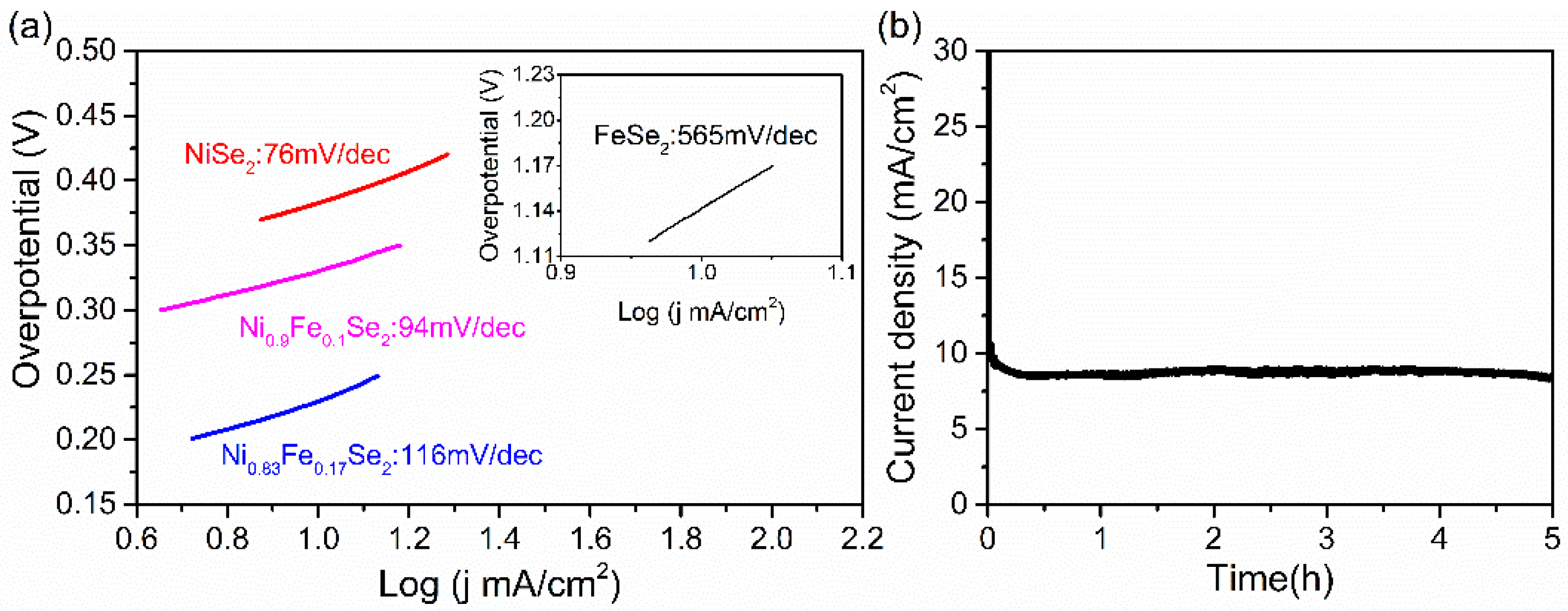
The Tafel slope is a fundamental parameter in electrocatalysis and holds significant importance. Its value directly reflects the ease of the electrode reaction—a lower Tafel slope indicates that a high current density can be achieved at a low overpotential, thereby greatly improving the efficiency of water electrolysis and demonstrating excellent catalytic performance. The OER kinetics were evaluated by converting polarization curves into Tafel plots (potential vs.
). As shown in
Figure 5a, the Tafel slope for Ni
0.9Fe
0.1Se
2 is
, which is lower than those of Ni
0.83Fe
0.17Se
2 (
) and FeSe
2 (
), but higher than that of NiSe
2 (
). It can be seen that the Tafel slope of the catalyst exhibits an increasing trend with higher iron doping content. A larger Tafel slope is detrimental to OER kinetics. Although doping trace amounts of iron into nickel selenide improves OER kinetics (as demonstrated by Ni
0.9Fe
0.1Se
2), the observed increase in Tafel slope with higher iron content indicates that the composition of this catalyst still requires further optimization.
Furthermore, the durability of a catalyst is a critical indicator for assessing its potential for practical applications. In this study, cyclic stability tests were conducted on the Ni
0.9Fe
0.1Se
2 sample. As shown in
Figure 5b, the results from chronoamperometric testing indicate that after 5 h of continuous operation, the current density decreased from an initial value of
to
. Although the degree of attenuation is relatively modest, it still suggests that the stability of the material requires further improvement, and the doping strategy needs additional optimization.
3. Materials and Methods
3.1. Chemicals and Materials
Oleylamine (OAm, C18H37N, ), tetralin (C10H12, ), Ni(acac)2 (Ni(C5H7O2)2, ), ferric sulfate (Fe2(SO4)3, ), ethanol (CH3CH2OH, ), isopropanol (C3H8O, ), n-hexane (C6H14, ) and n-octylamine (C8H19N, ) were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. Potassium hydroxide (KOH) was purchased from Aladdin Biochemical Technology Co., Ltd., Shanghai, China. Nafion solution () was purchased from Sigma-Aldrich® (Shanghai, China). High-purity argon (), high-purity oxygen () and deionized water () were used in the experiments. All chemical reagents were used without further purification.
3.2. The Synthesis of NiSe2
Dissolve of Ni(acac)2 in a mixed solution of OAm and tetralin. Preheat the mixture to under magnetic stirring and maintain this temperature for to form the precursor solution. After cooling to room temperature, disperse of selenium powder in a mixture of OAm and tetralin, then add this dispersion to the precursor solution. Stir for several minutes until a homogeneous black solution forms. Subsequently, heat the solution to and maintain for to synthesize NiSe2.
3.3. The Synthesis of FeSe2
FeSe2 was prepared using an analogous procedure to NiSe2 synthesis, replacing Ni(acac)2 with ferric sulfate (Fe2(SO4)3) as the metal precursor.
3.4. The Synthesis of Fe Doped NiSe2
After cooling as-synthesized NiSe2 to room temperature, add a dispersion of Fe2(SO4)3 in OAm and tetralin to the NiSe2 solution. Continue the reaction at for to obtain the Fe-doped NiSe2. By varying the amount of Fe2(SO4)3 added (, or ), Fex-NiSe2 samples with controlled doping levels were prepared.
All reactions were conducted in a three-necked round-bottomed flask under a continuous argon atmosphere. Finally, all products were washed twice with a mixed solvent of isopropanol and n-hexane, followed by ethanol washing, and dried under vacuum.
3.5. Characteristic Measurement
The phase composition of the sample was analyzed using powder X-ray diffraction (XRD) with the DX-2700 diffractometer. Sample characterization was performed using field emission scanning electron microscopy (FE-SEM, Hitachi S-4800, Hitachi High-Technologies Corporation, Tokyo, Japan). The surface elemental composition and chemical states of the sample were obtained by X-ray photoelectron spectroscopy (XPS) using a Kratos XSAM 800 (Kratos Analytical Ltd., Manchester, UK) system with an Al Kα X-ray source.
3.6. Preparation of the Working Electrode
To remove surface oxides generated during sample storage and enhance its dispersibility, the black powder sample was sonicated in n-octylamine solution () for h, followed by standing for h. After washing with deionized water, the product was uniformly dispersed in a mixed solution of isopropanol/water/Nafion (volume ratio ) to form a catalyst ink. Then, of the ink was drop-cast onto a diameter glassy carbon electrode (GCE) polished with Al2O3 powder, yielding a catalyst loading of approximately . After natural evaporation of deionized water and isopropanol and thorough drying of the sample on the electrode, a uniform catalyst layer was formed.
3.7. Electrochemical Measurements
All electrochemical measurements were performed at room temperature using a CHI 760e electrochemical workstation (CH Instruments, Inc., Shanghai, China) with a standard three-electrode configuration. Using an Ag/AgCl electrode (in KCl) as the reference electrode and a platinum wire as the counter electrode, the electrolyte was a KOH solution. Prior to testing, the sample was activated with cycles of CV scanning at a scan rate of to ensure a thorough evaluation of its electrochemical performance. During testing, oxygen was continuously bubbled into the electrolyte. Cyclic voltammetry (CV), linear sweep voltammetry (LSV), and Tafel measurements were performed at a rotation rate of with a scan rate of . The electrocatalytic stability of the samples was evaluated by the chronoamperometric (CA) stability test at fixed potential of vs. RHE.