Novel Biosynthetic Pathway for Nicotinamide Mononucleotide Production from Cytidine in Escherichia coli
Abstract
1. Introduction
2. Results and Discussion
2.1. Design and Validation of a Novel In Vitro Enzymatic Pathway for NMN Biosynthesis
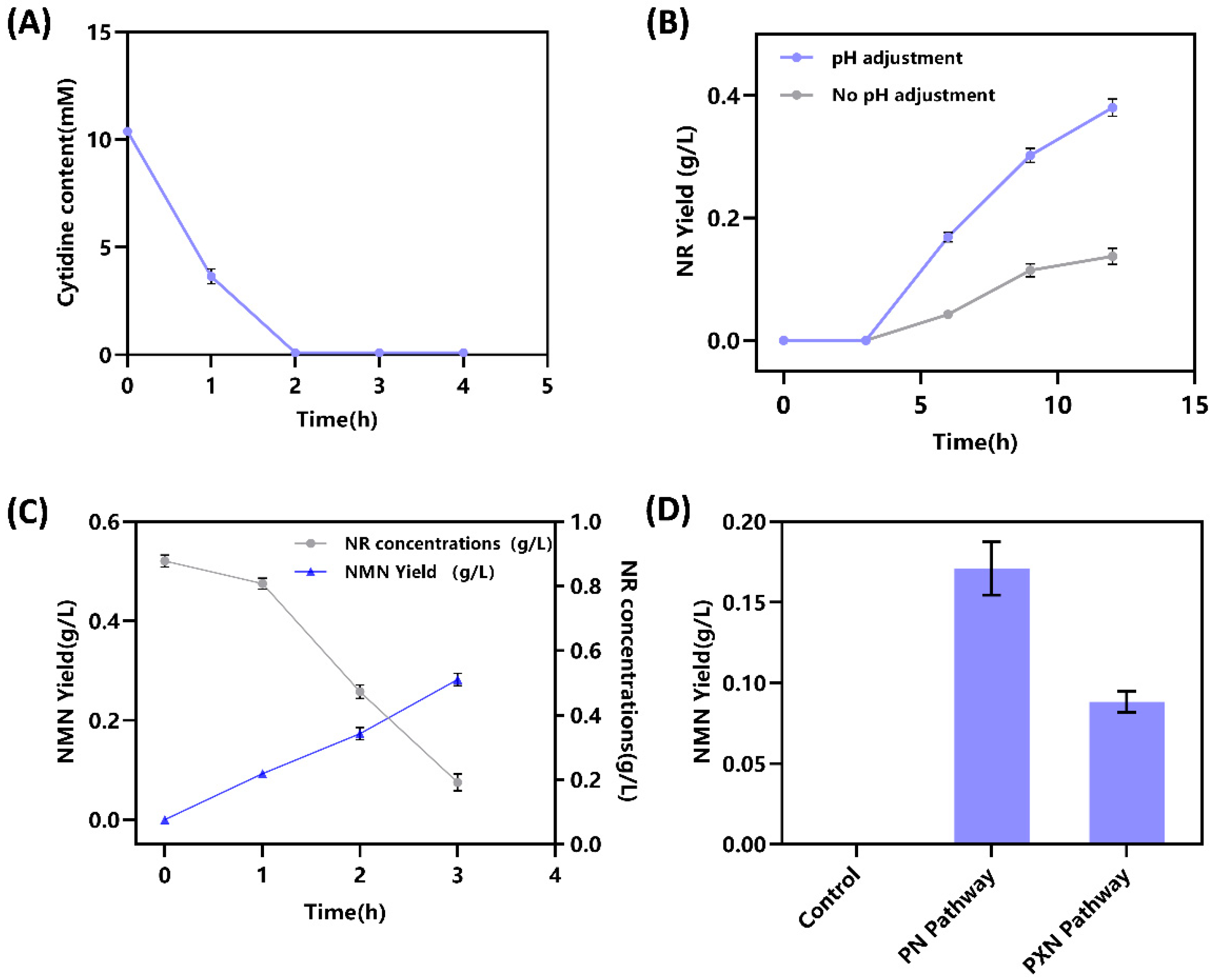
2.2. Enzyme Activity Validation and Optimization of In Vitro Cascade Reaction
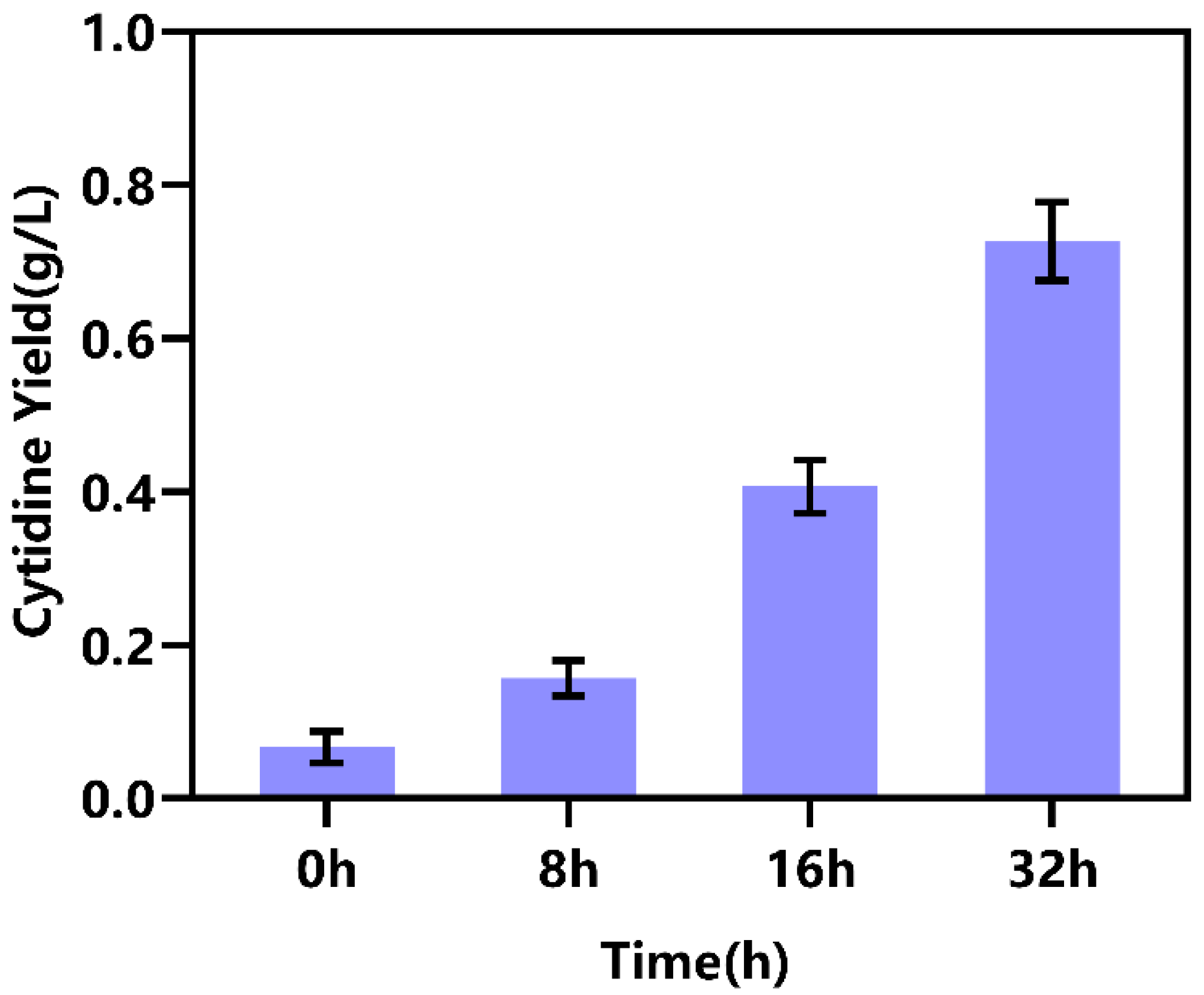
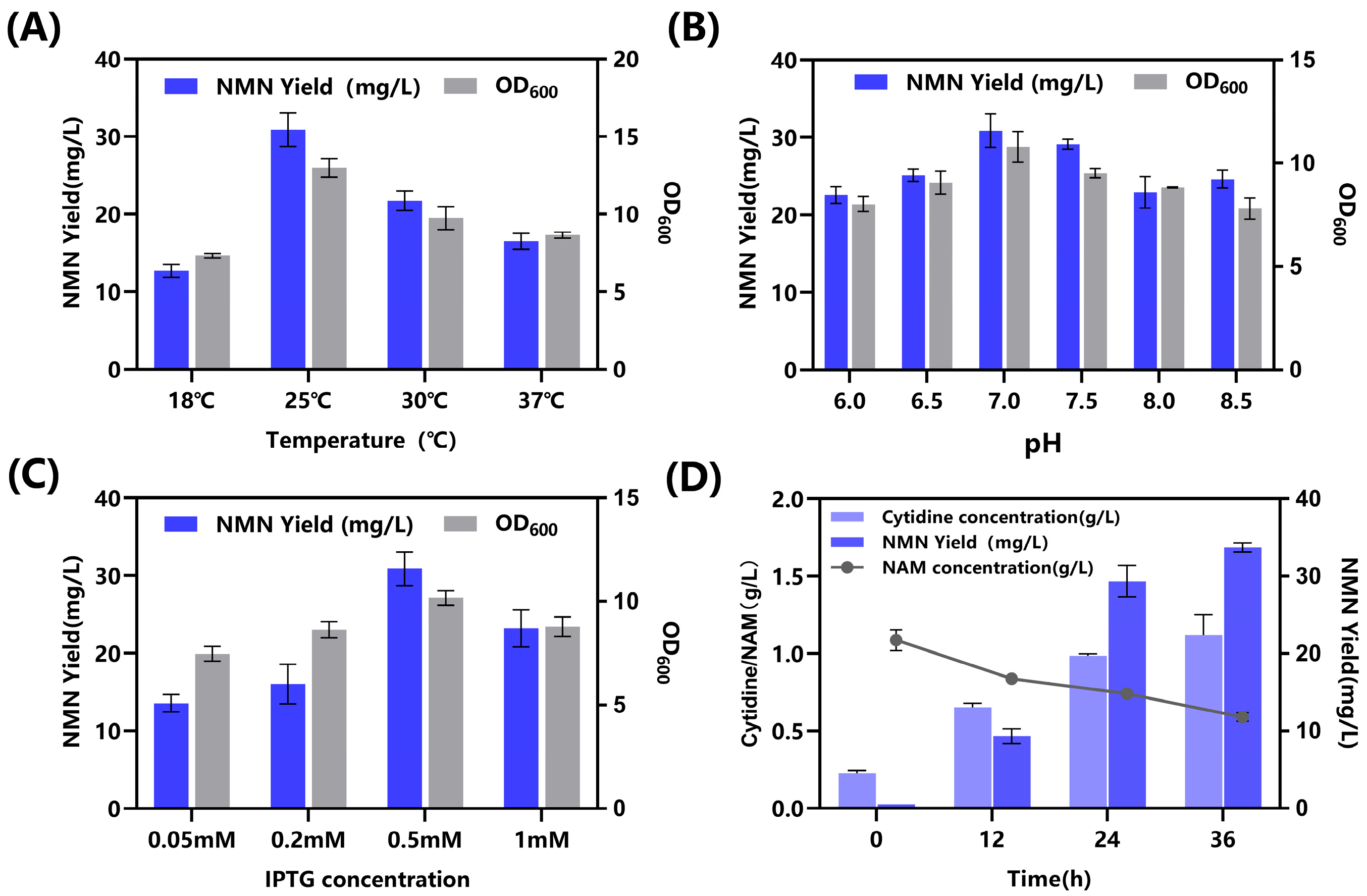
2.3. Intracellular Implementation of NMN Biosynthesis in Engineered E. coli
3. Materials and Methods
3.1. Plasmid Construction and Strains Used
3.2. Optimizing Cultivation and Expressing Proteins for Recombinant E. coli
3.3. Protein Purification and SDS-PAGE Assessment
3.4. Verification of Enzyme Activities in the Artificial NMN Synthesis Pathway
3.5. Multienzyme Cascade Catalysis Method for NMN Production
3.6. Recombinant E. coli via Shake Flask Fermentation
3.7. Analysis Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, Z.; Li, N.; Yu, S.; Zhang, W.; Zhang, T.; Zhou, J. Systematic Engineering of Escherichia coli for Efficient Production of Nicotinamide Mononucleotide from Nicotinamide. ACS Synth. Biol. 2022, 11, 2979–2988. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, B.; Duan, C.; Sun, B.; Yang, J.; Yang, S. Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system. Appl. Environ. Microbiol. 2015, 81, 2506–2514. [Google Scholar] [CrossRef]
- Zhou, C.L.; Feng, J.; Wang, J.; Hao, N.; Wang, X.; Chen, K.Q. Design of an in vitro multienzyme cascade system for the biosynthesis of nicotinamide mononucleotide. Catal. Sci. Technol. 2022, 12, 1080–1091. [Google Scholar] [CrossRef]
- Shoji, S.; Yamaji, T.; Makino, H.; Ishii, J.; Kondo, A. Metabolic design for selective production of nicotinamide mononucleotide from glucose, nicotinamide. Metab. Eng. 2021, 65, 167–177. [Google Scholar] [CrossRef]
- Black, W.B.; Aspacio, D.; Bever, D.; King, E.; Zhang, L.; Li, H. Metabolic engineering of Escherichia coli for optimized biosynthesis of nicotinamide mononucleotide, a noncanonical redox cofactor. Microb. Cell Factories 2020, 19, 150. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, C.; Huang, J.; Tao, Y.; Ke, C.; Yang, X. Advances in physiological activities, synthesis of beta-nicotinamide mononucleotide. Chin. J. Biotechnol. 2023, 39, 516–536. [Google Scholar]
- Huang, Z.; Wang, X.; Li, N.; Song, F.; Zhou, J. Systematic engineering of Escherichia coli for efficient production of nicotinamide riboside from nicotinamide, 3-cyanopyridine. Bioresour. Technol. 2023, 377, 128953. [Google Scholar] [CrossRef]
- Zhou, M.L.; Li, Y.; Che, H.T.; Sun, Y.Q.; Wang, S.; Zhan, Y.Y.; Cai, D.B.; Chen, S.W. Metabolic Engineering of Bacillus licheniformis for the Bioproduction of Nicotinamide Riboside from Nicotinamide, Glucose. ACS Sustain. Chem. Eng. 2023, 11, 6201–6210. [Google Scholar] [CrossRef]
- Revollo, J.R.; Grimm, A.A.; Imai, S.-I. The regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/visfatin in mammals. Curr. Opin. Gastroenterol. 2007, 23, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Li, Z.Q.; Miller, E.S. Vibrio Phage KVP40 Encodes a Functional NAD+ Salvage Pathway. J. Bacteriol. 2017, 199, e00855-16. [Google Scholar] [CrossRef]
- Palmer, R.D.; Elnashar, M.M.; Vaccarezza, M. Precursor comparisons for the upregulation of nicotinamide adenine dinucleotide. Novel approaches for better aging. Aging Med. 2021, 4, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Reiten, O.K.; Wilvang, M.A.; Mitchell, S.J.; Hu, Z.; Fang, E.F. Preclinical, clinical evidence of NAD(+) precursors in health, disease, ageing. Mech. Ageing Dev. 2021, 199, 111567. [Google Scholar] [CrossRef]
- Hong, W.; Mo, F.; Zhang, Z.; Huang, M.; Wei, X. Nicotinamide Mononucleotide: A Promising Molecule for Therapy of Diverse Diseases by Targeting NAD+ Metabolism. Front. Cell Dev. Biol. 2020, 8, 246. [Google Scholar] [CrossRef]
- Shukla, A.; Afsar, M.; Kumar, N.; Kumar, S.; Ramachandran, R. Structure based identification of first-in-class fragment inhibitors that target the NMN pocket of M. tuberculosis NAD(+)-dependent DNA ligase A. J. Struct. Biol. 2021, 213, 107655. [Google Scholar] [CrossRef]
- Maharjan, A.; Singhvi, M.; Kafle, S.R.; Kim, B.S. Enhanced production of nicotinamide mononucleotide by high cell density culture of engineered Escherichia coli. Process Biochem. 2023, 131, 264–271. [Google Scholar] [CrossRef]
- Zheng, C.; Li, Y.; Wu, X.; Gao, L.; Chen, X. Advances in the Synthesis, Physiological Metabolic Regulation of Nicotinamide Mononucleotide. Nutrients 2024, 16, 2354. [Google Scholar] [CrossRef]
- Paul, R.; Patra, M.D.; Sen, U. Crystal structure of apo, ligand bound vibrio cholerae ribokinase (Vc-RK): Role of monovalent cation induced activation, structural flexibility in sugar phosphorylation. Adv. Exp. Med. Biol. 2015, 842, 293–307. [Google Scholar]
- Hamamoto, T.; Noguchi, T.; Midorikawa, Y. Phosphopentomutase of Bacillus stearothermophilus TH6-2: The enzyme, its gene ppm. Biosci. Biotechnol. Biochem. 1998, 62, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, H.M.; Zaghloul, T.I.; Zhang, Y.H. A simple assay for determining activities of phosphopentomutase from a hyperthermophilic bacterium Thermotoga maritima. Anal. Biochem. 2016, 501, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Walther, T.; Baylac, A.; Alkim, C.; Vax, A.; Cordier, H.; François, J.M. The PGM3 gene encodes the major phosphoribomutase in the yeast Saccharomyces cerevisiae. FEBS Lett. 2012, 586, 4114–4118. [Google Scholar] [CrossRef]
- WielgusKutrowska, B.; Kulikowska, E.; Wierzchowski, J.; Bzowska, A.; Shugar, D. Nicotinamide riboside, an unusual, non-typical, substrate of purified purine-nucleoside phosphorylases. Eur. J. Biochem. 1997, 243, 408–414. [Google Scholar] [CrossRef]
- Feng, R.; Yan, Z.; Wei, G.; Wu, C.; Chen, F.; Zhang, A.; Xu, S.; Wang, X.; Chen, K. Establishing a novel pathway for the biosynthesis of nicotinamide mononucleotide. Enzym. Microb. Technol. 2025, 188, 110633. [Google Scholar] [CrossRef]
- Liu, K.F.; Chen, X.L.; Wu, J.; Song, W.; Wei, W.Q.; Liu, L.M.; Gao, C. Multivariate Modular Metabolic Engineering for High Titer Uridine Triphosphate Production in Escherichia coli. Acs Sustain. Chem. Eng. 2023, 12, 85–95. [Google Scholar] [CrossRef]
- Dong, H.; Liu, Y.; Zu, X.; Li, N.; Li, F.; Zhang, D. An enzymatic assay for high-throughput screening of cytidine-producing microbial strains. PLoS ONE 2015, 10, e0121612. [Google Scholar] [CrossRef]
- Iglesias, L.E.; Lewkowicz, E.S.; Medici, R.; Bianchi, P.; Iribarren, A.M. Biocatalytic approaches applied to the synthesis of nucleoside prodrugs. Biotechnol. Adv. 2015, 33, 412–434. [Google Scholar] [CrossRef] [PubMed]
- Hove-Jensen, B.; Rosenkrantz, T.J.; Haldimann, A.; Wanner, B.L. Escherichia coli phnN, encoding ribose 1,5-bisphosphokinase activity (phosphoribosyl diphosphate forming): Dual role in phosphonate degradation and NAD biosynthesis pathways. J. Bacteriol. 2003, 185, 2793–2801. [Google Scholar] [CrossRef]
- Dong, W.R.; Sun, C.C.; Zhu, G.; Hu, S.H.; Xiang, L.X.; Shao, J.Z. New function for Escherichia coli xanthosine phophorylase (xapA): Genetic, biochemical evidences on its participation in NAD(+) salvage from nicotinamide. BMC Microbiol. 2014, 14, 29. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Cui, L. Optimizing Biosynthesis of Nicotinamide Mononucleotide, a Potential Nutritional Supplement, by E. coli. Curr. Top. Nutraceutical Res. 2023, 21, 198–207. [Google Scholar] [CrossRef]
- Cheng, F.; Li, K.-X.; Wu, S.-S.; Liu, H.-Y.; Li, H.; Shen, Q.; Xue, Y.-P.; Zheng, Y.-G. Biosynthesis of Nicotinamide Mononucleotide: Synthesis Method, Enzyme, Biocatalytic System. J. Agric. Food Chem. 2024, 72, 3302–3313. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Y.J.; Wang, L.; Liu, W.; Liu, Y.; Peng, C.; Zhao, Z.K. Engineering Escherichia coli Nicotinic Acid Mononucleotide Adenylyltransferase for Fully Active Amidated NAD Biosynthesis. Appl. Environ. Microbiol. 2017, 83, e00692-17. [Google Scholar] [CrossRef]


| Strains or Plasmids | Description | Source |
|---|---|---|
| Strains | ||
| E. coli BL21 (DE3) | Used as host strain | Invitrogen |
| E. coli DH5α | Used as cloning strain | Invitrogen |
| E. coli KQ_BG001 | Used as host strain for NMN biosynthesis | Laboratory preservation |
| Plasmids | ||
| pET-28a (+) | Cloning vector, KanR | General bio |
| pRSFDuet-1 | With T7 promoter, KanR | General bio |
| pTrc99a | With trc promoter, AmpR | General bio |
| pRSFDuet-trc | With trc promoter, KanR | This study |
| Strains or Plasmids | Description | Source |
|---|---|---|
| Plasmids | ||
| pET28a-PyNP | pET-28a (+), with PyNP | This study |
| pET28a-XapA | pET-28a (+), with XapA | This study |
| pET28a-NRK | pET-28a (+), with NRK | This study |
| pRSFDuet-trc-PyNP | pRSFDuet-trc, with PyNP | This study |
| pTrc99a-NRK | pTrc99a, with NRK | This study |
| Primers | ||
| XapA-F | TCGCGGATCCGAATTCATGAGCCAGGTTCAGTTTAGCC | This study |
| XapA-R | GTGCGGCCGCAAGCTTTGCAATTTTACGCAGAAAACCGC | This study |
| NRK-F | TCGCGGATCCGAATTCATGACCACCACCAAAGTTAAACTGATTGCC | This study |
| NRK-R | GTGCGGCCGCAAGCTTATTTGCATCCAG | This study |
| PyNP-F | ATGAGAATGGTTGATATCATCACAAAAAAACAAAATGG | This study |
| PyNP-R | TTCCGTAATCACCGTATGCACAAGC | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Feng, R.; Liu, M.; Wang, X.; Chen, K.; Xu, S. Novel Biosynthetic Pathway for Nicotinamide Mononucleotide Production from Cytidine in Escherichia coli. Catalysts 2025, 15, 816. https://doi.org/10.3390/catal15090816
Yuan J, Feng R, Liu M, Wang X, Chen K, Xu S. Novel Biosynthetic Pathway for Nicotinamide Mononucleotide Production from Cytidine in Escherichia coli. Catalysts. 2025; 15(9):816. https://doi.org/10.3390/catal15090816
Chicago/Turabian StyleYuan, Jiaxiang, Rongchen Feng, Mingming Liu, Xin Wang, Kequan Chen, and Sheng Xu. 2025. "Novel Biosynthetic Pathway for Nicotinamide Mononucleotide Production from Cytidine in Escherichia coli" Catalysts 15, no. 9: 816. https://doi.org/10.3390/catal15090816
APA StyleYuan, J., Feng, R., Liu, M., Wang, X., Chen, K., & Xu, S. (2025). Novel Biosynthetic Pathway for Nicotinamide Mononucleotide Production from Cytidine in Escherichia coli. Catalysts, 15(9), 816. https://doi.org/10.3390/catal15090816






