Strontium-Promoted Ni-Catalyst Supported over MgO for Partial Oxidation of Methane: Unveiling a Cost-Effective Catalyst System for Fast Mitigation of Methane
Abstract
1. Introduction
2. Results and Discussion
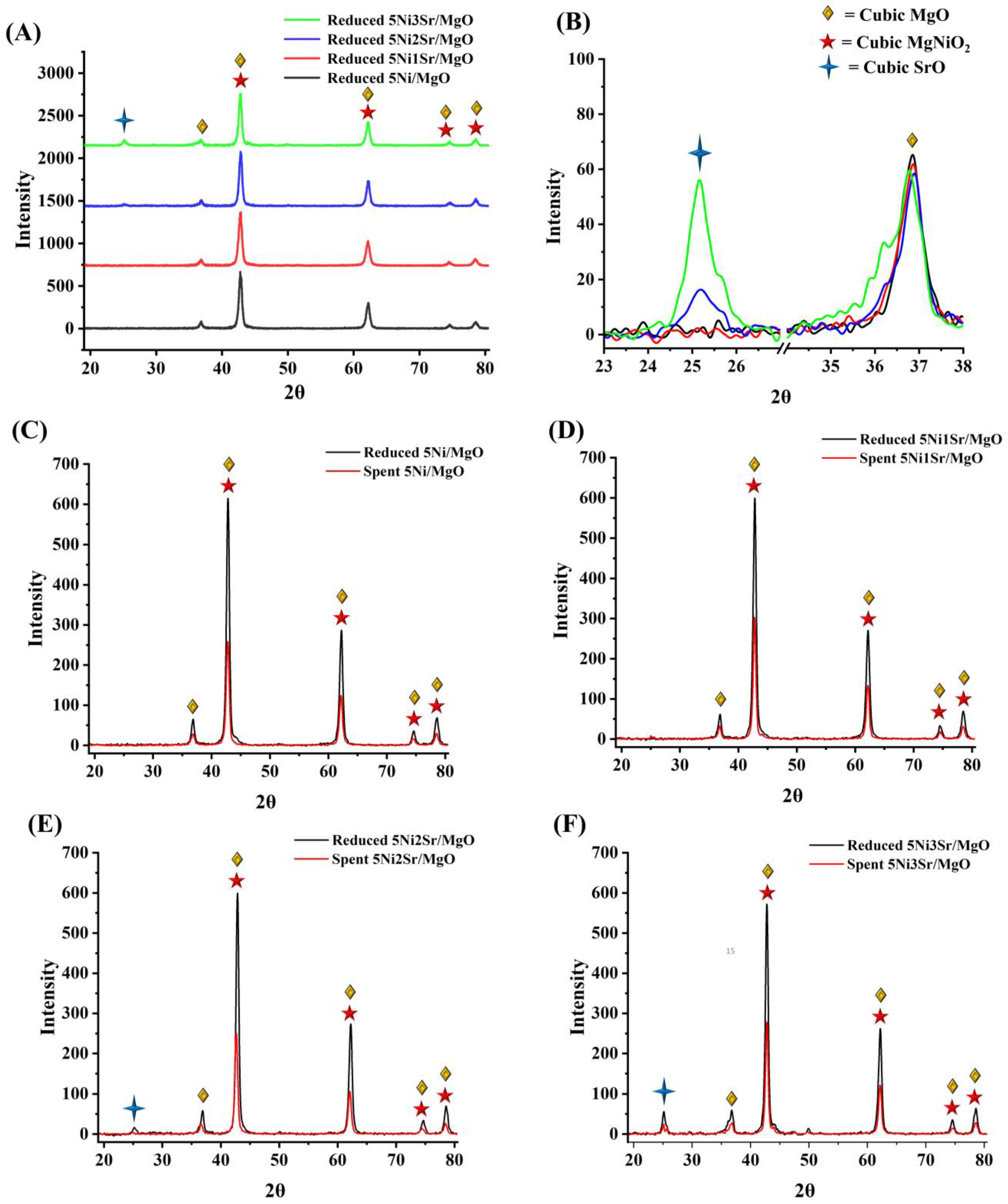
2.1. Characterization Results
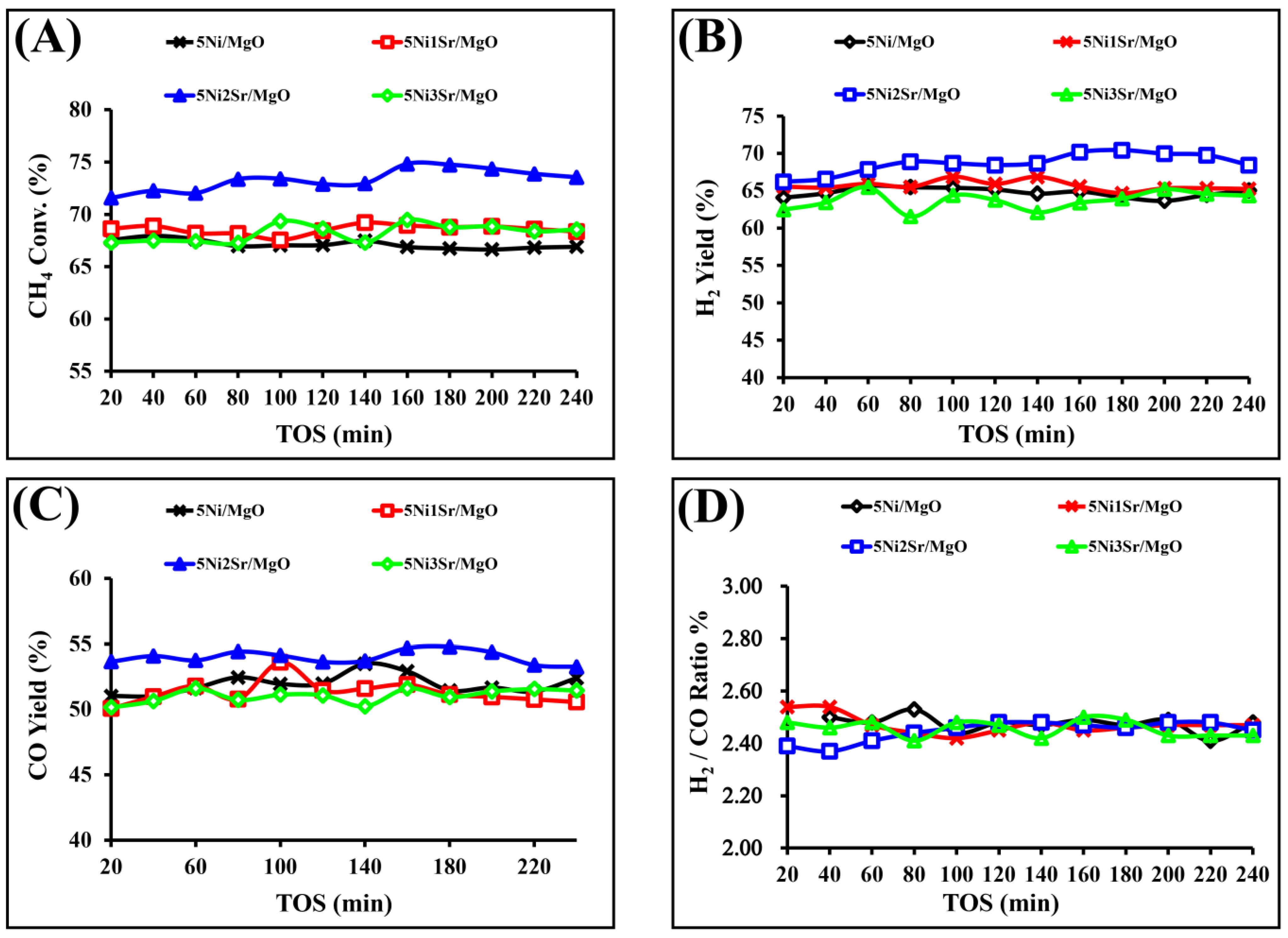
2.2. Results of Catalytic Activity and Discussion
3. Experimental Section
3.1. Resources
3.2. Catalyst Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nojedehi, P.; Heidari, M.; Ataei, A.; Nedaei, M.; Kurdestani, E. Environmental Assessment of Energy Production from Landfill Gas Plants by Using Long-Range Energy Alternative Planning (LEAP) and IPCC Methane Estimation Methods: A Case Study of Tehran. Sustain. Energy Technol. Assess. 2016, 16, 33–42. [Google Scholar] [CrossRef]
- Bousquet, P.; Ciais, P.; Miller, J.B.; Dlugokencky, E.J.; Hauglustaine, D.A.; Prigent, C.; Van der Werf, G.R.; Peylin, P.; Brunke, E.-G.; Carouge, C. Contribution of Anthropogenic and Natural Sources to Atmospheric Methane Variability. Nature 2006, 443, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Kammoun, R.; Naili, B.; Bejar, S. Application of a Statistical Design to the Optimization of Parameters and Culture Medium for α-Amylase Production by Aspergillus Oryzae CBS 819.72 Grown on Gruel (Wheat Grinding by-Product). Bioresour. Technol. 2008, 99, 5602–5609. [Google Scholar] [CrossRef]
- Elsgaard, L.; Olsen, A.B.; Petersen, S.O. Temperature Response of Methane Production in Liquid Manures and Co-Digestates. Sci. Total Environ. 2016, 539, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, K.J.; Banabdwin, K.M.; Abahussain, A.A.M.; Fakeeha, A.H.; Wazeer, I.; Abu-Dahrieh, J.K.; Hasnain Bakhtiar, S.U.; Kumar, R.; Al-Fatesh, A.S. The Role of Pore Architect, Reducibility and Silica-Alumina Ratio over Ni-Containing Molecular Sieves for Methane Partial Oxidation. Catal. Lett. 2025, 155, 93. [Google Scholar] [CrossRef]
- Sengodan, S.; Lan, R.; Humphreys, J.; Du, D.; Xu, W.; Wang, H.; Tao, S. Advances in Reforming and Partial Oxidation of Hydrocarbons for Hydrogen Production and Fuel Cell Applications. Renew. Sustain. Energy Rev. 2018, 82, 761–780. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, F.-Y.; Li, J.-M.; Weng, W.-Z.; Luo, C.-R.; Wang, M.-L.; Xia, W.-S.; Huang, C.-J.; Wan, H.-L. In Situ Raman Study on the Partial Oxidation of Methane to Synthesis Gas over Rh/Al2O3 and Ru/Al2O3 Catalysts. J. Catal. 2008, 256, 192–203. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, S.; Shan, J.; Nguyen, L.; Zhan, S.; Gu, X.; Tao, F. In Situ Surface Chemistries and Catalytic Performances of Ceria Doped with Palladium, Platinum, and Rhodium in Methane Partial Oxidation for the Production of Syngas. Acs Catal. 2013, 3, 2627–2639. [Google Scholar] [CrossRef]
- Jing, Q.S.; Zheng, X.M. Combined Catalytic Partial Oxidation and CO2 Reforming of Methane over ZrO2-Modified Ni/SiO2 Catalysts Using Fluidized-Bed Reactor. Energy 2006, 31, 2184–2192. [Google Scholar] [CrossRef]
- Lamber, R.; Schulz-Ekloff, G. Characterization of Microstructures in Nickel Alumina Catalysts by Analytical Electron Microscopy. Surf. Sci. 1991, 258, 107–118. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Xue, B. Catalytic Combustion of Methane over M (Ni, Co, Cu) Supported on Ceria–Magnesia. Fuel Process. Technol. 2009, 90, 652–656. [Google Scholar] [CrossRef]
- Alvarez-Galvan, C.; Melian, M.; Ruiz-Matas, L.; Eslava, J.L.; Navarro, R.M.; Ahmadi, M.; Roldan Cuenya, B.; Fierro, J.L.G. Partial Oxidation of Methane to Syngas over Nickel-Based Catalysts: Influence of Support Type, Addition of Rhodium, and Preparation Method. Front. Chem. 2019, 7, 104. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Hu, Y.H. Methane Partial Oxidation over NiO/MgO Solid Solution Catalysts. Appl. Catal. A Gen. 1999, 183, 85–92. [Google Scholar] [CrossRef]
- Troitzsch, U. TiO2-doped Zirconia: Crystal Structure, Monoclinic-tetragonal Phase Transition, and the New Tetragonal Compound Zr3TiO8. J. Am. Ceram. Soc. 2006, 89, 3201–3210. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Khan, W.U.; Al-Mubaddel, F.; Al-Fatesh, A.S.; Kasim, S.O.; Mahmud, S.L.; Al-Zahrani, A.A.; Siddiqui, M.R.H.; Fakeeha, A.H. Study of Partial Oxidation of Methane by Ni/Al2O3 Catalyst: Effect of Support Oxides of Mg, Mo, Ti and Y as Promoters. Molecules 2020, 25, 5029. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Kumar, R.; Fakeeha, A.H.; Kasim, S.O.; Khatri, J.; Ibrahim, A.A.; Arasheed, R.; Alabdulsalam, M.; Lanre, M.S.; Osman, A.I. Promotional Effect of Magnesium Oxide for a Stable Nickel-Based Catalyst in Dry Reforming of Methane. Sci. Rep. 2020, 10, 13861. [Google Scholar] [CrossRef] [PubMed]
- Titus, J.; Roussière, T.; Wasserschaff, G.; Schunk, S.; Milanov, A.; Schwab, E.; Wagner, G.; Oeckler, O.; Gläser, R. Dry Reforming of Methane with Carbon Dioxide over NiO–MgO–ZrO2. Catal. Today 2016, 270, 68–75. [Google Scholar] [CrossRef]
- Al-Anazi, A.; Bellahwel, O.; Abu-Dahrieh, J.; Ibrahim, A.A.; Santhosh, S.; Abasaeed, A.E.; Fakeeha, A.H.; Al-Fatesh, A.S. Promoter Impact on 5Ni/SAPO-5 Catalyst for H2 Production via Methane Partial Oxidation. Catal. 2024, 14, 316. [Google Scholar] [CrossRef]
- Song, J.H.; Han, S.J.; Yoo, J.; Park, S.; Kim, D.H.; Song, I.K. Hydrogen Production by Steam Reforming of Ethanol over Ni–X/Al2O3–ZrO2 (X= Mg, Ca, Sr, and Ba) Xerogel Catalysts: Effect of Alkaline Earth Metal Addition. J. Mol. Catal. A Chem. 2016, 415, 151–159. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Fakeeha, A.H.; Al-Fatesh, A.S. Enhancing Hydrogen Production by Dry Reforming Process with Strontium Promoter. Int. J. Hydrogen Energy 2014, 39, 1680–1687. [Google Scholar] [CrossRef]
- Anandhakumari, G.; Jayabal, P.; Balasankar, A.; Ramasundaram, S.; Oh, T.H.; Aruchamy, K.; Kallem, P.; Polisetti, V. Synthesis of Strontium Oxide-Zinc Oxide Nanocomposites by Co-Precipitation Method and Its Application for Degradation of Malachite Green Dye under Direct Sunlight. Heliyon 2023, 9, e20824. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Hussain, M.M.; Asiri, A.M. A Novel Approach towards Hydrazine Sensor Development Using SrO·CNT Nanocomposites. RSC Adv. 2016, 6, 65338–65348. [Google Scholar] [CrossRef]
- Ravikumar, R.; Jagadeshvaran, P.L.; Biju, R.; Binoy, L.; Raghavan, J.R.V.; Krishnakumar, T.S.; Indulal, C.R. Role of Polypyrrole-Based SrO–CuO Nanocomposite on Flame Retardancy and Heat Dissipation Applications. Chem. Pap. 2023, 77, 3413–3426. [Google Scholar] [CrossRef]
- Acharya, K.; Al-Fatesh, A.S.; Almutairi, G.; Fakeeha, A.H.; Ibrahim, A.A.; Abasaeed, A.E.; Siddiqui, M.R.H.; Kumar, R. The Role of Strontium as an Economic Promoter Over WO3 + ZrO2 Supported Ni Catalyst for H2 Production Through Dry Reforming of Methane. Catal. Lett. 2024, 154, 2023–2035. [Google Scholar] [CrossRef]
- Elnour, A.Y.; Abasaeed, A.E.; Fakeeha, A.H.; Ibrahim, A.A.; Alreshaidan, S.B.; Al-Fatesh, A.S. Dry Reforming of Methane (DRM) over Hydrotalcite-Based Ni-Ga/(Mg, Al) Ox Catalysts: Tailoring Ga Content for Improved Stability. Catalysts 2024, 14, 721. [Google Scholar] [CrossRef]
- Chen, L.F.; Wang, J.A.; Valenzuela, M.A.; Bokhimi, X.; Acosta, D.R.; Novaro, O. Hydrogen Spillover and Structural Defects in a PdO/Zirconia Nanophase Synthesized through a Surfactant-Templated Route. J. Alloys Compd. 2006, 417, 220–223. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Kumar, R.; Kasim, S.O.; Ibrahim, A.A.; Fakeeha, A.H.; Abasaeed, A.E.; Alrasheed, R.; Bagabas, A.; Chaudhary, M.L.; Frusteri, F. The Effect of Modifier Identity on the Performance of Ni-Based Catalyst Supported on γ-Al2O3 in Dry Reforming of Methane. Catal. Today 2020, 348, 236–242. [Google Scholar] [CrossRef]
- Gobara, H.M. Characterization and Catalytic Activity of NiO/Mesoporous Aluminosilicate AlSBA-15 in Conversion of Some Hydrocarbons. Egypt. J. Pet. 2012, 21, 1–10. [Google Scholar] [CrossRef]
- Meng, K.; Qi, Y.; Wen, L.U.; Zheyong, F.A.N.; Jinhua, F.E.I.; ZHENG, X.; Wheelock, T.D. Effect of Calcination Temperature on Characteristics and Performance of Ni/MgO Catalyst for CO2 Reforming of Toluene. Chinese, J. Catal. 2012, 33, 1508–1516. [Google Scholar] [CrossRef]
- Wen, Y.; Wei, C.H.U.; Jie, W.E.N.; Wenjing, S.U.N. Cerium Oxide Promoted Ni/MgO Catalyst for the Synthesis of Multi-Walled Carbon Nanotubes. Chin. J. Catal. 2011, 32, 1323–1328. [Google Scholar]
- Patel, N.; Al-Fatesh, A.S.; Bamatraf, N.A.; Osman, A.I.; Alreshaidan, S.B.; Fakeeha, A.H.; Wazeer, I.; Kumar, R. 5Ni/MgO and 5Ni/MgO + MOx (M= Zr, Ti, Al) Catalyst for Hydrogen Production via Dry Reforming of Methane: Promotor-Free, Cost-Effective, and Handy Catalyst System. Catal. Lett. 2024, 154, 3441–3456. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; Fajardo, H.V.; Dias, A.; Mezalira, D.Z.; Probst, L.F.D.; Stumpf, H.O.; Barros, W.P.; de Souza, G.P. Synthesis, Characterization and Catalytic Potential of MgNiO2 Nanoparticles Obtained from a Novel [MgNi(Opba)]n·9nH2O Chain. Ceram. Int. 2016, 42, 13635–13641. [Google Scholar] [CrossRef]
- Turner, R.C.; Hoffman, I.; Chen, D. Thermogravimetry of the Dehydration of Mg (OH)2. Can. J. Chem. 1963, 41, 243–251. [Google Scholar] [CrossRef]
- Gigantino, M.; Kiwic, D.; Steinfeld, A. Thermochemical Energy Storage via Isothermal Carbonation-Calcination Cycles of MgO-Stabilized SrO in the Range of 1000–1100 °C. Sol. Energy 2019, 188, 720–729. [Google Scholar] [CrossRef]
- André, L.; Abanades, S. Evaluation and Performances Comparison of Calcium, Strontium and Barium Carbonates during Calcination/Carbonation Reactions for Solar Thermochemical Energy Storage. J. Energy Storage 2017, 13, 193–205. [Google Scholar] [CrossRef]
- Mahon, D.; Claudio, G.; Eames, P. An Experimental Study of the Decomposition and Carbonation of Magnesium Carbonate for Medium Temperature Thermochemical Energy Storage. Energies 2021, 14, 1316. [Google Scholar] [CrossRef]
- Shahwan, T.; Erten, H.N. Characterization of Sr2+ Uptake on Natural Minerals of Kaolinite and Magnesite Using XRPD, SEM/EDS, XPS, and DRIFT. Radiochim. Acta 2005, 93, 225–232. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chen, C.-Y. Water Gas Shift Reaction for Hydrogen Production and Carbon Dioxide Capture: A Review. Appl. Energy 2020, 258, 114078. [Google Scholar] [CrossRef]
- Akbari, E.; Alavi, S.M.; Rezaei, M. Synthesis Gas Production over Highly Active and Stable Nanostructured NiMgOAl2O3 Catalysts in Dry Reforming of Methane: Effects of Ni Contents. Fuel 2017, 194, 171–179. [Google Scholar] [CrossRef]
- Wachter, P.; Hödl, P.; Raic, J.; Gaber, C.; Demuth, M.; Hochenauer, C. Towards Thermochemical Recuperation Applying Combined Steam Reforming and Partial Oxidation of Methane: Thermodynamic and Experimental Considerations. Energy Convers. Manag. 2022, 251, 114927. [Google Scholar] [CrossRef]
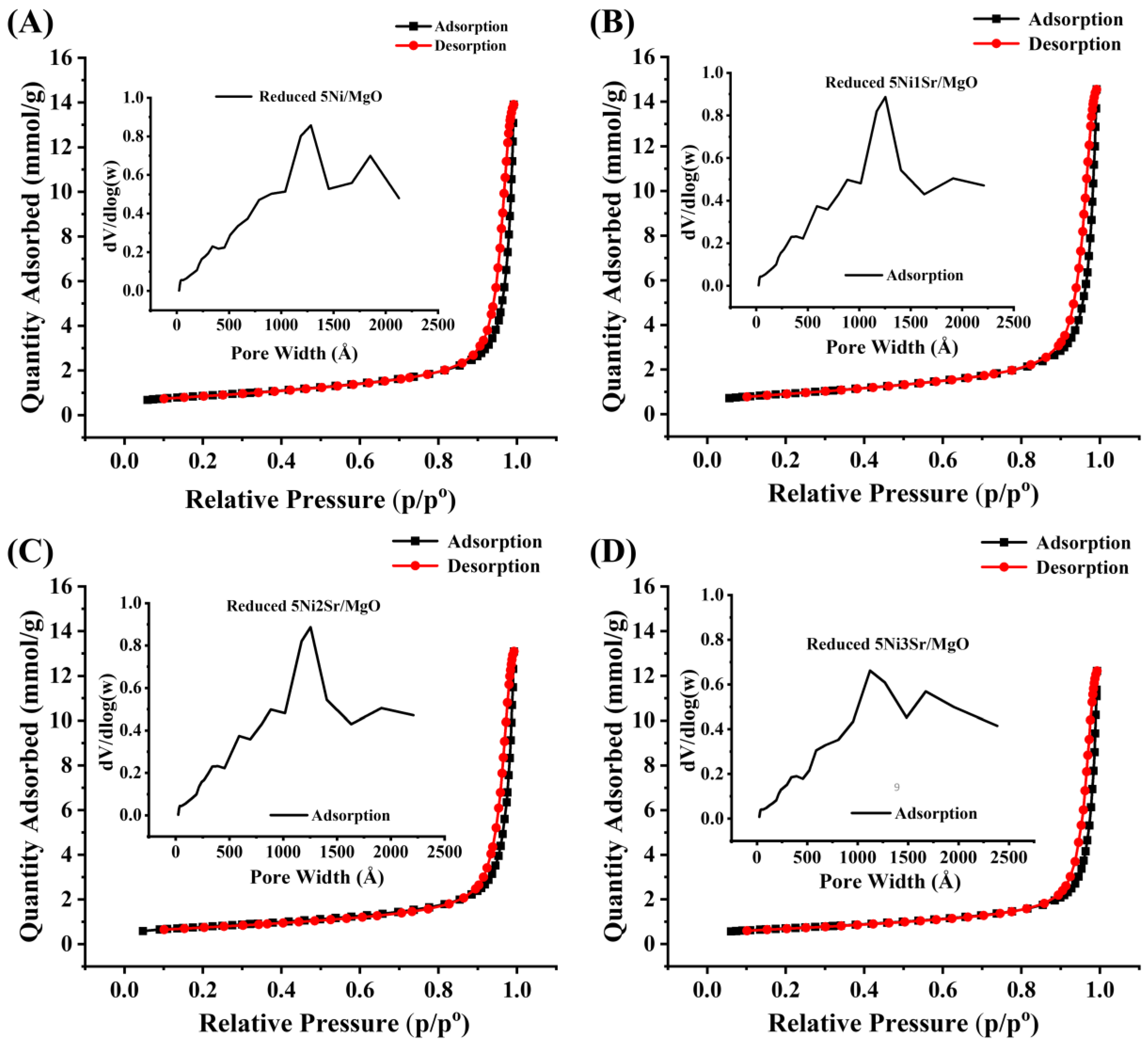


| Catalyst | Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| Reduced 5Ni/MgO | 68 | 0.48 | 34 |
| Reduced 5Ni1Sr/MgO | 72 | 0.50 | 33 |
| Reduced 5Ni2Sr/MgO | 61 | 0.45 | 35 |
| Reduced 5Ni3Sr/MgO | 55 | 0.42 | 38 |
| Spent 5Ni/MgO | 48 | 0.41 | 41 |
| Spent 5Ni2Sr/MgO | 40 | 0.36 | 44 |
| Spent 5Ni3Sr/MgO | 39 | 0.33 | 42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, F.A.A.; Chaudhary, K.J.; Ibrahim, A.A.; Alotaibi, N.N.; Alterary, S.S.; Fadhillah, F.; Kumar, R.; Al-Fatesh, A.S. Strontium-Promoted Ni-Catalyst Supported over MgO for Partial Oxidation of Methane: Unveiling a Cost-Effective Catalyst System for Fast Mitigation of Methane. Catalysts 2025, 15, 814. https://doi.org/10.3390/catal15090814
Ali FAA, Chaudhary KJ, Ibrahim AA, Alotaibi NN, Alterary SS, Fadhillah F, Kumar R, Al-Fatesh AS. Strontium-Promoted Ni-Catalyst Supported over MgO for Partial Oxidation of Methane: Unveiling a Cost-Effective Catalyst System for Fast Mitigation of Methane. Catalysts. 2025; 15(9):814. https://doi.org/10.3390/catal15090814
Chicago/Turabian StyleAli, Fekri Abdulraqeb Ahmed, Kirankumar J. Chaudhary, Ahmed A. Ibrahim, Nawaf N. Alotaibi, Seham S. Alterary, Farid Fadhillah, Rawesh Kumar, and Ahmed S. Al-Fatesh. 2025. "Strontium-Promoted Ni-Catalyst Supported over MgO for Partial Oxidation of Methane: Unveiling a Cost-Effective Catalyst System for Fast Mitigation of Methane" Catalysts 15, no. 9: 814. https://doi.org/10.3390/catal15090814
APA StyleAli, F. A. A., Chaudhary, K. J., Ibrahim, A. A., Alotaibi, N. N., Alterary, S. S., Fadhillah, F., Kumar, R., & Al-Fatesh, A. S. (2025). Strontium-Promoted Ni-Catalyst Supported over MgO for Partial Oxidation of Methane: Unveiling a Cost-Effective Catalyst System for Fast Mitigation of Methane. Catalysts, 15(9), 814. https://doi.org/10.3390/catal15090814








