Abstract
This study investigated the aerobic biodecolorization of azo dyes by Shewanella oneidensis MR-1. S. oneidensis MR-1 can rapidly degrade azo dyes under aerobic conditions, even at high concentrations of up to 270 mg/L, demonstrating remarkable dye decolorization capabilities. This decolorization efficiency persists even under high concentrations of oxygen. The introduction of different environmental metal ions led to either inhibitory or stimulatory effects on the decolorization of Methyl Orange and Amaranth. Furthermore, the addition of extracellular electron shuttles and electron scavengers confirmed that dyes were being reduced via electron transfer, and the decolorization capability of S. oneidensis MR-1 correlated with electron density. Our study unveils the rapid degradation ability of S. oneidensis MR-1 for dyes under aerobic conditions, which is closely linked to its electron transfer capacity. This research holds significant implications for a deeper understanding of the biodegradation mechanisms of azo dyes under aerobic conditions.
1. Introduction
Dye wastewater represents one of the most challenging pollution issues due to its recalcitrant nature and the presence of numerous toxic substances, severely compromising the environment essential for human existence [1]. Azo dyes, recognized as pivotal synthetic dyes, are commonly acknowledged as primary contaminants in textile effluents [2]. Owing to their robust stability and color diversity, azo dyes have found extensive application in various sectors, including textiles, food, pharmaceuticals, paper printing, and cosmetics [3]. Approximately 10% to 15% of azo dyes are discharged into wastewater during textile manufacturing processes due to inadequate utilization in industrial production [4]. Untreated azo dye effluents pose significant ecological and human health concerns owing to their recalcitrance and high toxicity if released directly into the environment [5]. In contrast to traditional chemical or physical methods, biodegradation has emerged as an environmentally friendly and cost-effective approach for dye wastewater treatment, posing no harm to the environment [6]. Biodegradation relies on the active metabolic activities of microorganisms capable of breaking down complex dye molecules into simpler metabolites, facilitating dye degradation and removal. Therefore, the biodegradation of azo dyes by microorganisms with high metabolic potential is highly desirable [7].
Several microorganisms, such as Bacillus firmus [8], Halomonas sp. strain A55 [9], and Dyella ginsengisoli LA-4 [10], have demonstrated strong dye decolorization capabilities under anaerobic conditions. S. oneidensis MR-1 is one of a kind of bacteria with an extremely diverse respiratory metabolism, exhibiting a remarkable diversity of electron acceptors [11,12]. This bacterium employs diverse respiratory strategies: aerobic respiration with oxygen (O2) as the terminal electron acceptor and anaerobic respiration using alternatives (e.g., Fe3+ or fumarate). These alternative terminal electron acceptors encompass but are not limited to, nitrate, nitrite, sulfate, azo dyes, and triphenylmethane [7,13,14]. During anaerobic dye degradation, S. oneidensis MR-1 reduces Methyl Orange (MO) and naphthol green B as electron acceptors through the Mtr respiratory pathway [15,16]. However, research on the dye decolorization capacity of S. oneidensis MR-1 has been primarily confined to anaerobic conditions and focused on individual or mixed dyes. Whether S. oneidensis MR-1 is capable of dye decolorization under aerobic conditions remains unclear. Furthermore, there are currently no reports on the biodecolorization of azo dyes by S. oneidensis MR-1 under aerobic conditions.
This study investigates the potential of S. oneidensis MR-1 to rapidly extracellularly degrade MO and Am under aerobic conditions. The impact of dye concentration, O2 levels, environmental metal ions, excess electron shuttles, and electron scavengers on the reduction of MO and Amaranth (Am) by S. oneidensis MR-1 is explored. Additionally, the aerobic reduction mechanism of MO and Am by S. oneidensis MR-1 is investigated. This discovery provides critical insights for a deeper understanding of the biodegradation mechanisms of azo dyes under aerobic conditions. Furthermore, this research aids in the development of more-efficient biodegradation technologies and enhances their applicability in bioremediation.
2. Results and Discussion
2.1. Decolorization of MO and Am by S. oneidensis MR-1
Dye wastewater in practical scenarios often occurs in aerobic environments. Nevertheless, studies on the aerobic decolorization of dyes by exoelectrogenic bacteria have been notably limited. Thus, this study delves into the aerobic decolorization capabilities of S. oneidensis MR-1 towards MO and Am. As illustrated in Figure 1, 90 mg/L solutions of MO and Am turned colorless after incubating with S. oneidensis MR-1 for 60 and 90 min, respectively. Furthermore, the UV–visible spectra (250–700 nm) of MO and Am were analyzed. After decolorization, the absorption peaks at 465 nm (Figure 1A) and 520 nm (Figure 1B) for MO and Am, respectively, vanished. The disappearance of these peaks at 465 nm and 520 nm reflects the cleavage of conjugated azo bonds in MO and Am [17,18], potentially causing a blue shift in the UV absorption spectrum. Additionally, the presence of sodium lactate, glucose, and yeast extract in the culture medium, serving as electron donors, facilitated dye decolorization (Figure S1). Figure S1 shows that S. oneidensis MR-1 completely lost dye degradation capability in the absence of lactate or glucose, demonstrating their essential role in activating the aerobic degradation pathway. Specifically, lactate and glucose sustain S. oneidensis MR-1 metabolism, supporting its pollutant degradation under stress. The rapid decolorization observed (60–90 min) contrasts with reported anaerobic degradation rates for similar dyes (typically hours to days) [16], highlighting a potential advantage of aerobic metabolism in accelerating dye breakdown.
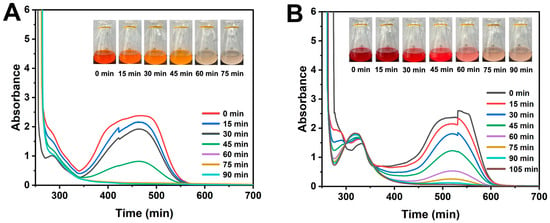
Figure 1.
Evolution of UV–vis spectra of decolorization of MO (A) and Am (B) at different times by S. oneidensis MR-1 cells under aerobic conditions. The concentrations of both MO and Am were 90 mg/L.
Previous studies on dye degradation by S. oneidensis MR-1 have predominantly focused on anaerobic or electrochemically assisted conditions [19,20], in which extracellular electron transfer (EET) pathways play a crucial role. However, the mechanisms of aerobic decolorization remain less understood. The findings demonstrate that S. oneidensis MR-1 can effectively degrade azo dyes even in fully aerobic environments, suggesting the involvement of alternative enzymatic pathways, such as azoreductases or laccases, which are known to function under oxygen-rich conditions [21]. This expands the potential applications of S. oneidensis MR-1 beyond traditional bioelectrochemical systems, making it suitable for conventional aerobic wastewater treatment. Notably, this discovery underscores the potential of harnessing microbial activity to achieve rapid and efficient degradation of environmental contaminants in natural settings. These findings unequivocally demonstrate that S. oneidensis MR-1 is capable of azo dye decolorization under aerobic conditions. The reaction products of decolorization may align with previous research outcomes [7,18].
2.2. Effect of Initial Dye Concentration and O2 Levels on MO and Am Decolorization
Due to the toxicity of high concentrations of azo dyes and their degradation products, the initial dye concentration in the culture medium may significantly impact dye decolorization [22]. Therefore, the decolorization efficiency of MO and Am at different initial concentrations was investigated. As shown in Figure 2A,B, with increasing initial dye concentrations, decolorization efficiency gradually decreased. Notably, when the concentrations of MO and Am exceeded 150 mg/L, the decolorization efficiency significantly decreased. This phenomenon suggests that S. oneidensis MR-1 cells require more time to adapt to the stress induced by high concentrations of MO and Am. Nevertheless, S. oneidensis MR-1 cells exhibited a high decolorization capability even under concentrations as high as 270 mg/L, achieving a decolorization efficiency of 96.8% after 160 min of incubation. These results signify the high tolerance of S. oneidensis MR-1 towards MO and Am dyes and their degradation products. Furthermore, heat-treatment studies were conducted to investigate the contribution of bacterial biofilm adsorption to the total dye decolorization. According to the results (Figure 2A,B), no dye degradation was observed in the heat-treated systems, indicating that azo dyes could not be degraded without the active biological system, and the decolorization of azo dyes was not attributable to physical adsorption by the biofilm. It is noteworthy that the decolorization efficiency surpasses that reported for most dye-degrading microorganisms [16,18,23,24]. Control experiments with heat-inactivated bacterial cells, incubated for 160 min, showed no dye degradation (Figure 2A,B), confirming the lack of reducing capability in dead bacterial cells. These results provided conclusive evidence that the decolorization of dyes by S. oneidensis MR-1 is primarily attributed to biological degradation rather than to adsorption [25]. This performance surpasses that of many reported dye-degrading strains (e.g., Pseudomonas sp., ~77% decolorization at 240 mg/L after 44 h [26]; Escherichia coli, ~75% at 20 mg/L under anaerobic conditions [27]). The exceptional tolerance of S. oneidensis MR-1 might stem from its unique respiratory versatility, including cytochrome c-mediated electron shuttling and robust stress response systems [28]. Notably, its ability to maintain high efficiency under aerobic conditions (unlike most exoelectrogens) positions it as a promising candidate for industrial wastewater treatment. The new culture medium used in this study has a perfect degradation ability when combined with S. oneidensis MR-1 (Figure 2A,B), surpassing existing biodegradation systems (Table 1).
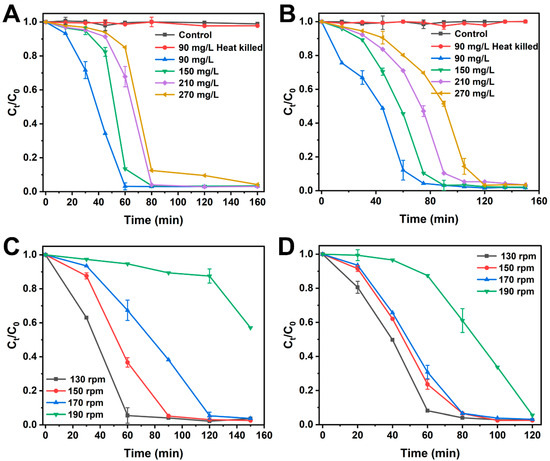
Figure 2.
Effect of operating parameters on MO and Am decolorization by S. oneidensis MR-1. (A) Initial MO dye concentration; (B) initial Am dye concentration; effect of different O2 concentrations on MO (C) and Am (D) degradation. Error bars represent the data range for quintuplicate cultures. For points lacking apparent error bars, the bars were smaller than the points.

Table 1.
The dye degradation efficiency of S. oneidensis MR-1 compared with that of other reported biological systems.
Previous studies have shown that most biodegradation of dyes occurs under anaerobic conditions, illustrating that O2 is a key factor affecting dye decolorization [9,10]. It is evident that the presence of O2 leverages the electrons released by S. oneidensis MR-1, thereby inhibiting dye degradation. However, in this study, the effect of oxygen was eliminated using a specially prepared medium, and the effect of different oxygen concentrations on dye degradation was tested. As depicted in Figure 2C,D, S. oneidensis MR-1 exhibited decolorization capabilities at different shaker speeds. Notably, for both MO and Am, the maximum decolorization efficiency was attained at 130 rpm. For MO degradation, the decolorization efficiency gradually decreased with an increase in O2 concentration. S. oneidensis MR-1 was still able to decolorize MO at 190 rpm, but a longer adaptation period of 120 min was required. This might be attributed to the preference of bacteria for growth at higher shaking speeds [30]. Similarly, the degradation of Am as a function of O2 concentration had a similar effect to that of MO, but the range of decolorization efficiency of Am at 130–170 rpm was smaller than that of MO. Furthermore, at 190 rpm, S. oneidensis MR-1 exhibited a shorter adaptation period of 40 min for Am degradation, possibly indicating that Am possesses a stronger electron-accepting capability than MO. These results underscore the robust decolorization capability of S. oneidensis MR-1 even under high O2 concentrations.
2.3. Effect of Metal Ions on MO and Am Decolorization
It is noteworthy that wastewater often contains various metal ions, such as Cd2+ and Fe3+, which have the potential to impact aerobic decolorization by microorganisms [31]. In this study, the effect of metal ions within the ranges of 1–4 mM Fe3+ and 0.2–0.8 mM Cd2+ on the decolorization of 90 mg/L dyes was investigated. In this context, when no other metal ions were added, approximately 98.5% of MO and 96.9% of Am were degraded within 90 min (Figure 3A,B). The addition of Fe3+ had a minor enhancing effect on MO decolorization but did not significantly promote Am decolorization, indicating that Fe3+ has a small promotion effect on the dye degradation performance of S. oneidensis MR-1. Conversely, the presence of Cd2+ markedly inhibited dye decolorization, with higher Cd2+ concentrations resulting in longer decolorization times (Figure 3C,D). For MO degradation, the addition of Cd2+ led to a sharp decrease in decolorization efficiency.
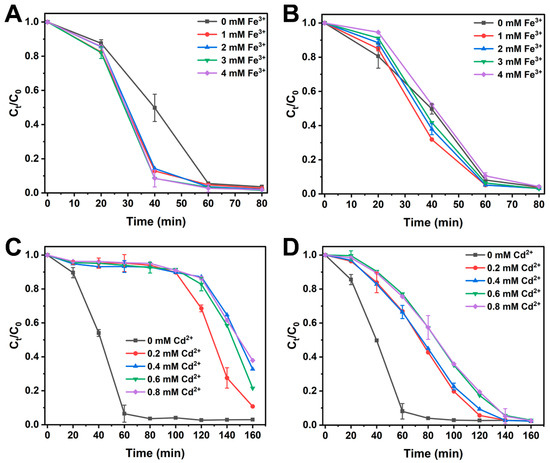
Figure 3.
Effect of the dosed Fe3+ on the MO (A) and Am (B) reduction by S. oneidensis MR-1. Effect of the dosed Cd2+ on the MO (C) and Am (D) reduction by S. oneidensis MR-1.
Interestingly, S. oneidensis MR-1 was still capable of degrading MO even in the presence of varying Cd2+ concentrations, but a longer adaptation period of 100 min was required (Figure 3C). This might be attributed to the cytotoxicity of the heavy metal Cd2+, leading to reduced activity of S. oneidensis MR-1 [32]. As illustrated in Figure 3C, Cd2+ exerted a similar impact on MO decolorization within 140 min when Cd2+ concentrations exceeded 0.4 mM, indicating that high Cd2+ concentrations surpassed the bacterial tolerance. Evidently, the presence of Cd2+ also reduced the decolorization efficiency of Am, albeit with a weaker inhibitory effect than that for MO (Figure 3D). For Am, Cd2+ merely extended the decolorization time without altering the adaptation period of S. oneidensis MR-1 (20 min) compared with the control without Cd2+. Interestingly, at a Cd2+ concentration of 0.8 mM, MO achieved a decolorization efficiency of 62.2% within 160 min, while Am was completely degraded. This indicates that S. oneidensis MR-1 retains strong dye degradation capabilities even under conditions of high concentration of heavy metal ions. The metal tolerance exhibited here surpasses that of many reported dye-degrading bacteria (e.g., Escherichia coli showing growth inhibition at Cd2+) [33], highlighting S. oneidensis MR-1’s unique suitability for complex wastewater. This advantage may stem from its facultative anaerobic metabolism allowing the flexible use of alternative electron acceptors when metal-disrupted aerobic respiration occurs [28]. In summary, the overall results suggest that the decolorization ability and electron transfer pathways of S. oneidensis MR-1 under aerobic conditions may be subject to inhibition by heavy metal ions. However, this inhibition does not impede the complete degradation of dyes. Effective strategies to mitigate or diminish the inhibitory effects of metal ions on bacterial dye degradation require further investigation.
2.4. Effect of Riboflavin and Electron Scavenger on MO and Am Decolorization
Numerous studies have confirmed that S. oneidensis MR-1 can secrete flavin as an electron shuttle, expediting the reduction of extracellular electron acceptors [16]. Therefore, experiments with additional riboflavin dosages were conducted to investigate whether extracellular electron mediators could enhance the decolorization of azo dyes by S. oneidensis MR-1. As depicted in Figure 4A,B, the addition of riboflavin significantly accelerated the biodegradation of MO and Am. With increasing riboflavin concentration, decolorization rates improved. When 0.8 μM riboflavin was added, the removal efficiency of MO increased 2.6-fold after 15 min of incubation and 3.8-fold after 25 min (Figure 4A). In alignment with MO, the Am degradation exhibited similar effects, increasing 1.8-fold after 15 min and 3.4-fold after 25 min (Figure 4B). Since riboflavin is primarily reduced in the outer membrane via the Mtr pathway [34] and serves as an electron shuttle between the cell surface and extracellular electron acceptors, it expedites dye decolorization. Consequently, the reduction of dyes is attributed to electrons released from the cell surface rather than to specific enzymes. These results indicate that MO and Am primarily decolorize extracellularly. Thus, dyes can be directly reduced by the outer membrane cytochromes MtrC/OmcA or indirectly via riboflavin serving as an electron shuttle. In theory, electron acceptors with higher redox potentials are more likely to acquire electrons from the cell surface. However, despite O2 having a higher redox potential than MO and Am, the reduction of these dyes was not inhibited by O2. This suggests that lactate and glucose in combination promote S. oneidensis MR-1 to exhibit potent reduction reactions. Furthermore, the sulfonic groups at the para-position of azo bonds in MO and Am act as strong electron-withdrawing groups through resonance, facilitating electron transfer to the dyes [35]. Therefore, the selectivity of S. oneidensis MR-1 for extracellular electron acceptors is not only related to redox potentials but may also be related to other factors such as molecular structure and absorbance [36].
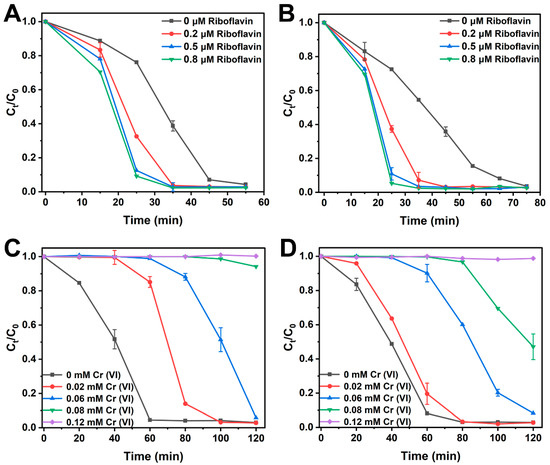
Figure 4.
Effect of the dosed riboflavin on the MO (A) and Am (B) decolorization by S. oneidensis. Effect of the dosed electron scavenger on the MO (C) and Am (D) decolorization by S. oneidensis MR-1.
To further corroborate that the degradation of dyes is mediated by S. oneidensis MR-1 through the release of extracellular electrons, the electron scavenger Cr(VI) was introduced into the solution. Cr(VI) is widely used in photocatalytic research as an electron scavenger and has been employed to study electron transfer processes [37]. Results from the electron scavenger study (Figure 4C,D) indicate that, with increasing Cr(VI) concentration, the degradation efficiency of MO and Am was significantly inhibited, suggesting that the presence of Cr(VI) disrupts the interaction between extracellular electrons released by S. oneidensis MR-1 and the dyes. Electrons from S. oneidensis MR-1 were entirely quenched by 0.4 mM Cr(VI), leading to no observable degradation of MO and Am. Notably, as seen in Figure 4C,D, MO decolorization was more sensitive to Cr(VI) than that of Am, implying that Am requires fewer electrons for decolorization. This result indicates that the decolorization of MO and Am is mediated by the extracellular electron reduction by bacteria rather than by the potential involvement of azoreductases.
2.5. Proposed Mechanism
Research indicates that the Mtr respiratory pathway, composed of c-type cytochromes CymA, MtrA, MtrB, and MtrC/OmcA, is a vital transmembrane electron transfer conduit in dissimilatory metal-reducing bacteria, such as S. oneidensis MR-1 [38]. Through this pathway, S. oneidensis MR-1 can utilize extracellular electron acceptors for anaerobic growth. Recently, it has been discovered that the Mtr pathway is responsible for the reduction of azo dyes rather than potential azoreductases [15]. Similarly, the results are consistent with those studied in this work (Figure S2). Therefore, it is interesting to know whether the Mtr pathway also plays a role in the MO and Am decolorization or not. To elucidate the MO and Am reduction mechanisms by S. oneidensis under aerobic conditions, the decolorization abilities of S. oneidensis MR-1 wild type and Mtr pathway mutants were investigated. The MO and Am removal efficiencies obtained by Mtr mutants were significantly lower than those obtained by the wild-type strain (Figure S3). The mutant without MtrA, CymA, or MtrC/OmcA showed lower decolorization efficiency for MO (2.3%, 36.1%, and 5.1%) and Am (0%, 18.5%, and 0%) after 75 min incubation than that of the wide type. Because the CymA complex exists on the inner membrane surface, this result suggests that MO and Am should have been decolorized by extracellular reduction. This implies the potential presence of additional electron transfer pathways, independent of Mtr-CymA, which may play a role in dye degradation.
Additionally, prior studies have suggested that the decolorization of dyes by S. oneidensis MR-1 occurs under anaerobic conditions, excluding the involvement of O2 [16]. Therefore, based on the aforementioned results, we hypothesize that under aerobic conditions, the biodecolorization of MO and Am by S. oneidensis MR-1 might also proceed through the Mtr pathway (Figure 5). The inner membrane c-type cytochrome CymA within S. oneidensis MR-1 is indispensable. During the decolorization of MO and Am, electrons from the inner membrane quinone pool are relayed to the Mtr respiratory pathway through CymA. MO and Am can be directly reduced via MtrC/OmcA or indirectly through flavins. In this cultivation system, even in the presence of O2, S. oneidensis MR-1 is still prompted to transfer electrons to the dyes, resulting in their decolorization. Furthermore, it is plausible that the combined action of lactate and glucose may stimulate the unique degradation pathways of S. oneidensis MR-1. For instance, electrons from CymA could also be transferred through other conduits composed of MtrE and DmsF (a homolog of MtrB) traversing the outer membrane [34]. In summary, under aerobic conditions, the exact pathways through which electrons are transmitted during azo dye reduction remain to be further investigated.
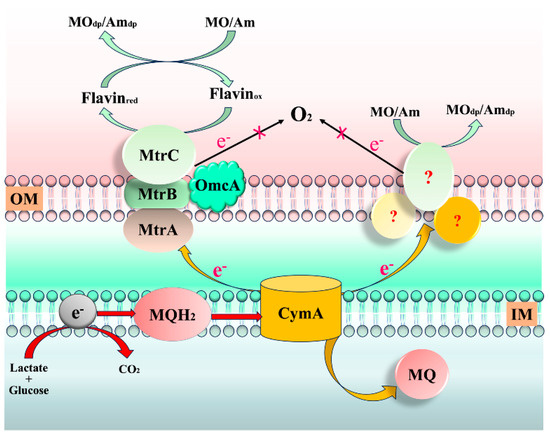
Figure 5.
Proposed aerobic degradation mechanisms of MO and Am by S. oneidensis MR-1. OM: outer membrane; IM: inner membrane; MO/Amdp: MO or Am degradation products. Arrows represent the pathway of electron flow.
3. Materials and Methods
3.1. Bacterial Strain and Cultivation
The wild-type S. oneidensis MR-1 strain utilized in this study was kindly provided by Professor Yangchun Yong from Jiangsu University [39]. Additionally, the S. oneidensis MR-1 Mtr respiratory pathway mutant strains used in this study were kindly provided by Prof. Hanqing Yu from the University of Science and Technology of China [15]. The strain was authenticated and cultivated in Luria–Bertani (LB) medium under aerobic conditions at 30 °C until reaching a stable phase.
3.2. Decolorization Medium Composition and Dye Assessment
Bacterial cells cultivated in LB were harvested by centrifugation at 8500 rpm for 3 min, for subsequent aerobic decolorization experiments using MO and Am. The medium employed for aerobic dye decolorization experiments consisted of 0.3 g/L sodium lactate, 0.3 g/L glucose, 10 g/L Na2HPO4·12H2O, 3 g/L KH2PO4, 0.5 g/L NaCl, and 0.2 g/L yeast extract. Cultures were inoculated into 100 mL conical flasks containing 50 mL of decolorization medium, and 90 mg/L of either MO or Am was added. Decolorization studies were conducted at 30 °C in a shaker (130 rpm). The initial optical density of the bacterial culture was adjusted to 1 at 600 nm using a UV–visible spectrophotometer (Shimadzu, UV2600, Kyoto City, Japan). Additionally, UV–visible spectrophotometry was employed to measure the absorbance values of MO (465 nm) and Am (520 nm). Decolorization efficiency was calculated according to the following formula:
where C0 refers to the initial absorbance at 464 nm and 520 nm, and Ct refers to the absorbance measured in the degradation. Each experiment was carried out in quintuplicate.
Decolorization efficiency (%) = 100 × (1 − Ct/C0)
3.3. Experimental Design for Biological Decolorization
The impact of various operational parameters, including dye concentration (90, 150, 210, and 270 mg/L), O2 concentration (130 rpm, 150 rpm, 170 rpm, and 190 rpm), and environmental metal ions (Fe3+ and Cd2+), on dye decolorization was systematically investigated. Furthermore, the effect of extracellular electron shuttles on the decolorization of azo dyes by S. oneidensis MR-1 was assessed by supplementing the culture medium with different concentrations of riboflavin (0, 0.2, 0.5, 0.8, and 1 μM) and the electron scavenger Cr(VI) (0, 0.02, 0.06, 0.08, and 0.4 mM). The addition of varying concentrations of proteinase K (0, 20, 30, and 40 μg/mL) was employed to evaluate the impact of degrading enzymes on dye degradation. This allowed for a comprehensive examination of the role of extracellular electron shuttling in the dye degradation process. Moreover, the potential aerobic decolorization mechanism was elucidated using S. oneidensis MR-1 as the model organism.
4. Conclusions
The ability of S. oneidensis MR-1 to decolorize azo dyes under aerobic conditions was studied for the first time. Remarkably, under the presence of O2, S. oneidensis MR-1 achieved complete degradation of both MO and Am at concentrations as high as 270 mg/L within a mere 160 min. The addition of Fe3+ and Cd2+ promoted and inhibited the decolorization ability of S. oneidensis MR-1, respectively. The addition of extracellular electron shuttles significantly accelerated decolorization, while the incorporation of extracellular electron scavengers distinctly hindered the process. These findings underscore that S. oneidensis MR-1 can rapidly decolorize dyes under aerobic conditions, with the decolorization capacity intricately linked to electricity production rather than to the hypothesized terminal reductases. This research holds the potential to enhance our understanding of the biodegradation mechanisms of azo dyes under aerobic conditions. Furthermore, as this degradation process occurs under aerobic conditions, it has the potential to offer substantial cost savings for wastewater treatment.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal15080796/s1: Figure S1: Effect of different electron donors in decolorization medium (DM) on MO (A) and Am (B) decolorization; Figure S2: Effect of proteinase K in MO (A) and Am (B) degradation; Figure S3: (A) Difference in MO decolorization efficiencies by S. oneidensis MR-1 and Mtr pathway mutants. (B) Difference in Am decolorization efficiencies by S. oneidensis MR-1 and Mtr pathway mutants.
Author Contributions
Y.W.: Conceptualization, Validation, Software, Writing—original draft. Y.L.: Validation, Data curation, Formal analysis, Writing—review and editing. L.C.: Validation, Supervision. D.C. and X.Z. (Xiaojun Zhang): Validation, Formal analysis, Writing—review and editing. M.Z. and X.Z. (Xianchun Zong): Resources, Methodology, Writing—review and editing. The manuscript was written through the contributions of all the authors. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities (grant number 2572020DP07 and 2572020DR08), the Doctoral Research Launch Fund Project of Mudanjiang Normal University (MNUB202411 and MNUB202410), and the Basic Research Fees of Universities in Heilongjiang Province, China (grant number 1454QN021 and 1454QN020).
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors thank Yangchun Yong at the Jiangsu University for providing the S. oneidensis MR-1 strain. And the authors thank Hanqing Yu at the University of Science and Technology of China for providing S. oneidensis MR-1 Mtr respiratory pathway mutant strains.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Solayman, H.M.; Hossen, M.A.; Aziz, A.A.; Yahya, N.Y.; Leong, K.H.; Sim, L.C.; Monir, M.U.; Zoh, K.-D. Performance evaluation of dye wastewater treatment technologies: A review. J. Environ. Chem. Eng. 2023, 11, 109610. [Google Scholar] [CrossRef]
- Maljaei, A.; Arami, M.; Mahmoodi, N.M. Decolorization and aromatic ring degradation of colored textile wastewater using indirect electrochemical oxidation method. Desalination 2009, 249, 1074–1078. [Google Scholar] [CrossRef]
- Guo, G.; Li, X.; Tian, F.; Liu, T.; Yang, F.; Ding, K.; Liu, C.; Chen, J.; Wang, C. Azo dye decolorization by a halotolerant consortium under microaerophilic conditions. Chemosphere 2020, 244, 125510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Fan, J.; Li, W.; Lens, P.N.L.; Shi, W. Low salinity enhances azo dyes degradation in aerobic granular sludge systems: Performance and mechanism analysis. Bioresour. Technol. 2023, 372, 128678. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; An, X.; Li, H.; Lai, F.; Yuan, E.; Xia, X.; Zhang, Q. Detoxification of azo dye Direct Black G by thermophilic Anoxybacillus sp. PDR2 and its application potential in bioremediation. Ecotox. Environ. Safe. 2021, 214, 112084. [Google Scholar] [CrossRef]
- Oturkar, C.C.; Nemade, H.N.; Mulik, P.M.; Patole, M.S.; Hawaldar, R.R.; Gawai, K.R. Mechanistic investigation of decolorization and degradation of Reactive Red 120 by Bacillus lentus BI377. Bioresour. Technol. 2011, 102, 758–764. [Google Scholar] [CrossRef]
- Cai, P.-J.; Xiao, X.; He, Y.-R.; Li, W.-W.; Chu, J.; Wu, C.; He, M.-X.; Zhang, Z.; Sheng, G.-P.; Lam, M.H.-W.; et al. Anaerobic biodecolorization mechanism of methyl orange by Shewanella oneidensis MR-1. Appl. Microbiol. Biotechnol. 2012, 93, 1769–1776. [Google Scholar] [CrossRef]
- Ogugbue, C.J.; Morad, N.; Sawidis, T.; Oranusi, N.A. Decolorization and partial mineralization of a polyazo dye by Bacillus firmus immobilized within tubular polymeric gel. 3 Biotech 2012, 2, 67–78. [Google Scholar] [CrossRef]
- Guadie, A.; Gessesse, A.; Xia, S. Halomonas sp. strain A55, a novel dye decolorizing bacterium from dye-uncontaminated Rift Valley Soda lake. Chemosphere 2018, 206, 59–69. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, J.; Jia, Y.; Chen, J. Biodecolorization of Acid Red GR by a newly isolated Dyella ginsengisoli LA-4 using response surface methodology. J. Hazard. Mater. 2010, 181, 602–608. [Google Scholar] [CrossRef]
- Hau, H.H.; Gralnick, J.A. Ecology and Biotechnology of the Genus Shewanella. Annu. Rev. Microbiol. 2007, 61, 237–258. [Google Scholar] [CrossRef]
- Yang, C.; Aslan, H.; Zhang, P.; Zhu, S.; Xiao, Y.; Chen, L.; Khan, N.; Boesen, T.; Wang, Y.; Liu, Y.; et al. Carbon dots-fed Shewanella oneidensis MR-1 for bioelectricity enhancement. Nat. Commun. 2020, 11, 1379. [Google Scholar] [CrossRef]
- Liang, Z.; Shen, N.; Huang, H.; Chen, Y. Evaluating decolorization capacity about alginate encapsulation system of Shewanella oneidensis MR-1 mingled with conductive materials. Environ. Technol. Innov. 2021, 21, 101344. [Google Scholar] [CrossRef]
- Chen, C.H.; Chang, C.F.; Liu, S.M. Partial degradation mechanisms of malachite green and methyl violet B by Shewanella decolorationis NTOU1 under anaerobic conditions. J. Hazard. Mater. 2010, 177, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Xu, C.-C.; Wu, Y.-M.; Cai, P.-J.; Li, W.-W.; Du, D.-L.; Yu, H.-Q. Biodecolorization of Naphthol Green B dye by Shewanella oneidensis MR-1 under anaerobic conditions. Bioresour. Technol. 2012, 110, 86–90. [Google Scholar] [CrossRef]
- Cao, D.-M.; Xiao, X.; Wu, Y.-M.; Ma, X.-B.; Wang, M.-N.; Wu, Y.-Y.; Du, D.-L. Role of electricity production in the anaerobic decolorization of dye mixture by exoelectrogenic bacterium Shewanella oneidensis MR-1. Bioresour. Technol. 2013, 136, 176–181. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, J.; Meng, X.; Fu, S.Q.; Wang, J.; Jin, R.; Lv, H. Decolorization of azo dyes by marine Shewanella strains under saline conditions. Appl. Microbiol. Biotechnol. 2013, 97, 4187–4197. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Berghahn, E.; Ilha, V.; Granada, C.E. Biodegradation potential of Citrobacter cultures for the removal of amaranth and congo red azo dyes. Int. J. Environ. Sci. Technol. 2019, 16, 6863–6872. [Google Scholar] [CrossRef]
- Wu, Y.; Xiao, X.; Xu, C.; Cao, D.; Du, D. Decolorization and detoxification of a sulfonated triphenylmethane dye aniline blue by Shewanella oneidensis MR-1 under anaerobic conditions. Appl. Microbiol. Biotechnol. 2013, 97, 7439–7446. [Google Scholar] [CrossRef]
- Xiao, X.; Li, T.-T.; Lu, X.-R.; Feng, X.-L.; Han, X.; Li, W.-W.; Li, Q.; Yu, H.-Q. A simple method for assaying anaerobic biodegradation of dyes. Bioresour. Technol. 2018, 251, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Banerjee, A.; Chakraborty, N.; Soren, K.; Chakraborty, P.; Bandopadhyay, R. Structural-functional analyses of textile dye degrading azoreductase, laccase and peroxidase: A comparative in silico study. Electron. J. Biotechnol. 2020, 43, 48–54. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Ferreira, L.F.R.; Hussain, C.M.; Mulla, S.I.; Bharagava, R.N. Degradation mechanism and toxicity reduction of methyl orange dye by a newly isolated bacterium Pseudomonas aeruginosa MZ520730. J. Water Process Eng. 2021, 43, 102300. [Google Scholar] [CrossRef]
- Fu, L.; Bai, Y.-N.; Lu, Y.-Z.; Ding, J.; Zhou, D.; Zeng, R.J. Degradation of organic pollutants by anaerobic methane-oxidizing microorganisms using methyl orange as example. J. Hazard. Mater. 2019, 364, 264–271. [Google Scholar] [CrossRef]
- Huang, S.; Tang, J.; Liu, X.; Dong, G.; Zhou, S. Fast Light-Driven Biodecolorization by a Geobacter sulfurreducens–CdS Biohybrid. ACS Sustain. Chem. Eng. 2019, 7, 15427–15433. [Google Scholar] [CrossRef]
- Asad, S.; Amoozegar, M.A.; Pourbabaee, A.A.; Sarbolouki, M.N.; Dastgheib, S.M.M. Decolorization of textile azo dyes by newly isolated halophilic and halotolerant bacteria. Bioresour. Technol. 2007, 98, 2082–2088. [Google Scholar] [CrossRef] [PubMed]
- Ambika; Kumar, V.; Jamwal, A.; Kumar, V.; Singh, D. Green bioprocess for degradation of synthetic dyes mixture using consortium of laccase-producing bacteria from Himalayan niches. J. Environ. Manag. 2022, 310, 114764. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, W.; Lv, Z.; Liu, J.; Zhang, W.; Zhou, J.; Xin, F.; Ma, J.; Jiang, M. Surface Display of Bacterial Laccase CotA on Escherichia coli Cells and its Application in Industrial Dye Decolorization. Mol. Biotechnol. 2018, 60, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Qi, Y.; Xu, C.; Yang, Y.; Wang, J. Transcriptome and metabolome responses of Shewanella oneidensis MR-1 to methyl orange under microaerophilic and aerobic conditions. Appl. Microbiol. Biotechnol. 2017, 101, 3463–3472. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Zhang, F.; Li, J.; Li, D.-B.; Liu, D.-F.; Li, W.-W.; Yu, H.-Q. Exclusive Extracellular Bioreduction of Methyl Orange by Azo Reductase-Free Geobacter sulfurreducens. Environ. Sci. Technol. 2017, 51, 8616–8623. [Google Scholar] [CrossRef] [PubMed]
- Montero-Lobato, Z.; Ramos-Merchante, A.; Fuentes, J.L.; Sayago, A.; Fernández-Recamales, Á.; Martínez-Espinosa, R.M.; Vega, J.M.; Vílchez, C.; Garbayo, I. Optimization of Growth and Carotenoid Production by Haloferax mediterranei Using Response Surface Methodology. Mar. Drugs 2018, 16, 372. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Singla, S.; Sharma, M.; Singh, D.P.; Prasad, R.; Thakur, V.K.; Singh, J. Kinetic Study of the Biodegradation of Acephate by Indigenous Soil Bacterial Isolates in the Presence of Humic Acid and Metal Ions. Biomolecules 2020, 10, 433. [Google Scholar] [CrossRef]
- Cui, D.; Wang, J.; Wang, H.; Yang, Y.; Zhao, M. The cytotoxicity of endogenous CdS and Cd2+ ions during CdS NPs biosynthesis. J. Hazard. Mater. 2021, 409, 124485. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Bai, L.; Wang, J.; Zhao, N.; Cui, D.; Zhao, M. Low-Toxicity Self-Photosensitized Biohybrid Systems for Enhanced Light-Driven H2 Production. Int. J. Mol. Sci. 2024, 25, 3085. [Google Scholar] [CrossRef]
- Coursolle, D.; Baron, D.B.; Bond, D.R.; Gralnick, J.A. The Mtr Respiratory Pathway Is Essential for Reducing Flavins and Electrodes in Shewanella oneidensis. J. Bacteriol. 2010, 192, 467–474. [Google Scholar] [CrossRef]
- Hsueh, C.-C.; Chen, B.-Y. Comparative study on reaction selectivity of azo dye decolorization by Pseudomonas luteola. J. Hazard. Mater. 2007, 141, 842–849. [Google Scholar] [CrossRef]
- Shi, Z.; Zachara, J.M.; Shi, L.; Wang, Z.; Moore, D.A.; Kennedy, D.W.; Fredrickson, J.K. Redox Reactions of Reduced Flavin Mononucleotide (FMN), Riboflavin (RBF), and Anthraquinone-2,6-disulfonate (AQDS) with Ferrihydrite and Lepidocrocite. Environ. Sci. Technol. 2012, 46, 11644–11652. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Ng, T.W.; An, T.; Li, G.; Li, Y.; Yip, H.Y.; Zhao, H.; Lu, A.; Wong, P.-K. A Recyclable Mineral Catalyst for Visible-Light-Driven Photocatalytic Inactivation of Bacteria: Natural Magnetic Sphalerite. Environ. Sci. Technol. 2013, 47, 11166–11173. [Google Scholar] [CrossRef] [PubMed]
- Hartshorne, R.S.; Reardon, C.L.; Ross, D.; Nuester, J.; Clarke, T.A.; Gates, A.J.; Mills, P.C.; Fredrickson, J.K.; Zachara, J.M.; Shi, L.; et al. Characterization of an electron conduit between bacteria and the extracellular environment. Proc. Natl. Acad. Sci. USA 2009, 106, 22169–22174. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Wang, Y.Z.; Fang, Z.; Shi, Y.T.; Cheng, Q.W.; Chen, Y.X.; Shi, W.D.; Yong, Y.C. Single cell electron collectors for highly efficient wiring-up electronic abiotic/biotic interfaces. Nat. Commun. 2020, 11, 4087. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).