Synthesis of Niacin from 3-Cyanopyridine with Recombinant Escherichia coli Carrying afnitA Nitrilase in a Deep Eutectic Solvent System
Abstract
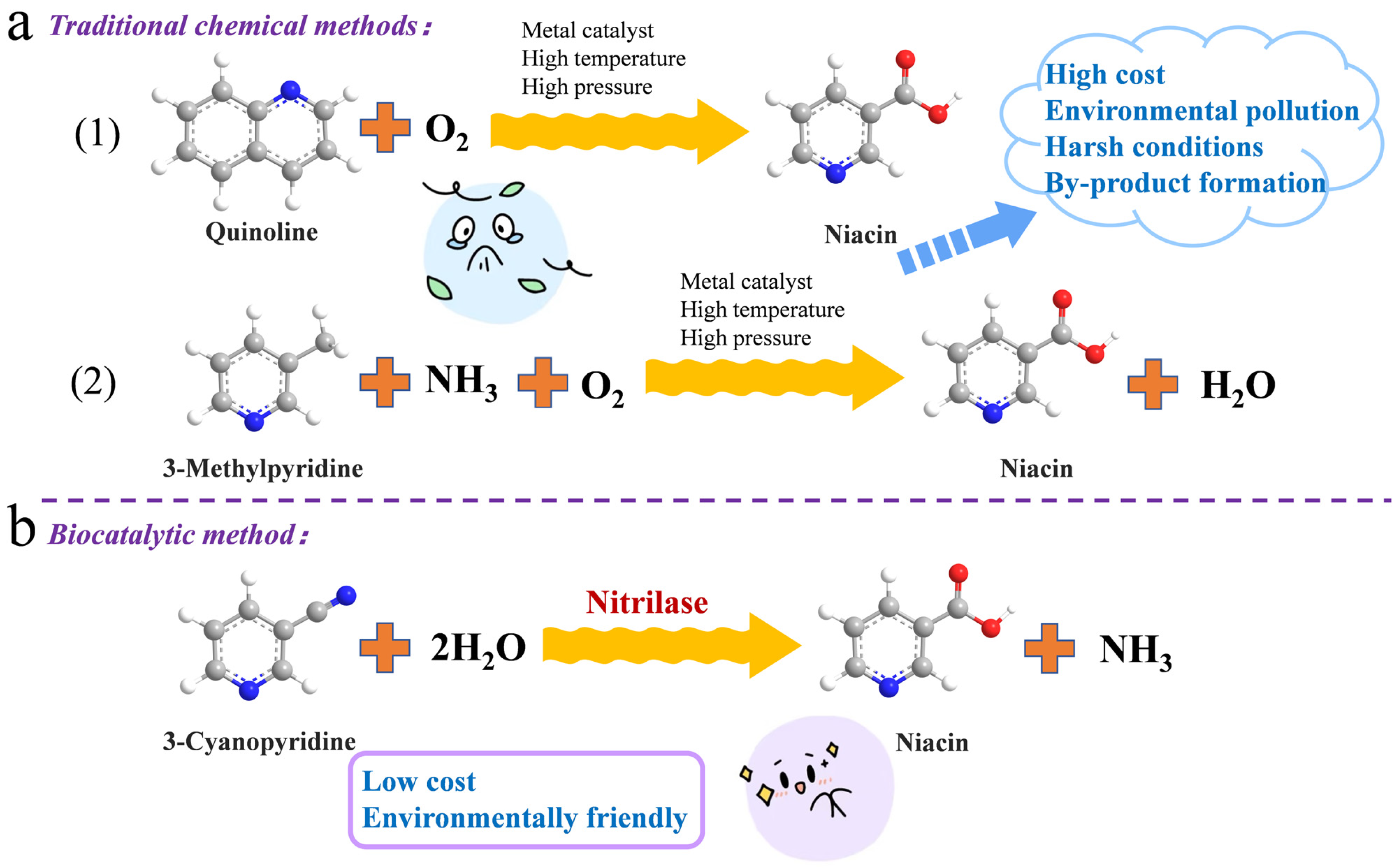
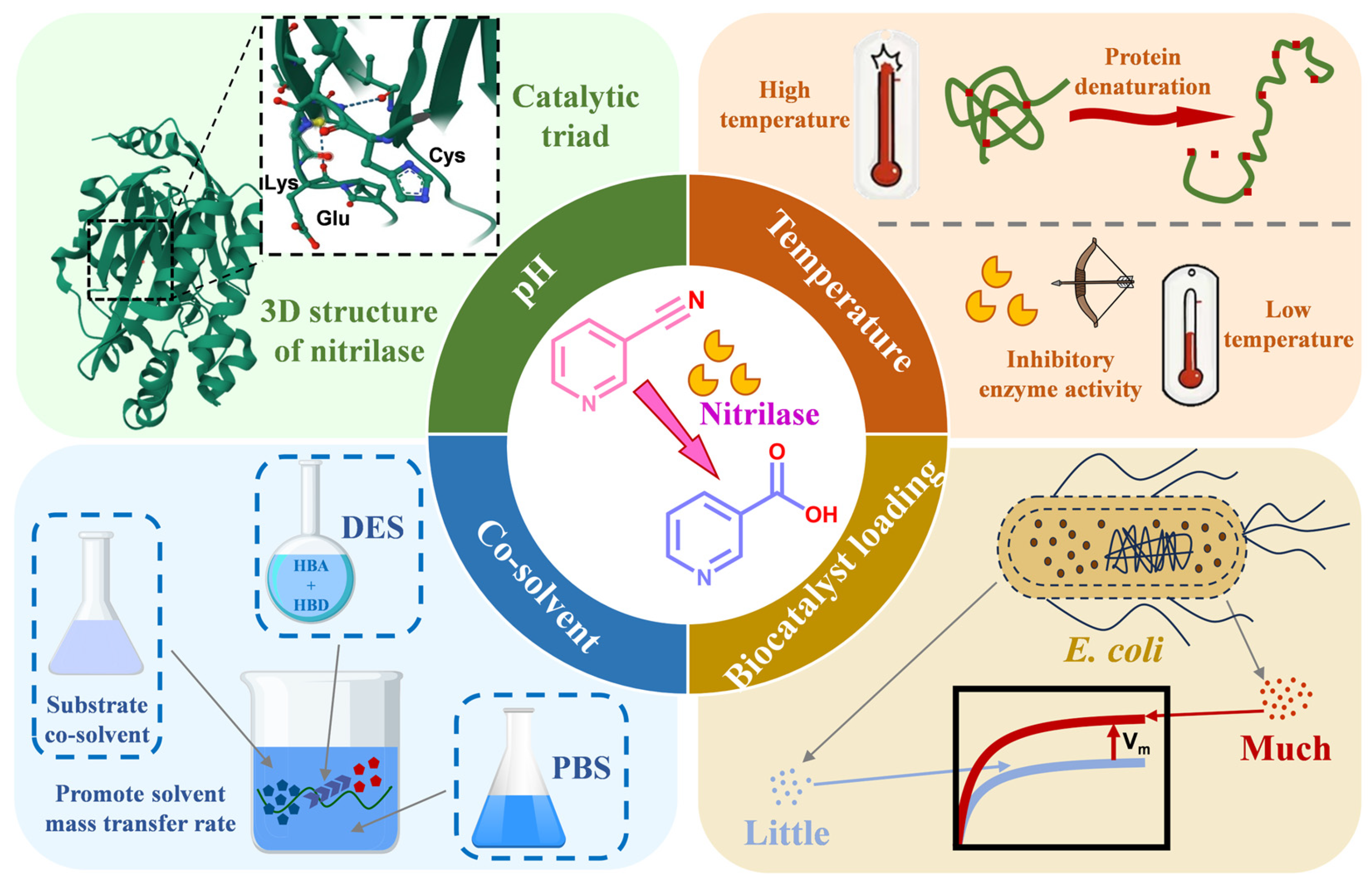
1. Introduction
2. Results and Discussion
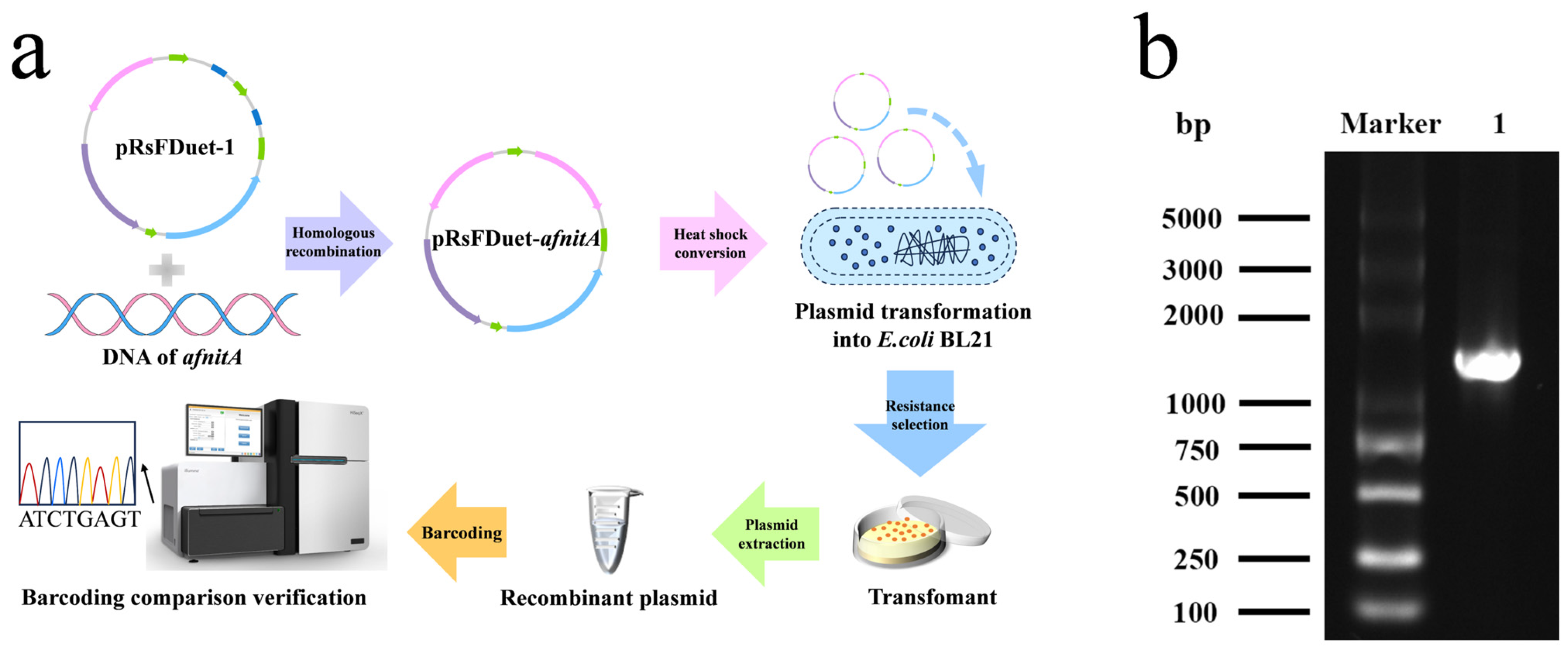
2.1. Construction of Recombinant E. coli Expressing Nitrilase AfnitA
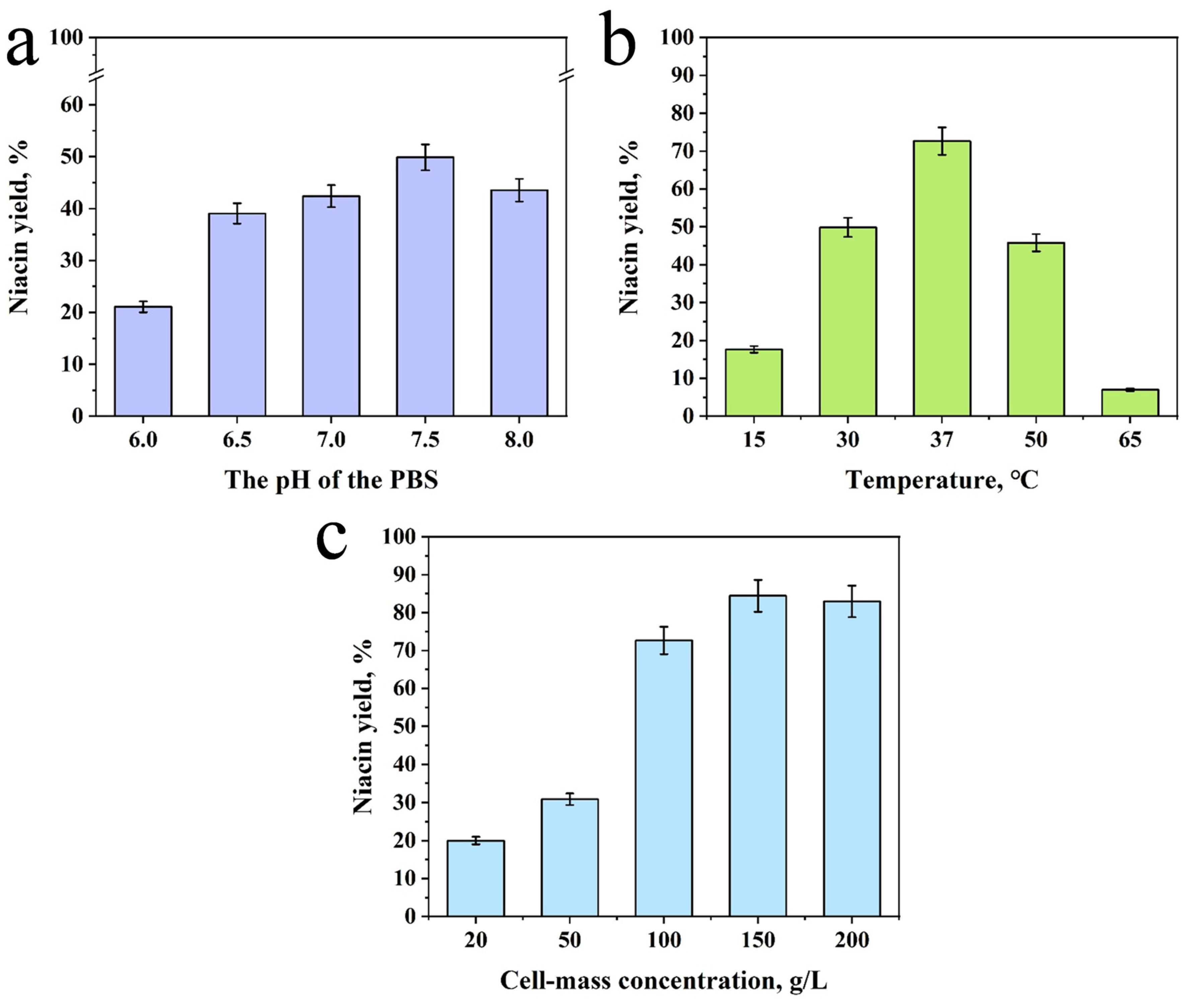
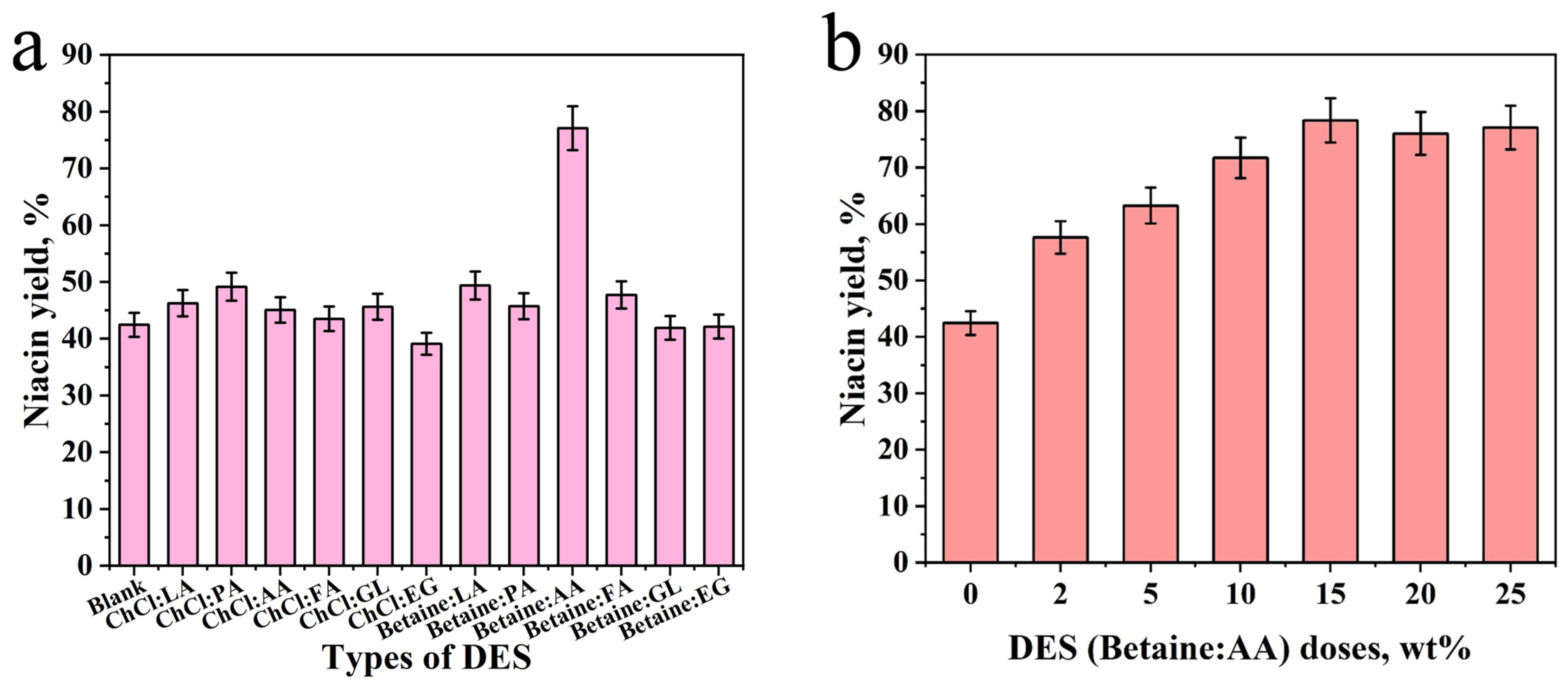
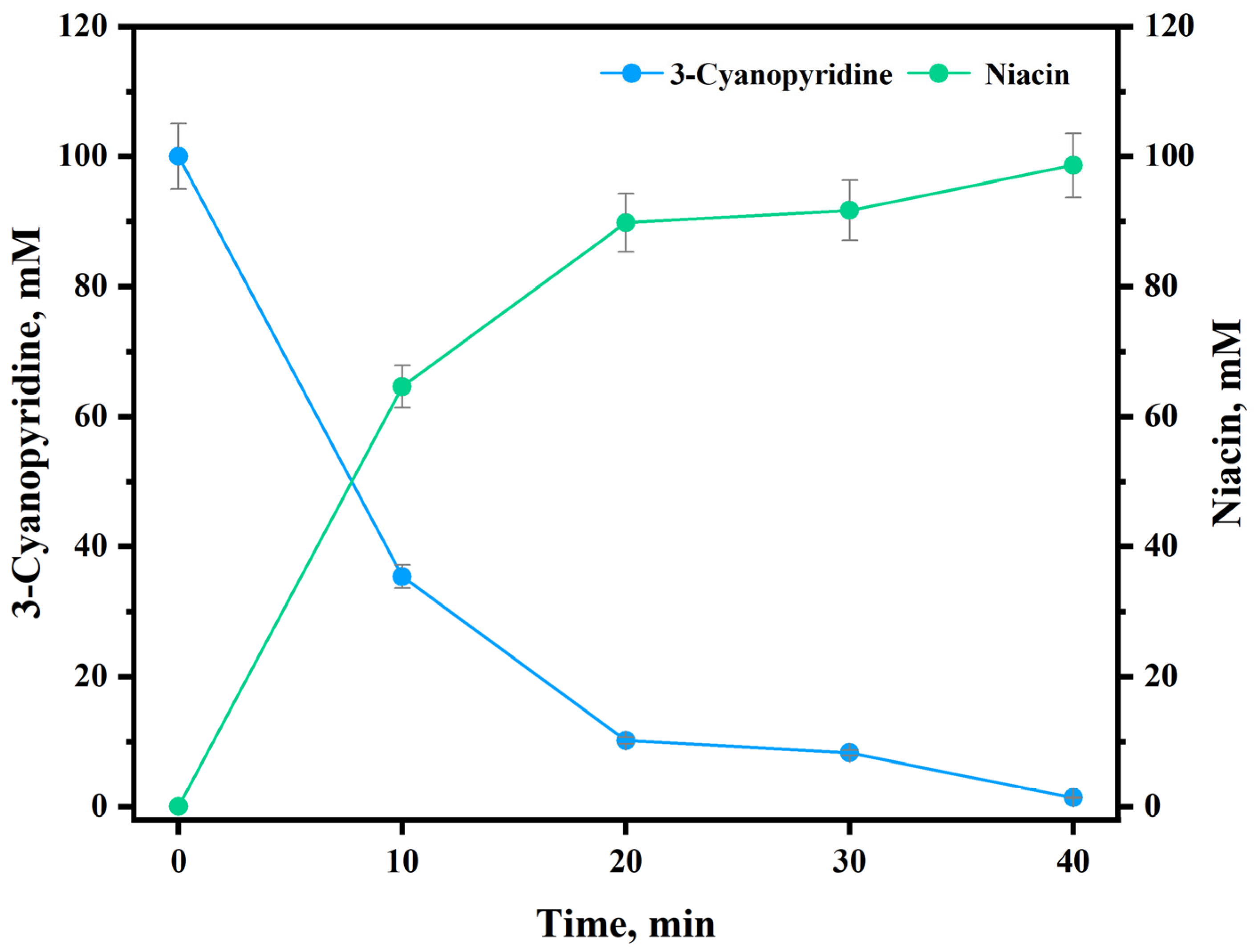
2.2. Whole-Cell Biocatalysis by Nitrilase Through Single Factor Analysis
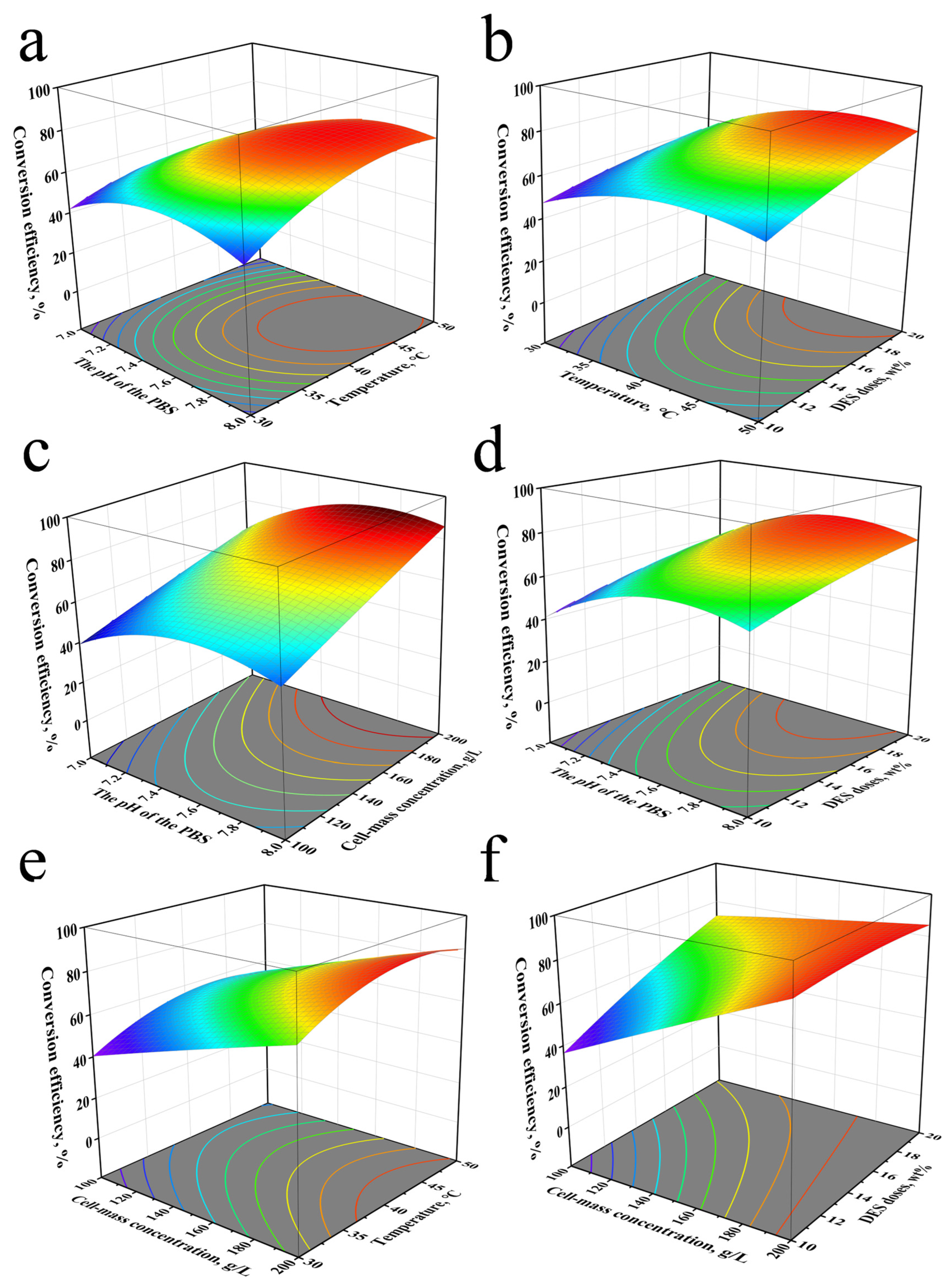
2.3. Optimization of the Nitrilase-Mediated Reaction via Response Surface Analysis
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of the DES
3.3. Construction and Culture of Nitrilase AfnitA Engineering Strain
3.4. Single-Factor Analysis
3.5. Box–Behnken Design Analysis
3.6. Analytical Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gasperi, V.; Sibilano, M.; Savini, I.; Catani, M.V. Niacin in the central nervous system: An update of biological aspects and clinical applications. Int. J. Mol. Sci. 2019, 20, 974. [Google Scholar] [CrossRef]
- Campbell, J.M. Supplementation with NAD+ and its precursors to prevent cognitive decline across disease contexts. Nutrients 2022, 14, 3231. [Google Scholar] [CrossRef] [PubMed]
- Wuerch, E.; Urgoiti, G.R.; Yong, V.W. The promise of Niacin in neurology. Neurotherapeutics 2023, 20, 1037–1054. [Google Scholar] [CrossRef]
- Ferrell, M.; Wang, Z.; Anderson, J.T.; Li, X.S.; Witkowski, M.; DiDonato, J.A.; Hilser, J.R.; Hartiala, J.A.; Haghikia, A.; Cajka, T.; et al. A terminal metabolite of niacin promotes vascular inflammation and contributes to cardiovascular disease risk. Nat. Med. 2024, 30, 424–434. [Google Scholar] [CrossRef]
- Cardoso-Lezama, I.; Fuentes-Figueroa, M.; Ramos-Tovar, E.; Márquez-Quiroga, L.V.; Ortiz-Fernández, A.; Vargas-Pozada, E.E.; Arellanes-Robledo, J.; Tsutsumi, V.; Muriel, P. Nicotinic acid attenuates experimental non-alcoholic steatohepatitis by inhibiting the NLRP3 inflammasome/pyroptosis pathway. Biochem. Pharmacol. 2023, 216, 115762. [Google Scholar] [CrossRef]
- Harris, E. High Niacin levels may raise Cardiovascular disease risk. J. Am. Med. Assoc. 2024, 331, 1001. [Google Scholar] [CrossRef]
- Lisicki, D.; Nowak, K.; Orlińska, B. Methods to produce nicotinic acid with potential industrial applications. Materials 2022, 15, 765. [Google Scholar] [CrossRef] [PubMed]
- Monika; Sheetal; Thakur, N.; Chand Bhalla, T. Biotransformation of 3-cyanopyridine to nicotinic acid using whole-cell nitrilase of Gordonia terrae mutant MN12. Bioprocess Biosyst. Eng. 2023, 46, 195–206. [Google Scholar] [CrossRef]
- Raja, R.; Thomas, J.M.; Greenhill-Hooper, M.; Ley, S.V.; Almeida Paz, F.A. Facile, one-step production of niacin (vitamin B3) and other nitrogen-containing pharmaceutical chemicals with a single-site heterogeneous catalyst. Chemistry 2008, 14, 2340–2348. [Google Scholar] [CrossRef]
- Dai, A.D.; Wu, Z.M.; Zheng, R.C.; Zheng, Y.G. Constitutive expression of nitrilase from Rhodococcus zopfii for efficient biosynthesis of 2-chloronicotinic acid. 3 Biotech 2022, 12, 50. [Google Scholar] [CrossRef]
- Martínková, L.; Rucká, L.; Nešvera, J.; Pátek, M. Recent advances and challenges in the heterologous production of microbial nitrilases for biocatalytic applications. World J. Microbiol. Biotechnol. 2017, 33, 8. [Google Scholar] [CrossRef]
- Shen, J.D.; Cai, X.; Liu, Z.Q.; Zheng, Y.G. Nitrilase: A promising biocatalyst in industrial applications for green chemistry. Crit. Rev. Biotechnol. 2021, 41, 72–93. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, J.; Jiang, S.; Wei, D. Recent research advancements on regioselective nitrilase: Fundamental and applicative aspects. Appl. Microbiol. Biotechnol. 2019, 103, 6393–6405. [Google Scholar] [CrossRef]
- Di, J.; Li, Q.; Ma, C.; He, Y.-C. An efficient and sustainable furfurylamine production from biomass-derived furfural by a robust mutant ω-transaminase biocatalyst. Bioresour. Technol. 2023, 369, 128425. [Google Scholar] [CrossRef]
- Teepakorn, C.; Zajkoska, P.; Cwicklinski, G.; De Berardinis, V.; Zaparucha, A.; Nonglaton, G.; Anxionnaz-Minvielle, Z. Nitrilase immobilization and transposition from a micro-scale batch to a continuous process increase the nicotinic acid productivity. Biotechnol. J. 2021, 16, e2100010. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Hua, D.; Jiang, Z.; Han, X.; Xue, Y.; Zheng, Y. A integrated process for nitrilase-catalyzed asymmetric hydrolysis and easy biocatalyst recycling by introducing biocompatible biphasic system. Bioresour. Technol. 2021, 320, 124392. [Google Scholar] [CrossRef] [PubMed]
- Monika; Sheetal; Thakur, N.; Bhalla, T.C. An improved process for synthesis of nicotinic acid using hyper induced whole cell nitrilase of Gordonia terrae MTCC8139. 3 Biotech 2022, 12, 303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, C.; Ge, Y.; Meng, Q.; Zhang, Y. Constitutive secretory expression and characterization of nitrilase from Alcaligenes faecalis in Pichia pastoris for production of R-mandelic acid. Biotechnol. Appl. Biochem. 2022, 69, 587–595. [Google Scholar] [CrossRef]
- Gong, J.S.; Lu, Z.M.; Li, H.; Shi, J.S.; Zhou, Z.M.; Xu, Z.H. Nitrilases in nitrile biocatalysis: Recent progress and forthcoming research. Microb. Cell Factories 2012, 11, 142. [Google Scholar] [CrossRef]
- Lancaster, L.; Abdallah, W.; Banta, S.; Wheeldon, I. Engineering enzyme microenvironments for enhanced biocatalysis. Chem. Soc. Rev. 2018, 47, 5177–5186. [Google Scholar] [CrossRef]
- Jin, L.Q.; Guo, D.J.; Li, Z.T.; Liu, Z.Q.; Zheng, Y.G. Immobilization of nitrilase on bioinspired silica for efficient synthesis of 2-hydroxy-4-(methylthio) butanoic acid from 2-hydroxy-4-(methylthio) butanenitrile. J. Ind. Microbiol. Biotechnol. 2016, 43, 585–593. [Google Scholar] [CrossRef]
- Tang, X.L.; Li, Y.Y.; Mao, Y.; Zheng, R.C.; Zheng, Y.G. Improvement of multicatalytic properties of nitrilase from Paraburkholderia graminis for efficient biosynthesis of 2-chloronicotinic acid. Biotechnol. Bioeng. 2022, 119, 3421–3431. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Z.M.; Hao, C.L.; Tang, X.L.; Zheng, R.C.; Zheng, Y.G. Highly regio- and enantioselective synthesis of chiral intermediate for pregabalin using one-pot bienzymatic cascade of nitrilase and amidase. Appl. Microbiol. Biotechnol. 2019, 103, 5617–5626. [Google Scholar] [CrossRef]
- Xu, P.; Zheng, G.W.; Zong, M.H.; Li, N.; Lou, W.Y. Recent progress on deep eutectic solvents in biocatalysis. Bioresour. Bioprocess. 2017, 4, 34. [Google Scholar] [CrossRef]
- Ling, J.K.U.; Hadinoto, K. Deep eutectic solvent as green solvent in extraction of biological macromolecules: A review. Int. J. Mol. Sci. 2022, 23, 3381. [Google Scholar] [CrossRef]
- Cotroneo-Figueroa, V.P.; Gajardo-Parra, N.F.; López-Porfiri, P.; Leiva, Á.; Gonzalez-Miquel, M.; Garrido, J.M.; Canales, R.I. Hydrogen bond donor and alcohol chain length effect on the physicochemical properties of choline chloride based deep eutectic solvents mixed with alcohols. J. Mol. Liq. 2022, 345, 116986. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Yao, L.; Qian, M.; Li, Z.; Han, Y.; Bai, S.; Lee, M. pH-responsive switchable deep eutectic solvents to mediate pretreatment method for trace analysis of triazole fungicides in peel wastes. Food Chem. 2023, 411, 135486. [Google Scholar] [CrossRef]
- Gao, Q.; Tang, Z.; He, Y.-C. Valorization of wheat straw through enhancement of cellulose accessibility, xylan elimination and lignin removal by choline chloride:p-toluenesulfonic acid pretreatment. Int. J. Biol. Macromol. 2025, 301, 140335. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, Y.-C.; Ma, C. Elevating biotransamiantion of pineapple peel-derived furfural into furfurylamine by recombinant Escherichia coli carrying pyruvate decarboxylase and mutant ω-transaminase with low loading of amine donor. Food Biosci. 2024, 62, 105164. [Google Scholar] [CrossRef]
- Swebocki, T.; Barras, A.; Abderrahmani, A.; Haddadi, K.; Boukherroub, R. Deep eutectic solvents comprising organic acids and their application in (bio)medicine. Int. J. Mol. Sci. 2023, 24, 8492. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Z.; Tang, W.; Ma, C.; He, Y.-C. Exploration of biomass fractionation and lignin removal for enhancing enzymatic digestion of wheat-stalk through deep eutectic solvent cetyl trimethyl ammonium chloride:Lactic acid treatment. Int. J. Biol. Macromol. 2025, 306, 141460. [Google Scholar] [CrossRef] [PubMed]
- Morozova, O.V.; Vasil’eva, I.S.; Shumakovich, G.P.; Zaitseva, E.A.; Yaropolov, A.I. Deep eutectic solvents for biotechnology applications. Biochemistry 2023, 88, S150–S175. [Google Scholar] [CrossRef] [PubMed]
- Gotor-Fernández, V.; Paul, C.E. Deep eutectic solvents for redox biocatalysis. J. Biotechnol. 2019, 293, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Pätzold, M.; Siebenhaller, S.; Kara, S.; Liese, A.; Syldatk, C.; Holtmann, D. Deep eutectic solvents as efficient solvents in biocatalysis. Trends Biotechnol. 2019, 37, 943–959. [Google Scholar] [CrossRef]
- Cheng, Q.B.; Zhang, L.W. Highly efficient enzymatic preparation of Daidzein in deep eutectic solvents. Molecules 2017, 22, 186. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Jurinjak Tušek, A.; VinkoviĿ, M.; RadoševiĿ, K.; Gaurina SrĿek, V.; RadojĿiĿ RedovnikoviĿ, I. Cholinium-based deep eutectic solvents and ionic liquids for lipase-catalyzed synthesis of butyl acetate. J. Mol. Catal. B Enzym. 2015, 122, 188–198. [Google Scholar] [CrossRef]
- Zhao, H.; Baker, G.A.; Holmes, S. Protease activation in glycerol-based deep eutectic solvents. J. Mol. Catal. B Enzym. 2011, 72, 163–167. [Google Scholar] [CrossRef]
- Khodaverdian, S.; Dabirmanesh, B.; Heydari, A.; Dashtban-moghadam, E.; Khajeh, K.; Ghazi, F. Activity, stability and structure of laccase in betaine based natural deep eutectic solvents. Int. J. Biol. Macromol. 2018, 107, 2574–2579. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, C.; Li, Q.; Li, Q.; He, Y.C. Efficient chemoenzymatic valorization of biobased D-fructose into 2,5-bis(hydroxymethyl)furan with deep eutectic solvent Lactic acid:Betaine and Pseudomonas putida S12 whole cells. Bioresour. Technol. 2022, 344, 126299. [Google Scholar] [CrossRef]
- Liu, X.-J.; Ma, B.-D.; Wu, X.-M.; Xu, Y. Highly efficient biosynthesis of nicotinic acid by immobilized whole cells of E. coli expressing nitrilase in semi-continuous packed-bed bioreactor. Catalysts 2023, 13, 371. [Google Scholar] [CrossRef]
- Singh, R.; Banerjee, A.; Kaul, P.; Barse, B.; Banerjee, U.C. Release of an enantioselective nitrilase from Alcaligenes faecalis MTCC 126: A comparative study. Bioprocess Biosyst. Eng. 2005, 27, 415–424. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, H.; Zhao, D.; Liu, R.; Liu, S.; Fu, J.; Zhang, Y.; Ding, H. Review on catalytic oxidation of VOCs at ambient temperature. Int. J. Mol. Sci. 2022, 23, 13739. [Google Scholar] [CrossRef]
- He, W.; Chen, B.; Yin, B.; Ye, J.; He, Y. Efficient biosynthesis of Monacolin J through enzymatic hydrolysis using a recombinant Lovastatin hydrolase. Processes 2024, 12, 2006. [Google Scholar] [CrossRef]
- Bisirri, E.A.; Kaar, J.L.; Schwartz, D.K. High-throughput screening identifies anionic polymer supports that improve enzyme activity at low pH and high temperature. Langmuir 2025, 41, 5335–5346. [Google Scholar] [CrossRef]
- Kabir, M.F.; Ju, L.-K. On optimization of enzymatic processes: Temperature effects on activity and long-term deactivation kinetics. Process Biochem. 2023, 130, 734–746. [Google Scholar] [CrossRef]
- Prentice, E.J.; Hicks, J.; Ballerstedt, H.; Blank, L.M.; Liáng, L.N.L.; Schipper, L.A.; Arcus, V.L. The inflection point hypothesis: The relationship between the temperature dependence of enzyme-catalyzed reaction rates and microbial growth rates. Biochemistry 2020, 59, 3562–3569. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.E.; Andersen, M.B.; Kara, S. A deep eutectic solvent thermomorphic multiphasic system for biocatalytic applications. Angew. Chem.—Int. Ed. 2022, 61, e202203823. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-X.; Zhao, L.-Q.; Wang, J.; Song, G.-L.; Liu, H.-M.; Cheng, H.; Yang, Z. Improving whole-cell biocatalysis by addition of deep eutectic solvents and natural deep eutectic solvents. ACS Sustain. Chem. Eng. 2017, 5, 5713–5722. [Google Scholar] [CrossRef]
- Hamed, R.; Alnadi, S.H.; Awadallah, A. The effect of enzymes and sodium lauryl sulfate on the surface tension of dissolution media: Toward understanding the solubility and dissolution of Carvedilol. AAPS PharmSciTech 2020, 21, 146. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H.; Liu, S.; Wang, Y.; Mu, T.; Xue, Z. Kamlet–Taft parameters of deep eutectic solvents and their relationship with dissolution of main lignocellulosic components. Ind. Eng. Chem. Res. 2023, 62, 11723–11734. [Google Scholar] [CrossRef]
- Xia, Q.; Liu, Y.; Meng, J.; Cheng, W.; Chen, W.; Liu, S.; Liu, Y.; Li, J.; Yu, H. Multiple hydrogen bond coordination in three-constituent deep eutectic solvents enhances lignin fractionation from biomass. Green Chem. 2018, 20, 2711–2721. [Google Scholar] [CrossRef]
- Santos, E.D.C.; de Menezes, L.H.S.; Santos, C.S.; Santana, P.V.B.; Soares, G.A.; Tavares, I.M.C.; Freitas, J.S.; de Souza-Motta, C.M.; Bezerra, J.L.; da Costa, A.M.; et al. High-throughput screening for distinguishing nitrilases from nitrile hydratases in Aspergillus and application of a Box-Behnken design for the optimization of nitrilase. Biotechnol. Appl. Biochem. 2022, 69, 2081–2090. [Google Scholar] [CrossRef]
- Selvaraj, C.; Rudhra, O.; Alothaim, A.S.; Alkhanani, M.; Singh, S.K. Structure and chemistry of enzymatic active sites that play a role in the switch and conformation mechanism. Adv. Protein Chem. Struct. Biol. 2022, 130, 59–83. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, A.F.C.; Pacheco, F.C.; Pereira, G.Z.; Paiva, P.H.C.; Lelis, C.A.; Tribst, A.A.L.; Leite Júnior, B.R.d.C. Structural changes induced by ultrasound in proteases and their consequences on the hydrolysis of pumpkin seed proteins and the multifunctional properties of hydrolysates. Food Bioprod. Process. 2024, 144, 13–21. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, C.; Zhang, T.; He, Y.-C.; Ma, C. Efficient bio-oxidation of biomass-derived furan-2,5-dicarbaldehyde to 5-formyl-2-furoic acid and 2,5-furandicarboxylic acid via whole-cell biocatalysis. Bioresour. Technol. 2025, 421, 132201. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.Q.; Liu, Z.Q.; Xu, J.M.; Zheng, Y.G. Biosynthesis of nicotinic acid from 3-cyanopyridine by a newly isolated Fusarium proliferatum ZJB-09150. World J. Microbiol. Biotechnol. 2013, 29, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Badoei-Dalfard, A.; Karami, Z.; Ramezani-pour, N. Bench scale production of nicotinic acid using a newly isolated Stenotrophomonas maltophilia AC21 producing highly-inducible and versatile nitrilase. J. Mol. Catal. B Enzym. 2016, 133, S552–S559. [Google Scholar] [CrossRef]
- Silva Teixeira, C.S.; Sousa, S.F.; Cerqueira, N. An Unsual Cys-Glu-Lys catalytic triad is responsible for the catalytic mechanism of the nitrilase superfamily: A QM/MM study on Nit2. Chemphyschem 2021, 22, 796–804. [Google Scholar] [CrossRef]
- van der Ent, F.; Skagseth, S.; Lund, B.A.; Sočan, J.; Griese, J.J.; Brandsdal, B.O.; Åqvist, J. Computational design of the temperature optimum of an enzyme reaction. Sci. Adv. 2023, 9, eadi0963. [Google Scholar] [CrossRef]
- Bilal, M.; Rashid, E.U.; Zdarta, J.; dos Santos, J.C.S.; Fernandes, P.C.B.; Cheng, H.; Jesionowski, T. Engineering magnetic nanobiocatalytic systems with multipurpose functionalities for biocatalysis, biotechnology and bioprocess applications. Sustain. Chem. Pharm. 2022, 30, 100866. [Google Scholar] [CrossRef]
- Held, C.; Stolzke, T.; Knierbein, M.; Jaworek, M.W.; Luong, T.Q.; Winter, R.; Sadowski, G. Cosolvent and pressure effects on enzyme-catalysed hydrolysis reactions. Biophys. Chem. 2019, 252, 106209. [Google Scholar] [CrossRef]
- Fotiadou, R.; Bellou, M.G.; Spyrou, K.; Yan, F.; Rudolf, P.; Gournis, D.; Stamatis, H. Effect of deep eutectic solvents on the biocatalytic properties of β-glucosidase@ZnOFe nano-biocatalyst. Sustain. Chem. Pharm. 2022, 30, 100886. [Google Scholar] [CrossRef]
- Zhang, Z.-G.; Yi, Y.; Lin, J.-C.; Chen, D.; Ji, X.-J. One-pot two-step biocatalytic upgrading of 5-hydroxymethylfurfural to 2, 5-furandicarboxylic acid (FDCA) through a sequential oxidation process. Sustain. Chem. Pharm. 2024, 39, 101616. [Google Scholar] [CrossRef]
- Tang, Z.; Fan, B.; Tang, W.; He, Y.-C.; Ma, C. Comprehensive understanding of co-producing fermentable sugar, furfural, and xylo-oligosaccharides through the pretreatment with CTAB-based deep eutectic solvent containing Brønsted and Lewis acid. Chem. Eng. J. 2024, 488, 150637. [Google Scholar] [CrossRef]
- Froger, A.; Hall, J.E. Transformation of plasmid DNA into E. coli using the heat shock method. J. Vis. Exp. 2007, 253. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Xu, J.H.; He, Y.C.; Ouyang, L.M.; Liu, Y.Y. Cloning and biochemical properties of a highly thermostable and enantioselective nitrilase from Alcaligenes sp. ECU0401 and its potential for (R)-(−)-mandelic acid production. Bioprocess Biosyst. Eng. 2011, 34, 315–322. [Google Scholar] [CrossRef]
- Ferreira, N.; Viana, T.; Henriques, B.; Tavares, D.S.; Jacinto, J.; Colónia, J.; Pinto, J.; Pereira, E. Application of response surface methodology and box–behnken design for the optimization of mercury removal by Ulva sp. J. Hazard. Mater. 2023, 445, 130405. [Google Scholar] [CrossRef]
- Salem-Bekhit, M.M.; Riad, O.K.M.; Selim, H.; Tohamy, S.T.K.; Taha, E.I.; Al-Suwayeh, S.A.; Shazly, G.A. Box-Behnken design for assessing the efficiency of Aflatoxin M1 detoxification in milk using Lactobacillus rhamnosus and Saccharomyces cerevisiae. Life 2023, 13, 1667. [Google Scholar] [CrossRef]
- Mahmoud, B.S.; McConville, C. Box-Behnken Design of experiments of polycaprolactone nanoparticles loaded with Irinotecan hydrochloride. Pharmaceutics 2023, 15, 1271. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Ma, C.; He, Y.-C. Chemobiocatalytic transfromation of biomass into furfurylamine with mixed amine donor in an eco-friendly medium. Bioresour. Technol. 2023, 387, 129638. [Google Scholar] [CrossRef]
| Constituents | HBA/HBD Molar Ratio | Viscosity (mPa⋅S) | Surface Tension (mN/m) | K-T Parameters | ||
|---|---|---|---|---|---|---|
| π* | α | β | ||||
| ChCl:LA | 1:2 | 137.11 | 45.60 | 0.80 | 0.69 | 0.48 |
| ChCl:PA | 1:2 | 97 | 31.09 | 0.77 | 0.55 | 0.71 |
| ChCl:AA | 1:2 | 59 | 29.48 | 0.53 | 0.64 | 0.43 |
| ChCl:FA | 1:2 | 88.38 | 44.88 | 0.43 | 1.7 | 0.76 |
| ChCl:GL | 1:2 | 284.89 | 44.62 | 1.08 | 0.65 | 0.88 |
| ChCl:EG | 1:2 | 89 | 43.68 | 1.03 | 0.68 | 0.87 |
| Betaine:LA | 1:2 | 376 | 58.06 | 1.37 | 0.59 | 0.67 |
| Betaine:PA | 1:2 | 232.6 | 33.76 | 1.24 | 0.9 | 0.64 |
| Betaine:AA | 1:2 | 488 | 41.03 | 1.34 | 0.84 | 0.53 |
| Betaine:FA | 1:2 | 274.84 | 57.70 | 1.26 | 0.73 | 0.78 |
| Betaine:GL | 1:2 | 2623.22 | 49.82 | 1.06 | 0.62 | 0.97 |
| Betaine:EG | 1:2 | 104 | 51.70 | 1.03 | 0.64 | 0.51 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 6950.75 | 14 | 496.48 | 8.47 | 0.0001 |
| A (The pH of the PBS) | 768.80 | 1 | 768.80 | 13.12 | 0.0028 |
| B (Cell-mass concentration) | 2846.23 | 1 | 2846.23 | 48.57 | <0.0001 |
| C (Temperature) | 551.08 | 1 | 551.08 | 9.40 | 0.0084 |
| D (DES doses) | 1004.30 | 1 | 1004.30 | 17.14 | 0.0010 |
| AB | 25.30 | 1 | 25.30 | 0.4317 | 0.5218 |
| AC | 119.25 | 1 | 119.25 | 2.03 | 0.1756 |
| AD | 0.7656 | 1 | 0.7656 | 0.0131 | 0.9106 |
| BC | 0.0121 | 1 | 0.0121 | 0.0002 | 0.9887 |
| BD | 315.24 | 1 | 315.24 | 5.38 | 0.0360 |
| CD | 18.23 | 1 | 18.23 | 0.3111 | 0.5858 |
| A2 | 898.04 | 1 | 898.04 | 15.32 | 0.0016 |
| B2 | 1.18 | 1 | 1.18 | 0.0201 | 0.8892 |
| C2 | 561.04 | 1 | 561.04 | 9.57 | 0.0079 |
| D2 | 23.36 | 1 | 23.36 | 0.3986 | 0.5380 |
| Residual | 820.41 | 14 | 58.60 | ||
| Lack of fit | 412.27 | 10 | 41.23 | 0.4040 | 0.8882 |
| Pure error | 408.14 | 4 | 102.04 | ||
| Cor total | 7771.16 | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Fan, B.; Fan, W.; He, Y. Synthesis of Niacin from 3-Cyanopyridine with Recombinant Escherichia coli Carrying afnitA Nitrilase in a Deep Eutectic Solvent System. Catalysts 2025, 15, 794. https://doi.org/10.3390/catal15080794
Zhou J, Fan B, Fan W, He Y. Synthesis of Niacin from 3-Cyanopyridine with Recombinant Escherichia coli Carrying afnitA Nitrilase in a Deep Eutectic Solvent System. Catalysts. 2025; 15(8):794. https://doi.org/10.3390/catal15080794
Chicago/Turabian StyleZhou, Jingyi, Bo Fan, Wenyan Fan, and Yucai He. 2025. "Synthesis of Niacin from 3-Cyanopyridine with Recombinant Escherichia coli Carrying afnitA Nitrilase in a Deep Eutectic Solvent System" Catalysts 15, no. 8: 794. https://doi.org/10.3390/catal15080794
APA StyleZhou, J., Fan, B., Fan, W., & He, Y. (2025). Synthesis of Niacin from 3-Cyanopyridine with Recombinant Escherichia coli Carrying afnitA Nitrilase in a Deep Eutectic Solvent System. Catalysts, 15(8), 794. https://doi.org/10.3390/catal15080794









