ZnO-Based Photocatalysts: Synergistic Effects of Material Modifications and Machine Learning Optimization
Abstract
1. Introduction
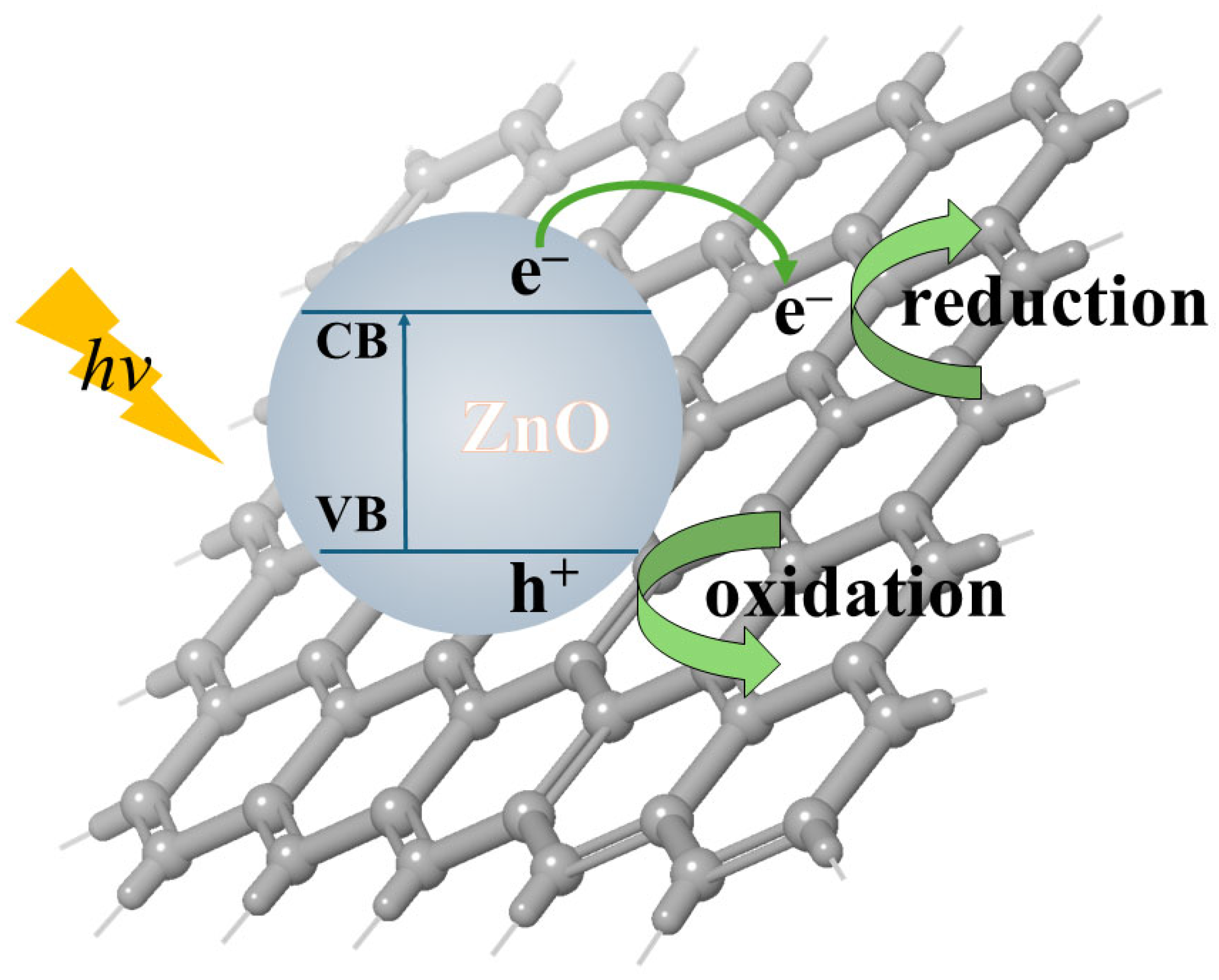
2. Fundamental Aspects of ZnO Photocatalysis
3. Strategies for ZnO Modification in Composite Photocatalysts
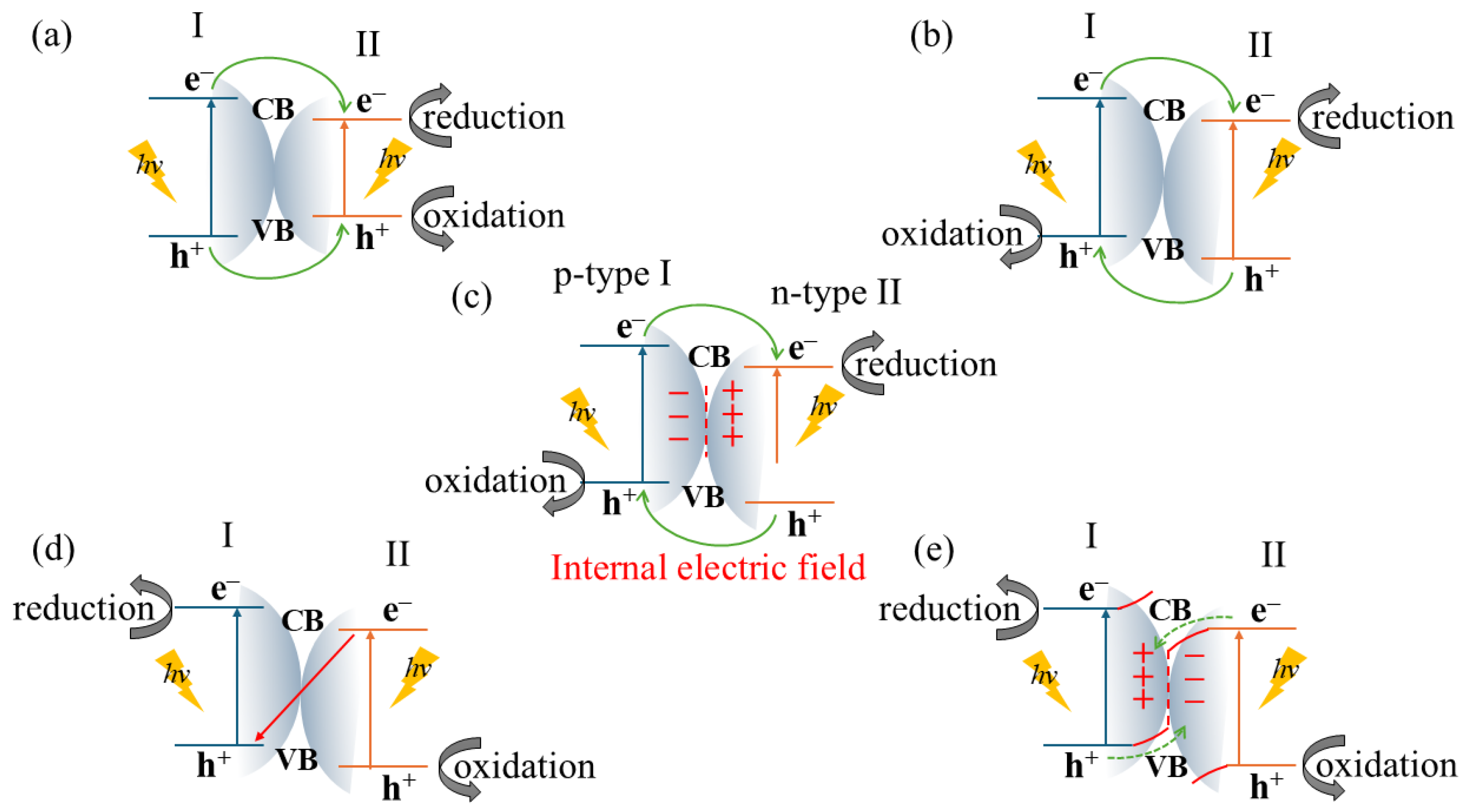
3.1. Metal–Oxide Composites
3.2. Carbon-Based Composites
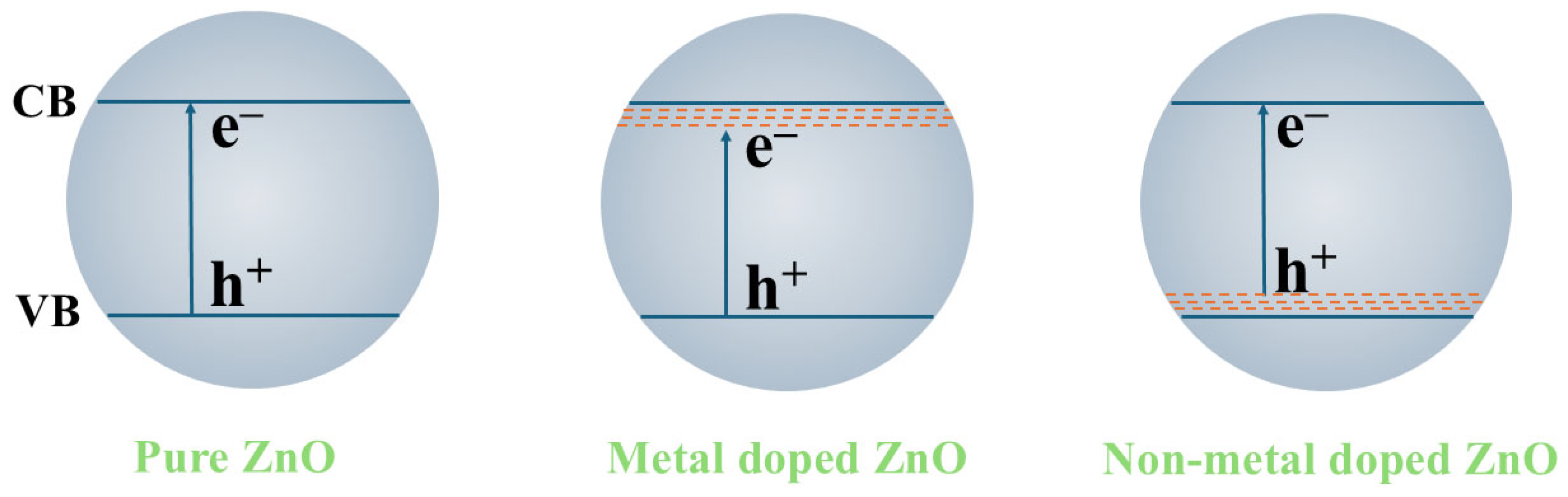
3.3. Metal and Non-Metal Doping
3.4. Metal–Organic Framework and Polymer Composites
4. Modeling of ZnO-Based Materials for Photocatalysis Based on Atomistic Calculations
4.1. Atomistic Calculations in Materials Science—Methodological Considerations
4.1.1. Overview of Atomistic Modeling Approaches
4.1.2. Molecular vs. Periodic Modeling in ZnO Systems
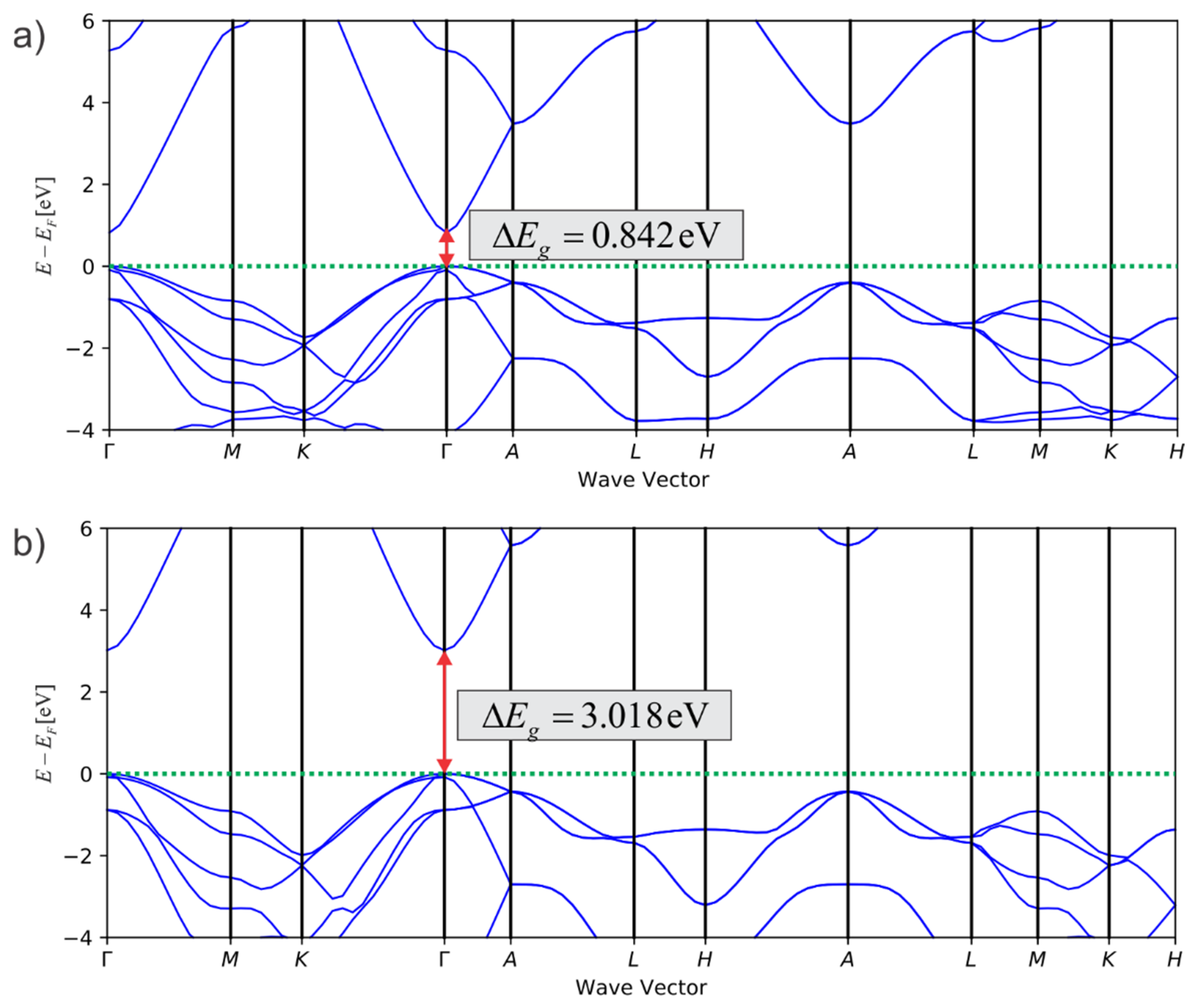
4.1.3. Selection of Density Functionals and Band Gap Corrections in DFT
4.1.4. Van Der Waals Forces and Noncovalent Interactions
4.2. Applications of Atomistic Calculations in ZnO-Based Materials for Photocatalysis
4.3. Tools for Atomistic Calculations
4.4. Examples of Studies on ZnO-Based Photocatalytic Materials Complemented by Atomistic Calculations
5. Modeling of ZnO-Based Photocatalysts for Photocatalysis Based on Machine Learning
5.1. Machine Learning Techniques in Materials Science
- Supervised learning, where models are trained on labeled data;
- Unsupervised learning, which explores hidden patterns in unlabeled data;
- Reinforcement learning, which learns through trial and error by interacting with an environment.
5.2. Machine Learning Applications in ZnO-Based Photocatalysts: From Property Prediction to Reaction Modeling
5.3. Examples of Data-Driven Predictions in Photocatalysis Using ZnO-Based Materials
6. Environmental Applications and Future Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pichat, P.; Herrmann, J.-M.; Courbon, H.; Disdier, J.; Mozzanega, M.-N. Photocatalytic Oxidation of Various Compounds over TiO2 and Other Semiconductor Oxides; Mechanistic Considerations. Can. J. Chem. Eng. 1982, 60, 27–32. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.d.M.R.; Lopes, P.R.M.; de Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial Action and Target Mechanisms of Zinc Oxide Nanoparticles against Bacterial Pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef]
- Uribe-López, M.C.; Hidalgo-López, M.C.; López-González, R.; Frías-Márquez, D.M.; Núñez-Nogueira, G.; Hernández-Castillo, D.; Alvarez-Lemus, M.A. Photocatalytic Activity of ZnO Nanoparticles and the Role of the Synthesis Method on Their Physical and Chemical Properties. J. Photochem. Photobiol. Chem. 2021, 404, 112866. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, S.; Sharma, K.; Thakur, K.; Choudhary, K.; Patial, A.; Tamana; Dhiman, V. Green Approach to La2O3/ZnO Nanocomposites Synthesis via Jatropha Curcas Latex: Implications in Photocatalysis. JCIS Open 2025, 18, 100140. [Google Scholar] [CrossRef]
- Dwidar, E.A.; Foda, F.M.; AbdEl-Aleem, E.M. Application of ZnO Nanoparticles for Wastewater Treatment and Antimicrobial Activity. Benha J. Appl. Sci. 2023, 8, 67–74. [Google Scholar] [CrossRef]
- Wahyuni, E.T.; Diantariani, N.P.; Kartini, I.; Kuncaka, A. Enhancement of the Photostability and Visible Photoactivity of ZnO Photocatalyst Used for Reduction of Cr(VI) Ions. Results Eng. 2022, 13, 100351. [Google Scholar] [CrossRef]
- Huang, M.; Lian, J.; Si, R.; Wang, L.; Pan, X.; Liu, P. Spatial Separation of Electrons and Holes among ZnO Polar {0001} and {1010} Facets for Enhanced Photocatalytic Performance. ACS Omega 2022, 7, 26844–26852. [Google Scholar] [CrossRef]
- Nazim, V.S.; El-Sayed, G.M.; Amer, S.M.; Nadim, A.H. Optimization of Metal Dopant Effect on ZnO Nanoparticles for Enhanced Visible LED Photocatalytic Degradation of Citalopram: Comparative Study and Application to Pharmaceutical Cleaning Validation. Sustain. Environ. Res. 2023, 33, 39. [Google Scholar] [CrossRef]
- Rangel, R.; Ramos-Corona, A.; Espino, J.; Quintana, P.; Bartolo-Pérez, P.; García, R. Effect of Nitrogen Doping in GO as Support in ZnO/GO-N Compounds and Their Photocatalytic Assessment to Degrade the Lignin Molecule. Catalysts 2023, 13, 69. [Google Scholar] [CrossRef]
- Mirzaeifard, Z.; Shariatinia, Z.; Jourshabani, M.; Rezaei Darvishi, S.M. ZnO Photocatalyst Revisited: Effective Photocatalytic Degradation of Emerging Contaminants Using S-Doped ZnO Nanoparticles under Visible Light Radiation. Ind. Eng. Chem. Res. 2020, 59, 15894–15911. [Google Scholar] [CrossRef]
- Alhebshi, A.; Sharaf Aldeen, E.; Mim, R.S.; Tahir, B.; Tahir, M. Recent Advances in Constructing Heterojunctions of Binary Semiconductor Photocatalysts for Visible Light Responsive CO2 Reduction to Energy Efficient Fuels: A Review. Int. J. Energy Res. 2022, 46, 5523–5584. [Google Scholar] [CrossRef]
- Ben Saber, N.; Mezni, A.; Alrooqi, A.; Altalhi, T. A Review of Ternary Nanostructures Based Noble Metal/Semiconductor for Environmental and Renewable Energy Applications. J. Mater. Res. Technol. 2020, 9, 15233–15262. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Reddy, P.A.K.; Shim, J.; Byon, C. Visible-Light-Driven Photocatalytic Activity of SnO2–ZnO Quantum Dots Anchored on g-C3N4 Nanosheets for Photocatalytic Pollutant Degradation and H2 Production. ACS Omega 2018, 3, 7587–7602. [Google Scholar] [CrossRef]
- Kassahun, S.K.; Tiruneh, S.N.; Mohammed, S.A.; Kim, H. Core Shell Fe3O4/SiO2/N-TiO2 for Photodegradation of 4-Nitrophenol under Sunlight: A Machine Learning Process Optimization. J. Water Process Eng. 2025, 72, 107639. [Google Scholar] [CrossRef]
- Dashti, A.; Navidpour, A.H.; Amirkhani, F.; Zhou, J.L.; Altaee, A. Application of Machine Learning Models to Improve the Prediction of Pesticide Photodegradation in Water by ZnO-Based Photocatalysts. Chemosphere 2024, 362, 142792. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Nancy; Singh, G.; Acevedo, R.; Singh, J. Recent Advancements in ZnO Nanoparticles: AI and Metaheuristic-Enhanced Applications in Photocatalysis, Energy Storage, and Environmental Remediation. In Metaheuristics-Based Materials Optimization; Elsevier: Amsterdam, The Netherlands, 2025; pp. 357–383. ISBN 978-0-443-29162-3. [Google Scholar]
- Zhu, C.; Wang, X. Nanomaterial ZnO Synthesis and Its Photocatalytic Applications: A Review. Nanomaterials 2025, 15, 682. [Google Scholar] [CrossRef] [PubMed]
- Baig, A.; Siddique, M.; Panchal, S. A Review of Visible-Light-Active Zinc Oxide Photocatalysts for Environmental Application. Catalysts 2025, 15, 100. [Google Scholar] [CrossRef]
- Hezam, A.; Drmosh, Q.A.; Ponnamma, D.; Bajiri, M.A.; Qamar, M.; Namratha, K.; Zare, M.; Nayan, M.B.; Onaizi, S.A.; Byrappa, K. Strategies to Enhance ZnO Photocatalyst’s Performance for Water Treatment: A Comprehensive Review. Chem. Rec. 2022, 22, e202100299. [Google Scholar] [CrossRef]
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A Review on ZnO: Fundamental Properties and Applications. Mater. Today Proc. 2022, 49, 3028–3035. [Google Scholar] [CrossRef]
- Coleman, V.A.; Jagadish, C. Basic Properties and Applications of ZnO. In Zinc Oxide Bulk, Thin Films and Nanostructures; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–20. ISBN 978-0-08-044722-3. [Google Scholar]
- Harun, K.; Salleh, N.A.; Deghfel, B.; Yaakob, M.K.; Mohamad, A.A. DFT+U Calculations for Electronic, Structural, and Optical Properties of ZnO Wurtzite Structure: A Review. Results Phys. 2020, 16, 102829. [Google Scholar] [CrossRef]
- Snedeker, L.P.; Risbud, A.S.; Masala, O.; Zhang, J.P.; Seshadri, R. Organic Phase Conversion of Bulk (Wurtzite) ZnO to Nanophase (Wurtzite and Zinc Blende) ZnO. Solid State Sci. 2005, 7, 1500–1505. [Google Scholar] [CrossRef]
- Chergui, Y. Zinc Oxide: Wurtzite Structure Properties under Isobaric and Isothermal Ensembles—A Computational Calculation. In Nanotechnology and Nanomaterials; Rovisco, A., Pimentel, A., Eds.; IntechOpen: London, UK, 2025; Volume 9, ISBN 978-0-85466-602-7. [Google Scholar]
- Zhu, Q.; Zhang, K.; Li, D.; Li, N.; Xu, J.; Bahnemann, D.W.; Wang, C. Polarization-Enhanced Photocatalytic Activity in Non-Centrosymmetric Materials Based Photocatalysis: A Review. Chem. Eng. J. 2021, 426, 131681. [Google Scholar] [CrossRef]
- Baranov, A.N.; Sokolov, P.S.; Tafeenko, V.A.; Lathe, C.; Zubavichus, Y.V.; Veligzhanin, A.A.; Chukichev, M.V.; Solozhenko, V.L. Nanocrystallinity as a Route to Metastable Phases: Rock Salt ZnO. Chem. Mater. 2013, 25, 1775–1782. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, K.; Thakur, N.; Chauhan, S.; Chauhan, M.S. The Effect of Shape and Size of ZnO Nanoparticles on Their Antimicrobial and Photocatalytic Activities: A Green Approach. Bull. Mater. Sci. 2020, 43, 20. [Google Scholar] [CrossRef]
- Borysiewicz, M.A. ZnO as a Functional Material, a Review. Crystals 2019, 9, 505. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Enaganti, P.K.; Amreen, K.; Goel, S. Integrated Temperature Controlling Platform to Synthesize ZnO Nanoparticles and Its Deposition on Al-Foil for Biosensing. IEEE Sens. J. 2021, 21, 9538–9545. [Google Scholar] [CrossRef]
- Nam, Y.; Lim, J.H.; Ko, K.C.; Lee, J.Y. Photocatalytic Activity of TiO2 Nanoparticles: A Theoretical Aspect. J. Mater. Chem. A 2019, 7, 13833–13859. [Google Scholar] [CrossRef]
- Malek, M.F.; Mamat, M.H.; Soga, T.; Rahman, S.A.; Abu Bakar, S.; Ismail, A.S.; Mohamed, R.; Alrokayan, S.A.H.; Khan, H.A.; Mahmood, M.R. Thickness-Controlled Synthesis of Vertically Aligned c-Axis Oriented ZnO Nanorod Arrays: Effect of Growth Time via Novel Dual Sonication Sol–Gel Process. Jpn. J. Appl. Phys. 2016, 55, 01AE15. [Google Scholar] [CrossRef]
- Mungchamnankit, A.; Eiamchai, P.; Chananonnawathorn, C.; Limwichean, S.; Horprathum, M.; Thongmee, A.; Sukplang, P. Effect of Annealing Temperature on ZnO Nanorods Prepared by Hydrothermal Process. Adv. Mater. Res. 2014, 979, 204–207. [Google Scholar] [CrossRef]
- Alqarni, L.S.; Alghamdi, A.M.; Elamin, N.Y.; Rajeh, A. Enhancing the Optical, Electrical, Dielectric Properties and Antimicrobial Activity of Chitosan/Gelatin Incorporated with Co-Doped ZnO Nanoparticles: Nanocomposites for Use in Energy Storage and Food Packaging. J. Mol. Struct. 2024, 1297, 137011. [Google Scholar] [CrossRef]
- Bao, R.; Wang, C.; Dong, L.; Yu, R.; Zhao, K.; Wang, Z.L.; Pan, C. Flexible and Controllable Piezo-Phototronic Pressure Mapping Sensor Matrix by ZnO NW/p-Polymer LED Array. Adv. Funct. Mater. 2015, 25, 2884–2891. [Google Scholar] [CrossRef]
- Peng, H.Y.; Li, G.P.; Ye, J.Y.; Wei, Z.P.; Zhang, Z.; Wang, D.D.; Xing, G.Z.; Wu, T. Electrode Dependence of Resistive Switching in Mn-Doped ZnO: Filamentary versus Interfacial Mechanisms. Appl. Phys. Lett. 2010, 96, 192113. [Google Scholar] [CrossRef]
- Raha, S.; Ahmaruzzaman, M.d. ZnO Nanostructured Materials and Their Potential Applications: Progress, Challenges and Perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wageh, S.; Al-Ghamdi, A.; Yu, J. New Understanding on the Different Photocatalytic Activity of Wurtzite and Zinc-Blende CdS. Appl. Catal. B Environ. 2016, 192, 101–107. [Google Scholar] [CrossRef]
- Kamenev, K.V.; Courac, A.; Sokolov, P.S.; Baranov, A.N.; Sharikov, F.Y.; Solozhenko, V.L. Heat Capacities of Nanostructured Wurtzite and Rock Salt ZnO: Challenges of ZnO Nano-Phase Diagram. Solids 2021, 2, 121–128. [Google Scholar] [CrossRef]
- Meng, L.; Vu, T.V.; Criscenti, L.J.; Ho, T.A.; Qin, Y.; Fan, H. Theoretical and Experimental Advances in High-Pressure Behaviors of Nanoparticles. Chem. Rev. 2023, 123, 10206–10257. [Google Scholar] [CrossRef]
- Leitner, J.; Kamrádek, M.; Sedmidubský, D. Thermodynamic Properties of Rock-Salt ZnO. Thermochim. Acta 2013, 572, 1–5. [Google Scholar] [CrossRef]
- Das, A.; Saini, C.P.; Singh, D.; Ahuja, R.; Kaur, A.; Aliukov, S.; Shukla, D.; Singh, F. High Temperature-Mediated Rocksalt to Wurtzite Phase Transformation in Cadmium Oxide Nanosheets and Its Theoretical Evidence. Nanoscale 2019, 11, 14802–14819. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A Comprehensive Review of ZnO Materials and Devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Wang, J.; Chen, R.; Xiang, L.; Komarneni, S. Synthesis, Properties and Applications of ZnO Nanomaterials with Oxygen Vacancies: A Review. Ceram. Int. 2018, 44, 7357–7377. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, D.; Bastia, S.; Chaudhary, Y.S. Band-Structure Tunability via the Modulation of Excitons in Semiconductor Nanostructures: Manifestation in Photocatalytic Fuel Generation. Nanoscale 2023, 15, 10939–10974. [Google Scholar] [CrossRef] [PubMed]
- Sakellaris, C.N.; Papakondylis, A.; Mavridis, A. Ab Initio Study of the Electronic Structure of Zinc Oxide and Its Ions, ZnO0,±. Ground and Excited States. J. Phys. Chem. A 2010, 114, 9333–9341. [Google Scholar] [CrossRef] [PubMed]
- Lavín, A.; Sivasamy, R.; Mosquera, E.; Morel, M.J. High Proportion ZnO/CuO Nanocomposites: Synthesis, Structural and Optical Properties, and Their Photocatalytic Behavior. Surf. Interfaces 2019, 17, 100367. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A Review of ZnO Nanoparticles as Solar Photocatalysts: Synthesis, Mechanisms and Applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Akhter, R.; Hussain, S.; Beura, S.; Uddin, S.M.N.; Maktedar, S.S. Hydrothermal Carbonization Assisted Lignocellulose Derived Formation of ZnO/Hydrochar Composites through Morphology Evolution for an Efficient Photo and Electrocatalytic Performance. New J. Chem. 2025, 49, 2453–2472. [Google Scholar] [CrossRef]
- Bhapkar, A.R.; Bhame, S. A Review on ZnO and Its Modifications for Photocatalytic Degradation of Prominent Textile Effluents: Synthesis, Mechanisms, and Future Directions. J. Environ. Chem. Eng. 2024, 12, 112553. [Google Scholar] [CrossRef]
- Dhamorikar, R.S.; Lade, V.G.; Kewalramani, P.V.; Bindwal, A.B. Review on Integrated Advanced Oxidation Processes for Water and Wastewater Treatment. J. Ind. Eng. Chem. 2024, 138, 104–122. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R.K. Zinc Oxide Based Photocatalysis: Tailoring Surface-Bulk Structure and Related Interfacial Charge Carrier Dynamics for Better Environmental Applications. RSC Adv. 2015, 5, 3306–3351. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Shi, B.-Y.; Yao, C.-B.; Wang, Z.-M.; Wang, X.; Jiang, C.-H.; Feng, L.-F.; Song, Y.-L. Size and Morphology Modulation in ZnO Nanostructures for Nonlinear Optical Applications: A Review. ACS Appl. Nano Mater. 2023, 6, 9975–10014. [Google Scholar] [CrossRef]
- Kang, Z.; Si, H.; Zhang, S.; Wu, J.; Sun, Y.; Liao, Q.; Zhang, Z.; Zhang, Y. Interface Engineering for Modulation of Charge Carrier Behavior in ZnO Photoelectrochemical Water Splitting. Adv. Funct. Mater. 2019, 29, 1808032. [Google Scholar] [CrossRef]
- Li, B.; Wang, R.; Shao, X.; Shao, L.; Zhang, B. Synergistically Enhanced Photocatalysis from Plasmonics and a Co-Catalyst in Au@ZnO–Pd Ternary Core–Shell Nanostructures. Inorg. Chem. Front. 2017, 4, 2088–2096. [Google Scholar] [CrossRef]
- Li, J.; Wu, N. Semiconductor-Based Photocatalysts and Photoelectrochemical Cells for Solar Fuel Generation: A Review. Catal. Sci. Technol. 2015, 5, 1360–1384. [Google Scholar] [CrossRef]
- Mendioroz, P.; Casoni, A.I.; Volpe, M.A.; Gerbino, D.C. Xanthone Synthesis through Catalysis: Exploring the Green Limits of Homogeneous and Heterogeneous Methods. Eur. J. Org. Chem. 2025, 28, e202401027. [Google Scholar] [CrossRef]
- Toe, C.Y.; Tsounis, C.; Zhang, J.; Masood, H.; Gunawan, D.; Scott, J.; Amal, R. Advancing Photoreforming of Organics: Highlights on Photocatalyst and System Designs for Selective Oxidation Reactions. Energy Environ. Sci. 2021, 14, 1140–1175. [Google Scholar] [CrossRef]
- Parrino, F.; Livraghi, S.; Giamello, E.; Ceccato, R.; Palmisano, L. Role of Hydroxyl, Superoxide, and Nitrate Radicals on the Fate of Bromide Ions in Photocatalytic TiO2 Suspensions. ACS Catal. 2020, 10, 7922–7931. [Google Scholar] [CrossRef]
- Satyam, S.; Patra, S. The Evolving Landscape of Advanced Oxidation Processes in Wastewater Treatment: Challenges and Recent Innovations. Processes 2025, 13, 987. [Google Scholar] [CrossRef]
- He, W.; Jia, H.; Cai, J.; Han, X.; Zheng, Z.; Wamer, W.G.; Yin, J.-J. Production of Reactive Oxygen Species and Electrons from Photoexcited ZnO and ZnS Nanoparticles: A Comparative Study for Unraveling Their Distinct Photocatalytic Activities. J. Phys. Chem. C 2016, 120, 3187–3195. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Taofeek Popoola, L.; Aderibigbe, E.I. Solar Photocatalytic Degradation of Organic Pollutants in Textile Industry Wastewater by ZnO/Pumice Composite Photocatalyst. J. Environ. Chem. Eng. 2020, 8, 103907. [Google Scholar] [CrossRef]
- Pujara, A.; Sharma, R.; Samriti; Bechelany, M.; Mishra, Y.K.; Prakash, J. Novel Zinc Oxide 3D Tetrapod Nano-Microstructures: Recent Progress in Synthesis, Modification and Tailoring of Optical Properties for Photocatalytic Applications. Mater. Adv. 2025, 6, 2123–2153. [Google Scholar] [CrossRef]
- Santhy, K.; Thorat, S.; Pukale, P.; Salot, M.; Avasthi, G.; Mandal, D.; Pramanick, A.K.; Singh, S.G.; Chaudhury, S.K. Eco-Friendly Synthesis of High-Purity Nanocrystalline ZnO Powder for Supercapacitance Applications. Ceram. Int. 2025, 51, 17657–17670. [Google Scholar] [CrossRef]
- Ning, S.; Wei, Z.; Chen, Z.; Wu, T.; Zhang, L. Curvature and Defect Formation Synergistically Promote the Photocatalysis of ZnO Slabs. Chin. Chem. Lett. 2025, 36, 111057. [Google Scholar] [CrossRef]
- Mediouni, N.; Guillard, C.; Dappozze, F.; Khrouz, L.; Parola, S.; Colbeau-Justin, C.; Amara, A.B.H.; Rhaiem, H.B.; Jaffrezic-Renault, N.; Namour, P. Impact of Structural Defects on the Photocatalytic Properties of ZnO. J. Hazard. Mater. Adv. 2022, 6, 100081. [Google Scholar] [CrossRef]
- Orlita, M.; Basko, D.M.; Zholudev, M.S.; Teppe, F.; Knap, W.; Gavrilenko, V.I.; Mikhailov, N.N.; Dvoretskii, S.A.; Neugebauer, P.; Faugeras, C.; et al. Observation of Three-Dimensional Massless Kane Fermions in a Zinc-Blende Crystal. Nat. Phys. 2014, 10, 233–238. [Google Scholar] [CrossRef]
- Uykur, E.; Li, W.; Kuntscher, C.A.; Dressel, M. Optical Signatures of Energy Gap in Correlated Dirac Fermions. Npj Quantum Mater. 2019, 4, 19. [Google Scholar] [CrossRef]
- Vazinishayan, A.; Hairi Yazdi, M.R. Correlation between Mechanical and Optical Properties of ZnO Nanowire. Optik 2021, 234, 166545. [Google Scholar] [CrossRef]
- Tüzemen, E.Ş.; Eker, S.; Kavak, H.; Esen, R. Dependence of Film Thickness on the Structural and Optical Properties of ZnO Thin Films. Appl. Surf. Sci. 2009, 255, 6195–6200. [Google Scholar] [CrossRef]
- Shingange, K.; Tshabalala, Z.P.; Dhonge, B.P.; Ntwaeaborwa, O.M.; Motaung, D.E.; Mhlongo, G.H. 0D to 3D ZnO Nanostructures and Their Luminescence, Magnetic and Sensing Properties: Influence of pH and Annealing. Mater. Res. Bull. 2017, 85, 52–63. [Google Scholar] [CrossRef]
- Ayoub, I.; Kumar, V.; Abolhassani, R.; Sehgal, R.; Sharma, V.; Sehgal, R.; Swart, H.C.; Mishra, Y.K. Advances in ZnO: Manipulation of Defects for Enhancing Their Technological Potentials. Nanotechnol. Rev. 2022, 11, 575–619. [Google Scholar] [CrossRef]
- Zheng, J.; Siegel, R.W.; Toney, C.G. Polymer Crystalline Structure and Morphology Changes in Nylon-6/ZnO Nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 1033–1050. [Google Scholar] [CrossRef]
- McCluskey, M.D.; Jokela, S.J. Defects in ZnO. J. Appl. Phys. 2009, 106, 071101. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Mahmoud, S.A.; Mohamed, A.A. Advances in Metal Oxide Composites for Sustainable Environmental Remediation. Inorg. Chem. Commun. 2025, 179, 114641. [Google Scholar] [CrossRef]
- Yang, G.; Yan, Z.; Xiao, T. Preparation and Characterization of SnO2/ZnO/TiO2 Composite Semiconductor with Enhanced Photocatalytic Activity. Appl. Surf. Sci. 2012, 258, 8704–8712. [Google Scholar] [CrossRef]
- Chiang, Y.-J.; Lin, C.-C. Photocatalytic Decolorization of Methylene Blue in Aqueous Solutions Using Coupled ZnO/SnO2 Photocatalysts. Powder Technol. 2013, 246, 137–143. [Google Scholar] [CrossRef]
- Ghamarpoor, R.; Fallah, A.; Jamshidi, M. A Review of Synthesis Methods, Modifications, and Mechanisms of ZnO/TiO2-Based Photocatalysts for Photodegradation of Contaminants. ACS Omega 2024, 9, 25457–25492. [Google Scholar] [CrossRef] [PubMed]
- Çırak, B.B.; Caglar, B.; Kılınç, T.; Morkoç Karadeniz, S.; Erdoğan, Y.; Kılıç, S.; Kahveci, E.; Ercan Ekinci, A.; Çırak, Ç. Synthesis and Characterization of ZnO Nanorice Decorated TiO2 Nanotubes for Enhanced Photocatalytic Activity. Mater. Res. Bull. 2019, 109, 160–167. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Ghollasimood, S.; Ahmadpour, N.; Homaeigohar, S. Biosynthesis of the ZnO/SnO2 Nanoparticles and Characterization of Their Photocatalytic Potential for Removal of Organic Water Pollutants. J. Photochem. Photobiol. Chem. 2022, 425, 113662. [Google Scholar] [CrossRef]
- Belay, H.; Abebe, B.; Tsegaye, D.; Ravikumar, C.R.; Reddy, S.G.; Murthy, H.C.A. Chemistry of Iron and Copper Co-Doped Zinc Oxide: Reduction and Degradation of Pollutants. Catal. Sci. Technol. 2023, 13, 5005–5016. [Google Scholar] [CrossRef]
- Wang, R.; Hao, Q.; Feng, J.; Wang, G.-C.; Ding, H.; Chen, D.; Ni, B. Enhanced Separation of Photogenerated Charge Carriers and Catalytic Properties of ZnO-MnO2 Composites by Microwave and Photothermal Effect. J. Alloys Compd. 2019, 786, 418–427. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Wang, C.; Ni, H.; Dai, J.; Liu, T.; Wu, Z.; Chen, X.; Dong, Z.; Qian, J.; Wu, Z. Comparison of Highly Active Type-I and Type-II Heterojunction Photocatalytic Composites Synthesized by Electrospinning for Humic Acid Degradation. Chem. Phys. Lett. 2022, 803, 139815. [Google Scholar] [CrossRef]
- Das, A.; Patra, M.; Kumar, P.M.; Bhagavathiachari, M.; Nair, R.G. Role of Type II Heterojunction in ZnO–In2O3 Nanodiscs for Enhanced Visible-Light Photocatalysis through the Synergy of Effective Charge Carrier Separation and Charge Transport. Mater. Chem. Phys. 2021, 263, 124431. [Google Scholar] [CrossRef]
- Shabbir, A.; Sardar, S.; Mumtaz, A. Mechanistic Investigations of Emerging Type-II, Z-Scheme and S-Scheme Heterojunctions for Photocatalytic Applications—A Review. J. Alloys Compd. 2024, 1003, 175683. [Google Scholar] [CrossRef]
- Vo, N.T.T.; You, S.-J.; Pham, M.-T.; Pham, V.V. A Green Synthesis Approach of P-n CuO/ZnO Junctions for Multifunctional Photocatalysis towards the Degradation of Contaminants. Environ. Technol. Innov. 2023, 32, 103285. [Google Scholar] [CrossRef]
- Zhu, L.; Li, H.; Xia, P.; Liu, Z.; Xiong, D. Hierarchical ZnO Decorated with CeO2 Nanoparticles as the Direct Z-Scheme Heterojunction for Enhanced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2018, 10, 39679–39687. [Google Scholar] [CrossRef]
- Jiang, Z.; Cheng, B.; Zhang, L.; Zhang, Z.; Bie, C. A Review on ZnO-Based S-Scheme Heterojunction Photocatalysts. Chin. J. Catal. 2023, 52, 32–49. [Google Scholar] [CrossRef]
- Tomić, J.; Malinović, N. Titanium Dioxide Photocatalyst: Present Situation and Future Approaches. AIDASCO Rev. 2023, 1, 26–30. [Google Scholar] [CrossRef]
- Yildirim, F.; Canpolat, N.; Aydogan, S.; Yilmaz, M. Investigating the Synergistic Effect of ZnO/GO Nanocomposite Films for Methylene Blue Removal. J. Am. Ceram. Soc. 2024, 107, 6091–6107. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, Z.; Zhang, M.; Qin, Z.; Cao, S.; Zhong, F.; Li, S.; Duan, H.M.; Zhang, J. Improved Gas-Sensitive Properties by a Heterojunction of Hollow Porous Carbon Microtubes Derived from Sycamore Fibers. ACS Sustain. Chem. Eng. 2021, 9, 14345–14352. [Google Scholar] [CrossRef]
- Tyagi, S.; Kumar Singh, P.; Kumar Tiwari, A.; Pain, P. Optimization and Comparison of Photovoltaic Parameters of Zinc Oxide (ZnO)/Graphene Oxide (GO) and Zinc Oxide (ZnO)/Carbon Quantum Dots (CQDs) Hybrid Solar Cell Using Firefly Algorithm for Application in Solar Trigeneration System in Commercial Buildings. Sustain. Energy Technol. Assess. 2021, 47, 101357. [Google Scholar] [CrossRef]
- Liu, S.; Wang, R.; Ma, C.; Yang, D.; Li, D.; Lewandowski, Z. Improvement of Electrochemical Performance via Enhanced Reactive Oxygen Species Adsorption at ZnO–NiO@rGO Carbon Felt Cathodes in Photosynthetic Algal Microbial Fuel Cells. Chem. Eng. J. 2020, 391, 123627. [Google Scholar] [CrossRef]
- Karaca, C.; Eroğlu, Z.; Karaca, S. Anthraquinone-Rich Rheum Ribes L. as a Source of Nitrogen-Doped Carbon Quantum Dots for ZnO-Based S-Scheme Heterojunction Photocatalysts in Tetracycline Degradation. J. Environ. Chem. Eng. 2025, 13, 115999. [Google Scholar] [CrossRef]
- Mohapatra, L.; Cheon, D.; Yoo, S.H. Carbon-Based Nanomaterials for Catalytic Wastewater Treatment: A Review. Molecules 2023, 28, 1805. [Google Scholar] [CrossRef] [PubMed]
- Saranya, M.; Ramachandran, R.; Wang, F. Graphene-Zinc Oxide (G-ZnO) Nanocomposite for Electrochemical Supercapacitor Applications. J. Sci. Adv. Mater. Devices 2016, 1, 454–460. [Google Scholar] [CrossRef]
- Yildiz, G.; Bolton-Warberg, M.; Awaja, F. Graphene and Graphene Oxide for Bio-Sensing: General Properties and the Effects of Graphene Ripples. Acta Biomater. 2021, 131, 62–79. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Sysoev, V.V.; Brzhezinskaya, M.; Solomatin, M.A.; Gabrelian, V.S.; Kirilenko, D.A.; Stolyarova, D.Y.; Saveliev, S.D.; Shvidchenko, A.V.; Cherviakova, P.D.; et al. Rationalizing Graphene–ZnO Composites for Gas Sensing via Functionalization with Amines. Nanomaterials 2024, 14, 735. [Google Scholar] [CrossRef]
- Rashid, H.; Muhammad, E.; Hassan, M.; Rauf, A.; Bashir, A.; Ali, W.; Jan, T.; Bahadur, A.; Iqbal, S.; Mahmood, S.; et al. Synthesis, Structural and Photocatalytic Properties of ZnO-CuO, ZnO-Graphene and ZnO-CuO-Graphene Nanocomposites. Polyhedron 2025, 273, 117471. [Google Scholar] [CrossRef]
- Xu, J.; Cui, Y.; Han, Y.; Hao, M.; Zhang, X. ZnO-Graphene Composites with High Photocatalytic Activities under Visible Light. RSC Adv. 2016, 6, 96778–96784. [Google Scholar] [CrossRef]
- Rashko, M.N.; Hamad, S.M.; Barzinjy, A.A.; Hamad, A.H. Mechanical Properties of Carbon Nanotubes (CNTs): A Review. Eurasian J. Sci. Eng. Technol. 2022, 8, 54–68. [Google Scholar] [CrossRef]
- CNT-ZnO Core-Shell Photoanodes for Photoelectrochemical Water Splitting. Available online: https://www.mdpi.com/2079-6412/12/1/47 (accessed on 14 May 2025).
- Phin, H.-Y.; Ong, Y.-T.; Sin, J.-C. Effect of Carbon Nanotubes Loading on the Photocatalytic Activity of Zinc Oxide/Carbon Nanotubes Photocatalyst Synthesized via a Modified Sol-Gel Method. J. Environ. Chem. Eng. 2020, 8, 103222. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, S.H.; Jeon, D.; Lee, S.-N. High-Performance Sol–Gel-Derived CNT-ZnO Nanocomposite-Based Photodetectors with Controlled Surface Wrinkles. Materials 2024, 17, 5325. [Google Scholar] [CrossRef]
- Mmelesi, O.K.; Mguni, L.L.; Li, F.; Nkosi, B.; Liu, X. Recent Development in Fluorescent Carbon Quantum Dots-Based Photocatalysts for Water and Energy Applications. Mater. Sci. Semicond. Process. 2024, 181, 108661. [Google Scholar] [CrossRef]
- Moridon, S.N.F.; Anuar, N.A.; Mohammad, N.Z.; Nordin, N.A.; Mohamed, M.A. Boosting Charge Transfer and Photoelectrochemical Activity in ZnO Photoelectrodes with Quantum Dot Carbon Decorations. J. Photochem. Photobiol. Chem. 2025, 461, 116141. [Google Scholar] [CrossRef]
- Dhayal, M.; Sharma, S.; Jakhar, P.; Sain, Y.; Sharma, H. Carbon-Supported Photocatalysts: A Sustainable Approach for Enhanced Environmental Remediation. Comments Inorg. Chem. 2025, 1–55. [Google Scholar] [CrossRef]
- Sajid, M.; Iram, G.; Nawaz, A.; Qayyum, W.; Farhan, A.; Qamar, M.A.; Nawaz, H.; Shahid, A. Carbon-Based Nanomaterials: Synthesis, Types and Fuel Applications: A Mini-Review. Rev. Inorg. Chem. 2025, 45, 125–149. [Google Scholar] [CrossRef]
- Priyadharsan, A.; Ranjith, R.; Karmegam, N.; Thennarasu, G.; Ragupathy, S.; Hwan Oh, T.; Ramasundaram, S. Effect of Metal Doping and Non-Metal Loading on Light Energy Driven Degradation of Organic Dye Using ZnO Nanocatalysts. Chemosphere 2023, 330, 138708. [Google Scholar] [CrossRef]
- Aftab, S.; Shabir, T.; Shah, A.; Nisar, J.; Shah, I.; Muhammad, H.; Shah, N.S. Highly Efficient Visible Light Active Doped ZnO Photocatalysts for the Treatment of Wastewater Contaminated with Dyes and Pathogens of Emerging Concern. Nanomaterials 2022, 12, 486. [Google Scholar] [CrossRef]
- Mahdavi, R.; Talesh, S.S.A. Sol-Gel Synthesis, Structural and Enhanced Photocatalytic Performance of Al Doped ZnO Nanoparticles. Adv. Powder Technol. 2017, 28, 1418–1425. [Google Scholar] [CrossRef]
- Ivanova, T.; Harizanova, A.; Koutzarova, T.; Vertruyen, B.; Closset, R. Sol–Gel Synthesis of ZnO:Li Thin Films: Impact of Annealing on Structural and Optical Properties. Crystals 2024, 14, 6. [Google Scholar] [CrossRef]
- Demircan, G.; Gurses, E.F.; Aktas, B.; Yalcin, S.; Acikgoz, A.; Ceyhan, G.; Balak, M.V. Sol–Gel Synthesis of Si-ZnO, Ti-ZnO and Si-Ti-ZnO Thin FIlms: Impact of Si and Ti Content on Structural and Optical Properties. Mater. Today Commun. 2023, 34, 105234. [Google Scholar] [CrossRef]
- Ruilin, L.; Yee, K.Y.; Salleh, N.A.; Deghfel, B.; Zakaria, Z.; Yaakob, M.K.; Ong, H.R.; Rahiman, W.; Akbulut, H.; Wang, D.; et al. Hydrothermal Synthesis of Manganese Doped Zinc Oxide Wurtzite Nanoparticles for Supercapacitors—A Brief Review. Inorg. Chem. Commun. 2024, 170, 113311. [Google Scholar] [CrossRef]
- Sanjana Devi, V.S.; Balraj, B.; Siva, C.; Amuthameena, S. Green Hydrothermal Synthesis of Ga Doping Derived 3D ZnO Nanosatellites for High Sensitive Gas Sensors. Sens. Actuators B Chem. 2023, 379, 133215. [Google Scholar] [CrossRef]
- Naik, E.I.; Naik, H.S.B.; Sarvajith, M.S.; Pradeepa, E. Co-Precipitation Synthesis of Cobalt Doped ZnO Nanoparticles: Characterization and Their Applications for Biosensing and Antibacterial Studies. Inorg. Chem. Commun. 2021, 130, 108678. [Google Scholar] [CrossRef]
- Anandan, M.; Dinesh, S.; Christopher, B.; Krishnakumar, N.; Krishnamurthy, B.; Ayyar, M. Multifaceted Investigations of Co-Precipitated Ni-Doped ZnO Nanoparticles: Systematic Study on Structural Integrity, Optical Interplay and Photocatalytic Performances. Phys. B Condens. Matter 2024, 674, 415597. [Google Scholar] [CrossRef]
- Qi, K.; Xing, X.; Zada, A.; Li, M.; Wang, Q.; Liu, S.; Lin, H.; Wang, G. Transition Metal Doped ZnO Nanoparticles with Enhanced Photocatalytic and Antibacterial Performances: Experimental and DFT Studies. Ceram. Int. 2020, 46, 1494–1502. [Google Scholar] [CrossRef]
- Esbergenova, A.; Hojamberdiev, M.; Kadirova, Z.C.; Sugai, Y.; Mamatkulov, S.; Jalolov, R.; Kong, D.; Qin, X.; Daminova, S.S.; Ruzimuradov, O.; et al. Interlinking the Fe Doping Concentration, Optoelectronic Properties, and Photocatalytic Performance of ZnO Nanostructures. Curr. Appl. Phys. 2024, 67, 18–29. [Google Scholar] [CrossRef]
- Khumphon, J.; Ahmed, R.; Imboon, T.; Giri, J.; Chattham, N.; Mohammad, F.; Kityakarn, S.; Mangala Gowri, V.; Thongmee, S. Boosting Photocatalytic Activity in Rhodamine B Degradation Using Cu-Doped ZnO Nanoflakes. ACS Omega 2025, 10, 9337–9350. [Google Scholar] [CrossRef]
- Jiang, W.; Loh, H.; Low, B.Q.L.; Zhu, H.; Low, J.; Heng, J.Z.X.; Tang, K.Y.; Li, Z.; Loh, X.J.; Ye, E.; et al. Role of Oxygen Vacancy in Metal Oxides for Photocatalytic CO2 Reduction. Appl. Catal. B Environ. 2023, 321, 122079. [Google Scholar] [CrossRef]
- Yelpale, A.M.; Patil, V.L.; Tarwal, N.L.; Yadav, A.A.; Dhavale, R.P.; Vhangutte, P.P.; Bhange, D.S.; Ghorpade, R.A.; Dalavi, D.S. Ag-Doped ZnO Nanostructures Synthesized via Co-Precipitation Method for Enhanced Photodegradation of Crystal Violet Dye. Mater. Sci. Eng. B 2025, 314, 118038. [Google Scholar] [CrossRef]
- Shah, R.; Khan, D.; Al-Anazi, A.; Ahmad, W.; Ullah, I.; Shah, N.S.; Khan, J.A. Non-Metal Doped ZnO and TiO2 Photocatalysts for Visible Light Active Degradation of Pharmaceuticals and Hydrogen Production: A Review. Appl. Catal. O Open 2025, 204, 207043. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, W.; Li, Q.; Liu, H.; Wang, X. Preparations and Applications of Zinc Oxide Based Photocatalytic Materials. Adv. Sens. Energy Mater. 2023, 2, 100069. [Google Scholar] [CrossRef]
- Huong, L.M.; Dat, N.M.; Hoai Nam, N.T.; An, H.; Hai, N.D.; Phu Hung, P.N.; Anh Minh, N.C.; Vu, N.H.; Nhiem, L.T.; Thang, N.T.; et al. Enhanced Sunlight-Driven Photocatalysis of Non-Metal Doped Zinc Oxide via Wet Impregnation for the Removal of Organic Compounds. Environ. Nanotechnol. Monit. Manag. 2024, 22, 100990. [Google Scholar] [CrossRef]
- Joy, A.; Viswanathan, M.R.; Vijayan, B.K.; Silva, C.G.; Basheer, I.; Sugathan, S.; Mohamed, P.A.; Solaiappan, A.; Shereef, A. Solar Photocatalysts: Non-Metal (C, N, and S)-Doped ZnO Synthesized through an Industrially Sustainable in Situ Approach for Environmental Remediation Applications. RSC Adv. 2024, 14, 21655–21667. [Google Scholar] [CrossRef]
- Maulana, F.; Engge, Y.; Nurhuda, M.; Istiroyah; Hakim, L.; Juwono, A.M. Boosting Hydrogen Production through Water Splitting: N, Ni, and N-Ni Doped ZnO Photocatalysts. J. Electrochem. Soc. 2024, 171, 046505. [Google Scholar] [CrossRef]
- Macías-Sánchez, J.J.; Hinojosa-Reyes, L.; Caballero-Quintero, A.; de la Cruz, W.; Ruiz-Ruiz, E.; Hernández-Ramírez, A.; Guzmán-Mar, J.L. Synthesis of Nitrogen-Doped ZnO by Sol–Gel Method: Characterization and Its Application on Visible Photocatalytic Degradation of 2,4-D and Picloram Herbicides. Photochem. Photobiol. Sci. 2015, 14, 536–542. [Google Scholar] [CrossRef]
- Shelar, S.G.; Mahajan, V.K.; Patil, S.P.; Sonawane, G.H. Effect of Doping Parameters on Photocatalytic Degradation of Methylene Blue Using Ag Doped ZnO Nanocatalyst. SN Appl. Sci. 2020, 2, 820. [Google Scholar] [CrossRef]
- Mariyappillai, V.; Shiyamala, C.; Abisheik, T.; Tiffany, M.; Pandiyan, V.; Senthilraja, A.; Afzal, M.; Barmavatu, P.; Shanmugaraj, K.; Balu, K. Zr-Modified ZnO Nanoparticles: Optimized Photocatalytic Degradation and Antibacterial Efficiency for Pollution Control. Ceram. Int. 2025, 51, 23003–23020. [Google Scholar] [CrossRef]
- Khammar, F.; Boukerche, S.; Djaber, S.; Boublia, A.; Messabhia, A.; Gharbi, A.; Ferkous, H.; Gomez, C.V.; Bellucci, S.; Albrahim, M.; et al. Synthesis, Characterization, and Photocatalytic Efficiency of Mg-Doped ZnO Nanoparticles for Basic Fuchsin Dye Degradation: Experimental and Theoretical Insights. Inorg. Chem. Commun. 2025, 176, 114274. [Google Scholar] [CrossRef]
- khavar, C.A.H.; Mahjoub, A.; Bayat Rizi, M. Low Temperature One-Pot Synthesis of Cu-Doped ZnO/Al2O3 Composite by a Facile Rout for Rapid Methyl Orange Degradation. J. Photochem. Photobiol. B 2017, 175, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Mohtar, S.S.; Aziz, F.; Ismail, A.F.; Sambudi, N.S.; Abdullah, H.; Rosli, A.N.; Ohtani, B. Impact of Doping and Additive Applications on Photocatalyst Textural Properties in Removing Organic Pollutants: A Review. Catalysts 2021, 11, 1160. [Google Scholar] [CrossRef]
- Abou Zeid, S.; Leprince-Wang, Y. Advancements in ZnO-Based Photocatalysts for Water Treatment: A Comprehensive Review. Crystals 2024, 14, 611. [Google Scholar] [CrossRef]
- Wee, L.H.; Janssens, N.; Sree, S.P.; Wiktor, C.; Gobechiya, E.; Fischer, R.A.; Kirschhock, C.E.A.; Martens, J.A. Local Transformation of ZIF-8 Powders and Coatings into ZnO Nanorods for Photocatalytic Application. Nanoscale 2014, 6, 2056–2060. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Darabdhara, J.; Ahmaruzzaman, M. ZnO-Based Cu Metal–Organic Framework (MOF) Nanocomposite for Boosting and Tuning the Photocatalytic Degradation Performance. Environ. Sci. Pollut. Res. 2023, 30, 95673–95691. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Harraz, F.A.; Jalalah, M.; Alsaiari, M.; Al-Sayari, S.A.; Al-Assiri, M.S. Polythiophene Doped ZnO Nanostructures Synthesized by Modified Sol-Gel and Oxidative Polymerization for Efficient Photodegradation of Methylene Blue and Gemifloxacin Antibiotic. Mater. Today Commun. 2020, 24, 101048. [Google Scholar] [CrossRef]
- Subudhi, S.; Prakash Tripathy, S.; Parida, K. Metal Oxide Integrated Metal Organic Frameworks (MO@MOF): Rational Design, Fabrication Strategy, Characterization and Emerging Photocatalytic Applications. Inorg. Chem. Front. 2021, 8, 1619–1636. [Google Scholar] [CrossRef]
- Sun, J.; Wei, Q.; Song, P.; Yang, Z.; Wang, Q. Porous ZnO Cubes Derived from Metal–Organic Frameworks with Excellent Sensing Performance Triethylamine. J. Mater. Sci. Mater. Electron. 2020, 31, 838–847. [Google Scholar] [CrossRef]
- Abazari, R.; Mahjoub, A.R. Amine-Functionalized Al-MOF#@yxSm2O3–ZnO: A Visible Light-Driven Nanocomposite with Excellent Photocatalytic Activity for the Photo-Degradation of Amoxicillin. Inorg. Chem. 2018, 57, 2529–2545. [Google Scholar] [CrossRef]
- Darabdhara, J.; Roy, S.; Ahmaruzzaman, M. Efficient Photocatalytic Degradation of an Organic Dye by the Fabrication of a Novel Ternary Composite Based on Zeolitic Imidazolate Framework via a Facile in-Situ Synthetic Approach. Inorg. Chem. Commun. 2023, 152, 110694. [Google Scholar] [CrossRef]
- Liu, N.; Shang, Q.; Gao, K.; Cheng, Q.; Pan, Z. Construction of ZnO/ZIF-9 Heterojunction Photocatalyst: Enhanced Photocatalytic Performance and Mechanistic Insight. New J. Chem. 2020, 44, 6384–6393. [Google Scholar] [CrossRef]
- Lei, Z.; Wu, Y.; Tang, L.; Chen, J. Preparation of MOF-Zn@ZnO Composite and Its Properties in SBR. Polym. Compos. 2022, 43, 8749–8760. [Google Scholar] [CrossRef]
- Nakagaki, S.; Ferreira, G.; Ucoski, G.; Dias De Freitas Castro, K. Chemical Reactions Catalyzed by Metalloporphyrin-Based Metal-Organic Frameworks. Molecules 2013, 18, 7279–7308. [Google Scholar] [CrossRef]
- Yang, X.; Wen, Z.; Wu, Z.; Luo, X. Synthesis of ZnO/ZIF-8 Hybrid Photocatalysts Derived from ZIF-8 with Enhanced Photocatalytic Activity. Inorg. Chem. Front. 2018, 5, 687–693. [Google Scholar] [CrossRef]
- Iqbal, T.; Irfan, M.; Ramay, S.M.; Gaithan, H.M.; Mahmood, A.; Saleem, M. Investigations on ZnO/Polymer Nanocomposite Thin Film for Polymer Based Devices. Mater. Res. Express 2019, 6, 075322. [Google Scholar] [CrossRef]
- Devaraju, A.; Sivasamy, P.; Loganathan, G.B. Mechanical Properties of Polymer Composites with ZnO Nano-Particle. Mater. Today Proc. 2020, 22, 531–534. [Google Scholar] [CrossRef]
- Podasca, V.-E.; Damaceanu, M.-D. ZnO-Ag Based Polymer Composites as Photocatalysts for Highly Efficient Visible-Light Degradation of Methyl Orange. J. Photochem. Photobiol. Chem. 2021, 406, 113003. [Google Scholar] [CrossRef]
- Di Mauro, A.; Cantarella, M.; Nicotra, G.; Pellegrino, G.; Gulino, A.; Brundo, M.V.; Privitera, V.; Impellizzeri, G. Novel Synthesis of ZnO/PMMA Nanocomposites for Photocatalytic Applications. Sci. Rep. 2017, 7, 40895. [Google Scholar] [CrossRef] [PubMed]
- Guedri, A.; Zaabat, M.; Boudine, B.; Hafdallah, A. Synthesis, Characterization, Structural, and Optical Properties of Polyvinyl Chloride/Zinc Oxide Nanocomposite Films for Photocatalysis Application. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4884–4894. [Google Scholar] [CrossRef]
- Borjigin, T.; Schmitt, M.; Morlet-Savary, F.; Xiao, P.; Lalevée, J. Low-Cost and Recyclable Photocatalysts: Metal Oxide/Polymer Composites Applied in the Catalytic Breakdown of Dyes. Photochem 2022, 2, 733–751. [Google Scholar] [CrossRef]
- Ward, L.; Wolverton, C. Atomistic Calculations and Materials Informatics: A Review. Curr. Opin. Solid State Mater. Sci. 2017, 21, 167–176. [Google Scholar] [CrossRef]
- Baskes, M.I.; Angelo, J.E.; Bisson, C.L. Atomistic Calculations of Composite Interfaces. Model. Simul. Mater. Sci. Eng. 1994, 2, 505. [Google Scholar] [CrossRef]
- Hourahine, B.; Aradi, B.; Blum, V.; Bonafé, F.; Buccheri, A.; Camacho, C.; Cevallos, C.; Deshaye, M.Y.; Dumitrică, T.; Dominguez, A.; et al. DFTB+, a Software Package for Efficient Approximate Density Functional Theory Based Atomistic Simulations. J. Chem. Phys. 2020, 152, 124101. [Google Scholar] [CrossRef]
- Bielenica, A.; Beegum, S.; Mary, Y.S.; Mary, Y.S.; Thomas, R.; Armaković, S.; Armaković, S.J.; Madeddu, S.; Struga, M.; Van Alsenoy, C. Experimental and Computational Analysis of 1-(4-Chloro-3-Nitrophenyl)-3-(3,4-Dichlorophenyl)Thiourea. J. Mol. Struct. 2020, 1205, 127587. [Google Scholar] [CrossRef]
- Jensen, J.H. Molecular Modeling Basics; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-1-4200-7527-4. [Google Scholar]
- Armstrong, G.S.J.; Khokhlova, M.A.; Labeye, M.; Maxwell, A.S.; Pisanty, E.; Ruberti, M. Dialogue on Analytical and Ab Initio Methods in Attoscience. Eur. Phys. J. D 2021, 75, 209. [Google Scholar] [CrossRef]
- Pokluda, J.; Černý, M.; Šob, M.; Umeno, Y. Ab Initio Calculations of Mechanical Properties: Methods and Applications. Prog. Mater. Sci. 2015, 73, 127–158. [Google Scholar] [CrossRef]
- Thomas, R.; Hossain, M.; Mary, Y.S.; Resmi, K.S.; Armaković, S.; Armaković, S.J.; Nanda, A.K.; Ranjan, V.K.; Vijayakumar, G.; Van Alsenoy, C. Spectroscopic Analysis and Molecular Docking of Imidazole Derivatives and Investigation of Its Reactive Properties by DFT and Molecular Dynamics Simulations. J. Mol. Struct. 2018, 1158, 156–175. [Google Scholar] [CrossRef]
- Hossain, M.; Thomas, R.; Mary, Y.S.; Resmi, K.S.; Armaković, S.; Armaković, S.J.; Nanda, A.K.; Vijayakumar, G.; Alsenoy, C.V. Understanding Reactivity of Two Newly Synthetized Imidazole Derivatives by Spectroscopic Characterization and Computational Study. J. Mol. Struct. 2018, 1158, 176–196. [Google Scholar] [CrossRef]
- Mary, Y.S.; Miniyar, P.B.; Mary, Y.S.; Resmi, K.S.; Panicker, C.Y.; Armaković, S.; Armaković, S.J.; Thomas, R.; Sureshkumar, B. Synthesis and Spectroscopic Study of Three New Oxadiazole Derivatives with Detailed Computational Evaluation of Their Reactivity and Pharmaceutical Potential. J. Mol. Struct. 2018, 1173, 469–480. [Google Scholar] [CrossRef]
- Mary, Y.S.; Mary, Y.S.; Thomas, R.; Narayana, B.; Samshuddin, S.; Sarojini, B.K.; Armaković, S.; Armaković, S.J.; Pillai, G.G. Theoretical Studies on the Structure and Various Physico-Chemical and Biological Properties of a Terphenyl Derivative with Immense Anti-Protozoan Activity. Polycycl. Aromat. Compd. 2021, 41, 825–840. [Google Scholar] [CrossRef]
- Beegum, S.; Mary, Y.S.; Mary, Y.S.; Thomas, R.; Armaković, S.; Armaković, S.J.; Zitko, J.; Dolezal, M.; Van Alsenoy, C. Exploring the Detailed Spectroscopic Characteristics, Chemical and Biological Activity of Two Cyanopyrazine-2-Carboxamide Derivatives Using Experimental and Theoretical Tools. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2020, 224, 117414. [Google Scholar] [CrossRef]
- Celebi, A.T.; Jamali, S.H.; Bardow, A.; Vlugt, T.J.H.; Moultos, O.A. Finite-Size Effects of Diffusion Coefficients Computed from Molecular Dynamics: A Review of What We Have Learned so Far. Mol. Simul. 2021, 47, 831–845. [Google Scholar] [CrossRef]
- Ding, H.; Wang, H.; Qu, X.; Varveri, A.; Gao, J.; You, Z. Towards an Understanding of Diffusion Mechanism of Bio-Rejuvenators in Aged Asphalt Binder through Molecular Dynamics Simulation. J. Clean. Prod. 2021, 299, 126927. [Google Scholar] [CrossRef]
- Christensen, A.S.; Kubař, T.; Cui, Q.; Elstner, M. Semiempirical Quantum Mechanical Methods for Noncovalent Interactions for Chemical and Biochemical Applications. Chem. Rev. 2016, 116, 5301–5337. [Google Scholar] [CrossRef]
- Thiel, W. Semiempirical Quantum–Chemical Methods. WIREs Comput. Mol. Sci. 2014, 4, 145–157. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods IV: Extension of MNDO, AM1, and PM3 to More Main Group Elements. J. Mol. Model. 2004, 10, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.P. Calculation of the Geometry of a Small Protein Using Semiempirical Methods. J. Mol. Struct. THEOCHEM 1997, 401, 195–205. [Google Scholar] [CrossRef]
- Stewart, J.J.P.; Stewart, A.C. A Semiempirical Method Optimized for Modeling Proteins. J. Mol. Model. 2023, 29, 284. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods VI: More Modifications to the NDDO Approximations and Re-Optimization of Parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods V: Modification of NDDO Approximations and Application to 70 Elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods II. Applications. J. Comput. Chem. 1989, 10, 221–264. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods I. Method. J. Comput. Chem. 1989, 10, 209–220. [Google Scholar] [CrossRef]
- Bannwarth, C.; Caldeweyher, E.; Ehlert, S.; Hansen, A.; Pracht, P.; Seibert, J.; Spicher, S.; Grimme, S. Extended Tight-Binding Quantum Chemistry Methods. WIREs Comput. Mol. Sci. 2021, 11, e1493. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB—An Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef] [PubMed]
- Ehlert, S.; Stahn, M.; Spicher, S.; Grimme, S. Robust and Efficient Implicit Solvation Model for Fast Semiempirical Methods. J. Chem. Theory Comput. 2021, 17, 4250–4261. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Bannwarth, C.; Shushkov, P. A Robust and Accurate Tight-Binding Quantum Chemical Method for Structures, Vibrational Frequencies, and Noncovalent Interactions of Large Molecular Systems Parametrized for All Spd-Block Elements (Z = 1–86). J. Chem. Theory Comput. 2017, 13, 1989–2009. [Google Scholar] [CrossRef] [PubMed]
- Froitzheim, T.; Müller, M.; Hansen, A.; Grimme, S. G-xTB: A General-Purpose Extended Tight-Binding Electronic Structure Method for the Elements H to Lr (Z=1–103) 2025. ChemRxiv 2025. [Google Scholar] [CrossRef]
- Axelrod, S.; Schwalbe-Koda, D.; Mohapatra, S.; Damewood, J.; Greenman, K.P.; Gómez-Bombarelli, R. Learning Matter: Materials Design with Machine Learning and Atomistic Simulations. Acc. Mater. Res. 2022, 3, 343–357. [Google Scholar] [CrossRef]
- Ceriotti, M. Unsupervised Machine Learning in Atomistic Simulations, between Predictions and Understanding. J. Chem. Phys. 2019, 150, 150901. [Google Scholar] [CrossRef]
- Behler, J. Perspective: Machine Learning Potentials for Atomistic Simulations. J. Chem. Phys. 2016, 145, 170901. [Google Scholar] [CrossRef]
- Herrera-Viedma, E.; Pasi, G.; Lopez-Herrera, A.G.; Porcel, C. Evaluating the Information Quality of Web Sites: A Methodology Based on Fuzzy Computing with Words. J. Am. Soc. Inf. Sci. Technol. 2006, 57, 538–549. [Google Scholar] [CrossRef]
- Fenza, G.; Gallo, M.; Loia, V.; Orciuoli, F.; Herrera-Viedma, E. Data Set Quality in Machine Learning: Consistency Measure Based on Group Decision Making. Appl. Soft Comput. 2021, 106, 107366. [Google Scholar] [CrossRef]
- Menzel, J.P.; Kloppenburg, M.; Belić, J.; de Groot, H.J.M.; Visscher, L.; Buda, F. Efficient Workflow for the Investigation of the Catalytic Cycle of Water Oxidation Catalysts: Combining GFN-xTB and Density Functional Theory. J. Comput. Chem. 2021, 42, 1885–1894. [Google Scholar] [CrossRef]
- Collins, E.M.; Raghavachari, K. Effective Molecular Descriptors for Chemical Accuracy at DFT Cost: Fragmentation, Error-Cancellation, and Machine Learning. J. Chem. Theory Comput. 2020, 16, 4938–4950. [Google Scholar] [CrossRef]
- Kronik, L.; Tkatchenko, A. Understanding Molecular Crystals with Dispersion-Inclusive Density Functional Theory: Pairwise Corrections and Beyond. Acc. Chem. Res. 2014, 47, 3208–3216. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys Rev Lett 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Lambert, D.S.; O’Regan, D.D. Use of DFT+U+J with Linear Response Parameters to Predict Non-Magnetic Oxide Band Gaps with Hybrid-Functional Accuracy. Phys. Rev. Res. 2023, 5, 013160. [Google Scholar] [CrossRef]
- Fabrizio, K.; Le, K.N.; Andreeva, A.B.; Hendon, C.H.; Brozek, C.K. Determining Optical Band Gaps of MOFs. ACS Mater. Lett. 2022, 4, 457–463. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, Q.-D.; Liu, D.; Liang, J. Effectively Improving the Accuracy of PBE Functional in Calculating the Solid Band Gap via Machine Learning. Comput. Mater. Sci. 2021, 198, 110699. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid Functionals Based on a Screened Coulomb Potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-Energy-Loss Spectra and the Structural Stability of Nickel Oxide: An LSDA+U Study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Cococcioni, M.; de Gironcoli, S. Linear Response Approach to the Calculation of the Effective Interaction Parameters in the LDA+U Method. Phys. Rev. B 2005, 71, 035105. [Google Scholar] [CrossRef]
- Garrity, K.F.; Bennett, J.W.; Rabe, K.M.; Vanderbilt, D. Pseudopotentials for High-Throughput DFT Calculations. Comput. Mater. Sci. 2014, 81, 446–452. [Google Scholar] [CrossRef]
- Jansen, T.; Brocks, G.; Bokdam, M. Phase Transitions of LaMnO3 and SrRuO3 from DFT+U Based Machine Learning Force Fields Simulations. Phys. Rev. B 2023, 108, 235122. [Google Scholar] [CrossRef]
- Macke, E.; Timrov, I.; Marzari, N.; Ciacchi, L.C. Orbital-Resolved DFT+U for Molecules and Solids. J. Chem. Theory Comput. 2024, 20, 4824–4843. [Google Scholar] [CrossRef] [PubMed]
- Goh, E.S.; Mah, J.W.; Yoon, T.L. Effects of Hubbard Term Correction on the Structural Parameters and Electronic Properties of Wurtzite ZnO. Comput. Mater. Sci. 2017, 138, 111–116. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Marques, M.; Teles, L.K. Slater Half-Occupation Technique Revisited: The LDA-1/2 and GGA-1/2 Approaches for Atomic Ionization Energies and Band Gaps in Semiconductors. AIP Adv. 2011, 1, 032119. [Google Scholar] [CrossRef]
- Slater, J.C.; Johnson, K.H. Self-Consistent-Field Xα Cluster Method for Polyatomic Molecules and Solids. Phys. Rev. B 1972, 5, 844–853. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Marques, M.; Teles, L.K. Approximation to Density Functional Theory for the Calculation of Band Gaps of Semiconductors. Phys. Rev. B 2008, 78, 125116. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate Spin-Dependent Electron Liquid Correlation Energies for Local Spin Density Calculations: A Critical Analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. III. Role Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Smith, D.G.A.; Burns, L.A.; Patkowski, K.; Sherrill, C.D. Revised Damping Parameters for the D3 Dispersion Correction to Density Functional Theory. J. Phys. Chem. Lett. 2016, 7, 2197–2203. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Caldeweyher, E.; Ehlert, S.; Hansen, A.; Neugebauer, H.; Spicher, S.; Bannwarth, C.; Grimme, S. A Generally Applicable Atomic-Charge Dependent London Dispersion Correction. J. Chem. Phys. 2019, 150, 154122. [Google Scholar] [CrossRef] [PubMed]
- Caldeweyher, E.; Bannwarth, C.; Grimme, S. Extension of the D3 Dispersion Coefficient Model. J. Chem. Phys. 2017, 147, 034112. [Google Scholar] [CrossRef]
- Goerigk, L. Treating London-Dispersion Effects with the Latest Minnesota Density Functionals: Problems and Possible Solutions. J. Phys. Chem. Lett. 2015, 6, 3891–3896. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- Valero, R.; Costa, R.; de P. R. Moreira, I.; Truhlar, D.G.; Illas, F. Performance of the M06 Family of Exchange-Correlation Functionals for Predicting Magnetic Coupling in Organic and Inorganic Molecules. J. Chem. Phys. 2008, 128, 114103. [Google Scholar] [CrossRef]
- Jacquemin, D.; Perpète, E.A.; Ciofini, I.; Adamo, C.; Valero, R.; Zhao, Y.; Truhlar, D.G. On the Performances of the M06 Family of Density Functionals for Electronic Excitation Energies. J. Chem. Theory Comput. 2010, 6, 2071–2085. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Van Der Giessen, E.; Schultz, P.A.; Bertin, N.; Bulatov, V.V.; Cai, W.; Csányi, G.; Foiles, S.M.; Geers, M.G.D.; González, C.; Hütter, M.; et al. Roadmap on Multiscale Materials Modeling. Model. Simul. Mater. Sci. Eng. 2020, 28, 043001. [Google Scholar] [CrossRef]
- Scandolo, S.; Giannozzi, P.; Cavazzoni, C.; de Gironcoli, S.; Pasquarello, A.; Baroni, S. First-Principles Codes for Computational Crystallography in the Quantum-ESPRESSO Package. Z. Für Krist. Cryst. Mater. 2005, 220, 574–579. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baseggio, O.; Bonfà, P.; Brunato, D.; Car, R.; Carnimeo, I.; Cavazzoni, C.; de Gironcoli, S.; Delugas, P.; Ferrari Ruffino, F.; et al. Quantum ESPRESSO toward the Exascale. J. Chem. Phys. 2020, 152, 154105. [Google Scholar] [CrossRef] [PubMed]
- Giannozzi, P.; Andreussi, O.; Brumme, T.; Bunau, O.; Buongiorno Nardelli, M.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced Capabilities for Materials Modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A Modular and Open-Source Software Project for Quantum Simulations of Materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Turney, J.M.; Simmonett, A.C.; Parrish, R.M.; Hohenstein, E.G.; Evangelista, F.A.; Fermann, J.T.; Mintz, B.J.; Burns, L.A.; Wilke, J.J.; Abrams, M.L.; et al. Psi4: An Open-Source Ab Initio Electronic Structure Program. WIREs Comput. Mol. Sci. 2012, 2, 556–565. [Google Scholar] [CrossRef]
- Parrish, R.M.; Burns, L.A.; Smith, D.G.A.; Simmonett, A.C.; DePrince, A.E.I.; Hohenstein, E.G.; Bozkaya, U.; Sokolov, A.Y.; Di Remigio, R.; Richard, R.M.; et al. Psi4 1.1: An Open-Source Electronic Structure Program Emphasizing Automation, Advanced Libraries, and Interoperability. J. Chem. Theory Comput. 2017, 13, 3185–3197. [Google Scholar] [CrossRef]
- Smith, D.G.A.; Burns, L.A.; Simmonett, A.C.; Parrish, R.M.; Schieber, M.C.; Galvelis, R.; Kraus, P.; Kruse, H.; Di Remigio, R.; Alenaizan, A.; et al. PSI4 1.4: Open-Source Software for High-Throughput Quantum Chemistry. J. Chem. Phys. 2020, 152, 184108. [Google Scholar] [CrossRef]
- Lu, T. A Comprehensive Electron Wavefunction Analysis Toolbox for Chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Van Der Waals Potential: An Important Complement to Molecular Electrostatic Potential in Studying Intermolecular Interactions. J. Mol. Model. 2020, 26, 315. [Google Scholar] [CrossRef]
- Lu, T.; Manzetti, S. Wavefunction and Reactivity Study of Benzo[a]Pyrene Diol Epoxide and Its Enantiomeric Forms. Struct. Chem. 2014, 25, 1521–1533. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, S.; Rahimi, P.; Khedri, M.; Heydari, R.; Mirzaei, M.; Bahrami, A.; Akhlaghian, F.; Taghipoor, M. Evaluation of Machine Learning and Molecular Dynamics Models for Photocatalytic Water Decontamination. Process Saf. Environ. Prot. 2025, 195, 106780. [Google Scholar] [CrossRef]
- Armaković, S.; Armaković, S.J. Atomistica.Online—Web Application for Generating Input Files for ORCA Molecular Modelling Package Made with the Anvil Platform. Mol. Simul. 2023, 49, 117–123. [Google Scholar] [CrossRef]
- Armaković, S.; Armaković, S.J. Online and Desktop Graphical User Interfaces for Xtb Programme from Atomistica.Online Platform. Mol. Simul. 2024, 50, 560–570. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Shermo: A General Code for Calculating Molecular Thermochemistry Properties. Comput. Theor. Chem. 2021, 1200, 113249. [Google Scholar] [CrossRef]
- Wang, P.; Song, B.; Zhao, G. Novel two-dimensional ZnO materials for enhanced photocatalytic hydrogen evolution performance. Appl. Surf. Sci. 2025, 697, 163068. [Google Scholar] [CrossRef]
- Soussi, A.; Ait Hssi, A.; Boulkaddat, L.; Asbayou, A.; Labchir, N.; Elfanaoui, A.; Markazi, R.; Bouabid, K.; Ihlal, A.; Taleb, A. Structural, Optical and Electronic Properties of La-Doped Zno Thin Films: Experimental Study and DFT Calculations. Phys. B Condens. Matter 2022, 643, 414181. [Google Scholar] [CrossRef]
- Kumar, A.; Gorai, D.K.; Kumar, B.; Kundu, T.K.; Ahmad, M.I. Impact of Al–Tl co-doping on the optoelectronic properties of ZnO thin film: Experimental and DFT approach. Mater. Chem. Phys. 2025, 341, 130908. [Google Scholar] [CrossRef]
- Ullah, I.; Neder, R.B.; Khan, M.I.; Din, I.U.; Parwaz, H.; Sufian, S. Ligand-capped pristine and doped ZnO2 nanoparticles for enhanced photocatalytic methylene blue degradation: A DFT-supported study. Ceram. Int. 2025, 51, 17061–17078. [Google Scholar] [CrossRef]
- Obijiofor, O.C.; Novikov, A.S. Exploring the Role of Density Functional Theory in the Design of Gold Nanoparticles for Targeted Drug Delivery: A Systematic Review. J. Mol. Model. 2025, 31, 186. [Google Scholar] [CrossRef] [PubMed]
- Goniakowski, J.; Menon, S.; Laurens, G.; Lam, J. Nonclassical Nucleation of Zinc Oxide from a Physically Motivated Machine-Learning Approach. J. Phys. Chem. C 2022, 126, 17456–17469. [Google Scholar] [CrossRef]
- Bzdok, D.; Krzywinski, M.; Altman, N. Machine Learning: A Primer. Nat. Methods 2017, 14, 1119–1120. [Google Scholar] [CrossRef]
- Choudhary, K.; DeCost, B.; Chen, C.; Jain, A.; Tavazza, F.; Cohn, R.; Park, C.W.; Choudhary, A.; Agrawal, A.; Billinge, S.J.L.; et al. Recent Advances and Applications of Deep Learning Methods in Materials Science. Npj Comput. Mater. 2022, 8, 59. [Google Scholar] [CrossRef]
- Rodrigues, J.F.; Florea, L.; De Oliveira, M.C.F.; Diamond, D.; Oliveira, O.N. Big Data and Machine Learning for Materials Science. Discov. Mater. 2021, 1, 12. [Google Scholar] [CrossRef]
- Jordan, M.I.; Mitchell, T.M. Machine Learning: Trends, Perspectives, and Prospects. Science 2015, 349, 255–260. [Google Scholar] [CrossRef]
- Xu, P.; Ji, X.; Li, M.; Lu, W. Small Data Machine Learning in Materials Science. Npj Comput. Mater. 2023, 9, 42. [Google Scholar] [CrossRef]
- Balachandran, P.V. Machine Learning Guided Design of Functional Materials with Targeted Properties. Comput. Mater. Sci. 2019, 164, 82–90. [Google Scholar] [CrossRef]
- Butler, K.T.; Davies, D.W.; Cartwright, H.; Isayev, O.; Walsh, A. Machine Learning for Molecular and Materials Science. Nature 2018, 559, 547–555. [Google Scholar] [CrossRef]
- Schmidt, J.; Marques, M.R.G.; Botti, S.; Marques, M.A.L. Recent Advances and Applications of Machine Learning in Solid-State Materials Science. Npj Comput. Mater. 2019, 5, 83. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Titanium Dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2023, 13, 26. [Google Scholar] [CrossRef]
- Mobarak, M.H.; Mimona, M.A.; Islam, M.A.; Hossain, N.; Zohura, F.T.; Imtiaz, I.; Rimon, M.I.H. Scope of Machine Learning in Materials Research—A Review. Appl. Surf. Sci. Adv. 2023, 18, 100523. [Google Scholar] [CrossRef]
- Hart, G.L.W.; Mueller, T.; Toher, C.; Curtarolo, S. Machine Learning for Alloys. Nat. Rev. Mater. 2021, 6, 730–755. [Google Scholar] [CrossRef]
- Gao, C.; Min, X.; Fang, M.; Tao, T.; Zheng, X.; Liu, Y.; Wu, X.; Huang, Z. Innovative Materials Science via Machine Learning. Adv. Funct. Mater. 2022, 32, 2108044. [Google Scholar] [CrossRef]
- Mai, H.; Le, T.C.; Chen, D.; Winkler, D.A.; Caruso, R.A. Machine Learning for Electrocatalyst and Photocatalyst Design and Discovery. Chem. Rev. 2022, 122, 13478–13515. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A Materials Genome Approach to Accelerating Materials Innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef]
- Stanev, V.; Oses, C.; Kusne, A.G.; Rodriguez, E.; Paglione, J.; Curtarolo, S.; Takeuchi, I. Machine Learning Modeling of Superconducting Critical Temperature. Npj Comput. Mater. 2018, 4, 29. [Google Scholar] [CrossRef]
- Legrain, F.; Carrete, J.; van Roekeghem, A.; Curtarolo, S.; Mingo, N. How Chemical Composition Alone Can Predict Vibrational Free Energies and Entropies of Solids. Chem. Mater. 2017, 29, 6220–6227. [Google Scholar] [CrossRef]
- Isayev, O.; Oses, C.; Toher, C.; Gossett, E.; Curtarolo, S.; Tropsha, A. Universal Fragment Descriptors for Predicting Properties of Inorganic Crystals. Nat. Commun. 2017, 8, 15679. [Google Scholar] [CrossRef]
- Zitnick, C.L.; Chanussot, L.; Das, A.; Goyal, S.; Heras-Domingo, J.; Ho, C.; Hu, W.; Lavril, T.; Palizhati, A.; Riviere, M.; et al. An Introduction to Electrocatalyst Design Using Machine Learning for Renewable Energy Storage. Available online: https://arxiv.org/abs/2010.09435v1 (accessed on 22 May 2025).
- Chelghoum, H.; Nasrallah, N.; Tahraoui, H.; Seleiman, M.F.; Bouhenna, M.M.; Belmeskine, H.; Zamouche, M.; Djema, S.; Zhang, J.; Mendil, A.; et al. Eco-Friendly Synthesis of ZnO Nanoparticles for Quinoline Dye Photodegradation and Antibacterial Applications Using Advanced Machine Learning Models. Catalysts 2024, 14, 831. [Google Scholar] [CrossRef]
- Akyildiz, H.I.; Yigit, E.; Arat, A.B.; Islam, S. A Machine Learning Approach for the Estimation of Photocatalytic Activity of ALD ZnO Thin Films on Fabric Substrates. J. Photochem. Photobiol. Chem. 2024, 448, 115308. [Google Scholar] [CrossRef]
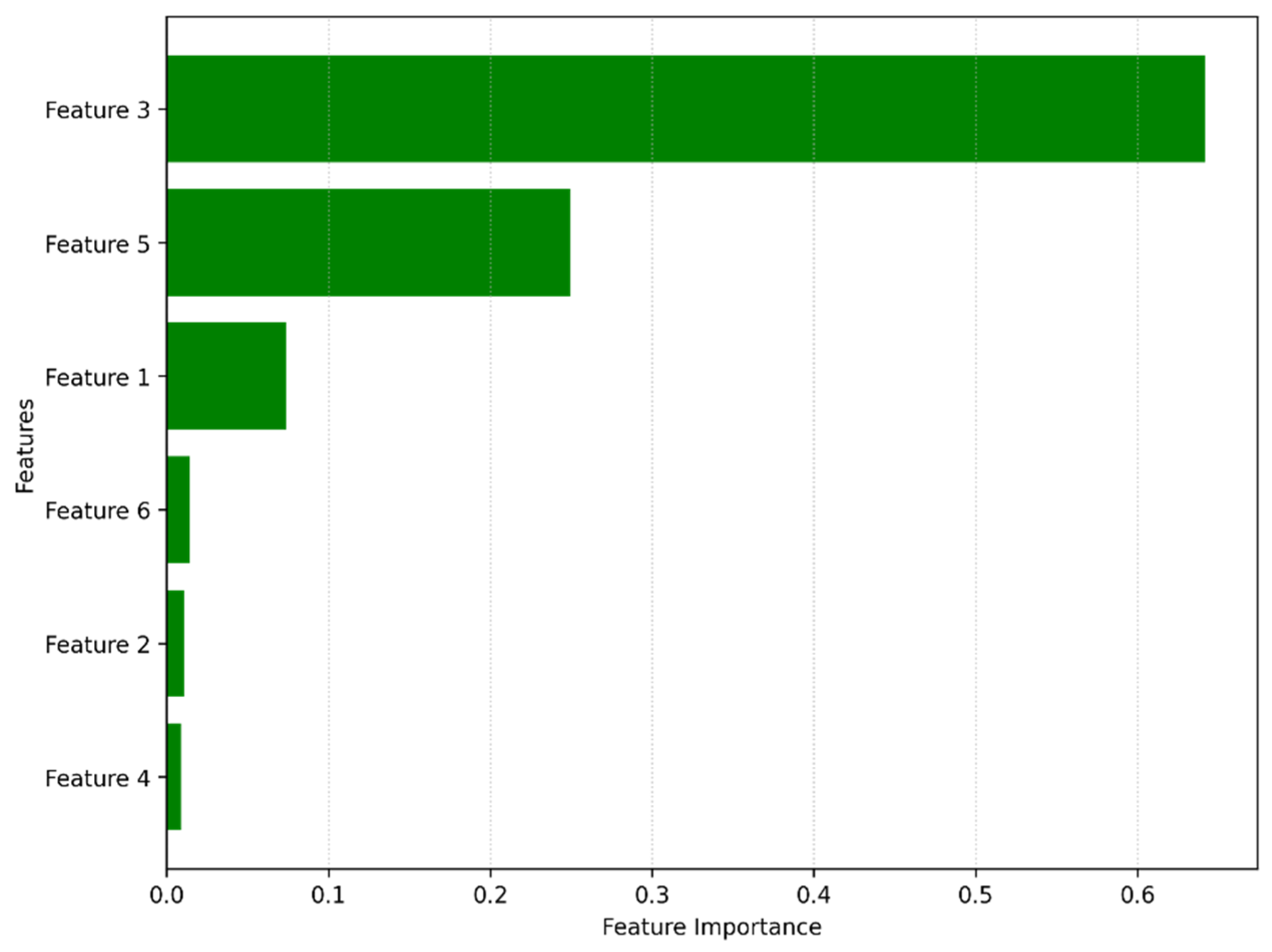
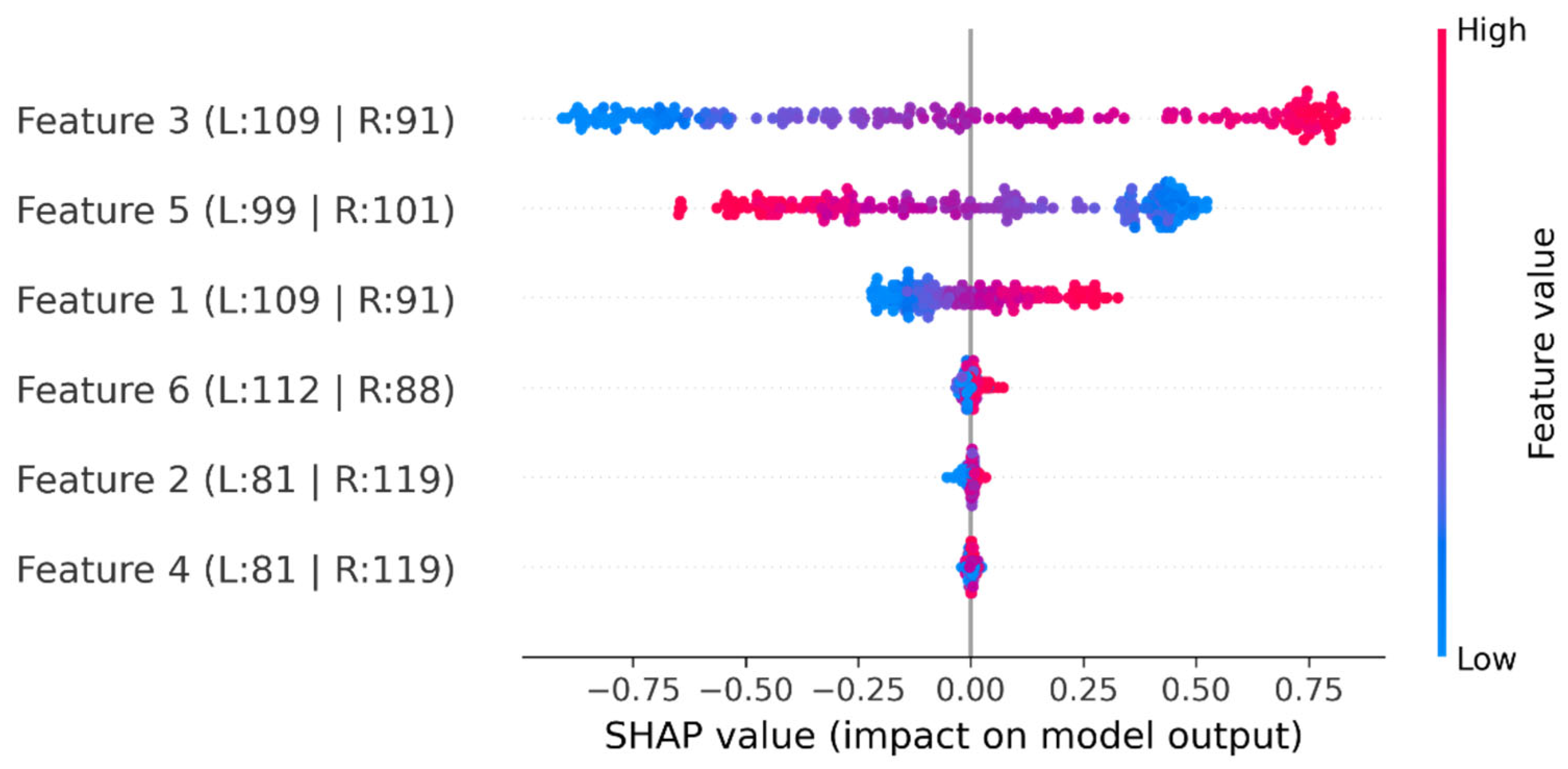
- Qayyum, F.; Khan, M.A.; Kim, D.-H.; Ko, H.; Ryu, G.-A. Explainable AI for Material Property Prediction Based on Energy Cloud: A Shapley-Driven Approach. Materials 2023, 16, 7322. [Google Scholar] [CrossRef]
- Tan, S.; Wang, R.; Song, G.; Qi, S.; Zhang, K.; Zhao, Z.; Yin, Q. Machine Learning and Shapley Additive Explanation-Based Interpretable Prediction of the Electrocatalytic Performance of N-Doped Carbon Materials. Fuel 2024, 355, 129469. [Google Scholar] [CrossRef]
- Ali, H.; Yasir, M.; Haq, H.U.; Guler, A.C.; Masar, M.; Khan, M.N.A.; Machovsky, M.; Sedlarik, V.; Kuritka, I. Machine Learning Approach for Photocatalysis: An Experimentally Validated Case Study of Photocatalytic Dye Degradation. J. Environ. Manag. 2025, 386, 125683. [Google Scholar] [CrossRef] [PubMed]
- Ezeakunne, C.; Lamichhane, B.; Kattel, S. Integrating Density Functional Theory with Machine Learning for Enhanced Band Gap Prediction in Metal Oxides. Phys. Chem. Chem. Phys. 2025, 27, 5338–5358. [Google Scholar] [CrossRef]
- Li, K.; Du, H.; Liu, L.; Yang, H.; Fang, J.; Li, D. Research Progress of Machine Learning in the Field of Photocatalysis Applications. J. Ind. Eng. Chem. 2025, in press. [Google Scholar] [CrossRef]
- Levine, D.S.; Shuaibi, M.; Spotte-Smith, E.W.C.; Taylor, M.G.; Hasyim, M.R.; Michel, K.; Batatia, I.; Csányi, G.; Dzamba, M.; Eastman, P.; et al. The Open Molecules 2025 (OMol25) Dataset, Evaluations, and Models 2025. arXiv 2025, arXiv:2505.08762. [Google Scholar]
- Wood, B.M.; Dzamba, M.; Fu, X.; Gao, M.; Shuaibi, M.; Barroso-Luque, L.; Abdelmaqsoud, K.; Gharakhanyan, V.; Kitchin, J.R.; Levine, D.S.; et al. UMA: A Family of Universal Models for Atoms 2025. arXiv 2025, arXiv:2506.23971. [Google Scholar]
- Rohatgi, A. WebPlotDigitizer. Available online: https://automeris.io/docs/ (accessed on 1 August 2025).
- Esmaeili, A.; Pourranjabar Hasan Kiadeh, S.; Ebrahimian Pirbazari, A.; Esmaeili Khalil Saraei, F.; Ebrahimian Pirbazari, A.; Derakhshesh, A.; Tabatabai-Yazdi, F.-S. CdS Nanocrystallites Sensitized ZnO Nanosheets for Visible Light Induced Sonophotocatalytic/Photocatalytic Degradation of Tetracycline: From Experimental Results to a Generalized Model Based on Machine Learning Methods. Chemosphere 2023, 332, 138852. [Google Scholar] [CrossRef]
- Lamouadene, H.; El Kassaoui, M.; El Yadari, M.; El Kenz, A.; Benyoussef, A. Exploring Modeling Techniques for Predicting Band Gaps of Doped-ZnO: A Machine Learning Approach. Chem. Phys. 2025, 591, 112603. [Google Scholar] [CrossRef]
- Navarro-López, D.E.; Perfecto-Avalos, Y.; Zavala, A.; de Luna, M.A.; Sanchez-Martinez, A.; Ceballos-Sanchez, O.; Tiwari, N.; López-Mena, E.R.; Sanchez-Ante, G. Unraveling the Complex Interactions: Machine Learning Approaches to Predict Bacterial Survival against ZnO and Lanthanum-Doped ZnO Nanoparticles. Antibiotics 2024, 13, 220. [Google Scholar] [CrossRef]
- Keller, N.; Ivanez, J.; Highfield, J.; Ruppert, A.M. Photo-/Thermal Synergies in Heterogeneous Catalysis: Towards Low-Temperature (Solar-Driven) Processing for Sustainable Energy and Chemicals. Appl. Catal. B Environ. 2021, 296, 120320. [Google Scholar] [CrossRef]
- Singh, S.; Gade, J.V.; Verma, D.K.; Elyor, B.; Jain, B. Exploring ZnO Nanoparticles: UV–Visible Analysis and Different Size Estimation Methods. Opt. Mater. 2024, 152, 115422. [Google Scholar] [CrossRef]
- Srivastava, S.K. Recent Advances in Removal of Pharmaceutical Pollutants in Wastewater Using Metal Oxides and Carbonaceous Materials as Photocatalysts: A Review. RSC Appl. Interfaces 2024, 1, 340–429. [Google Scholar] [CrossRef]
- Thanh, P.N.; Phung, V.-D.; Nguyen, T.B.H. Recent Advances and Future Trends in Metal Oxide Photocatalysts for Removal of Pharmaceutical Pollutants from Wastewater: A Comprehensive Review. Environ. Geochem. Health 2024, 46, 364. [Google Scholar] [CrossRef] [PubMed]
- Kumari, H.; Sonia; Suman; Ranga, R.; Chahal, S.; Devi, S.; Sharma, S.; Kumar, S.; Kumar, P.; Kumar, S.; et al. A Review on Photocatalysis Used For Wastewater Treatment: Dye Degradation. Water Air Soil Pollut. 2023, 234, 349. [Google Scholar] [CrossRef] [PubMed]
- Halim, O.M.A.; Mustapha, N.H.; Mohd Fudzi, S.N.; Azhar, R.; Zanal, N.I.N.; Nazua, N.F.; Nordin, A.H.; Mohd Azami, M.S.; Mohd Ishak, M.A.; Ismail, W.I.N.W.; et al. A Review on Modified ZnO for the Effective Degradation of Methylene Blue and Rhodamine B. Results Surf. Interfaces 2025, 18, 100408. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Feng, Y.; Wang, L.; Bian, Z.; Li, H.; Wang, Z.L. Fluid Eddy Induced Piezo-Promoted Photodegradation of Organic Dye Pollutants in Wastewater on ZnO Nanorod Arrays/3D Ni Foam. Mater. Today 2017, 20, 501–506. [Google Scholar] [CrossRef]
- Khan, M.S.; Asif, M.I.; Asif, M.; Khan, M.R.; Mustafa, G.; Adeel, M. Nanomaterials for the Catalytic Degradation and Detection of Microplastics: A Review. Top. Catal. 2025, 68, 796–813. [Google Scholar] [CrossRef]
- Md Noor, S.F.; Paiman, S.H.; Nordin, A.H.; Ngadi, N.; Nik Malek, N.A.N.; Abd Hamed, N.K. Solid- and Aqueous-Phase Approaches on Zinc Oxide-Based Photocatalytic System for Degradation of Plastics and Microplastics: A Review. Chem. Eng. Res. Des. 2024, 201, 194–208. [Google Scholar] [CrossRef]
- Sayem, M.A.; Hossen Suvo, M.A.; Syed, I.M.; Bhuiyan, M.A. Effective Adsorption and Visible Light Driven Enhanced Photocatalytic Degradation of Rhodamine B Using ZnO Nanoparticles Immobilized on Graphene Oxide Nanosheets. Results Phys. 2024, 58, 107471. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, E.; Hong, Z.L.; Ahmed, W.; Elhissi, A.; Khalid, N.R. Photocatalytic, Sonocatalytic and Sonophotocatalytic Degradation of Rhodamine B Using ZnO/CNTs Composites Photocatalysts. Ultrason. Sonochem. 2014, 21, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Pei, C.C.; Leung, W.W.-F. Photocatalytic Degradation of Rhodamine B by TiO2/ZnO Nanofibers under Visible-Light Irradiation. Sep. Purif. Technol. 2013, 114, 108–116. [Google Scholar] [CrossRef]
- Isai, K.A.; Shrivastava, V.S. Photocatalytic Degradation of Methylene Blue Using ZnO and 2%Fe–ZnO Semiconductor Nanomaterials Synthesized by Sol–Gel Method: A Comparative Study. SN Appl. Sci. 2019, 4, 251–262. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, E.; Hong, Z.L.; Xu, J.F.; Khalid, N.R.; Elhissi, A.; Ahmed, W. A Facile One-Step Approach to Synthesizing ZnO/Graphene Composites for Enhanced Degradation of Methylene Blue under Visible Light. Appl. Surf. Sci. 2013, 274, 273–281. [Google Scholar] [CrossRef]
- Stanley, R.; Jebasingh, J.A.; Vidyavathy, S.M.; Stanley, P.K.; Ponmani, P.; Shekinah, M.E.; Vasanthi, J. Excellent Photocatalytic Degradation of Methylene Blue, Rhodamine B and Methyl Orange Dyes by Ag-ZnO Nanocomposite under Natural Sunlight Irradiation. Optik 2021, 231, 166518. [Google Scholar] [CrossRef]
- Al-Gharibi, M.A.; Kyaw, H.H.; Al-Sabahi, J.N.; Zar Myint, M.T.; Al-Sharji, Z.A.; Al-Abri, M.Z. Silver Nanoparticles Decorated Zinc Oxide Nanorods Supported Catalyst for Photocatalytic Degradation of Paracetamol. Mater. Sci. Semicond. Process. 2021, 134, 105994. [Google Scholar] [CrossRef]
- Ivanova, D.; Tzvetkov, G.; Kaneva, N. Degradation of Paracetamol in Distilled and Drinking Water via Ag/ZnO Photocatalysis under UV and Natural Sunlight. Water 2023, 15, 3549. [Google Scholar] [CrossRef]
- Hassan, F.; Backer, S.N.; Almanassra, I.W.; Ali Atieh, M.; Elbahri, M.; Shanableh, A. Solar-Matched S-Scheme ZnO/g-C3N4 for Visible Light-Driven Paracetamol Degradation. Sci. Rep. 2024, 14, 12220. [Google Scholar] [CrossRef]
- Kaur, S.; Pal, B. Superior Visible-Light Absorbing Ag@ZnO Nanorods Hybrid Photocatalyst for Efficient Decomposition of Commercial Pharmaceuticals Tetracycline and Amoxicillin. J. Water Process Eng. 2024, 58, 104765. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Wei, S.; Wu, Y.; Zheng, Y.; Yuan, F.; Hou, J. Study on Photocatalytic Degradation of Amoxicillin in Wastewater by Bi2WO6/Nano-ZnO. Opt. Mater. 2022, 123, 111835. [Google Scholar] [CrossRef]
- Charafi, S.; Janani, F.Z.; Elhalil, A.; Abdennouri, M.; Sadiq, M.; Barka, N. Optimization of Photocatalytic Degradation of Amoxicillin by ZnO-TiO2 Heterojunction under UV-Visible Irradiation. Clean. Chem. Eng. 2025, 11, 100183. [Google Scholar] [CrossRef]
- Uheida, A.; Mejía, H.G.; Abdel-Rehim, M.; Hamd, W.; Dutta, J. Visible Light Photocatalytic Degradation of Polypropylene Microplastics in a Continuous Water Flow System. J. Hazard. Mater. 2021, 406, 124299. [Google Scholar] [CrossRef]
- Daher, E.A.; Hammoud, Y.; Robert, C.L.; Paz Lopez, C.V.; Riachi, B.; Hamd, W. Natural Sunlight-Driven Photocatalytic Degradation of Polypropylene Microplastics over ZnO Nanorods. Environ. Res. 2025, 279, 121836. [Google Scholar] [CrossRef]
- Zia, M.; Faisal, S.; Shams, D.F.; Anjum, F.; Saeed, M.; Shah, Z.; Nadhman, A. Degradation of Polyethylene Plastic by Non-Embedded Visible-Light Iron-Doped Zinc Oxide Nanophotocatalyst. Appl. Sci. Converg. Technol. 2021, 30, 87–91. [Google Scholar] [CrossRef]
- Lam, S.-M.; Sin, J.-C.; Zeng, H.; Lin, H.; Li, H.; Chai, Y.-Y.; Choong, M.-K.; Mohamed, A.R. Green Synthesis of Fe-ZnO Nanoparticles with Improved Sunlight Photocatalytic Performance for Polyethylene Film Deterioration and Bacterial Inactivation. Mater. Sci. Semicond. Process. 2021, 123, 105574. [Google Scholar] [CrossRef]
- Lin, S.; Chen, Y.; Li, H.; Wang, W.; Wang, Y.; Wu, M. Application of Metal-Organic Frameworks and Their Derivates for Thermal-Catalytic C1 Molecules Conversion. iScience 2024, 27, 109656. [Google Scholar] [CrossRef]
- Fan, X.; Meng, X.-M.; Zhang, X.-H.; Wu, S.-K.; Lee, S.-T. Formation of ZnS/SiO2 Nanocables. Appl. Phys. Lett. 2005, 86, 173111. [Google Scholar] [CrossRef]
- Darmawan, A.; Zakiyah, H.; Muhtar, H.; Elma, M. Graphene Oxide/Silicon Dioxide (GO/SiO2) Hybrid Coating on Zirconia Ceramic for Sustainable Water Desalination. Ceram. Int. 2024, 50, 47232–47243. [Google Scholar] [CrossRef]
- Tyagi, S.; Singh, P.K.; Tiwari, A.K. Optimization of Graphene Oxide Layer Thickness of ZnO-Based Hybrid Solar Cell Using SCAPS 1D: A Comparative Study on ZnO/GO and ZnO/SiO2 Hybrid Cells. In Sustainable Technology and Advanced Computing in Electrical Engineering; Lecture Notes in Electrical Engineering; Mahajan, V., Chowdhury, A., Padhy, N.P., Lezama, F., Eds.; Springer Nature: Singapore, 2022; Volume 939, pp. 785–800. ISBN 978-981-19436-3-8. [Google Scholar]
- Prasert, A.; Sontikaew, S.; Sriprapai, D.; Chuangchote, S. Polypropylene/ZnO Nanocomposites: Mechanical Properties, Photocatalytic Dye Degradation, and Antibacterial Property. Materials 2020, 13, 914. [Google Scholar] [CrossRef] [PubMed]
- Zailan, S.N.; Bouaissi, A.; Mahmed, N.; Abdullah, M.M.A.B. Influence of ZnO Nanoparticles on Mechanical Properties and Photocatalytic Activity of Self-Cleaning ZnO-Based Geopolymer Paste. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2007–2016. [Google Scholar] [CrossRef]
- Ahn, S.; Hong, M.; Sundararajan, M.; Ess, D.H.; Baik, M.-H. Design and Optimization of Catalysts Based on Mechanistic Insights Derived from Quantum Chemical Reaction Modeling. Chem. Rev. 2019, 119, 6509–6560. [Google Scholar] [CrossRef]
- Shambhawi; Mohan, O.; Choksi, T.S.; Lapkin, A.A. The Design and Optimization of Heterogeneous Catalysts Using Computational Methods. Catal. Sci. Technol. 2024, 14, 515–532. [Google Scholar] [CrossRef]
- Suvarna, M.; Pérez-Ramírez, J. Embracing Data Science in Catalysis Research. Nat. Catal. 2024, 7, 624–635. [Google Scholar] [CrossRef]
- Kumar, A. The Role of Simulation and Modeling in Artificial Intelligence: A Review. Int. J. Model. Simul. Sci. Comput. 2024, 15, 2430002. [Google Scholar] [CrossRef]
- Liu, J.; Liang, L.; Su, B.; Wu, D.; Zhang, Y.; Wu, J.; Fu, C. Transformative Strategies in Photocatalyst Design: Merging Computational Methods and Deep Learning. J. Mater. Inform. 2024, 4, 33. [Google Scholar] [CrossRef]
- Rangineni, S. An Analysis of Data Quality Requirements for Machine Learning Development Pipelines Frameworks. Int. J. Comput. Trends Technol. 2023, 71, 16–27. [Google Scholar] [CrossRef]
- Shahzad, K.; Mardare, A.I.; Hassel, A.W. Accelerating Materials Discovery: Combinatorial Synthesis, High-Throughput Characterization, and Computational Advances. Sci. Technol. Adv. Mater. Methods 2024, 4, 2292486. [Google Scholar] [CrossRef]
- Badini, S.; Regondi, S.; Pugliese, R. Unleashing the Power of Artificial Intelligence in Materials Design. Materials 2023, 16, 5927. [Google Scholar] [CrossRef]
- Aliferis, C.; Simon, G. Overfitting, Underfitting and General Model Overconfidence and Under-Performance Pitfalls and Best Practices in Machine Learning and AI. In Health Informatics; Springer International Publishing: Cham, Switzerland, 2024; pp. 477–524. ISBN 978-3-031-39354-9. [Google Scholar]
- Barbierato, E.; Gatti, A. The Challenges of Machine Learning: A Critical Review. Electronics 2024, 13, 416. [Google Scholar] [CrossRef]
- Stoller, M.; Ochando-Pulido, J.M. ZnO Nano-Particles Production Intensification by Means of a Spinning Disk Reactor. Nanomaterials 2020, 10, 1321. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.B.N.; Jakmunee, J.; Mishra, Y.K.; Prakash, J. ZnO Based 0–3D Diverse Nano-Architectures, Films and Coatings for Biomedical Applications. J. Mater. Chem. B 2024, 12, 2950–2984. [Google Scholar] [CrossRef] [PubMed]
- Gulab, H.; Fatima, N.; Tariq, U.; Gohar, O.; Irshad, M.; Khan, M.Z.; Saleem, M.; Ghaffar, A.; Hussain, M.; Khaliq Jan, A.; et al. Advancements in Zinc Oxide Nanomaterials: Synthesis, Properties, and Diverse Applications. Nano-Struct. Nano-Objects 2024, 39, 101271. [Google Scholar] [CrossRef]
- Hartlieb, K.J.; Raston, C.L.; Saunders, M. Controlled Scalable Synthesis of ZnO Nanoparticles. Chem. Mater. 2007, 19, 5453–5459. [Google Scholar] [CrossRef]




| Physical Parameters | Values |
|---|---|
| Stable phase at 300 K | Wurtzite |
| Lattice constants | a = b = 0.32495 nm; c = 0.52069 nm |
| Melting point | 1975 °C |
| Density | 5.66 g/cm3 |
| Band gap (direct) | 3.37 eV |
| Refractive index | 2.01 |
| Hole effective mass | 0.59 |
| Electron effective mass | 0.24 |
| Exciton binding energy | 60 meV |
| Static dielectric constant | 8.656 |
| No. | Material | Pollutant | Radiation Source | Degradation Efficiency | Reference |
|---|---|---|---|---|---|
| 1 | ZnO | Rhodamine B | Visible light | 20%, 60 min | [279] |
| 2 | ZnO/carbon nanotubes | Rhodamine B | Sunlight | 50%, 60 min | [280] |
| 3 | TiO2/ZnO nanofibers | Rhodamine B | Visible light | 100%, 60 min | [281] |
| 4 | ZnO | Methylene blue | Visible light | 86%, 180 min | [282] |
| 5 | ZnO/graphene composites | Methylene blue | Visible light | 100%, 90 min | [283] |
| 6 | Ag/ZnO | Methylene blue | Sunlight | 98%, 30 min | [284] |
| 7 | ZnO | Paracetamol | Visible light | 54%, 240 min | [285] |
| 8 | Ag/ZnO | Paracetamol | Sunlight | 80%, 240 min | [286] |
| 9 | ZnO/g-C3N4 | Paracetamol | Visible light | 90%, 60 min | [287] |
| 10 | ZnO | Amoxicillin | Visible light | 38%, 90 min | [288] |
| 11 | Bi2WO6/nano-ZnO | Amoxicillin | Visible light | 93%, 120 min | [289] |
| 12 | ZnO/TiO2 | Amoxicillin | Visible light | 94%, 210 min | [290] |
| 13 | ZnO | Polypropylene | Visible light | 40%, 3600 min | [291] |
| 14 | ZnO nanorods | Polypropylene | Sunlight | 100%, 11,760 min | [292] |
| 15 | ZnO | Polyethylene | Visible light | 15%, 10,080 min | [293] |
| 16 | Fe-ZnO | Polyethylene | Sunlight | 40%, 7200 min | [294] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armaković, S.J.; Armaković, S.; Bilić, A.; Savanović, M.M. ZnO-Based Photocatalysts: Synergistic Effects of Material Modifications and Machine Learning Optimization. Catalysts 2025, 15, 793. https://doi.org/10.3390/catal15080793
Armaković SJ, Armaković S, Bilić A, Savanović MM. ZnO-Based Photocatalysts: Synergistic Effects of Material Modifications and Machine Learning Optimization. Catalysts. 2025; 15(8):793. https://doi.org/10.3390/catal15080793
Chicago/Turabian StyleArmaković, Sanja J., Stevan Armaković, Andrijana Bilić, and Maria M. Savanović. 2025. "ZnO-Based Photocatalysts: Synergistic Effects of Material Modifications and Machine Learning Optimization" Catalysts 15, no. 8: 793. https://doi.org/10.3390/catal15080793
APA StyleArmaković, S. J., Armaković, S., Bilić, A., & Savanović, M. M. (2025). ZnO-Based Photocatalysts: Synergistic Effects of Material Modifications and Machine Learning Optimization. Catalysts, 15(8), 793. https://doi.org/10.3390/catal15080793











