Silicalite-1 Zeolite-Supported Cu Nanoparticles for Ethanol Dehydrogenation: Influence of Silanols
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Catalysts
2.2. Catalytic Performance
3. Materials and Methods
3.1. Reagent and Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zabed, H.; Sahu, J.N.; Suely, A.; Boyce, A.N.; Faruq, G. Bioethanol production from renewable sources: Current perspectives and technological progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- Yao, X.; Li, T.; Chung, S.; Ruiz-Martínez, J. Advances in the catalytic conversion of ethanol into nonoxygenated added-value chemicals. Adv. Mater. 2024, 36, 2406472. [Google Scholar] [CrossRef] [PubMed]
- Gérardy, R.; Debecker, D.P.; Estager, J.; Luis, P.; Monbaliu, J.C.M. Continuous flow upgrading of selected C2-C6 platform chemicals derived from biomass. Chem. Rev. 2020, 120, 7219–7347. [Google Scholar] [CrossRef]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of biomass: Deriving more value from waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef]
- Shindell, D.; Smith, C.J. Climate and air-quality benefits of a realistic phase-out of fossil fuels. Nature 2019, 573, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, B.; Yuan, H.; Sun, Y.; Yang, D.; Cui, X.; Shi, F. The catalytic dehydrogenation of ethanol by heterogeneous catalysts. Catal. Sci. Technol. 2021, 11, 1652–1664. [Google Scholar] [CrossRef]
- Li, X.; Kant, A.; He, Y.; Thakkar, H.V.; Atanga, M.A.; Rezaei, F.; Ludlow, D.K.; Rownaghi, A.A. Light olefins from renewable resources: Selective catalytic dehydration of bioethanol to propylene over zeolite and transition metal oxide catalysts. Catal. Today 2016, 276, 62–77. [Google Scholar] [CrossRef]
- Wu, X.; Fang, G.; Tong, Y.; Jiang, D.; Liang, Z.; Leng, W.; Liu, L.; Tu, P.; Wang, H.; Ni, J.; et al. Catalytic upgrading of ethanol to n-butanol: Progress in catalyst development. ChemSusChem 2018, 11, 71–85. [Google Scholar] [CrossRef]
- Pomalaza, G.; Capron, M.; Ordomsky, V.; Dumeignil, F. Recent breakthroughs in the conversion of ethanol to butadiene. Catalysts 2016, 6, 203. [Google Scholar] [CrossRef]
- Yamamoto, T.; Mine, H.; Katada, S.; Tone, T. Direct ethyl acetate synthesis from ethanol over amorphous-, monoclinic-, tetragonal ZrO2 supported copper catalysts prepared from the same zirconium source. Appl. Catal. B 2023, 327, 122433. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y. Recent advances in catalytic conversion of ethanol to chemicals. ACS Catal. 2014, 4, 1078–1090. [Google Scholar] [CrossRef]
- Pang, J.; Yin, M.; Wu, P.; Li, X.; Li, H.; Zheng, M.; Zhang, T. Advances in catalytic dehydrogenation of ethanol to acetaldehyde. Green Chem. 2021, 23, 7902–7916. [Google Scholar] [CrossRef]
- Phung, T.K. Copper-based catalysts for ethanol dehydrogenation and dehydrogenative coupling into hydrogen, acetaldehyde and ethyl acetate. Int. J. Hydrogen Energy 2022, 47, 42234–42249. [Google Scholar] [CrossRef]
- He, L.; Zhou, B.; Sun, D.; Li, W.; Lv, W.; Wang, J.; Liang, Y.; Lu, A. Catalytic conversion of ethanol to oxygen-containing value-added chemicals. ACS Catal. 2023, 13, 11291–11304. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, W.; Gan, T.; Chu, X.; Zhu, W.; Zhang, W.; Wang, D.; Liu, G. Enhancing stability of industrial Cu-based catalysts under harsh reduction reaction conditions with silica armor protection. Chem. Eng. J. 2024, 500, 157331. [Google Scholar] [CrossRef]
- Pampararo, G.; Hlavenková, Z.; Styskalik, A.; Debecker, D.P. Suppressing on-stream deactivation of CuSiO2 catalysts in the dehydrogenation of bioethanol to acetaldehyde. Catal. Sci. Technol. 2024, 14, 4912–4926. [Google Scholar] [CrossRef]
- Guo, J.; Pang, J.; Yin, M.; Feng, L.; Liu, S.; Wu, P.; Zheng, M. Diamine-assistant synthesis of Cu@MFI catalysts for ethanol dehydrogenation. ChemCatChem 2024, 16, e202400269. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, H.; Yang, Z.; Liu, S.; Yang, T.; Wang, L.; Sun, Y.; Wang, X.; Li, Q.; Tian, P. Spatial confinement effect of zeolite Silicalite-1 on dispersing high loading Cu nanoparticles and their superior ethanol dehydrogenation catalytic performance. Appl. Catal. B 2025, 367, 125099. [Google Scholar] [CrossRef]
- Tian, C.; Yue, Y.; Miao, C.; Hua, W.; Gao, Z. Mesoporous silica-encapsulated Cu nanoparticles with enhanced performance for ethanol dehydrogenation to acetaldehyde. ACS Sustain. Chem. Eng. 2025, 13, 1401–1408. [Google Scholar] [CrossRef]
- Tian, C.; Yue, Y.; Miao, C.; Hua, W.; Gao, Z. Dehydrogenation of ethanol to acetaldehyde catalyzed by Cu nanoparticles supported on nanorod-shaped La2O2CO3. Appl. Catal. A 2025, 702, 120334. [Google Scholar] [CrossRef]
- Tang, F.; Li, W.; Wang, D.; Lu, A. Synergistic roles of hexagonal boron nitride-supported Cu0 and Cu+ species in dehydrogenation of ethanol to acetaldehyde: A computational mechanistic study. J. Phys. Chem. C 2023, 127, 11014–11025. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, L.; Lu, A. Highly selective copper catalyst supported on mesoporous carbon for the dehydrogenation of ethanol to acetaldehyde. ChemCatchem 2015, 7, 2846–2852. [Google Scholar] [CrossRef]
- Zhukova, A.I.; Chuklina, S.G.; Maslenkova, S.A. Study of Cu modified Zr and Al mixed oxides in ethanol conversion: The structure-catalytic activity relationship. Catal. Today 2021, 379, 159–165. [Google Scholar] [CrossRef]
- Campisano, I.S.P.; Rodella, C.B.; Sousa, Z.S.B.; Henriques, C.A.; da Silva, V.T. Influence of thermal treatment conditions on the characteristics of Cu-based metal oxides derived from hydrotalcite-like compounds and their performance in bio-ethanol dehydrogenation to acetaldehyde. Catal. Today 2018, 306, 111–120. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, Y.; Zhou, R.; Chang, Z.; Hou, Z. Co-production of hydrogen and acetaldehyde from ethanol over a highly dispersed Cu catalyst. Fuel 2022, 321, 123980. [Google Scholar] [CrossRef]
- Ausavasukhi, A.; Krukrathok, N.; Singthaisong, P. Thermal transformation of copper incorporated hydrotalcite-derived oxides and their catalytic activity for ethanol dehydrogenation. J. Ind. Eng. Chem. 2023, 117, 371–385. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, H.R.; Jaenicke, S.; Chuah, G.K. Highly efficient and robust Cu catalyst for non-oxidative dehydrogenation of ethanol to acetaldehyde and hydrogen. J. Catal. 2020, 389, 19–28. [Google Scholar] [CrossRef]
- Pang, J.; Zheng, M.; Wang, C.; Yang, X.; Liu, H.; Liu, X.; Sun, J.; Wang, Y.; Zhang, T. Hierarchical echinus-like Cu-MFI catalysts for ethanol dehydrogenation. ACS Catal. 2020, 10, 13624–13629. [Google Scholar] [CrossRef]
- Pang, J.; Yin, M.; Wu, P.; Song, L.; Li, X.; Zhang, T.; Zheng, M. Redispersion and stabilization of Cu/MFI catalysts by the encapsulation method for ethanol dehydrogenation. ACS Sustain. Chem. Eng. 2023, 11, 3297–3305. [Google Scholar] [CrossRef]
- Finger, P.H.; Osmari, T.A.; Cabral, N.M.; Bueno, J.M.C.; Marcel, J.; Gallo, R. Direct synthesis of Cu supported on mesoporous silica: Tailoring the Cu loading and the activity for ethanol dehydrogenation. Catal. Today 2021, 381, 26–33. [Google Scholar] [CrossRef]
- Lin, L.; Cao, P.; Pang, J.; Wang, Z.; Jiang, Q.; Su, Y.; Chen, R.; Wu, Z.; Zheng, M.; Luo, W. Zeolite-encapsulated Cu nanoparticles with enhanced performance for ethanol dehydrogenation. J. Catal. 2022, 413, 565–574. [Google Scholar] [CrossRef]
- Liu, H.; Chang, Z.; Fu, J.; Hou, Z. A CuZn-BTC derived stable Cu/ZnO@SiO2 catalyst for ethanol dehydrogenation. Appl. Catal. B 2023, 324, 122194. [Google Scholar] [CrossRef]
- Klinthongchai, Y.; Prichanont, S.; Praserthdam, P.; Jongsomjit, B. Synthesis, characteristics and application of mesocellular foam carbon (MCF-C) as catalyst for dehydrogenation of ethanol to acetaldehyde. J. Environ. Chem. Eng. 2020, 8, 103752. [Google Scholar] [CrossRef]
- Tian, C.; Yue, Y.; Miao, C.; Hua, W.; Gao, Z. Cu/MgO as an efficient new catalyst for the non-oxidative dehydrogenation of ethanol into acetaldehyde. Catalysts 2024, 14, 541. [Google Scholar] [CrossRef]
- Cassinelli, W.H.; Martins, L.; Passos, A.R.; Pulcinelli, S.H.; Rochet, A.; Briois, V.; Santilli, C.V. Correlation between structural and catalytic properties of copper supported on porous alumina for the ethanol dehydrogenation reaction. ChemCatChem 2015, 7, 1668–1677. [Google Scholar] [CrossRef]
- Kokotailo, G.T.; Lawton, S.L.; Olson, D.H.; Meier, W.M. Structure of synthetic zeolite ZSM-5. Nature 1978, 272, 437–438. [Google Scholar] [CrossRef]
- Yue, Y.; Gu, L.; Zhou, Y.; Liu, H.; Yuan, P.; Zhu, H.; Bai, Z.; Bao, X. Template-free synthesis and catalytic applications of microporous and hierarchical ZSM-5 zeolites from natural aluminosilicate minerals. Ind. Eng. Chem. Res. 2017, 56, 10069–10077. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.T. Increasing selective CO2 adsorption on amine-grafted SBA-15 by increasing silanol density. J. Phys. Chem. C 2011, 115, 21264–21272. [Google Scholar] [CrossRef]
- Peng, P.; Moldovan, S.; Vicente, A.; Ruaux, V.; Debost, M.; Hu, H.; Aleksandrov, H.A.; Vayssilov, G.N.; Yan, Z.; Mintova, S. Synthesis of nanosized MFI zeolites using Cu-containing complexes. Microporous Mesoporous Mater. 2023, 357, 112625. [Google Scholar] [CrossRef]
- Piva, D.; Fang, G.; Ghojavand, S.; Dalena, F.; AlHajjar, N.; De Waele, V.; Ordomsky, V.; Khodakov, A.; Tayeb, K.B.; Fernandes, T.; et al. Role of hydroxyl groups in Zn-containing nanosized MFI zeolite for the photocatalytic oxidation of methane. ChemSusChem 2025, 18, e202401656. [Google Scholar] [CrossRef]
- Medeiros-Costa, I.C.; Dib, E.; Dubray, F.; Moldovan, S.; Gilson, J.; Dath, J.; Nesterenko, N.; Aleksandrov, H.A.; Vayssilov, G.N.; Mintova, S. Unraveling the effect of silanol defects on the insertion of single site Mo in the MFI zeolite framework. Inorg. Chem. 2022, 61, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Palčić, A.; Moldovan, S.; El Siblani, H.; Vicente, A.; Valtchev, V. Defect sites in zeolites: Origin and healing. Adv. Sci. 2022, 9, 2104414. [Google Scholar] [CrossRef]
- Luo, Y.; Wei, C.; Miao, C.; Yue, Y.; Hua, W.; Gao, Z. Isobutane dehydrogenation assisted by CO2 over Silicalite-1-supported ZnO catalysts: Influence of support crystallite size. Chin. J. Chem. 2020, 38, 703–708. [Google Scholar] [CrossRef]
- Bolis, V.; Busco, C.; Bordiga, S.; Ugliengo, P.; Lamberti, C.; Zecchina, A. Calorimetric and IR spectroscopic study of the interaction of NH3 with variously prepared defective silicalites-Comparison with Ab initio computational data. Appl. Surf. Sci. 2002, 196, 56–70. [Google Scholar] [CrossRef]
- Karbowiak, T.; Saada, M.A.; Rigolet, S.; Ballandras, A.; Weber, G.; Bezverkhyy, I.; Soulard, M.; Patarin, J.; Bellat, J.P. New insights in the formation of silanol defects in silicalite-1 by water intrusion under high pressure. Phys. Chem. Chem. Phys. 2010, 12, 11454–11466. [Google Scholar] [CrossRef]
- Heitmann, G.P.; Dahlhoff, G.; Hölderich, W.F. Catalytically active sites for the Beckmann rearrangement of cyclohexanone oxime to ε-caprolactam. J. Catal. 1999, 186, 12–19. [Google Scholar] [CrossRef]
- Barbera, K.; Bonino, F.; Bordiga, S.; Janssens, T.V.W.; Beato, P. Structure-deactivation relationship for ZSM-5 catalysts governed by framework defects. J. Catal. 2011, 280, 196–205. [Google Scholar] [CrossRef]
- Liu, G.; Liu, J.; He, N.; Miao, C.; Wang, J.; Xin, Q.; Guo, H. Silicalite-1 zeolite acidification by zinc modification and its catalytic properties for isobutane conversion. RSC Adv. 2018, 8, 18663–18671. [Google Scholar] [CrossRef]
- Zhao, D.; Tian, X.; Doronkin, D.E.; Han, S.; Kondratenko, V.A.; Grunwaldt, J.; Perechodjuk, A.; Vuong, T.H.; Rabeah, J.; Eckelt, R.; et al. In situ formation of ZnOx species for efficient propane dehydrogenation. Nature 2021, 599, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Kong, X.; Peng, B.; Li, L.; Fang, Z.; Zhu, Y. Efficient Cu catalyst for 5-hydroxymethylfurfural hydrogenolysis by forming Cu–O–Si bonds. Catal. Sci. Technol. 2020, 10, 7323–7330. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, F.; Kang, H.; Chen, J.; Zhang, X.; Xia, C. Highly dispersed silica-supported copper nanoparticles prepared by precipitation-gel method: A simple by efficient and stable catalyst for glycerol hydrogenolysis. Chem. Mater. 2008, 20, 5090–5099. [Google Scholar] [CrossRef]
- Sato, A.G.; Volanti, D.P.; Meira, D.M.; Damyanova, S.; Longo, E.; Bueno, J.M.C. Effect of the ZrO2 phase on the structure and behavior of supported Cu catalysts for ethanol conversion. J. Catal. 2013, 307, 1–17. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, L.; Li, W.; Li, W.; Si, R.; Schüth, F.; Lu, A. Cu supported on thin carbon layer-coated porous SiO2 for efficient ethanol dehydrogenation. Catal. Sci. Technol. 2018, 8, 472–479. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, D.; Liu, W.; Zhang, M.; Lou, Y.; Wang, Z.; Tang, Q.; Yang, J. Uniformly dispersed Cu nanoparticles over mesoporous silica as a highly selective and recyclable ethanol dehydrogenation catalyst. Catalysts 2022, 12, 1049. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Y.; Cui, X.; Wang, G.; Cen, Y.; Deng, T.; Yan, W.; Gao, J.; Zhu, S.; Olsbye, U.; et al. A highly stable copper-based catalyst for clarifying the catalytic roles of Cu0 and Cu+ species in methanol dehydrogenation. Angew. Chem. Int. Ed. 2018, 57, 1836–1840. [Google Scholar] [CrossRef]
- Chen, L.; Guo, P.; Qiao, M.; Yan, S.; Li, H.; Shen, W.; Xu, H.; Fan, K. Cu/SiO2 catalysts prepared by the ammonia evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2008, 257, 172–180. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, W.; Rehman, M.U.; Liu, W.; Xu, Y.; Huang, H.; Wang, S.; Zhao, Y.; Mei, D.; Ma, X. Copper phyllosilicate nanotube catalysts for the chemosynthesis of cyclohexane via hydrodeoxygenation of phenol. ACS Catal. 2022, 12, 4724–4736. [Google Scholar] [CrossRef]
- Huang, J.; Ding, T.; Ma, K.; Cai, J.; Sun, Z.; Tian, Y.; Jiang, Z.; Zhang, J.; Zheng, L.; Li, X. Modification of Cu/SiO2 catalysts by La2O3 to quantitatively tune Cu+-Cu0 dual sites with improved catalytic activities and stabilities for dimethyl ether steam reforming. ChemCatChem 2018, 10, 3862–3871. [Google Scholar] [CrossRef]
- Xu, C.; Chen, G.; Zhao, Y.; Liu, P.; Duan, X.; Gu, L.; Fu, G.; Yuan, Y.; Zheng, N. Interfacing with silica boosts the catalysis of copper. Nat. Commun. 2018, 9, 3367. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Tian, J.; Chen, W.; Guan, Y.; Xu, H.; Li, X.; Wu, H.; Wu, P. One-pot synthesized core/shell structured zeolite@copper catalysts for selective hydrogenation of ethylene carbonate to methanol and ethylene glycol. Green Chem. 2019, 21, 5414–5426. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, R.; Sodesawa, T.; Yuma, K.I.; Obata, Y. Distinction between surface and bulk oxidation of Cu through N2O decomposition. J. Catal. 2000, 196, 195–199. [Google Scholar] [CrossRef]
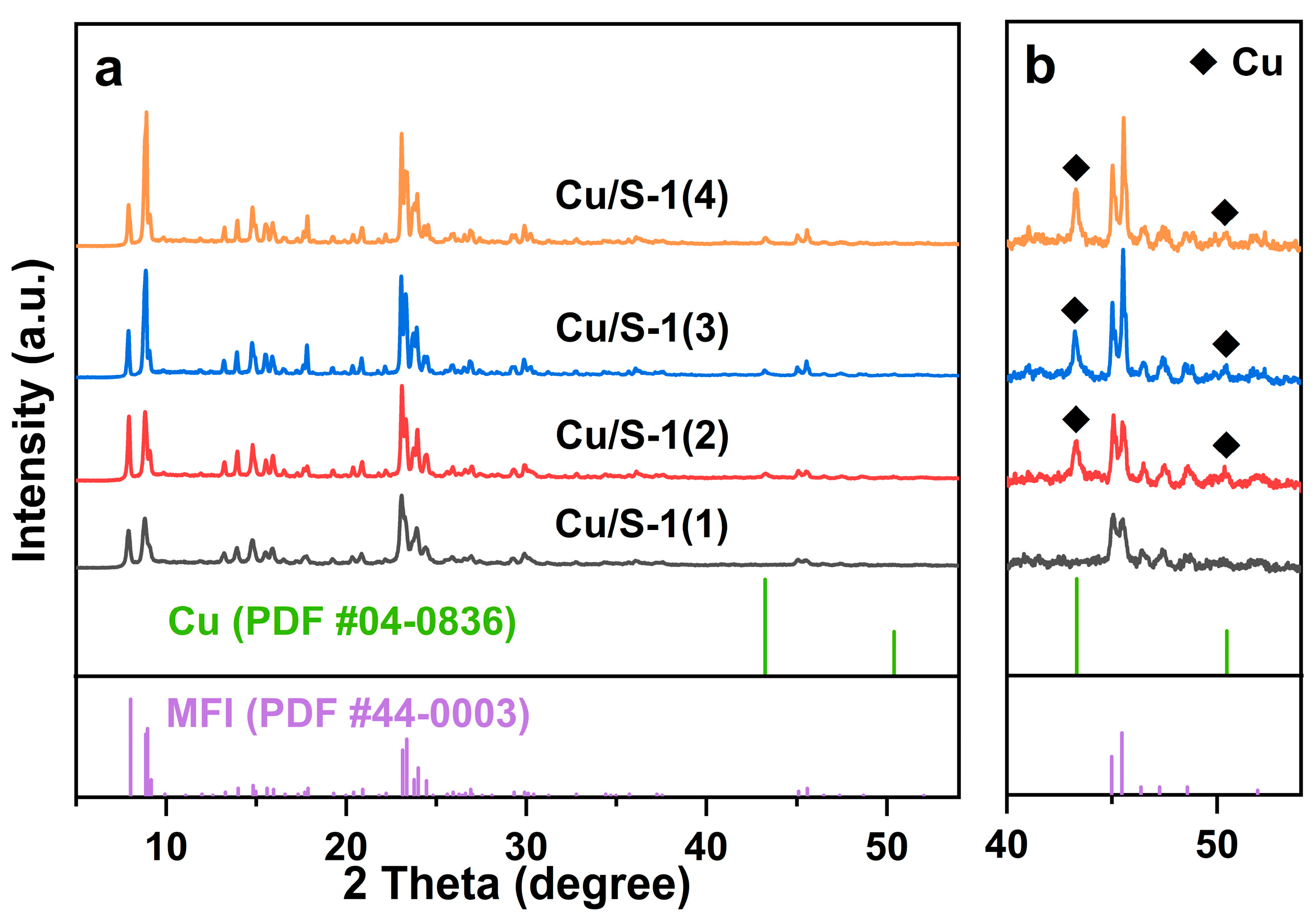
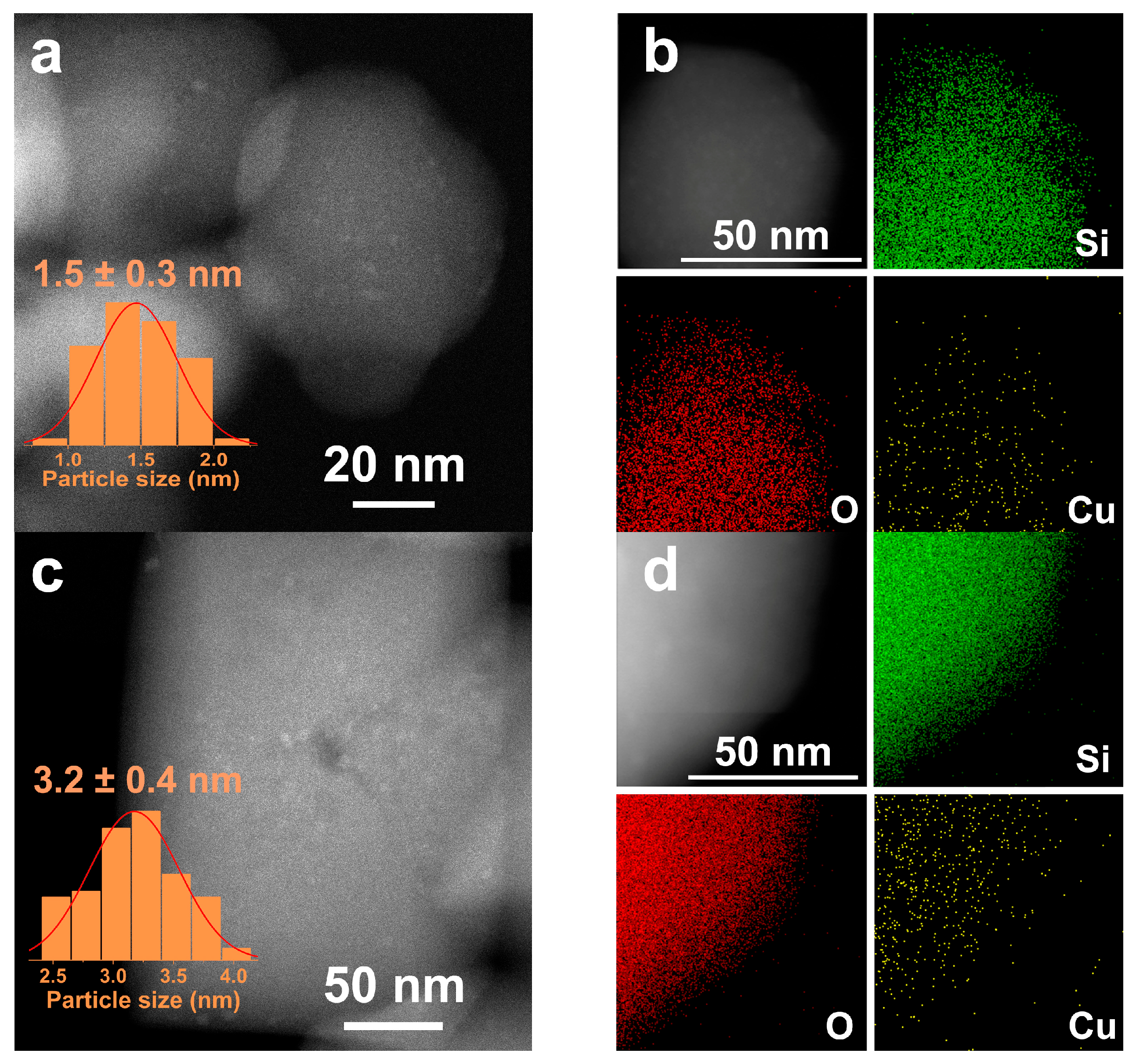
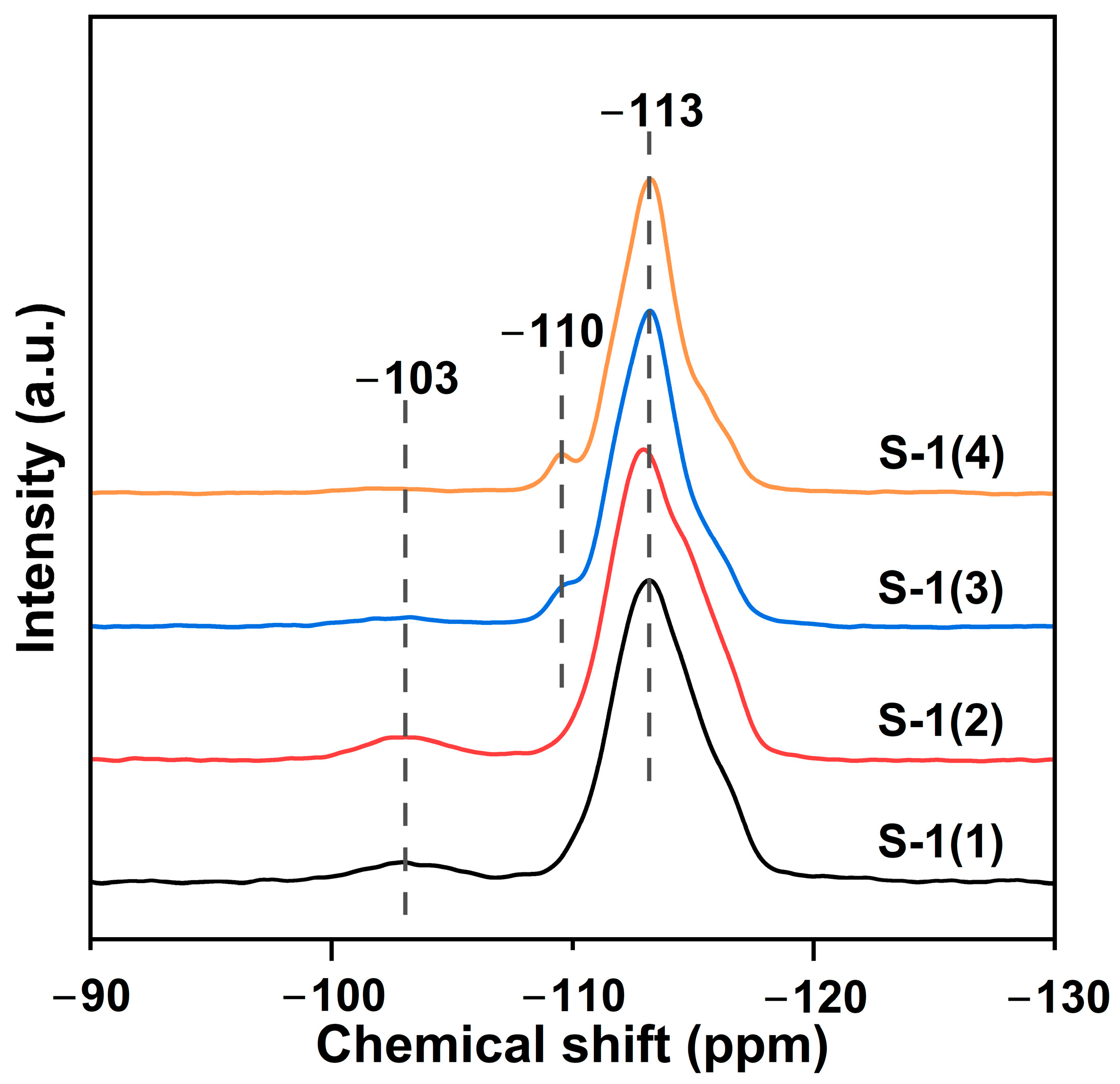
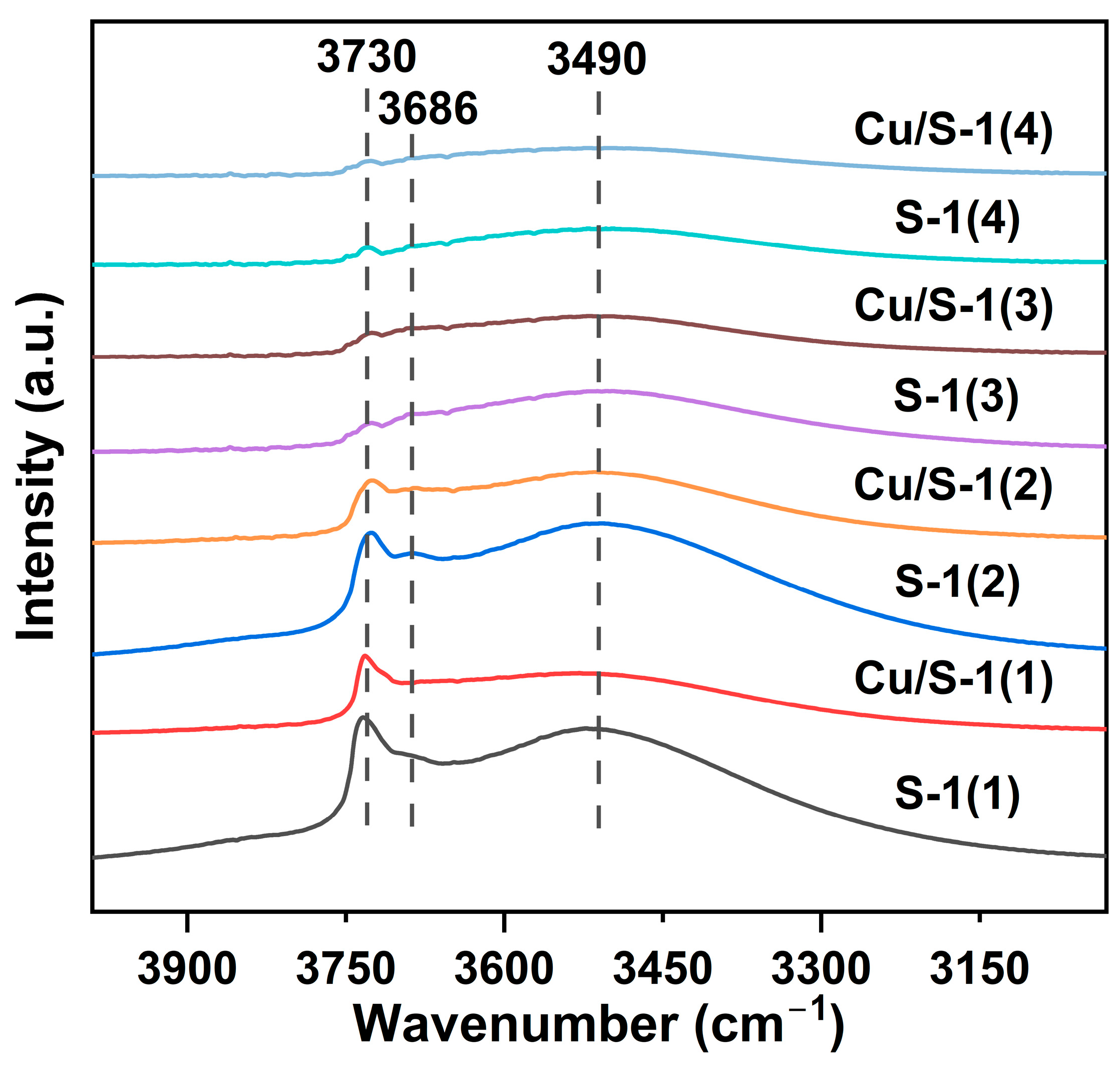
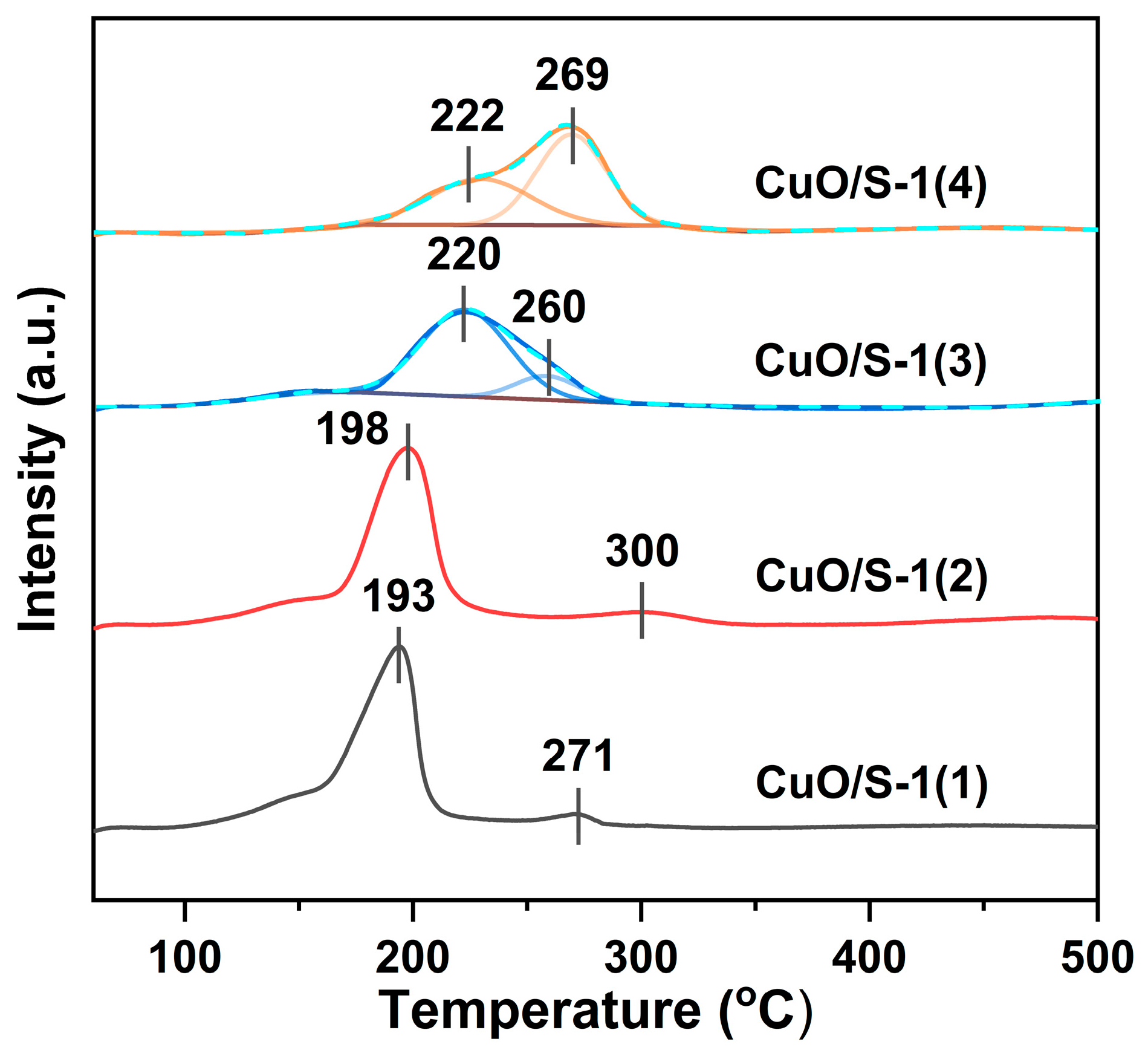
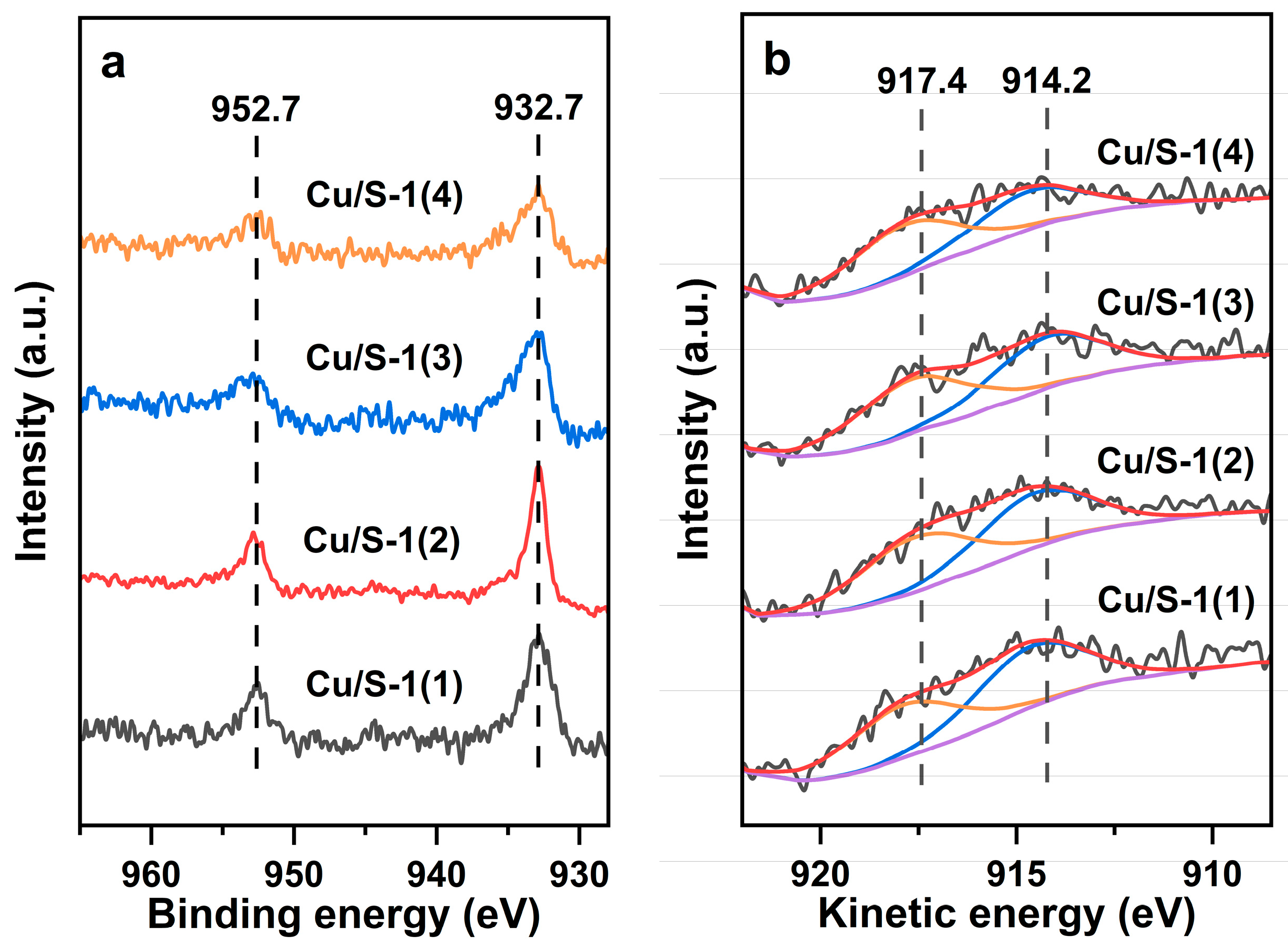
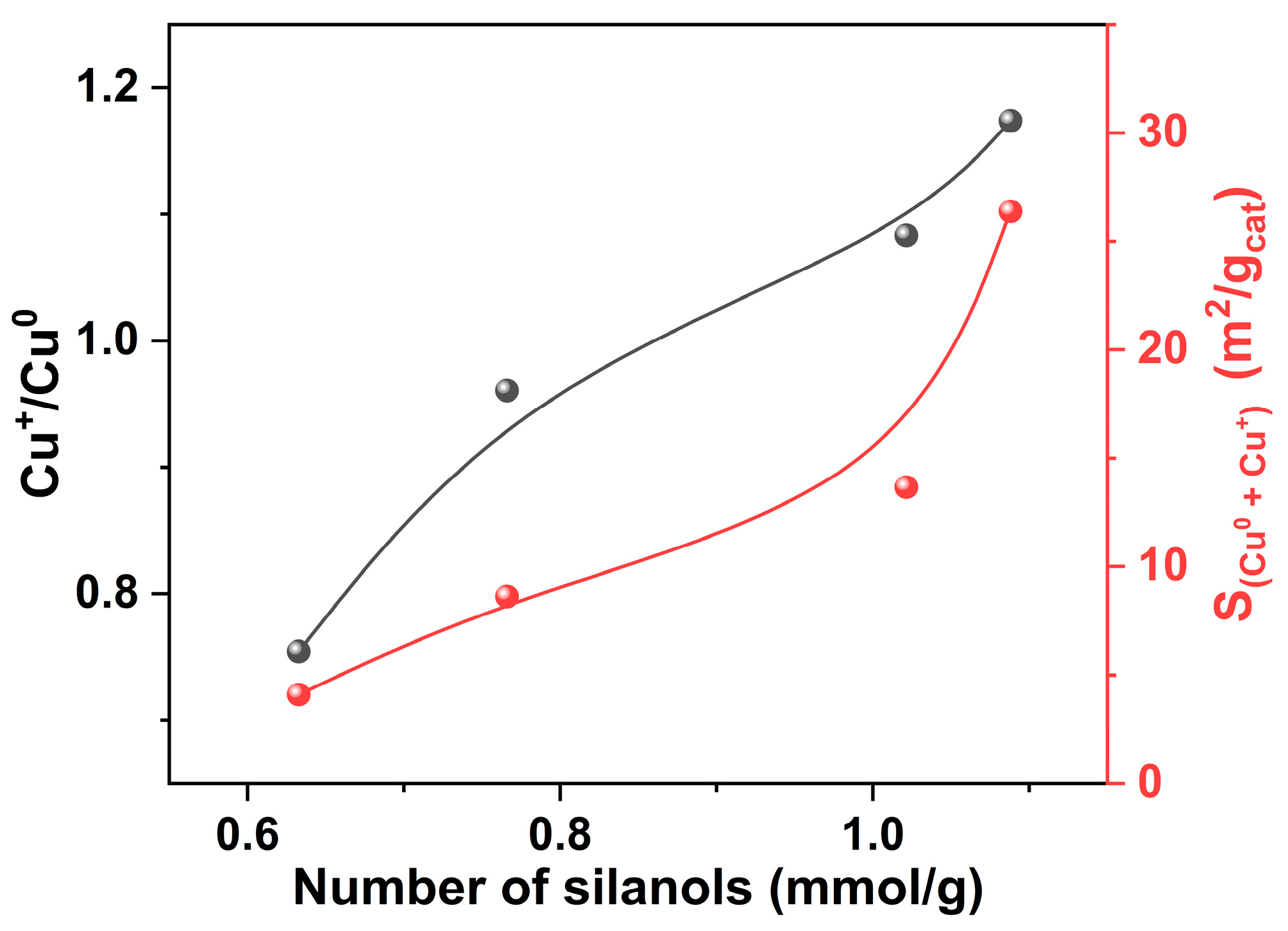

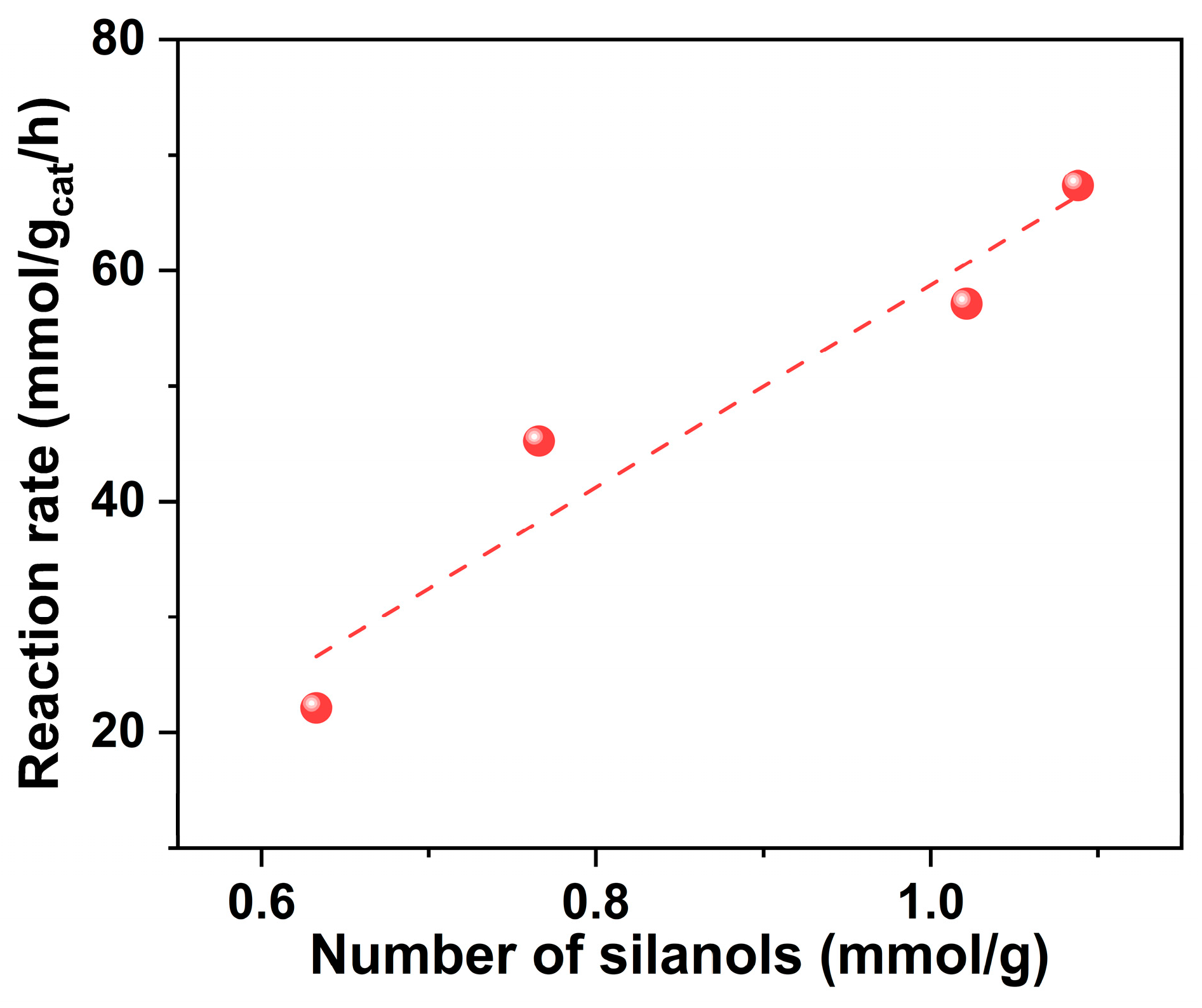

| Sample | SBET | Sext | Smicro | Vmicro | n(OH) | Q3/Q4 |
|---|---|---|---|---|---|---|
| (m2/g) | (m2/g) a | (m2/g) a | (cm3/g) a | (mmol/g) b | Ratio c | |
| S-1(1) | 491 | 148 | 343 | 0.143 | 1.09 | 0.108 |
| S-1(2) | 437 | 93 | 344 | 0.148 | 1.02 | 0.100 |
| S-1(3) | 405 | 79 | 326 | 0.143 | 0.77 | 0.073 |
| S-1(4) | 400 | 75 | 325 | 0.142 | 0.63 | 0.050 |
| Catalyst | Cu Loading | SBET | Sext | Smicro | Vmicro | DCu | dCu | Cu+/Cu0 | SCu0 | SCu+ |
|---|---|---|---|---|---|---|---|---|---|---|
| (wt%) a | (m2/g) | (m2/g) b | (m2/g) b | (cm3/g) b | (%) c | (nm) c | Ratio d | (m2/g) c | (m2/g) e | |
| Cu/S-1(1) | 3.03 | 408 | 72 | 336 | 0.140 | 65 | 1.5 | 1.17 | 12.2 | 14.2 |
| Cu/S-1(2) | 3.02 | 409 | 72 | 337 | 0.145 | 32 | 3.1 | 1.08 | 6.6 | 7.1 |
| Cu/S-1(3) | 3.03 | 366 | 47 | 319 | 0.141 | 22 | 4.5 | 0.96 | 4.4 | 4.2 |
| Cu/S-1(4) | 3.02 | 382 | 61 | 321 | 0.141 | 12 | 8.3 | 0.75 | 2.3 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, C.; Tian, C.; Yue, Y.; Tang, G.; Hua, W.; Gao, Z. Silicalite-1 Zeolite-Supported Cu Nanoparticles for Ethanol Dehydrogenation: Influence of Silanols. Catalysts 2025, 15, 787. https://doi.org/10.3390/catal15080787
He C, Tian C, Yue Y, Tang G, Hua W, Gao Z. Silicalite-1 Zeolite-Supported Cu Nanoparticles for Ethanol Dehydrogenation: Influence of Silanols. Catalysts. 2025; 15(8):787. https://doi.org/10.3390/catal15080787
Chicago/Turabian StyleHe, Chaofan, Chao Tian, Yinghong Yue, Gangfeng Tang, Weiming Hua, and Zi Gao. 2025. "Silicalite-1 Zeolite-Supported Cu Nanoparticles for Ethanol Dehydrogenation: Influence of Silanols" Catalysts 15, no. 8: 787. https://doi.org/10.3390/catal15080787
APA StyleHe, C., Tian, C., Yue, Y., Tang, G., Hua, W., & Gao, Z. (2025). Silicalite-1 Zeolite-Supported Cu Nanoparticles for Ethanol Dehydrogenation: Influence of Silanols. Catalysts, 15(8), 787. https://doi.org/10.3390/catal15080787







