Abstract
The selective dehydrogenation of ethanol to acetaldehyde is an efficient alternative to biomass valorization. Herein, a series of Cu catalysts supported on Silicalite-1 zeolites with tunable contents of surface silanols and the same Cu loading of 3 wt% were synthesized by an impregnation method. The parent Silicalite-1 supports and as-synthesized Cu/S-1 catalysts were characterized by N2 adsorption, XRD, SEM, TEM, TGA, DRIFT, 29Si MAS NMR, XPS, and TPR. The Cu dispersion and Cu species distribution of Cu/S-1 catalysts can be modulated by engineering the amount of silanol groups on the support. More silanols present on the surfaces of parent Silicalite-1 supports can promote the Cu dispersion, and lead to a higher Cu+/Cu0 molar ratio arising from strong interfacial interaction between Cu species and silanols on the Silicalite-1 support via the formation of Si-O-Cu bonds. Thus, higher catalytic activity is achieved.
1. Introduction
Currently, the attention to environmental issues is rising, with increasing in-depth research focused on renewable energy [1]. Among these research initiatives, renewable biomass energy has become a research hotspot due to its unique advantages [2]. As a product of biomass with high productivity and versatility [3,4,5], ethanol is primarily used as a renewable fuel additive and serves as a key platform chemical for upgrading into various high-value chemicals and fuels, including acetaldehyde, propene, n-butanol, butadiene, and ethyl acetate [6,7,8,9,10,11]. The direct dehydrogenation of ethanol is of great significance, as acetic acid, trichloroacetaldehyde, and other important chemicals can be produced by using acetaldehyde. Thus, selective ethanol dehydrogenation to acetaldehyde has attracted increasing interest due to its fundamental and technological importance [6,12,13,14,15,16,17,18,19,20].
In the ethanol dehydrogenation reaction, the cleavage of O-H and C-H bonds represents the rate-determining step [6,12]. Previous studies have shown that copper catalysts exhibit distinctive activity and selectivity in this process owing to their superior capability of O-H and C-H cleavages [21,22,23]. Because of this ability combined with their low price, Cu-based catalysts have received significant attention. Various kinds of supports were developed to disperse Cu nanoparticles (NPs), such as mixed metal oxides possessing both acidic and basic sites [24,25,26], silica-based materials with relatively weak acid sites including amorphous silica, mesoporous silica, and Silicalite-1 zeolite [27,28,29,30,31,32], carbon materials exhibiting inert surface properties [22,33], and basic oxides [20,34]. The Cu+ species are considered to be the most active sites for ethanol dehydrogenation [28,31,35]. Thus, maintaining a high proportion of Cu+ species in the catalyst can enhance the activity. Pang et al. [28] found that Silicalite-1 zeolite-supported copper catalysts displayed high catalytic stability for ethanol dehydrogenation with high activity. The formation of Cu-O-Si bonds between Cu species and silanol groups of the Silicalite-1 support stabilizes the Cu+ species, which plays a pivotal role in their superior performance.
However, to our knowledge, there has been no report about a detailed investigation of the impact of the number of silanols on Silicalite-1 zeolites on the catalytic activity of a supported Cu catalyst for the dehydrogenation of ethanol. In this work, Silicalite-1 zeolites with tunable amounts of surface silanols were prepared via a hydrothermal method, and used as supports to prepare copper catalysts by an impregnation method. The influences of silanols of Silicalite-1 supports on Cu species and thereby catalytic activity for ethanol dehydrogenation were studied.
2. Results and Discussion
2.1. Characterization of Catalysts
The XRD patterns of parent Silicatlite-1 supports presented in Figure S1 show typical diffraction peaks of MFI topological structure (PDF #44-0003) [36,37]. As depicted in Figure 1, in addition to the typical diffraction peaks of MFI crystals, two peaks at 2θ = 43.3° and 50.4° can be observed for Cu/S-1(2), Cu/S-1(3), and Cu/S-1(4) catalysts, which are indexed to the (111) and (200) planes of Cu crystallites, respectively (PDF #04-0836). No reflections assigned to metallic Cu can be discernible for the Cu/S-1(1) catalyst. The intensity of the Cu (111) peak increases slightly in the order of Cu/S-1(2) < Cu/S-1(3) < Cu/S-1(4). The XRD observation indicates that the Cu nanoparticle size follows the order of Cu/S-1(1) < Cu/S-1(2) < Cu/S-1(3) < Cu/S-1(4).
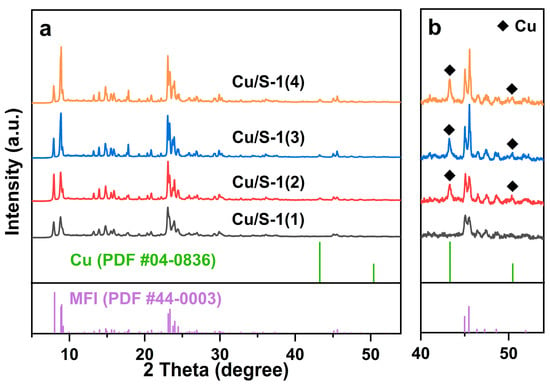
Figure 1.
(a) XRD patterns of the Cu/S-1 catalysts. (b) Magnification of the portion (2θ = 40–54°) of the XRD patterns for these catalysts.
As shown in Figure S2, the SEM images of parent Silicatlite-1 supports show that both S-1(1) and S-1(2) samples exhibit a predominantly uniform and spherical morphology, with grain sizes of approximately 70 and 260 nm, respectively. The S-1(3) and S-1(4) samples possess both submicron-sized and micron-sized grains. For the synthesis of Silicalite-1 zeolites, increasing the concentration of OH− (i.e., higher TPAOH/SiO2 ratio and lower H2O/SiO2 ratio) can accelerate the dissolution of Si, thus leading to the generation of Silicalite-1 with smaller crystallite size. The N2 sorption isotherms and BJH adsorption pore size distributions of the parent Silicatlite-1 supports are illustrated in Figure S3. The N2 adsorption isotherms are of type I as classified by IUPAC recommendations, indicating their microporous character. For the S-1 support, a significant hysteresis at p/p0 between 0.9 and 1.0 was observed, suggesting the existence of additional mesopores contributing from intercrystalline voids in this sample. The mesopore size is centered at 30.3 nm, as shown in Figure S3b. The BET surface area follows the order of S-1(1) > S-1(2) > S-1(3) ≈ S-1(4), while the micropore surface area and volume are similar for differently prepared Silicalite-1 zeolites (Table 1). The external surface area decreases in the order of S-1(1) > S-1(2) > S-1(3) > S-1(4), indicating that a Silicalite-1 zeolite with smaller crystallite size possesses a higher external surface area.

Table 1.
Textural properties of parent Silicalite-1 supports.
The HAADF-STEM image analysis of Cu/S-1(1) and Cu/S-1(2) as representative catalysts reveals that well-dispersed Cu nanoparticles with average sizes of ~1.5 and ~3.2 nm, respectively, are formed (Figure 2a,c), which are consistent with the average particle sizes estimated by N2O titration (Table 2). The corresponding EDX mapping analysis clearly shows that Cu is uniformly distributed on the S-1(1) and S-1(2) supports (Figure 2b,d). An even distribution of Cu on S-1(3) and S-1(4) was also observed (Figure S4).
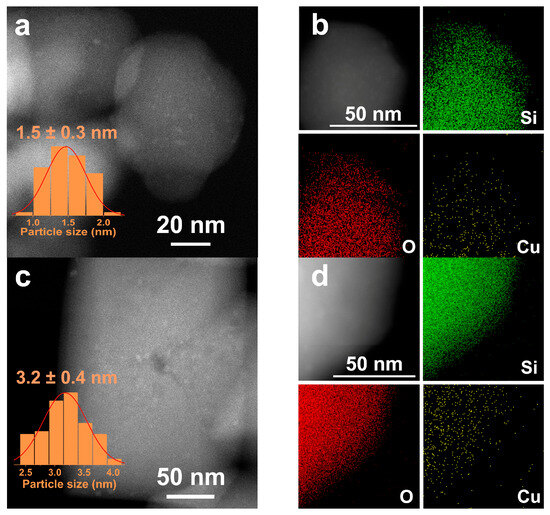
Figure 2.
HAADF-STEM images of (a) Cu/S-1(1) and (c) Cu/S-1(2). EDX mapping analysis of (b) Cu/S-1(1) and (d) Cu/S-1(2). The insets of (a,c) are the Cu NPs size distributions.

Table 2.
Physicochemical properties of the Cu/S-1 catalysts.
In comparison with parent Silicatlite-1 supports, the corresponding Cu/S-1 catalysts show a major loss of the external surface area and yet a limited decrease in the micropore surface area and volume after Cu addition (Table 1 and Table 2). The above observation indicates that most supported Cu NPs are located on the external surfaces of differently prepared Silicalite-1 zeolites [31]. The elemental analysis of ICP-AES indicates that the actual Cu loadings of Cu/S-1 catalysts are equivalent to the theoretical Cu content (3 wt%), as shown in Table 2.
The amount of surface silanols on parent Silicatlite-1 supports was estimated by thermogravimetry in a N2 flow. Figure S5 illustrates the TGA and DTG curves. According to the result from a previous study [38], the steep weight loss below 100 °C and slow weight loss between 100 and 200 °C are attributed, respectively, to the removal of physically adsorbed and chemically adsorbed water. The weight loss between 200 and 850 °C is attributed to the dehydroxylation by condensation of silanols. Based on the weight loss, the estimated silanol numbers for S-1(1), S-1(2), S-1(3), and S-1(4) are 1.09, 1.02, 0.77, and 0.63 mmol/g, respectively, as shown in Table 1.
29Si MAS NMR can offer information about the local environment of Si atoms in the MFI-type zeolite framework. Figure 3 illustrates the 29Si MAS NMR spectra of differently prepared Silicalite-1 zeolites. The peaks at −113 and −103 ppm are attributed to the Q4 (Si-[(OSi)4]) and Q3 (HO-Si-[(OSi)3]) structures in Silicalite-1, respectively [39,40]. The additional peak at −110 ppm observed for S-1(3) and S-1(4) is also assigned to the Q4 species [40]. The presence of a Q3 signal indicates the presence of silanol defects, while the low-resolution Q4 signal is also a typical manifestation of silanol defects in the Silicalite-1 zeolite framework [41]. As listed in Table 1, the peak area ratio of Q3 to Q4 shows a progressive decrease from samples S-1(1) to S-1(4), which indicates that the amount of silanol groups gradually decreases. This result is in accordance with the quantitative data of surface silanols determined by TGA (Table 1). For the synthesis of Silicalite-1 zeolites, the positive charge of the structure-directing agent (TPA+) is balanced by siloxy defects (≡Si–O−). Thus, an increased concentration of hydroxide anions (i.e., higher TPAOH/SiO2 ratio and lower H2O/SiO2 ratio), along with an increased concentration of cations (TPA+), contributes to the generation of a higher number of framework defects as more silanols get deprotonated to compensate the charge of cations [42].
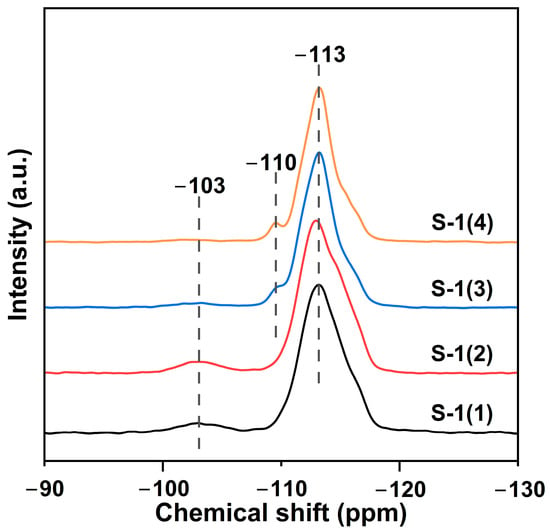
Figure 3.
29Si NMR spectra of parent Silicatlite-1 supports.
To gain a deep insight into the nature of surface silanols on parent Silicatlite-1 supports and their interaction with Cu species, additional investigation was performed using DRIFT spectra. Figure 4 reveals the presence of three types of silanol groups (Si-OH). The intense and sharp peak at 3730 cm−1 is assigned to isolated silanols on the external surface of the Silicalite-1 zeolite, while the small peak observed at 3686 cm−1 is associated with vicinal silanols within the micropores [43,44,45,46,47,48]. The nest silanols are interacted by multiple silanol groups through extended hydrogen bonds, corresponding to the intense and wide peak at 3490 cm−1 [43,44,46,47,49]. The peak intensities of surface silanols follow the order of S-1(1) > S-1(2) > S-1(3) > S-1(4), suggesting that the amount of silanol groups decreases in this order, which is in agreement with the results of TGA and 29Si MAS NMR. After loading of Cu NPs, an obvious decrease in the peak intensities of three types of silanols were observed when comparing with the bare Silicalite-1 supports, affording a decrease by 51%, 47%, 32%, and 19%, respectively, for Cu/S-1(1), Cu/S-1(2), Cu/S-1(3), and Cu/S-1(4). This result indicates that a portion of surface Si-OH groups were occupied by Cu species via the formation of Si-O-Cu bonds [28,50], revealing the strong interaction between Cu species and silanols on the surfaces of Silicalite-1 supports. The formation of Si-O-Cu bonds stabilizes the Cu+ species.
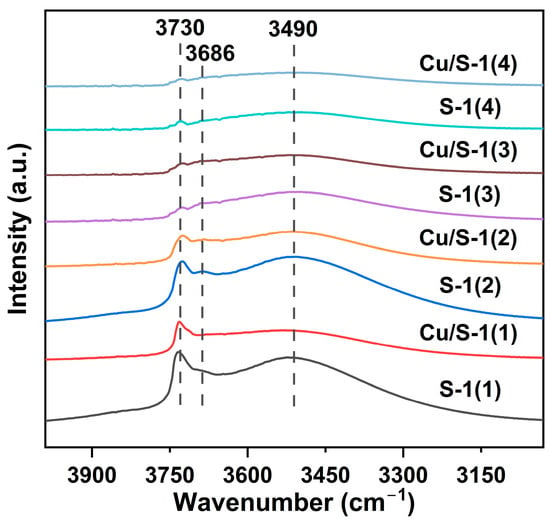
Figure 4.
DRIFT spectra of parent Silicalite-1 supports and Cu/S-1 catalysts.
To investigate the reducibility of CuO/S-1 precursors, H2-TPR analysis was performed, and the results are illustrated in Figure 5. For CuO/S-1(1) and CuO/S-1(2), an intense peak at ca. 200 °C coupled with a small reduction peak at relatively high temperature can be found. The low-temperature peak and high-temperature one can be attributed to the reduction of dispersed CuO with small size and bulk CuO with large size, respectively [28,51]. Differently, over CuO/S-1(3) and CuO/S-1(4), two reduction peaks are overlapped. The large proportion of high-temperature peaks observed for CuO/S-1(3) and CuO/S-1(4) suggests the larger CuO particles. Thus, CuO particles supported on Silicalite-1 zeolite with less silanols have larger size. The overlap of two reduction peaks could be associated with the larger CuO particles on CuO/S-1(3) and CuO/S-1(4). To gauge the Cu dispersion and exposed surface area, an N2O titration experiment was conducted. As shown in Table 2, the copper dispersion of Cu/S-1(1), Cu/S-1(2), Cu/S-1(3), and Cu/S-1(4) decreases gradually from 65% to 12%. Accordingly, the surface area of Cu0 follows the same order. Combining the amount of silanols on parent Silicalite-1 supports, it can be concluded that more silanols present on the surface of a parent Silicalite-1 zeolite favor the dispersion of copper on the support.
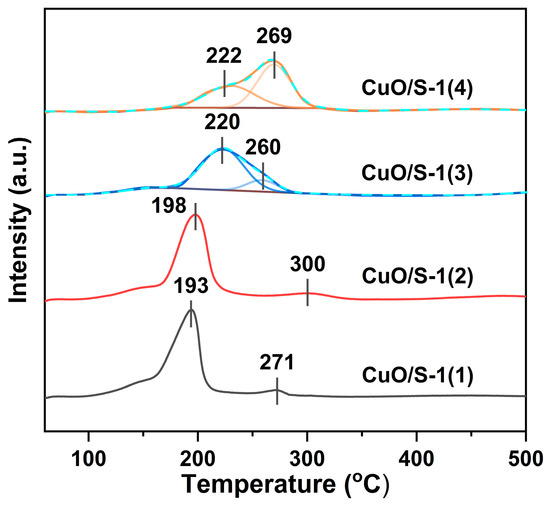
Figure 5.
H2-TPR profiles of CuO/S-1 precursors.
The Cu/S-1 catalysts were further investigated by XPS to reveal the chemical state of Cu species on differently prepared Silicalite-1 zeolites. The XPS survey spectra and C 1s and Si 2p XPS spectra are illustrated in Figures S6 and S7, respectively. Figure 6a illustrates the Cu 2p XPS spectra of the catalysts. Two peaks can be observed at 932.7 eV and 952.7 eV, which are attributed to Cu0 and/or Cu+ species of Cu 2p3/2 and Cu 2p1/2, respectively [27,28,29,52,53,54]. To further distinguish and quantify Cu0 and Cu+ species which have similar binding energy, the Cu LMM X-ray-induced AES (XAES) analysis was performed, and the results are illustrated in Figure 6b. The Auger signal was deconvoluted into two symmetrical peaks. The peak at 914.2 eV signifies Cu+ species, while the one at 917.4 eV denotes Cu0 species [27,28,55,56]. The Cu+/Cu0 molar ratio, calculated according to the corresponding areas of deconvoluted peaks, follows the order of Cu/S-1(1) (1.17) > Cu/S-1(2) (1.08) > Cu/S-1(3) (0.96) > Cu/S-1(4) (0.75). Based on the Cu0 surface area and Cu+/Cu0 molar ratio, the surface area of Cu+ was calculated [57,58]. It was found that the Cu+ surface area decreases in the order of Cu/S-1(1) > Cu/S-1(2) > Cu/S-1(3) > Cu/S-1(4). More silanols of the Silicalite-1 support are conducive to anchoring Cu+ species. Hence, the Cu species distribution of Silicalite-1-supported Cu catalysts can be tailored by engineering the amount of silanols on the support. The above findings reveal the co-existence of Cu0 and Cu+ species in the Cu/S-1 catalysts. Cu+ species may adhere to Cu particles [29,53]. The appearance of Cu+ species confirms strong interfacial interaction between Cu species and the Silicalite-1 support through the formation of Si-O-Cu bonds [28,29,50,59,60].
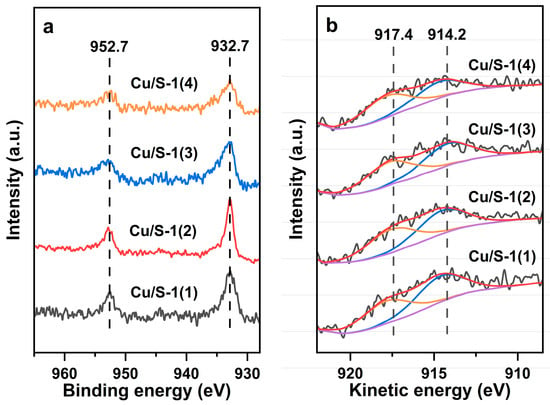
Figure 6.
(a) Cu 2p XPS spectra and (b) Cu LMM XAES spectra of the Cu/S-1 catalysts.
By combining TGA, N2O titration, and Cu LMM XAES results, it can be found that the Cu+/Cu0 ratios and total surface areas of Cu0 and Cu+ for the Cu/S-1 catalysts increase with the amount of silanols present on parent Silicalite-1 supports (Figure 7). To sum up, more silanols present on the surfaces of parent Silicalite-1 supports can promote Cu dispersion, and lead to higher Cu+/Cu0 ratios and surface areas of Cu0 and Cu+. Thus, higher catalytic activity can be expected.
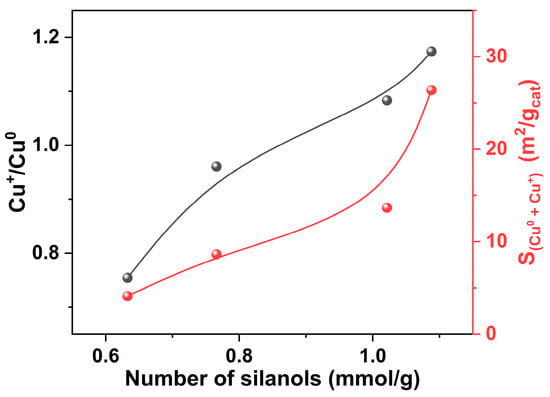
Figure 7.
Relationships of Cu+/Cu0 molar ratios determined by Cu LMM XAES and the total surface areas of Cu0 and Cu+ for the Cu/S-1 catalysts, with the number of silanols on parent Silicalite-1 supports.
2.2. Catalytic Performance
The catalytic dehydrogenation of ethanol to acetaldehyde over Silicalite-1 zeolite-supported Cu catalysts was investigated at 250 °C, and the results are shown in Figure 8. The ethanol conversion decreases in the order of Cu/S-1(1) (77.6%) > Cu/S-1(2) (65.8%) > Cu/S-1(3) (52.1%) > Cu/S-1(4) (25.5%). Combined with the amount of silanols on parent Silicalite-1 supports, it can be found that the more silanols bare Silicalite-1 supports have, the more active supported Cu catalysts are. A positive linear correlation between the reaction rate and the number of silanols on the support was established, as illustrated in Figure 9. The acetaldehyde selectivities of Cu/S-1(1), Cu/S-1(2), Cu/S-1(3) and Cu/S-1(4) increase gradually from 92.0% to 96.8%. Besides the main product acetaldehyde, small amounts of byproducts were observed, including acetone, butyraldehyde, ethyl acetate, ethylene, and butanol.
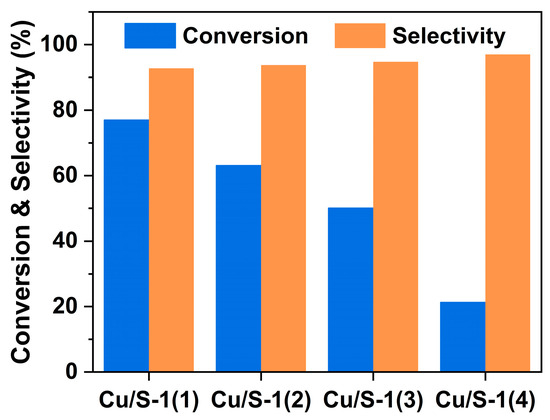
Figure 8.
Catalytic behavior of Cu/S-1 catalysts for ethanol dehydrogenation. Reaction conditions: 250 °C, WHSV = 4 h−1, after 1 h of the reaction.
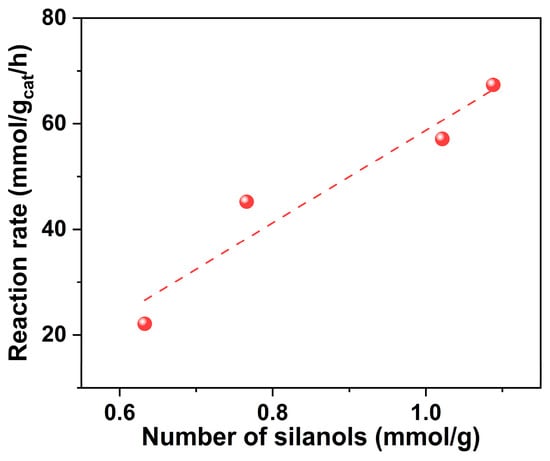
Figure 9.
Relationship of the reaction rates of Cu/S-1 catalysts at 250 °C with the number of silanols on parent Silicalite-1 supports. The reaction rate is defined as gram of converted ethanol/(gram of catalyst)/(contact time).
We have correlated the reaction rates of Cu/S-1 catalysts with their Cu+/Cu0 ratios and the total surface areas of Cu0 and Cu+. As illustrated in Figure 10, the reaction rates are positively correlated with the Cu+/Cu0 ratios and total surface areas of Cu0 and Cu+ for the Cu/S-1 catalysts. On the basis of DFT calculations, Pang et al. have proposed that both Cu+ and Cu0 species are active for the ethanol dehydrogenation [28]. Cu+ species served as active sites for C-H bond cleavage which is the rate-controlling step in ethanol dehydrogenation. Simultaneously, the generated hydrogen atoms were transferred to Cu0 sites for combination to release H2. Obviously, our results (Figure 10) are in good agreement with the reaction mechanism proposed by Pang et al. [28].
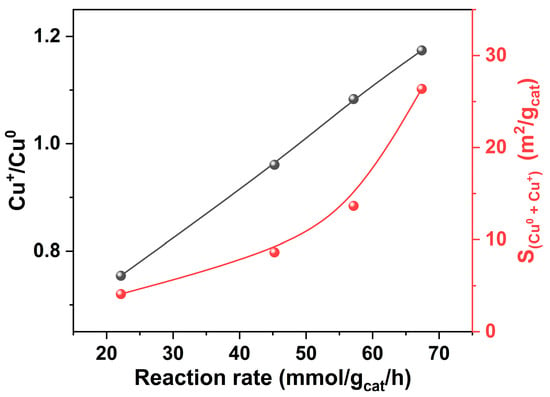
Figure 10.
Relationships of the reaction rates of Cu/S-1 catalysts at 250 °C with their Cu+/Cu0 molar ratios determined by Cu LMM XAES and the total surface areas of Cu0 and Cu+. The reaction rate has the same definition as that in Figure 9.
3. Materials and Methods
3.1. Reagent and Materials
The tetraethyl orthosilicate (TEOS), tetrapropylammonium hydroxide (TPAOH), and ethanol were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). The Cu(NO3)2·3H2O was purchased from Macklin Biochemical Co. Ltd. (Shanghai, China). All the chemicals were of analytical grade and used as received.
3.2. Catalyst Preparation
Four kinds of Silicalite-1 zeolites with different contents of OH groups designated as S-1(1), S-1(2), S-1(3), and S-1(4) were synthesized via a hydrothermal method. They were synthesized with the same procedure upon varying the TPAOH/SiO2 ratio, H2O/SiO2 ratio, and crystallization time.
Synthesis of S-1(1): TEOS and TPAOH (40 wt%) were added to deionized water, resulting in a molar composition of 1.0SiO2: 0.25TPAOH: 8.0H2O. The solution was stirred at 80 °C for 24 h to form a gel, which was then transferred to a 100 mL Teflon-lined stainless-steel autoclave, and heated at 170 °C for 24 h. Subsequently, the obtained solid was washed with deionized water, dried at 100 °C overnight, and finally calcined in air at 550 °C for 6 h.
Synthesis of S-1(2): TEOS and TPAOH (25 wt%) were added to deionized water, resulting in a molar composition of 1.0SiO2: 0.18TPAOH: 15.4H2O. The solution was stirred at room temperature for 6 h to form a gel, which was then transferred to a 100 mL Teflon-lined stainless-steel autoclave, and heated at 170 °C for 48 h, followed by the same treatment as above.
Synthesis of S-1(3): The procedure was the same as that of S-1(2), except that the molar composition was changed to 1.0SiO2: 0.12TPAOH: 19.2H2O, and the crystallization time was changed to 72 h.
Synthesis of S-1(4): The procedure was the same as that of S-1(2), except that the molar composition was changed to 1.0SiO2: 0.15TPAOH: 44.2H2O.
The Silicalite-1 zeolite-supported Cu catalysts (labelled as Cu/S-1) were synthesized by an impregnation method. A certain amount of Cu(NO3)2·3H2O was dissolved in deionized water. Subsequently, a certain amount of Silicalite-1 zeolites were added to the solution, which were dried under an infrared lamp. The resulting solids were dried at 100 °C overnight, and then calcined at 500 °C for 4 h in air to yield the CuO/S-1 precursors. The CuO/S-1 precursors were reduced at 300 °C for 1 h in a flow of 10 vol% H2/Ar to yield the Cu/S-1 catalysts with 3 wt% nominal Cu mass loading, which was calculated according to the formula:
3.3. Catalyst Characterization
X-ray diffraction (XRD) patterns were measured on a D2 PHASER diffractometer (Bruker, Billercia, MA, USA) with Cu Kα radiation at 30 kV and 10 mA. Scanning electron microscopy (SEM) images were acquired on a Nova NanoSem 450 microscope (FEI, Hillsboro, OR, USA). The high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) images and elemental mapping were obtained on a Tecnai G2 F20 S-TWIN microscope (FEI, Hillsboro, OR, USA). The BET surface areas and micropore volumes were measured by nitrogen adsorption at −196 °C using a Tristar 3020 apparatus (Micromeritics, Atlanta, GA, USA). The samples were dehydrated under a vacuum at 300 °C for 5 h before the measurement. Thermogravimetric analysis (TGA) was conducted in a N2 flow from room temperature to 900 °C at a ramp rate of 10 °C/min using an SDT Q600 instrument (TA, New Castle, DE, USA). 29Si magic angle spinning nuclear magnetic resonance (MAS NMR) measurements were conducted on an Avance III 400 WB spectrometer (Bruker, Billercia, MA, USA) operating at a resonance frequency of 400 MHz. Diffuse reflectance infrared Fourier transform (DRIFT) spectra were collected using an iS50 spectrometer (Thermo Fisher, Waltham, MA, USA), which was equipped with a heating accessory. The samples were pretreated for 1 h in a He flow at 450 °C, followed by cooling to 300 °C under flowing He. Then, DRIFT spectra were measured at 300 °C. The background spectrum was collected at 300 °C using KBr as the reference material. The X-ray photoelectron spectroscopy (XPS) and Auger electron spectroscopy (AES) measurements were conducted using an AXIS Kratos Supra+ spectrometer (Kratos, Trafford, GM, UK), excited by an Al Kα radiation source. The charge increase was balanced using a built-in electron flood gun during measurements. The binding energy values were calibrated against the adventitious C 1s core level at 284.6 eV. The actual Cu loadings of the catalysts were determined by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) using an Optima 8000 instrument (Perkin-Elmer, Waltham, MA, USA).
The H2 temperature-programed reduction (H2-TPR) analysis was conducted using an AutoChem II instrument (Micromeritics, Atlanta, GA, USA). For this process, 0.1 g of CuO/S-1 (40–60 mesh) was pretreated at 300 °C under a He flow for 1 h, followed by cooling to 60 °C in flowing He. The gas was changed to 10 vol% H2/Ar (30 mL/min). The temperature was then ramped up to 500 °C at a rate of 10 °C/min. The Cu dispersion was determined by N2O oxidation and then by H2 titration on the same apparatus [61]. Next, 0.1 g of CuO/S-1 (40–60 mesh) were initially reduced at 300 °C for 1 h in a 10 vol% H2/Ar flow (30 mL/min), followed by cooling to 60 °C. Then, the gas was switched to 20 vol% N2O/Ar (30 mL/min) and kept at this temperature for 1 h to oxidize surface Cu atoms to Cu2O, followed by sweeping with He (30 mL/min) for 1 h. Finally, the same H2-TPR experiment was conducted as above. A1 and A2 denote the hydrogen consumption amount in the first and second H2-TPR experiments, respectively. The copper dispersion (DCu), the area of surface Cu0 (SCu0), and average volume-surface diameter (dCu) were calculated according to the equations:
where NA is Avogadro’s constant, MCu is Cu molar mass (63.46 g/mol), 1.4 × 1019 is the number of Cu atoms/m2, and ρCu is the copper density (8.92 g/cm3).
3.4. Catalytic Tests
The dehydrogenation of ethanol was conducted in a flow-type fixed-bed reactor under ambient pressure, with a quartz tube internal diameter of 6 mm. For this process, 0.17 g of CuO/S-1 (40–60 mesh) was in situ reduced at 300 °C for 1 h under a flow of 10 vol% H2/Ar, followed by cooling to a reaction temperature of 250 °C. Then, a gas mixture (5 vol% ethanol and 95 vol% N2) with a total flow rate of 120 mL/min was passed through the reactor. Ethanol was introduced into the reactor through a bubbler, with a weight hourly space velocity (WHSV) of 4 h−1. The analysis of the products was conducted online using a gas chromatograph equipped with a flame ionization detector and an FFAP capillary column (30 m × 0.32 mm × 0.25 µm). The calculation formulas of ethanol conversion and acetaldehyde selectivity are as follows:
4. Conclusions
In this contribution, a series of Cu/S-1 catalysts with 3 wt% Cu loading were prepared by an impregnation method using differently synthesized Silicalite-1 zeolites with tunable amounts of surface silanols as supports. The Cu dispersion and Cu+/Cu0 molar ratio of Cu/S-1 catalysts can be tailored by engineering the amount of silanols on the support. Cu0 and Cu+ species co-exist in the Cu/S-1 catalysts. The occurrence of Cu+ species originates from strong interfacial interaction between Cu species and silanols on the Silicalite-1 support through the formation of Si-O-Cu bonds. The amount of surface silanols on the parent Silicalite-1 was found to correlate positively with Cu dispersion and Cu+/Cu0 ratio. Thus, the Cu/S-1 catalyst with more silanols on the parent Silicalite-1 support exhibits a higher activity for ethanol dehydrogenation to acetaldehyde.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal15080787/s1: Figure S1: XRD patterns of parent Silicatlite-1 supports, Figure S2: SEM images of (a) S-1(1), (b) S-1(2), (c) S-1(3), and (d) S-1(4), Figure S3: (a) N2 sorption isotherms and (b) BJH adsorption pore size distributions of parent Silicatlite-1 supports, Figure S4: EDX mapping analysis of (a) Cu/S-1(3) and (b) Cu/S-1(4), Figure S5: TGA and DTG curves of (a) S-1(1), (b) S-1(2), (c) S-1(3), and (d) S-1(4), Figure S6: XPS survey spectra of the Cu/S-1 catalysts, Figure S7: (a) C 1s and (b) Si 2p XPS spectra of the Cu/S-1 catalysts.
Author Contributions
C.H.: investigation, data curation, writing—original draft. C.T.: investigation, data curation. Y.Y.: methodology, formal analysis. G.T.: data curation, formal analysis. W.H.: conceptualization, supervision, writing—review & editing, project administration. Z.G.: validation, formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China, grant number 22072027.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zabed, H.; Sahu, J.N.; Suely, A.; Boyce, A.N.; Faruq, G. Bioethanol production from renewable sources: Current perspectives and technological progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- Yao, X.; Li, T.; Chung, S.; Ruiz-Martínez, J. Advances in the catalytic conversion of ethanol into nonoxygenated added-value chemicals. Adv. Mater. 2024, 36, 2406472. [Google Scholar] [CrossRef] [PubMed]
- Gérardy, R.; Debecker, D.P.; Estager, J.; Luis, P.; Monbaliu, J.C.M. Continuous flow upgrading of selected C2-C6 platform chemicals derived from biomass. Chem. Rev. 2020, 120, 7219–7347. [Google Scholar] [CrossRef]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of biomass: Deriving more value from waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef]
- Shindell, D.; Smith, C.J. Climate and air-quality benefits of a realistic phase-out of fossil fuels. Nature 2019, 573, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, B.; Yuan, H.; Sun, Y.; Yang, D.; Cui, X.; Shi, F. The catalytic dehydrogenation of ethanol by heterogeneous catalysts. Catal. Sci. Technol. 2021, 11, 1652–1664. [Google Scholar] [CrossRef]
- Li, X.; Kant, A.; He, Y.; Thakkar, H.V.; Atanga, M.A.; Rezaei, F.; Ludlow, D.K.; Rownaghi, A.A. Light olefins from renewable resources: Selective catalytic dehydration of bioethanol to propylene over zeolite and transition metal oxide catalysts. Catal. Today 2016, 276, 62–77. [Google Scholar] [CrossRef]
- Wu, X.; Fang, G.; Tong, Y.; Jiang, D.; Liang, Z.; Leng, W.; Liu, L.; Tu, P.; Wang, H.; Ni, J.; et al. Catalytic upgrading of ethanol to n-butanol: Progress in catalyst development. ChemSusChem 2018, 11, 71–85. [Google Scholar] [CrossRef]
- Pomalaza, G.; Capron, M.; Ordomsky, V.; Dumeignil, F. Recent breakthroughs in the conversion of ethanol to butadiene. Catalysts 2016, 6, 203. [Google Scholar] [CrossRef]
- Yamamoto, T.; Mine, H.; Katada, S.; Tone, T. Direct ethyl acetate synthesis from ethanol over amorphous-, monoclinic-, tetragonal ZrO2 supported copper catalysts prepared from the same zirconium source. Appl. Catal. B 2023, 327, 122433. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y. Recent advances in catalytic conversion of ethanol to chemicals. ACS Catal. 2014, 4, 1078–1090. [Google Scholar] [CrossRef]
- Pang, J.; Yin, M.; Wu, P.; Li, X.; Li, H.; Zheng, M.; Zhang, T. Advances in catalytic dehydrogenation of ethanol to acetaldehyde. Green Chem. 2021, 23, 7902–7916. [Google Scholar] [CrossRef]
- Phung, T.K. Copper-based catalysts for ethanol dehydrogenation and dehydrogenative coupling into hydrogen, acetaldehyde and ethyl acetate. Int. J. Hydrogen Energy 2022, 47, 42234–42249. [Google Scholar] [CrossRef]
- He, L.; Zhou, B.; Sun, D.; Li, W.; Lv, W.; Wang, J.; Liang, Y.; Lu, A. Catalytic conversion of ethanol to oxygen-containing value-added chemicals. ACS Catal. 2023, 13, 11291–11304. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, W.; Gan, T.; Chu, X.; Zhu, W.; Zhang, W.; Wang, D.; Liu, G. Enhancing stability of industrial Cu-based catalysts under harsh reduction reaction conditions with silica armor protection. Chem. Eng. J. 2024, 500, 157331. [Google Scholar] [CrossRef]
- Pampararo, G.; Hlavenková, Z.; Styskalik, A.; Debecker, D.P. Suppressing on-stream deactivation of CuSiO2 catalysts in the dehydrogenation of bioethanol to acetaldehyde. Catal. Sci. Technol. 2024, 14, 4912–4926. [Google Scholar] [CrossRef]
- Guo, J.; Pang, J.; Yin, M.; Feng, L.; Liu, S.; Wu, P.; Zheng, M. Diamine-assistant synthesis of Cu@MFI catalysts for ethanol dehydrogenation. ChemCatChem 2024, 16, e202400269. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, H.; Yang, Z.; Liu, S.; Yang, T.; Wang, L.; Sun, Y.; Wang, X.; Li, Q.; Tian, P. Spatial confinement effect of zeolite Silicalite-1 on dispersing high loading Cu nanoparticles and their superior ethanol dehydrogenation catalytic performance. Appl. Catal. B 2025, 367, 125099. [Google Scholar] [CrossRef]
- Tian, C.; Yue, Y.; Miao, C.; Hua, W.; Gao, Z. Mesoporous silica-encapsulated Cu nanoparticles with enhanced performance for ethanol dehydrogenation to acetaldehyde. ACS Sustain. Chem. Eng. 2025, 13, 1401–1408. [Google Scholar] [CrossRef]
- Tian, C.; Yue, Y.; Miao, C.; Hua, W.; Gao, Z. Dehydrogenation of ethanol to acetaldehyde catalyzed by Cu nanoparticles supported on nanorod-shaped La2O2CO3. Appl. Catal. A 2025, 702, 120334. [Google Scholar] [CrossRef]
- Tang, F.; Li, W.; Wang, D.; Lu, A. Synergistic roles of hexagonal boron nitride-supported Cu0 and Cu+ species in dehydrogenation of ethanol to acetaldehyde: A computational mechanistic study. J. Phys. Chem. C 2023, 127, 11014–11025. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, L.; Lu, A. Highly selective copper catalyst supported on mesoporous carbon for the dehydrogenation of ethanol to acetaldehyde. ChemCatchem 2015, 7, 2846–2852. [Google Scholar] [CrossRef]
- Zhukova, A.I.; Chuklina, S.G.; Maslenkova, S.A. Study of Cu modified Zr and Al mixed oxides in ethanol conversion: The structure-catalytic activity relationship. Catal. Today 2021, 379, 159–165. [Google Scholar] [CrossRef]
- Campisano, I.S.P.; Rodella, C.B.; Sousa, Z.S.B.; Henriques, C.A.; da Silva, V.T. Influence of thermal treatment conditions on the characteristics of Cu-based metal oxides derived from hydrotalcite-like compounds and their performance in bio-ethanol dehydrogenation to acetaldehyde. Catal. Today 2018, 306, 111–120. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, Y.; Zhou, R.; Chang, Z.; Hou, Z. Co-production of hydrogen and acetaldehyde from ethanol over a highly dispersed Cu catalyst. Fuel 2022, 321, 123980. [Google Scholar] [CrossRef]
- Ausavasukhi, A.; Krukrathok, N.; Singthaisong, P. Thermal transformation of copper incorporated hydrotalcite-derived oxides and their catalytic activity for ethanol dehydrogenation. J. Ind. Eng. Chem. 2023, 117, 371–385. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, H.R.; Jaenicke, S.; Chuah, G.K. Highly efficient and robust Cu catalyst for non-oxidative dehydrogenation of ethanol to acetaldehyde and hydrogen. J. Catal. 2020, 389, 19–28. [Google Scholar] [CrossRef]
- Pang, J.; Zheng, M.; Wang, C.; Yang, X.; Liu, H.; Liu, X.; Sun, J.; Wang, Y.; Zhang, T. Hierarchical echinus-like Cu-MFI catalysts for ethanol dehydrogenation. ACS Catal. 2020, 10, 13624–13629. [Google Scholar] [CrossRef]
- Pang, J.; Yin, M.; Wu, P.; Song, L.; Li, X.; Zhang, T.; Zheng, M. Redispersion and stabilization of Cu/MFI catalysts by the encapsulation method for ethanol dehydrogenation. ACS Sustain. Chem. Eng. 2023, 11, 3297–3305. [Google Scholar] [CrossRef]
- Finger, P.H.; Osmari, T.A.; Cabral, N.M.; Bueno, J.M.C.; Marcel, J.; Gallo, R. Direct synthesis of Cu supported on mesoporous silica: Tailoring the Cu loading and the activity for ethanol dehydrogenation. Catal. Today 2021, 381, 26–33. [Google Scholar] [CrossRef]
- Lin, L.; Cao, P.; Pang, J.; Wang, Z.; Jiang, Q.; Su, Y.; Chen, R.; Wu, Z.; Zheng, M.; Luo, W. Zeolite-encapsulated Cu nanoparticles with enhanced performance for ethanol dehydrogenation. J. Catal. 2022, 413, 565–574. [Google Scholar] [CrossRef]
- Liu, H.; Chang, Z.; Fu, J.; Hou, Z. A CuZn-BTC derived stable Cu/ZnO@SiO2 catalyst for ethanol dehydrogenation. Appl. Catal. B 2023, 324, 122194. [Google Scholar] [CrossRef]
- Klinthongchai, Y.; Prichanont, S.; Praserthdam, P.; Jongsomjit, B. Synthesis, characteristics and application of mesocellular foam carbon (MCF-C) as catalyst for dehydrogenation of ethanol to acetaldehyde. J. Environ. Chem. Eng. 2020, 8, 103752. [Google Scholar] [CrossRef]
- Tian, C.; Yue, Y.; Miao, C.; Hua, W.; Gao, Z. Cu/MgO as an efficient new catalyst for the non-oxidative dehydrogenation of ethanol into acetaldehyde. Catalysts 2024, 14, 541. [Google Scholar] [CrossRef]
- Cassinelli, W.H.; Martins, L.; Passos, A.R.; Pulcinelli, S.H.; Rochet, A.; Briois, V.; Santilli, C.V. Correlation between structural and catalytic properties of copper supported on porous alumina for the ethanol dehydrogenation reaction. ChemCatChem 2015, 7, 1668–1677. [Google Scholar] [CrossRef]
- Kokotailo, G.T.; Lawton, S.L.; Olson, D.H.; Meier, W.M. Structure of synthetic zeolite ZSM-5. Nature 1978, 272, 437–438. [Google Scholar] [CrossRef]
- Yue, Y.; Gu, L.; Zhou, Y.; Liu, H.; Yuan, P.; Zhu, H.; Bai, Z.; Bao, X. Template-free synthesis and catalytic applications of microporous and hierarchical ZSM-5 zeolites from natural aluminosilicate minerals. Ind. Eng. Chem. Res. 2017, 56, 10069–10077. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.T. Increasing selective CO2 adsorption on amine-grafted SBA-15 by increasing silanol density. J. Phys. Chem. C 2011, 115, 21264–21272. [Google Scholar] [CrossRef]
- Peng, P.; Moldovan, S.; Vicente, A.; Ruaux, V.; Debost, M.; Hu, H.; Aleksandrov, H.A.; Vayssilov, G.N.; Yan, Z.; Mintova, S. Synthesis of nanosized MFI zeolites using Cu-containing complexes. Microporous Mesoporous Mater. 2023, 357, 112625. [Google Scholar] [CrossRef]
- Piva, D.; Fang, G.; Ghojavand, S.; Dalena, F.; AlHajjar, N.; De Waele, V.; Ordomsky, V.; Khodakov, A.; Tayeb, K.B.; Fernandes, T.; et al. Role of hydroxyl groups in Zn-containing nanosized MFI zeolite for the photocatalytic oxidation of methane. ChemSusChem 2025, 18, e202401656. [Google Scholar] [CrossRef]
- Medeiros-Costa, I.C.; Dib, E.; Dubray, F.; Moldovan, S.; Gilson, J.; Dath, J.; Nesterenko, N.; Aleksandrov, H.A.; Vayssilov, G.N.; Mintova, S. Unraveling the effect of silanol defects on the insertion of single site Mo in the MFI zeolite framework. Inorg. Chem. 2022, 61, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Palčić, A.; Moldovan, S.; El Siblani, H.; Vicente, A.; Valtchev, V. Defect sites in zeolites: Origin and healing. Adv. Sci. 2022, 9, 2104414. [Google Scholar] [CrossRef]
- Luo, Y.; Wei, C.; Miao, C.; Yue, Y.; Hua, W.; Gao, Z. Isobutane dehydrogenation assisted by CO2 over Silicalite-1-supported ZnO catalysts: Influence of support crystallite size. Chin. J. Chem. 2020, 38, 703–708. [Google Scholar] [CrossRef]
- Bolis, V.; Busco, C.; Bordiga, S.; Ugliengo, P.; Lamberti, C.; Zecchina, A. Calorimetric and IR spectroscopic study of the interaction of NH3 with variously prepared defective silicalites-Comparison with Ab initio computational data. Appl. Surf. Sci. 2002, 196, 56–70. [Google Scholar] [CrossRef]
- Karbowiak, T.; Saada, M.A.; Rigolet, S.; Ballandras, A.; Weber, G.; Bezverkhyy, I.; Soulard, M.; Patarin, J.; Bellat, J.P. New insights in the formation of silanol defects in silicalite-1 by water intrusion under high pressure. Phys. Chem. Chem. Phys. 2010, 12, 11454–11466. [Google Scholar] [CrossRef]
- Heitmann, G.P.; Dahlhoff, G.; Hölderich, W.F. Catalytically active sites for the Beckmann rearrangement of cyclohexanone oxime to ε-caprolactam. J. Catal. 1999, 186, 12–19. [Google Scholar] [CrossRef]
- Barbera, K.; Bonino, F.; Bordiga, S.; Janssens, T.V.W.; Beato, P. Structure-deactivation relationship for ZSM-5 catalysts governed by framework defects. J. Catal. 2011, 280, 196–205. [Google Scholar] [CrossRef]
- Liu, G.; Liu, J.; He, N.; Miao, C.; Wang, J.; Xin, Q.; Guo, H. Silicalite-1 zeolite acidification by zinc modification and its catalytic properties for isobutane conversion. RSC Adv. 2018, 8, 18663–18671. [Google Scholar] [CrossRef]
- Zhao, D.; Tian, X.; Doronkin, D.E.; Han, S.; Kondratenko, V.A.; Grunwaldt, J.; Perechodjuk, A.; Vuong, T.H.; Rabeah, J.; Eckelt, R.; et al. In situ formation of ZnOx species for efficient propane dehydrogenation. Nature 2021, 599, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Kong, X.; Peng, B.; Li, L.; Fang, Z.; Zhu, Y. Efficient Cu catalyst for 5-hydroxymethylfurfural hydrogenolysis by forming Cu–O–Si bonds. Catal. Sci. Technol. 2020, 10, 7323–7330. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, F.; Kang, H.; Chen, J.; Zhang, X.; Xia, C. Highly dispersed silica-supported copper nanoparticles prepared by precipitation-gel method: A simple by efficient and stable catalyst for glycerol hydrogenolysis. Chem. Mater. 2008, 20, 5090–5099. [Google Scholar] [CrossRef]
- Sato, A.G.; Volanti, D.P.; Meira, D.M.; Damyanova, S.; Longo, E.; Bueno, J.M.C. Effect of the ZrO2 phase on the structure and behavior of supported Cu catalysts for ethanol conversion. J. Catal. 2013, 307, 1–17. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, L.; Li, W.; Li, W.; Si, R.; Schüth, F.; Lu, A. Cu supported on thin carbon layer-coated porous SiO2 for efficient ethanol dehydrogenation. Catal. Sci. Technol. 2018, 8, 472–479. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, D.; Liu, W.; Zhang, M.; Lou, Y.; Wang, Z.; Tang, Q.; Yang, J. Uniformly dispersed Cu nanoparticles over mesoporous silica as a highly selective and recyclable ethanol dehydrogenation catalyst. Catalysts 2022, 12, 1049. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Y.; Cui, X.; Wang, G.; Cen, Y.; Deng, T.; Yan, W.; Gao, J.; Zhu, S.; Olsbye, U.; et al. A highly stable copper-based catalyst for clarifying the catalytic roles of Cu0 and Cu+ species in methanol dehydrogenation. Angew. Chem. Int. Ed. 2018, 57, 1836–1840. [Google Scholar] [CrossRef]
- Chen, L.; Guo, P.; Qiao, M.; Yan, S.; Li, H.; Shen, W.; Xu, H.; Fan, K. Cu/SiO2 catalysts prepared by the ammonia evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2008, 257, 172–180. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, W.; Rehman, M.U.; Liu, W.; Xu, Y.; Huang, H.; Wang, S.; Zhao, Y.; Mei, D.; Ma, X. Copper phyllosilicate nanotube catalysts for the chemosynthesis of cyclohexane via hydrodeoxygenation of phenol. ACS Catal. 2022, 12, 4724–4736. [Google Scholar] [CrossRef]
- Huang, J.; Ding, T.; Ma, K.; Cai, J.; Sun, Z.; Tian, Y.; Jiang, Z.; Zhang, J.; Zheng, L.; Li, X. Modification of Cu/SiO2 catalysts by La2O3 to quantitatively tune Cu+-Cu0 dual sites with improved catalytic activities and stabilities for dimethyl ether steam reforming. ChemCatChem 2018, 10, 3862–3871. [Google Scholar] [CrossRef]
- Xu, C.; Chen, G.; Zhao, Y.; Liu, P.; Duan, X.; Gu, L.; Fu, G.; Yuan, Y.; Zheng, N. Interfacing with silica boosts the catalysis of copper. Nat. Commun. 2018, 9, 3367. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Tian, J.; Chen, W.; Guan, Y.; Xu, H.; Li, X.; Wu, H.; Wu, P. One-pot synthesized core/shell structured zeolite@copper catalysts for selective hydrogenation of ethylene carbonate to methanol and ethylene glycol. Green Chem. 2019, 21, 5414–5426. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, R.; Sodesawa, T.; Yuma, K.I.; Obata, Y. Distinction between surface and bulk oxidation of Cu through N2O decomposition. J. Catal. 2000, 196, 195–199. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).