Integrating Carbon-Coated Cu/Cu2O Nanoparticles with Biochars Enabled Efficient Capture and Electrocatalytic Reduction of CO2
Abstract
1. Introduction
2. Results and Discussion
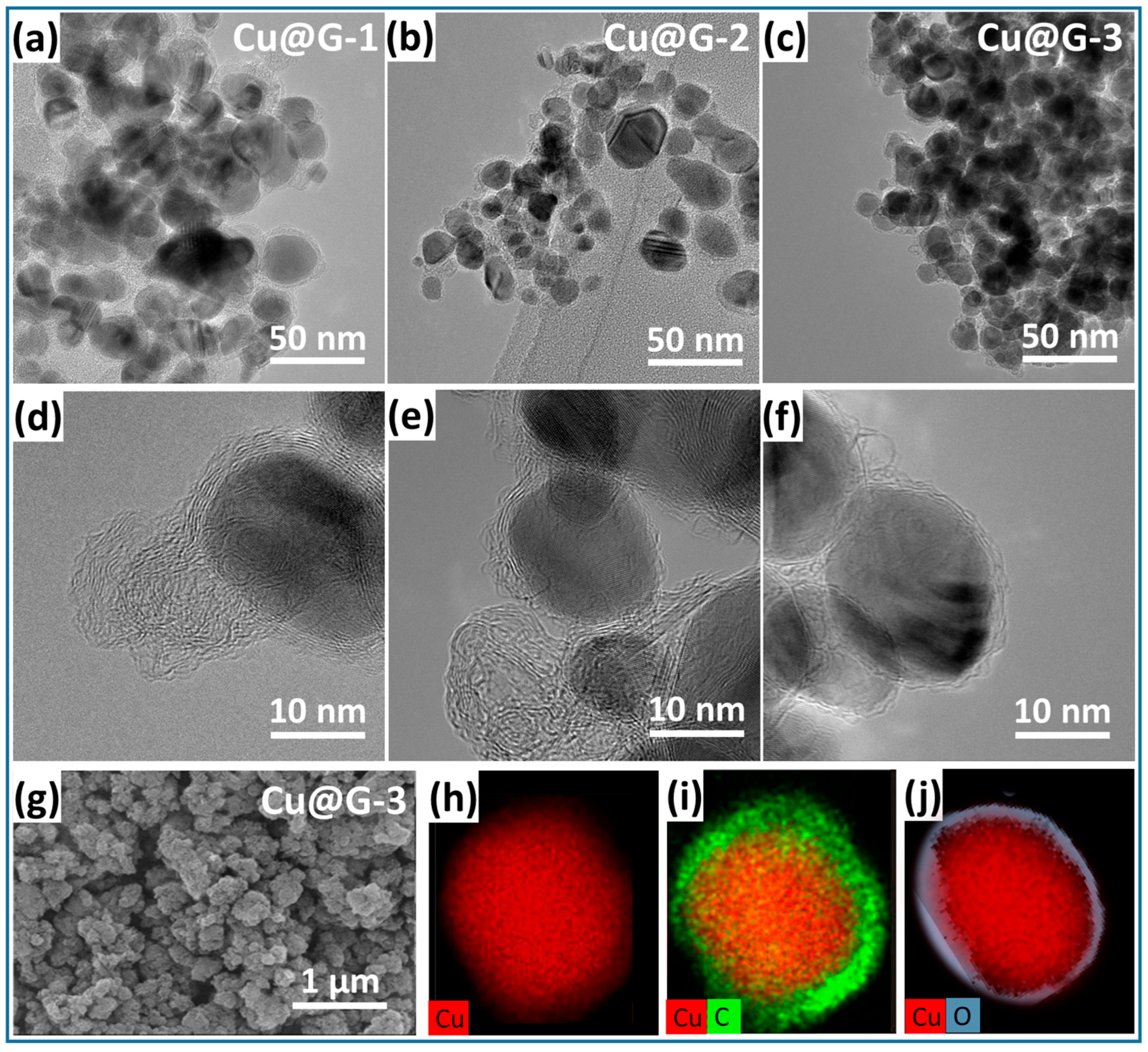
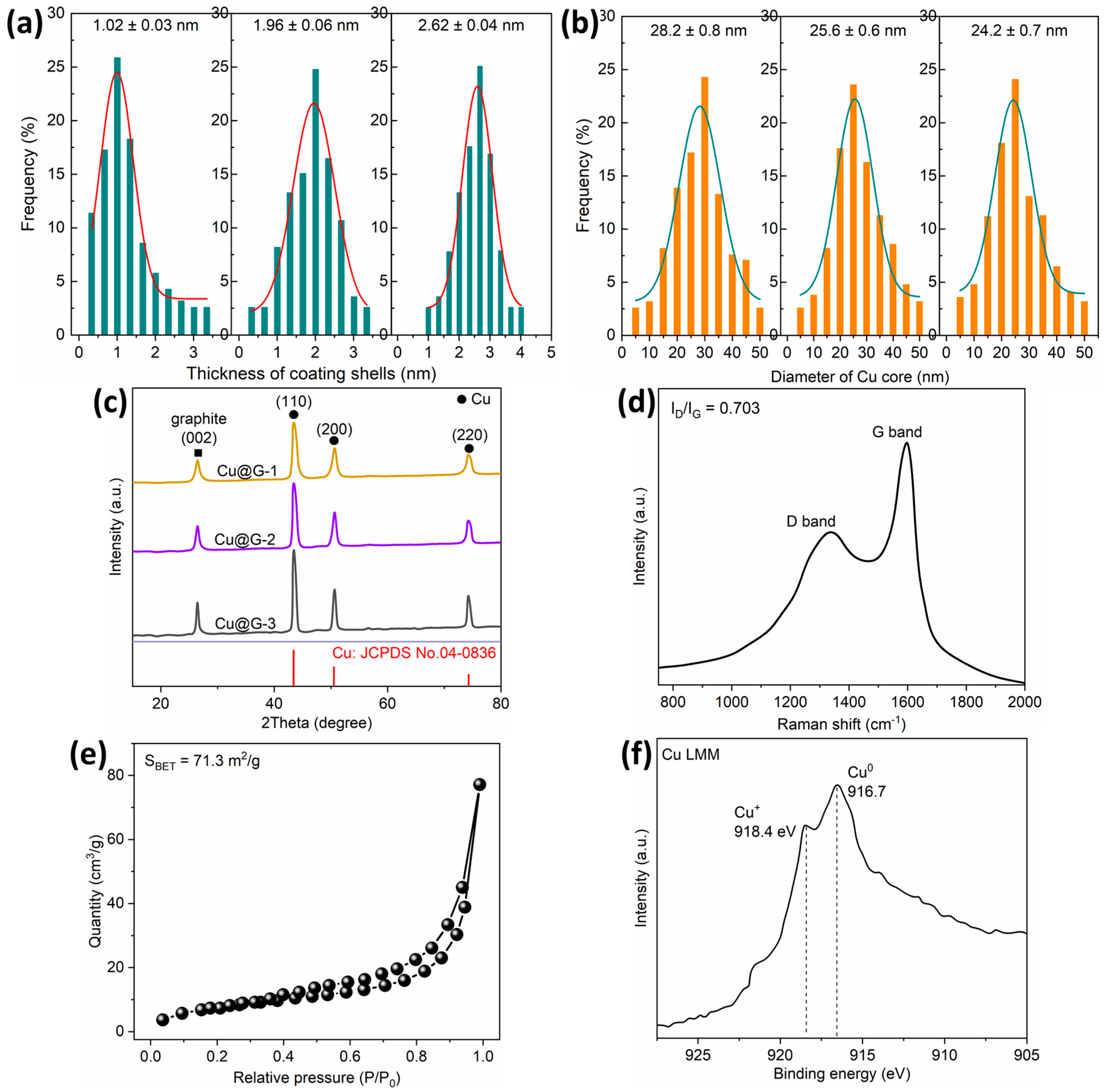
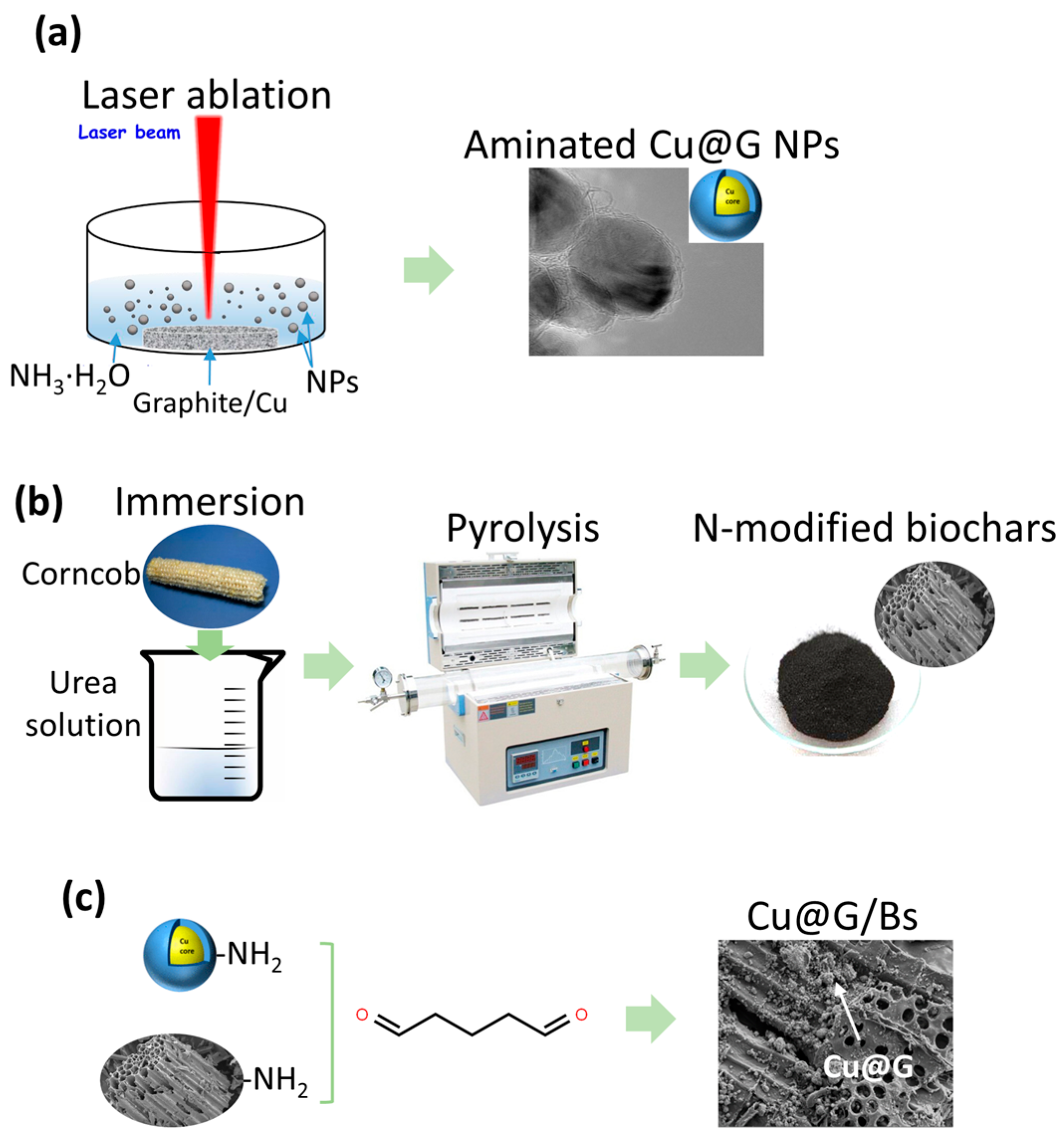
2.1. Characterization of Laser-Ablated Cu@G NPs
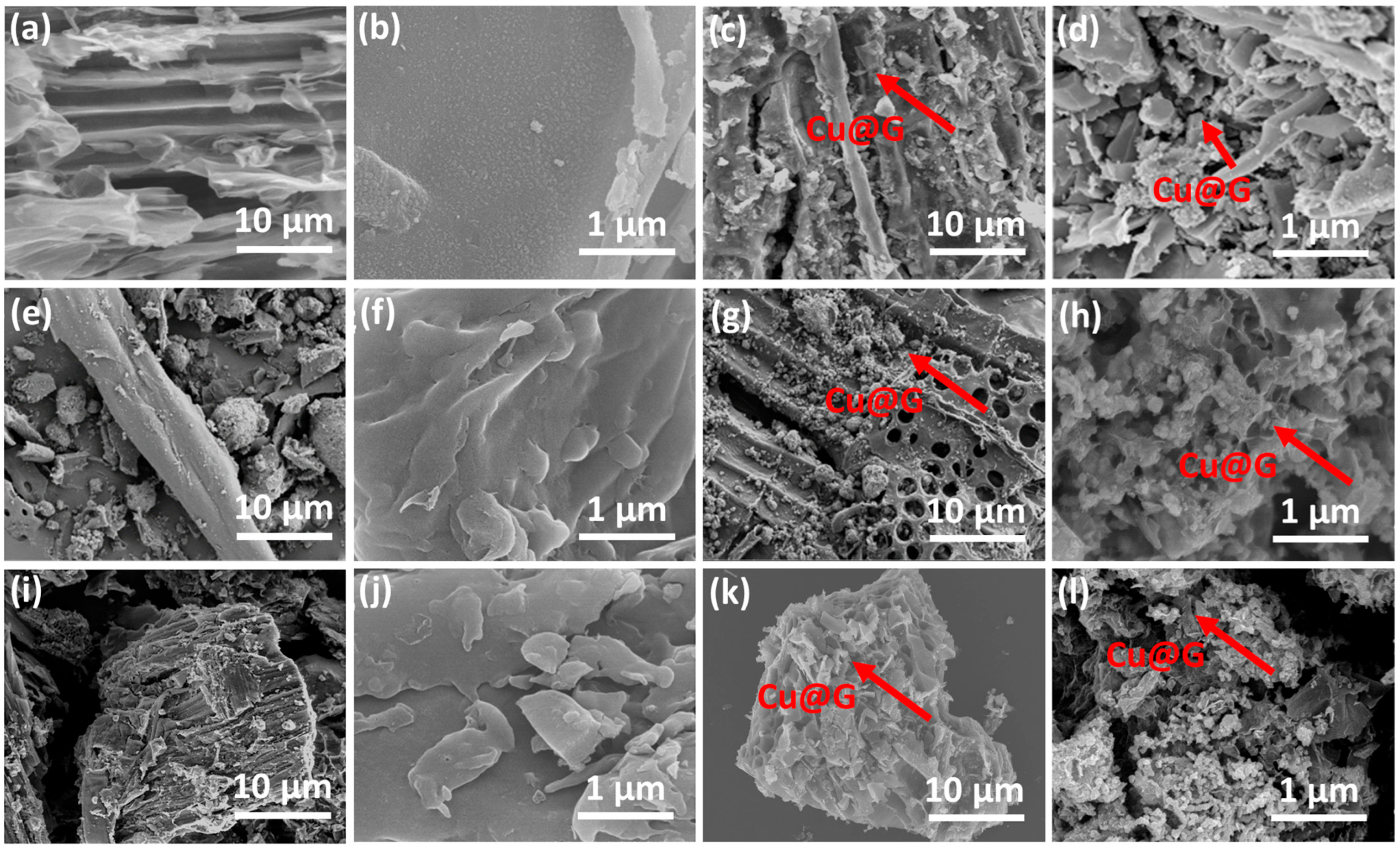
2.2. Characterization of Cu@G/Bs
2.3. Sorption of CO2 on Cu@G/Bs
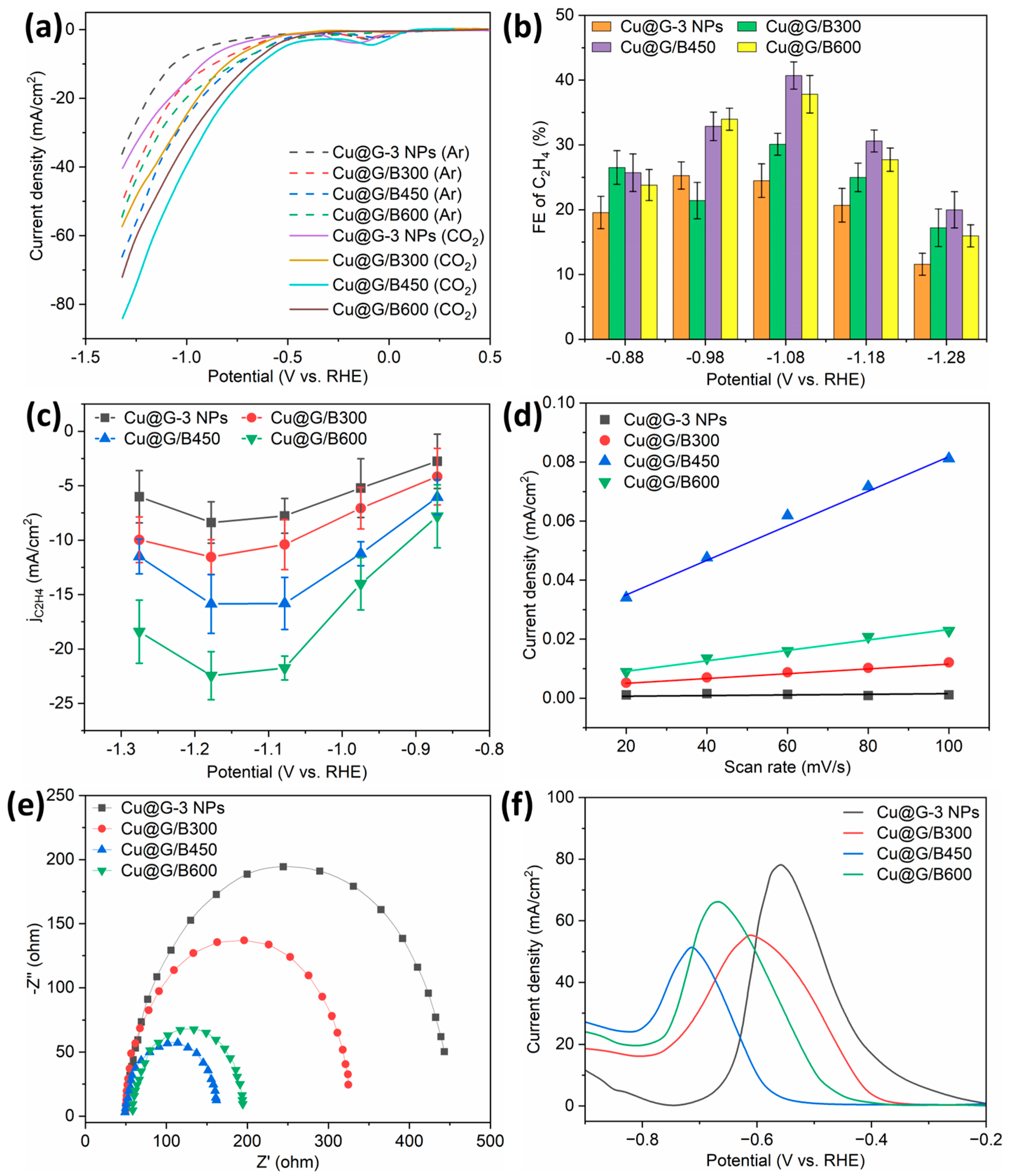
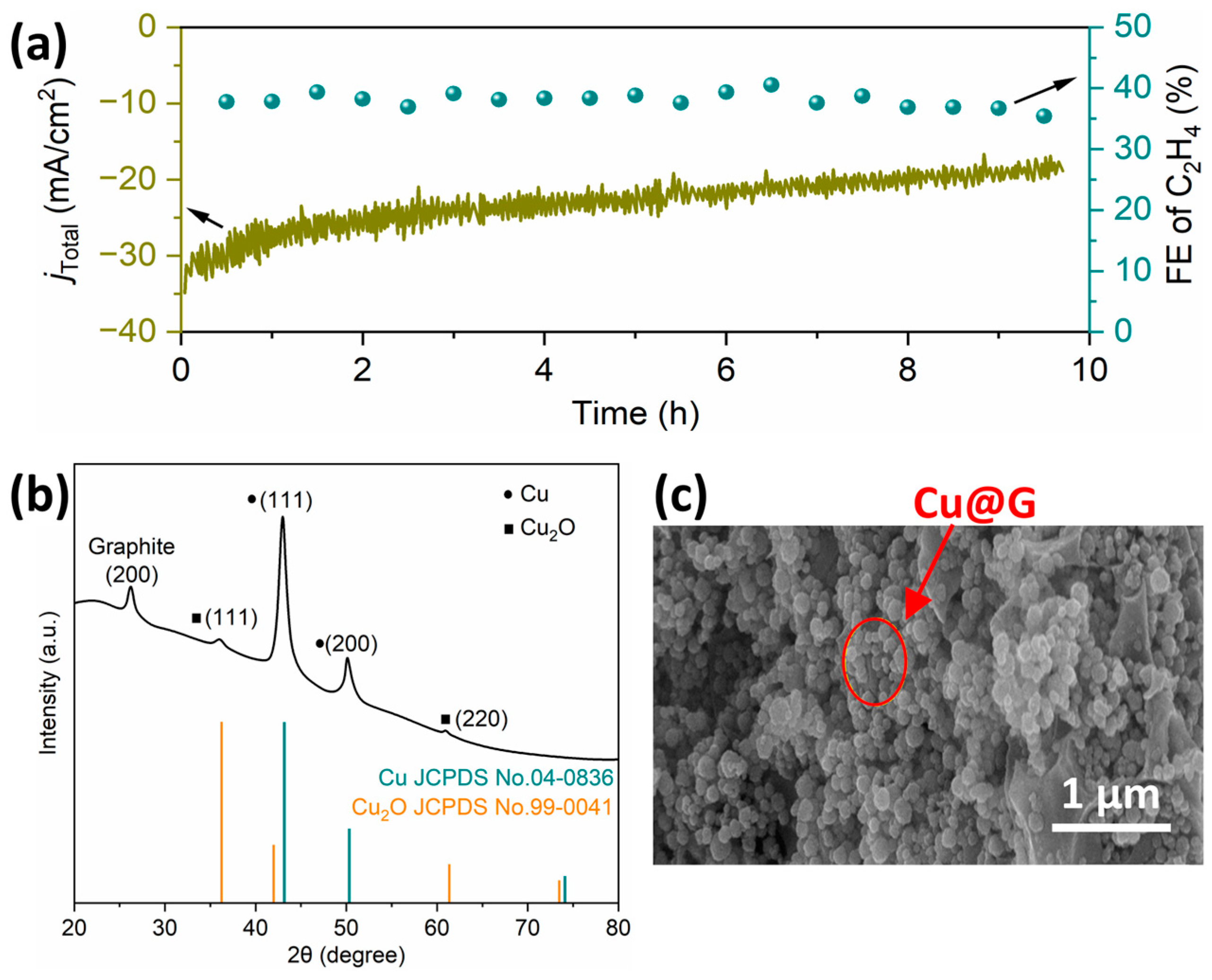
2.4. Evaluation of Electroreduction Performance
3. Materials and Methods
3.1. Materials
3.2. Fabrication of Cu@G/Bs
3.3. Characterization
3.4. Adsorption Experiments of CO2
3.5. Electrochemical Experiments
3.5.1. Electrode Preparation
3.5.2. Electrochemical Activity Measurement
3.5.3. Reduction of CO2 and Stability Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peter, S.C. Reduction of CO2 to Chemicals and Fuels: A Solution to Global Warming and Energy Crisis. ACS Energy Lett. 2018, 3, 1557–1561. [Google Scholar] [CrossRef]
- Tong, D.; Zhang, Q.; Liu, F.; Geng, G.; Zheng, Y.; Xue, T.; Hong, C.; Wu, R.; Qin, Y.; Zhao, H.; et al. Current Emissions and Future Mitigation Pathways of Coal-Fired Power Plants in China from 2010 to 2030. Environ. Sci. Technol. 2018, 52, 12905–12914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fan, Q.; Xia, R.; Meyer, T.J. CO2 Reduction: From Homogeneous to Heterogeneous Electrocatalysis. Acc. Chem. Res. 2020, 53, 255–264. [Google Scholar] [CrossRef]
- Zheng, H.; Yang, Z.; Luo, L.; Cheng, J.; Jin, Y.; Gao, Q.; Fan, M.; Zhao, Z.; Kong, X.; Zhang, X.; et al. Engineering the Stable BiOx Species for Efficient Electroreduction of CO2 into Formic Acid at Ampere-Level Current. Nano Lett. 2025, 25, 8750–8757. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, B.; Wang, Q.; Hu, H.; Xia, R.; Zhu, J.; Sun, Q.; Wu, Z.; Miao, R.K.; Guan, Q.; et al. Dynamic Activation of Edge-Hosted Co–N4 Sites for Energy-Efficient Electrochemical CO2 Reduction at Industry-Level Current Density. ACS Catal. 2025, 15, 8540–8550. [Google Scholar] [CrossRef]
- Baek, S.; Gutierrez-Portocarrero, S.; Gerulskis, R.; Minteer, S.D.; German, S.R.; White, H.S. Detection of CO2 Locally Generated by Formate Dehydrogenase Using Carbonate Ion-Selective Micropipette Electrodes. ACS Nano 2025, 19, 13240–13249. [Google Scholar] [CrossRef] [PubMed]
- Naz, R.; Tahir, M. Recent Developments in Metal-free Materials for Photocatalytic and Electrocatalytic Carbon Dioxide Conversion into Value Added Products. Energy Fuels 2025, 39, 6127–6150. [Google Scholar] [CrossRef]
- Hazari, N.; Shafaat, H.S.; Yang, J.Y. Transforming Highly Oxidized and Reduced Carbon Feedstocks: Strategies for Catalytic CO2 and CH4 Valorization. Acc. Chem. Res. 2024, 57, 3451–3453. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, W.; Zhuang, Y.; Zhang, K.; Zhao, Q.; Xiao, D.; Liu, S.; Liu, Z.; Zhang, Y. High-Entropy Metal Sulfide Promises High-Performance Carbon Dioxide Reduction. ACS Appl. Mater. Interfaces 2024, 16, 66211–66218. [Google Scholar] [CrossRef]
- Jolly, B.J.; Pung, M.J.; Liu, C. Integrated electrochemical CO2 reduction and hydroformylation. Dalton Trans. 2024, 53, 18834–18838. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Sun, H.; Gao, L.; Jin, X.; Li, Y.; Lv, Z.; Xu, L.; Liu, W.; Sun, X. Current Status and Perspectives of Dual-Atom Catalysts Towards Sustainable Energy Utilization. Nano-Micro Lett. 2024, 16, 139. [Google Scholar] [CrossRef]
- Hou, J.; Xu, B.; Lu, Q. Influence of electric double layer rigidity on CO adsorption and electroreduction rate. Nat. Commun. 2024, 15, 1926. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Masana, J.J.; Qiu, M.; Yu, Y. Cu−based bimetallic sites’ p-d orbital hybridization promotes CO asymmetric coupling conversion to C2 products. Mater. Today Phys. 2024, 48, 101565. [Google Scholar] [CrossRef]
- Nellaiappan, S.; Sharma, S. Substitution of Zinc(II) in Nickel(II) Oxide as Proficient Copper-Free Catalysts for Selective CO2 Electroreduction. ACS Appl. Energy Mater. 2019, 2, 2998–3003. [Google Scholar] [CrossRef]
- Lin, L.; Liu, T.; Xiao, J.; Li, H.; Wei, P.; Gao, D.; Nan, B.; Si, R.; Wang, G.; Bao, X. Enhancing CO2 Electroreduction to Methane with a Cobalt Phthalocyanine and Zinc–Nitrogen–Carbon Tandem Catalyst. Angew. Chem. Int. Ed. Engl. 2020, 59, 22408–22413. [Google Scholar] [CrossRef] [PubMed]
- Kornienko, N.; Zhao, Y.; Kley, C.S.; Zhu, C.; Kim, D.; Lin, S.; Chang, C.J.; Yaghi, O.M.; Yang, P. Metal–Organic Frameworks for Electrocatalytic Reduction of Carbon Dioxide. J. Am. Chem. Soc. 2015, 137, 14129–14135. [Google Scholar] [CrossRef]
- Al-Attas, T.A.; Marei, N.N.; Yong, X.; Yasri, N.G.; Thangadurai, V.; Shimizu, G.; Siahrostami, S.; Kibria, G. Ligand-Engineered Metal–Organic Frameworks for Electrochemical Reduction of Carbon Dioxide to Carbon Monoxide. ACS Catal. 2021, 11, 7350–7357. [Google Scholar] [CrossRef]
- Lin, S.; Diercks, C.S.; Zhang, Y.-B.; Kornienko, N.; Nichols, E.M.; Zhao, Y.; Paris, A.R.; Kim, D.; Yang, P.; Yaghi, O.M.; et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 2015, 349, 1208–1213. [Google Scholar] [CrossRef]
- Liu, H.; Chu, J.; Yin, Z.; Cai, X.; Zhuang, L.; Deng, H. Covalent Organic Frameworks Linked by Amine Bonding for Concerted Electrochemical Reduction of CO2. Chem 2018, 4, 1696–1709. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Zhao, J.; Xiong, Z.; Zhao, Y.; Zhang, J. PtCu alloy cocatalysts for efficient photocatalytic CO2 reduction into CH4 with 100% selectivity. Catal. Sci. Technol. 2022, 12, 3454–3463. [Google Scholar] [CrossRef]
- Fu, X.; Zhu, A.; Chen, X.; Zhang, S.; Wang, M.; Yuan, M. Stabilization of Cu/Ni Alloy Nanoparticles with Graphdiyne Enabling Efficient CO2 Reduction. Chem. Res. Chin. Univ. 2021, 37, 1328–1333. [Google Scholar] [CrossRef]
- Yu, J.; Liu, S.; Mu, X.; Yang, G.; Luo, X.; Lester, E.; Wu, T. Cu-ZrO2 catalysts with highly dispersed Cu nanoclusters derived from ZrO2@ HKUST-1 composites for the enhanced CO2 hydrogenation to methanol. Chem. Eng. J. 2021, 419, 129656. [Google Scholar] [CrossRef]
- Fu, Y.; Xie, Q.; Wu, L.; Luo, J. Crystal facet effect induced by different pretreatment of Cu2O nanowire electrode for enhanced electrochemical CO2 reduction to C2+ products. Chin. J. Catal. 2022, 43, 1066–1073. [Google Scholar] [CrossRef]
- Xin, Z.; Yuan, Z.; Liu, J.; Wang, X.; Shen, K.; Chen, Y.; Lan, Y.-Q. Cu cluster embedded porous nanofibers for high-performance CO2 electroreduction. Chin. Chem. Lett. 2022, 34, 107458. [Google Scholar] [CrossRef]
- Quan, W.; Lin, Y.; Luo, Y.; Huang, Y. Electrochemical CO2 Reduction on Cu: Synthesis-Controlled Structure Preference and Selectivity. Adv. Sci. 2021, 8, 2101597. [Google Scholar] [CrossRef]
- Xu, H.; Rebollar, D.; He, H.; Chong, L.; Liu, Y.; Liu, C.; Sun, C.-J.; Li, T.; Muntean, J.V.; Winans, R.E.; et al. Highly selective electrocatalytic CO2 reduction to ethanol by metallic clusters dynamically formed from atomically dispersed copper. Nat. Energy 2020, 5, 623–632. [Google Scholar] [CrossRef]
- Liang, H.-Q.; Zhao, S.; Hu, X.-M.; Ceccato, M.; Skrydstrup, T.; Daasbjerg, K. Hydrophobic Copper Interfaces Boost Electroreduction of Carbon Dioxide to Ethylene in Water. ACS Catal. 2021, 11, 958–966. [Google Scholar] [CrossRef]
- Le, T.; Salavati-Fard, T.; Wang, B. Plasmonic Energetic Electrons Drive CO2 Reduction on Defective Cu2O. ACS Catal. 2023, 13, 6328–6337. [Google Scholar] [CrossRef]
- Yu, W.-B.; Yi, M.; Fu, H.-H.; Pei, M.-J.; Liu, Y.; Xu, B.-M.; Li, Y.; Yan, W.; Zhang, J. Dandelion-Like Nanostructured Cu/Cu2O Heterojunctions with Fast Diffusion Channels Enabling Rapid Photocatalytic Pollutant Removal. ACS Appl. Nano Mater. 2023, 6, 2928–2941. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Starsich, F.; Dasargyri, A.; Wurnig, M.C.; Krumeich, F.; Boss, A.; Leroux, J.; Pratsinis, S.E. Photothermal Killing of Cancer Cells by the Controlled Plasmonic Coupling of Silica-Coated Au/Fe2O3 Nanoaggregates. Adv. Funct. Mater. 2014, 24, 2818–2827. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, J.; Ding, Q.; Lin, D.; Dong, R.; Yang, L.; Liu, J. Sea-urchin-like Fe3O4@C@Ag particles: An efficient SERS substrate for detection of organic pollutants. Nanoscale 2013, 5, 5887–5895. [Google Scholar] [CrossRef] [PubMed]
- Riedel, R.; Mahr, N.; Yao, C.; Wu, A.; Yang, F.; Hampp, N. Synthesis of gold–silica core–shell nanoparticles by pulsed laser ablation in liquid and their physico-chemical properties towards photothermal cancer therapy. Nanoscale 2020, 12, 3007–3018. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tang, H.; Yang, H.; Cao, Y.; Zhou, T.; Wang, G. Steering electrochemical CO2 reduction selectivity toward CH4 or C2H4 on N-doped carbon-coated Cu/Cu2O composite catalysts. ACS Catal. 2024, 14, 15088–15095. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hong, D.; Lee, J.C.; Kim, H.G.; Lee, S.; Shin, S.; Kim, B.; Lee, H.; Kim, M.; Oh, J.; et al. Quasi-graphitic carbon shell-induced Cu confinement promotes electrocatalytic CO2 reduction toward C2+ products. Nat. Commun. 2021, 12, 3765. [Google Scholar] [CrossRef]
- Cardona, J.E.M.; Louaguef, D.; Gaffet, E.; Ashammakhi, N.; Alem, H. Review of core/shell nanostructures presenting good hyperthermia properties for cancer therapy. Mater. Chem. Front. 2021, 5, 6429–6443. [Google Scholar] [CrossRef]
- Gong, T.; Wang, X.; Zhu, H.; Wen, C.; Ma, Q.; Li, X.; Li, M.; Guo, R.; Liang, W. Folic acid–maltodextrin polymer coated magnetic graphene oxide as a NIR-responsive nano-drug delivery system for chemo-photothermal synergistic inhibition of tumor cells. RSC Adv. 2023, 13, 12609–12617. [Google Scholar] [CrossRef]
- Li, F.; Qian, Q.; Yan, F.; Yuan, G. Nitrogen-doped porous carbon microspherules as supports for preparing monodisperse nickel nanoparticles. Carbon 2006, 44, 128–132. [Google Scholar] [CrossRef]
- Zou, R.; Liu, Q.; He, G.; Yuen, M.F.; Xu, K.; Hu, J.; Parkin, I.P.; Lee, C.; Zhang, W. Nanoparticles Encapsulated in Porous Carbon Matrix Coated on Carbon Fibers: An Ultrastable Cathode for Li-Ion Batteries. Adv. Energy Mater. 2016, 7, 1601363. [Google Scholar] [CrossRef]
- He, X.; Peng, Z.; Shen, P.; Miao, C.; Xie, J.; Deng, C.; Kougias, P.G.; Shen, D.; Lin, R. Promoting Multi-Carbon Fatty Acids Production from Microbial Chain Elongation of CO2/H2 with Carbonaceous Materials. ACS Sustain. Chem. Eng. 2025, 13, 6720–6734. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, X.; Yang, X.-B.; Peng, P.; Li, Z.; Jin, C. Enhanced Transformation of Cr(VI) by Heterocyclic-N within Nitrogen-Doped Biochar: Impact of Surface Modulatory Persistent Free Radicals (PFRs). Environ. Sci. Technol. 2020, 54, 8123–8132. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Jiang, Y.; Zhao, Z.; Wang, L.; Chen, J.; Ren, S.; Liu, Z.; Zhang, Y.; Tsang, D.C.W.; Crittenden, J.C. pH Dependence of Arsenic Oxidation by Rice-Husk-Derived Biochar: Roles of Redox-Active Moieties. Environ. Sci. Technol. 2019, 53, 9034–9044. [Google Scholar] [CrossRef]
- Guo, S.; Li, Y.; Wang, Y.; Wang, L.; Sun, Y.; Liu, L. Recent advances in biochar-based adsorbents for CO2 capture. Carbon Capture Sci. Technol. 2022, 4, 100059. [Google Scholar] [CrossRef]
- Khan, A.; Rashid, A.; Younas, R.; Chong, R. A chemical reduction approach to the synthesis of copper nanoparticles. Int. Nano Lett. 2015, 6, 21–26. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Q.; Fang, Q.; Li, W.; Wei, H.; Zhao, T.; Zhang, Y.; Huang, G.; Cui, C.; Zhang, K. Preparation of nickel nanoparticle-decorated graphene/copper composites with enhanced interfacial bonding and heat dissipation properties. J. Alloys Compd. 2025, 1028, 180696. [Google Scholar] [CrossRef]
- Yook, J.Y.; Jun, J.; Kwak, S. Amino functionalization of carbon nanotube surfaces with NH3 plasma treatment. Appl. Surf. Sci. 2010, 256, 6941–6944. [Google Scholar] [CrossRef]
- Al Mesfer, M.K.; Danish, M. Breakthrough adsorption study of activated carbons for CO2 separation from flue gas. J. Environ. Chem. Eng. 2018, 6, 4514–4524. [Google Scholar] [CrossRef]
- Shin, H.K.; Kim, C.; Talkner, P.; Lee, E.K. Brownian motion from molecular dynamics. Chem. Phys. 2010, 375, 316–326. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, J.; Qi, Y.; Zhang, J.; Guo, T.; Wang, H. Locust leaves-derived biochar coupled CuxO composites for efficient electrocatalytic CO2 reduction. Fuel 2024, 372, 132245. [Google Scholar] [CrossRef]
- Dong, L.; Feng, D.; Zhang, Y.; Wang, Z.; Zhao, Y.; Du, Q.; Gao, J.; Sun, S. Mechanism of biochar-Cu-based catalysts construction and its electrochemical CO2 reduction performance. Carbon Capture Sci. Technol. 2024, 13, 100250. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Zhou, Y.; Zhu, S.; Cui, J.; Song, B.; Zhu, W. High-Performance Electrocatalytic Reduction of Carbon Dioxide to Carbon Monoxide Using Biochar-Supported Gold Nanoparticles. Energy Fuels 2025, 39, 9907–9916. [Google Scholar] [CrossRef]
- Lei, F.; Liu, W.; Sun, Y.; Xu, J.; Liu, K.; Liang, L.; Yao, T.; Pan, B.; Wei, S.; Xie, Y. Metallic tin quantum sheets confined in graphene toward high-efficiency carbon dioxide electroreduction. Nat. Commun. 2016, 7, 12697. [Google Scholar] [CrossRef]
- Zhang, S.; Kang, P.; Meyer, T.J. Nanostructured tin catalysts for selective electrochemical reduction of carbon dioxide to formate. J. Am. Chem. Soc. 2014, 136, 1734–1737. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, X.; Tan, Y.; Liu, A.; Luo, F.; Zhang, M.; Zeng, L.; Zhang, Y. The in situ growth of Cu2O with a honeycomb structure on a roughed graphite paper for the efficient electroreduction of CO2 to C2H4. Catal. Sci. Technol. 2021, 11, 6742–6749. [Google Scholar] [CrossRef]
- Chu, S.; Yan, X.; Choi, C.; Hong, S.; Robertson, A.W.; Masa, J.; Han, B.; Jung, Y.; Sun, Z. Stabilization of Cu+ by tuning a CuO–CeO2 interface for selective electrochemical CO2 reduction to ethylene. Green Chem. 2020, 22, 6540–6546. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, Y.; Wen, X.; Guo, X.; Wei, R.; Gao, L.; Pan, X.; Zhang, J.; Xiao, G. Modulable Cu(0)/Cu(I)/Cu(II) sites of Cu/C catalysts derived from MOF for highly selective CO2 electroreduction to hydrocarbons. Vacuum 2023, 215, 112231. [Google Scholar] [CrossRef]
- Ning, H.; Mao, Q.; Wang, W.; Yang, Z.; Wang, X.; Zhao, Q.; Song, Y.; Wu, M. N-doped reduced graphene oxide supported Cu2O nanocubes as high active catalyst for CO2 electroreduction to C2H4. J. Alloys Compd. 2019, 785, 7–12. [Google Scholar] [CrossRef]
- Jeon, Y.E.; Na Ko, Y.; Kim, J.; Choi, H.; Lee, W.; Kim, Y.E.; Lee, D.; Kim, H.Y.; Park, K.T. Selective production of ethylene from CO2 over CuAg tandem electrocatalysts. J. Ind. Eng. Chem. 2022, 116, 191–198. [Google Scholar] [CrossRef]
- Cheng, Y.-S.; Chu, X.-P.; Ling, M.; Li, N.; Wu, K.-L.; Wu, F.-H.; Li, H.; Yuan, G.; Wei, X.-W. An MOF-derived copper@nitrogen-doped carbon composite: The synergistic effects of N-types and copper on selective CO2 electroreduction. Catal. Sci. Technol. 2019, 9, 5668–5675. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Q.; Xie, Q.; Wan, L.; Zhao, Y.; Zhang, X.; Luo, J. Selective electrochemical reduction of carbon dioxide to ethylene on a copper hydroxide nitrate nanostructure electrode. Nanoscale 2020, 12, 17013–17019. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Z.; Wei, X.; Liu, S.; Guo, P.; Han, P.; Wang, H.; Zhang, J.; Lu, X.; Wei, B. Promotion of electrochemical CO2 reduction to ethylene on phosphorus-doped copper nanocrystals with stable Cuδ+ sites. Appl. Surf. Sci. 2021, 544, 148965. [Google Scholar] [CrossRef]
- Wang, Z.; Shang, Y.; Chen, H.; Cao, S.; Zhu, Q.; Liu, S.; Wei, S.; Lu, X. Toward highly active electrochemical CO2 reduction to C2H4 by copper hydroxyphosphate. J. Solid State Electrochem. 2023, 27, 1279–1287. [Google Scholar] [CrossRef]
- Chang, Z.; Huo, S.-J.; Zhang, W.; Fang, J.; Wang, H. The Tunable and Highly Selective Reduction Products on Ag@Cu Bimetallic Catalysts Toward CO2 Electrochemical Reduction Reaction. J. Phys. Chem. C 2017, 121, 11368–11379. [Google Scholar] [CrossRef]


| Samples | Total SA (m2/g) | Micropore SA (m2/g) | Average Pore Diameter | Total Pore Volume (cm3/g) | Micropore Volume (cm3/g) |
|---|---|---|---|---|---|
| B300 | 4.1 | 0 | 16.8 | 0.0197 | 0 |
| B450 | 67.4 | 15.9 | 12.5 | 0.1084 | 0.0361 |
| B600 | 165.3 | 83.2 | 9.4 | 0.3016 | 0.1437 |
| Cu@G/B300 | 31.8 | 0 | 14.2 | 0.0428 | 0 |
| Cu@G/B450 | 75.2 | 16.4 | 10.7 | 0.0983 | 0.0331 |
| Cu@G/B600 | 155.9 | 71.2 | 7.8 | 0.2896 | 0.1364 |
| Samples | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qmax (mg/g) | kL (L/mg) | R2 | n (mg/L) | KF (L/mg) | R2 | |
| Cu@G/B300 | 61.11 | 0.0467 | 0.989 | 2.2862 | 7.2495 | 0.991 |
| Cu@G/B450 | 77.82 | 0.0602 | 0.992 | 2.8043 | 13.7593 | 0.979 |
| Cu@G/B600 | 107.03 | 0.0559 | 0.994 | 2.7005 | 17.5947 | 0.978 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.; Zhou, X.; Zeng, F. Integrating Carbon-Coated Cu/Cu2O Nanoparticles with Biochars Enabled Efficient Capture and Electrocatalytic Reduction of CO2. Catalysts 2025, 15, 767. https://doi.org/10.3390/catal15080767
Hong Y, Zhou X, Zeng F. Integrating Carbon-Coated Cu/Cu2O Nanoparticles with Biochars Enabled Efficient Capture and Electrocatalytic Reduction of CO2. Catalysts. 2025; 15(8):767. https://doi.org/10.3390/catal15080767
Chicago/Turabian StyleHong, Yutong, Xiaokai Zhou, and Fangang Zeng. 2025. "Integrating Carbon-Coated Cu/Cu2O Nanoparticles with Biochars Enabled Efficient Capture and Electrocatalytic Reduction of CO2" Catalysts 15, no. 8: 767. https://doi.org/10.3390/catal15080767
APA StyleHong, Y., Zhou, X., & Zeng, F. (2025). Integrating Carbon-Coated Cu/Cu2O Nanoparticles with Biochars Enabled Efficient Capture and Electrocatalytic Reduction of CO2. Catalysts, 15(8), 767. https://doi.org/10.3390/catal15080767






