A Quinary-Metallic High-Entropy Electrocatalyst with Driving of Cocktail Effect for Enhanced Oxygen Evolution Reaction
Abstract
1. Introduction
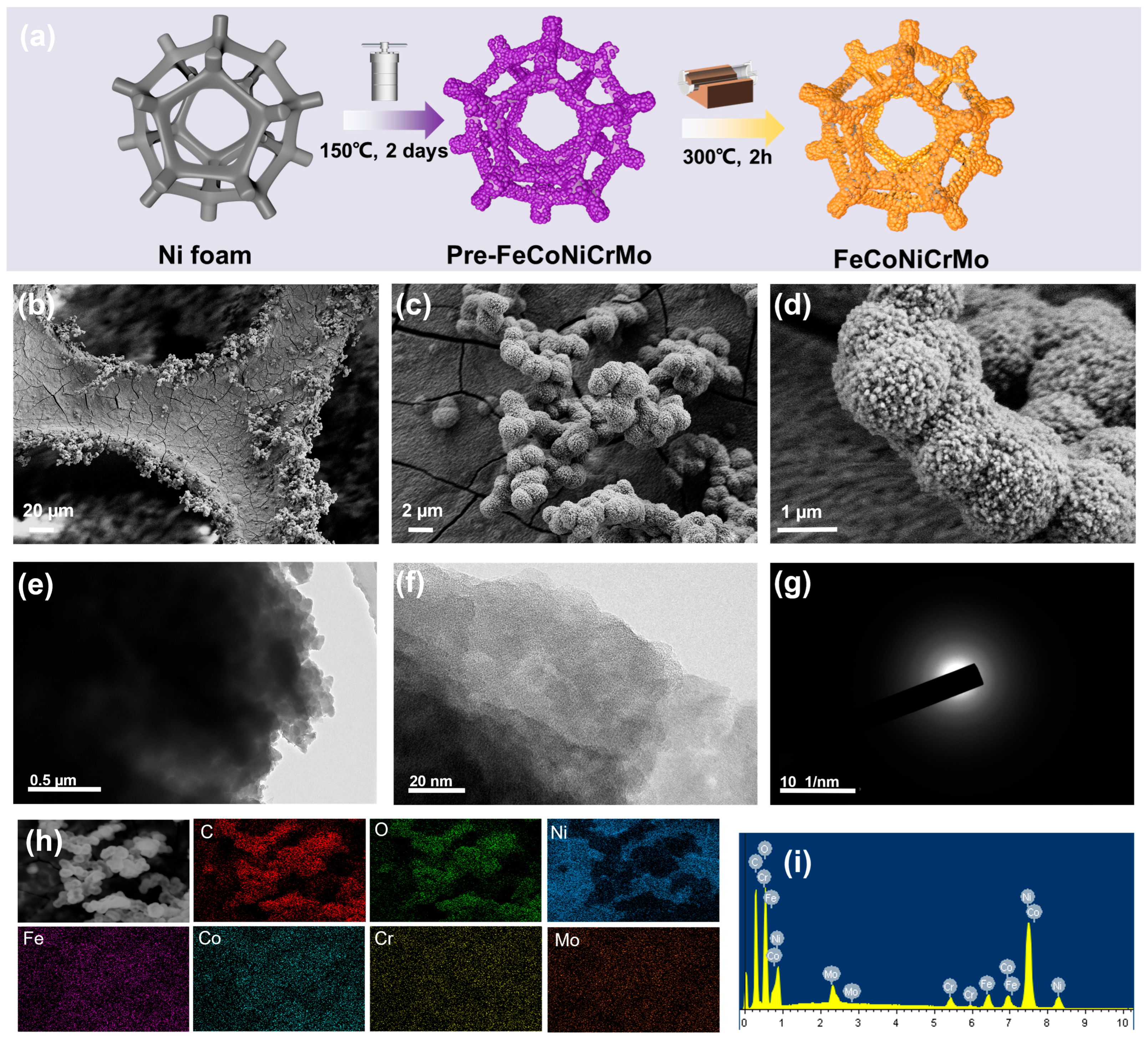
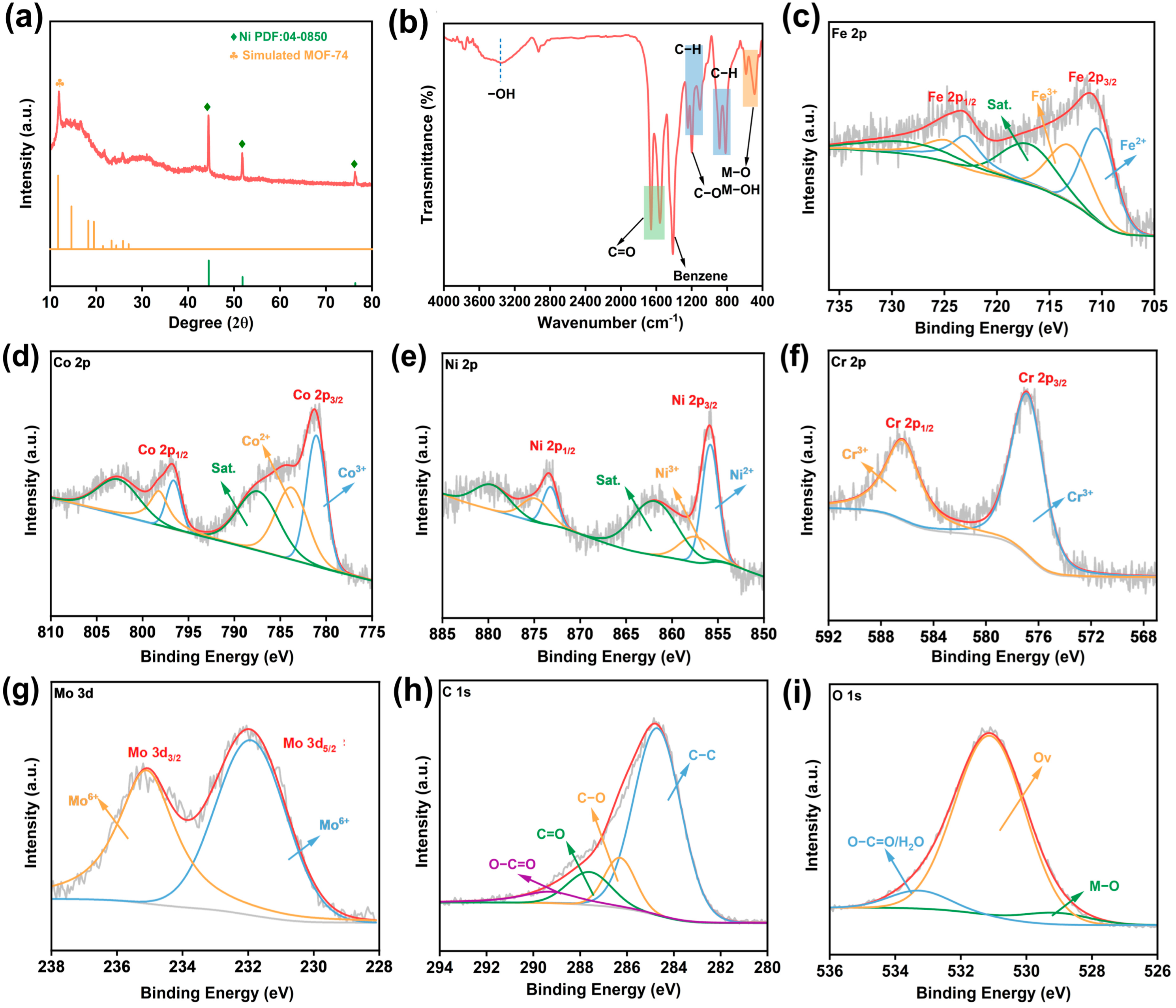
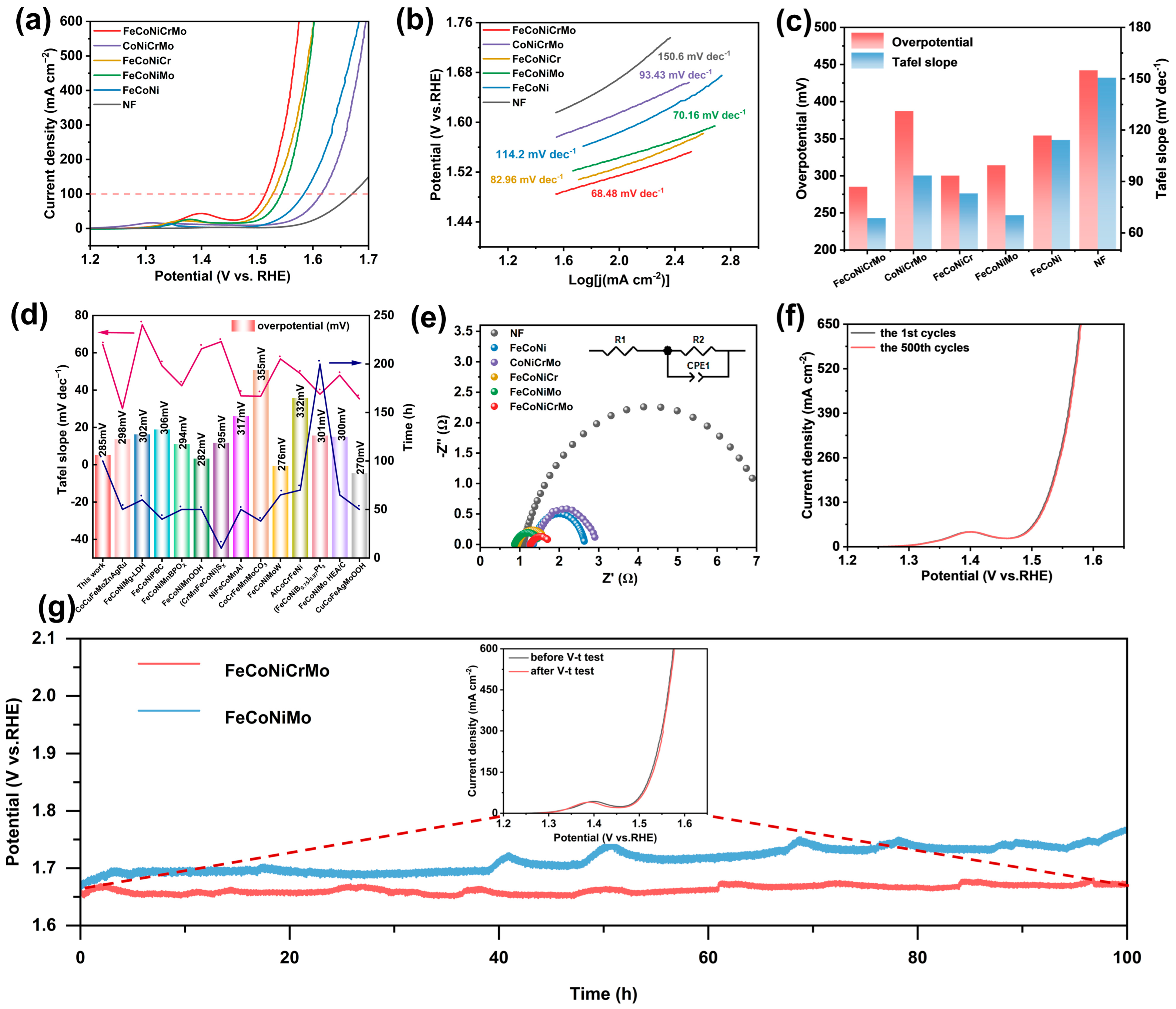
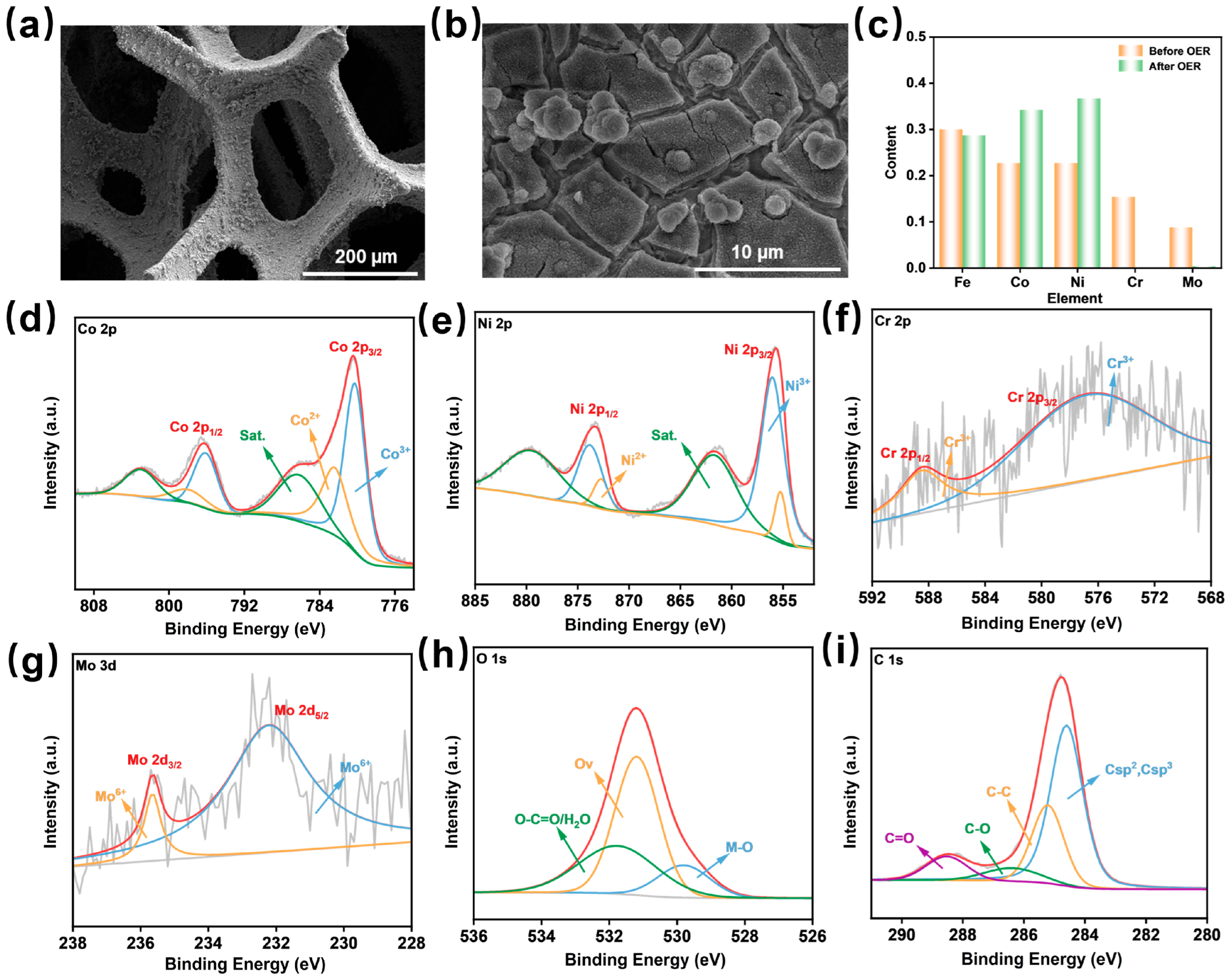
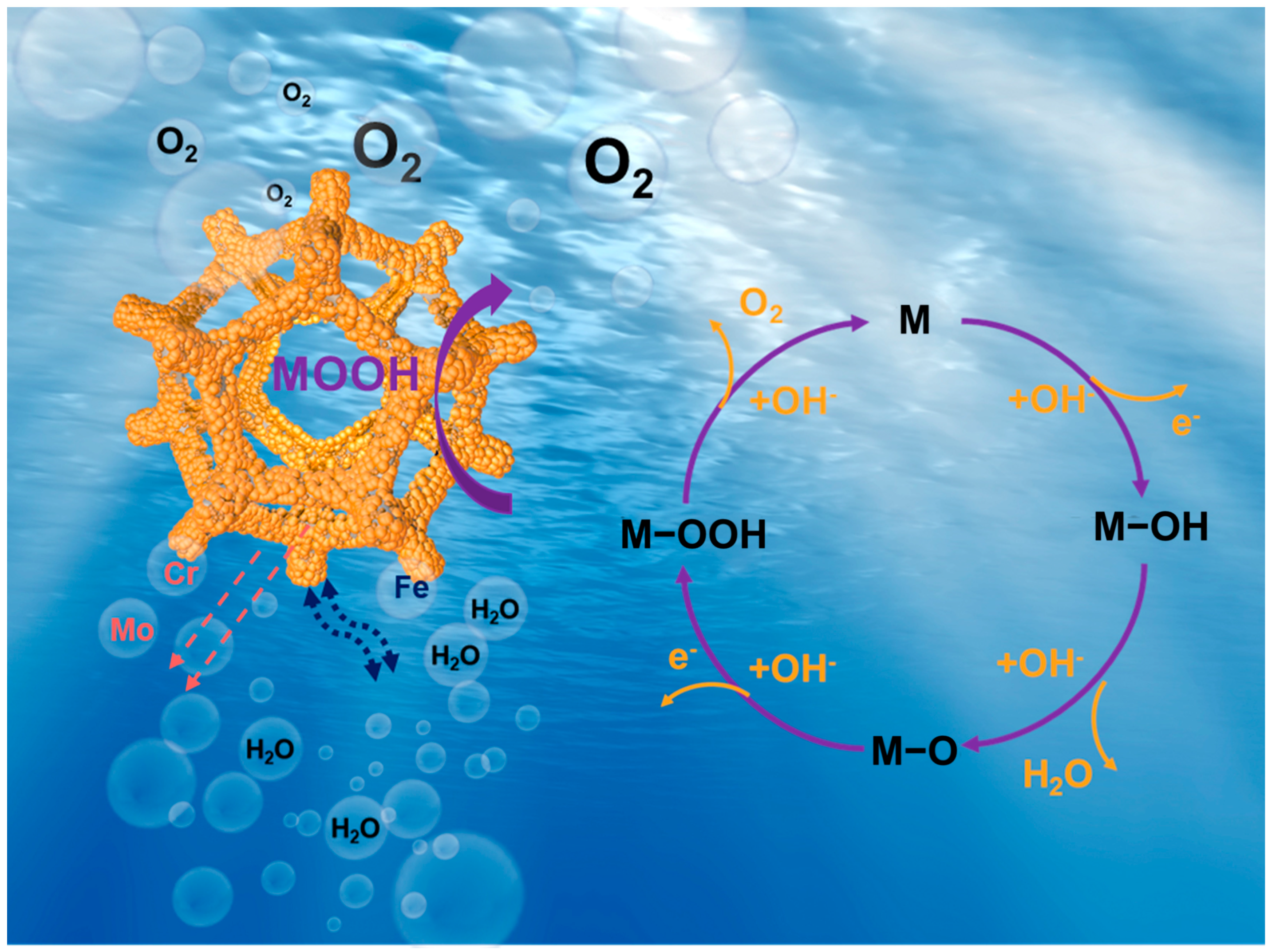
2. Results and Discussion
3. Experimental Section
3.1. Electrocatalyst Synthesis
3.2. Materials Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Wang, X.; Liu, K.; Sun, H.; Sun, D.; Huang, K.; Tang, Y.; Xing, W.; Li, H.; Fu, G. Reinforcing Co—O Covalency via Ce(4f)─O(2p)─Co(3d) Gradient Orbital Coupling for High-Efficiency Oxygen Evolution. Adv. Mater. 2023, 35, 2302462. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Liu, K.; Zhu, Z.; Guo, H.; Li, M.; Du, H.; Sun, D.; Li, H.; Huang, K.; et al. Ce-Induced Differentiated Regulation of Co Sites via Gradient Orbital Coupling for Bifunctional Water-Splitting Reactions. Adv. Funct. Mater. 2023, 13, 2301162. [Google Scholar] [CrossRef]
- Wu, T.; Sun, S.; Song, J.; Xi, S.; Du, Y.; Chen, B.; Sasangka, W.A.; Liao, H.; Gan, C.L.; Scherer, G.G.; et al. Iron-facilitated dynamic active-site generation on spinel CoAl2O4 with self-termination of surface reconstruction for water oxidation. Nat. Catal. 2019, 2, 763–772. [Google Scholar] [CrossRef]
- Wang, X.; Liu, B.; Zhang, Y.; Butburee, T.; Ostrikov, K.; Wang, S.; Huang, W. Development of ABO4-type photoanodes for photoelectrochemical water splitting. EcoEnergy 2023, 1, 108–153. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Yu, W.L.; Zhao, J.; Fu, J.Y.; Dong, B.; Wang, F.L.; Yu, J.F.; Liu, C.G.; Chai, Y.M. Self-supported FexNi1-xMoO4 with synergistic morphology and composition for efficient overall water splitting at large current density. Chin. Chem. Lett. 2023, 34, 107422. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Q.; Yan, B.; You, B.; Zheng, J.; Feng, L.; Zhang, C.; Jiang, S.; Chen, W.; He, S. Facet Engineering of Advanced Electrocatalysts Toward Hydrogen/Oxygen Evolution Reactions. Nano-Micro Lett. 2023, 15, 52. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.S.; Jang, G.Y.; Zhang, K.; Park, J.H. Transition metal carbide-based nanostructures for electrochemical hydrogen and oxygen evolution reactions. EcoEnergy 2023, 1, 344–374. [Google Scholar] [CrossRef]
- Fan, R.Y.; Xie, J.Y.; Liu, H.J.; Wang, H.Y.; Li, M.X.; Yu, N.; Luan, R.N.; Chai, Y.M.; Dong, B. Directional regulating dynamic equilibrium to continuously update electrocatalytic interface for oxygen evolution reaction. Chem. Eng. J. 2022, 431, 134040. [Google Scholar] [CrossRef]
- Yu, K.; Yang, H.; Zhang, H.; Huang, H.; Wang, Z.; Kang, Z.; Liu, Y.; Menezes, P.W.; Chen, Z. Immobilization of Oxyanions on the Reconstructed Heterostructure Evolved from a Bimetallic Oxysulfide for the Promotion of Oxygen Evolution Reaction. Nano-Micro Lett. 2023, 15, 186. [Google Scholar] [CrossRef]
- Chen, X.; Xu, K.; Li, J.; Wang, X.; Zhao, T.; Liu, F.; Yu, M.; Cheng, F. Ni3S2@NiFePx electrode with dual-anion-modulated layer for efficient and stable oxygen evolution. Chin. Chem. Lett. 2023, 34, 108713. [Google Scholar] [CrossRef]
- He, R.; Wang, C.; Feng, L. Amorphous FeCoNi-S as efficient bifunctional electrocatalysts for overall water splitting reaction. Chin. Chem. Lett. 2023, 34, 107241. [Google Scholar] [CrossRef]
- Han, N.; Zhang, W.; Guo, W.; Pan, H.; Jiang, B.; Xing, L.; Tian, H.; Wang, G.; Zhang, X.; Fransaer, J. Designing Oxide Catalysts for Oxygen Electrocatalysis: Insights from Mechanism to Application. Nano-Micro Lett. 2023, 15, 185. [Google Scholar] [CrossRef]
- Liu, K.K.; Meng, Z.; Fang, Y.; Jiang, H.L. Conductive MOFs for electrocatalysis and electrochemical sensor. eScience 2023, 3, 100133. [Google Scholar] [CrossRef]
- Liu, Y.R.; Hu, W.H.; Han, G.Q.; Dong, B.; Li, X.; Shang, X.; Chai, Y.M.; Liu, Y.Q.; Liu, C.G. Novel CoP Hollow Prisms as Bifunctional Electrocatalysts for Hydrogen Evolution Reaction in Acid media and Overall Water-splitting in Basic media. Electrochim. Acta 2016, 220, 98–106. [Google Scholar] [CrossRef]
- Yu, N.; Ma, Y.; Ren, J.K.; Zhang, Z.J.; Liu, H.J.; Nan, J.; Li, Y.C.; Chai, Y.M.; Dong, B. High negative voltage activating perovskite oxide with bi-vacancy synergistic regulation for water oxidation. Chem. Eng. J. 2023, 478, 147415. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Cao, Z.; Kozlov, S.M.; García de Arquer, F.P.; Dinh, C.T.; Li, J.; Wang, Z.; Zheng, X.; Zhang, L.; et al. High-valence metals improve oxygen evolution reaction performance by modulating 3d metal oxidation cycle energetics. Nat. Catal. 2020, 3, 985–992. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Yu, N.; Jin, Z.Y.; Yu, W.L.; Zhang, Y.S.; Li, Y.C.; Zhou, Y.L.; Chai, Y.M.; Dong, B. Amorphous high entropy oxides based on polyoxometalate clusters to accelerate surface reconstruction for oxygen evolution reaction. Colloids Surf. A Physicochem. Eng. Asp. 2024, 684, 133073. [Google Scholar] [CrossRef]
- Li, X.; Yan, K.L.; Rao, Y.; Dong, B.; Shang, X.; Han, G.Q.; Chi, J.Q.; Hu, W.H.; Liu, Y.R.; Chai, Y.M.; et al. Electrochemically activated NiSe-NixSy hybrid nanorods as efficient electrocatalysts for oxygen evolution reaction. Electrochim. Acta 2016, 220, 536–544. [Google Scholar] [CrossRef]
- Liu, D.; Yan, X.X.; Guo, P.F.; Yang, Y.X.; He, Y.F.; Liu, J.; Chen, J.; Pan, H.G.; Wu, R.B. Inert Mg Incorporation to Break the Activity/Stability Relationship in High-Entropy Layered Hydroxides for Electrocatalytic Oxygen Evolution Reaction. ACS Catal. 2023, 13, 7698–7706. [Google Scholar] [CrossRef]
- Xiao, H.; Shin, H.; Goddard, W.A. Synergy between Fe and Ni in the optimal performance of (Ni,Fe)OOH catalysts for the oxygen evolution reaction. Proc. Natl. Acad. Sci. USA 2018, 115, 5872–5877. [Google Scholar] [CrossRef]
- Trotochaud, L.; Young, S.L.; Ranney, J.K.; Boettcher, S.W. Nickel–Iron Oxyhydroxide Oxygen-Evolution Electrocatalysts: The Role of Intentional and Incidental Iron Incorporation. J. Am. Chem. Soc. 2014, 136, 6744–6753. [Google Scholar] [CrossRef]
- Yu, N.; Lv, J.Y.; Guo, Z.C.; Tian, X.J.; Zhang, Y.S.; Li, W.J.; Zhou, Y.L.; Chai, Y.M.; Dong, B. Strong Lewis acid-induced self-healing of loose FeOOH for alkaline oxygen evolution. Chem. Eng. J. 2024, 487, 150253. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Xin, Y.; Li, S.; Qian, Y.; Zhu, W.; Yuan, H.; Jiang, P.; Guo, R.; Wang, L. High-Entropy Alloys as a Platform for Catalysis: Progress, Challenges, and Opportunities. ACS Catal. 2020, 10, 11280–11306. [Google Scholar] [CrossRef]
- Wang, X.; Zuo, Y.; Horta, S.; He, R.; Yang, L.; Ostovari Moghaddam, A.; Ibáñez, M.; Qi, X.; Cabot, A. CoFeNiMnZnB as a High-Entropy Metal Boride to Boost the Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2022, 14, 48212–48219. [Google Scholar] [CrossRef]
- Theibault, M.J.; McCormick, C.R.; Lang, S.; Schaak, R.E.; Abruña, H.D. High Entropy Sulfide Nanoparticles as Lithium Polysulfide Redox Catalysts. ACS Nano 2023, 17, 18402–18410. [Google Scholar] [CrossRef]
- Mei, Y.; Feng, Y.; Zhang, C.; Zhang, Y.; Qi, Q.; Hu, J. High-Entropy Alloy with Mo-Coordination as Efficient Electrocatalyst for Oxygen Evolution Reaction. ACS Catal. 2022, 12, 10808–10817. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, C.; Zhang, Z.; Qi, Q.; Zhang, Y.; Hu, J. Constructing high entropy alloy/MoC heterostructure as efficient and stable catalysts for oxygen evolution reaction. Appl. Catal. A Gen. 2024, 681, 119780. [Google Scholar] [CrossRef]
- Wan, Q.; Zhang, Y.; Qi, Q.; Feng, H.; Feng, Y.; Bai, F.; Zhang, C.; Hu, J. Hydrothermal synthesis of FeCoNi/V3O5 heterojunctions as highly efficient electrocatalysts for the oxygen evolution reaction. Mater. Lett. 2024, 365, 136485. [Google Scholar] [CrossRef]
- Hu, J.; Guo, T.; Zhong, X.; Li, J.; Mei, Y.; Zhang, C.; Feng, Y.; Sun, M.; Meng, L.; Wang, Z.; et al. In Situ Reconstruction of High-Entropy Heterostructure Catalysts for Stable Oxygen Evolution Electrocatalysis under Industrial Conditions. Adv. Mater. 2024, 36, 2310918. [Google Scholar] [CrossRef]
- Li, H.; Lai, J.; Li, Z.; Wang, L. Multi-Sites Electrocatalysis in High-Entropy Alloys. Adv. Funct. Mater. 2021, 31, 2106715. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, H.-F.; Wang, K.-Y.; Chen, Z.-J.; Li, L.; Peng, J.; Peng, X.; Huang, Y.-J.; Yu, H.-B. Three factors make bulk high-entropy alloys as effective electrocatalysts for oxygen evolution. Mater. Futures 2023, 2, 045101. [Google Scholar] [CrossRef]
- Ren, J.T.; Chen, L.; Wang, H.Y.; Yuan, Z.Y. High-entropy alloys in electrocatalysis: From fundamentals to applications. Chem. Soc. Rev. 2023, 52, 8319–8373. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gao, L.; Chen, H.; Wang, H.; Zhang, J.; Li, X.; Duo, F.; Guan, G. Fabrication of amorphous FeCoNiCuMnPx high-entropy phosphide/carbon composites with a heterostructured fusiform morphology for efficient oxygen evolution reaction. J. Mater. Sci. Technol. 2024, 168, 62–70. [Google Scholar] [CrossRef]
- Baluk, M.A.; Trzebiatowska, P.J.; Pieczyńska, A.; Makowski, D.; Kroczewska, M.; Łuczak, J.; Zaleska-Medynska, A. A new strategy for PET depolymerization: Application of bimetallic MOF-74 as a selective catalyst. J. Environ. Manag. 2024, 363, 121360. [Google Scholar] [CrossRef]
- Li, D.; Li, Z.; Zou, R.; Shi, G.; Huang, Y.; Yang, W.; Yang, W.; Liu, C.; Peng, X. Coupling overall water splitting and biomass oxidation via Fe-doped Ni2P@C nanosheets at large current density. Appl. Catal. B Environ. Energy 2022, 307, 121170. [Google Scholar] [CrossRef]
- Karthikeyan, N.; Joseph Prince, J.; Ramalingam, S.; Periandy, S. Electronic [UV–Visible] and vibrational [FT-IR, FT-Raman] investigation and NMR–mass spectroscopic analysis of terephthalic acid using quantum Gaussian calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 139, 229–242. [Google Scholar] [CrossRef]
- Yu, N.; Liu, H.J.; Ren, J.K.; Zhang, Z.J.; Ma, Y.; Zhai, X.J.; Liu, D.P.; Chai, Y.M.; Dong, B. Dual roles derived from lattice incorporation of Cl- in ultrathin LiCoO2-xClx for water elelctrolysis. Fuel 2024, 357, 129786. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Shen, M.; Wang, Z.; Zheng, R.; Sun, H.; Liu, Y.; Wang, D.; Liu, C. Highly efficient oxygen evolution reaction enabled by phosphorus-boron facilitating surface reconstruction of amorphous high-entropy materials. J. Colloid Interface Sci. 2022, 628, 242–251. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Li, Y.; Shao, G.; Jia, Z.; Shen, B. Non-noble metal-based amorphous high-entropy oxides as efficient and reliable electrocatalysts for oxygen evolution reaction. Nano Res. 2022, 15, 8751–8759. [Google Scholar] [CrossRef]
- Triolo, C.; Moulaee, K.; Ponti, A.; Pagot, G.; Noto, V.D.; Pinna, N.; Neri, G.; Santangelo, S. Spinel-Structured High-Entropy Oxide Nanofibers as Electrocatalysts for Oxygen Evolution in Alkaline Solution: Effect of Metal Combination and Calcination Temperature. Adv. Funct. Mater. 2024, 34, 2306375. [Google Scholar] [CrossRef]
- Triolo, C.; Schweidler, S.; Lin, L.; Pagot, G.; Noto, V.D.; Breitung, B.; Santangelo, S. Evaluation of electrospun spinel-type high-entropy (Cr0.2Mn0.2Fe0.2Co0.2Ni0.2)3O4, (Cr0.2Mn0.2Fe0.2Co0.2Zn0.2)3O4 and (Cr0.2Mn0.2Fe0.2Ni0.2Zn0.2)3O4 oxide nanofibers as electrocatalysts for oxygen evolution in alkaline medium. Energy Adv. 2023, 2, 667–678. [Google Scholar] [CrossRef]
- Ponti, A.; Triolo, C.; Petrovicˇova`, B.; Ferretti, A.M.; Pagot, G.; Xu, W.L.; Noto, V.D.; Pinna, N.; Santangelo, S. Structure and magnetism of electrospun porous high-entropy (Cr1/5Mn1/5Fe1/5Co1/5Ni1/5)3O4, (Cr1/5Mn1/5Fe1/5Co1/5Zn1/5)3O4 and (Cr1/5Mn1/5Fe1/5Ni1/5Zn1/5)3O4 spinel oxide nanofibers. Phys. Chem. Chem. Phys. 2023, 25, 2212–2226. [Google Scholar] [CrossRef]
- Xu, N.; Yu, N.; Jin, Z.Y.; Zhou, Y.N.; Zhang, Y.S.; Tan, J.L.; Zhou, Y.L.; Chai, Y.M.; Dong, B. Boosting activity on molten salt-synthesized Ce doped cobalt hydroxyl nitrate nanorods by oxygen vacancies for efficient oxygen evolution. Fuel 2024, 365, 131214. [Google Scholar] [CrossRef]
- Feng, J.H.; Zhang, T.; Yang, Z.; Qian, K.Y.; Wang, X.; Song, W.Q.; Qiao, P.Z.; Ding, J.; Hu, W.B. Sub-5 nm amorphous iridium oxide nanosheets with synergistic multi-element doping for enhanced acidic oxygen evolution electrocatalysis. Chem. Eng. J. 2025, 503, 158213. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, C.; Hou, J.; Ma, W.; Jian, T.; Yan, S.; Liu, H. Duplex Interpenetrating-Phase FeNiZn and FeNi3 Heterostructure with Low-Gibbs Free Energy Interface Coupling for Highly Efficient Overall Water Splitting. Nano-Micro Lett. 2023, 15, 95. [Google Scholar] [CrossRef]
- Yu, N.; Luan, R.N.; Gu, C.Y.; Dong, Y.L.; Han, G.Q.; Hu, H.; Liu, B.; Chai, Y.M.; Dong, B. Surface S doping induced ladder-regulation of lattice oxygen on the vertical FeCoOOH for water oxidation. Fuel 2024, 376, 132757. [Google Scholar] [CrossRef]
- He, L.; Wang, N.; Sun, B.; Zhong, L.; Yao, M.; Hu, W.; Komarneni, S. High-entropy FeCoNiMn (oxy)hydroxide as high-performance electrocatalyst for OER and boosting clean carrier production under quasi-industrial condition. J. Clean. Prod. 2022, 356, 131680. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Su, Y.-H.; Lin, C.-C.; Ting, J.-M. Self-Reconstruction of Sulfate-Containing High Entropy Sulfide for Exceptionally High-Performance Oxygen Evolution Reaction Electrocatalyst. Adv. Funct. Mater. 2021, 31, 2106229. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, F.; Song, X.; Cai, W.; Ren, J.; Yang, H.; Bao, N. A novel septenary high-entropy (oxy)hydroxide electrocatalyst for boosted oxygen evolution reaction. J. Materiomics 2024, 10, 348–354. [Google Scholar] [CrossRef]
- Feng, P.F.; Song, G.J.; Zhang, W.J.; Zheng, H.; Li, B.W.; Zhou, S.F.; Liu, Y.Q.; Wu, G.S.; Ma, L.C. Interfacial Improvement of Carbon Fiber/Epoxy Composites by Incorporating Superior and Versatile Multiscale Gradient Modulus Intermediate Layer with Rigid-flexible Hierarchical Structure. Chin. J. Polym. Sci. 2021, 39, 896–905. [Google Scholar] [CrossRef]
- Cui, M.; Yang, C.; Li, B.; Dong, Q.; Wu, M.; Hwang, S.; Xie, H.; Wang, X.; Wang, G.; Hu, L. High-Entropy Metal Sulfide Nanoparticles Promise High-Performance Oxygen Evolution Reaction. Adv. Energy Mater. 2021, 11, 2002887. [Google Scholar] [CrossRef]
- Guan, S.; Xu, B.; Wu, J.; Han, J.; Guan, T.; Yang, Y.; Li, K.; Wang, J. High-entropy materials based on deep eutectic solvent for boosting oxygen evolution reaction. Fuel 2024, 358, 130315. [Google Scholar] [CrossRef]
- Lu, C.; Li, M.; Zhang, X.; Hou, H.; Li, X.; Yang, X.; Liu, X.; Ding, Y.; Hou, J.; Wang, Y. Low-temperature synthesized amorphous quasi-high-entropy carbonate electrocatalyst with superior surface self-optimization for efficient water oxidation. Ceram. Int. 2023, 49, 12156–12165. [Google Scholar] [CrossRef]
- He, R.; Yang, L.; Zhang, Y.; Jiang, D.; Lee, S.; Horta, S.; Liang, Z.; Lu, X.; Ostovari Moghaddam, A.; Li, J.; et al. A 3d-4d-5d High Entropy Alloy as a Bifunctional Oxygen Catalyst for Robust Aqueous Zinc–Air Batteries. Adv. Mater. 2023, 35, 2303719. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, K.; Zhang, B.; Xia, J.; Wu, J.; Zhang, Z.; Huang, Y. Corrosion engineering on AlCoCrFeNi high-entropy alloys toward highly efficient electrocatalysts for the oxygen evolution of alkaline seawater. Int. J. Miner. Metall. Mater. 2023, 30, 1922–1932. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Liu, Y.; Jia, Z.; Wang, Q.; Sun, L.; Zhang, L.C.; Kruzic, J.J.; Lu, J.; Shen, B. Defect Engineering of a High-Entropy Metallic Glass Surface for High-Performance Overall Water Splitting at Ampere-Level Current Densities. Adv. Mater. 2023, 35, 2303439. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, W.; Bao, N.; Yang, H. Implanting an Electron Donor to Enlarge the d–p Hybridization of High-Entropy (Oxy)hydroxide: A Novel Design to Boost Oxygen Evolution. Adv. Mater. 2022, 34, 2110511. [Google Scholar] [CrossRef]
- Yi, L.; Xiao, S.; Wei, Y.; Li, D.; Wang, R.; Guo, S.; Hu, W. Free-standing high-entropy alloy plate for efficient water oxidation catalysis: Structure/composition evolution and implication of high-valence metals. Chem. Eng. J. 2023, 469, 144015. [Google Scholar] [CrossRef]
- Larsson, A.; Grespi, A.; Abbondanza, G.; Eidhagen, J.; Gajdek, D.; Simonov, K.; Yue, X.; Lienert, U.; Hegedüs, Z.; Jeromin, A.; et al. The Oxygen Evolution Reaction Drives Passivity Breakdown for Ni–Cr–Mo Alloys. Adv. Mater. 2023, 35, 2304621. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Dai, C.; Liu, Z.; Luo, S.; Ge, J.; Duan, Y.; Chen, G.; Wei, C.; Chen, R.R.; Wang, J.; et al. Methanol electro-oxidation to formate on iron-substituted lanthanum cobaltite perovskite oxides. eScience 2022, 2, 87–94. [Google Scholar] [CrossRef]
- Xu, S.J.; Zhou, Y.N.; Shen, G.P.; Dong, B. Ni(OH)2 Derived from NiS2 Induced by Reflux Playing Three Roles for Hydrogen/Oxygen Evolution Reaction. Chin. J. Struct. Chem. 2022, 41, 2208052–2208057. [Google Scholar]
- Chung, D.Y.; Lopes, P.P.; Farinazzo Bergamo Dias Martins, P.; He, H.; Kawaguchi, T.; Zapol, P.; You, H.; Tripkovic, D.; Strmcnik, D.; Zhu, Y.; et al. Dynamic stability of active sites in hydr(oxy)oxides for the oxygen evolution reaction. Nat. Energy 2020, 5, 222–230. [Google Scholar] [CrossRef]
- Fan, R.Y.; Zhou, Y.N.; Li, M.X.; Xie, J.Y.; Yu, W.L.; Chi, J.Q.; Wang, L.; Yu, J.F.; Chai, Y.M.; Dong, B. In situ construction of Fe(Co)OOH through ultra-fast electrochemical activation as real catalytic species for enhanced water oxidation. Chem. Eng. J. 2021, 426, 131943. [Google Scholar] [CrossRef]
- Anantharaj, S.; Kundu, S.; Noda, S. “The Fe Effect”: A review unveiling the critical roles of Fe in enhancing OER activity of Ni and Co based catalysts. Nano Energy 2021, 80, 105514. [Google Scholar] [CrossRef]
- Zhou, Y.N.; Dong, Y.W.; Wu, Y.; Dong, B.; Liu, H.J.; Zhai, X.J.; Han, G.Q.; Liu, D.P.; Chai, Y.M. Nitrate induced precise atom substitution and vacancies for overall water splitting. Chem. Eng. J. 2023, 463, 142380. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, J.-Y.; Zhang, Z.-J.; Zhang, H.; Nan, J.; Chen, Z.; Liu, X.; Han, F.; Chai, Y.-M.; Dong, B. A Quinary-Metallic High-Entropy Electrocatalyst with Driving of Cocktail Effect for Enhanced Oxygen Evolution Reaction. Catalysts 2025, 15, 744. https://doi.org/10.3390/catal15080744
Lv J-Y, Zhang Z-J, Zhang H, Nan J, Chen Z, Liu X, Han F, Chai Y-M, Dong B. A Quinary-Metallic High-Entropy Electrocatalyst with Driving of Cocktail Effect for Enhanced Oxygen Evolution Reaction. Catalysts. 2025; 15(8):744. https://doi.org/10.3390/catal15080744
Chicago/Turabian StyleLv, Jing-Yi, Zhi-Jie Zhang, Hao Zhang, Jun Nan, Zan Chen, Xin Liu, Fei Han, Yong-Ming Chai, and Bin Dong. 2025. "A Quinary-Metallic High-Entropy Electrocatalyst with Driving of Cocktail Effect for Enhanced Oxygen Evolution Reaction" Catalysts 15, no. 8: 744. https://doi.org/10.3390/catal15080744
APA StyleLv, J.-Y., Zhang, Z.-J., Zhang, H., Nan, J., Chen, Z., Liu, X., Han, F., Chai, Y.-M., & Dong, B. (2025). A Quinary-Metallic High-Entropy Electrocatalyst with Driving of Cocktail Effect for Enhanced Oxygen Evolution Reaction. Catalysts, 15(8), 744. https://doi.org/10.3390/catal15080744






