Role of Cation Nature in FAU Zeolite in Both Liquid-Phase and Gas-Phase Adsorption
Abstract
1. Introduction
2. Results and Discussion
2.1. Zeolite Characterization
2.2. Liquid-Phase Adsorption Studies
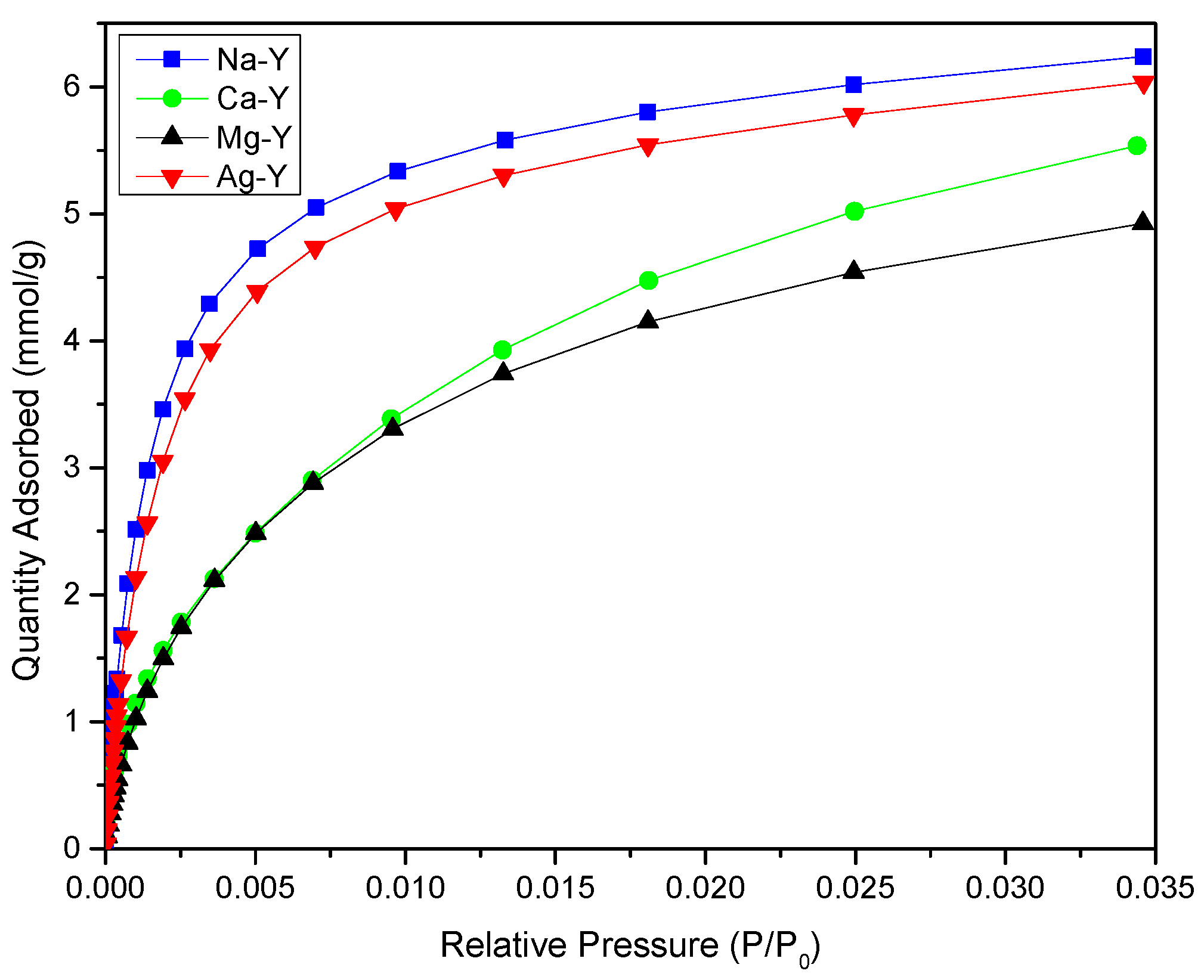
2.3. Gas-Phase Adsorption Studies
3. Materials and Methods
3.1. Characterization
3.2. Synthesis and Cation Exchange of Y Zeolite
3.3. Adsorption of Dye Molecules
3.4. CO2 Adsorption Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MB | Methylene Blue |
| XRD | X-ray Diffraction |
| BET | Brunauer–Emmett–Teller |
| SEM | Scanning Electron Microscopy |
References
- El-Bery, H.M.; Saleh, M.; El-Gendy, R.A.; Saleh, M.R.; Thabet, S.M. High Adsorption Capacity of Phenol and Methylene Blue Using Activated Carbon Derived from Lignocellulosic Agriculture Wastes. Sci. Rep. 2022, 12, 5499. [Google Scholar] [CrossRef]
- Li, Z.; Sellaoui, L.; Gueddida, S.; Dotto, G.L.; Ben Lamine, A.; Bonilla-Petriciolet, A.; Badawi, M. Adsorption of Methylene Blue on Silica Nanoparticles: Modelling Analysis of the Adsorption Mechanism via a Double Layer Model. J. Mol. Liq. 2020, 319, 114348. [Google Scholar] [CrossRef]
- Israfilov, N.; Soukup, K.; Louis, B.; Planeix, J.-M. MOF Side Chains as Sources of Supramolecular Interactions: Organic Pollutant Extraction from Water. New J. Chem. 2022, 46, 8967–8970. [Google Scholar] [CrossRef]
- Becker, A.; Israfilov, N.; Ehrstein, E.; Lara-Ibeas, I.; Planeix, J.-M.; Louis, B.; Le Calvé, S. Adsorption of Gaseous Formaldehyde on Y Zeolites and on Metal-Organic Frameworks. Microporous Mesoporous Mater. 2022, 343, 112136. [Google Scholar] [CrossRef]
- Deshpande, S.; Kheur, S.; Kheur, M.; Eyüboğlu, T.F.; Özcan, M. A Review on Zeolites and Their Applications in Dentistry. Curr. Oral Health Rep. 2023, 10, 36–42. [Google Scholar] [CrossRef]
- Tufail, M.K.; Ifrahim, M.; Rashid, M.; Ul Haq, I.; Asghar, R.; Uthappa, U.T.; Selvaraj, M.; Kurkuri, M. Chemistry of Zeolites and Zeolite Based Composite Membranes as a Cutting-Edge Candidate for Removal of Organic Dyes & Heavy Metal Ions: Progress and Future Directions. Sep. Purif. Technol. 2025, 354, 128739. [Google Scholar] [CrossRef]
- Tao, Z.; Tian, Y.; Hanif, A.; Chan, V.; Gu, Q.; Shang, J. Metal Cation-Exchanged LTA Zeolites for CO2/N2 and CO2/CH4 Separation: The Roles of Gas-Framework and Gas-Cation Interactions. Carbon Capture Sci. Technol. 2023, 8, 100126. [Google Scholar] [CrossRef]
- Derbe, T.; Temesgen, S.; Bitew, M. A Short Review on Synthesis, Characterization, and Applications of Zeolites. Adv. Mater. Sci. Eng. 2021, 2021, 6637898. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. Methylene Blue Dye: Toxicity and Potential Elimination Technology from Wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Asefa, M.T.; Feyisa, G.B. Comparative Investigation on Two Synthesizing Methods of Zeolites for Removal of Methylene Blue from Aqueous Solution. Int. J. Chem. Eng. 2022, 2022, 9378712. [Google Scholar] [CrossRef]
- Le, T.P.; Luong, H.V.T.; Nguyen, H.N.; Pham, T.K.T.; Trinh Le, T.L.; Tran, T.B.Q.; Ngo, T.N.M. Insight into Adsorption-Desorption of Methylene Blue in Water Using Zeolite NaY: Kinetic, Isotherm and Thermodynamic Approaches. Results Surf. Interfaces 2024, 16, 100281. [Google Scholar] [CrossRef]
- Majid, Z.; AbdulRazak, A.A.; Noori, W.A.H. Modification of Zeolite by Magnetic Nanoparticles for Organic Dye Removal. Arab. J. Sci. Eng. 2019, 44, 5457–5474. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Liu, J.; Zhang, Y. Facile One-Pot Synthesis of Novel Spherical Zeolite–Reduced Graphene Oxide Composites for Cationic Dye Adsorption. Ind. Eng. Chem. Res. 2014, 53, 13711–13717. [Google Scholar] [CrossRef]
- Sheemol, V.N.; Tyagi, B.; Jasra, R.V. Acylation of Toluene Using Rare Earth Cation Exchanged Zeolite β as Solid Acid Catalyst. J. Mol. Catal. A: Chem. 2004, 215, 201–208. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, J.; Li, G.; Hua, Y.; Sun, Y.; Hu, S.; Wen, X. Adsorption, Separation and Regeneration of Cation-Exchanged X Zeolites for LNG Purification: Li+, K+, Mg2+ and Ca2+. Microporous Mesoporous Mater. 2022, 340, 112032. [Google Scholar] [CrossRef]
- González-Crisostomo, J.C.; López-Juárez, R.; Yocupicio-Gaxiola, R.I.; Villanueva, E.; Zavala-Flores, E.; Petranovskii, V. Chabazite Synthesis and Its Exchange with Ti, Zn, Cu, Ag and Au for Efficient Photocatalytic Degradation of Methylene Blue Dye. Int. J. Mol. Sci. 2022, 23, 1730. [Google Scholar] [CrossRef]
- Chowdhury, S.; Bhattacharyya, K.G. Use of Cu(II)-Incorporated Zeolite Y for Decolourization of Dyes in Water: A Case Study with Aqueous Methylene Blue and Congo Red. SN Appl. Sci. 2019, 1, 87. [Google Scholar] [CrossRef]
- Colar, L.A.; Jakab, A.; Manea, F.; Pode, R.; Orha, C. Photocatalytic Performance of Ag-Modified Natural Zeolite Catalyst for Photocatalysis Degradation of Methylene Blue (MB) Under VIS Irradiation; WIT Press: Southampton, UK, 2012; pp. 335–344. [Google Scholar]
- Boer, D.G.; Langerak, J.; Pescarmona, P.P. Zeolites as Selective Adsorbents for CO2 Separation. ACS Appl. Energy Mater. 2023, 6, 2634–2656. [Google Scholar] [CrossRef]
- Castillo, J.M.; Silvestre-Albero, J.; Rodriguez-Reinoso, F.; Vlugt, T.J.H.; Calero, S. Water Adsorption in Hydrophilic Zeolites: Experiment and Simulation. Phys. Chem. Chem. Phys. 2013, 15, 17374. [Google Scholar] [CrossRef] [PubMed]
- Clatworthy, E.B.; Ghojavand, S.; Guillet-Nicolas, R.; Gilson, J.-P.; Llewellyn, P.L.; Nesterenko, N.; Mintova, S. Dynamic Adsorption of CO2 by CHA Zeolites—Size Matters. Chem. Eng. J. 2023, 471, 144557. [Google Scholar] [CrossRef]
- Walton, K.S.; Abney, M.B.; Douglas LeVan, M. CO2 Adsorption in Y and X Zeolites Modified by Alkali Metal Cation Exchange. Microporous Mesoporous Mater. 2006, 91, 78–84. [Google Scholar] [CrossRef]
- Yang, S.-T.; Kim, J.; Ahn, W.-S. CO2 Adsorption over Ion-Exchanged Zeolite Beta with Alkali and Alkaline Earth Metal Ions. Microporous Mesoporous Mater. 2010, 135, 90–94. [Google Scholar] [CrossRef]
- Tao, Z.; Tian, Y.; Ou, S.Y.; Gu, Q.; Shang, J. Direct Air Capture of CO2 by Metal Cation-exchanged LTA Zeolites: Effect of the Charge-to-size Ratio of Cations. AIChE J. 2023, 69, e18139. [Google Scholar] [CrossRef]
- Bernardon, C.; Louis, B.; Bénéteau, V.; Pale, P. Diels-Alder Reaction between Isoprene and Methyl Acrylate over Different Zeolites: Influence of Pore Topology and Acidity. ChemPlusChem 2013, 78, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Lutzweiler, G.; Zhang, Y.; Gens, F.; Echalard, A.; Ladam, G.; Hochart, J.; Janicot, T.; Mofaddel, N.; Louis, B. Deciphering the Role of Faujasite-Type Zeolites as a Cation Delivery Platform to Sustain the Functions of MC3T3-E1 Pre-Osteoblastic Cells. Mater. Adv. 2022, 3, 8616–8628. [Google Scholar] [CrossRef]
- Dyer, A. Ion-Exchange Properties of Zeolites and Related Materials. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2007; Volume 168, pp. 525–553. ISBN 978-0-444-53063-9. [Google Scholar]
- Dyer, A. Ion Exchange Capacity. In Verified Syntheses of Zeolitic Materials; Elsevier: Amsterdam, The Netherlands, 2001; pp. 67–68. ISBN 978-0-444-50703-7. [Google Scholar]
- Gellens, L.R.; Mortier, W.J.; Uytterhoeven, J.B. Oxidation and Reduction of Silver in Zeolite Y: A Structural Study. Zeolites 1981, 1, 85–90. [Google Scholar] [CrossRef]
- Shameli, K.; Mansor Bin Ahmad, M.; Mohsen, Z.; Yunis, W.Z.; Ibrahim, N.A. Fabrication of Silver Nanoparticles Doped in the Zeolite Framework and Antibacterial Activity. Int. J. Nanomed. 2011, 6, 331–341. [Google Scholar] [CrossRef]
- Min, J.G.; Kemp, K.C.; Hong, S.B. Silver ZK-5 Zeolites for Selective Ethylene/Ethane Separation. Sep. Purif. Technol. 2020, 250, 117146. [Google Scholar] [CrossRef]
- Yu, J.; Ye, S.; Xv, X.; Pan, L.; Lin, P.; Liao, H.; Wang, D. Thermal-Driven Formation of Silver Clusters Inside Na/Li FAUY Zeolites for Formaldehyde Detection. Nanomaterials 2022, 12, 3215. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Popovich, J.; Iannuzo, N.; Haydel, S.E.; Seo, D.-K. Silver-Ion-Exchanged Nanostructured Zeolite X as Antibacterial Agent with Superior Ion Release Kinetics and Efficacy against Methicillin-Resistant Staphylococcus aureus. ACS Appl. Mater. Interfaces 2017, 9, 39271–39282. [Google Scholar] [CrossRef]
- Min, J.G.; Kemp, K.C.; Kencana, K.S.; Hong, S.B. Silver-Exchanged CHA Zeolite as a CO2-Resistant Adsorbent for N2/O2 Separation. Microporous Mesoporous Mater. 2021, 323, 111239. [Google Scholar] [CrossRef]
- Gęsikiewicz-Puchalska, A.; Zgrzebnicki, M.; Michalkiewicz, B.; Kałamaga, A.; Narkiewicz, U.; Morawski, A.W.; Wrobel, R. Changes in Porous Parameters of the Ion Exchanged X Zeolite and Their Effect on CO2 Adsorption. Molecules 2021, 26, 7520. [Google Scholar] [CrossRef]
- Frising, T.; Leflaive, P. Extraframework Cation Distributions in X and Y Faujasite Zeolites: A Review. Microporous Mesoporous Mater. 2008, 114, 27–63. [Google Scholar] [CrossRef]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in Adsorption Processes: State of the Art and Future Prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.G.T.; Tao, R.; Van Zee, R.D. Porosity, Powder X-Ray Diffraction Patterns, Skeletal Density, and Thermal Stability of NIST Zeolitic Reference Materials RM 8850, RM 8851, and RM 8852. J. Res. Natl. Inst. Stan. 2021, 126, 126047. [Google Scholar] [CrossRef]
- Pan, L.; Ye, S.; Xv, X.; Lin, P.; Huang, R.; Wang, D. Zeolite-Encaged Luminescent Silver Nanoclusters. Materials 2023, 16, 3736. [Google Scholar] [CrossRef]
- Aspromonte, S.G.; Romero, A.; Boix, A.V.; Alonso, E. Hydrolysis of Cellulose to Glucose by Supercritical Water and Silver Mesoporous Zeolite Catalysts. Cellulose 2019, 26, 2471–2485. [Google Scholar] [CrossRef]
- Wang, L.; Lin, C.; Boldog, I.; Yang, J.; Janiak, C.; Li, J. Inverse Adsorption Separation of N2 O/CO2 in AgZK-5 Zeolite. Angew. Chem. Int. Ed. 2024, 63, e202317435. [Google Scholar] [CrossRef]
- Zheng, Y.; Vidal-Moya, A.; Hernández-Garrido, J.C.; Mon, M.; Leyva-Pérez, A. Silver-Exchanged Zeolite Y Catalyzes a Selective Insertion of Carbenes into C–H and O–H Bonds. J. Am. Chem. Soc. 2023, 145, 24736–24745. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.C.; Silva, M.P.; Gonçalves, R.; Pereira, M.F.R.; Botelho, G.; Fonseca, A.M.; Lanceros-Mendez, S.; Neves, I.C. Enhancement of the Dielectric Constant and Thermal Properties of α-Poly(Vinylidene Fluoride)/Zeolite Nanocomposites. J. Phys. Chem. C 2010, 114, 14446–14452. [Google Scholar] [CrossRef]
- Vanaja, M.; Paulkumar, K.; Baburaja, M.; Rajeshkumar, S.; Gnanajobitha, G.; Malarkodi, C.; Sivakavinesan, M.; Annadurai, G. Degradation of Methylene Blue Using Biologically Synthesized Silver Nanoparticles. Bioinorg. Chem. Appl. 2014, 2014, 742346. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Zharylkan, S.; Sultakhan, S.; Suleimenova, M.; Seitkhan, A.; Sailaukhanuly, Y.; Tulepov, M.; Tauanov, Z. Enhanced Adsorption of Mercury and Methylene Blue Using Silver and Surface Modified Zeolite-NaX Derived from Rice Husk. Eng. Sci. 2024, 31, 1241. [Google Scholar] [CrossRef]
- Awala, H.; Leite, E.; Saint-Marcel, L.; Clet, G.; Retoux, R.; Naydenova, I.; Mintova, S. Properties of Methylene Blue in the Presence of Zeolite Nanoparticles. New J. Chem. 2016, 40, 4277–4284. [Google Scholar] [CrossRef]
- Salimi, F.; Eskandari, M.; Karami, C. Investigation of Methylene Blue Adsorption in Wastewater Using Nano-Zeolite Modified with Copper. Desalin. Water Treat. 2017, 85, 206–214. [Google Scholar] [CrossRef]
- Hadda Aya, H.; Djamel, N.; Samira, A.; Otero, M.; Ali Khan, M. Optimizing Methylene Blue Adsorption Conditions on Hydrothermally Synthesized NaX Zeolite through a Full Two-Level Factorial Design. RSC Adv. 2024, 14, 23816–23827. [Google Scholar] [CrossRef]
- Nyankson, E.; Adjasoo, J.; Efavi, J.K.; Amedalor, R.; Yaya, A.; Manu, G.P.; Asare, K.; Amartey, N.A. Characterization and Evaluation of Zeolite A/Fe3O4 Nanocomposite as a Potential Adsorbent for Removal of Organic Molecules from Wastewater. J. Chem. 2019, 2019, 8090756. [Google Scholar] [CrossRef]
- Boudjema, L.; Assaf, M.; Salles, F.; Gassin, P.-M.; Martin-Gassin, G.; Zajac, J. Renewing Interest in Zeolites as Adsorbents for Capture of Cationic Dyes from Aqueous and Ethanolic Solutions: A Simulation-Based Insight into the Efficiency of Dye Adsorption in View of Wastewater Treatment and Valorization of Post-Sorption Materials. Molecules 2024, 29, 2952. [Google Scholar] [CrossRef]
- Jiao, D.; King, C.; Grossfield, A.; Darden, T.A.; Ren, P. Simulation of Ca2+ and Mg2+ Solvation Using Polarizable Atomic Multipole Potential. J. Phys. Chem. B 2006, 110, 18553–18559. [Google Scholar] [CrossRef] [PubMed]
- Chaib Draa, Y.M.; Ors, T.; Deroche, I.; Dejoie, C.; Paillaud, J.-L. 3D ED for the Localization of Cations in Potassium Exchanged and Partially Dehydrated Nano Y Zeolite†. CrystEngComm 2025, 27, 5095–5099. [Google Scholar] [CrossRef]
- Bénariac-Doumal, L.; Deroche, I.; Nouali, H.; Paillaud, J.-L.; Ors, T.; Fitch, A.N.; Dejoie, C. A Comparative Study of CO2 Adsorption in a Series of Zeolites. Phys. Chem. Chem. Phys. 2025, 27, 10621–10634. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Tian, T.; L’Hermitte, A.; Méndez, A.S.J.; Danaci, D.; Platero-Prats, A.E.; Petit, C. Using Silver Exchange to Achieve High Uptake and Selectivity for Propylene/Propane Separation in Zeolite Y. Chem. Eng. J. 2022, 446, 137104. [Google Scholar] [CrossRef]
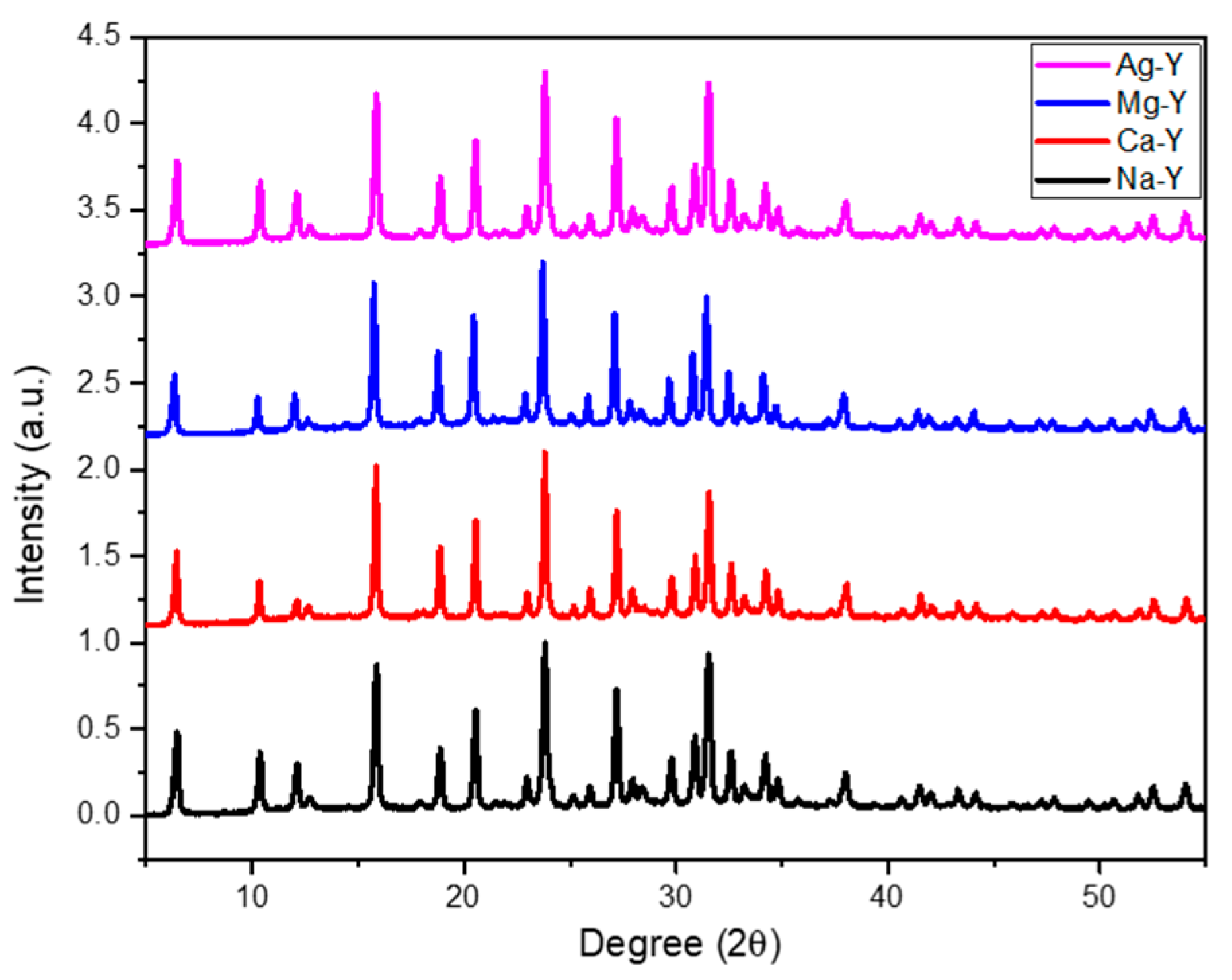
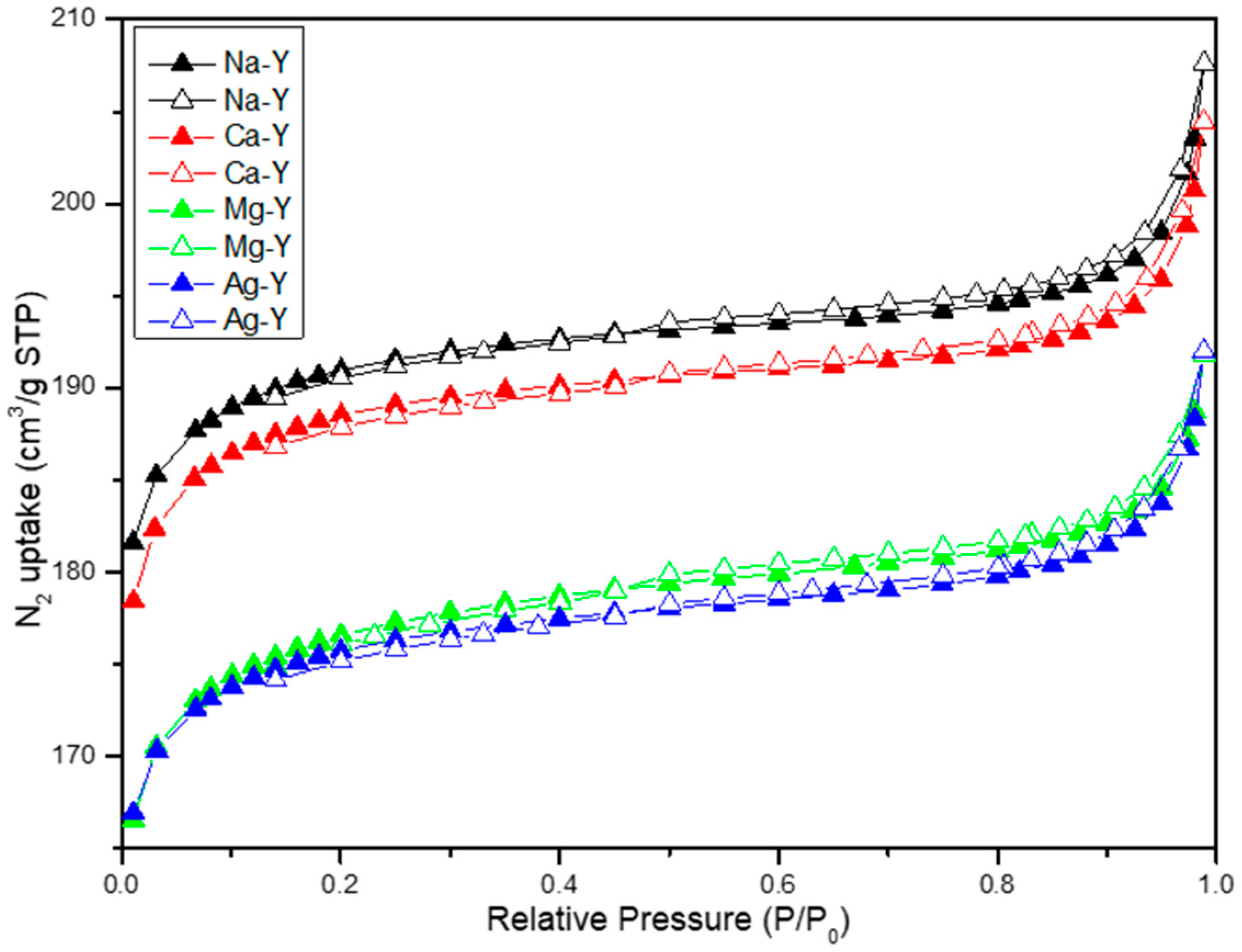
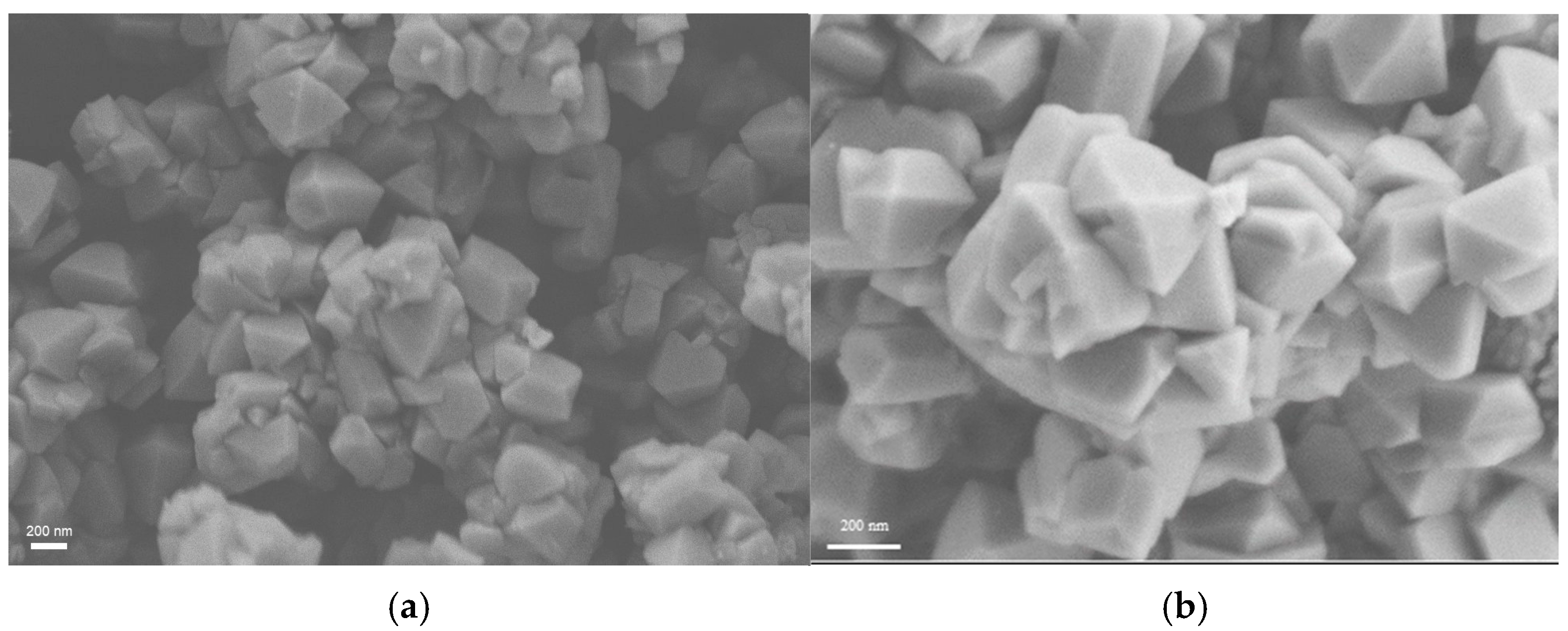
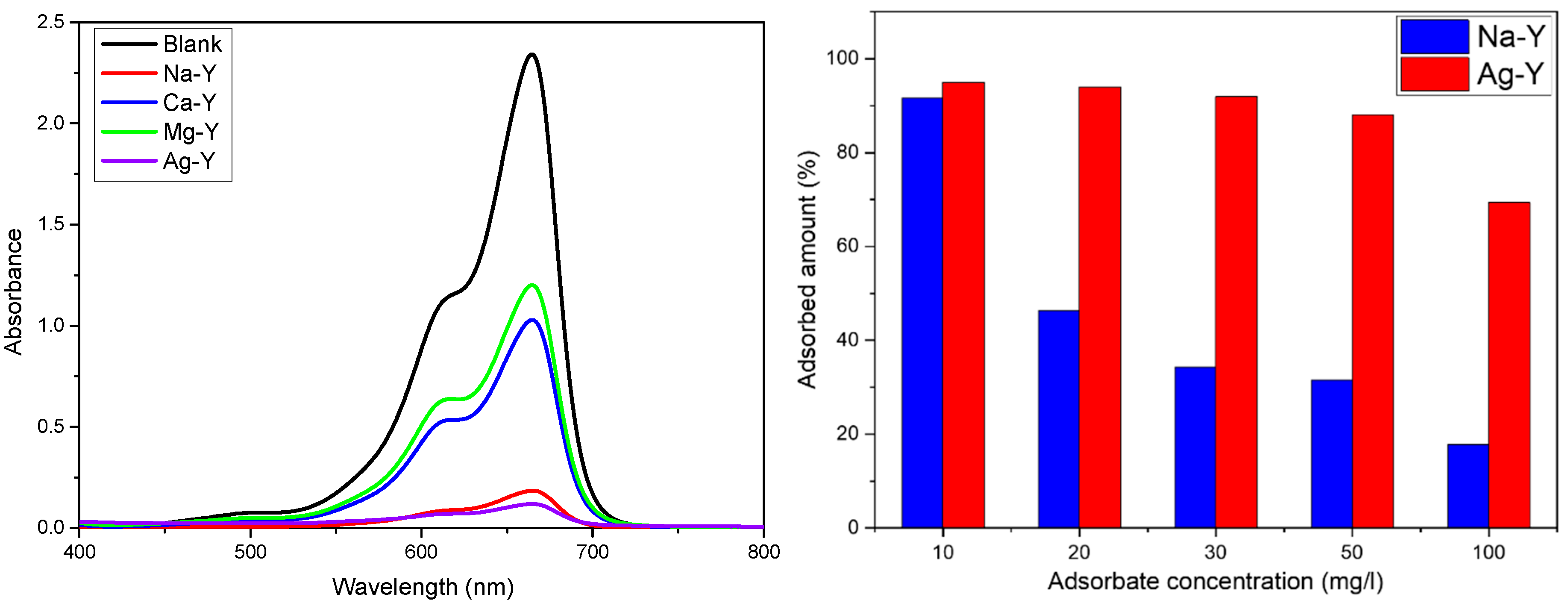
| Zeolite | Si/Al Ratio | Cation Content (wt%) | Exchange Degree (%) |
|---|---|---|---|
| Na-Y | 2.2 | 9.6 | - |
| Ca-Y | 2.1 | 6.9 | 80 |
| Mg-Y | 2.2 | 3.6 | 71 |
| Ag-Y | 2.2 | 3.2 | 7 |
| Zeolites | Surface Area (m2/g) | Vtotal (cm3/g) | Vmicro (cm3/g) | Adsorbed CO2 Quantity (mmol/g) | CO2 Adsorption Per m2 (µmol/m2) |
|---|---|---|---|---|---|
| Na-Y | 636 | 0.31 | 0.29 | 6.2 | 9.61 |
| Ca-Y | 598 | 0.30 | 0.28 | 5.3 | 8.86 |
| Mg-Y | 560 | 0.28 | 0.27 | 4.6 | 8.21 |
| Ag-Y | 557 | 0.28 | 0.26 | 5.8 | 10.4 |
| MB Concentration | Na-Y 5 Min./24 h | Ag-Y 5 Min./24 h |
|---|---|---|
| 10 mg/L | 66.3%/91.7% | 94.7%/95.0% |
| 20 mg/L | 34.1%/46.4% | 91.1%/94.0% |
| 30 mg/L | 27.2%/34.3% | 88.2%/92.0% |
| 50 mg/L | 25.4%/31.5% | 59.9%/88.1% |
| 100 mg/L | 12.8%/17.8% | 39.6%/69.4% |
| Kinetic Model | Constants | Na-Y 10 mg/L | Na-Y 20 mg/L | Ag-Y 30 mg/L | Ag-Y 50 mg/L | Ag-Y 100 mg/L |
|---|---|---|---|---|---|---|
| Pseudo-first-order | K1 (L/min) | 0.004177 | 0.002791 | 0.070828 | 0.069225 | 0.096555 |
| Qe (mg/g) | 3.484603 | 3.458684 | 3.701937 | 20.957285 | 64.414176 | |
| R2 | 0.591932 | 0.548921 | 0.644684 | 0.892314 | 0.994152 | |
| Pseudo-second-order | K2 (g/mg·min) | 0.005448 | 0.003573 | 0.121223 | 0.014924 | 0.004202 |
| Qe (mg/g) | 9.832053 | 9.964659 | 30.229127 | 46.268587 | 71.133793 | |
| R2 | 0.999017 | 0.997445 | 0.999994 | 0.999999 | 0.999991 |
| Isotherm Models | Constants | Na-Y 10 mg/L | Ag-Y 30 mg/L |
|---|---|---|---|
| Langmuir Model | Qm (mg/g) | 17.93 | 79.38 |
| KL (L/mg) | 0.324476 | 0.24768 | |
| RL | 0.2567 | 0.551 | |
| Freundlich Model | R2 | 0.994498 | 0.999307 |
| KF (mg/g) | 9.645987 | 16.62 | |
| 1/n | 0.135909 | 0.469567 | |
| R2 | 0.759577 | 0.945006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarbaliyev, B.; Israfilov, N.; Feyziyeva, S.; Lutzweiler, G.; Guliyeva, N.; Louis, B. Role of Cation Nature in FAU Zeolite in Both Liquid-Phase and Gas-Phase Adsorption. Catalysts 2025, 15, 734. https://doi.org/10.3390/catal15080734
Zarbaliyev B, Israfilov N, Feyziyeva S, Lutzweiler G, Guliyeva N, Louis B. Role of Cation Nature in FAU Zeolite in Both Liquid-Phase and Gas-Phase Adsorption. Catalysts. 2025; 15(8):734. https://doi.org/10.3390/catal15080734
Chicago/Turabian StyleZarbaliyev, Baylar, Nizami Israfilov, Shabnam Feyziyeva, Gaëtan Lutzweiler, Narmina Guliyeva, and Benoît Louis. 2025. "Role of Cation Nature in FAU Zeolite in Both Liquid-Phase and Gas-Phase Adsorption" Catalysts 15, no. 8: 734. https://doi.org/10.3390/catal15080734
APA StyleZarbaliyev, B., Israfilov, N., Feyziyeva, S., Lutzweiler, G., Guliyeva, N., & Louis, B. (2025). Role of Cation Nature in FAU Zeolite in Both Liquid-Phase and Gas-Phase Adsorption. Catalysts, 15(8), 734. https://doi.org/10.3390/catal15080734








