Abstract
Asymmetric synthesis of chiral hydroxysulfones, key pharmaceutical intermediates, is challenging. We report an efficient synthesis from readily available materials via a one-pot photo-biocatalytic cascade reaction in aqueous conditions, utilizing visible light as an energy source. This sustainable process achieves up to 84% yields and 99% ee. Engineered ketoreductase produces R-configured products with high conversion and enantioselectivity across diverse substrates. Molecular dynamics (MD) simulations explored enzyme–substrate interactions and their influence on reaction activity and stereoselectivity.
1. Introduction
Chiral hydroxysulfones are valuable synthons in organic synthesis due to their capacity to diversify molecular structures without racemization, thereby facilitating further functionalization of the α-carbon and the subsequent removal of the sulfonyl group [1,2]. They are also utilized in the synthesis of pharmaceutical molecules and biologically active compounds (Figure 1A) [3,4,5,6,7]. Various chemical synthetic methods have been employed to produce hydroxysulfones, including the asymmetric reduction of β-ketosulfones via borane reduction [8,9,10], transition metal-catalyzed asymmetric hydrogenation [11,12,13,14,15] and hydroxysulfonylation of alkenes [16,17], and asymmetric transfer hydrogenation [18,19,20,21,22,23]. The synthetic route involving the asymmetric addition to the aldehyde group of sulfur-containing compounds followed by oxidation with H2O2 to generate γ-hydroxysulfones has also been reported [24]. However, these methods often encounter limitations such as the necessity for high-pressure conditions, the use of complex chiral-ligand catalysts, or the risk of heavy metal contamination. Additionally, biocatalytic approaches have been developed for obtaining chiral hydroxysulfones. The dynamic kinetic resolution of racemic β- and γ-hydroxysulfones by lipase has been widely studied [25,26]. Nonetheless, these biocatalytic methods can be time-consuming and typically achieve only a 50% conversion rate. Another biocatalytic method using biocatalysts for the asymmetric reduction of ketosulfones has been gaining increasing attention due to the high selectivity and high atom economy [27,28].
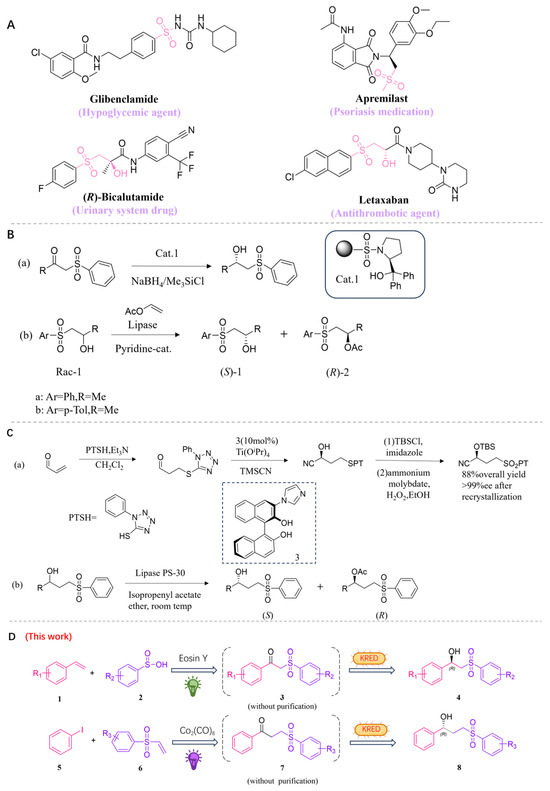
Figure 1.
(A) Representative bioactive chiral hydroxysulfones and derivatives. (B) Selected previous works for the synthesis of β-hydroxysulfones. (C) Selected previous works for the synthesis of γ-hydroxysulfones. (D) Our proposed one-pot photo-enzymatic cascade reactions for producing chiral β-hydroxysulfones and γ-hydroxysulfones in this work.
Ketoreductases (KREDs) are NAD(P)H-dependent enzymes that catalyze the reduction of prochiral ketones to their corresponding chiral alcohols. Enhanced through protein engineering to further improve their activity and selectivity, KREDs are increasingly utilized in the synthesis of active pharmaceutical intermediates (APIs) [29,30,31,32,33,34,35]. It is worth noting that KREDs convert nearly all substrate atoms into the desired chiral alcohol, minimizing waste [36,37]. In addition, KREDs typically use water as a solvent and operate under mild conditions, ensuring environmental friendliness [38].
Furthermore, ketosulfones are not commercially available and must be synthesized in advance. Therefore, there is a strong demand for the development of an environmentally friendly process to prepare prochiral ketosulfones from simple, readily available materials under mild conditions. Photocatalysis has become a potent method that utilizes visible light to induce unique reactivity, which cannot be achieved through thermal activation, by producing open-shell intermediates. Visible-light-driven photocatalysis provides a gentle, sustainable, and clean approach to chemical activation by transforming photonic energy into chemical energy [39,40,41,42,43,44]. Therefore, we aimed to develop a one-pot photo-biocatalytic cascade reaction, which benefits from the selectivity of enzymes and the reactivity of photocatalysts, to synthesize chiral hydroxysulfones [45,46]. In the consecutive one-pot process, the desired products can be obtained without the need for isolation and purification of intermediates. This approach minimizes production cycles and costs, saves time and resources, and reduces waste. In our group, we have been dedicated to using biocatalysts and photocatalysts to synthesize pharmaceutical molecules and potentially bioactive compounds in environmentally friendly ways, so we were motivated to embark on this study [47,48,49,50].
Within this context, we describe a mild and efficient one-pot cascade reaction for the direct synthesis of 4/8 from readily available styrene and benzene sulfinic acid/iodobenzene and phenyl vinyl sulfone by combining the KRED biocatalytic step with photocatalyzed sulfonylation/carbonylation reactions (Figure 1D). In contrast to the previous synthesis route for chiral β-hydroxysulfones (see Figure S4 in the Supporting Information), although the yield is slightly lower, our approach is performed at room temperature and utilizes only inexpensive visible light as the energy source, thereby minimizing energy consumption. Furthermore, our synthetic method operates under metal-free conditions, which reduces environmental pollution and mitigates the potential risk of water eutrophication. In addition, our method does not require purification of intermediates during the operation, minimizing the production cycle and cost, saving time and resources, and decreasing waste.
2. Results and Discussion
2.1. Reshaping the Substrate Binding Region of LkKRED
We initially synthesized 1-phenyl-3-(phenylsulfonyl) propan-1-one (3a) by employing styrene (1a) and benzene sulfinic acid (2a) within a photocatalytic system [51]. Subsequently, we optimized various conditions, including the selection of photocatalyst and solvent, leading to the establishment of optimal photocatalytic conditions (see Table S3 in the Supporting Information). Subsequently, we incorporated the catalytic system of wild-type (WT) ketoreductase from Lactobacillus kefir (LkKRED) into the photocatalytic reaction system, achieving a yield of 7% and enantiomeric excess (ee) value of 71% for compound 4a. The results indicate that LkKRED has the potential to catalyze the conversion of ketosulfone (3a) into chiral hydroxysulfone (4a). Consequently, we envisioned engineering the WT LkKRED to enhance both catalytic activity and enantioselectivity. The proposed catalytic mechanism of KRED involves a reduction step followed by a subsequent alkoxide protonation step (Figure 2A) [35]. To efficiently obtain LkKRED variants with the desired activity and enantioselectivity, we employed a semi-rational design approach, informed by the structural characteristics of LkKRED and the LkKRED-3a binding complex. The binding structure of LkKRED and 3a was elucidated by docking 3a with the WT LkKRED structure using AutoDock Vina. This analysis revealed a conformation of 3a that experiences significant steric hindrance within the small binding pocket (Figure 2B). Therefore, modification of the residues within the substrate binding pocket is necessary to facilitate the opening of the binding pocket and enhance the stereoselectivity of LkKRED.
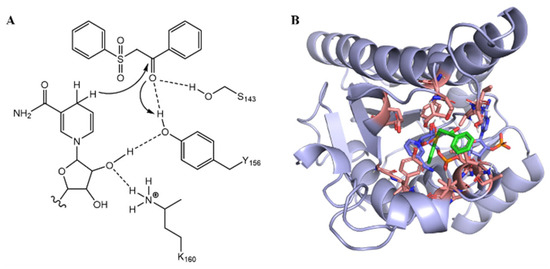
Figure 2.
(A) Proposed catalytic mechanism of 3a by LkKRED. (B) Molecular docking modes of 3a in LkKRED. The substrate (3a) is shown as green sticks; the NADPH as light purple sticks; the amino acid residues in the range of substrate 5 Å as pink sticks.
A total of 17 residues were identified within a 5 Å range of 3a, specifically A94, V95, S96, E145, T152, L153, G189, Y190, I191, T193, L195, V196, L199, E200, A202, S143, and Y156 (Figure 2B). In the first round of evolution, an alanine-scanning strategy was employed, wherein residues that were either alanine or glycine were mutated to tyrosine, while S143 and Y156 were excluded from mutagenesis due to their established roles in the catalytic triad. Following successful construction through site-directed mutagenesis, the catalytic activity and stereoselectivity of 15-site alanine mutants against 3a were evaluated within a whole-cell system (Table S2). Three critical positions (S96, L153, and Y190) were identified based on the results of alanine-scanning experiments, as mutations at these positions resulted in significant alterations in both catalytic activity and stereoselectivity (Figure 3). Notably, the catalytic activity of the Y190A mutant increased significantly, achieving up to a 14-fold enhancement (96% yield) compared to the WT LkKRED. Consequently, we initially conducted NNK codon saturation mutagenesis on the Y190 residue (Table S2). Encouragingly, the reaction yield of the Y190S variant reached 95%, accompanied by an ee of 93%. To further enhance the yield and enantioselectivity of the ketosulfone reduction reaction catalyzed by LkKRED, iterative saturation mutagenesis was performed on the S96 and L153 residues using Y190S as a template (Table S2). As illustrated in Figure 3, the iterative mutation of S96 based on Y190S demonstrated an improvement in both catalytic efficiency and enantioselectivity. The yield of the Y190S/S96T variant reached 99%, with the ee up to 99%. Among all Y190S/L153X mutations, Y190S/L153Q emerged as the most favorable mutation, yielding 95% with an ee up to 96%.
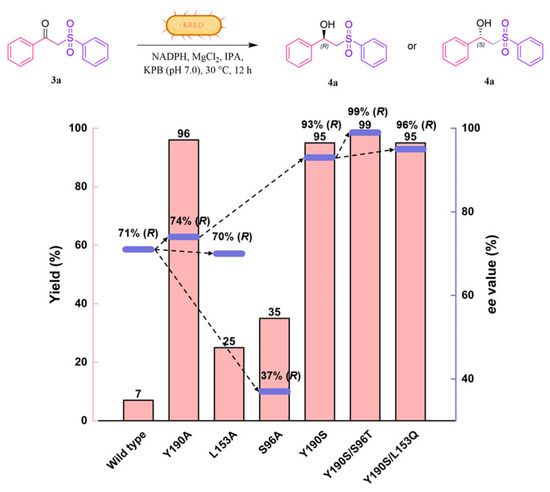
Figure 3.
Enantioselectivities (purple bar) and reaction yields (pink bar) of the evolved variants of LkKRED.
2.2. The One-Pot Photo-Enzymatic Cascade System of β and γ-Hydroxysulfones
The combination of the visible-light-catalyzed sulfonylation reaction with the biocatalytic ketone reduction was investigated as a one-pot cascade reaction. Components 1a and 2a were suspended in a solvent mixture of EtOH/H2O (1.6 mL: 0.4 mL) and treated with Eosin Y under a green LED, and 3a was generated within 24 h. The subsequent addition of KRED, NADPH, Mg2+, and IPA within potassium phosphate buffer (KPB) (pH = 7.0) to the reaction mixture facilitated the reduction of 3a to enantiomerically pure 4a with 84% yield and 99% ee. With Y190S/S96T-LkKRED in hand, the scope of this one-pot reaction was then expanded to a set of different substrates 1 and 2. As shown in Figure 4, the photo-enzymatic cascade reaction illustrated a broad substrate spectrum and showed high productivity and enantiomeric purity for all β-hydroxysulfones, regardless of whether the aromatic substitutions were present on the aryl moiety of styrene or benzenesulfinic acid. Most hydroxysulfones, including those with electron-withdrawing and electron-donating groups, displayed yields exceeding 65–84% and 80–99% ee. When the phenyl ring of benzenesulfinic acid was replaced with a methyl (4e) or ethyl group (4f), the ee significantly decreased. This reduction is likely due to the inability of the β-ketosulfone structure to fit well into the catalytic active site of KRED without the phenyl ring. Moreover, compared to 4k, the ee value of 4l was lower, potentially because the meta-methoxy substituent hindered KRED’s access to the carbonyl group. Overall, whether the phenyl ring was substituted with electron-withdrawing or electron-donating groups had little effect on ee values. Conversely, with respect to yield, electron-donating substituents consistently resulted in higher yields than their electron-withdrawing counterparts. Following the successful expansion of substrate scope for β-hydroxysulfones, we sought to determine whether Y190S/S96T-LkKRED is equally effective in reducing γ-ketosulfone and the derivatives. To synthesize prochiral γ-ketosulfone, we conducted the carbonylation of iodobenzene (5a) with phenyl vinyl sulfone (6a) under purple LED irradiation. After optimizing the conditions, the yield of 7a reached 84% (see Table S4 in the Supporting Information). Subsequently, we performed a photo-enzymatic cascade reaction (Figure 5). All hydroxysulfones exhibited moderate yields (62–76%) and demonstrated good to excellent ee (77–99%). These results strongly suggest that the photo-enzyme catalytic platform we have developed holds considerable potential for practical applications.
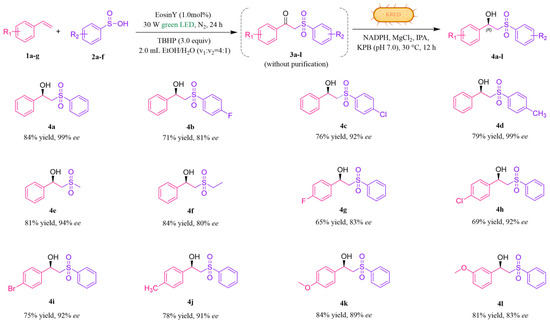
Figure 4.
Substrate scope of photo-enzymatic reaction of producing β-hydroxysulfones. Reaction conditions: 1a-g (0.2 mmol), 2a-f (0.3 mmol), Eosin Y (1.0 mol%), TBHP (0.6 mmol), EtOH (1.6 mL), and H2O (0.4 mL), stirred under green LED irradiation (30 W) at room temperature under a nitrogen atmosphere. Then, the reaction was carried out with 0.1 g/mL wet cell with Y190S/S96T LkKRED, 0.2 mM NADPH, 0.1 mM MgCl2, and 5% v/v IPA in 2 mL of 0.1 M KPB buffer and pH 7.0 at 30 °C and 220 rpm for 12 h.
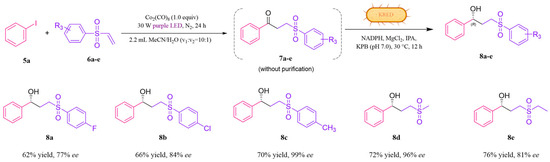
Figure 5.
Substrate scope of photo-enzymatic reaction of producing γ-hydroxysulfones. Reaction conditions: 5a (0.3 mmol), 6a-e (0.2 mmol), Co2(CO)8 (0.2 mmol), MeCN (2 mL), and H2O (0.2 mL), stirred under purple LED irradiation (30 W) at room temperature under a nitrogen atmosphere. Then the reaction was carried out with 0.1 g/mL wet cell with Y190S/S96T LkKRED, 0.2 mM NADPH, 0.1 mM MgCl2, and 5% v/v IPA in 2 mL of 0.1 M KPB buffer and pH 7.0 at 30 °C and 220 rpm for 12 h.
2.3. Molecular Dynamics Simulation Analysis of LKKRED and Its Mutants
Molecular dynamics (MD) simulations were conducted to study the interactions between the substrate and the enzyme’s active site, as well as their impact on reaction activity and stereoselectivity. Through molecular docking analysis (Figure 6), it was found that the S96T and Y190S mutations significantly improved the structure of the enzyme’s active site, thereby enhancing the reaction activity and stereoselectivity. The structural changes in the mutant allowed the rearrangement of amino acid residues surrounding the active site. Notably, the replacement of Tyr190 with Ser190 created more space, permitting better accommodation of the substrate (3a) within the active site and improving stereoselective control over the substrate. Additionally, the introduction of Thr96 strengthened the hydrogen bond interactions with both the substrate and NADPH, further stabilizing the substrate’s binding. Compared to the WT, the mutant formed a more complex hydrogen bond network and stronger hydrophobic interactions, particularly between residues such as I191 and L199 with the substrate, ensuring the precise positioning of the substrate in the active site. These enhanced interactions not only increased the binding affinity of the substrate but also improved the stereoselectivity of the reaction. Furthermore, NADPH, as a crucial cofactor, formed stronger hydrogen bond networks with the enzyme, optimizing electron transfer to the substrate and further increasing the reaction efficiency.
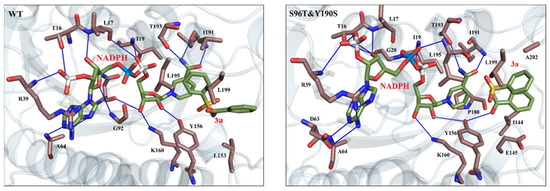
Figure 6.
Docking results of WT and mutant-type LkKRED with 3a. The substrate (3a) and NADPH are shown as light-green sticks; the key amino acid residues in the active site are shown as brown sticks.
As shown in Figure 7A, the substrate 3a is located within an active pocket formed by several amino acids. The RMSD curve in Figure 7B shows the conformational stability of the system over time. In the early stages of the simulation (approximately the first 20 ns), the RMSD values gradually increased, indicating that the system was transitioning from the initial conformation to a more stable one. Over time, the RMSD values stabilized at around 0.25 nm, suggesting that the system had reached a steady state. This means that in the latter half of the MD, the conformational fluctuations of the system were minimal, and the enzyme–substrate complex maintained a relatively stable binding state. Figure 7C shows the variation in key interaction distances over time. The black line (dCαA94-CαL195) represents the Cα atom distance between two residues (Ala94 and Leu195). Overall, the distance remained stable during the simulation, staying between 0.8 and 1.0 nm, indicating that the spatial relationship between these two residues was relatively fixed, with no significant structural changes. The red line (dHY156-O3a) represents the distance between the hydrogen atom of Tyr156 and the oxygen atom of the substrate 3a, which fluctuated considerably, especially during the first 20 ns. This suggests that Tyr156’s interaction with the substrate may have some dynamic nature, but it gradually stabilized in the latter half, with distances around 1.0–1.4 nm, indicating that the interaction between this residue and the substrate strengthened and stabilized over time. The green line (dHNADPH-C3a) represents the distance between the hydrogen atom of NADPH and the C atom of the substrate 3a, which stabilized at around 1.0–1.2 nm, suggesting that the interaction between NADPH and 3a became stronger over time, contributing to improved reaction efficiency. The blue line (dHS143-O3a) represents the distance between the hydrogen atom of Ser143 and the oxygen atom of the substrate 3a. This distance remained between 0.7 and 0.9 nm during the simulation, indicating that Ser143 formed a strong interaction with the substrate, facilitating its precise positioning.
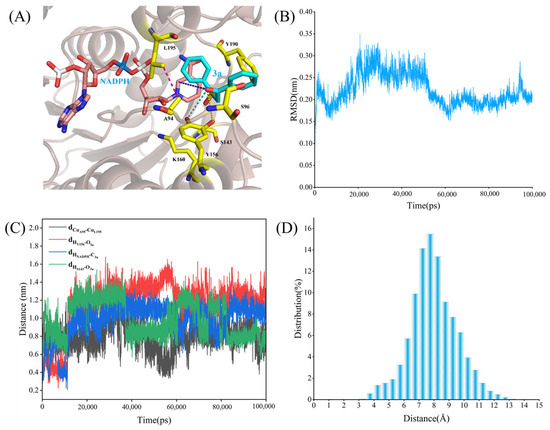
Figure 7.
Binding properties of the WT LkKRED and 3a. (A) The binding structure of the WT LkKRED and 3a. The NADPH is shown as pink sticks; the substrate (3a) is shown as cyan sticks; key amino acid residues are highlighted as yellow sticks. (B) The RMSD of the complex in the simulation process. (C) The distance fluctuation of the catalytic process characterization. (D) The distance distribution between A94 and L195.
In Figure 8A, it is evident that the introduction of Thr96 and Ser190 in the mutant altered the spatial structure of the active site, leading to a tighter binding between the substrate 3a and NADPH. This corresponds to the changes in interaction distances shown in Figure 8C, confirming that the spatial adaptation induced by the mutations improved the binding efficiency of the substrate. The RMSD graph (Figure 8B) shows that the RMSD values of the mutant are lower and more stable during the simulation, indicating that the structure of the mutated system is more stable. In contrast, the RMSD fluctuations in the WT were more pronounced, suggesting greater structural instability. This increased stability in the mutant helps ensure that the substrate remains more stably bound within the active site, thereby enhancing reaction efficiency. Figure 8C shows that the key interaction distances between the mutant and the substrate 3a, as well as NADPH, remained shorter and more stable, particularly the distances between Y156 and the O atom of 3a, NADPH, and C3a throughout the simulation. In contrast, the interaction distances in the WT fluctuated more, with particularly noticeable changes in the Cα distances (black line). The distance distribution in Figure 8D shows that the interaction distances between key residues in the mutant and 3a are more concentrated, especially within the 9–10 Å range, with a higher density. This indicates that the mutant binds more tightly and stably to the substrate, resulting in a more consistent orientation and binding mode during the reaction. In comparison, the WT distribution is broader and more dispersed, suggesting less stable substrate binding, which may lead to reduced reaction efficiency and selectivity. The concentrated distribution in the mutant provides a potential explanation for the improvement in reaction activity and stereoselectivity.
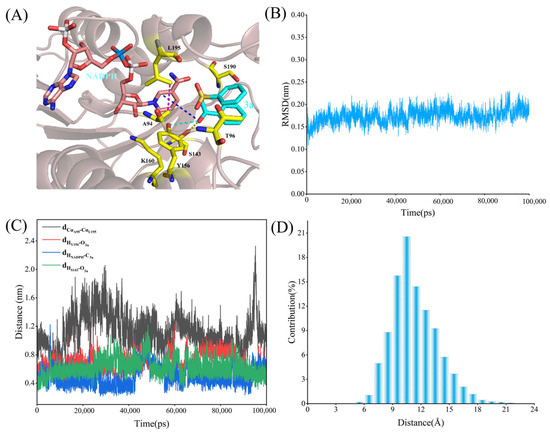
Figure 8.
Binding properties of mutant LkKRED and 3a. The NADPH is shown as pink sticks; the substrate (3a) is shown as cyan sticks; key amino acid residues are highlighted as yellow sticks. (A) Binding structure of Y190S/S96T LkKRED and 3a. (B) RMSD of the complex in the simulation process. (C) Distance fluctuation of the catalytic process characterization. (D) Distance distribution between A94 and L195.
3. Materials and Methods
3.1. Mutant Library Construction and Expression of KREDs
A total of 15 residues in the 5 Å range of 3a were selected for alanine scanning to determine the effect of these residues on the catalytic activity of the substrate. Residues proposed to affect the catalytic properties of LkKRED were further selected to establish a saturation mutagenesis library. The mutant with the highest elevated catalytic activity and stereoselectivity was selected as a template for iterative saturation mutagenesis to obtain the optimal mutant. The recombinant plasmid pET-LkKRED was constructed and preserved in our laboratory. All the site-directed mutagenesis and site-saturation mutagenesis for mutant library construction was performed using the one-step PCR approach with 2× SuperNova PCR Mix DNA polymerase. The 50 µL reaction mixtures typically contained 19.5 µL water, 25 µL 2× SuperNova PCR Mix, 0.5 µL (100 ng) of template DNA, 2.5 µL forward primer, and 2.5 µL reverse primer. A typical PCR cycle was as follows: The reaction began with an initial denaturing step for 300 s at 98 °C, followed by 30 cycles of 15 s at 98 °C, 15 s at 65 °C, and 210 s at 72 °C, and with a final extension step at 72 °C for 600 s. The PCR product (10 μL) digested for 1 h at 37 °C by the DMT enzyme (1 μL) was transformed into 100 μL of E. coli BL21 (DE3) competent cells, and single colonies were sent for sequencing. The strain with a verified sequence was inoculated into LB liquid medium containing 50 mg/L kanamycin and cultured overnight at 37 °C and 200 r/min. The cells were transferred into 0.1 L of the TB liquid medium with a 1% inoculation amount and cultured to OD600 of 0.6–0.8. IPTG with a final concentration of 0.1 mmol·L−1 was added to the medium. After induction at 24 °C for 16 h, the cells were collected by centrifugation.
3.2. Synthesis of Ketosulfones and Racemic Hydroxysulfones
Synthesis of β-ketosulfones and racemic β-hydroxysulfones. A 25 mL tube was charged with 1a-g (0.2 mmol), 2a-f (0.3 mmol), and Eosin Y (1 mol%). Then the tube was evacuated and backfilled with nitrogen (three times). TBHP (0.6 mmol, 3.0 equiv) in 2 mL of EtOH/H2O (v1/v2 = 4:1) was added by syringe under nitrogen. The solution was stirred at room temperature with the irradiation of a 30 W green LED for 24 h. Upon completion of the reaction, the mixture was quenched with 3 mL H2O and diluted with EtOAc (3 × 5 mL), and the solvent was then removed under vacuum. The residue was purified with a chromatography column on silica gel (gradient eluent of EtOAc/petroleum ether: 1/10 to 1/3) to give the corresponding products 3a-l. Then, an appropriate amount of ketosulfone was taken and reacted with NaBH4 in 0.5 mL CH3OH for 10 min. A total of 3 mL of H2O and 0.5 mL of EtOAc were added for extraction, and the solvent was then removed under vacuum to obtain β-hydroxysulfones.
Synthesis of γ-ketosulfones and racemic γ-hydroxysulfones. In a nitrogen-filled glovebox, a 25 mL glass tube was charged with Co2(CO)8 (0.2 mmol, 1.0 equiv), and MeCN (2.0 mL) was added into the glass tube. Then 5a (0.3 mmol, 1.5 equiv) and 6a-e (0.2 mmol, 1.0 equiv) were sequentially added into the glass tube and removed from the glovebox. Deionized H2O was added to the glass tube (0.2 mL) by a microinjector. The mixture was stirred under 30 W purple light irradiation at room temperature for 24 h. After the reaction completed, as monitored by TLC analysis (gradient eluent of EtOAc/petroleum ether: 1/10 to 1/3), the reaction mixture was concentrated in vacuo and purified by flash column chromatography on silica gel (gradient eluent of EtOAc/petroleum ether: 1/10 to 1/3) to afford the desired product 7a-e. Then, an appropriate amount of ketosulfone was taken and reacted with NaBH4 in 0.5 mL CH3OH for 10 min. A total of 3 mL of H2O and 0.5 mL of EtOAc were added for extraction, and the solvent was then removed under vacuum to obtain γ-hydroxysulfones.
3.3. Asymmetric Screening for the Variants
The asymmetric screening was conducted using a reaction system (1 mL) composed of potassium phosphate buffer (KPB) (100 mM, pH 7.0), 10 mM 3a, 100 μL DMSO, 0.2 mM NADPH, 100 mg wet cells, and 50 μL IPA. The mixture was reacted at 30 °C, 220 rpm for 12 h. The reaction mixture was extracted with EtOAc (2 × 1 mL). The combined organic layers were concentrated in vacuo. The product was analyzed by chiral HPLC for yield and ee values.
3.4. Procedure of the One-Pot Photo-Enzymatic Cascade System
Procedure of the one-pot photo-enzymatic cascade system of β-hydroxysulfones. A total of 0.2 mmol (1a-g), 0.3 mmol (2a-f), Eosin Y (1.0 mol%), and 0.6mmol TBHP were dissolved in a mixture of EtOH/H2O at a ratio of 1.6 mL: 0.4 mL and then stirred under a 30 W green LED for 24 h at room temperature under a nitrogen atmosphere. The synthesis of β-ketosulfones (3a-l) had been successfully achieved at this point. Then the reaction was carried out with 0.1 g/mL wet cell with Y190S/S96T LkKRED, 0.2 mM NADPH, 0.1 mM MgCl2, and 5% v/v IPA in 2 mL of 0.1 M KPB buffer and pH 7.0 at 30 °C and 220 rpm for 12 h. The mutants used for the photo-enzymatic cascade were Y190S/S96T-LkKRED. At the end of the cascade reaction, the product was extracted with ethyl acetate (3 × 2 mL). The organic layer was then concentrated in vacuo and purified by flash column chromatography on silica gel (petroleum ether/EtOAc = 1/10 − 1/4, v/v) to afford the desired product 4a-l. Weighing the purified 4a-l, the yield was calculated based on the theoretical yield extrapolated from 1a-g. The dried sample was then analyzed by chiral HPLC to determine the ee value of β-hydroxysulfones (4a-l).
Procedure of the one-pot photo-enzymatic cascade system of γ-hydroxysulfones. A total of 0.3 mmol (5a), 0.2 mmol (6a-e), and Co2(CO)8 (0.2 mmol) were dissolved in a mixture of MeCN/H2O at a ratio of 2.0 mL: 0.2 mL and then stirred under a 30 W purple LED for 24 h at room temperature under a nitrogen atmosphere. The synthesis of γ-ketosulfones (7a-e) has been successfully achieved at this point. Then the reaction was carried out with 0.1 g/mL wet cell with Y190S/S96T LkKRED, 0.2 mM NADPH, 0.1 mM MgCl2, and 5% v/v IPA in 2 mL of 0.1 M KPB buffer and pH 7.0 at 30 °C and 220 rpm for 12 h. The mutants used for the photo-enzymatic cascade were Y190S/S96T-LkKRED. At the end of the cascade reaction, the product was extracted with ethyl acetate (3 × 2 mL). The organic layer was then concentrated in vacuo and purified by flash column chromatography on silica gel (petroleum ether/EtOAc = 1/10 − 1/4, v/v) to afford the desired product 8a-e. Weighing the purified 8a-e, the yield was calculated based on the theoretical yield extrapolated from 6a-e. The dried sample was then analyzed by chiral HPLC to determine the ee value of γ-hydroxysulfones (8a-e).
3.5. Molecular Dynamics Simulations
Our study employed molecular dynamics (MD) simulations using the GROMACS 2023.2 software to simulate a 100 ns protein–ligand complex. The AMBER99SB-ILDN force field was selected to construct the protein topology files, while the Amber20 software and GAFF force field were used to generate the small molecule ligand topology files. A truncated octahedral TIP3P water box was added at a distance of 10 nm from the system, and Na/Cl ions were included to neutralize the system’s charge. Energy minimization was performed using 2500 steps of steepest descent and 2500 steps of conjugate gradient methods. The system was equilibrated with a 100 ps NVT ensemble simulation and a 100 ps NPT ensemble simulation at 368.15 K. Finally, a 100 ns MD ensemble simulation was conducted under periodic boundary conditions, with long-range electrostatic interactions calculated using the PME method, a non-bonded cutoff distance of 1 nm, a collision frequency of 2 ps, a pressure of 101.325 kPa, and a time step of 2 ps, saving trajectories every 10 ps.
4. Conclusions
In summary, we have developed an efficient, mild, sustainable, and highly enantioselective one-pot photo-biocatalytic cascade strategy for the synthesis of chiral β- or γ-hydroxysulfones, eliminating the need for isolation and purification of ketosulfones, thereby reducing costs and minimizing waste. By utilizing visible light as the sole energy source, this method produces a diverse array of chiral hydroxysulfones from readily available and simple starting materials in an aqueous medium, achieving high atom economy. More importantly, the engineered biocatalyst Y190S/S96T LkKRED has been demonstrated to effectively reduce ketosulfones selectively, yielding (R)-hydroxysulfones with excellent enantiomeric excess (ee). Various β-hydroxysulfones and γ-hydroxysulfones were synthesized with high yields (up to 84%) and impressive ee (up to 99%). Furthermore, molecular dynamics (MD) simulations were employed to investigate the mechanism by which Y190S/S96T LkKRED enhances both reaction activity and selectivity. Overall, the research on the development of chiral hydroxysulfones could pave the way for novel possibilities in photocatalytic and enzymatic organic synthesis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15080733/s1, Figure S1: (A) Green LED photoreactor used in sulfonylation reactions (commercially available); (B) purple LED photoreactor used in carbonylation reactions (commercially available); Figure S2: Determination of the absolute configuration of (R)-4d by HPLC; Figure S3: Optimization of the catalytic conditions of one-pot photo-enzymatic cascade reaction; Figure S4: Comparison of the strategy for synthesizing chiral β-hydroxysulfones developed by previous work4 and our work; Table S1: The primers used in this study; Table S2: The relative activities and stereoselectivities of wild-type (WT) and variants towards substrate 3a; Table S3: Optimization of the sulfonylation reaction conditions; Table S4: Optimization of the carbonylation reaction conditions; Table S5: Chiral HPLC methods for chiral hydroxysulfones. Experimental section and NMR data of β/γ-hydroxysulfones [14,51,52,53,54,55,56,57].
Author Contributions
Writing—original draft, Methodology, Investigation, Formal analysis, X.Q.; Software, Methodology, Investigation, Data curation, Q.P.; Data curation, Formal analysis, Y.D.; Writing—review and editing, Supervision, Funding acquisition, L.W. and Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Doctoral Graduate Students’ Scientific Research and Innovation Ability Project of Jilin Provincial Department of Education (Science and Technology) (JJKH20250075BS).
Data Availability Statement
The data presented in this study are available in Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fuchs, P.L.; Braish, T.F. Multiply convergent syntheses via conjugate-addition reactions to cycloalkenyl sulfones. Chem. Rev. 1986, 86, 903–917. [Google Scholar] [CrossRef]
- Gais, H.-J.; von der Weiden, I.; Fleischhauer, J.; Esser, J.; Raabe, G. Lipase catalyzed resolution of α-hydroxymethyl sulfones. Determination of absolute configuration by semiempirical calculation of CD spectra and verification by X-ray structure analysis. Tetrahedron Asymmetry 1997, 8, 3111–3123. [Google Scholar] [CrossRef]
- Catto, M.; Aliano, R.; Carotti, A.; Cellamare, S.; Palluotto, F.; Purgatorio, R.; De Stradis, A.; Campagna, F. Design, synthesis and biological evaluation of indane-2-arylhydrazinylmethylene-1,3-diones and indol-2-aryldiazenylmethylene-3-ones as β-amyloid aggregation inhibitors. Eur. J. Med. Chem. 2010, 45, 1359–1366. [Google Scholar] [CrossRef]
- Fujimoto, T.; Imaeda, Y.; Konishi, N.; Hiroe, K.; Kawamura, M.; Textor, G.P.; Aertgeerts, K.; Kubo, K. Discovery of a Tetrahy-dropyrimidin-2(1H)-one Derivative (TAK-442) as a Potent, Selective, and Orally Active Factor Xa Inhibitor. J. Med. Chem. 2010, 53, 3517–3531. [Google Scholar] [CrossRef]
- Shinozawa, E.; Nakayama, M.; Imura, Y. TAK-442, a Direct Factor Xa Inhibitor, Inhibits Monocyte Chemoattractant Protein 1 Production in Endothelial Cells via Involvement of Protease-Activated Receptor 1. Front. Pharmacol. 2018, 9, 1431. [Google Scholar] [CrossRef]
- Devasthale, P.; Wang, W.; Mignone, J.; Renduchintala, K.; Radhakrishnan, S.; Dhanapal, J.; Selvaraj, J.; Kuppusamy, R.; Pelleymounter, M.A.; Longhi, D.; et al. Non-basic azolotriazinone MCHR1 antagonists for the treatment of obesity: An empirical brain-exposures-driven candidate selection for in vivo efficacy studies. Bioorg. Med. Chem. Lett. 2015, 25, 4412–4418. [Google Scholar] [CrossRef]
- Narode, H.; Gayke, M.; Eppa, G.; Yadav, J.S. A Review on Synthetic Advances toward the Synthesis of Apremilast, an Anti-inflammatory Drug. Org. Process Res. Dev. 2021, 25, 1512–1523. [Google Scholar] [CrossRef]
- Choi, O.K.; Cho, B.T. An efficient synthesis of enantiopure 3-chlorostyrene oxide via oxazaborolidine-catalyzed reduction. Org. Prep. Proced. Int. 2000, 32, 493–497. [Google Scholar] [CrossRef]
- Cho, B.T.; Kim, D.J. Efficient synthesis of optically active β-hydroxy p-tolylsulfones with very high enantiomeric excess via CBS–oxazaborolidine-catalyzed borane reduction. Tetrahedron Asymmetry 2001, 12, 2043–2047. [Google Scholar] [CrossRef]
- Zhao, G.; Hu, J.-B.; Qian, Z.-S.; Yin, W.-X. Enantioselective reduction of β-keto sulfones using the NaBH4/Me3SiCl system catalyzed by polymer-supported chiral sulfonamide. Tetrahedron Asymmetry 2002, 13, 2095–2098. [Google Scholar] [CrossRef]
- Wan, X.; Meng, Q.; Zhang, H.; Sun, Y.; Fan, W.; Zhang, Z. An Efficient Synthesis of Chiral β-Hydroxy Sulfones via Ru-Catalyzed Enantioselective Hydrogenation in the Presence of Iodine. Org. Lett. 2007, 9, 5613–5616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-L.; Hou, X.-L.; Dai, L.-X.; Luo, Z.-B. Synthesis of a biferrocene diphosphine ligand with only planar chirality and its application in the Rh-catalyzed asymmetric hydrogenation of β-keto sulfones. Tetrahedron Asymmetry 2007, 18, 224–228. [Google Scholar] [CrossRef]
- Huang, X.-F.; Zhang, S.-Y.; Geng, Z.-C.; Kwok, C.-Y.; Liu, P.; Li, H.-Y.; Wang, X.-W. Asymmetric Hydrogenation of β-Keto Sulfonamides and β-Keto Sulfones with a Chiral Cationic Ruthenium Diamine Catalyst. Adv. Synth. Catal. 2013, 355, 2860–2872. [Google Scholar] [CrossRef]
- Cui, P.; Liu, Q.; Wang, J.; Liu, H.; Zhou, H. One-pot synthesis of chiral β-hydroxysulfones from alkynes via aerobic oxysulfonylation and asymmetric reduction in MeOH/H2O. Green Chem. 2019, 21, 634–639. [Google Scholar] [CrossRef]
- Tao, L.; Yin, C.; Dong, X.-Q.; Zhang, X. Efficient synthesis of chiral β-hydroxy sulfones via iridium-catalyzed hydrogenation. Org. Biomol. Chem. 2019, 17, 785–788. [Google Scholar] [CrossRef]
- Nobukazu, T. Aerobic Nickel-Catalyzed Hydroxysulfonylation of Alkenes Using Sodium Sulfinates. J. Org. Chem. 2015, 80, 7797–7802. [Google Scholar] [CrossRef]
- Rao, H.W.; Gao, C.; Jiang, L.L.; Ma, X.X.; Xu, R.J.; Li, J.; Dong, W.X.; Li, W.Y.; Zou, D.G. Aerobic Copper-Catalyzed Hydroxysulfonylation of Vinylarenes with Sodium Sulfinates. J. Org. Chem. 2025, 90, 1489–1500. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Yang, J.; Wang, T.; Shen, Z.; Zhang, Y. Dynamic kinetic resolution of β-keto sulfones via asymmetric transfer hydrogenation. Chem. Commun. 2009, 5, 571–573. [Google Scholar] [CrossRef]
- Zhang, D.; Cheng, T.; Zhao, Q.; Xu, J.; Liu, G. Highly Enantioselective One-Pot Synthesis of Chiral β-Hydroxy Sulfones via Asymmetric Transfer Hydrogenation in an Aqueous Medium. Org. Lett. 2014, 16, 5764–5767. [Google Scholar] [CrossRef]
- Wang, J.; Wu, L.; Hu, X.; Liu, R.; Jin, R.; Liu, G. One-pot synthesis of optically pure β-hydroxy sulfones via a heterogeneous ruthenium/diamine-promoted nucleophilic substitution-asymmetric transfer hydrogenation tandem process. Catal. Sci. Technol. 2017, 7, 4444–4450. [Google Scholar] [CrossRef]
- Vyas, V.K.; Srivastava, P.; Bhatt, P.; Shende, V.; Ghosh, P.; Bhanage, B.M. Highly Enantioselective One-Pot Synthesis of Chiral β-Heterosubstituted Alcohols via Ruthenium–Prolinamide-Catalyzed Asymmetric Transfer Hydrogenation. ACS Omega 2018, 3, 12737–12745. [Google Scholar] [CrossRef]
- Xiong, Z.; Pei, C.; Xue, P.; Lv, H.; Zhang, X. Highly enantioselective transfer hydrogenation of racemic α-substituted β-keto sulfonamides via dynamic kinetic resolution. Chem. Commun. 2018, 54, 3883–3886. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Lv, N.; Tan, C.; Cheng, T.; Liu, G. A Compartmentalized-type Bifunctional Magnetic Catalyst for One-pot Aerobic Oxysulfonylation and Asymmetric Transfer Hydrogenation. ChemCatChem 2021, 13, 909–915. [Google Scholar] [CrossRef]
- Friestad, G.K.; Sreenilayam, G. Versatile configuration-encoded strategy for rapid synthesis of 1,5-polyol stereoisomers. Org. Lett. 2010, 12, 5016–5019. [Google Scholar] [CrossRef]
- Kiełbasiński, P.; Rachwalski, M.; Mikołajczyk, M.; Moelands, M.A.H.; Zwanenburg, B.; Rutjes, F.P.J.T. Lipase-promoted dynamic kinetic resolution of racemic β-hydroxyalkyl sulfones. Tetrahedron Asymmetry 2005, 16, 2157–2160. [Google Scholar] [CrossRef]
- Jacobs, H.K.; Mueller, B.H.; Gopalan, A.S. Chiral γ and δ Hydroxysulfones via lipase catalyzed resolutions-synthesis of (R)(+)-4-hexanolide and (2R,5S)-2-methyl-5-hexanolide using intramolecular acylation. Tetrahedron 1992, 48, 8891–8898. [Google Scholar] [CrossRef]
- Lorraine, K.; King, S.; Greasham, R.; Chartrain, M. Asymmetric bioreduction of a ketosulfone to the corresponding trans-hydroxysulfone by the yeast Rhodotorula rubra MY 2169. Enzym. Microb. Technol. 1996, 19, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.; Tillyer, R.; King, A.; Dagneau, P.; Wang, X.; O’Shea, P.; Greasham, R.; Chartrain, M. Asymmetric Bioreduction of an Allylic Ketosulfone to its Corresponding (R)-Alcohol. Bio-Catal. Biotransform. 2000, 18, 471–477. [Google Scholar] [CrossRef]
- Huisman, G.W.; Liang, J.; Krebber, A. Practical chiral alcohol manufacture using ketoreductases. Curr. Opin. Chem. Biol. 2010, 14, 122–129. [Google Scholar] [CrossRef]
- Bornscheuer, U.T.; Huisman, G.W.; Kazlauskas, R.J.; Lutz, S.; Moore, J.C.; Robins, K. Engineering the third wave of biocataly-sis. Nature 2012, 485, 185–194. [Google Scholar] [CrossRef]
- Ni, Y.; Xu, J.-H. Biocatalytic ketone reduction: A green and efficient access to enantiopure alcohols. Biotechnol. Adv. 2012, 30, 1279–1288. [Google Scholar] [CrossRef]
- Hughes, G.; Lewis, J.C. Introduction: Biocatalysis in Industry. Chem. Rev. 2018, 118, 1–3. [Google Scholar] [CrossRef]
- Hollmann, F.; Opperman, D.J.; Paul, C.E. Biocatalytic Re-duction Reactions from a Chemist’s Perspective. Angew. Chem. Int. Ed. 2021, 60, 5644–5665. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, H.; Liu, J.; Huang, Z.; Chen, F. Application of Ketoreductase in Asymmetric Synthesis of Pharmaceuticals and Bioactive Molecules: An Update (2018–2020). Chem. Rec. 2021, 21, 1611–1630. [Google Scholar] [CrossRef]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic Synthesis for Industrial Applications. Angew. Chem. Int. Ed. 2021, 60, 88–119. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Liu, J.; Fang, J.; Jia, X.; Zheng, Z.; You, S.; Qin, B. Ketoreductase Catalyzed (Dynamic) Kinetic Resolution for Bi-omanufacturing of Chiral Chemicals. Front. Bioeng. Biotechnol. 2022, 10, 929784. [Google Scholar]
- Zheng, C.; Wang, Z.; Wang, Q.; Wang, S.; Lao, S.; He, J.; Chen, Z. Efficient preparation of the chiral intermediate of luliconazole with Lactobacillus kefir alcohol dehydrogenase through rational rearrangement of the substrate binding pocket. Mol. Catal. 2021, 509, 111639. [Google Scholar] [CrossRef]
- Yuan, Q.; Ma, L.; Kong, W.; Liu, J.; Zhang, S.; Yan, J.; Bai, J.; He, Y.; Zhou, L.; Liu, Y.; et al. Enzymatic synthesis of chiral alco-hols using ketoreductases. Catal. Rev. 2024, 67, 283–322. [Google Scholar] [CrossRef]
- Shaw, M.H.; Twilton, J.; MacMillan, D.W.C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898–6926. [Google Scholar] [CrossRef]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef]
- Narayanam, J.M.R.; Stephenson, C.R.J. Visible light photoredox catalysis: Applications in organic synthesis. Chem. Soc. Rev. 2011, 40, 102–113. [Google Scholar] [CrossRef]
- Marzo, L.; Pagire, S.K.; Reiser, O.; König, B. Visible-Light Photocatalysis: Does It Make a Difference in Organic Synthesis? Angew. Chem. Int. Ed. 2018, 57, 10034–10072. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef]
- Strieth-Kalthoff, F.; Glorius, F. Triplet Energy Transfer Photocatalysis: Unlocking the Next Level. Chem 2020, 6, 1888–1903. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, X.; Xia, Y.; Guo, X.; Guo, J.; Zhang, J.; Zhao, W.; Wu, Y.; Wang, J.; Zhong, F. Whole-cell-catalyzed hydrogena-tion/deuteration of aryl halides with a genetically repurposed photodehalogenase. Chem 2023, 9, 1897–1909. [Google Scholar] [CrossRef]
- Emmanuel, M.A.; Bender, S.G.; Bilodeau, C.; Carceller, J.M.; DeHovitz, J.S.; Fu, H.; Liu, Y.; Nicholls, B.T.; Ouyang, Y.; Page, C.G.; et al. Photobiocatalytic Strategies for Organic Synthesis. Chem. Rev. 2023, 123, 5459–5520. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhang, C.; Dai, Y.; Li, F.; Wang, L.; Chen, P.; Wang, Z. Hemoglobin: An efficient oxidase for the green synthesis of quinazoline derivatives by biocatalytic domino strategy. Mol. Catal. 2025, 570, 114680. [Google Scholar] [CrossRef]
- Li, F.; Xu, Y.; Liu, Y.; Kan, W.; Piao, Y.; Han, W.; Li, Z.; Wang, Z.; Wang, L. Switching engineered Vitreoscilla hemoglobin into carbene transferase for enantioselective SH insertion. Int. J. Biol. Macromol. 2024, 278, 134756. [Google Scholar] [CrossRef]
- Xie, H.; Li, F.; Xu, Y.; Wang, C.; Xu, Y.; Wu, J.; Li, Z.; Wang, Z.; Wang, L. Vitreoscilla hemoglobin: A natural carbene transfer catalyst for diastereo- and enantioselective synthesis of nitrile-substituted cyclopropanes. Green Chem. 2023, 25, 6853–6858. [Google Scholar] [CrossRef]
- Ma, J.; Li, F.; Wang, C.; Wang, Z.; Du, C.; Wang, L. Synthesis of Isothiocyanates from Primary Amines via Visible-Light Photocatalysis. Org. Lett. 2023, 25, 5692–5696. [Google Scholar] [CrossRef]
- Yang, D.; Huang, B.; Wei, W.; Li, J.; Lin, G.; Liu, Y.; Ding, J.; Sun, P.; Wang, H. Visible-light initiated direct oxysulfonylation of alkenes with sulfinic acids leading to β-ketosulfones. Green Chem. 2016, 18, 5630–5634. [Google Scholar] [CrossRef]
- Mou, Q.; Han, T.; Liu, M. Light-Driven Three-Component Carbonylation of Aryl Halides Using Abundant Metal Carbonyl. Org. Lett. 2024, 26, 2169–2174. [Google Scholar] [CrossRef] [PubMed]
- López-Agudo, M.; Ríos-Lombardía, N.; González-Sabín, J.; Lavandera, I.; Gotor-Fernández, V. Chemoenzymatic Oxosulfonylation-Bioreduction Sequence for the Stereoselective Synthesis of β-Hydroxy Sulfones. ChemSusChem 2022, 15, e202101313. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, W.; Huo, C. One-Pot Synthesis of β-Hydroxysulfones and Its Application in the Preparation of Anticancer Drug Bicalutamide. J. Org. Chem. 2017, 82, 10628–10634. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Gao, X.; Qian, D.; Wang, H.; Jia, X.; Zhang, W.; Qin, B.; You, S. Efficient synthesis of an apremilast precursor and chiral β-hydroxy sulfones via ketoreductase-catalyzed asymmetric reduction. Org. Biomol. Chem. 2022, 20, 2081–2085. [Google Scholar] [CrossRef]
- Jeremias, N.; Mohr, L.-M.; Bach, T. Intermolecular [2+2] Photocycloaddition of α,β-Unsaturated Sulfones: Catalyst-Free Reaction and Catalytic Variants. Org. Lett. 2021, 23, 5674–5678. [Google Scholar] [CrossRef]
- Nwaukwa, S.O.; Lee, S.; Keehn, P.M. Epoxide Ring Opening by α-Sulfonyl Carbanions. Synthesis of γ-Hydroxy Sulfones and Triflones. Synth. Commun. 1986, 16, 309–329. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).