Abstract
In our previous work, we fabricated Ni single-atoms within Beta zeolite (Ni1@Beta-NO3−) using NiNO3·6H2O as a metal precursor without any chelating agents, which exhibited exceptional performance in the selective hydrogenation of furfural. Owing to the confinement effect, the as-encapsulated nickel species appears in the form of Ni0 and Niδ+, which implies its feasibility in metal catalysis and coordination catalysis. In the study reported herein, we further explored the hydrogen production and olefin oligomerization performance of Ni1@Beta-NO3−. It was found that Ni1@Beta-NO3− demonstrated a high H2 generation turnover frequency (TOF) and low activation energy (Ea) in a sodium borohydride (NaBH4) hydrolysis reaction, with values of 331 min−1 and 30.1 kJ/mol, respectively. In ethylene dimerization, it exhibited a high butylene selectivity of 99.4% and a TOF as high as 5804 h−1. In propylene oligomerization, Ni1@Beta-NO3− demonstrated high selectivity (75.21%) of long-chain olefins (≥C6+), overcoming the problem of cracking reactions that occur during oligomerization using H-Beta. Additionally, as a comparison, the influence of the metal precursor (NiCl2) on the performance of the encapsulated Ni catalyst was also examined. This research expands the application scenarios of non-noble metal single-atom catalysts and provides significant assistance and potential for the production of H2 from hydrogen storage materials and the production of valuable chemicals.
1. Introduction
Metal nanoparticles and their compounds represent a significant category of industrial catalysts. However, their application has been severely restricted because of the issue of sintering and deactivation during thermal treatment or catalytic processes [1,2]. In recent years, encapsulating metal species within the micropores of zeolites has been employed to suppress the movement and aggregation of metal species through the confinement effect of the rigid zeolite framework. This strategy can significantly enhance their resistance to sintering [3,4]. Moreover, these catalysts inherit the unique shape-selective catalytic capabilities of zeolites. They have been extensively applied in reactions such as hydrogenation, oxidation, isomerization, cracking, and reforming, demonstrating superior activity, stability, and selectivity [5,6,7]. At present, to address the issue of low metal loading (<2 wt%) within zeolites, zeolite-encapsulated single-atom metal catalysts have been proposed and found to be highly efficient catalysts.
Hydrogen, as a promising alternative energy source, offers a viable solution to the increasingly pressing energy challenges. In recent years, liquid-phase hydrogen storage materials (e.g., borohydride and ammonia borane solution) have attracted extensive research interest because they can release hydrogen rapidly, conveniently, and controllably in aqueous solutions under the action of suitable catalysts [8,9]. Yu et al. [10] employed a direct hydrogen reduction strategy under in situ hydrothermal conditions to successfully encapsulate Rh single-atoms within Silicate-1 zeolite (Rh@S-1) for the hydrolysis of ammonia borane. The as-prepared Rh@S-1-H exhibited a TOF as high as 432 min−1, which is twice that of Rh nanoparticles on S-1 (195 min−1). The aforementioned reports highlight that noble metal-based catalysts encapsulated in zeolites exhibit superior hydrogen production performance. However, there are currently few reports on the use of non-noble metal single-atoms encapsulated in zeolites for hydrogen production reactions.
Light olefin (ethylene or propylene) oligomerization is a series–parallel reaction for the production of a wide range of linear and branched alkylene products (C4–C12) suitable as transportation fuels, which involves β-scission and co-oligomerization reaction pathways [11]. It has also been frequently used as a probe reaction to identify the confinement effects in zeolite catalysis [12]. Nozik et al. [13] found that the higher ethylene oligomerization activity and selectivity to even-carbon-numbered alkenes of Ga/H-MFI stems from cooperative effects between Ga3+ sites and Brønsted (B) acid sites (H+). Kitamura et al. [14] found that the Ni2+ cations grafted onto the silanols in dealuminated zeolites containing almost no zeolitic acid show high activity for ethylene oligomerization. Therefore, we infer that the combination of Ni single-atoms and acid sites in Beta zeolite should be a good choice for olefin oligomerization. In this study, we focused on the catalytic consequence of Ni1@Beta-NO3− in the hydrolysis of NaBH4, ethylene dimerization, and propylene oligomerization.
For the synthesis of Ni single-atom catalysts within zeolite, we previously fabricated Ni single-atoms within Beta zeolite (Ni1@Beta-NO3−) using NiNO3·6H2O as a metal precursor without any chelating agents [15]. The initial separate nucleation step allows for a strong interaction between Ni2+ cations and the alumino-silicate gel, resulting in the formation of nickel silicate and achieving high dispersion. This process is not only related to the pH but also dependent on the anions in the precursors. Escola et al. [16] prepared Ni/Beta using chloride, nitrate, acetylacetonate, and tris(ethylenediamine) nickel(II) chloride (TEDAN) as precursors. It was found that Ni nitrate and acetylacetonate led to smaller and more dispersed particles, thus closer to the acidic sites, driving higher hydrogenation and hydrocracking activity in the hydroreforming of low-density polyethylene thermal cracking oil. In contrast, TEDAN and nickel chloride resulted in larger nickel particles, which hindered the rapid hydrogenation of intermediate species, thus favoring the production of aromatics and branched hydrocarbons. In this study, we synthesized Ni@Beta-Cl− with NiCl2 as the metal precursor, following the same methods used for the Ni1@Beta-NO3− sample. Their catalytic consequence in propylene oligomerization was compared to point out the effect of anions for metal precursors in the encapsulation of Ni single-atoms within zeolite.
2. Results and Discussion
2.1. Hydrolysis of NaBH4
We previously found that, for the Ni1@Beta catalyst, the hydrogenation of furfural involves not only the activation of hydrogen but also the selective activation of furfural. It should be noted that single-atoms confined within micropores may be subject to diffusion effects, which may not fully reflect the characteristics of isolated single-atoms. To further ascertain the hydrogen activation capability of single-atoms, the NaBH4 hydrolysis reaction for hydrogen production is used as a probe to evaluate the performance of the single-atom Ni catalyst (Figure 1 and Table 1). As shown in Table 1, the hydrogen generation rates (HGRs) can be improved with the increase in reaction temperature. What is more, Ni1@Beta-NO3− exhibits higher HGRs than Ni/Beta-NO3− at the tested temperatures. In addition to HGR, Ea is a key parameter to evaluate the performance of catalysts for H2 generation. Kinetic studies revealed a much lower Ea on Ni1@Beta-NO3− of 30.1 kJ/mol than Ni/Beta-NO3− of 42.7 kJ/mol (Figure 1b). Additionally, compared with the other catalysts, even noble metal catalysts, Ni1@Beta-NO3− also shows a comparable or even lower Ea and a higher TOF value (331 min−1 at 298 K) than most of the reported catalysts (Figure 1a and Table 2) [17,18,19,20,21,22,23]. The high TOF and low Ea fully demonstrate that Ni single-atoms in Ni1@Beta-NO3− have superior catalytic activity for H2 generation from the hydrolysis of NaBH4.

Figure 1.
(a) TOF versus the Ea of the reported metal-based materials in the hydrolysis of NaBH4. MSN: mesoporous silica nanosphere. The numerals correspond to the reference numbers in Table 2 [17,18,19,20,21,22,23]. (b) Arrhenius plots of Ni1@Beta-NO3− and Ni/Beta-NO3− in the hydrolysis dehydrogenation of NaBH4.

Table 1.
Comparison of the catalytic hydrogen production rate of different samples.

Table 2.
A comparison of catalytic activities over various metal catalysts in the hydrolysis of NaBH4 measured in this work and the ones reported in the literature.
2.2. Ethylene Dimerization over Ni Single-Atom Catalysts
1-Butene is the most in-demand linear α-olefin. Ethylene dimerization to 1-Butene is regarded as a cost-effective route to produce 1-Butene compared to other techniques based on refinery fractionation and steam cracking [24]. The highly active homogeneous ethylene dimerization catalysts are characteristic of a common structural moiety, that is, Ni2+ coordinated with two imine groups and two other ligands (halogens) [25]. The catalytic activity of homogeneous Ni-based catalysts is up to 107 g/(molNi h), denoted as 107 h−1 [26], but the catalysts are difficult to separate from the reaction system. In contrast, heterogeneous Ni-based catalysts are easy to separate, but their activity is up to 105–106 h−1. Recently, Ni-containing zeolites with coordinately unsaturated Ni2+ centers were applied as effective catalysts for ethylene dimerization [27,28]. In our previous work, we found that Ni single-atoms (Ni1@Beta) have a superior ability to activate hydrogen, but due to the strong interaction between the framework and Ni, Ni single-atoms exhibit ionic characteristics. Many studies have reported that the B acid sites (BASs) in zeolites play an important role in the dimerization reaction [29,30,31]. In view of this, three types of protonated zeolites were employed for ethylene dimerization. Figure 2 shows the results of the ethylene dimerization of zeolite catalysts with AlEt2Cl as a co-catalyst. The H-Beta zeolite presents low catalytic activity with a TOF of 317 h−1. After incorporating Ni species into Beta zeolite, the catalytic activity is obviously enhanced. Specifically, Ni1@H-Beta-NO3− shows a TOF of 5804 h−1, which is about twice as much as that of Ni/H-Beta-NO3−. Additionally, compared with other heterogeneous Ni-based catalysts [32,33,34], Ni1@Beta-NO3− still exhibits the highest TOF under similar reaction conditions (Table 3). This is due to the fact that Ni1@H-Beta-NO3− possesses atomically dispersed Ni species, which provide extremely high percentages of exposed catalytically active surfaces to ensure high catalytic activity. Moreover, under the investigated conditions, Ni1@H-Beta-NO3− shows high selectivity for butene, affording 99.44% butene and 0.56% hexenes, without higher oligomers, which is also higher than the catalysts listed in Table 3. The high selectivity of butene can be ascribed to the steric limitation effect of the zeolite structure, which plays an important role in shape selectivity because shape selectivity is apparent, resulting in the formation of a broader range of low-carbon hydrocarbons (C4, C6, C8, etc.) when the active sites of the external surface are compromised.
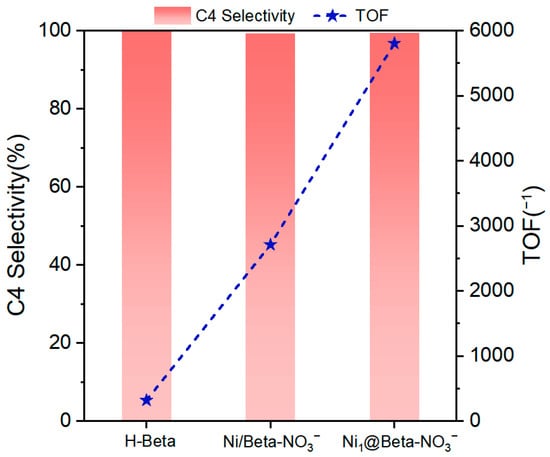
Figure 2.
The selectivity of C4 products and the TOF of H-Beta, Ni/H-Beta-NO3−, and Ni1@H-Beta-NO3−. Reaction conditions: catalyst mass = 0.1467 g, 5 mL Et2AlCl (1 M dissolved in toluene) as a co-catalyst, P = 1 MPa, T = 298 K, and t = 0.5 h.

Table 3.
A comparison of catalytic activities over various Ni-based catalysts in ethylene dimerization measured in this work and those reported in the literature.
2.3. Propylene Oligomerization
The ethylene dimerization above shows that the conversion of olefins depends on Ni as the active center; the BASs showed a bit of activity (Figure 2). We found that Ni single-atoms possess superior olefin conversion activity. It should also be noted that the BASs, as active sites for the isomerization, cracking, and aromatization of olefins, has been realized. Here, propylene oligomerization to larger α-olefins as a probe reaction was carried out, and the Ni1@Beta-NO3−, without ion-exchanging treatment with NH4Cl to generate BASs, was used as an oligomerization catalyst to explore the performance of single-atoms. Concurrently, as a comparison, the sample prepared by using the NiCl2 precursor was also included.
2.3.1. Catalytic Performance
Figure 3a,b show the relationship between gas hourly space velocity (GHSV) and propylene conversion, as well as the selectivity of various products over H-Beta and Ni1@Beta-NO3− at 453 K, respectively. When the GHSV decreases from 600 h−1 to 200 h−1, propylene conversion for both H-Beta and Ni1@Beta-NO3− increases by approximately 79%. This indicates that reducing the GHSV is beneficial for enhancing propylene conversion. The high conversion of H-Beta over Ni1@Beta-NO3− is attributed to the abundance of protonic acid sites in H-Beta. They enable the combination of propylene with protons, leading to dimerization and trimerization, yielding products including C6, C9, and so on. Concurrently, isomerization, β-scission, cyclization, and other reactions also take place. In contrast, the Ni1@Beta-NO3− sample possesses some Lewis acid sites (LASs) generated by the Ni species, but with few BASs. On the other hand, a low GHSV is detrimental to the formation of long-chain olefins because of the cracking reactions. For H-Beta, at higher GHSV, the short contact time between reactants and the catalyst leads to a decreased probability of cracking reactions of oligomerization products. Consequently, with the increasing GHSV, the selectivity of <C3, C4, and C5 shows a declining trend, while that of C6, C7, C8, and C9+ shows an upward trend. The pattern of selectivity variation for each product in Ni1@Beta-NO3− is largely consistent with that of H-Beta. It is noteworthy that in H-Beta, the selectivity for <C3 significantly increases at GHSV = 200 h−1, reaching 42.7%, higher than other products. For Ni1@Beta-NO3−, the selectivity for <C3 remains at a very low level, suggesting that the active sites for oligomerization and cracking reactions in H-Beta are primarily the BASs. The high acidity is prone to cause C6 cracking into C2 and C4. In contrast, in Ni1@Beta-NO3−, the reaction active sites are LASs, and C6 is less likely to undergo cracking to produce C4 and C2. Overall, H-Beta is more prone to cracking reactions compared to Ni1@Beta-NO3−.

Figure 3.
The curves of the conversion of propylene and product selectivity versus GHSV for (a) H-Beta and (b) the Ni1@Beta-NO3− sample; (c) the product selectivity of the reaction with similar conversions; (d) the product selectivity over Ni1@Beta-NO3− and Ni/Beta-NO3−.
Table 4 presents the yield of various products for different catalysts at similar conversion rates. Figure 3c presents the selectivity of various products for H-Beta, Ni1@Beta-NO3−, and Ni@Beta-Cl− at conversion of 13.9%, 19.3%, and 21.1%, respectively. It can be observed that for H-Beta, the selectivity for C4, C5, C6, and C7 is relatively similar, indicating that these are the primary products of the reaction. For Ni1@Beta-NO3− and Ni@Beta-Cl−, the selectivity for C7 is the highest, followed by C4, C5, C6, and C9+, with their selectivity being relatively close. This suggests that in H-Beta, C7 may crack to C3 and C4, or it may first polymerize with other products and then undergo cracking. In contrast, Ni1@Beta-NO3− and Ni@Beta-Cl− exhibit weaker acidity, which favors the formation of long-chain olefins, leading to the low selectivity for C4 and C5. Figure 3d illustrates the selectivity of the products for Ni1@Beta-NO3− and Ni/Beta-NO3− at conversion of 79.8% and 79.5%, respectively. Both of them primarily produce C4 and C5. The difference is that the selectivity for C4 is much greater than C5 in Ni1@Beta-NO3−, while they are similar in Ni/Beta-NO3−. This may be due to the fact that C4 and C5 primarily originate from the cracking of long-chain olefins (C9) at the metal active sites on Ni/Beta-NO3−. Ni1@Beta-NO3− has higher cracking activity because of Ni species, which possess both metallic and acidic properties. Consequently, C4 may not only originate from C9 but also potentially from C6, C7, and C8. In summary, introducing metal species into the zeolite channels can generate new LASs. It is helpful to overcome the cracking issues occurring during oligomerization using H-type zeolite, thereby enhancing the selectivity of the long-chain olefins.

Table 4.
Product yields of propylene polymerization.
From the aforementioned results, it can be observed that Ni1@Beta-NO3− and Ni@Beta-Cl− exhibit similar product selectivity. However, when GHVS = 400 h−1, the conversion of Ni@Beta-Cl− is only 21.1%, significantly lower than that of Ni1@Beta-NO3− (~40.6%). This discrepancy arises because the nickel species in Ni1@Beta-NO3− interact strongly with the framework, making them less reducible, whereas in Ni@Beta-Cl−, the majority of the nickel species can be reduced, reducing the active centers for the oligomerization of olefins to Ni+ or Ni2+ [35].
2.3.2. Discussion on the Effect of Metal Precursors for Ni@Beta Zeolite Synthesis
Figure 4a presents the X-ray diffraction (XRD) patterns of Na-Beta and Ni@Beta-Cl−. All samples exhibit characteristic peaks of Beta zeolite, indicating that the introduction of Ni species does not disrupt the structure of the Beta zeolite. No diffraction peaks related to Ni species are observed, suggesting that the lattice distortion of Beta zeolite after encapsulation with metal can be neglected. There are no significant Ni particles in the samples, indicating strong dispersion of Ni. According to the X-ray fluorescence (XRF) results in Table 5, after the introduction of Ni using the two-step crystallization method, the SiO2/Al2O3 of Ni@Beta-Cl− is higher than that of Na-Beta, indicating that the addition of Ni affects the crystallization process of the zeolite. It is also higher than that of Ni1@Beta-NO3−, suggesting that anions also play a non-negligible role in the crystallization process. Furthermore, the actual Ni content in Ni@Beta-Cl− is higher than the theoretical value, which is due to the fact that the introduction of metal precursors increases the difficulty of crystallization of the zeolite, and not all silicon species are converted into zeolite products during the conversion of alumino-silicate gel. Figure 4b presents the N2 adsorption–desorption isotherms for various samples. The isotherms for all samples are of the I type, characteristic of typical microporous materials, indicating a rich microporous structure. Compared to Na-Beta, the total surface area and total pore volume of Ni@Beta-Cl− and Ni1@Beta-NO3− both increased. This may be due to the fact that metal cations can participate in the crystallization process of zeolites, playing a role in the structural guidance and charge balance of the framework. Figure 4c presents the transmission electron microscopy (TEM) image and EDS mapping of Ni@Beta-Cl−. It can be observed that the two-step crystallization method did not result in the formation of large agglomerated particles after the introduction of Ni, indicating their uniform dispersion and encapsulation within the Beta zeolite. Additionally, the regular crystal structure of the zeolite suggests that the addition of Ni via the two-step crystallization method does not affect the crystal structure of the zeolite, consistent with the XRD results. The EDS results further demonstrate that the two-step crystallization method enables a uniform dispersion of Ni particles throughout the entire Beta zeolite crystal. The above results indicate that during the hydrothermal synthesis of zeolite, the in situ introduction of a metal precursor can effectively encapsulate the metal and maintain its small particle size and high dispersion.
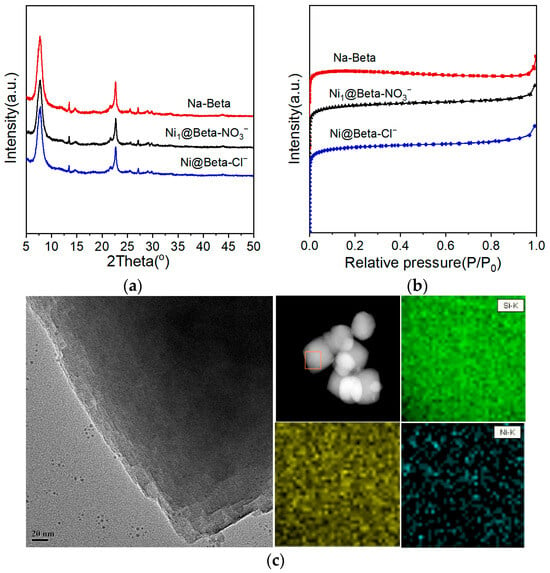
Figure 4.
(a) XRD patterns, (b) N2 adsorption–desorption isotherm of Na-Beta, Ni1@Beta-NO3−, and Ni@Beta-Cl−; (c) TEM images and EDS mapping of Si, Al, and Ni for Ni@Beta-Cl−.

Table 5.
Physicochemical properties of Na-Beta, Ni1@Beta-NO3−, and Ni@Beta-Cl−.
Pyridine adsorption (FT-IR-Py) and ammonia temperature-programmed desorption (NH3-TPD) were employed to analyze the acidity of the zeolite samples. As shown in Figure 5a, two absorption bands at 1450 cm−1 for LASs and 1545 cm−1 for BASs are observed for Na-Beta, Ni1@Beta-NO3−, and Ni@Beta-Cl−. Upon the introduction of Ni, the peak corresponding to the LASs significantly increased in intensity, while the peak associated with the BASs decreased, leading to a reduction in the ratio of B/L (Table 6). Further NH3-TPD analysis was conducted to investigate the quantity and strength of the acidic centers of the catalysts. As depicted in Figure 5b, all samples exhibit two desorption peaks, with the peak in the range of 453–483 K attributed to weak acid sites and the peak between 513 and 593 K corresponding to medium–strong acid sites. The total acidity of the Beta zeolite increased after the incorporation of Ni, which is attributed to the generation of new LASs associated with Ni2+ and NiO [36], consistent with the findings from the FT-IR-Py analysis. The differences in acid amount and strength between Ni@Beta-NO3− and Ni@Beta-Cl− arise from the fact that different anions alter the dissolution rate of the alumino-silicate gel and the size of the template agent.

Figure 5.
(a) FT-IR-Py and (b) NH3-TPD profiles of Na-Beta, Ni1@Beta-NO3−, and Ni@Beta-Cl−.

Table 6.
The FT-IR-Py and NH3-TPD results for Na-Beta, Ni1@Beta-NO3−, and Ni@Beta-Cl−.
A previous study has demonstrated that the excellent catalytic performance of Ni1@Beta-NO3− is closely related to the electronic properties of the isolated Ni sites [15]. The temperature-programmed reduction of hydrogen (H2-TPR) was conducted on Ni@Beta-Cl− and Ni1@Beta-NO3− (Figure 6a). Ni@Beta-Cl− exhibits two H2 consumption peaks at 753 K and 876 K, which are attributed to the reduction of NiO to metallic Ni [37,38] and the reduction of isolated Ni cations within the zeolite pores, respectively. However, the reduction temperature is significantly lower than that of Ni1@Beta-NO3− (923 K), indicating weaker metal–support interactions in Ni@Beta-Cl− compared to Ni1@Beta-NO3− [39,40]. Additionally, the calculated nickel reduction degrees for Ni@Beta-Cl− and Ni1@Beta-NO3− are determined to be 62.9% and 23.4%, respectively, also indicating a stronger metal–support interaction in Ni1@Beta-NO3− than that in Ni@Beta-Cl−. X-ray photoelectron spectroscopy (XPS) analysis was further utilized to characterize the electronic states of Ni in Ni1@Beta-NO3− and Ni@Beta-Cl−. The XPS spectrum of the Ni 2p3/2 for Ni1@Beta-NO3− (Figure 6b) only shows one characteristic peak at 856.1 eV, which is attributed to the Ni2+ species. Unlike Ni1@Beta-NO3−, Ni@Beta-Cl− exhibits two main peaks centered at 851.5 eV and 855.4 eV, corresponding to Ni0 and Ni2+ species, respectively [41,42]. This indicates that only a portion of the Ni species in Ni@Beta-Cl− carry a positive charge, suggesting a weaker interaction between Ni and O atoms. Notably, the peak of Ni2+ in Ni1@Beta-NO3− is shifted to a slightly higher binding energy compared to Ni@Beta-Cl−. This positive shift indicates a buildup of positive charges on the Ni species, which also points to enhanced electron interaction between Ni and O atoms. To further characterize the electronic configurations of Ni species in Ni1@Beta-NO3− and Ni@Beta-Cl−, Fourier transform infrared spectroscopy of CO adsorbed (FT-IR-CO) was conducted at low temperatures of −100 K, with CO being gradually introduced (Figure 6c,d). As for Ni@Beta-Cl−, the adsorption peaks at positions of 2020 and ~1900 cm−1 are attributed to linear and bridge adsorption of CO on metal Ni nanoparticles, respectively [43,44]. Notably, such two signals were not apparent for Ni1@Beta-NO3−, further confirming that the Ni species in Ni1@Beta-NO3− are atomically dispersed. The vibrational characteristics of CO adsorbed near 2168 cm−1 on Ni1@Beta-NO3− and Ni@Beta-Cl− are associated with the formation of carbonyl involving Na+ [45,46]. In addition, for Ni1@Beta-NO3−, a new peak appears at 2202 cm−1, which can be assigned to the adsorption of CO on isolated Ni2+ sites [28,47]. However, this was not observed in Ni@Beta-Cl−, indicating that the degree of dispersion of Ni species and the content of Ni2+ in Ni@Beta-Cl− are both lower than those in Ni1@Beta-NO3−.

Figure 6.
Characterization of Ni@Beta-Cl− and Ni1@Beta-NO3−. (a) H2-TPR profiles and (b) Ni 2p XPS spectra of Ni@Beta-Cl− and Ni1@Beta-NO3−. FT-IR-CO at low temperature (−100 K) on (c) Ni@Beta-Cl− and (d) Ni1@Beta-NO3−.
Based on the above results, the preparation process of Ni@Beta is influenced by the anionic species in the precursor solution. The anions present in the nickel precursor directly influence the SiO2/Al2O3 and pore structure, subsequently leading to variations in the content and charge environment of Ni. It was shown that the dispersion of Ni species and the content of Ni2+ in Ni@Beta-Cl− were both lower than those in Ni1@Beta-NO3−. Compared to Cl− anions, NO3− anions have a plane triangle structure, high electronegativity of oxygen atoms, and a strong ability to form hydrogen bonds with hydroxyl or water molecules. This means that they can easily interact with alumino-silicate gel. Accordingly, it is beneficial for the dispersion of their counter ions (Ni2+) because the movement of nitrate ions is accompanied by Ni2+ cations.
3. Materials and Methods
3.1. Materials for Catalyst Synthesis
Silica gel (99 wt% SiO2), tetraethylammonium hydroxide (TEAOH, 25 wt% in H2O), and diethylaluminum chloride (AlEt2Cl, A.R.) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China. Sodium hydroxide (NaOH, 99%) was obtained from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. Sodium metaaluminate (NaAlO2, 45 wt% Al2O3) was obtained from the Tianjin Jinke Fine Chemical Research Institute, Tianjin, China. Nickel (II) nitrate hexahydrate (Ni(NO3)2·6H2O, 98%) was obtained from Tianjin Fuchen Chemical Reagent Factory, Tianjin, China. Anhydrous Ni chloride (NiCl2, 98%) was obtained from Thermo Fisher Scientific Co., Ltd., Shanghai, China. Ammonium chloride (NH4Cl, 99.5%) was purchased from Shanghai Macklin Biochemical Co., Ltd., Shanghai, China. Sodium borohydride (NaBH4, 98%) was obtained from J&K scientific Co., Ltd., Beijing, China. Toluene was purchased from Tianjin Fuyu Chemical Co., Ltd., Tianjin, China. Ethylene was purchased from Zhangjiakou Chuangqi Gas Co., Ltd., Zhangjiakou, China. Propylene was obtained from Beijing Qianxi Jingcheng Gases Co., Ltd., Beijing, China. Ethanol (C2H5OH, 99%) was obtained from Beijing Tongguang Fine Chemical Co., Ltd., Beijing, China. The above materials were used without further purification.
3.2. Catalyst Preparation
3.2.1. Synthesis of Beta Zeolite
The Beta zeolite was prepared using a conventional hydrothermal synthesis method. In a typical process, 27.9 g TEAOH and 0.45 g NaOH were added to deionized water and stirred until a clear solution was formed. Subsequently, 0.8 g NaAlO2 was added and stirring was continued for 30 min. Then, 15 g silica gel was slowly added to the above solution and stirred for 3 h to achieve a homogeneous alumino-silicate gel with a molar composition of SiO2/Al2O3/TEAOH/H2O/Na2O = 1/0.014/0.19/7.21/0.042. The as-prepared gel was transferred into a 100 mL Teflon-lined stainless-steel autoclave, placed in an oven at 413 K, and crystallized for 48 h. After crystallization, the solid products were separated via centrifugation, washed with deionized water until the pH of the filtrate was ~7, and dried at 373 K for 12 h. Finally, the Beta zeolite was obtained through calcination in the air at 823 K for 6 h with a ramping rate of 2 K min−1).
3.2.2. Synthesis of Ni1@Beta-NO3−
For the synthesis of Ni1@Beta-NO3−, an alumino-silicate gel with a molar composition of SiO2/Al2O3/TEAOH/H2O/Na2O = 1/0.014/0.19/7.21/0.042 was produced. It was placed in an autoclave at 273 K for 24 h and then the autoclave was cooled to ambient temperature. Next, the 0.75 g Ni(NO3)2 precursor was dissolved into 1 g deionized water and added to the autoclave. The autoclave was sealed again, heated to 413 K, and kept at this temperature for 36 h. The as-obtained solid product was filtered, washed with water several times, and then dried at 373 K overnight, followed by calcination at 823 K for 6 h. Finally, the sample was reduced at 30 mL min−1 10% H2/N2 flow at 773 K (ramping rate: 5 K min−1) for 2 h. Before exposure to air, all of the samples were passivated at 303 K for 1 h under 30 mL min–1 1% O2/N2 flow.
3.2.3. Synthesis of Ni@Beta-Cl−
Ni@Beta-Cl− was prepared with the same procedure as Ni1@Beta-NO3− using 0.61 g NiCl2 as a precursor.
3.2.4. Synthesis of Ni/Beta-NO3−
Ni/Beta was prepared using an impregnation method. In a typical procedure, 5 g Beta zeolite was slowly added to a beaker containing 40 mL of a Ni(NO3)2 solution (0.021 mol L−1). The mixture was stirred uniformly at 333 K for approximately 6 h until the solvent evaporated. Subsequently, the obtained sample was dried overnight at 373 K. The calcination, reduction, and passivation steps were identical to those used for Ni@Beta-Cl−.
3.2.5. Synthesis of H-Beta, Ni1@H-Beta-NO3−, and Ni@H-Beta-Cl−
The Beta, Ni1@Beta-NO3−, and Ni@Beta-Cl− zeolites were treated with ammonium ion-exchange in 1 mol/L NH4Cl solution at 363 K for 2 h. The ion-exchanged zeolites were filtered and washed with deionized water and then calcined at 823 K for 6 h. The ion-exchanging process was repeated twice to obtain H-Beta, Ni1@H-Beta-NO3−, and Ni@H-Beta-Cl−.
3.3. Catalyst Characterization
XRD analysis was conducted using a Bruker D8 Advance diffractometer with a Cu-Kα radiation source (40 kV, 40 mA) (Malvern Panalytical, Malvern, UK). The elemental composition of the zeolite samples was determined via XRF analysis, which was conducted on a PANalytical AxiosMAX analyzer (Malvern Panalytical, Malvern, UK). TEM images of the zeolite samples were obtained using a FEI Tecnai G2 F20 microscope equipped with an EDS spectrometer (FEI Company, Hillsboro, OR, USA). The textural properties of zeolite samples were recorded via N2-sorption using a Kubo X1000 setup (Bjbuilder, Beijing, China) operating at 77 K. Before sorption measurements, the samples were treated at 623 K for 4 h under vacuum to eliminate adsorbed substances. The BET equation was used to calculate the specific surface area of the zeolite sample. The micropore surface area and the micropore volume were calculated using the t-plot method. XPS analysis was performed using a Fluorolog-Tau3 spectrometer (HORIBA, Kyoto, Japan), with the C 1s peak at 284.6 eV used as a reference for calibration. The H2-TPR and NH3-TPD were conducted on a chemisorption instrument (Huasi, Yueyang, China, DAS-7000), which was equipped with a TCD detector. FT-IR-Py and FT-IR-CO were performed using a Bruker Tensor II equipped with an MCT detector, produced by Bruker (Billerica, MA, USA).
3.4. Catalyst Evaluation
3.4.1. Hydrolysis of NaBH4
Hydrolysis of NaBH4 was conducted in a 150 mL small reactor with a quartz glass inner sleeve at 298 K under atmospheric pressure. The outlet of the reactor is connected to a CS200-A flow meter, which is connected to a computer to monitor the HGR (mL/min) during the hydrolysis process. In a typical manner, 0.05 g catalyst and 25 mL deionized water were introduced into the glass container and thoroughly mixed by using a magnetic stirrer. The reactor was purged with N2 for 30 min before the reaction started, then heated to the reaction temperature, and 25 mL 75 mM NaBH4 solution (corresponding to a maximum amount of H2 gas of 7.5 mmol = 168 mL at 298 K) was introduced into the glass container via a syringe.
The TOF was calculated based on the quantity of Ni metals in the catalysts when the conversion of NaBH4 reached 100%. The calculation equation used was as below:
where is the mole number of released H2, nNi is the mole number of Ni atoms in the catalyst based on the bulk composition, and t is the completion time of the reaction in minutes.
For kinetic studies, hydrolytic dehydrogenation of the NaBH4 reaction was also carried out at 293, 303, 308, and 313 K in order to obtain the Ea. The Ea value can be calculated via the following Arrhenius equation:
where r is the HGR, T is the hydrolysis temperature in Kelvins, R is the gas constant (8.314 J mol−1 K−1), and k0 signifies the pre-exponential factor. By fitting the Arrhenius plot of lnr versus 1/T, the slope can be clearly obtained via −Ea/R.
3.4.2. Ethylene Dimerization
Ethylene dimerization was performed in a 100 mL stainless steel autoclave, which was purged three times with N2 and once with 1 atm ethylene. Then, the as-prepared catalysts and AlEt2Cl were dispersed into toluene to prepare a suspension and injected into the reactor under N2. Next, the autoclave was pressurized with ethylene to 10 atm and kept constant during the reaction. After 0.5 h of reaction, the reactor was cooled to 253 K with ice-cold ethanol solution and depressurized. An organic layer was separated from the reaction mixture and analyzed via gas chromatography (GC).
The TOF was calculated according to the following equation:
where (g) is the mass of C2H4 consumed, which is equal to the yield of oligomer products (g). M (g/mol) is the molar mass of C2H4, nNi is the mole of Ni atoms in the catalyst based on bulk composition, and t is the reaction time (h).
3.4.3. Propylene Oligomerization
Propylene oligomerization was carried out in a stainless steel fixed-bed reactor (inner diameter: 10 mm, length: 360 mm). In general, the catalyst was pressed into sheets at 20 MPa and then crushed and screened into particles (0.250–0.425 mm). Finally, the catalyst was loaded into the reactor. The catalyst was reduced by hydrogen gas (10 mol% H2/N2, 30 mL/min) at 573 K (ramping rate 2 K/min) for 2 h. Mixed gas (25 mol% propylene/N2) was fed into the reactor, and numerical results were obtained under the reaction condition that the reactive temperature was maintained at 453 K, the operation pressure was 1 atm, and GHSV = 200–600 h−1 was varied. The products were measured using an online GC (HuiFen, Beijing, China, GC-7820) equipped with an HP-PONA capillary column connected to a flame ionization detector (FID). The conversion (X) of propylene and selectivity (S) of the products were calculated as follows:
where i represents the product (i ≤ C3, C4, C5, C6, C7, C8, and C9), ni represents the number of carbon atoms in product i, and ci represents the molar concentration of product i.
4. Conclusions
In summary, benefiting from a unique coordination environment and maximum atomic utilization of encaged Ni single-atoms within Beta zeolite, compared with other heterogeneous catalysts, the obtained Ni1@Beta catalyst not only exhibits good catalytic activity and selectivity in hydrogen generation reactions (TOF = 331 min−1 and Ea = 30.1 kJ/mol) and the dimerization of ethylene (butylene selectivity = 99.4% and TOF = 5804 h−1) but also propylene oligomerization (long-chain olefin (≥C6+) selectivity = 75.21%). These findings provide a general and promising configuration of metal atoms. The prepared catalysts will be of great potential for hydrogen production and the synthesis of clean liquid fuels involving metal or metal ions as active sites. Moreover, the effect of the anions of metal precursors for encapsulation has been identified. Compared with Ni(NO3)2, the introduction of NiCl2 leads to a weaker interaction between Ni species and the zeolite framework, and larger nickel clusters are formed.
Author Contributions
Writing—review and editing, writing—original draft preparation, investigation, and data curation, Y.Z. (Yitong Zhao); writing—original draft preparation, methodology, investigation, and formal analysis, M.L.; data curation and methodology, Y.N.; supervision, Y.Z. (Ying Zhang); writing—review and editing, supervision, project administration, and funding acquisition, Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CNPC Innovation Fund (2021DQ02-0702).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Prieto, G.; Zečević, J.; Friedrich, H.; de Jong, K.P.; de Jongh, P.E. Towards stable catalysts by controlling collective properties of supported metal nanoparticles. Nat. Mater. 2012, 12, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Meng, X.; Xiao, F.S. New Strategies for the Preparation of Sinter-Resistant Metal-Nanoparticle-Based Catalysts. Adv. Mater. 2019, 31, e1901905. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, C.; Wang, L.; Wang, L.; Xiao, F.S. Zeolite Fixed Metal Nanoparticles: New Perspective in Catalysis. Acc. Chem. Res. 2021, 54, 2579–2590. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L.; Xiao, F.-S. Metal@Zeolite Hybrid Materials for Catalysis. ACS Cent. Sci. 2020, 6, 1685–1697. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, Z.-P.; Lv, X.; Chen, L.; Yuan, Z.-Y. Ultrasmall PtZn bimetallic nanoclusters encapsulated in silicalite-1 zeolite with superior performance for propane dehydrogenation. J. Catal. 2020, 385, 61–69. [Google Scholar] [CrossRef]
- Javed, M.; Cheng, S.; Zhang, G.; Amoo, C.C.; Wang, J.; Lu, P.; Lu, C.; Xing, C.; Sun, J.; Tsubaki, N. A facile solvent-free synthesis strategy for Co-imbedded zeolite-based Fischer-Tropsch catalysts for direct gasoline production. Chin. J. Catal. 2020, 41, 604–612. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, T.; Hou, H.; Yin, J.; Wan, H.; Sun, X.; Zhang, Q.; Sun, F.; Wei, Y.; Dong, M.; et al. Regioselective hydroformylation of propene catalysed by rhodium-zeolite. Nature 2024, 629, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Astruc, D. Recent developments of nanocatalyzed liquid-phase hydrogen generation. Chem. Soc. Rev. 2021, 50, 3437–3484. [Google Scholar] [CrossRef] [PubMed]
- Eppinger, J.; Huang, K.-W. Formic Acid as a Hydrogen Energy Carrier. ACS Energy Lett. 2016, 2, 188–195. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, N.; Zhang, T.; Bai, R.; Mayoral, A.; Zhang, P.; Zhang, Q.; Terasaki, O.; Yu, J. Zeolite-Encaged Single-Atom Rhodium Catalysts: Highly-Efficient Hydrogen Generation and Shape-Selective Tandem Hydrogenation of Nitroarenes. Angew. Chem. Int. Ed. 2019, 58, 18570–18576. [Google Scholar] [CrossRef] [PubMed]
- Wulfers, M.J.; Lobo, R.F. Assessment of mass transfer limitations in oligomerization of butene at high pressure on H-beta. Appl. Catal. A Gen. 2015, 505, 394–401. [Google Scholar] [CrossRef]
- Kilburn, L.; Salomé Rivera, D.; Bickel Rogers, E.E.; Gounder, R.; Hibbitts, D.D. Assessing the Influence of Void Environment in MFI Zeolites on Propene Oligomerization Kinetics Using a Combined Computational and Experimental Approach. ACS Catal. 2025, 15, 7121–7137. [Google Scholar] [CrossRef]
- Nozik, D.; Bell, A.T. Role of Ga3+ Sites in Ethene Oligomerization over Ga/H-MFI. ACS Catal. 2022, 12, 14173–14184. [Google Scholar] [CrossRef]
- Kitamura, H.; Sumi, T.; Kubota, S.; Kokuryo, S.; Tamura, K.; Miyake, K.; Uchida, Y.; Miyamoto, M.; Nishiyama, N. Stable and selective conversion of ethylene to propylene and butylene using Ni-loaded dealuminated Beta zeolite catalyst. Appl. Catal. A Gen. 2023, 668, 119429. [Google Scholar] [CrossRef]
- Liu, M.; Miao, C.; Fo, Y.; Wang, W.; Ning, Y.; Chu, S.; Song, W.; Zhang, Y.; Liu, J.; Wu, Z.; et al. Chelating-agent-free incorporation of isolated Ni single-atoms within BEA zeolite for enhanced biomass hydrogenation. Chin. J. Catal. 2025, 71, 308–318. [Google Scholar] [CrossRef]
- Escola, J.M.; Serrano, D.P.; Aguado, J.; Briones, L. Hydroreforming of the LDPE Thermal Cracking Oil over Hierarchical Ni/Beta Catalysts with Different Ni Particle Size Distributions. Ind. Eng. Chem. Res. 2015, 54, 6660–6668. [Google Scholar] [CrossRef]
- Zahmakıran, M.; Ayvalı, T.; Akbayrak, S.; Çalışkan, S.; Çelik, D.; Özkar, S. Zeolite framework stabilized nickel(0) nanoparticles: Active and long-lived catalyst for hydrogen generation from the hydrolysis of ammonia-borane and sodium borohydride. Catal. Today 2011, 170, 76–84. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Chang, H.-A. Efficient hydrogen production from NaBH4 hydrolysis catalyzed by a magnetic cobalt/carbon composite derived from a zeolitic imidazolate framework. Chem. Eng. J. 2016, 296, 243–251. [Google Scholar] [CrossRef]
- Rakap, M.; Özkar, S. Intrazeolite cobalt(0) nanoclusters as low-cost and reusable catalyst for hydrogen generation from the hydrolysis of sodium borohydride. Appl. Catal. B Environ. 2009, 91, 21–29. [Google Scholar] [CrossRef]
- Luo, C.; Fu, F.; Yang, X.; Wei, J.; Wang, C.; Zhu, J.; Huang, D.; Astruc, D.; Zhao, P. Highly Efficient and Selective Co@ZIF-8 Nanocatalyst for Hydrogen Release from Sodium Borohydride Hydrolysis. ChemCatChem 2019, 11, 1643–1649. [Google Scholar] [CrossRef]
- Zahmakiran, M.; Ozkar, S. Zeolite-Confined Ruthenium(0) Nanoclusters Catalyst: Record Catalytic Activity, Reusability, and Lifetime in Hydrogen Generation from the Hydrolysis of Sodium Borohydride. Langmuir 2009, 25, 2667–2678. [Google Scholar] [CrossRef] [PubMed]
- Tuan, D.D.; Lin, K.-Y.A. Ruthenium supported on ZIF-67 as an enhanced catalyst for hydrogen generation from hydrolysis of sodium borohydride. Chem. Eng. J. 2018, 351, 48–55. [Google Scholar] [CrossRef]
- Irum, M.; Zaheer, M.; Friedrich, M.; Kempe, R. Mesoporous silica nanosphere supported platinum nanoparticles (Pt@MSN): One-pot synthesis and catalytic hydrogen generation. RSC Adv. 2016, 6, 10438–10441. [Google Scholar] [CrossRef]
- Chen, C.; Alalouni, M.R.; Dong, X.; Cao, Z.; Cheng, Q.; Zheng, L.; Meng, L.; Guan, C.; Liu, L.; Abou-Hamad, E.; et al. Highly Active Heterogeneous Catalyst for Ethylene Dimerization Prepared by Selectively Doping Ni on the Surface of a Zeolitic Imidazolate Framework. J. Am. Chem. Soc. 2021, 143, 7144–7153. [Google Scholar] [CrossRef] [PubMed]
- Metzger, E.D.; Brozek, C.K.; Comito, R.J.; Dincă, M. Selective Dimerization of Ethylene to 1-Butene with a Porous Catalyst. ACS Cent. Sci. 2016, 2, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Q.; Solan, G.A.; Sun, W.-H. Recent advances in Ni-mediated ethylene chain growth: Nimine-donor ligand effects on catalytic activity, thermal stability and oligo-/polymer structure. Coord. Chem. Rev. 2017, 350, 68–83. [Google Scholar] [CrossRef]
- Wang, L.; Ke, J.; Chai, Y.; Wu, G.; Wang, C.; Li, L. Additive-Free Ethylene Dimerization Over Well-Defined Nickel-Zeolite Catalysts. Angew. Chem. Int. Ed. 2025, 64, e202502563. [Google Scholar] [CrossRef] [PubMed]
- Martínez Gómez-Aldaraví, A.; Paris, C.; Moliner, M.; Martínez, C. Design of bi-functional Ni-zeolites for ethylene oligomerization: Controlling Ni speciation and zeolite properties by one-pot and post-synthetic Ni incorporation. J. Catal. 2023, 426, 140–152. [Google Scholar] [CrossRef]
- Shirazi, L.; Jamshidi, E.; Ghasemi, M.R. The effect of Si/Al ratio of ZSM-5 zeolite on its morphology, acidity and crystal size. Cryst. Res. Technol. 2008, 43, 1300–1306. [Google Scholar] [CrossRef]
- Gabrienko, A.A.; Danilova, I.G.; Arzumanov, S.S.; Pirutko, L.V.; Freude, D.; Stepanov, A.G. Direct Measurement of Zeolite Brønsted Acidity by FTIR Spectroscopy: Solid-State 1H MAS NMR Approach for Reliable Determination of the Integrated Molar Absorption Coefficients. J. Phys. Chem. C 2018, 122, 25386–25395. [Google Scholar] [CrossRef]
- Qiu, P.; Lunsford, J.H.; Rosynek, M.P. Characterization of Ga/ZSM-5 for the catalytic aromatization of dilute ethylene streams. Catal. Lett. 1998, 52, 27–42. [Google Scholar] [CrossRef]
- Ning, Y.; Li, C.; Zhao, B.; Min, W.; Li, X.; Zhang, Y. Unprecedented High Activity of One-Dimensional Nickel-Based Coordination Polymer for Ethylene Dimerization. Appl. Organomet. Chem. 2025, 39, e70019. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Han, Y.; Sheng, D.; Shan, D.; Liu, X.; Cheng, A. Ultrathin Nickel-Based Metal–Organic Framework Nanosheets as Reusable Heterogeneous Catalyst for Ethylene Dimerization. ACS Appl. Nano Mater. 2018, 2, 136–142. [Google Scholar] [CrossRef]
- Lallemand, M.; Finiels, A.; Fajula, F.; Hulea, V. Catalytic oligomerization of ethylene over Ni-containing dealuminated Y zeolites. Appl. Catal. A Gen. 2006, 301, 196–201. [Google Scholar] [CrossRef]
- Sohn, J.R.; Park, W.C.; Kim, H.W. Characterization of Nickel Sulfate Supported on γ-Al2O3 for Ethylene Dimerization and Its Relationship to Acidic Properties. J. Catal. 2002, 209, 69–74. [Google Scholar] [CrossRef]
- Kostyniuk, A.; Bajec, D.; Likozar, B. Catalytic hydrogenation, hydrocracking and isomerization reactions of biomass tar model compound mixture over Ni-modified zeolite catalysts in packed bed reactor. Renew. Energy 2021, 167, 409–424. [Google Scholar] [CrossRef]
- Segobia, D.J.; Trasarti, A.F.; Apesteguía, C.R. Effect of the catalyst preparation method on the performance of Ni-supported catalysts for the synthesis of saturated amines from nitrile hydrogenation. Chin. J. Catal. 2019, 40, 1693–1703. [Google Scholar] [CrossRef]
- Moussa, S.; Concepción, P.; Arribas, M.A.; Martínez, A. The nature of active Ni sites and the role of Al species in the oligomerization of ethylene on mesoporous Ni-Al-MCM-41 catalysts. Appl. Catal. A Gen. 2020, 608, 117831. [Google Scholar] [CrossRef]
- Yan, P.; Xi, S.; Peng, H.; Mitchell, D.R.G.; Harvey, L.; Drewery, M.; Kennedy, E.M.; Zhu, Z.; Sankar, G.; Stockenhuber, M. Facile and Eco-Friendly Approach To Produce Confined Metal Cluster Catalysts. J. Am. Chem. Soc. 2023, 145, 9718–9728. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.J.; Louis, B.; Lam, Y.L.; Pereira, M.M. Ni-ZSM-5 catalysts: Detailed characterization of metal sites for proper catalyst design. J. Catal. 2010, 269, 103–109. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, X.; Su, Y.; Zhao, Y.; Qiao, B. Catalytic propane dehydrogenation by anatase supported Ni single-atom catalysts. Chin. J. Catal. 2024, 57, 105–113. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, S.; Zhang, A.; Song, C.; Shi, C.; Guo, X. Hollow zeolite encapsulated Ni–Pt bimetals for sintering and coking resistant dry reforming of methane. J. Mater. Chem. A 2015, 3, 16461–16468. [Google Scholar] [CrossRef]
- Resini, C.; Venkov, T.; Hadjiivanov, K.; Presto, S.; Riani, P.; Marazza, R.; Ramis, G.; Busca, G. An FTIR study of the dispersed Ni species on Ni-YSZ catalysts. Appl. Catal. A Gen. 2009, 353, 137–143. [Google Scholar] [CrossRef]
- Moussa, S.; Concepción, P.; Arribas, M.A.; Martínez, A. Nature of Active Nickel Sites and Initiation Mechanism for Ethylene Oligomerization on Heterogeneous Ni-beta Catalysts. ACS Catal. 2018, 8, 3903–3912. [Google Scholar] [CrossRef]
- Aleksandrov, H.A.; Zdravkova, V.R.; Mihaylov, M.Y.; Petkov, P.S.; Vayssilov, G.N.; Hadjiivanov, K.I. Precise Identification of the Infrared Bands of the Polycarbonyl Complexes on Ni–MOR Zeolite by12C16O–13C18O Coadsorption and Computational Modeling. J. Phys. Chem. C 2012, 116, 22823–22831. [Google Scholar] [CrossRef]
- Cairon, O.; Bellat, J.-P. Macroscopic and Molecular Insights from CO Adsorption on NaY Zeolite: A Combined FTIR and Manometric Study. J. Phys. Chem. C 2012, 116, 11195–11199. [Google Scholar] [CrossRef]
- Ma, R.; Gao, J.; Kou, J.; Dean, D.P.; Breckner, C.J.; Liang, K.; Zhou, B.; Miller, J.T.; Zou, G. Insights into the Nature of Selective Nickel Sites on Ni/Al2O3 Catalysts for Propane Dehydrogenation. ACS Catal. 2022, 12, 12607–12616. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).