Boosting Dual Hydrogen Electrocatalysis with Pt/NiMo Catalysts: Tuning the Ni/Mo Ratio and Minimizing Pt Usage
Abstract
1. Introduction
2. Results and Discussion
2.1. Electrochemical Characterization of the Materials
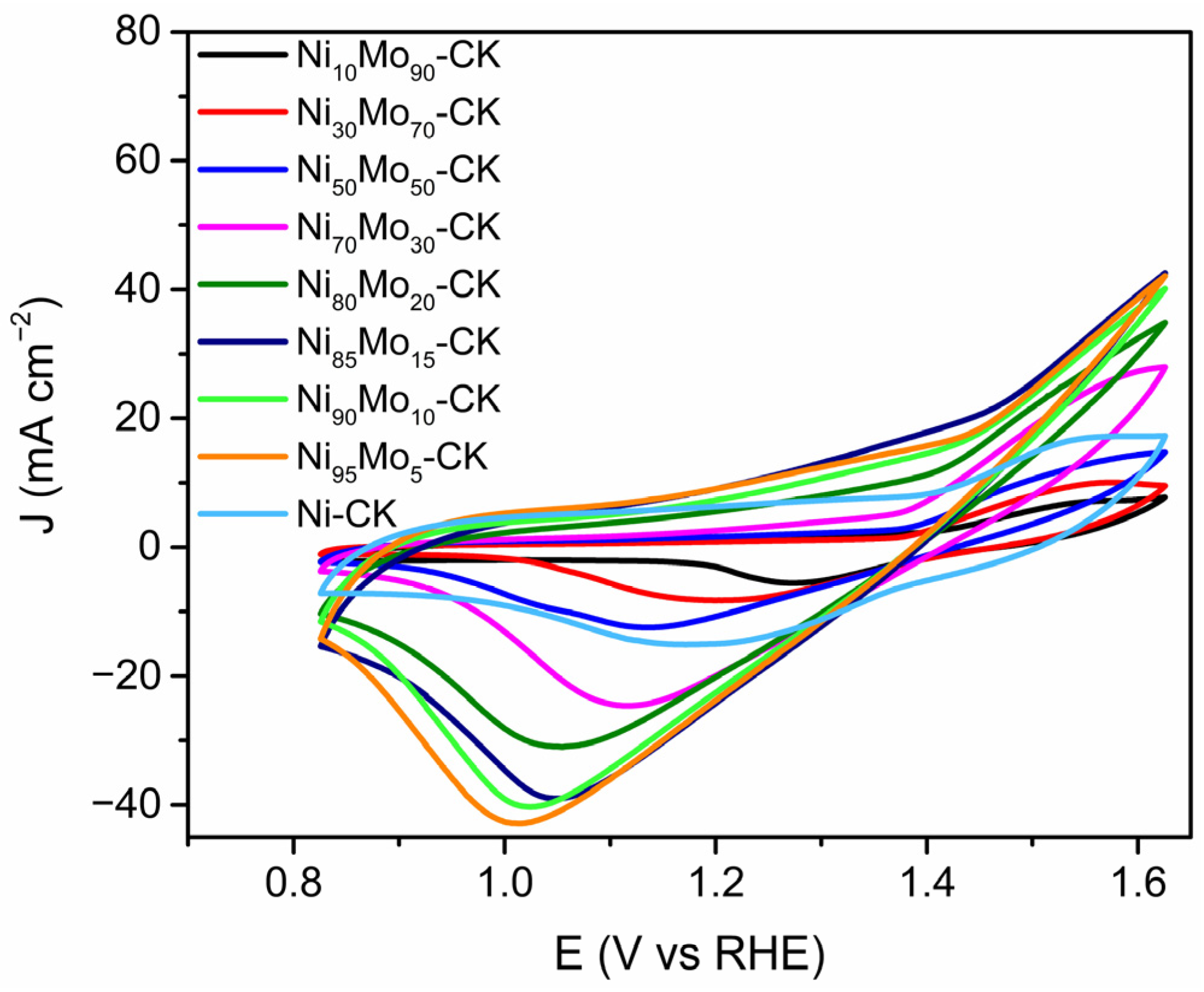
2.1.1. Electrochemical Active Surface Area (ECSA) Determination of Ni by β-NiOOH Method
2.1.2. Active Surface Area of the Pt-CK and Pt/Ni90Mo10-CK Materials
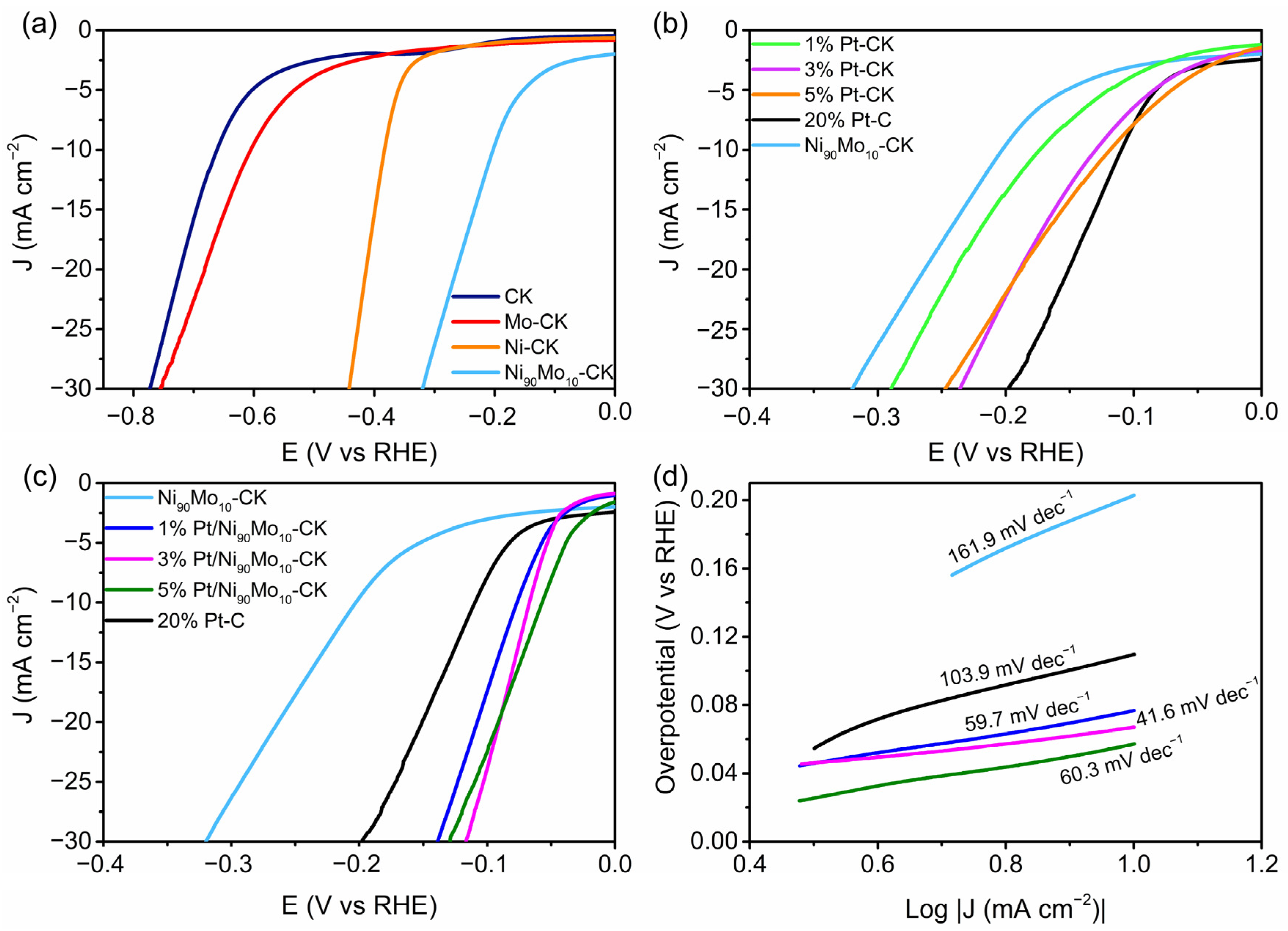
2.1.3. Electrocatalytic Activity for the Hydrogen Evolution Reaction (HER)
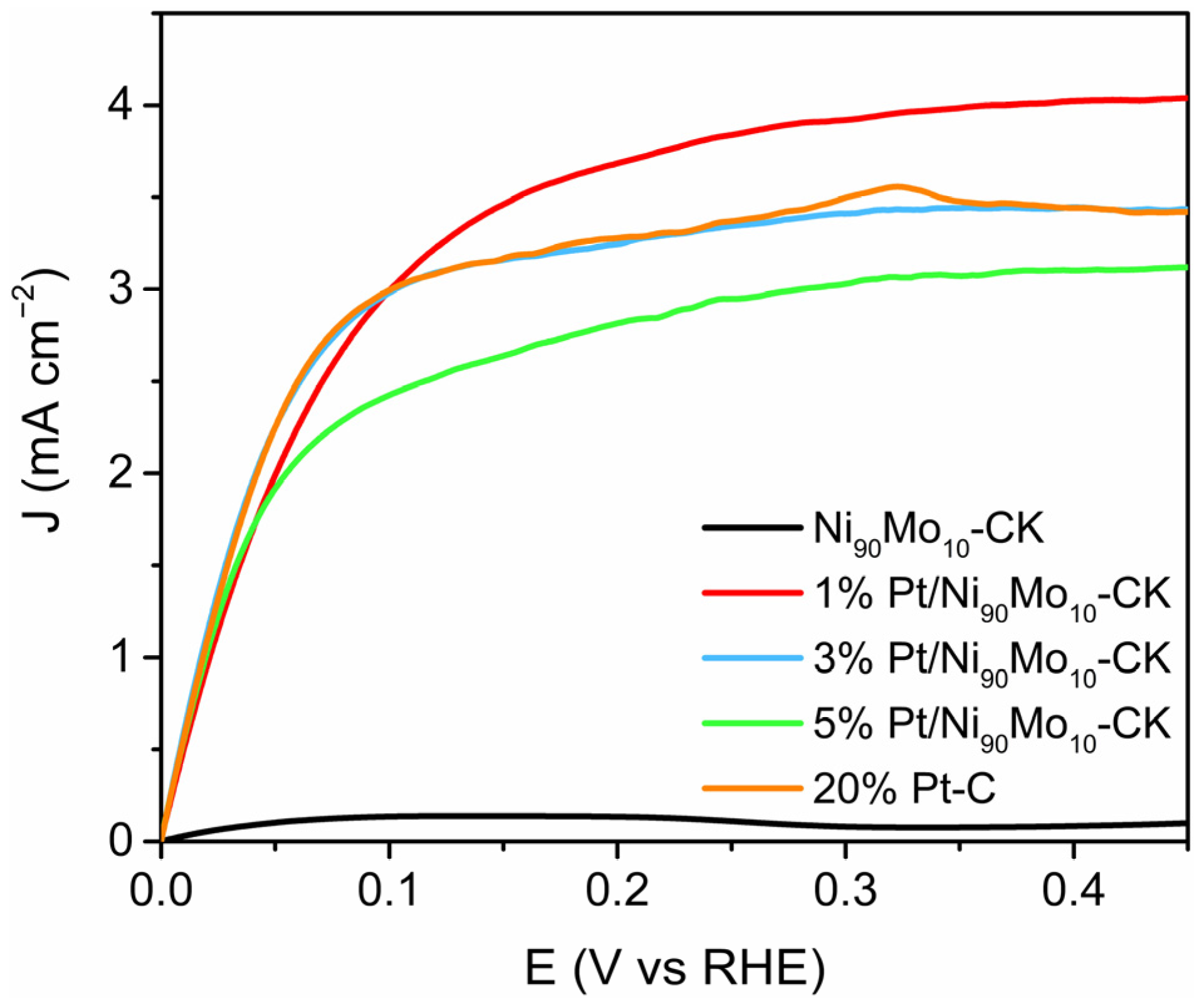
2.1.4. Electrocatalytic Activity for the Hydrogen Oxidation Reaction (HOR)
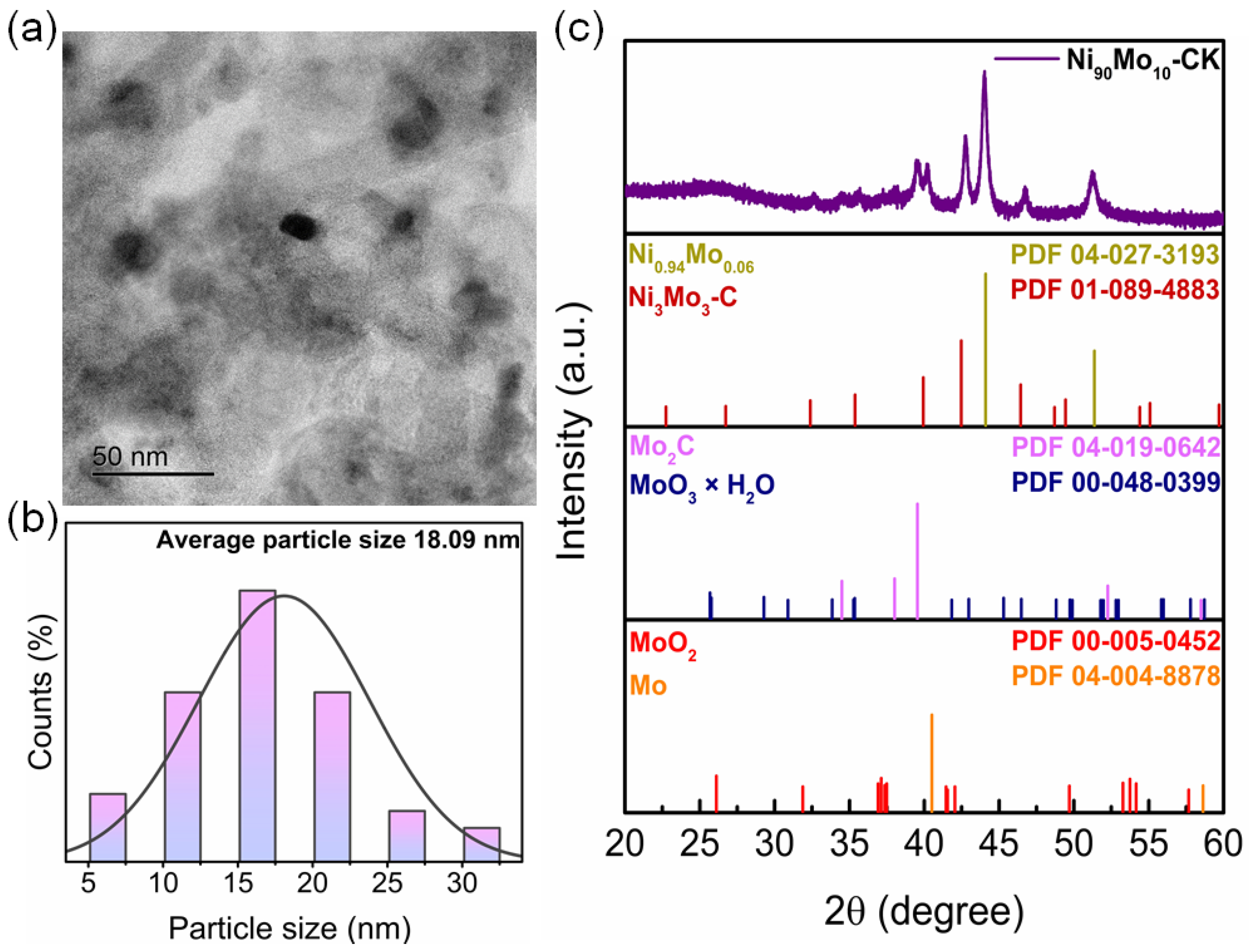
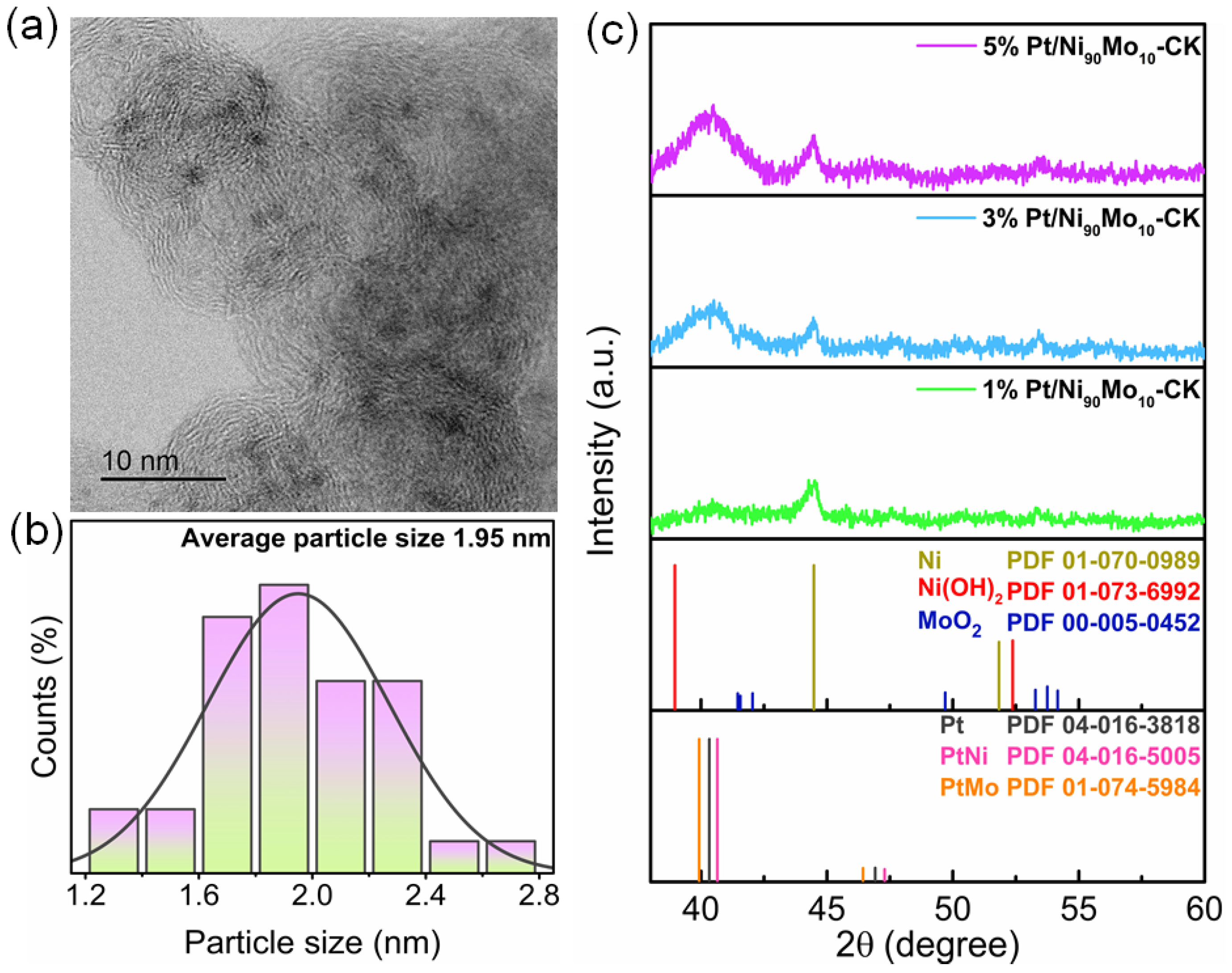
2.2. Physicochemical Characterization of Catalysts
3. Materials and Methods
3.1. Reagents
3.2. Synthesis of the Ni-CK, Mo-C, Ni90Mo10-CK, and NixMo100−x-CK
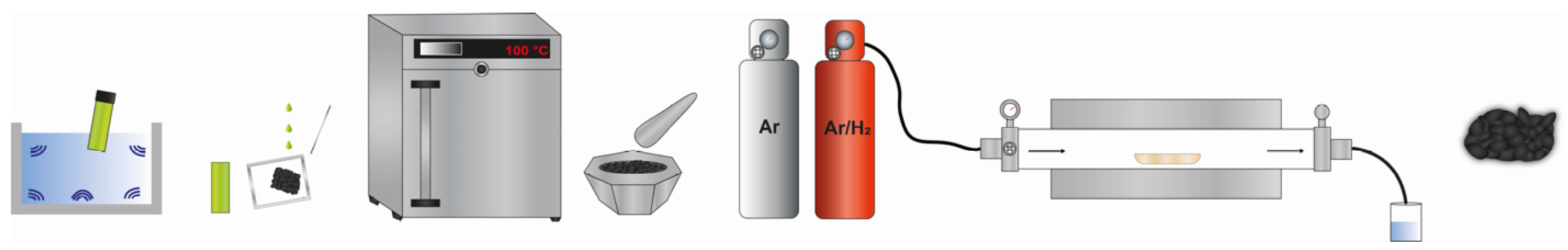
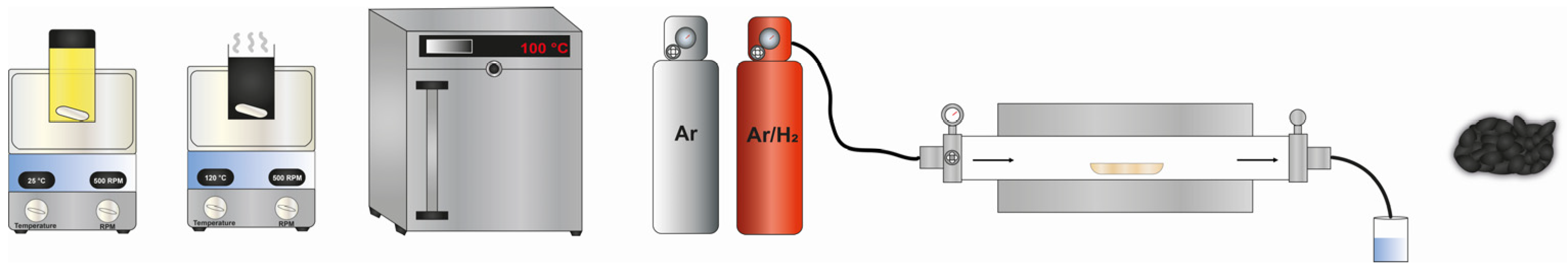
3.3. Synthesis of the Pt/Ni90Mo10-CK
3.4. Electrochemical Measurement
3.5. Physicochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morales-Guio, C.G.; Stern, L.-A.; Hu, X. Nanostructured Hydrotreating Catalysts for Electrochemical Hydrogen Evolution. Chem. Soc. Rev. 2014, 43, 6555. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Yang, M.; Jiang, J.; He, X.; Zou, J.; Xiong, Z.; Liao, G.; Liu, S. Recent Advances of MXenes as Electrocatalysts for Hydrogen Evolution Reaction. npj 2D Mater. Appl. 2021, 5, 78. [Google Scholar] [CrossRef]
- Alsawat, M.; Alshehri, N.A.; Shaltout, A.A.; Ahmed, S.I.; Al-Malki, H.M.O.; Das, M.R.; Boukherroub, R.; Amin, M.A.; Ibrahim, M.M. Enhanced Alkaline Hydrogen Evolution on Gd1.0/Ndx (x = 0.5, 1.0, 3.0, and 6.0%)-Doped TiO2 Bimetallic Electrocatalysts. Catalysts 2023, 13, 1192. [Google Scholar] [CrossRef]
- Wang, C.; Shang, H.; Xu, H.; Du, Y. Nanoboxes Endow Non-Noble-Metal-Based Electrocatalysts with High Efficiency for Overall Water Splitting. J. Mater. Chem. A 2021, 9, 857–874. [Google Scholar] [CrossRef]
- Cai, W.; Liu, X.; Wang, L.; Wang, B. Design and Synthesis of Noble Metal–Based Electrocatalysts Using Metal–Organic Frameworks and Derivatives. Mater. Today Nano 2022, 17, 100144. [Google Scholar] [CrossRef]
- Singh, V.K.; Gupta, U.; Mukherjee, B.; Chattopadhyay, S.; Das, S. MoS2 Nanosheets on MoNi4/MoO2 Nanorods for Hydrogen Evolution. ACS Appl. Nano Mater. 2021, 4, 886–896. [Google Scholar] [CrossRef]
- Badreldin, A.; Abusrafa, A.E.; Abdel-Wahab, A. Oxygen-Deficient Cobalt-Based Oxides for Electrocatalytic Water Splitting. ChemSusChem 2021, 14, 10–32. [Google Scholar] [CrossRef]
- Cao, X.; Wang, T.; Jiao, L. Transition-Metal (Fe, Co, and Ni)-Based Nanofiber Electrocatalysts for Water Splitting. Adv. Fiber Mater. 2021, 3, 210–228. [Google Scholar] [CrossRef]
- Nivetha, R.; Sajeev, A.; Mary Paul, A.; Gothandapani, K.; Gnanasekar, S.; Bhardwaj, P.; Jacob, G.; Sellappan, R.; Raghavan, V.; N, K.C.; et al. Cu Based Metal Organic Framework (Cu-MOF) for Electrocatalytic Hydrogen Evolution Reaction. Mater. Res. Express 2020, 7, 114001. [Google Scholar] [CrossRef]
- Nadar, A.; Banerjee, A.M.; Pai, M.R.; Antony, R.P.; Patra, A.K.; Sastry, P.U.; Donthula, H.; Tewari, R.; Tripathi, A.K. Effect of Mo Content on Hydrogen Evolution Reaction Activity of Mo2C/C Electrocatalysts. Int. J. Hydrog. Energy 2020, 45, 12691–12701. [Google Scholar] [CrossRef]
- Anantharaj, S.; Kundu, S.; Noda, S. Progress in Nickel Chalcogenide Electrocatalyzed Hydrogen Evolution Reaction. J. Mater. Chem. A 2020, 8, 4174–4192. [Google Scholar] [CrossRef]
- Du, W.; Shi, Y.; Zhou, W.; Yu, Y.; Zhang, B. Unveiling the In Situ Dissolution and Polymerization of Mo in Ni4Mo Alloy for Promoting the Hydrogen Evolution Reaction. Angew. Chemie Int. Ed. 2021, 60, 7051–7055. [Google Scholar] [CrossRef]
- Yao, R.; Zhou, Y.; Shi, H.; Wan, W.; Zhang, Q.; Gu, L.; Zhu, Y.; Wen, Z.; Lang, X.; Jiang, Q. Nanoporous Surface High-Entropy Alloys as Highly Efficient Multisite Electrocatalysts for Nonacidic Hydrogen Evolution Reaction. Adv. Funct. Mater. 2021, 31, 2009613. [Google Scholar] [CrossRef]
- Song, J.; Jin, Y.Q.; Zhang, L.; Dong, P.; Li, J.; Xie, F.; Zhang, H.; Chen, J.; Jin, Y.; Meng, H.; et al. Phase-Separated Mo–Ni Alloy for Hydrogen Oxidation and Evolution Reactions with High Activity and Enhanced Stability. Adv. Energy Mater. 2021, 11, 2003511. [Google Scholar] [CrossRef]
- Xu, X.; Yu, X.; Guo, K.; Dong, L.; Miao, X. Alkaline Media Regulated NiFe-LDH-Based Nickel–Iron Phosphides toward Robust Overall Water Splitting. Catalysts 2023, 13, 198. [Google Scholar] [CrossRef]
- Regmi, Y.N.; Waetzig, G.R.; Duffee, K.D.; Schmuecker, S.M.; Thode, J.M.; Leonard, B.M. Carbides of Group IVA, VA and VIA Transition Metals as Alternative HER and ORR Catalysts and Support Materials. J. Mater. Chem. A 2015, 3, 10085–10091. [Google Scholar] [CrossRef]
- Meyer, S.; Nikiforov, A.V.; Petrushina, I.M.; Köhler, K.; Christensen, E.; Jensen, J.O.; Bjerrum, N.J. Transition Metal Carbides (WC, Mo2C, TaC, NbC) as Potential Electrocatalysts for the Hydrogen Evolution Reaction (HER) at Medium Temperatures. Int. J. Hydrog. Energy 2015, 40, 2905–2911. [Google Scholar] [CrossRef]
- Wan, C.; Regmi, Y.N.; Leonard, B.M. Multiple Phases of Molybdenum Carbide as Electrocatalysts for the Hydrogen Evolution Reaction. Angew. Chemie Int. Ed. 2014, 126, 6525–6528. [Google Scholar] [CrossRef]
- Dubouis, N.; Grimaud, A. The Hydrogen Evolution Reaction: From Material to Interfacial Descriptors. Chem. Sci. 2019, 10, 9165–9181. [Google Scholar] [CrossRef]
- Peng, L.; Liao, M.; Zheng, X.; Nie, Y.; Zhang, L.; Wang, M.; Xiang, R.; Wang, J.; Li, L.; Wei, Z. Accelerated Alkaline Hydrogen Evolution on M(OH)X/M-MoPOX (M = Ni, Co, Fe, Mn) Electrocatalysts by Coupling Water Dissociation and Hydrogen Ad-Desorption Steps. Chem. Sci. 2020, 11, 2487–2493. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Z.; Yang, F.; Li, J.; Liu, Z.; Ren, W.; Zhang, S.; Liu, B. Stabilized Hydroxide-Mediated Nickel-Based Electrocatalysts for High-Current-Density Hydrogen Evolution in Alkaline Media. Energy Environ. Sci. 2021, 14, 4610–4619. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.; Shen, T.; Wu, C.; Zu, L.; Zhang, L. Porous NiFe Alloys Synthesized via Freeze Casting as Bifunctional Electrocatalysts for Oxygen and Hydrogen Evolution Reaction. Int. J. Hydrog. Energy 2021, 46, 37736–37745. [Google Scholar] [CrossRef]
- Lima, D.W.; Trombetta, F.; Paula Benvenutti, P.; Teixeira, S.R.; Martini, E.M.A. Effect of Different Carbon Supports for Ni Particles for the HER in Tetra-alkylammonium-sulfonic Acid Media. Int. J. Energy Res. 2019, 43, 7352–7363. [Google Scholar] [CrossRef]
- Hu, T.; Fan, Y.; Ye, Y.; Cai, Y.; Liu, J.; Ma, Y.; Li, P.; Liang, C. Laser Irradiation-Induced Pt-Based Bimetallic Alloy Nanostructures without Chemical Reducing Agents for Hydrogen Evolution Reaction. Catalysts 2023, 13, 1018. [Google Scholar] [CrossRef]
- Popov, A.A.; Afonnikova, S.D.; Varygin, A.D.; Bauman, Y.I.; Trenikhin, M.V.; Plyusnin, P.E.; Shubin, Y.V.; Vedyagin, A.A.; Mishakov, I.V. Pt1−xNix Alloy Nanoparticles Embedded in Self-Grown Carbon Nanofibers: Synthesis, Properties and Catalytic Activity in HER. Catalysts 2023, 13, 599. [Google Scholar] [CrossRef]
- Kabir, S.; Lemire, K.; Artyushkova, K.; Roy, A.; Odgaard, M.; Schlueter, D.; Oshchepkov, A.; Bonnefont, A.; Savinova, E.; Sabarirajan, D.C.; et al. Platinum Group Metal-Free NiMo Hydrogen Oxidation Catalysts: High Performance and Durability in Alkaline Exchange Membrane Fuel Cells. J. Mater. Chem. A 2017, 5, 24433–24443. [Google Scholar] [CrossRef]
- Park, J.; Jang, J.; Lee, A.; Kim, S.; Lee, S.; Yoo, S.J.; Lee, J. Effect of Support for Non-Noble NiMo Electrocatalyst in Alkaline Hydrogen Oxidation. Adv. Sustain. Syst. 2022, 6, 2100226. [Google Scholar] [CrossRef]
- Duan, Y.; Yu, Z.-Y.; Yang, L.; Zheng, L.-R.; Zhang, C.-T.; Yang, X.-T.; Gao, F.-Y.; Zhang, X.-L.; Yu, X.; Liu, R.; et al. Bimetallic Nickel-Molybdenum/Tungsten Nanoalloys for High-Efficiency Hydrogen Oxidation Catalysis in Alkaline Electrolytes. Nat. Commun. 2020, 11, 4789. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tian, C.; Zhai, Y.; Li, X.; Li, J.; Chen, H.; Cheng, L.; Zhao, H.; Dai, P. Ternary MoWNi Alloy as a Bifunctional Catalyst for Alkaline Hydrogen Oxidation and Evolution Reactions. Catalysts 2025, 15, 15. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, T.; Yu, S.; Yang, H.; Sun, Q.; Zheng, J.Y. Constructing Stable MoOx-NiSx Film via Electrodeposition and Hydrothermal Method for Water Splitting. Catalysts 2023, 13, 1426. [Google Scholar] [CrossRef]
- Kamel, M.M.; Abd-Ellah, A.A.; Alhadhrami, A.; Ibrahim, M.M.; Anwer, Z.M.; Shata, S.S.; Mostafa, N.Y. Ni-Mo Nanostructure Alloys as Effective Electrocatalysts for Green Hydrogen Production in an Acidic Medium. RSC Adv. 2025, 15, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Peng, Z.; Jia, D.; Wang, Y.; Wu, W.; Deng, P.; Xu, M.; Xu, X.; Jia, G.; Ye, W.; et al. Interfacial Electronic Rearrangement and Synergistic Catalysis for Alkaline Water Splitting in Carbon-Encapsulated Ni (111)/Ni3C (113) Heterostructures. Catalysts 2022, 12, 1367. [Google Scholar] [CrossRef]
- Kim, H.; Park, H.; Tran, D.S.; Kim, S.-K. Facile Fabrication of Amorphous NiMo Catalysts for Alkaline Hydrogen Oxidation Reaction. J. Ind. Eng. Chem. 2021, 94, 309–316. [Google Scholar] [CrossRef]
- Xu, Z.; Hao, M.; Liu, X.; Ma, J.; Wang, L.; Li, C.; Wang, W. Co(OH)2 Nanoflowers Decorated α-NiMoO4 Nanowires as a Bifunctional Electrocatalyst for Efficient Overall Water Splitting. Catalysts 2022, 12, 1417. [Google Scholar] [CrossRef]
- Neumüller, D.; Rafailović, L.D.; Pašti, I.A.; Griesser, T.; Gammer, C.; Eckert, J. Revealing the Role of Mo Leaching in the Structural Transformation of NiMo Thin Film Catalysts upon Hydrogen Evolution Reaction. Small 2024, 20, 2402200. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, H.; Gao, W.; Xue, W.; Liu, Z.; Huang, J.; Pan, X.; Huang, Y. Surface-Engineered PtNi-O Nanostructure with Record-High Performance for Electrocatalytic Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2018, 140, 9046–9050. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, G.; Gao, T.; Chen, Y.; Liao, H.; Guo, X.; Li, H.; Liu, R.; Dou, M.; Nan, S.; et al. Effect of Atomic Ordering Transformation of PtNi Nanoparticles on Alkaline Hydrogen Evolution: Unexpected Superior Activity of the Disordered Phase. J. Phys. Chem. C 2020, 124, 5036–5045. [Google Scholar] [CrossRef]
- He, Y.; Hu, J.; Yin, W.; Xing, Y.; Hu, F.; Wei, H.; Wei, H.; Li, J. Disordered and Ultrafine PtNiMo Alloy for Superior Electrocatalytic Hydrogen Evolution in Alkaline. Mater. Chem. Phys. 2023, 301, 127585. [Google Scholar] [CrossRef]
- Cossar, E.; Houache, M.S.E.; Zhang, Z.; Baranova, E.A. Comparison of Electrochemical Active Surface Area Methods for Various Nickel Nanostructures. J. Electroanal. Chem. 2020, 870, 114246. [Google Scholar] [CrossRef]
- Ciapina, E.G.; Santos, S.F.; Gonzalez, E.R. Electrochemical CO Stripping on Nanosized Pt Surfaces in Acid Media: A Review on the Issue of Peak Multiplicity. J. Electroanal. Chem. 2018, 815, 47–60. [Google Scholar] [CrossRef]
- Danisman, B.; Zhang, G.-R.; Baumunk, A.F.; Yang, J.; Brummel, O.; Darge, P.; Mayrhofer, K.J.J.; Libuda, J.; Ledendecker, M.; Etzold, B.J.M. Strong Activity Changes Observable during the First Pretreatment Cycles of Trimetallic PtNiMo/C Catalysts. ChemElectroChem 2023, 10, e202300109. [Google Scholar] [CrossRef]
- Ordóñez, L.C.; Roquero, P.; Sebastian, P.J.; Ramírez, J. CO Oxidation on Carbon-Supported PtMo Electrocatalysts: Effect of the Platinum Particle Size. Int. J. Hydrog. Energy 2007, 32, 3147–3153. [Google Scholar] [CrossRef]
- Cruz-Reyes, I.; Trujillo-Navarrete, B.; García-Tapia, K.; Salazar-Gastélum, M.I.; Paraguay-Delgado, F.; Félix-Navarro, R.M. Pd/MnO2 as a Bifunctional Electrocatalyst for Potential Application in Alkaline Fuel Cells. Fuel 2020, 27, 118470. [Google Scholar] [CrossRef]
- Sadeghi, E.; Chamani, S.; Erdem, E.; Peighambardoust, N.S.; Aydemir, U. NiMo/CoMoO4 Heterostructure with Confined Oxygen Vacancy for Active and Durable Alkaline Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2023, 6, 7658–7671. [Google Scholar] [CrossRef]
- Wei, H.; Xi, Q.; Chen, X.; Guo, D.; Ding, F.; Yang, Z.; Wang, S.; Li, J.; Huang, S. Molybdenum Carbide Nanoparticles Coated into the Graphene Wrapping N-Doped Porous Carbon Microspheres for Highly Efficient Electrocatalytic Hydrogen Evolution Both in Acidic and Alkaline Media. Adv. Sci. 2018, 5, 1700733. [Google Scholar] [CrossRef]
- Hussain, S.; Muhammad, S.; Faizan, M.; Nam, K.-W.; Kim, H.-S.; Vikraman, D.; Jung, J. Hierarchical Mo2C@CNT Hybrid Structure Formation for the Improved Lithium-Ion Battery Storage Performance. Nanomaterials 2021, 11, 2195. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Qiu, Y.; Liu, S.; Fan, J.; Guo, X. Improving Oxygen Vacancies by Cobalt Doping in MoO2 Nanorods for Efficient Electrocatalytic Hydrogen Evolution Reaction. Nano Sel. 2021, 2, 2148–2158. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.; Cao, B.; Jiang, J.; Gao, R.; Zhang, J. Few Layered N, P Dual-Doped Carbon-Encapsulated Ultrafine MoP Nanocrystal/MoP Cluster Hybrids on Carbon Cloth: An Ultrahigh Active and Durable 3D Self-Supported Integrated Electrode for Hydrogen Evolution Reaction in a Wide PH Range. Adv. Funct. Mater. 2018, 28, 1801527. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Jia, S.; Xu, H.; Zang, J.; Lu, J.; Xu, X. A Hybrid of NiMo-Mo2C/C as Non-Noble Metal Electrocatalyst for Hydrogen Evolution Reaction in an Acidic Solution. Electrochim. Acta 2016, 222, 747–754. [Google Scholar] [CrossRef]
- Kurys, Y.I.; Mazur, D.O.; Koshechko, V.G.; Pokhodenko, V.D. Nanocomposite Based on N,P-Doped Reduced Graphene Oxide, Mo2C, and Mo2N as Efficient Electrocatalyst for Hydrogen Evolution in a Wide PH Range. Electrocatalysis 2021, 12, 469–477. [Google Scholar] [CrossRef]
- Hou, H.; Wang, W.; Kang, Q.; Wang, J.; Chen, Z.; Wang, Y.; Cui, Y.; Yu, Y.; Zhu, J.; Yan, H.; et al. Undoped MoOX with Oxygen-Rich Vacancies as Hole Transport Material for Efficient Indoor/Outdoor Organic Solar Cells. Nano Energy 2024, 131, 110173. [Google Scholar] [CrossRef]
- Salunkhe, P.; Kekuda, D. Effect of Annealing Temperature on the Physical Properties of NiO Thin Films and ITO/NiO/Al Schottky Diodes. J. Mater. Sci. Mater. Electron. 2022, 33, 21060–21074. [Google Scholar] [CrossRef]
- Seo, K.; Jeong, S.-M.; Lim, T.; Ju, S. Continuous Hydrogen Regeneration through the Oxygen Vacancy Control of Metal Oxides Using Microwave Irradiation. RSC Adv. 2018, 8, 37958–37964. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Li, W.; Liu, L. Vertically Aligned Porous Nickel (II) Hydroxide Nanosheets Supported on Carbon Paper with Long-Term Oxygen Evolution Performance. Chem.—An Asian J. 2017, 12, 543–551. [Google Scholar] [CrossRef]
- Jiang, Y.; Fu, T.; Liu, J.; Zhao, J.; Li, B.; Chen, Z. Molten Salt Synthesis of Carbon-Supported Pt–Rare Earth Metal Nanoalloy Catalysts for Oxygen Reduction Reaction. RSC Adv. 2022, 12, 4805–4812. [Google Scholar] [CrossRef]
- Ratke, L.; Gurikov, P. Specific Surface Area. In The Chemistry and Physics of Aerogels; Gurikov, P., Ratkd, L., Eds.; Cambridge University Press: Cambridge, UK, 2021; pp. 236–267. [Google Scholar]
- Soo, J.Z.; Riaz, A.; Zhang, D.; Jagadish, C.; Tan, H.H.; Karuturi, S. Enhancing the Hydrogen Evolution Reaction Performance of Solution-Corroded NiMo via Plasma Modification. Chem. Mater. 2024, 36, 4164–4173. [Google Scholar] [CrossRef]
- Zhang, T.; Debow, S.; Song, F.; Qian, Y.; Creasy, W.R.; DeLacy, B.G.; Rao, Y. Interface Catalysis of Nickel Molybdenum (NiMo) Alloys on Two-Dimensional (2D) MXene for Enhanced Hydrogen Electrochemistry. J. Phys. Chem. Lett. 2021, 12, 11361–11370. [Google Scholar] [CrossRef]
- Yang, C.; Zhou, L.; Yan, T.; Bian, Y.; Hu, Y.; Wang, C.; Zhang, Y.; Shi, Y.; Wang, D.; Zhen, Y.; et al. Synergistic Mechanism of Ni(OH)2/NiMoS Heterostructure Electrocatalyst with Crystalline/Amorphous Interfaces for Efficient Hydrogen Evolution over All PH Ranges. J. Colloid Interface Sci. 2022, 606, 1004–1013. [Google Scholar] [CrossRef]
- Chen, Q.; Cao, Z.; Du, G.; Kuang, Q.; Huang, J.; Xie, Z.; Zheng, L. Excavated Octahedral Pt-Co Alloy Nanocrystals Built with Ultrathin Nanosheets as Superior Multifunctional Electrocatalysts for Energy Conversion Applications. Nano Energy 2017, 39, 582–589. [Google Scholar] [CrossRef]
- Wu, W.; Tang, Z.; Wang, K.; Liu, Z.; Li, L.; Chen, S. Peptide Templated AuPt Alloyed Nanoparticles as Highly Efficient Bi-Functional Electrocatalysts for Both Oxygen Reduction Reaction and Hydrogen Evolution Reaction. Electrochim. Acta 2018, 260, 168–176. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, L.; Xu, G.-L.; Zhu, S.; Wang, Q.; Gu, M.; Zhang, X.; Sun, C.; Balbuena, P.B.; Amine, K.; et al. The Role of Ru in Improving the Activity of Pd toward Hydrogen Evolution and Oxidation Reactions in Alkaline Solutions. ACS Catal. 2019, 9, 9614–9621. [Google Scholar] [CrossRef]
- Mosallaei, H.; Hadadzadeh, H.; Ensafi, A.A.; Mousaabadi, K.Z.; Weil, M.; Foelske, A.; Sauer, M. Evaluation of HER and OER Electrocatalytic Activity over RuO2–Fe2O3 Nanocomposite Deposited on HrGO Nanosheets. Int. J. Hydrogen Energy 2023, 48, 1813–1830. [Google Scholar] [CrossRef]
- Du, Z.; Wang, Y.; Li, J.; Liu, J. Facile Fabrication of Pt–Ni Alloy Nanoparticles Supported on Reduced Graphene Oxide as Excellent Electrocatalysts for Hydrogen Evolution Reaction in Alkaline Environment. J. Nanoparticle Res. 2019, 21, 13. [Google Scholar] [CrossRef]
- de la Cruz-Cruz, J.J.; Domínguez-Crespo, M.A.; Ramírez-Meneses, E.; Torres-Huerta, A.M.; Brachetti-Sibaja, S.B.; Rodríguez-Salazar, A.E.; Pastor, E.; González-Sánchez, L.E. Data Supporting the in Situ Synthesis by Organometallic Method of Vulcan Supported PdNi Nanostructures for Hydrogen Evolution Reaction in Alkaline Solution. Data Br. 2022, 42, 108256. [Google Scholar] [CrossRef]





| Notation | Composition | Notation | Composition |
|---|---|---|---|
| Mo-CK | Mo on CK | 1% Pt-CK | 1 wt% Pt on CK |
| Ni-CK | Ni on CK | 3% Pt-CK | 3 wt% Pt on CK |
| Ni10Mo90-CK | Ni/Mo = 10:90 on CK | 5% Pt-CK | 5 wt% Pt on CK |
| Ni30Mo70-CK | Ni/Mo = 30:70 on CK | 1% Pt/Ni90Mo10-CK | 1 wt% Pt on Ni90Mo10-CK |
| Ni50Mo50-CK | Ni/Mo = 50:50 on CK | 3% Pt/Ni90Mo10-CK | 3 wt% Pt on Ni90Mo10-CK |
| Ni70Mo30-CK | Ni/Mo = 70:30 on CK | 5% Pt/Ni90Mo10-CK | 5 wt% Pt on Ni90Mo10-CK |
| Ni80Mo20-CK | Ni/Mo = 80:20 on CK | 20% Pt-C | commercial 20 wt% Pt on carbon |
| Ni85Mo15-CK | Ni/Mo = 85:15 on CK | ||
| Ni90Mo10-CK | Ni/Mo = 90:10 on CK | ||
| Ni95Mo5-CK | Ni/Mo = 95:5 on CK |
| Material | Qβ-NiOOH (mC) | LNi (mg) | ECSANi (cm2) | ECSANi (cm2 g−1) |
|---|---|---|---|---|
| Ni10Mo90-CK | 0.33 | 0.02 | 0.80 | 3.73 |
| Ni30Mo70-CK | 1.31 | 0.06 | 3.13 | 4.84 |
| Ni50Mo50-CK | 1.92 | 0.10 | 4.58 | 4.25 |
| Ni70Mo30-CK | 3.36 | 0.15 | 8.00 | 5.30 |
| Ni80Mo20-CK | 3.42 | 0.17 | 8.16 | 4.73 |
| Ni85Mo15-CK | 3.56 | 0.18 | 8.47 | 4.62 |
| Ni90Mo10-CK | 4.40 | 0.19 | 10.47 | 5.39 |
| Ni95Mo5-CK | 4.76 | 0.20 | 11.33 | 5.53 |
| Ni-CK | 1.48 | 0.21 | 3.54 | 1.64 |
| Material | QCO (mC) | LPt (mg) | ECSAPt (cm2) | ECSAPt (cm2 g−1) |
|---|---|---|---|---|
| 1% Pt-CK | 0.180 | 0.002 | 0.428 | 19.877 |
| 3% Pt-CK | 0.200 | 0.006 | 0.476 | 7.362 |
| 5% Pt-CK | 0.234 | 0.010 | 0.557 | 5.174 |
| 1% Pt/Ni90Mo10-CK | 0.700 | 0.002 | 1.670 | 77.470 |
| 3% Pt/Ni90Mo10-CK | 1.010 | 0.006 | 2.420 | 37.480 |
| 5% Pt/Ni90Mo10-CK | 1.770 | 0.010 | 4.230 | 39.290 |
| 20% Pt/C | 11.400 | 0.041 | 27.142 | 66.202 |
| Material | Tafel Slope (mV dec−1) | Jo (mA cm−2) |
|---|---|---|
| Ni90Mo10-CK | - | - |
| 1% Pt/Ni90Mo10-CK | 40.00 | 0.92 |
| 3% Pt/Ni90Mo10-CK | 32.90 | 1.03 |
| 5% Pt/Ni90Mo10-CK | 38.84 | 1.04 |
| 20% Pt-C | 34.80 | 1.04 |
| Material | Surface Area (m2 g−1) | Total Pore Volume (cm3 g−1) | Average Pore Size (nm) |
|---|---|---|---|
| CK | 759.52 | 1.14 | 6.03 |
| Ni-CK | 297.73 | 0.42 | 5.64 |
| Mo-CK | 6.25 | 0.01 | 7.62 |
| Ni90Mo10-CK | 51.99 | 0.09 | 7.56 |
| 3% Pt/Ni90Mo10-CK | 124.69 | 0.25 | 8.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabanillas-Esparza, L.F.; Reynoso-Soto, E.A.; Trujillo-Navarrete, B.; Alcántar-Vázquez, B.; Silva-Carrillo, C.; Félix-Navarro, R.M. Boosting Dual Hydrogen Electrocatalysis with Pt/NiMo Catalysts: Tuning the Ni/Mo Ratio and Minimizing Pt Usage. Catalysts 2025, 15, 633. https://doi.org/10.3390/catal15070633
Cabanillas-Esparza LF, Reynoso-Soto EA, Trujillo-Navarrete B, Alcántar-Vázquez B, Silva-Carrillo C, Félix-Navarro RM. Boosting Dual Hydrogen Electrocatalysis with Pt/NiMo Catalysts: Tuning the Ni/Mo Ratio and Minimizing Pt Usage. Catalysts. 2025; 15(7):633. https://doi.org/10.3390/catal15070633
Chicago/Turabian StyleCabanillas-Esparza, Luis Fernando, Edgar Alonso Reynoso-Soto, Balter Trujillo-Navarrete, Brenda Alcántar-Vázquez, Carolina Silva-Carrillo, and Rosa María Félix-Navarro. 2025. "Boosting Dual Hydrogen Electrocatalysis with Pt/NiMo Catalysts: Tuning the Ni/Mo Ratio and Minimizing Pt Usage" Catalysts 15, no. 7: 633. https://doi.org/10.3390/catal15070633
APA StyleCabanillas-Esparza, L. F., Reynoso-Soto, E. A., Trujillo-Navarrete, B., Alcántar-Vázquez, B., Silva-Carrillo, C., & Félix-Navarro, R. M. (2025). Boosting Dual Hydrogen Electrocatalysis with Pt/NiMo Catalysts: Tuning the Ni/Mo Ratio and Minimizing Pt Usage. Catalysts, 15(7), 633. https://doi.org/10.3390/catal15070633








