Oxidation of 4-Methylpyridine on Vanadium-Based Catalysts Modified with Titanium and Manganese
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Characterization of Catalysts
2.2. Surface Morphology and Textural Properties
2.3. Analysis of Bonding and Oxide Phases
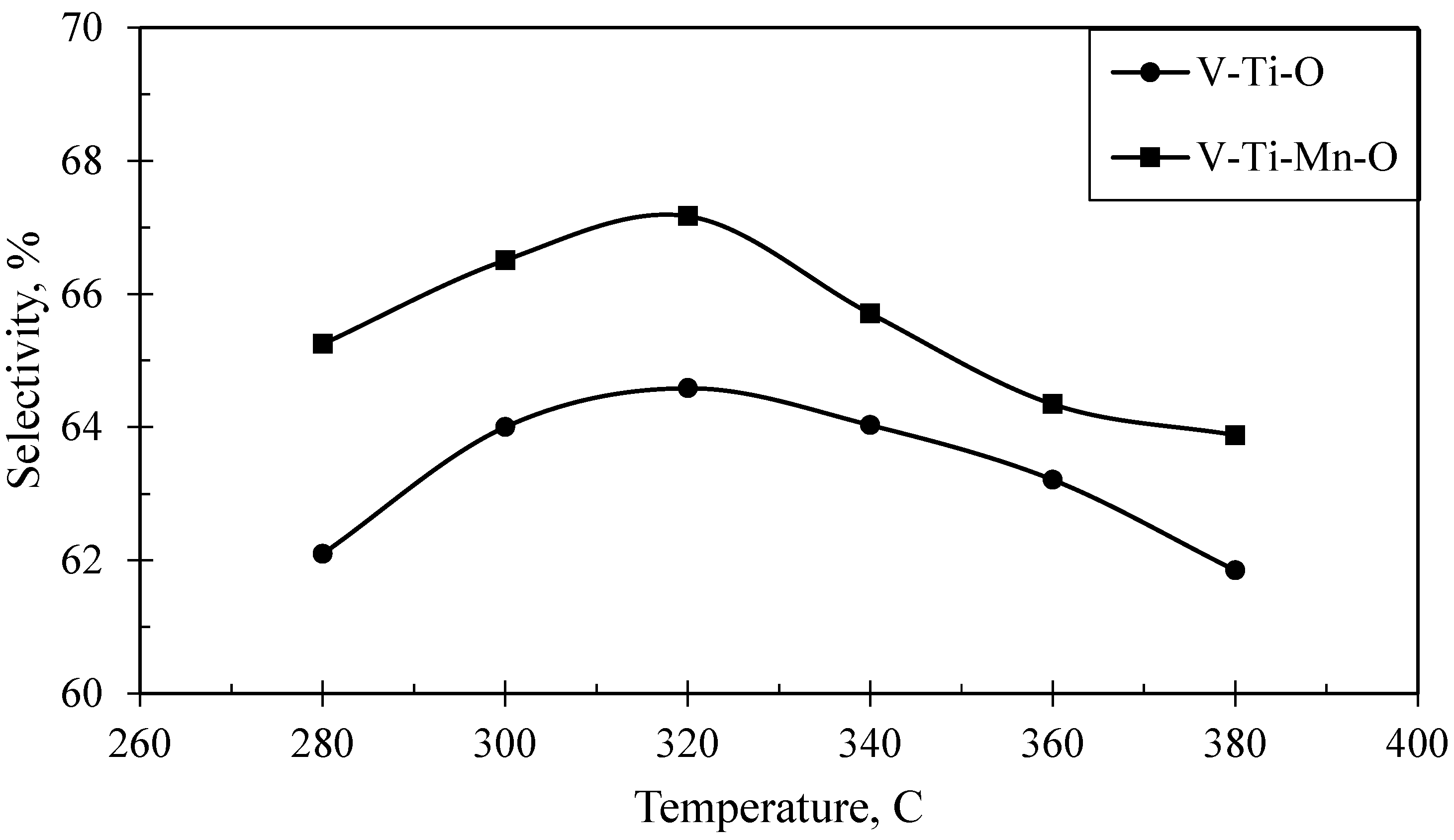
2.4. Catalytic Performance
3. Materials and Methods
3.1. Materials
3.2. Catalyst Synthesis
3.3. Experimental Procedure
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| INA | Isonicotinic Acid |
| CA | Catalytic Activity |
| XRD | X-ray Diffraction |
| SEM | Scanning Electron Microscopy |
| 4-MP | 4-methylpyridine |
| EG | Ethylene Glycol |
| CAc | Citric Acid |
References
- Isoniazid. In Dictionary of Toxicology; Springer: Singapore, 2024; p. 530. [CrossRef]
- Everett, H.M.; Barker, R.S. Process for Preparing Isonicotinic Acid. U.S. Patent Application No. US2748137A, 29 May 1956. Available online: https://patents.google.com/patent/US2748137A/en (accessed on 4 April 2023).
- Suvorov, B.V.; Bukeykhanov, N.R. Oxidation Reactions in Organic Synthesis. In Khimiya; Chemistry: Moscow, Russia, 1978; Volume 200. [Google Scholar]
- Chuk, R. Technology development in nicotinate production. Appl. Cat. A Gen. 2005, 280, 75–82. [Google Scholar] [CrossRef]
- Vorobyev, V.B.; Mikhaylovskaya, T.P.; Yugai, O.K.; Serebryanskaya, A.P.; Kurmakyzy, R. Vapour-phase oxidation of 3- and 4-methylpyridines on vanadium oxide catalysts modified by titanium and chromium oxides. Chem. J. Kaz. 2019, 1, 113–124. [Google Scholar]
- Sembaev, D.K.; Vorobyov, P.B.; Kurmakizy, R.; Nikitina, A.I.; Serebryanskaya, A.P. Catalytic oxidation of 3- and 4-methylpyridines. In Proceedings of the International Scientific Conference “Chemistry and Applications of Natural and Synthetic Biologically Active Compounds”, Almaty, Kazakhstan, 16–17 October 2004; pp. 215–217. [Google Scholar]
- Vorobyev, P.; Mikhailovskaya, T.; Yugay, O.; Serebryanskaya, A.; Chukhno, N.; Imangazy, A. Catalytic oxidation of 4-methylpyridine on modified vanadium oxide catalysts. Iran. J. Chem. Chem. Eng. 2018, 37, 81. Available online: https://www.researchgate.net/publication/330874472_Catalytic_Oxidation_of_4-Methylpyridine_on_Modified_Vanadium_Oxide_Catalysts (accessed on 4 April 2023).
- Vorobyev, P.B.; Gabdrakipov, V.Z.; Mikhaylovskaya, T.P.; Sembaev, D.K. Reactivity of isomeric picolines under oxidative ammonolysis on a vanadium oxide catalyst. Rus. J. Gen. Chem. 2001, 71, 650–652. [Google Scholar] [CrossRef]
- Langeslay, R.R.; Kaphan, D.M.; Marshall, C.L.; Stair, P.C.; Sattelberger, A.P.; Delferro, M. Catalytic Applications of Vanadium: A Mechanistic Perspective. Chem. Rev. 2018, 119, 2128–2191. [Google Scholar] [CrossRef]
- Védrine, J.C. Gas phase heterogeneous partial oxidation reactions. In Metal Oxides in Heterogeneous Catalysis; Elsevier: Amsterdam, The Netherlands, 2018; pp. 211–286. [Google Scholar] [CrossRef]
- Guerrero-Pérez, M.O. V-Containing Mixed Oxide Catalysts for Reduction–Oxidation-Based Reactions with Environmental Applications: A Short Review. Cat. 2018, 8, 564. [Google Scholar] [CrossRef]
- Wang, N.L.; Qiu, J.E.; Wu, J.; You, K.Y.; Luo, H.A. A Comparison of the Redox Properties of Bulk Vanadium Mixed Oxide Catalysts. Cat. Lett. 2015, 145, 1792–1797. [Google Scholar] [CrossRef]
- Benavente, E.; Aliaga, J.; González, G. Vanadium Oxides in Photocatalysis, Including Bare Oxides and VOx-based Organic–Inorganic Nanocomposites. In Vanadium Catalysis; Royal Society of Chemistry: Cambridge, UK, 2020; pp. 340–373. [Google Scholar] [CrossRef]
- Monfort, O.; Petrisková, P. Binary and Ternary Vanadium Oxides: General Overview, Physical Properties, and Photochemical Processes for Environmental Applications. Processes 2021, 9, 214. [Google Scholar] [CrossRef]
- Wachs, I.E. Catalysis Science of Supported Vanadium Oxide Catalysts. Dalton Trans. 2013, 42, 11145–11160. [Google Scholar] [CrossRef]
- Hoelderich, W. Verfahren Zur Herstellung von Isonikotinsaeure. Germany Patent Application No. DE10146088A1, 19 September 2001. Available online: https://patents.google.com/patent/DE10146088A1/de (accessed on 13 March 2023).
- Yugay, O.; Mikhailovskaya, T.; Sembaev, D.; Vorobyev, P. Oxidation of 3- and 4-methylpyridines on vanadia-anatase and vanadia-rutile catalysts. Eur. Chem. Tech. 2012, 1, 337–342. [Google Scholar] [CrossRef]
- Vorobyev, P.B.; Saurambayeva, L.I.; Mikhailovskaya, T.P.; Yugay, O.K.; Serebryanskaya, A.P.; Shlygina, I.A. Vapor-phase oxidation of β-picoline to nicotinic acid over V2O5 and modified vanadium oxide catalysts. J. Appl. Chem. 2014, 87, 894–901. [Google Scholar]
- Dimesso, L. Pechini Processes: An Alternate Approach of the Sol–Gel Method, Preparation, Properties, and Applications. In Handbook of Sol-Gel Science and Technology; Springer: Cham, Switzerland, 2016; pp. 1–22. [Google Scholar] [CrossRef]
- Sunde, T.O.L.; Grande, T.; Aksnes, M.A. Modified Pechini Synthesis of Oxide Powders and Thin Films. In Handbook of Sol-Gel Science and Technology; Springer: Cham, Switzerland, 2016; pp. 1–30. [Google Scholar] [CrossRef]
- Hajizadeh-Oghaz, M.H.; Razavi, S.R.; Estarki, L.M. Estarki, Large-scale synthesis of YSZ nanopowder by Pechini method. Bull. Mat. Sci. 2014, 37, 969–973. [Google Scholar] [CrossRef]
- Gawande, M.B.; Pandey, R.K.; Jayaram, R.V. Role of Mixed Metal Oxides in Catalysis Science: Versatile Applications in Organic Synthesis. Cat. Sci. Tech. 2012, 2, 1113–1125. [Google Scholar] [CrossRef]
- Reddy, C.V.; Reddy, I.N.; Koutavarapu, R.; Reddy, K.R.; Saleh, T.A.; Aminabhavi, T.M.; Shim, J. Novel edge-capped ZrO2 nanoparticles onto V2O5 nanowires for efficient photosensitized reduction of chromium (Cr (VI)), photoelectrochemical solar water splitting, and electrochemical energy storage applications. Chem. Eng. J. 2022, 430, 132988. [Google Scholar] [CrossRef]
- Chen, S.; Xiong, F.; Huang, W. Surface Chemistry and Catalysis of Oxide Model Catalysts from Single Crystals to Nanocrystals. Surf. Sci. Rep. 2019, 74, 100471. [Google Scholar] [CrossRef]
- Inumaru, K.; Misono, M.; Okuhara, T. Structure and Catalysis of Vanadium Oxide Overlayers on Oxide Supports. Appl. Cat. A Gen. 1997, 149, 133–149. [Google Scholar] [CrossRef]
- Zhao, L.; Lin, S.; Bi, K.; Liang, C.; Du, Y.; Liu, J.; Yang, H.; Fan, D.; Wang, Y.; Lei, M. Manganese vanadium oxide hollow microspheres: A novel electrocatalyst for oxygen reduction reaction. J. Solid State Electrochem. 2017, 21, 1743–1749. [Google Scholar] [CrossRef]
- Sandhya, G.L.; Dhananjaya, M.; Prakash, N.G.; Lakshminarayana, A.; Rosaiah, P.; Hussain, O.M. Structural and Supercapacitive Performance of V2O5 Thin Films Prepared By DC Magnetron Sputtering. IOSR J. Appl. Chem. 2017, 10, 64–69. [Google Scholar] [CrossRef]
- Post, J.E.; McKeown, D.A.; Heaney, P.J. Raman spectroscopy study of manganese oxides: Layer structures. Am. Mineral. 2021, 106, 351–366. [Google Scholar] [CrossRef]
- Nuguid, R.J.G.; Ferri, D.; Marberger, A.; Nachtegaal, M.; Kröcher, O. Modulated-Excitation Raman Spectroscopy of V2O5/TiO2: Mechanistic Insights into the Selective Catalytic Reduction of NO by NH3. ACS Cat. 2019, 9, 6814–6820. [Google Scholar] [CrossRef]
- Gupta, S.K.; Desai, R.; Jha, P.K.; Sahoo, S.; Kirin, D. Titanium dioxide synthesized using titanium chloride: Size effect study using Raman spectroscopy and photoluminescence. J. Raman Spectrosc. 2009, 40, 1401–1407. [Google Scholar] [CrossRef]
- Carvalho Costa, R.C.; de Sá Oliveira, F.M.; Ferreira, A.P.; Cappa de Oliveira, L.F. Raman microscopy as a tool for the surface characterization of V2O5/TiO2 catalysts before and after reaction with H2O2. Quarks Braz. Electron. J. Phys. Chem. Mater. Sci. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Understanding Polymorphic Phase Transformation Behavior during Growth of Nanocrystalline Aggregates: Insights from TiO2. J. Phys. Chem. B. 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Holmgren Rondahl, S.; Pointurier, F.; Ahlinder, L.; Ramebäck, H.; Marie, O.; Ravat, B.; Delaunay, F.; Young, E.; Blagojevic, N.; Hester, J.R.; et al. Comparing results of X-ray diffraction, µ-Raman spectroscopy and neutron diffraction when identifying chemical phases in seized nuclear material, during a comparative nuclear forensics exercise. J. Radioanal. Nucl. Chem. 2018, 315, 395–408. [Google Scholar] [CrossRef]
- Li, G.; Richter, C.P.; Milot, R.L.; Cai, L.; Schmuttenmaer, C.A.; Crabtree, R.H.; Brudvig, G.W.; Batista, V.S. Synergistic effect between anatase and rutile TiO2 nanoparticles in dye-sensitized solar cells. Dalton Trans. 2009, 45, 10078–10085. [Google Scholar] [CrossRef]
- Gargasya, Y.; Gish, M.K.; Nair, V.V.; Johnson, J.C.; Law, M. Evaluation of nanostructured β-Mn2V2O₇ thin films as photoanodes for photoelectrochemical water oxidation. Chem. Mater. 2021, 33, 7743–7754. [Google Scholar] [CrossRef]
- Risch, M.; Stoerzinger, K.A.; Han, B.; Regier, T.Z.; Peak, D.; Sayed, S.Y.; Wei, C.; Xu, Z.; Shao-Horn, Y. Redox processes of manganese oxide in catalyzing oxygen evolution and reduction: An in situ soft X-ray absorption spectroscopy study. J. Phys. Chem. C. 2017, 121, 17682–17692. [Google Scholar] [CrossRef]
- Yan, Y.; Mao, L.; Zhao, X.; Xiao, Y.; Dong, G. A relationship between the V4+/V5+ ratio and the surface dispersion, surface acidity, and redox performance of V2O5–WO3/TiO2 SCR catalysts. RSC Adv. 2018, 8, 31081. [Google Scholar] [CrossRef]
- Koust, S.; Reinecke, B.N.; Adamsen, K.C.; Beinik, I.; Handrup, K.; Li, Z.; Moses, P.G.; Schnadt, J.; Lauritsen, J.V.; Wendt, S. Coverage-dependent oxidation and reduction of vanadium supported on anatase TiO2(101). J. Catal. 2018, 360, 118–126. [Google Scholar] [CrossRef]
- Chan, Z.M.; Kitchaev, D.A.; Weker, J.N.; Schnedermann, C.; Lim, K.; Ceder, G.; Tumas, W.; Toney, M.F.; Nocera, D.G. Electrochemical trapping of metastable Mn3+ ions for activation of MnO2 oxygen evolution catalysts. Proc. Natl. Acad. Sci. USA 2018, 115, E5261–E5268. [Google Scholar] [CrossRef]
- Han, S.J.; Ameen, M.; Hanifah, M.F.R.; Aqsha, A.; Bilad, M.R.; Jaafar, J.; Kheawhom, S. Catalytic evaluation of nanoflower structured manganese oxide electrocatalyst for oxygen reduction in alkaline media. Catalysts. 2020, 10, 822. [Google Scholar] [CrossRef]
- Buzayev, N.A.; Kadirbekov, K.A.; Tolemisova, D.K.; Basbayeva, G.S. Oxidation of 4-methylpyridine on V-Cr-O catalyst. Chem. J. Kaz. 2024, 3, 114–123. [Google Scholar] [CrossRef]
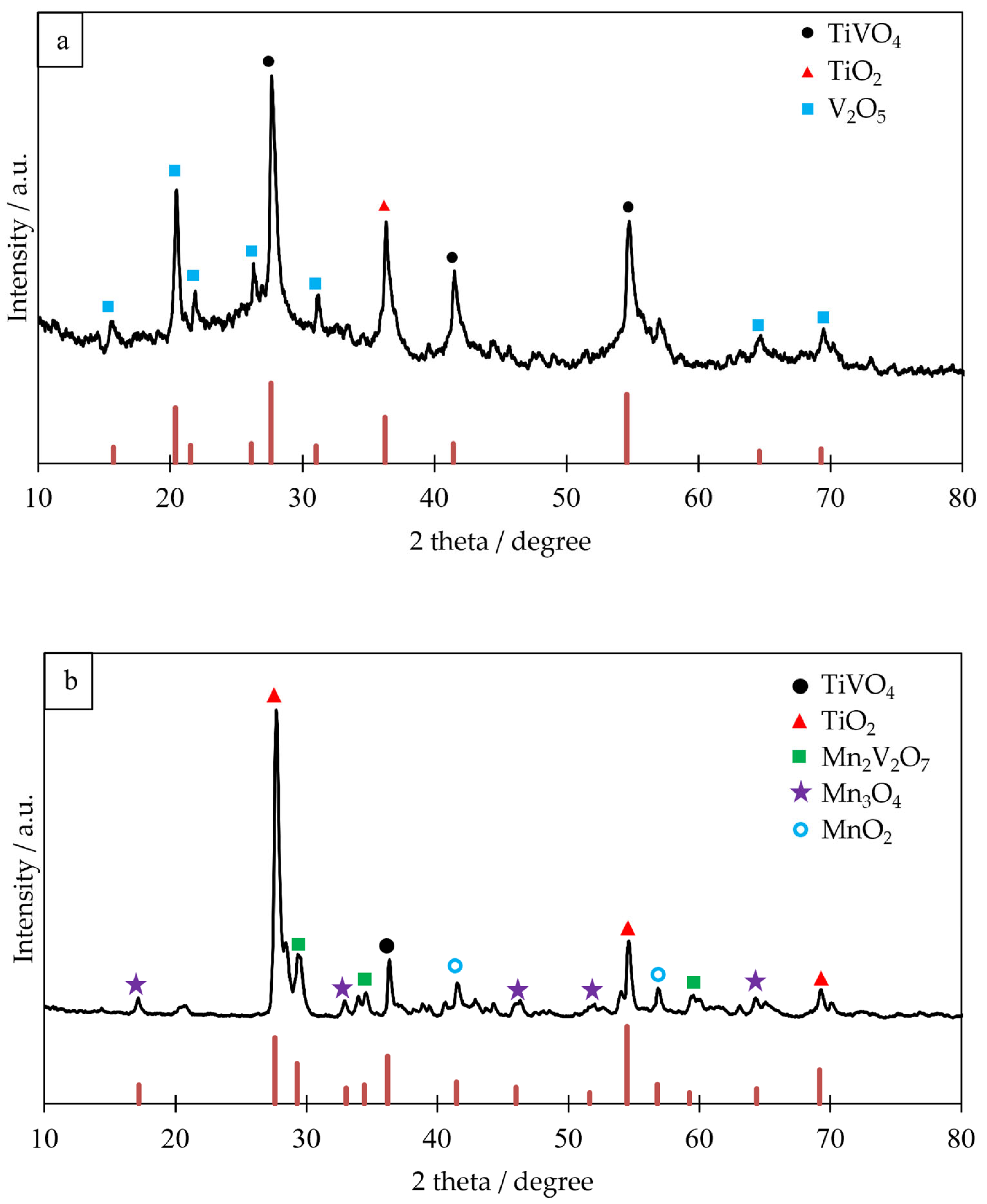
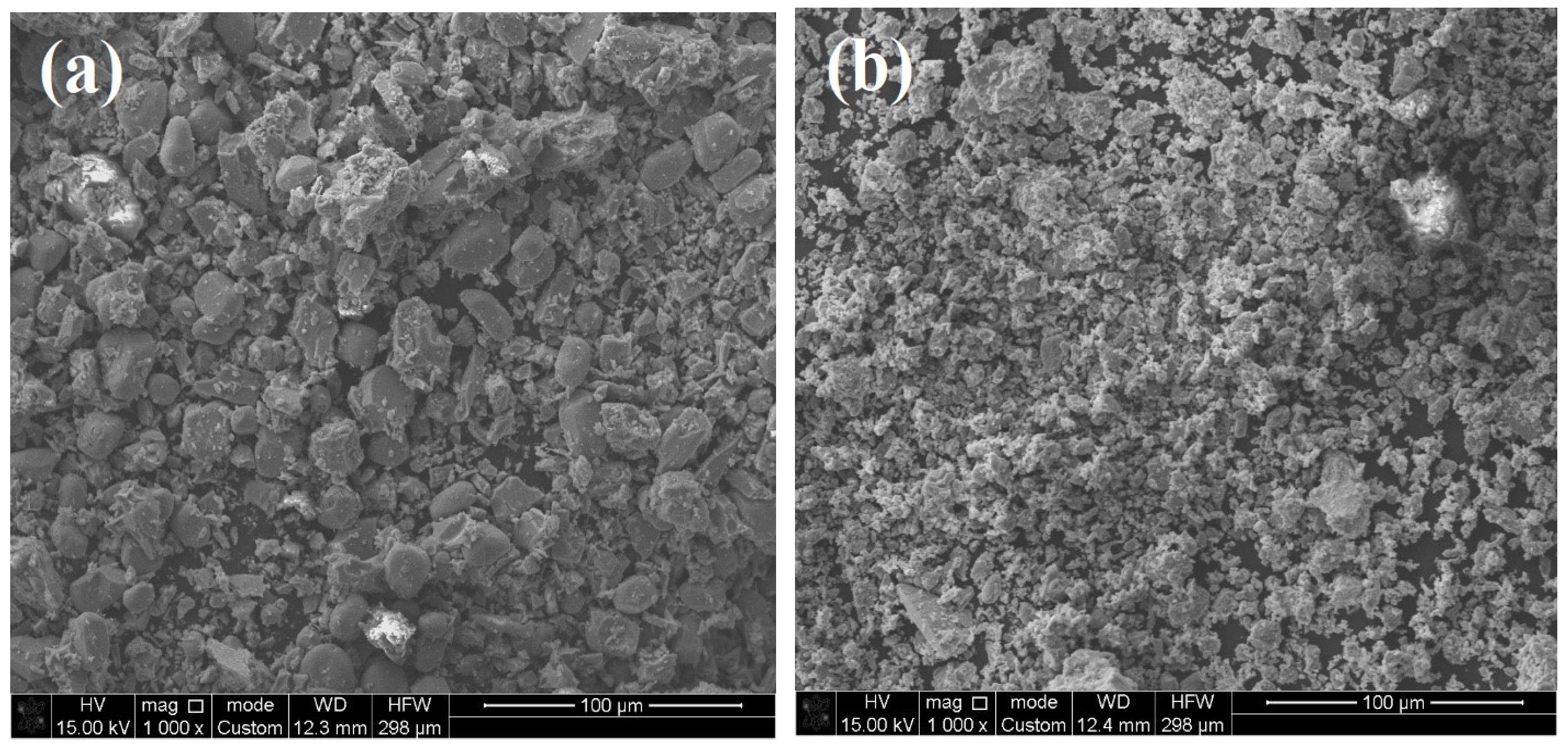
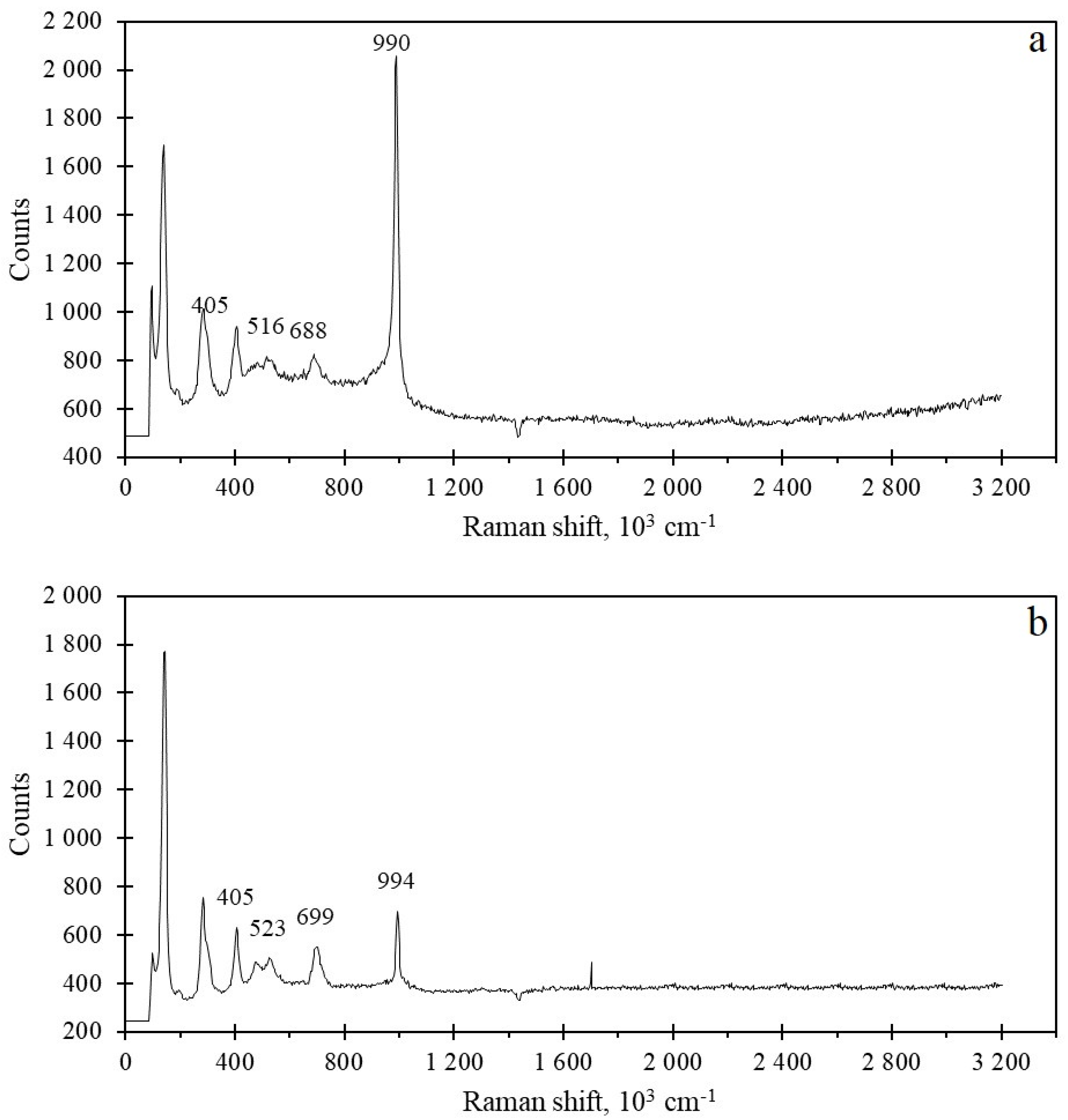
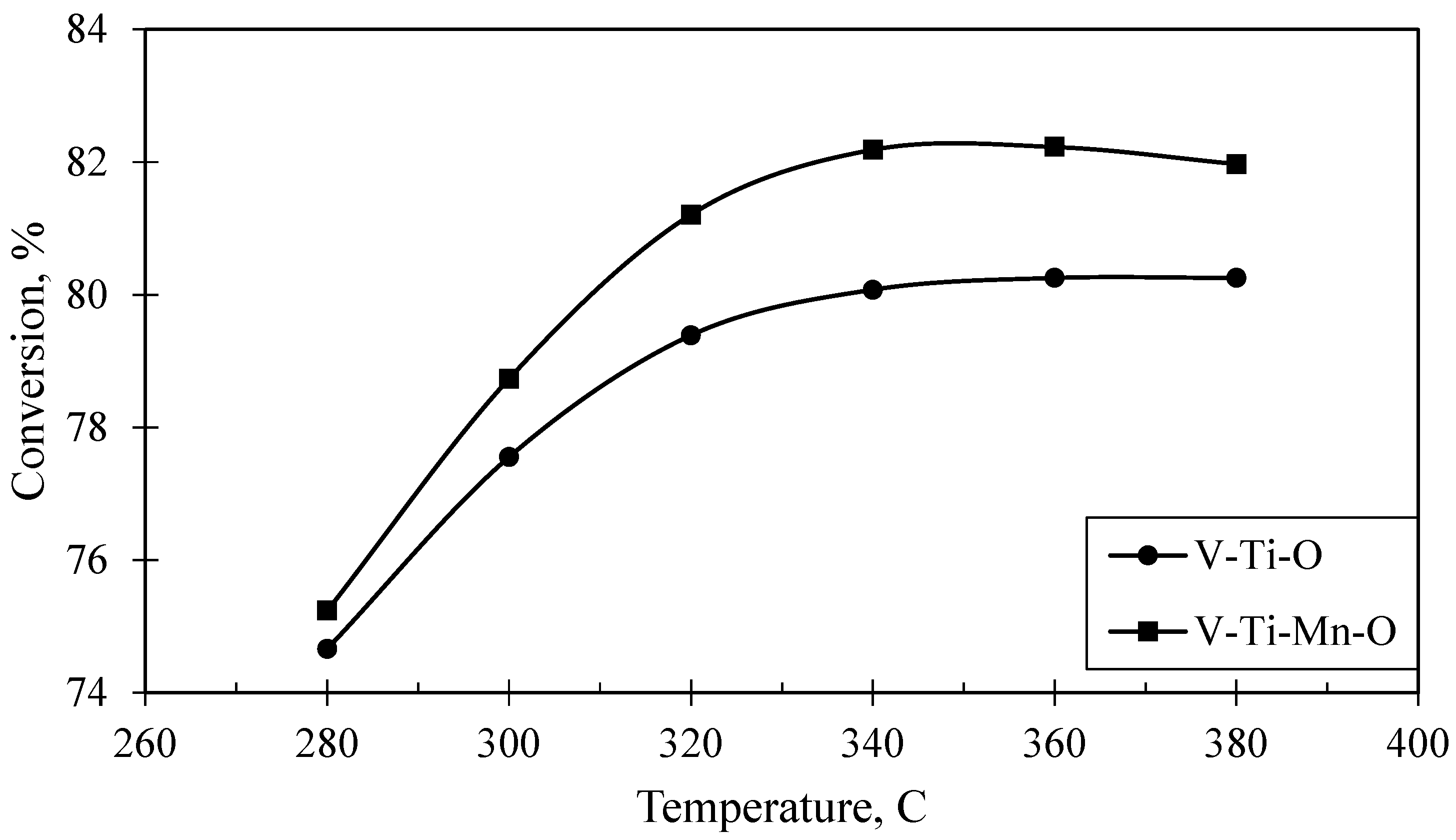
| Catalysts | V-Ti-O (Fresh) | V-Ti-O (Used) | V-Ti-Mn-O (Fresh) | V-Ti-Mn-O (Used) | |
|---|---|---|---|---|---|
| Parameter | |||||
| BET surface area, m2/g | 2.05 | 1.82 | 44.60 | 40.72 | |
| Temperature, °C | Conversion, % | INA Selectivity, % | Oxidation Products | ||
|---|---|---|---|---|---|
| Pyridine | Pyridine-4-Carboxaldehyde | Others | |||
| (a) | |||||
| 280 | 74.66 | 62.10 | 12.07 | 5.49 | 10.74 |
| 300 | 77.55 | 64.00 | 12.37 | 5.64 | 9.91 |
| 320 | 79.39 | 64.59 | 12.69 | 5.40 | 10.01 |
| 340 | 80.08 | 64.03 | 13.04 | 5.94 | 9.83 |
| 360 | 80.26 | 63.21 | 13.79 | 6.28 | 9.46 |
| 380 | 80.26 | 61.85 | 13.95 | 6.28 | 10.39 |
| (b) | |||||
| 280 | 75.24 | 65.25 | 10.65 | 4.76 | 10.74 |
| 300 | 78.74 | 66.51 | 11.64 | 5.26 | 9.47 |
| 320 | 81.21 | 67.17 | 11.95 | 5.40 | 9.32 |
| 340 | 82.19 | 65.71 | 12.11 | 5.52 | 10.56 |
| 360 | 82.23 | 64.34 | 13.63 | 5.86 | 9.83 |
| 380 | 81.97 | 63.88 | 13.82 | 5.89 | 9.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadirbekov, K.; Buzayev, N.; Tussupkaliyev, Y.; Oshakbayev, M. Oxidation of 4-Methylpyridine on Vanadium-Based Catalysts Modified with Titanium and Manganese. Catalysts 2025, 15, 625. https://doi.org/10.3390/catal15070625
Kadirbekov K, Buzayev N, Tussupkaliyev Y, Oshakbayev M. Oxidation of 4-Methylpyridine on Vanadium-Based Catalysts Modified with Titanium and Manganese. Catalysts. 2025; 15(7):625. https://doi.org/10.3390/catal15070625
Chicago/Turabian StyleKadirbekov, Kairat, Nurdaulet Buzayev, Yersin Tussupkaliyev, and Mels Oshakbayev. 2025. "Oxidation of 4-Methylpyridine on Vanadium-Based Catalysts Modified with Titanium and Manganese" Catalysts 15, no. 7: 625. https://doi.org/10.3390/catal15070625
APA StyleKadirbekov, K., Buzayev, N., Tussupkaliyev, Y., & Oshakbayev, M. (2025). Oxidation of 4-Methylpyridine on Vanadium-Based Catalysts Modified with Titanium and Manganese. Catalysts, 15(7), 625. https://doi.org/10.3390/catal15070625







