Efficiency of Microwave-Assisted Surface Grafting of Ni and Zn Clusters on TiO2 as Cocatalysts for Solar Light Degradation of Cyanotoxins
Abstract
1. Introduction
2. Results and Discussion
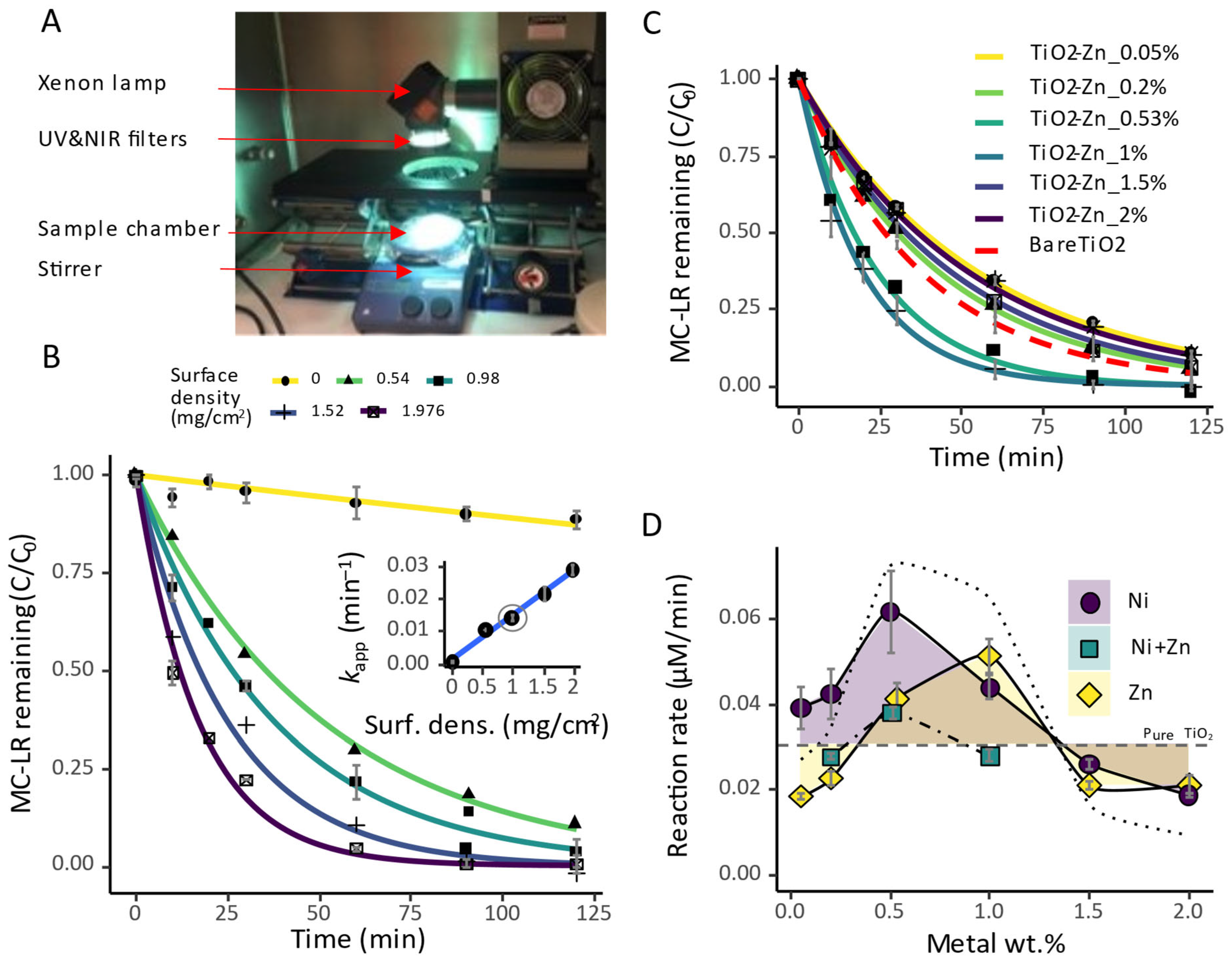
2.1. Photocatalytic Activities
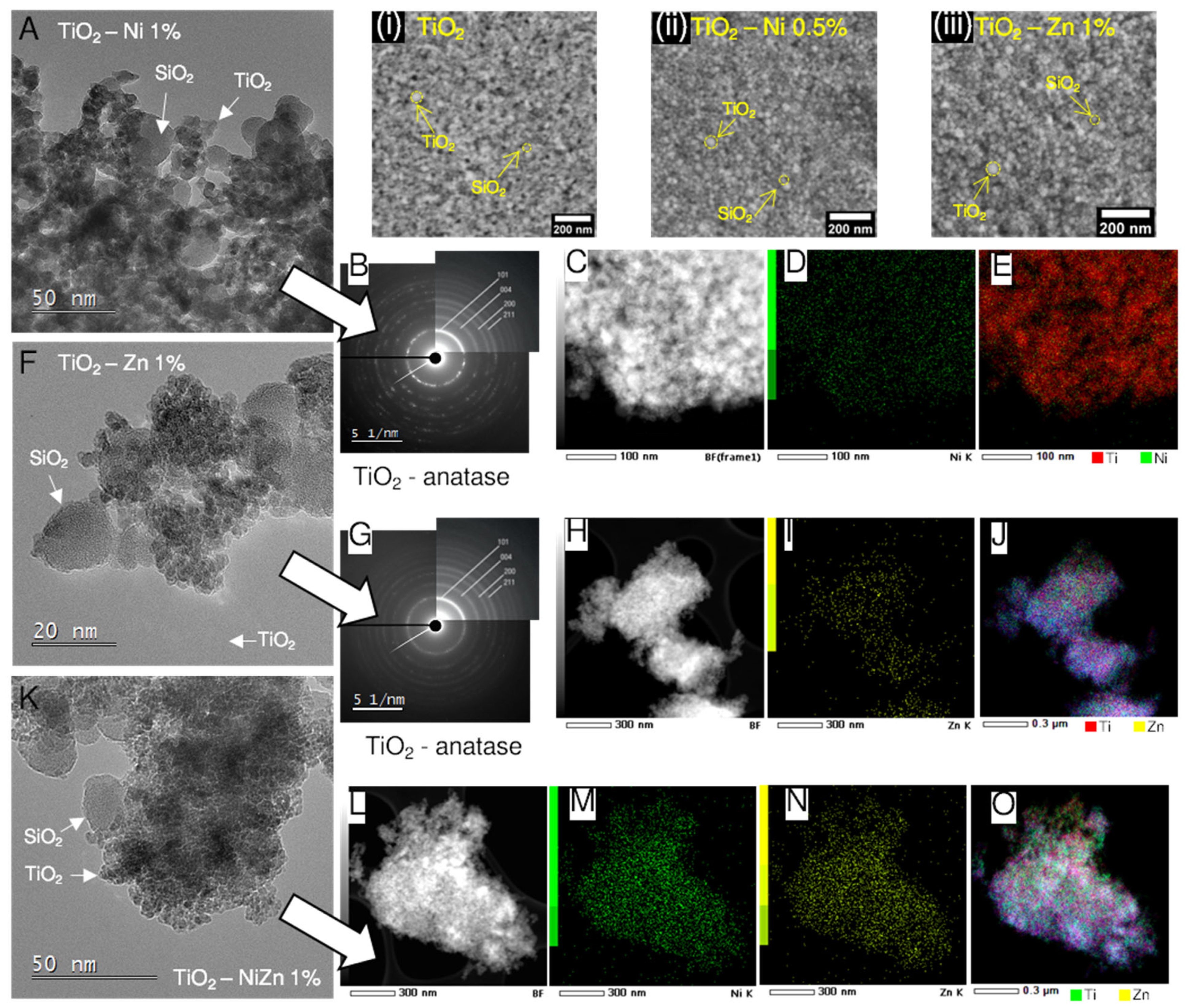
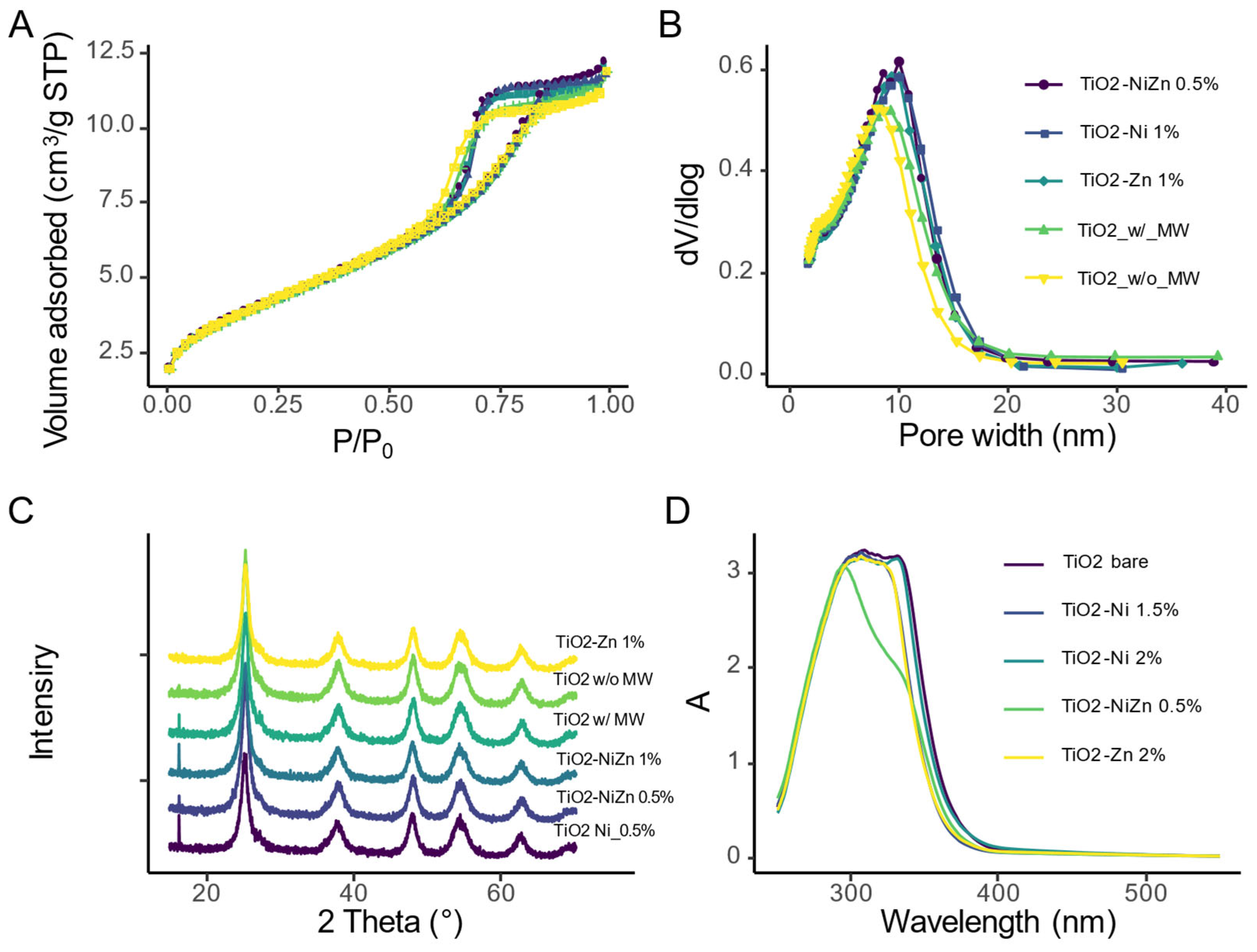
2.2. Morphorogical and Structural Characterization
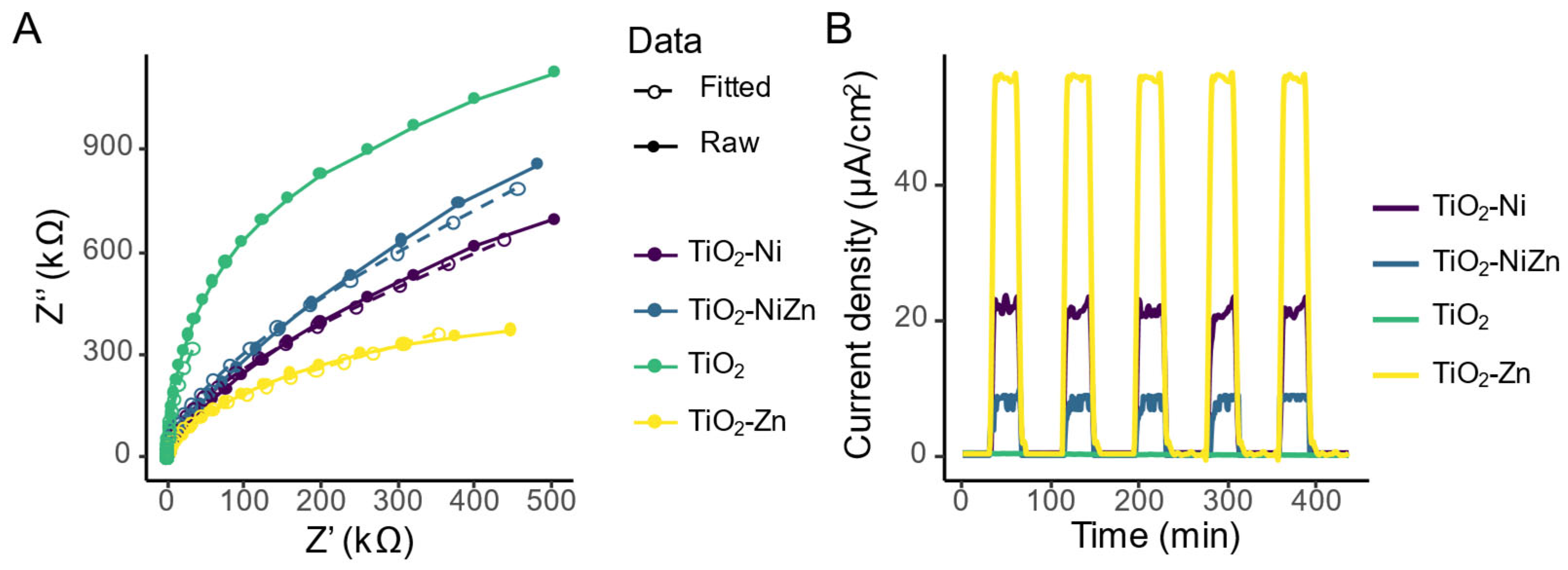
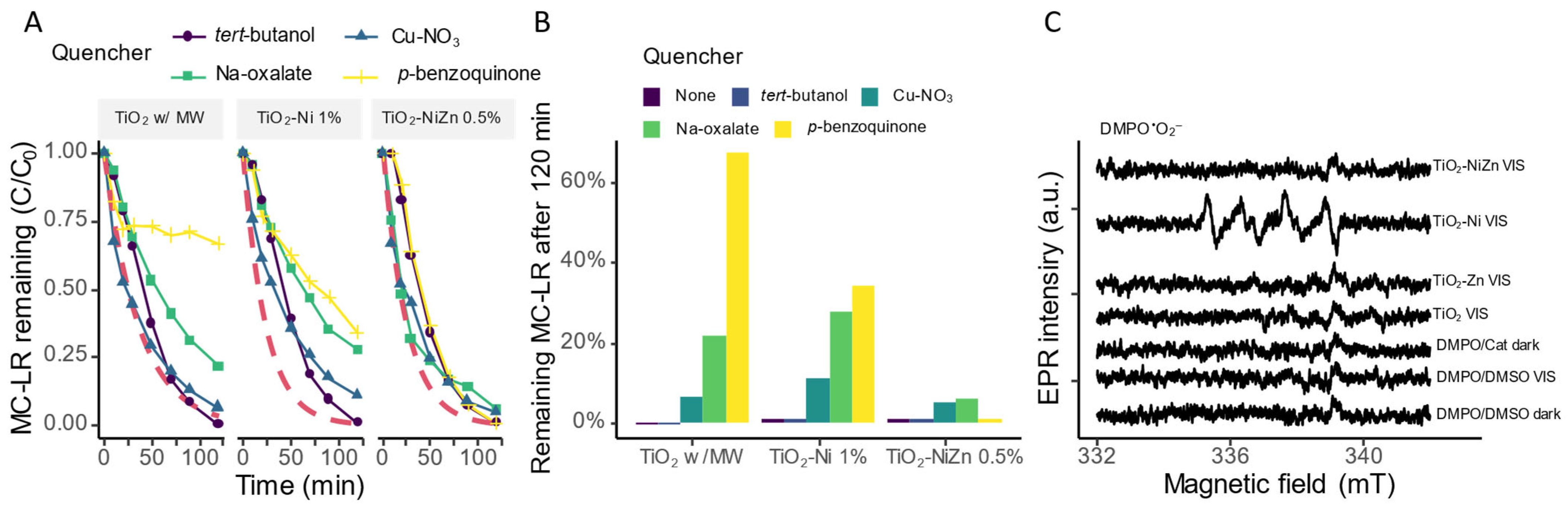
2.3. Study of Active Species
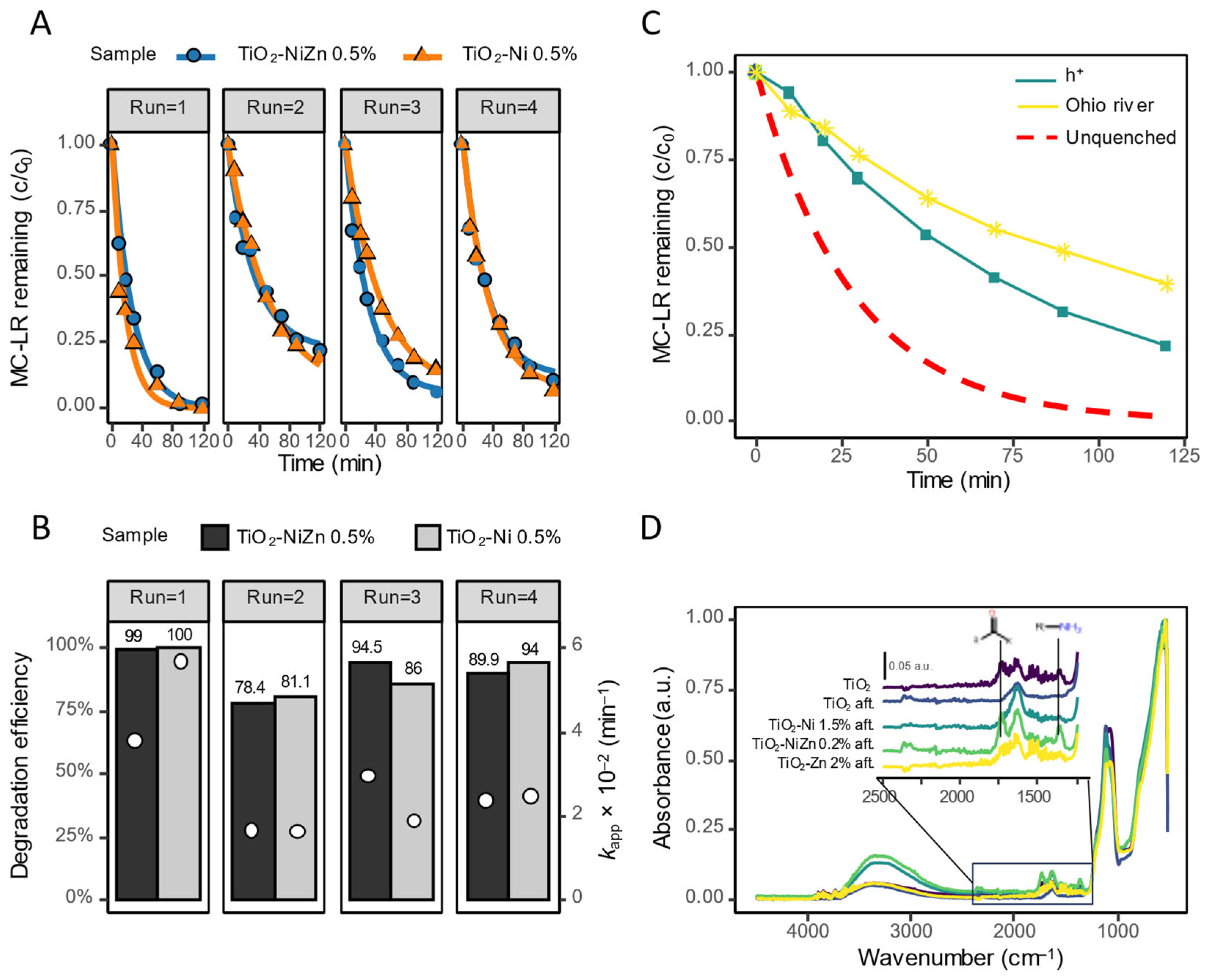
2.4. Recyclability and River Water Test
3. Materials and Methods
3.1. Preparation of Materials
3.2. Characterization
3.3. Photocatalytic Activity Tests
3.4. Analytical Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Guidelines for Drinking Water Quality, 2nd ed.; Health Criteria and Other Supporting Information; WHO: Geneva, Switzerland, 1998. [Google Scholar]
- He, X.; Wang, A.; Wu, P.; Tang, S.; Zhang, Y.; Li, L.; Ding, P. Photocatalytic Degradation of Microcystin-LR by Modified TiO2 Photocatalysis: A Review. Sci. Total Environ. 2020, 743, 140694. [Google Scholar] [CrossRef]
- Wu, S.; Lv, J.; Wang, F.; Duan, N.; Li, Q.; Wang, Z. Photocatalytic Degradation of Microcystin-LR with a Nanostructured Photocatalyst Based on Upconversion nanoparticles@TiO2 Composite under Simulated Solar Lights. Sci. Rep. 2017, 7, 14435. [Google Scholar] [CrossRef]
- Fotiou, T.; Triantis, T.M.; Kaloudis, T.; O’Shea, K.E.; Dionysiou, D.D.; Hiskia, A. Assessment of the Roles of Reactive Oxygen Species in the UV and Visible Light Photocatalytic Degradation of Cyanotoxins and Water Taste and Odor Compounds Using C-TiO2. Water Res. 2016, 90, 52–61. [Google Scholar] [CrossRef]
- Campos, A.; Vasconcelos, V. Molecular Mechanisms of Microcystin Toxicity in Animal Cells. Int. J. Mol. Sci. 2010, 11, 268–287. [Google Scholar] [CrossRef]
- Chintalapati, P.; Mohseni, M. Degradation of Cyanotoxin Microcystin-LR in Synthetic and Natural Waters by Chemical-Free UV/VUV Radiation. J. Hazard. Mater. 2020, 381, 120921. [Google Scholar] [CrossRef]
- Kerketta, U.; Tesler, A.B.; Schmuki, P. Single-Atom Co-Catalysts Employed in Titanium Dioxide Photocatalysis. Catalysts 2022, 12, 1223. [Google Scholar] [CrossRef]
- Zaera, F. Role of Metal Cocatalysts in the Photocatalytic Production of Hydrogen from Water Revisited. Energy Fuels 2025, 39, 2422–2434. [Google Scholar] [CrossRef]
- Šuligoj, A.; Cerc Korošec, R.; Žerjav, G.; Novak Tušar, N.; Lavrenčič Štangar, U. Solar-Driven Photocatalytic Films: Synthesis Approaches, Factors Affecting Environmental Activity, and Characterization Features. Top. Curr. Chem. 2022, 380, 51. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual Cocatalysts in TiO2 Photocatalysis. Adv. Mater. 2019, 31, 1807660. [Google Scholar] [CrossRef]
- Čižmar, T.; Lavrenčič Štangar, U.; Fanetti, M.; Arčon, I. Effects of Different Copper Loadings on the Photocatalytic Activity of TiO2-SiO2 Prepared at a Low Temperature for the Oxidation of Organic Pollutants in Water. ChemCatChem 2018, 10, 2982–2993. [Google Scholar] [CrossRef]
- Pozan, G.S.; Isleyen, M.; Gokcen, S. Transition Metal Coated TiO2 Nanoparticles: Synthesis, Characterization and Their Photocatalytic Activity. Appl. Catal. B Environ. 2013, 140–141, 537–545. [Google Scholar] [CrossRef]
- Toledo-Antonio, J.A.; Piccirillo, C.; Rozman, N.; Pullar, R.C.; Seabra, M.P.; Škapin, A.S.; Castro, P.M.L.; Labrincha, J.A. Effects of Cu, Zn and Cu-Zn Addition on the Microstructure and Antibacterial and Photocatalytic Functional Properties of Cu-Zn Modified TiO2 Nano-Heterostructures. J. Photochem. Photobiol. A Chem. 2016, 330, 44–54. [Google Scholar] [CrossRef]
- Pliekhova, O.; Arčon, I.; Pliekhov, O.; Tušar, N.N.; Štangar, U.L. Cu and Zr Surface Sites in the Photocatalytic Activity of TiO2 Nanoparticles. Environ. Sci. Pollut. Res. 2017, 24, 12571–12581. [Google Scholar] [CrossRef]
- Šuligoj, A.; Arčon, I.; Mazaj, M.; Dražić, G.; Arčon, D.; Cool, P.; Štangar, U.L.; Tušar, N.N. Surface Modified Titanium Dioxide Using Transition Metals: Nickel as a Winning Transition Metal for Solar Light Photocatalysis. J. Mater. Chem. A 2018, 6, 9882–9892. [Google Scholar] [CrossRef]
- Fan, L.; Long, J.; Gu, Q.; Huang, H.; Lin, H.; Wang, X. Single-Site Nickel-Grafted Anatase TiO2 for Hydrogen Production: Toward Understanding the Nature of Visible-Light Photocatalysis. J. Catal. 2014, 320, 147–159. [Google Scholar] [CrossRef]
- Nussbaum, M.; Shaham-Waldmann, N.; Paz, Y. Synergistic Photocatalytic Effect in Fe,Nb-Doped BiOCl. J. Photochem. Photobiol. A Chem. 2014, 290, 11–21. [Google Scholar] [CrossRef]
- Hegde, V.I.; Aykol, M.; Kirklin, S.; Wolverton, C. The Phase Stability Network of All Inorganic Materials. Sci. Adv. 2020, 6, eaay5606. [Google Scholar] [CrossRef]
- Ravbar, M.; Maver, K.; Knaflič, T.; Arčon, I.; Novak Tušar, N.; Lavrenčič Štangar, U.; Šuligoj, A. Nickel-Decorated ZnO Nanoparticles for Effective Solar Reduction of Hexavalent Chromium and Removal of Selected Pharmaceuticals. Appl. Surf. Sci. 2025, 681, 161463. [Google Scholar] [CrossRef]
- Pelaez, M.; Falaras, P.; Likodimos, V.; O’Shea, K.; de la Cruz, A.A.; Dunlop, P.S.M.; Byrne, J.A.; Dionysiou, D.D. Use of Selected Scavengers for the Determination of NF-TiO2 Reactive Oxygen Species during the Degradation of Microcystin-LR under Visible Light Irradiation. J. Mol. Catal. A Chem. 2016, 425, 183–189. [Google Scholar] [CrossRef]
- Baghbanzadeh, M.; Carbone, L.; Cozzoli, P.D.; Kappe, C.O. Microwave-Assisted Synthesis of Colloidal Inorganic Nanocrystals. Angew. Chem. Int. Ed. 2011, 50, 11312–11359. [Google Scholar] [CrossRef]
- Tu, W.; Liu, H. Rapid Synthesis of Nanoscale Colloidal Metal Clusters by Microwave Irradiation. J. Mater. Chem. 2000, 10, 2207–2211. [Google Scholar] [CrossRef]
- Tsuji, M.; Matsumoto, K.; Jiang, P.; Matsuo, R.; Tang, X.L.; Kamarudin, K.S.N. Roles of Pt Seeds and Chloride Anions in the Preparation of Silver Nanorods and Nanowires by Microwave-Polyol Method. Colloids Surf. A Physicochem. Eng. Asp. 2008, 316, 266–277. [Google Scholar] [CrossRef]
- Oseghe, E.O.; Ofomaja, A.E. Facile Microwave Synthesis of Pine Cone Derived C-Doped TiO2 for the Photodegradation of Tetracycline Hydrochloride under Visible-LED Light. J. Environ. Manag. 2018, 223, 860–867. [Google Scholar] [CrossRef]
- Li, M.; Wang, M.; Zhu, L.; Li, Y.; Yan, Z.; Shen, Z.; Cao, X. Facile Microwave Assisted Synthesis of N-Rich Carbon Quantum Dots/Dual-Phase TiO2 Heterostructured Nanocomposites with High Activity in CO2 Photoreduction. Appl. Catal. B Environ. 2018, 231, 269–276. [Google Scholar] [CrossRef]
- Han, E.; Vijayarangamuthu, K.; Youn, J.; Park, Y.-K.; Jung, S.-C.; Jeon, K.-J. Degussa P25 TiO2 Modified with H2O2 under Microwave Treatment to Enhance Photocatalytic Properties. Catal. Today 2018, 303, 305–312. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Hedhili, M.N.; Ahmed, E.; Shi, L.; Wang, P. Microwave-Assisted Self-Doping of TiO2 Photonic Crystals for Efficient Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2014, 6, 691–696. [Google Scholar] [CrossRef]
- Kete, M.; Pavlica, E.; Fresno, F.; Bratina, G.; Štangar, U.L. Highly Active Photocatalytic Coatings Prepared by a Low-Temperature Method. Environ. Sci. Pollut. Res. 2014, 21, 11238–11249. [Google Scholar] [CrossRef]
- Tasbihi, M.; Călin, I.; Šuligoj, A.; Fanetti, M.; Lavrenčič Štangar, U. Photocatalytic Degradation of Gaseous Toluene by Using TiO2 Nanoparticles Immobilized on Fiberglass Cloth. J. Photochem. Photobiol. A Chem. 2017, 336, 89–97. [Google Scholar] [CrossRef]
- Kong, L.; Wang, C.; Wan, F.; Zheng, H.; Zhang, X. Synergistic Effect of Surface Self-Doping and Fe Species-Grafting for Enhanced Photocatalytic Activity of TiO2 under Visible-Light. Appl. Surf. Sci. 2017, 396, 26–35. [Google Scholar] [CrossRef]
- Balayeva, N.O.; Fleisch, M.; Bahnemann, D.W. Surface-Grafted WO3/TiO2 Photocatalysts: Enhanced Visible-Light Activity towards Indoor Air Purification. Catal. Today 2018, 313, 63–71. [Google Scholar] [CrossRef]
- Zhang, M.; Xiong, J.; Yang, H.; Wen, Z.; Chen, R.; Cheng, G. Surface Potential/Wettability and Interface Charge Transfer Engineering of Copper-Oxide (Cu–MOx, M = W, Ti, and Ce) Hybrids for Efficient Wastewater Treatment through Adsorption–Photocatalysis Synergy. Ind. Eng. Chem. Res. 2020, 59, 15454–15463. [Google Scholar] [CrossRef]
- Benz, D.; Felter, K.M.; Köser, J.; Thöming, J.; Mul, G.; Grozema, F.C.; Hintzen, H.T.; Kreutzer, M.T.; Van Ommen, J.R. Assessing the Role of Pt Clusters on TiO2 (P25) on the Photocatalytic Degradation of Acid Blue 9 and Rhodamine B. J. Phys. Chem. C 2020, 124, 8269–8278. [Google Scholar] [CrossRef]
- Dvoranová, D.; Barbieriková, Z.; Brezová, V. Radical Intermediates in Photoinduced Reactions on TiO2 (An EPR Spin Trapping Study). Molecules 2014, 19, 17279–17304. [Google Scholar] [CrossRef]
- Khachatryan, L.; Vejerano, E.; Lomnicki, S.; Dellinger, B. Environmentally Persistent Free Radicals (EPFRs). 1. Generation of Reactive Oxygen Species in Aqueous Solutions. Environ. Sci. Technol. 2011, 45, 8559–8566. [Google Scholar] [CrossRef]
- Wood, P.M. The Potential Diagram for Oxygen at pH 7. Biochem. J. 1988, 253, 287–289. [Google Scholar] [CrossRef]
- Chang, C.-W.; Huo, X.; Lin, T.-F. Exposure of Microcystis Aeruginosa to Hydrogen Peroxide and Titanium Dioxide under Visible Light Conditions: Modeling the Impact of Hydrogen Peroxide and Hydroxyl Radical on Cell Rupture and Microcystin Degradation. Water Res. 2018, 141, 217–226. [Google Scholar] [CrossRef]
- Ateia, M.; Alalm, M.G.; Awfa, D.; Johnson, M.S.; Yoshimura, C. Modeling the Degradation and Disinfection of Water Pollutants by Photocatalysts and Composites: A Critical Review. Sci. Total Environ. 2020, 698, 134197. [Google Scholar] [CrossRef]
- Šuligoj, A.; Lavrenčič Štangar, U.; Novak Tušar, N. Photocatalytic Air-Cleaning Using TiO2 Nanoparticles in Porous Silica Substrate. Chem. Pap. 2014, 68, 1265–1272. [Google Scholar] [CrossRef]
- Launer, P.J. Infrared Analysis of Organosilicon Compounds: Spectra-Structure Correlations. In Silicon Compounds: Register and Review; Petrarch Systems: Bristol, PA, USA, 1987; pp. 100–103. [Google Scholar]
- Maučec, D.; Šuligoj, A.; Ristić, A.; Dražić, G.; Pintar, A.; Tušar, N.N. Titania versus Zinc Oxide Nanoparticles on Mesoporous Silica Supports as Photocatalysts for Removal of Dyes from Wastewater at Neutral pH. Catal. Today 2018, 310, 32–41. [Google Scholar] [CrossRef]
- Pichat, P. Photocatalysis and Water Purification: From Fundamentals to Recent Applications, 1st ed.; Pichat, P., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; ISBN 9783527329878. [Google Scholar]
- Iguchi, S.; Teramura, K.; Hosokawa, S.; Tanaka, T. Effect of the Chloride Ion as a Hole Scavenger on the Photocatalytic Conversion of CO2 in an Aqueous Solution over Ni–Al Layered Double Hydroxides. Phys. Chem. Chem. Phys. 2015, 17, 17995–18003. [Google Scholar] [CrossRef]
| Parameter | Amount | Unit |
|---|---|---|
| Bromide | 0.04 | mg/L |
| NO3 | 0.91 | mg/L |
| pH | 7.8 | / |
| Turbidity | 66 | NTU |
| Phosphorus | 0.16 | mg/L |
| Chloride | 19.5 | mg/L |
| TDS | 171 | mg/L |
| PO4 | 0.125 | mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šuligoj, A.; Nadagouda, M.; Žerjav, G.; Pintar, A.; Dionysiou, D.D.; Tušar, N.N. Efficiency of Microwave-Assisted Surface Grafting of Ni and Zn Clusters on TiO2 as Cocatalysts for Solar Light Degradation of Cyanotoxins. Catalysts 2025, 15, 590. https://doi.org/10.3390/catal15060590
Šuligoj A, Nadagouda M, Žerjav G, Pintar A, Dionysiou DD, Tušar NN. Efficiency of Microwave-Assisted Surface Grafting of Ni and Zn Clusters on TiO2 as Cocatalysts for Solar Light Degradation of Cyanotoxins. Catalysts. 2025; 15(6):590. https://doi.org/10.3390/catal15060590
Chicago/Turabian StyleŠuligoj, Andraž, Mallikarjuna Nadagouda, Gregor Žerjav, Albin Pintar, Dionysios D. Dionysiou, and Nataša Novak Tušar. 2025. "Efficiency of Microwave-Assisted Surface Grafting of Ni and Zn Clusters on TiO2 as Cocatalysts for Solar Light Degradation of Cyanotoxins" Catalysts 15, no. 6: 590. https://doi.org/10.3390/catal15060590
APA StyleŠuligoj, A., Nadagouda, M., Žerjav, G., Pintar, A., Dionysiou, D. D., & Tušar, N. N. (2025). Efficiency of Microwave-Assisted Surface Grafting of Ni and Zn Clusters on TiO2 as Cocatalysts for Solar Light Degradation of Cyanotoxins. Catalysts, 15(6), 590. https://doi.org/10.3390/catal15060590









_Dionysiou.jpg)



