Defect-Engineered Silicalite-1 Monoliths for Enhanced Hydrophobicity in Room-Temperature Tritium Oxidation
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of the Binder Amount
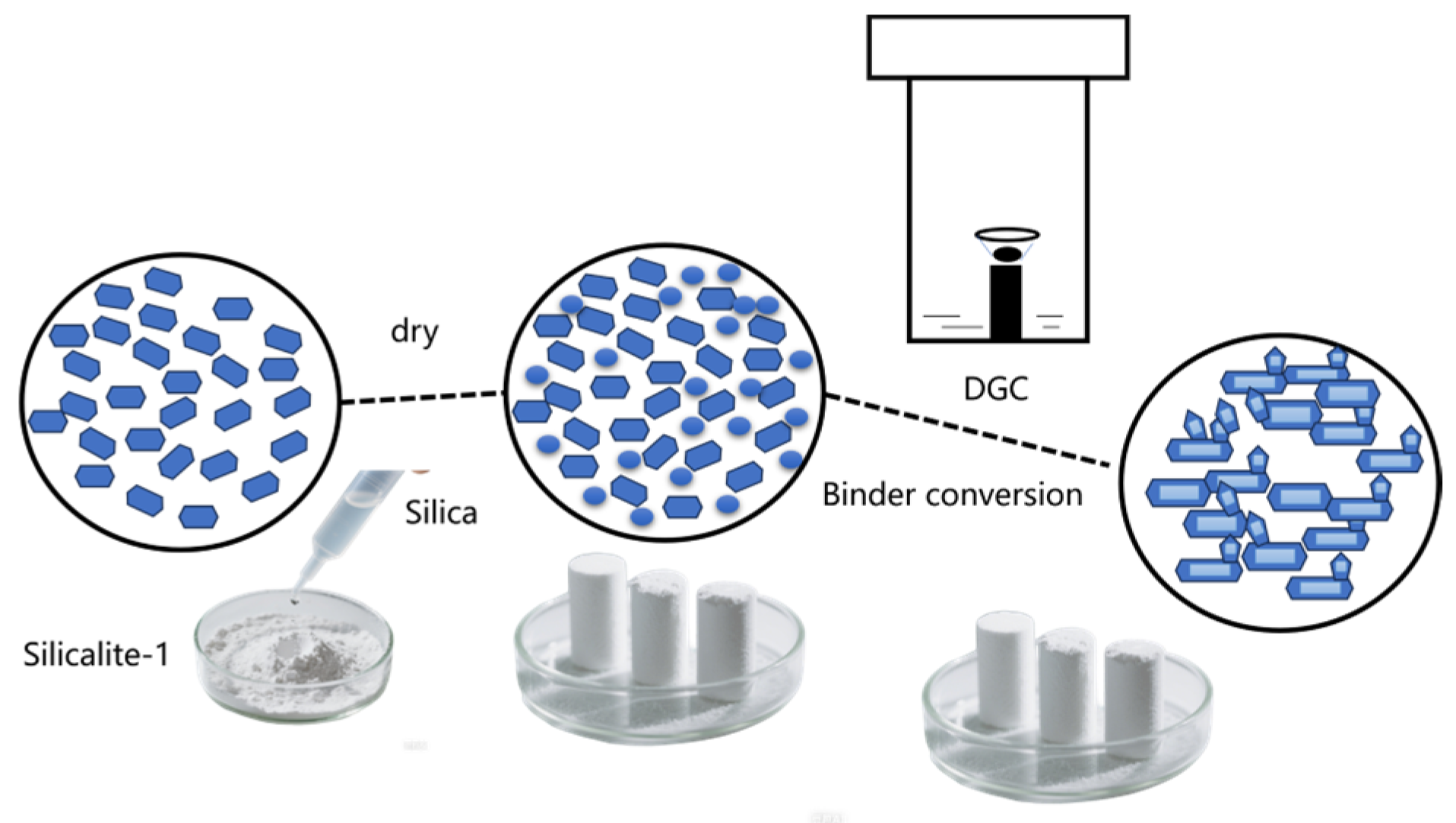
2.2. Characterization of Binder Conversion Process (Secondary Crystalization)
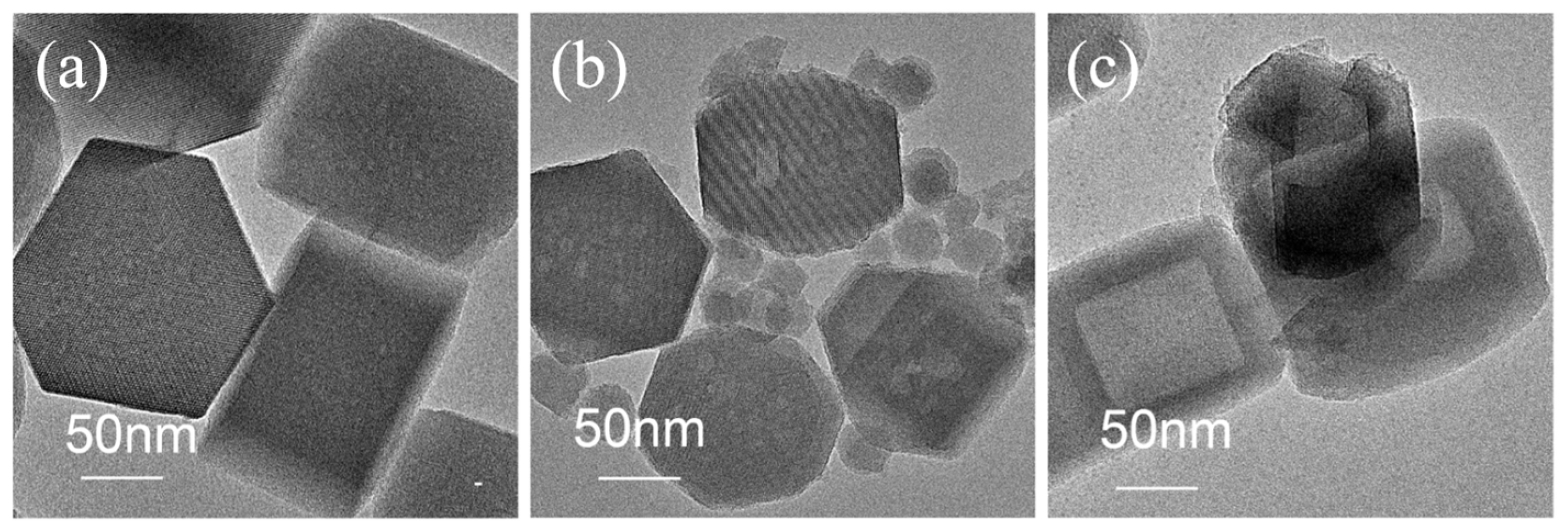
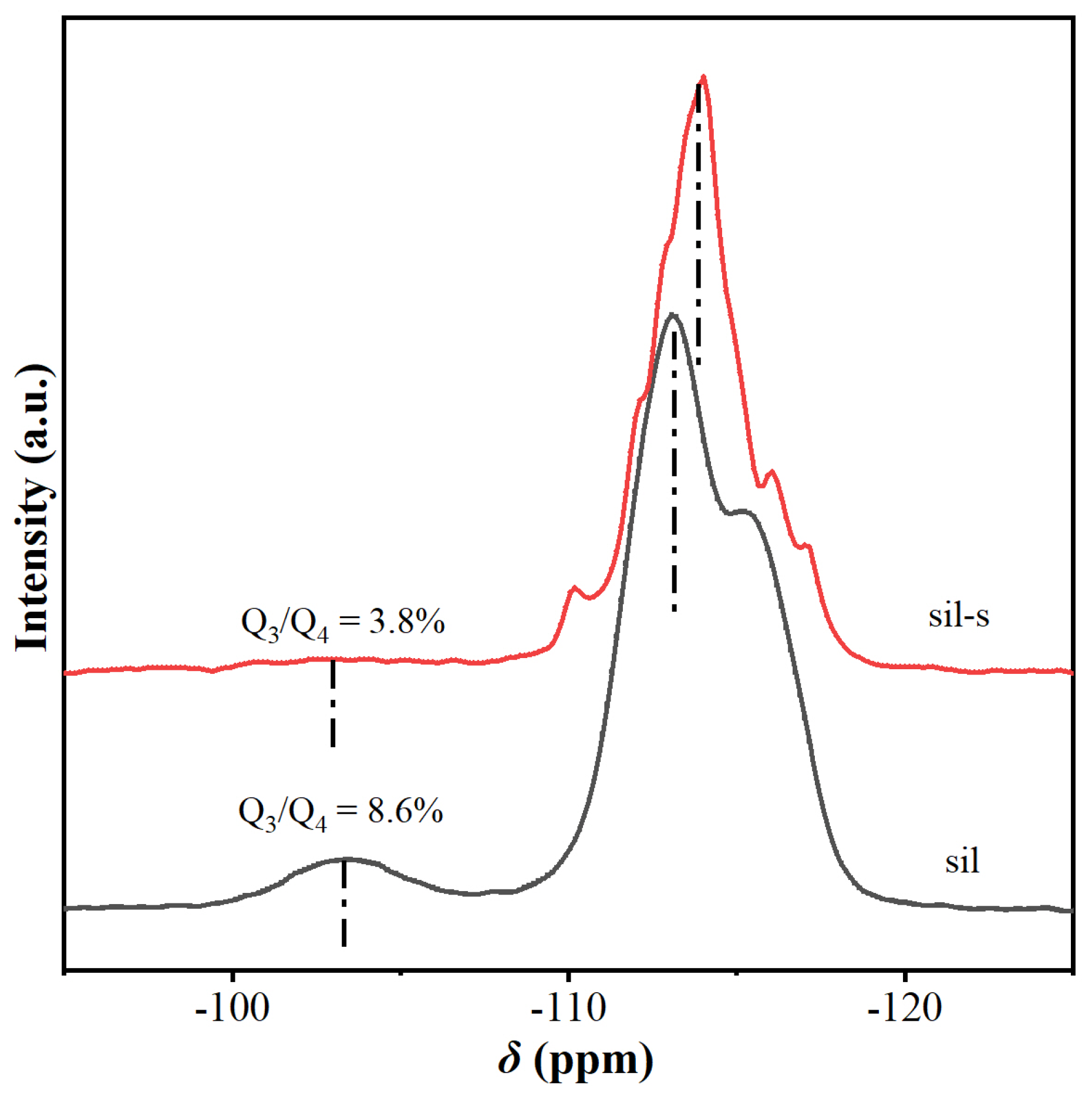
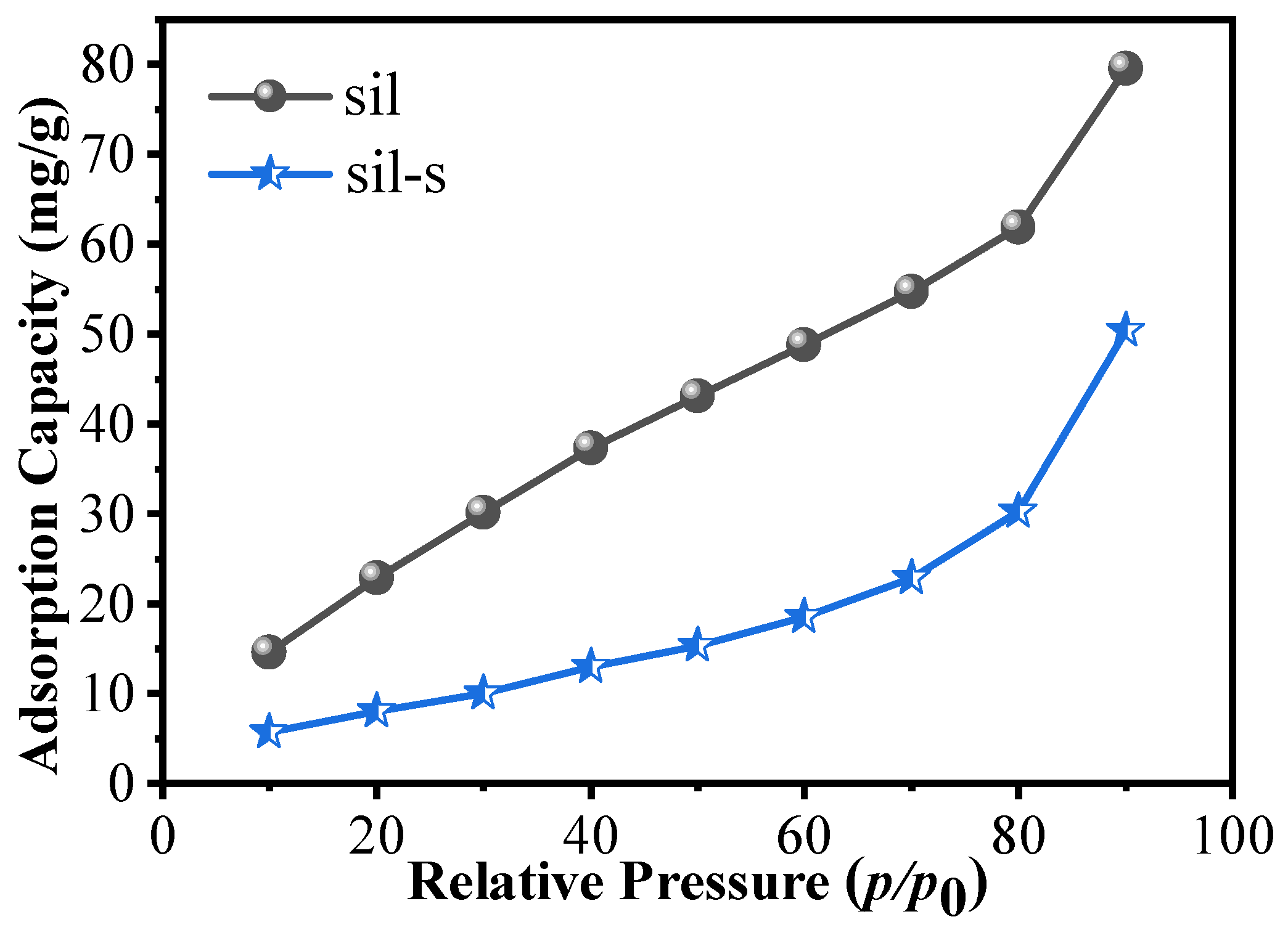
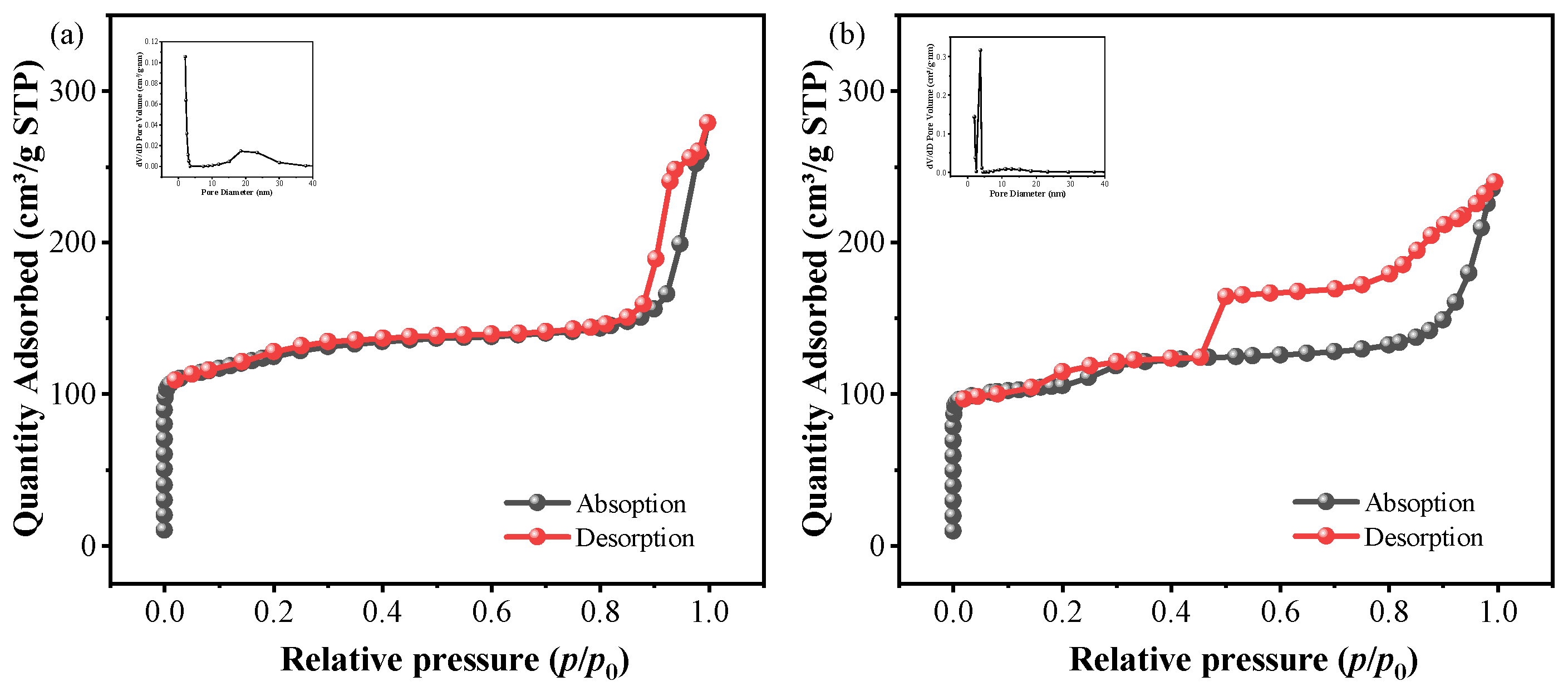
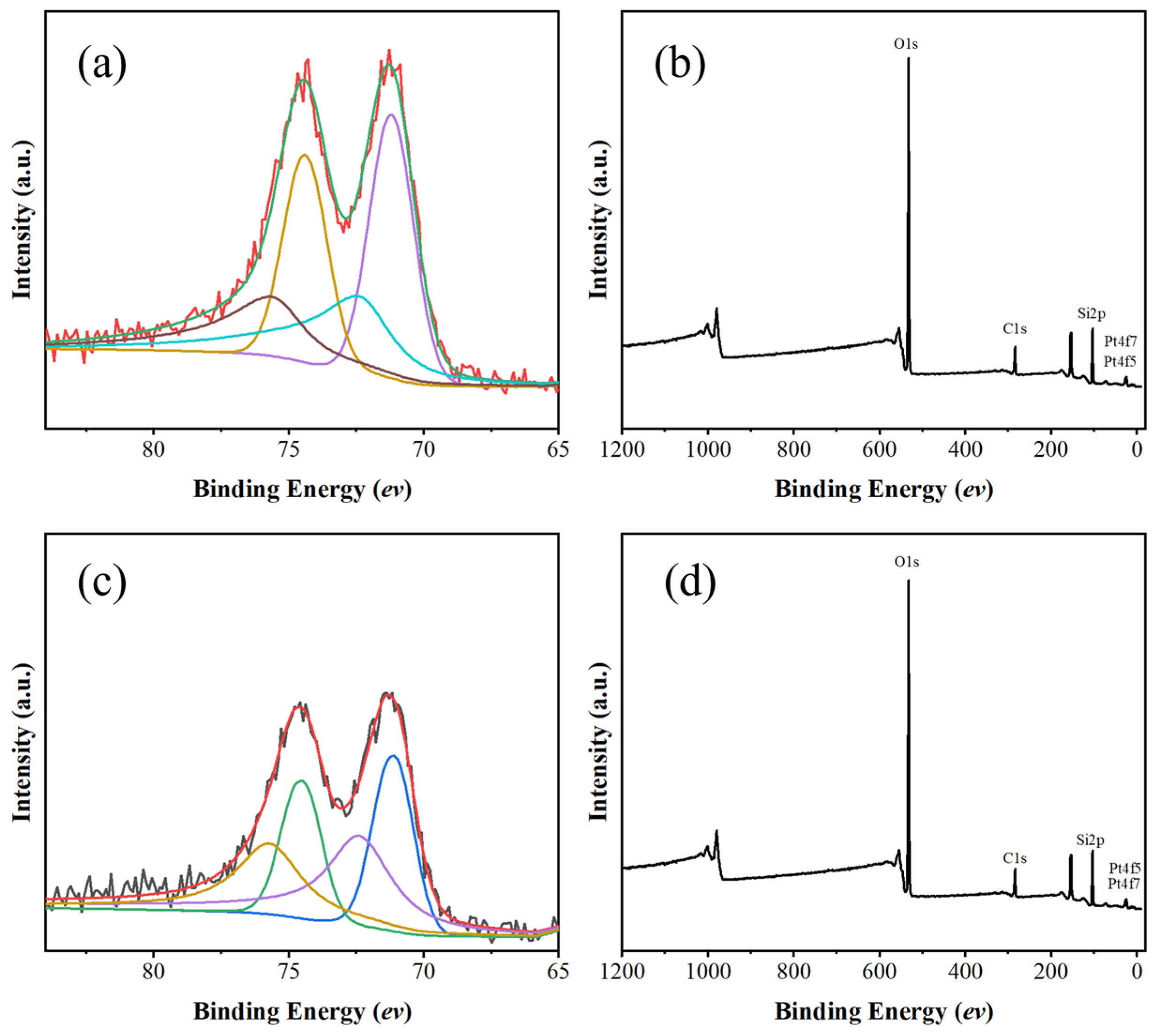
2.3. Comparison of Sil and Sil-s with Regard to Defects and Hydrophobicity
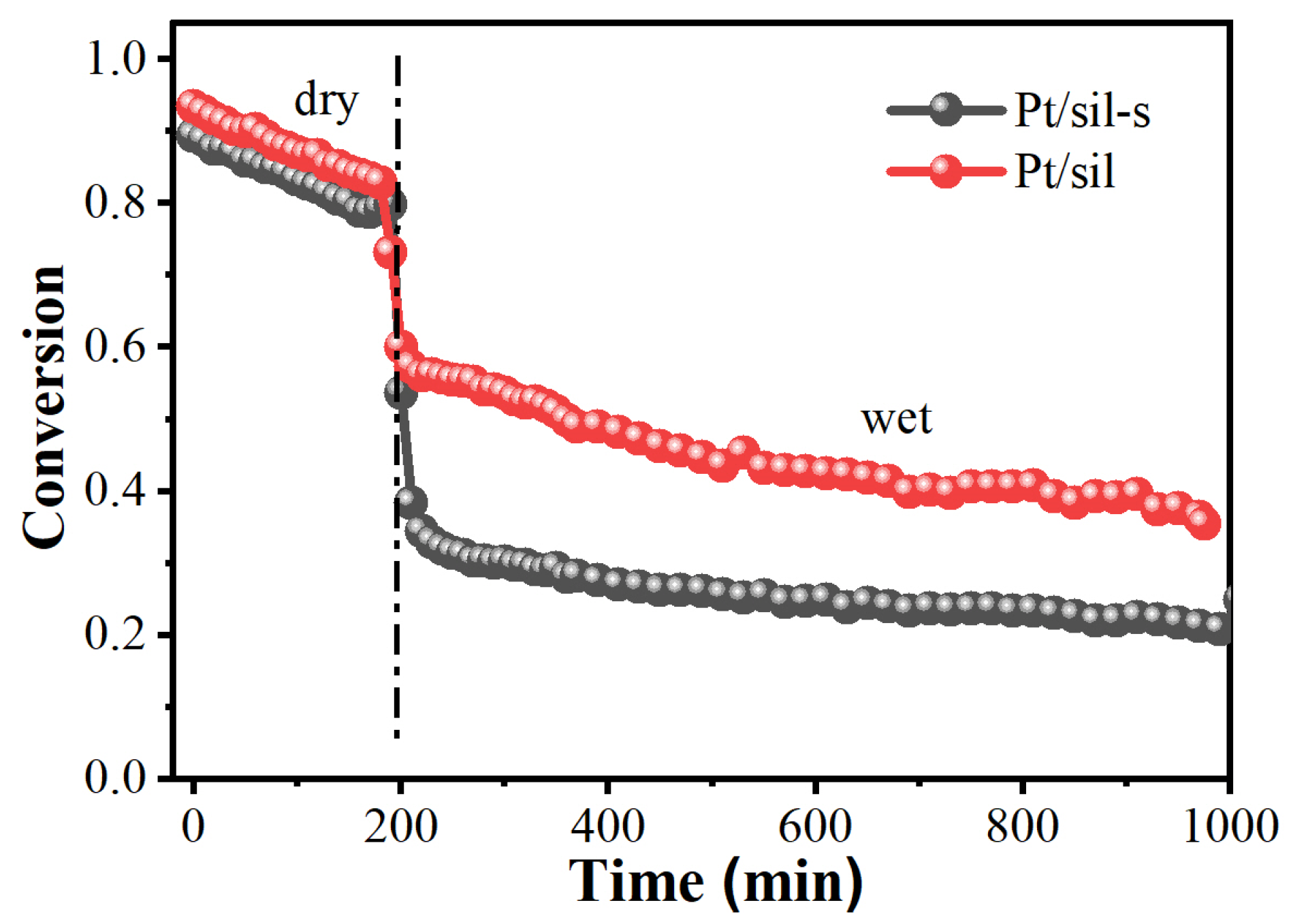
2.4. Pt/Sil and Pt/Sil-s Catalysts and Their Catalytic Performance in Hydrogen Oxidation Reaction
3. Materials and Methods
3.1. Synthesis of Silicalite-1
3.2. Synthesis of Silicalite-1 Monolith
3.3. Preparation of Platinum Catalysts with Sil and Sil-s as the Supports
3.4. Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fiore, K. Nuclear Energy and Sustainability: Understanding ITER. Energy Policy 2006, 34, 3334–3341. [Google Scholar] [CrossRef]
- Rizzello, C.; Borgognoni, F.; Pinna, T.; Tosti, S. Review of Tritium Confinement and Atmosphere Detritiation System in Hot Cells Complex. Fusion Eng. Des. 2010, 85, 58–63. [Google Scholar] [CrossRef]
- Beloglazov, S.; Camp, P.; Hayashi, T.; Lepetit, L.; Perevezentsev, A.; Yamanishi, Y. Configuration and Operation of Detritiation Systems for ITER Tokamak Complex. Fusion Eng. Des. 2010, 85, 1670–1674. [Google Scholar] [CrossRef]
- Iwai, Y.; Sato, K.; Taniuchi, J.; Noguchi, H.; Kubo, H.; Harada, N.; Oshima, Y.; Yamanishi, T. Room-Temperature Reactor Packed with Hydrophobic Catalysts for the Oxidation of Hydrogen Isotopes Released in a Nuclear Facility. J. Nucl. Sci. Technol. 2011, 48, 1184–1192. [Google Scholar] [CrossRef]
- Xiao, C.; Yang, Y.; Zhou, X.; Du, Y.; Tan, Z. Humidity-Tolerant H2-O2 Recombination Platinum Catalyst for Mitigating Hydrogen Using Silicalite-1 as Support. Microporous Mesoporous Mater. 2019, 279, 395–399. [Google Scholar] [CrossRef]
- Iwai, Y.; Sato, K.; Yamanishi, T. Development of Pt/ASDBC Catalyst Applicable for Hydrogen Oxidation in the Presence of Saturated Water Vapor at Room Temperature. J. Plasma Fusion Res. 2010, 9, 332–337. [Google Scholar]
- Iwai, Y.; Sato, K.; Yamanishi, T. Development of Pt/ASDBC Catalyst for Room Temperature Recombiner of Atmosphere Detritiation System. Fusion Eng. Des. 2011, 86, 2164–2167. [Google Scholar] [CrossRef]
- Lakiss, L.; Gilson, J.-P.; Valtchev, V.; Mintova, S.; Vicente, A.; Vimont, A.; Bedard, R.; Abdo, S.; Bricker, J. Zeolites in a Good Shape: Catalyst Forming by Extrusion Modifies Their Performances. Microporous Mesoporous Mater. 2020, 299, 110114. [Google Scholar] [CrossRef]




| Proportions (Sil/SiO2) | Sil/g | Silica Sol/g | TPAOH/g (nTPAOH/nSiO2 = 0.1) |
|---|---|---|---|
| A (18%) | 1.6 | 0.72 | 0.244 |
| B (24%) | 1.6 | 0.96 | 0.325 |
| C (30%) | 1.6 | 1.20 | 0.407 |
| Sample | SBET (m2/g) | Vt (cm3/g) | Vmic (cm3/g) | Vmeso (cm3/g) | ravg (nm) |
|---|---|---|---|---|---|
| Pt/sil | 465.68 | 0.314 | 0.116 | 0.272 | 7.63 |
| Pt/sil-s | 416.93 | 0.285 | 0.115 | 0.320 | 4.72 |
| Cat. | Percent of Pt | Metal Dispersion (%) | Cube Crystallite Size (nm) |
|---|---|---|---|
| Pt/sil | 2 wt% | 7.16 | 13.19 |
| Pt/sil-s | 2 wt% | 7.43 | 12.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, B.; Wang, T.; Xiao, C. Defect-Engineered Silicalite-1 Monoliths for Enhanced Hydrophobicity in Room-Temperature Tritium Oxidation. Catalysts 2025, 15, 584. https://doi.org/10.3390/catal15060584
Yu B, Wang T, Xiao C. Defect-Engineered Silicalite-1 Monoliths for Enhanced Hydrophobicity in Room-Temperature Tritium Oxidation. Catalysts. 2025; 15(6):584. https://doi.org/10.3390/catal15060584
Chicago/Turabian StyleYu, Benlong, Tao Wang, and Chao Xiao. 2025. "Defect-Engineered Silicalite-1 Monoliths for Enhanced Hydrophobicity in Room-Temperature Tritium Oxidation" Catalysts 15, no. 6: 584. https://doi.org/10.3390/catal15060584
APA StyleYu, B., Wang, T., & Xiao, C. (2025). Defect-Engineered Silicalite-1 Monoliths for Enhanced Hydrophobicity in Room-Temperature Tritium Oxidation. Catalysts, 15(6), 584. https://doi.org/10.3390/catal15060584






