Support Effects on Fe- or Cu-Promoted Ni Catalysts Used in the Catalytic Deoxygenation of Tristearin to Fuel-like Hydrocarbons
Abstract
:1. Introduction
2. Results and Discussion
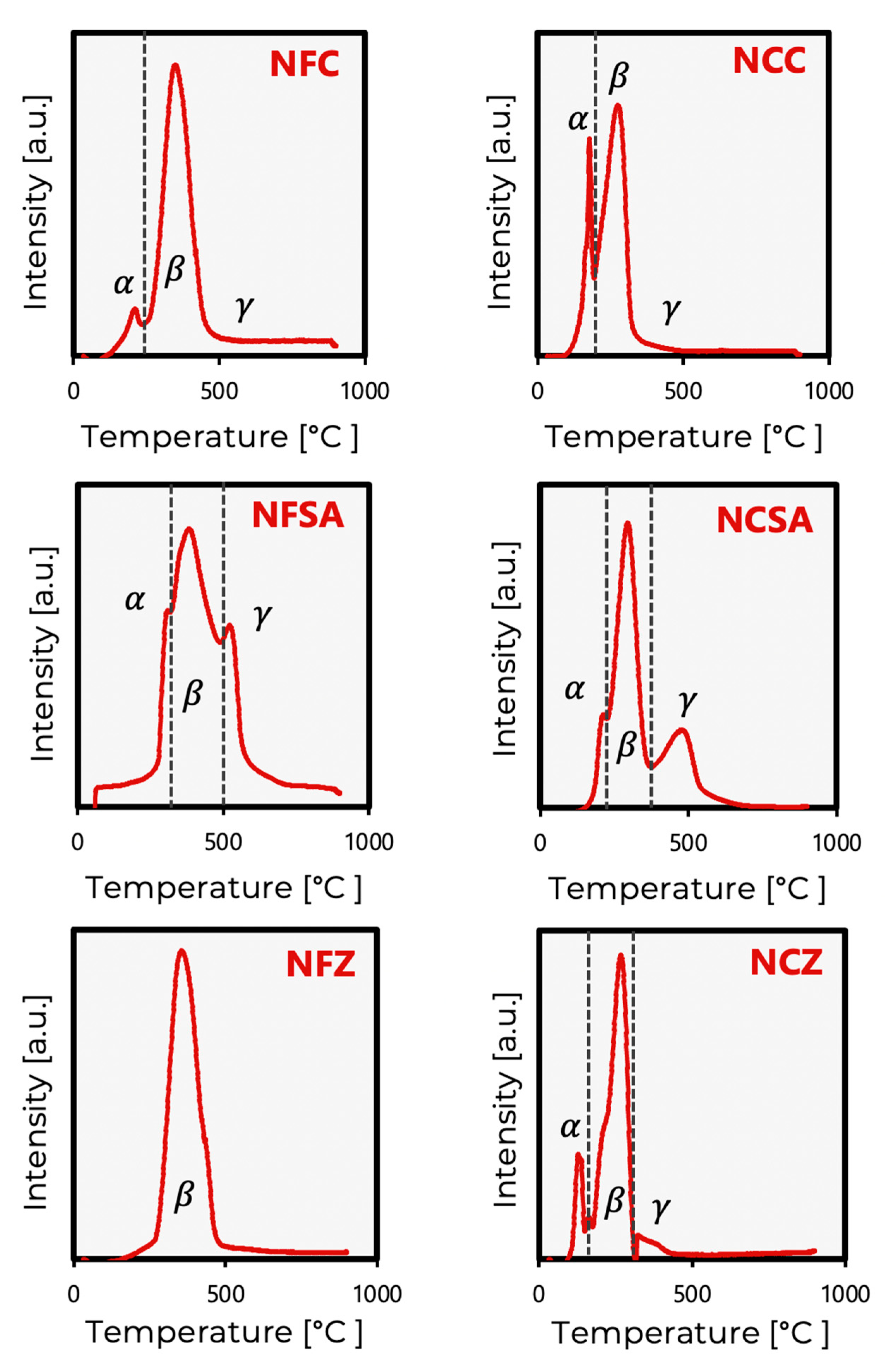
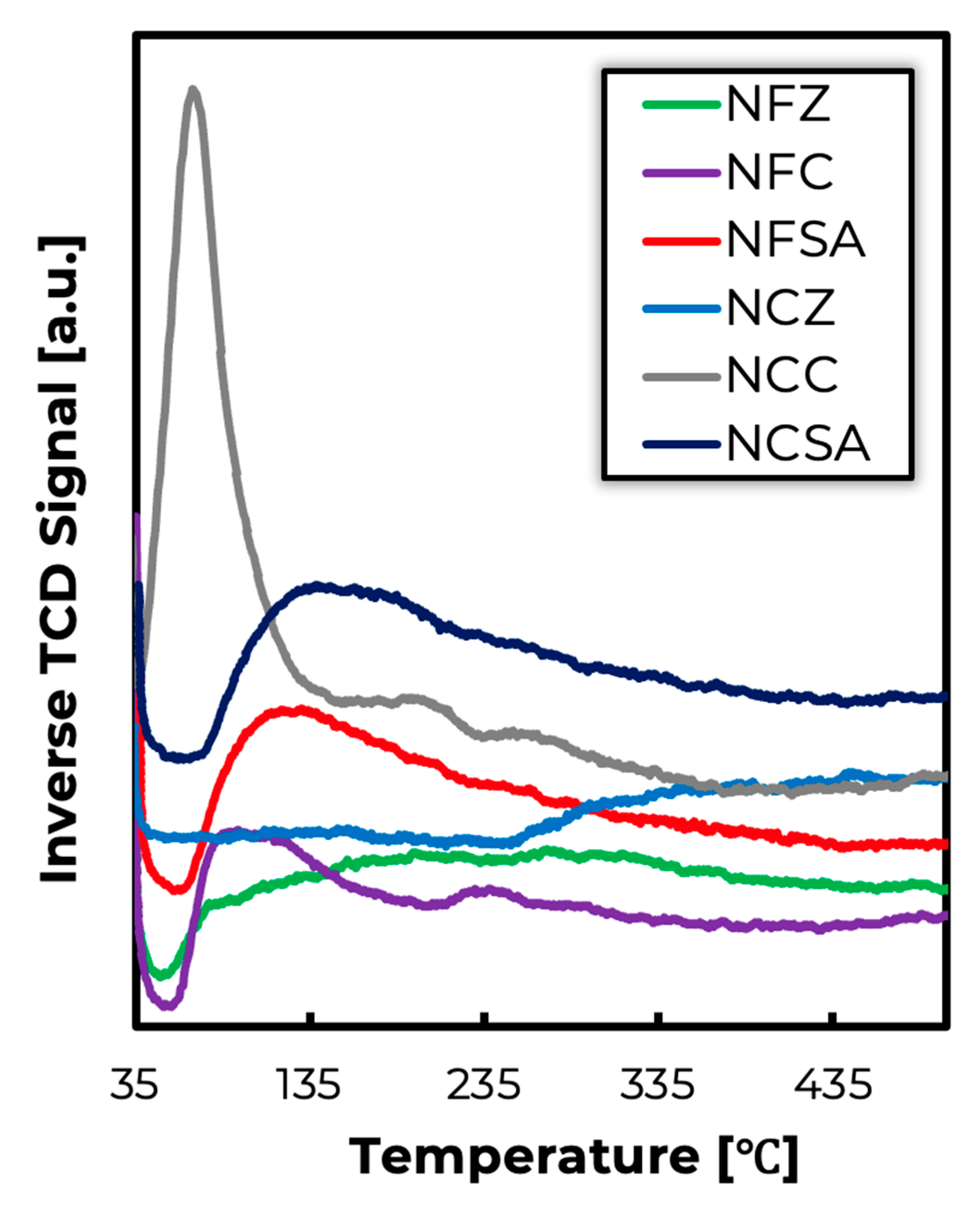
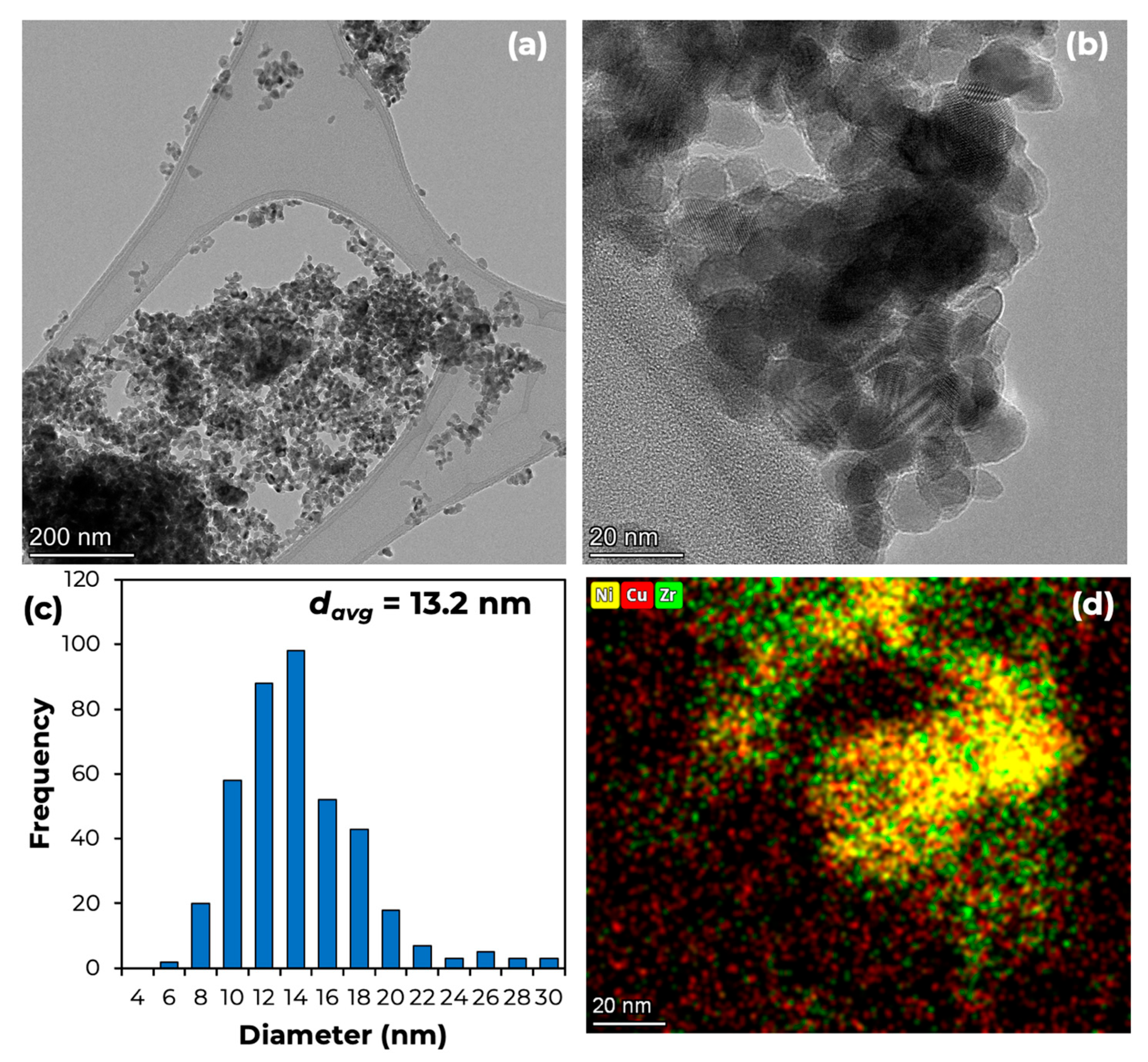
2.1. Fresh Catalyst Characterization
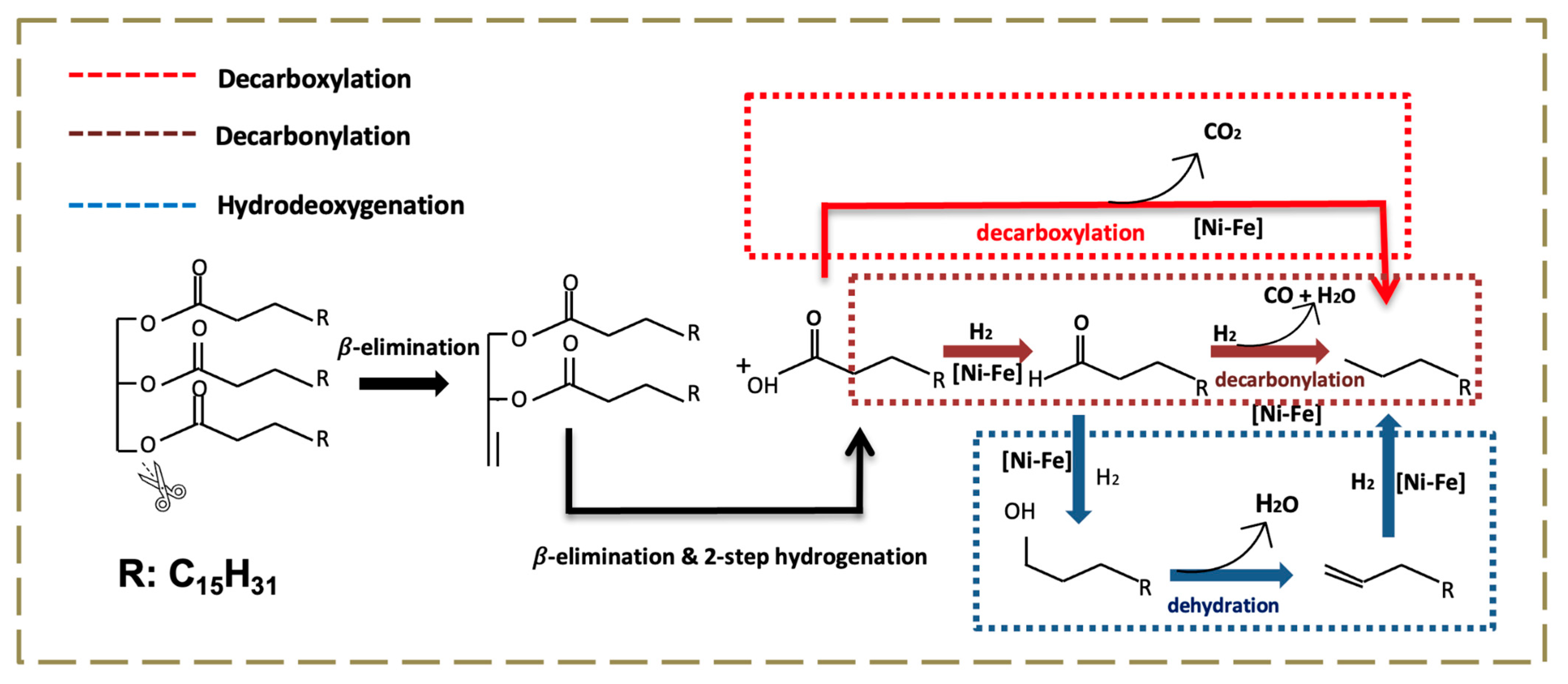
2.2. Tristearin Deoxygenation in Semi-Batch Mode
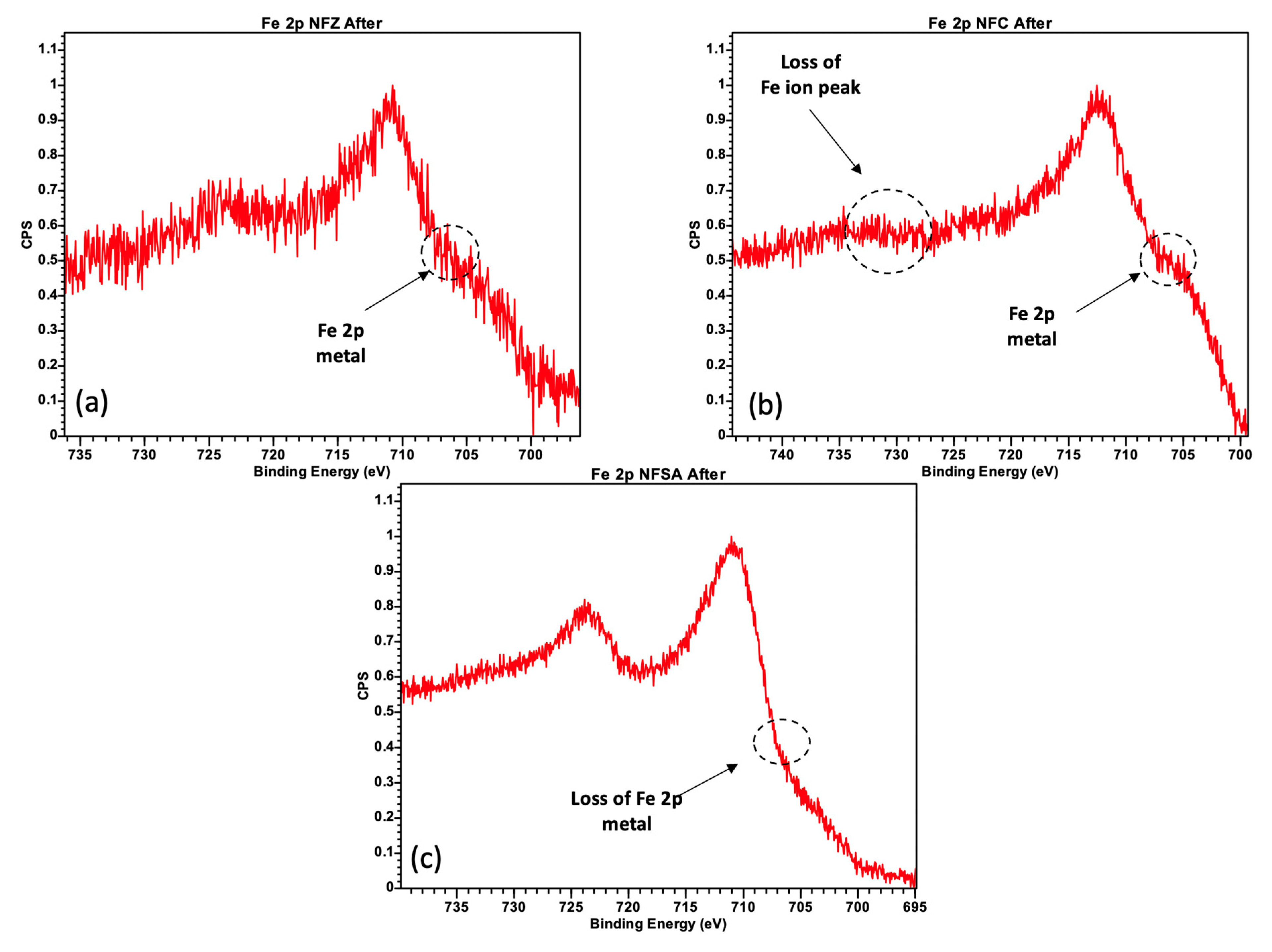
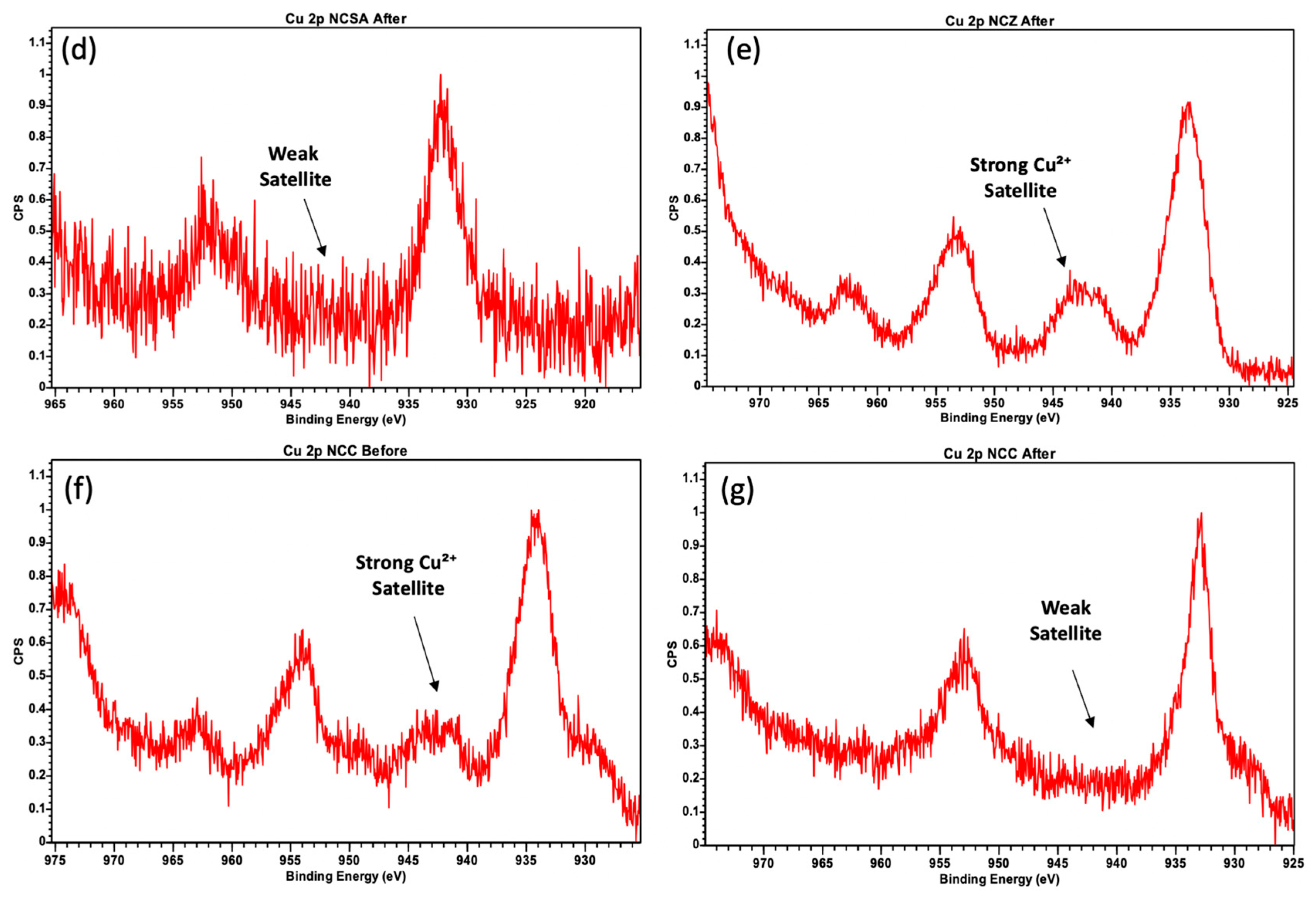
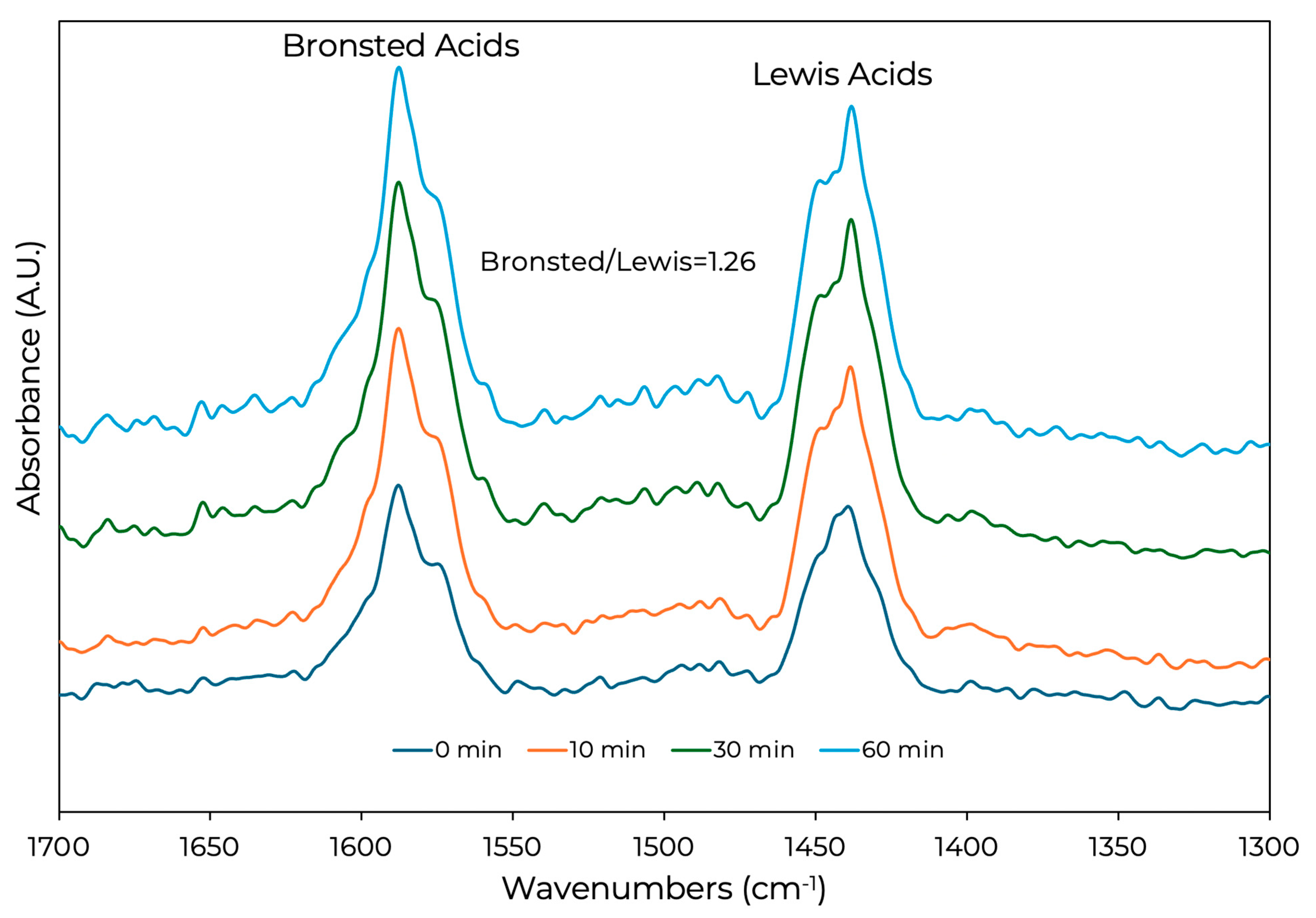
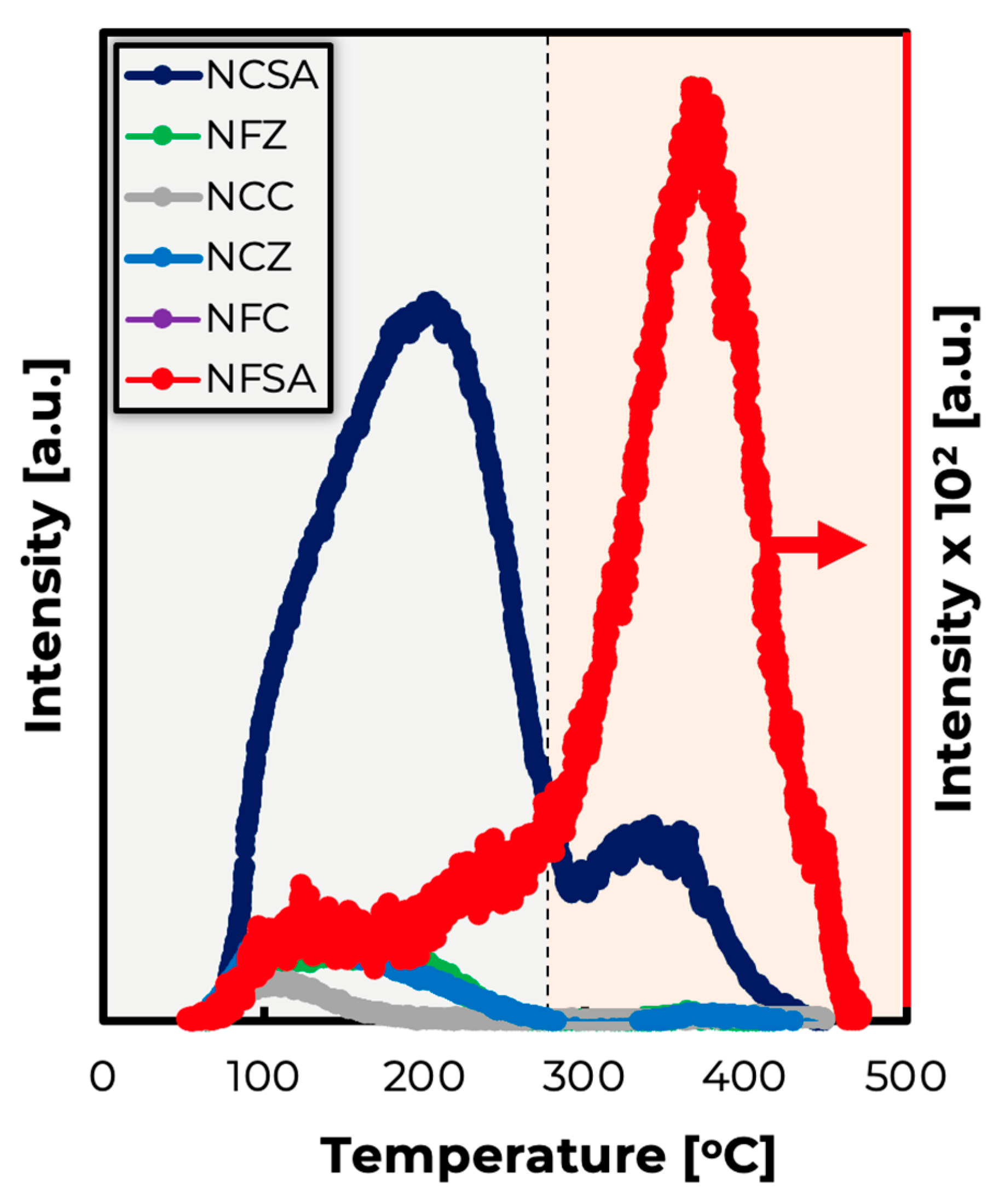
2.3. Spent Catalysts Characterization
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalyst Characterization
3.3. Deoxygenation in a Semi-Batch Mode
3.4. Liquid Product Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Umenweke, G.C.; Santillan-Jimenez, E. Hydrocarbon Biofuels; American Chemical Society: Washington, DC, USA, 2024. [Google Scholar]
- Azman, N.S.; Marliza, T.S.; Mijan, N.A.; Yun Hin, T.Y.; Khairuddin, N. Production of Biodiesel from Waste Cooking Oil via Deoxygenation Using Ni-Mo/Ac Catalyst. Processes 2021, 9, 750. [Google Scholar] [CrossRef]
- Loe, R.; Lavoignat, Y.; Maier, M.; Abdallah, M.; Morgan, T.; Qian, D.; Pace, R.; Santillan-Jimenez, E.; Crocker, M. Continuous Catalytic Deoxygenation of Waste Free Fatty Acid-Based Feeds to Fuel-Like Hydrocarbons Over a Supported Ni-Cu Catalyst. Catalysts 2019, 9, 123. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, H.; Zheng, Y. The role of cobalt and nickel in deoxygenation of vegetable oils. Appl. Catal. B Environ. 2014, 160–161, 415–422. [Google Scholar] [CrossRef]
- Zhou, K.; Du, X.; Zhou, L.; Yang, H.; Lei, X.; Zeng, Y.; Li, D.; Hu, C. The Deoxygenation of Jatropha Oil to High Quality Fuel via the Synergistic Catalytic Effect of Ni, W2C and WC Species. Catalysts 2021, 11, 469. [Google Scholar] [CrossRef]
- Kung, H.H.; Ko, E.I. Preparation of oxide catalysts and catalyst supports—A review of recent advances. Chem. Eng. J. Biochem. Eng. J. 1996, 64, 203–214. [Google Scholar] [CrossRef]
- Zulkepli, S.; Rahman, N.A.; Lee, H.V.; Cheng, C.K.; Chen, W.-H.; Juan, J.C. Synergistic effect of bimetallic Fe-Ni supported on hexagonal mesoporous silica for production of hydrocarbon-like biofuels via deoxygenation under hydrogen-free condition. Energy Convers. Manag. 2022, 273, 116371. [Google Scholar] [CrossRef]
- Simakova, I.; Simakova, O.; Mäki-Arvela, P.; Simakov, A.; Estrada, M.; Murzin, D.Y. Deoxygenation of palmitic and stearic acid over supported Pd catalysts: Effect of metal dispersion. Appl. Catal. A 2009, 355, 100. [Google Scholar] [CrossRef]
- Silva, G.C.R.; Qian, D.; Pace, R.; Heintz, O.; Caboche, G.; Santillan-Jimenez, E.; Crocker, M. Promotional Effect of Cu, Fe and Pt on the Performance of Ni/Al2O3 in the Deoxygenation of Used Cooking Oil to Fuel-Like Hydrocarbons. Catalysts 2020, 10, 91. [Google Scholar] [CrossRef]
- Snåre, M.; Kubičková, I.; Mäki-Arvela, P.; Eränen, K.; Murzin, D.Y. Heterogeneous catalytic deoxygenation of stearic acid for production of biodiesel. Ind. Eng. Chem. Res. 2006, 45, 5708. [Google Scholar] [CrossRef]
- Morgan, T.; Grubb, D.; Santillan-Jimenez, E.; Crocker, M. Conversion of Triglycerides to Hydrocarbons Over Supported Metal Catalysts. Top. Catal. 2010, 53, 820–829. [Google Scholar] [CrossRef]
- Loe, R.; Santillan-Jimenez, E.; Morgan, T.; Sewell, L.; Ji, Y.; Jones, S.; Isaacs, M.A.; Lee, A.F.; Crocker, M. Effect of Cu and Sn promotion on the catalytic deoxygenation of model and algal lipids to fuel-like hydrocarbons over supported Ni catalysts. Appl. Catal. B Environ. 2016, 191, 147–156. [Google Scholar] [CrossRef]
- Gosselink, R.W.; Xia, W.; Muhler, M.; de Jong, K.P.; Bitter, J.H. Enhancing the Activity of Pd on Carbon Nanofibers for Deoxygenation of Amphiphilic Fatty Acid Molecules through Support Polarity. ACS Catal. 2013, 3, 2397–2402. [Google Scholar] [CrossRef]
- Loe, R.; Santillan-Jimenez, E.; Crocker, M. Upgrading of Lipids to Fuel-like Hydrocarbons and Terminal Olefins via Decarbonylation/Decarboxylation. In Chemical Catalysts for Biomass Upgrading; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2020; pp. 497–528. [Google Scholar]
- Peng, B.; Yuan, X.; Zhao, C.; Lercher, J.A. Stabilizing Catalytic Pathways via Redundancy: Selective Reduction of Microalgae Oil to Alkanes. J. Am. Chem. Soc. 2012, 134, 9400–9405. [Google Scholar] [CrossRef]
- Zuo, H.L.; Liu, Q.; Wang, T.J.; Shi, N.; Liu, J.; Ma, L.L. Catalytic hydrodeoxygenation of vegetable oil over Ni catalysts to produce second-generation biodiesel. J. Fuel Chem. Technol. 2012, 40, 1067–1073. [Google Scholar]
- Kumar, P.; Yenumala, S.R.; Maity, S.K.; Shee, D. Kinetics of hydrodeoxygenation of stearic acid using supported nickel catalysts: Effects of supports. Appl. Catal. A Gen. 2014, 471, 28–38. [Google Scholar] [CrossRef]
- Maier, W.F.; Roth, W.; Thies, I.; Schleyer, P.V.R. Hydrogenolysis, IV. Gas phase decarboxylation of carboxylic acids. Chem. Berichte 1982, 115, 808–812. [Google Scholar] [CrossRef]
- Wang, F.; Pace, R.; Ji, Y.; Jiang, J.; Jiang, X.; Krystianiak, A.; Heintz, O.; Caboche, G.; Santillan-Jimenez, E.; Crocker, M. Effect of Pd promotion and catalyst support on the Ni-catalyzed deoxygenation of tristearin to fuel-like hydrocarbons. Renew. Energy 2022, 195, 1468–1479. [Google Scholar] [CrossRef]
- Peng, B.; Zhao, C.; Kasakov, S.; Foraita, S.; Lercher, J.A. Manipulating Catalytic Pathways: Deoxygenation of Palmitic Acid on Multifunctional Catalysts. Chem. A Eur. J. 2013, 19, 4732–4741. [Google Scholar] [CrossRef]
- Yakovlev, V.A.; Khromova, S.A.; Sherstyuk, O.V.; Dundich, V.O.; Ermakov, D.Y.; Novopashina, V.M.; Lebedev, M.Y.; Bulavchenko, O.; Parmon, V.N. Development of new catalytic systems for upgraded bio-fuels production from bio-crude-oil and biodiesel. Catal. Today 2009, 144, 362–366. [Google Scholar] [CrossRef]
- Khoshroo, G.; Sapi, A.; Imre, S.; Efremova, A.; Bali, H.; B. Ábrahámné, K.; Erdőhelyi, A.; Kukovecz, A.; Kónya, Z. Pure Ni-Based and Trimetallic Ni-Co-Fe Catalysts for the Dry Reforming of Methane: Effect of K Promoter and the Calcination Temperature. Catal. Lett. 2022, 153, 2755–2762. [Google Scholar] [CrossRef]
- Harris, P.J.F. Growth and structure of supported metal catalyst particles. Int. Mater. Rev. 1995, 40, 97–115. [Google Scholar] [CrossRef]
- Albero, J.; Garcia, H.; Corma, A. Temperature Dependence of Solar Light Assisted CO2 Reduction on Ni Based Photocatalyst. Top. Catal. 2016, 59, 787–791. [Google Scholar] [CrossRef]
- Kline, M.J.; Karunarathne, S.A.; Schwartz, T.J.; Wheeler, M.C. Hydrogenation of 2-methylnaphthalene over bi-functional Ni catalysts. Appl. Catal. A Gen. 2022, 630, 118462. [Google Scholar] [CrossRef]
- Seelam, P.K.; Balla, P.; Rajendiran, R.; Ravi, B.; Prathap, C.; Lassi, U.; Kim, S.; Vijayanand, P. Activity of Nickel Supported Over Nanorod-Shaped Strontium Hydroxyapatite Catalysts in Selective Methanation of CO & CO2. Top. Catal. 2023, 66, 1391–1405. [Google Scholar]
- Hongxia, Q.; Zhiqiang, W.; Hua, Y.; Lin, Z.; Xiaoyan, Y. Preparation and Characterization of NiO Nanoparticles by Anodic Arc Plasma Method. J. Nanomater. 2009, 479, 795928. [Google Scholar]
- Taghvaei, H.; Rahimpour, M.R.; Bruggeman, P. Catalytic hydrodeoxygenation of anisole over nickel supported on plasma treated alumina–silica mixed oxides. RSC Adv. 2017, 7, 30990–30998. [Google Scholar] [CrossRef]
- Gac, W.; Zawadzki, W.; Kuśmierz, M.; Słowik, G.; Grudziński, W. Neodymium promoted ceria and alumina supported nickel catalysts for CO2 methanation reaction. Appl. Surf. Sci. 2023, 631, 157542. [Google Scholar] [CrossRef]
- Zimmer, P.; Tschöpe, A.; Birringer, R. Temperature-Programmed Reaction Spectroscopy of Ceria- and Cu/Ceria-Supported Oxide Catalyst. J. Catal. 2002, 205, 339–345. [Google Scholar] [CrossRef]
- Ji, K.; Meng, F.; Xun, J.; Liu, P.; Zhang, K.; Li, Z.; Gao, J. Carbon Deposition Behavior of Ni Catalyst Prepared by Combustion Method in Slurry Methanation Reaction. Catalysts 2019, 9, 570. [Google Scholar] [CrossRef]
- Marczewski, M.; Migdał, A.; Marczewska, H. Acidity of the silica–alumina/BX3 superacids. Phys. Chem. Chem. Phys. 2003, 5, 423–429. [Google Scholar] [CrossRef]
- Hensen, E.J.M.; Poduval, D.G.; Degirmenci, V.; Ligthart, D.A.J.M.; Chen, W.; Maugé, F.; Rigutto, M.S.; van Veen, J.A.R. Acidity Characterization of Amorphous Silica–Alumina. J. Phys. Chem. C 2012, 116, 21416–21429. [Google Scholar] [CrossRef]
- Chizallet, C.; Raybaud, P. Pseudo-Bridging Silanols as Versatile Brønsted Acid Sites of Amorphous Aluminosilicate Surfaces. Angew. Chem. Int. Ed. 2009, 48, 2891–2893. [Google Scholar] [CrossRef] [PubMed]
- Castaño, P.; Pawelec, B.; Fierro, J.L.G.; Arandes, J.M.; Bilbao, J. Enhancement of pyrolysis gasoline hydrogenation over Pd-promoted Ni/SiO2–Al2O3 catalysts. Fuel 2007, 86, 2262–2274. [Google Scholar] [CrossRef]
- Rebba, E.; Ivanchenko, P.; Bordignon, S.; Samrout, O.E.; Chierotti, M.R.; Cesano, F.; Berlier, G. Exploring hydrophobic surface modifications in silica and alumina nanomaterials. Surf. Interfaces 2024, 51, 104535. [Google Scholar] [CrossRef]
- Song, H.; Ozkan, U.S. Changing the Oxygen Mobility in Co/Ceria Catalysts by Ca Incorporation: Implications for Ethanol Steam Reforming. J. Phys. Chem. A 2010, 114, 3796–3801. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, B.; Li, Y.; Xu, Y.; Shen, W. Hydrogen production by oxidative steam reforming of ethanol over an Ir/CeO2 catalyst. Catal. Commun. 2007, 8, 1588–1594. [Google Scholar] [CrossRef]
- Yang, F.; Bao, X.; Li, P.; Wang, X.; Cheng, G.; Chen, S.; Luo, W. Boosting Hydrogen Oxidation Activity of Ni in Alkaline Media through Oxygen-Vacancy-Rich CeO2/Ni Heterostructures. Angew. Chem. Int. Ed. 2019, 58, 14179–14183. [Google Scholar] [CrossRef]
- Lu, Z.; Yang, Z.; He, B.; Castleton, C.; Hermansson, K. Cu-doped ceria: Oxygen vacancy formation made easy. Chem. Phys. Lett. 2011, 510, 60–66. [Google Scholar] [CrossRef]
- Rao, M.; Raju, B.; Kamaraju, S. Unique influence of rare earth (Pr, Nd, and Er) oxide surface acidic texture over CeO2/γ-Al2O3 catalysts for selective production of styrene using CO2 flow. Res. Chem. Intermed. 2019, 45, 2749–2770. [Google Scholar]
- Kopelent, R.; van Bokhoven, J.A.; Szlachetko, J.; Edebeli, J.; Paun, C.; Nachtegaal, M.; Safonova, O.V. Catalytically Active and Spectator Ce3+ in Ceria-Supported Metal Catalysts. Angew. Chem. Int. Ed. 2015, 54, 8728–8731. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Santillan-Jimenez, E.; Crocker, M. Catalytic deoxygenation of fatty acids and their derivatives to hydrocarbon fuels via decarboxylation/decarbonylation. J. Chem. Technol. Biotechnol. 2012, 87, 1041–1050. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Zhang, J.; Wang, Q. Tuning the decarboxylation selectivity for deoxygenation of vegetable oil over Pt–Ni bimetal catalysts via surface engineering. Catal. Sci. Technol. 2018, 8, 1126–1133. [Google Scholar] [CrossRef]
- Morgan, T.; Santillan-Jimenez, E.; Harman-Ware, A.E.; Ji, Y.; Grubb, D.; Crocker, M. Catalytic deoxygenation of triglycerides to hydrocarbons over supported nickel catalysts. Chem. Eng. J. 2012, 189–190, 346–355. [Google Scholar] [CrossRef]


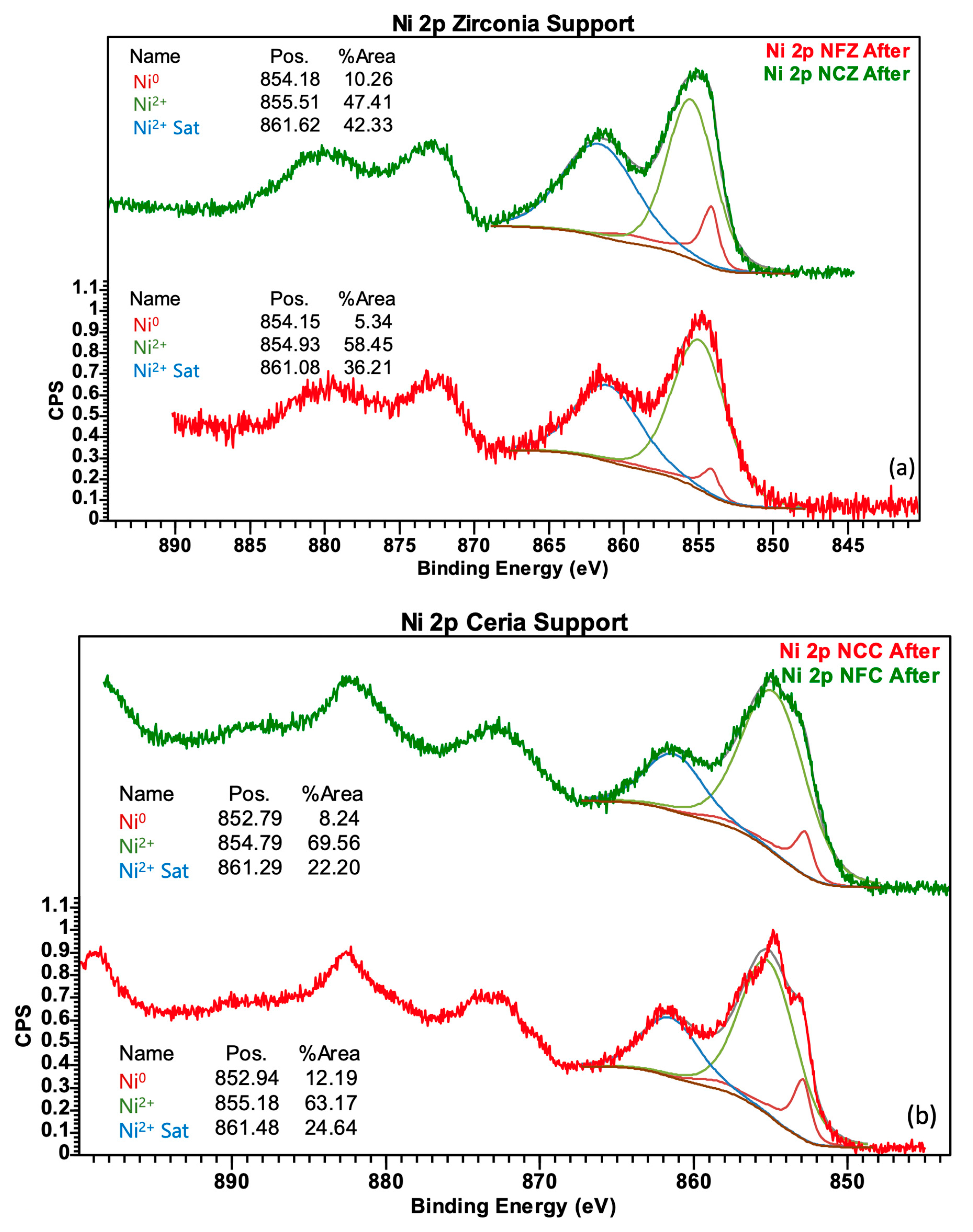

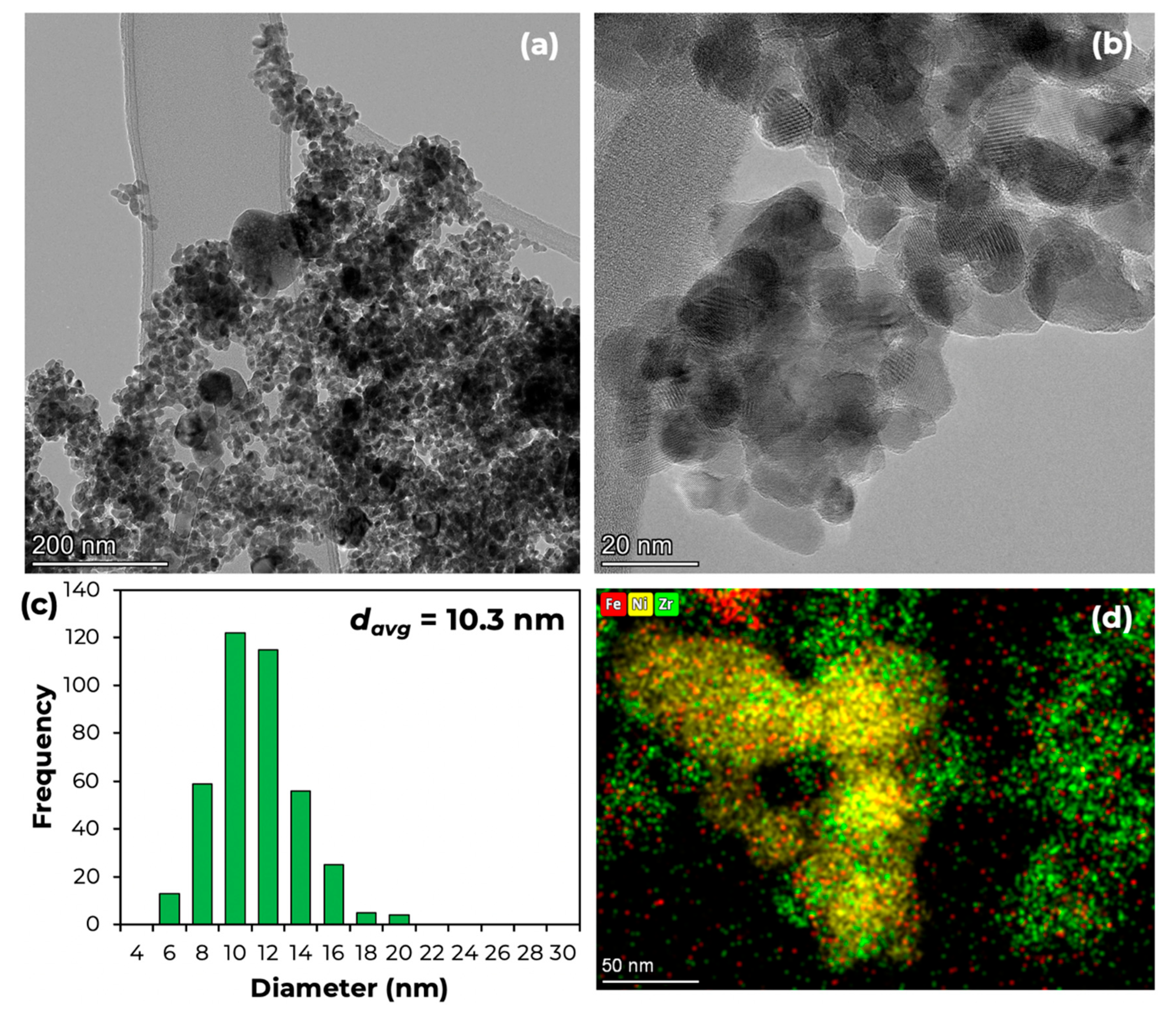
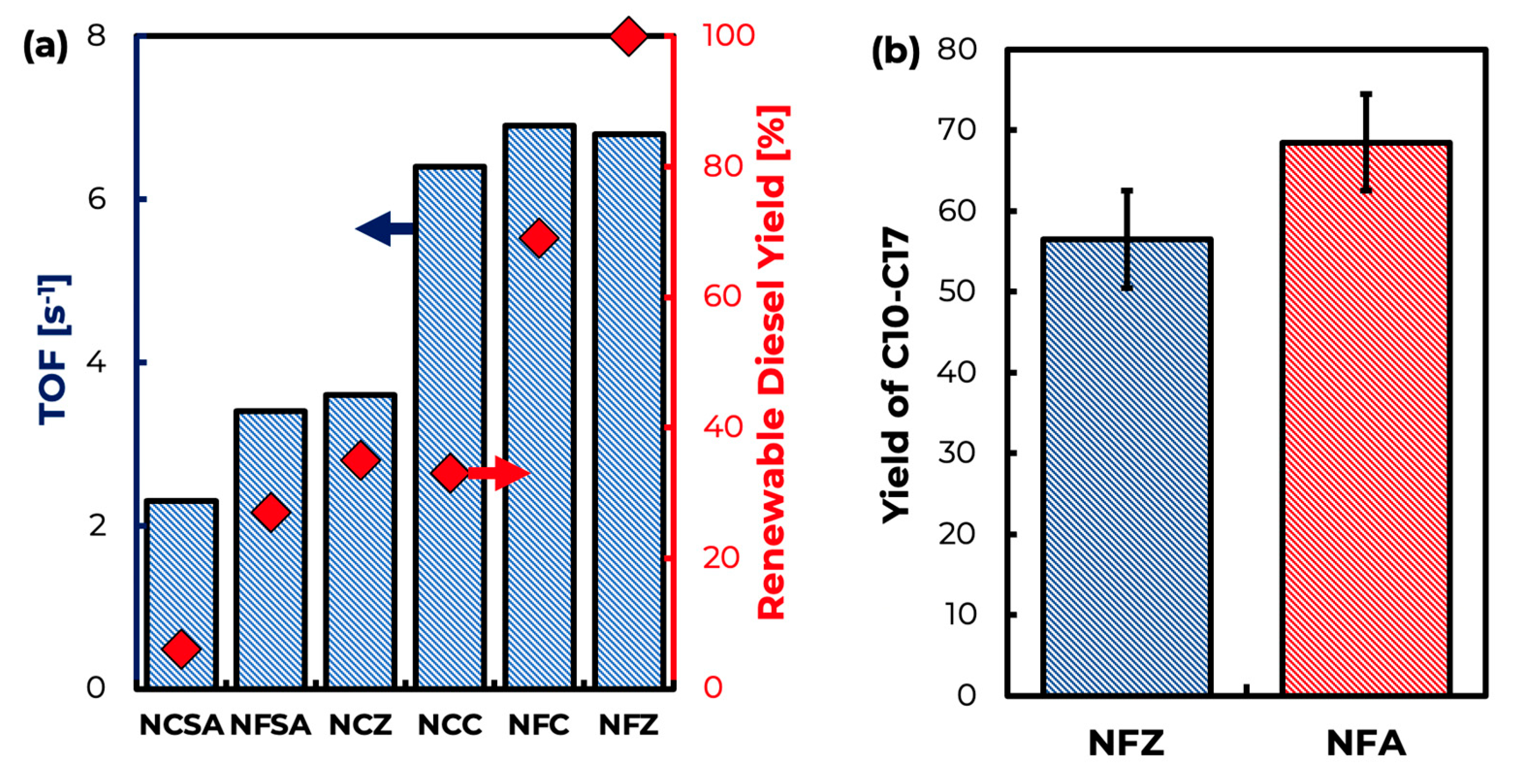

| Catalyst | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Width (nm) | NiO Particle Size (nm) |
|---|---|---|---|---|
| Ce0.8Pr0.2O2 Support | 74 | 0.14 | 6.8 | - |
| NFC | 41 | 0.06 | 5.3 | 10.4 |
| NCC | 45 | 0.08 | 6.6 | 20.5 |
| SiO2-Al2O3 Support | 517 | 0.73 | 4.8 | - |
| NFSA | 365 | 0.34 | 3.7 | 10.2 |
| NCSA | 208 | 0.22 | 4.0 | 10.4 |
| ZrO2 Support | 87 | 0.22 | 7.7 | - |
| NFZ | 50 | 0.13 | 8.7 | 10.7 |
| NCZ | 42 | 0.13 | 8.1 | 19.7 |
| Catalyst | H2 Uptake (cm3/g) | Ni Specific Surface Area (m2/g) | O2 Uptake (cm3/g) | Acidity (µmol/g) |
|---|---|---|---|---|
| NFC | 0.201 | 7.0 | 0.312 | 0.99 |
| NCC | 0.103 | 3.6 | 0.368 | 10.5 |
| NFSA | 0.157 | 5.5 | 0.159 | 3280 |
| NCSA | 0.051 | 1.8 | 0.197 | 215 |
| NFZ | 0.293 | 10.2 | 0.296 | 39.2 |
| NCZ | 0.194 | 6.8 | 0.329 | 33.5 |
| Catalyst | Ni (Ni0) * | C | O | Fe | Cu | Zr | Al | Si | Ce | Pr |
|---|---|---|---|---|---|---|---|---|---|---|
| NFZ | 6.3 (0.31) | 12.46 | 49.52 | 4.79 | - | 26.94 | - | - | - | - |
| NCZ | 8.9 (0.92) | 11.37 | 50.45 | - | 2.71 | 26.57 | - | - | - | - |
| NFC | 14.5 (1.18) | 36.17 | 32.84 | 7.65 | - | - | - | - | 7.79 | 1.05 |
| NCC | 15.3 (2.03) | 40 | 31.84 | - | 3.85 | - | - | - | 6.80 | 1.86 |
| NFSA | 4.36 (0.16) | 5.72 | 34.19 | 5.96 | - | - | 38.57 | 11.19 | - | - |
| NCSA | 2.77 (0.40) | 3.99 | 41.14 | - | 0.58 | - | 23.49 | 28.03 | - | - |
| Catalysts | Conversion (%) | Selectivity to (% yield of) C10–C18 | Selectivity to (% yield of) C17 | Selectivity to (% yield of) C18 | TOF (sec−1) a |
|---|---|---|---|---|---|
| NFC | 82 | 85 (69) | 61 (50) | 4 (3) | 6.9 |
| NCC | 37 | 89 (33) | 69 (25) | 0 (0) | 6.4 |
| NFSA | 29 | 93 (27) | 23 (7) | 48 (14) | 3.4 |
| NCSA | 30 | 20 (6) | 8 (2) | 8 (2) | 2.3 |
| NFZ | 100 | 100 (100) | 62 (62) | 7 (7) | 6.8 |
| NCZ | 37 | 94 (35) | 72 (27) | 0 (0) | 3.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umenweke, G.C.; Pace, R.; Récalt, T.; Heintz, O.; Caboche, G.; Santillan-Jimenez, E. Support Effects on Fe- or Cu-Promoted Ni Catalysts Used in the Catalytic Deoxygenation of Tristearin to Fuel-like Hydrocarbons. Catalysts 2025, 15, 501. https://doi.org/10.3390/catal15050501
Umenweke GC, Pace R, Récalt T, Heintz O, Caboche G, Santillan-Jimenez E. Support Effects on Fe- or Cu-Promoted Ni Catalysts Used in the Catalytic Deoxygenation of Tristearin to Fuel-like Hydrocarbons. Catalysts. 2025; 15(5):501. https://doi.org/10.3390/catal15050501
Chicago/Turabian StyleUmenweke, Great C., Robert Pace, Thomas Récalt, Olivier Heintz, Gilles Caboche, and Eduardo Santillan-Jimenez. 2025. "Support Effects on Fe- or Cu-Promoted Ni Catalysts Used in the Catalytic Deoxygenation of Tristearin to Fuel-like Hydrocarbons" Catalysts 15, no. 5: 501. https://doi.org/10.3390/catal15050501
APA StyleUmenweke, G. C., Pace, R., Récalt, T., Heintz, O., Caboche, G., & Santillan-Jimenez, E. (2025). Support Effects on Fe- or Cu-Promoted Ni Catalysts Used in the Catalytic Deoxygenation of Tristearin to Fuel-like Hydrocarbons. Catalysts, 15(5), 501. https://doi.org/10.3390/catal15050501







