Kinetic Understanding of the Enhanced Electroreduction of Nitrate to Ammonia for Co3O4–Modified Cu2+1O Nanowire Electrocatalyst
Abstract
1. Introduction
2. Results and Discussion
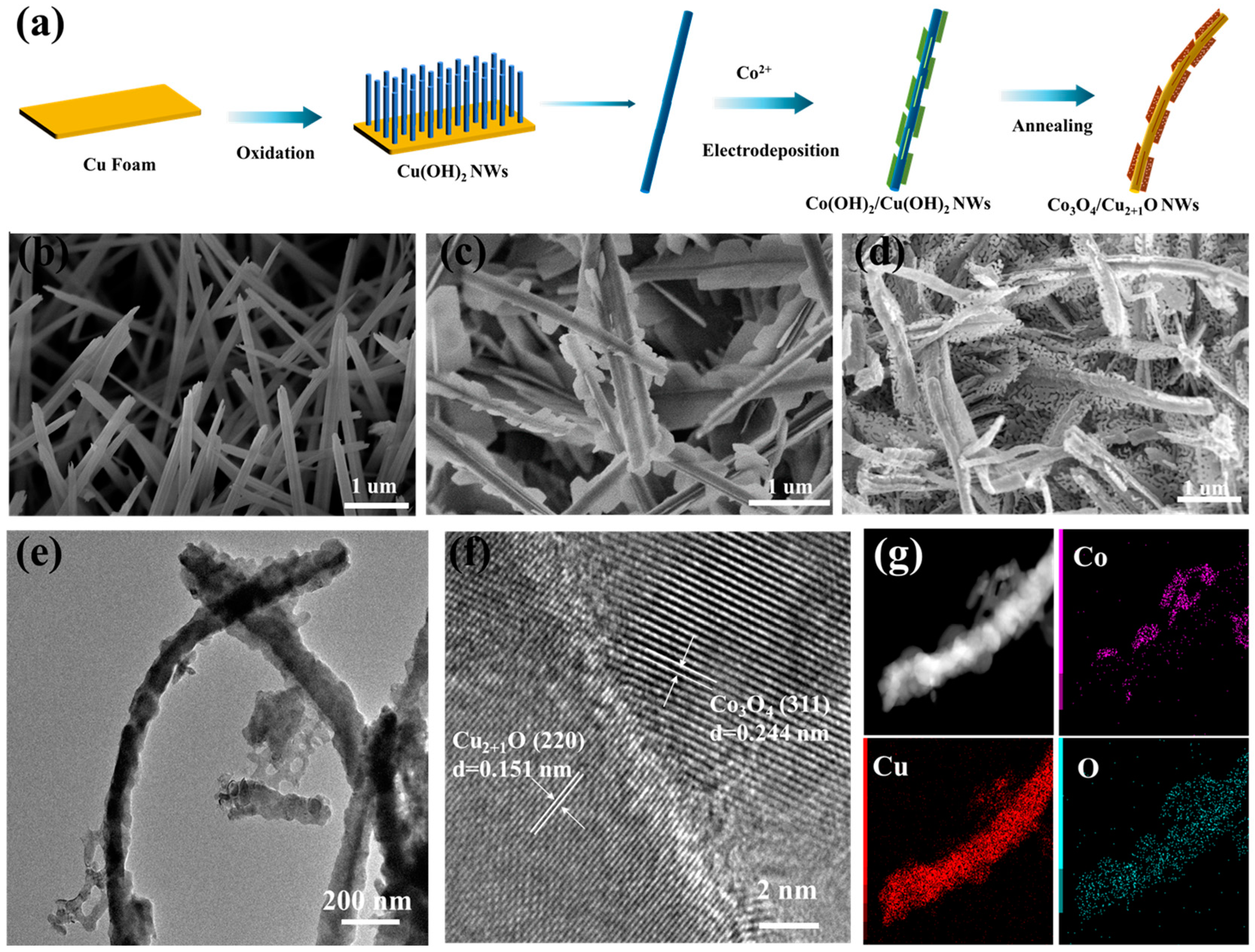
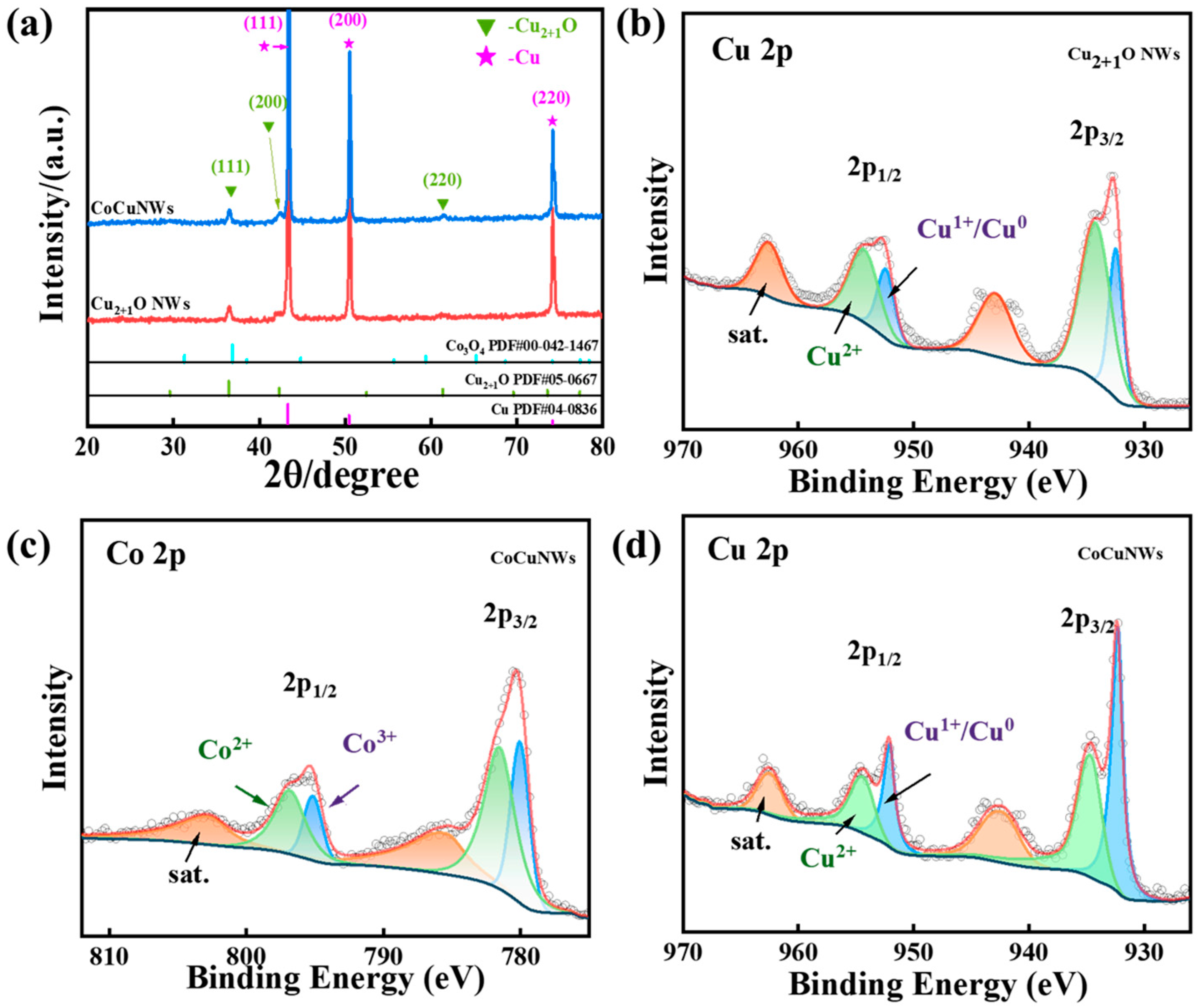
2.1. Material Characterizations
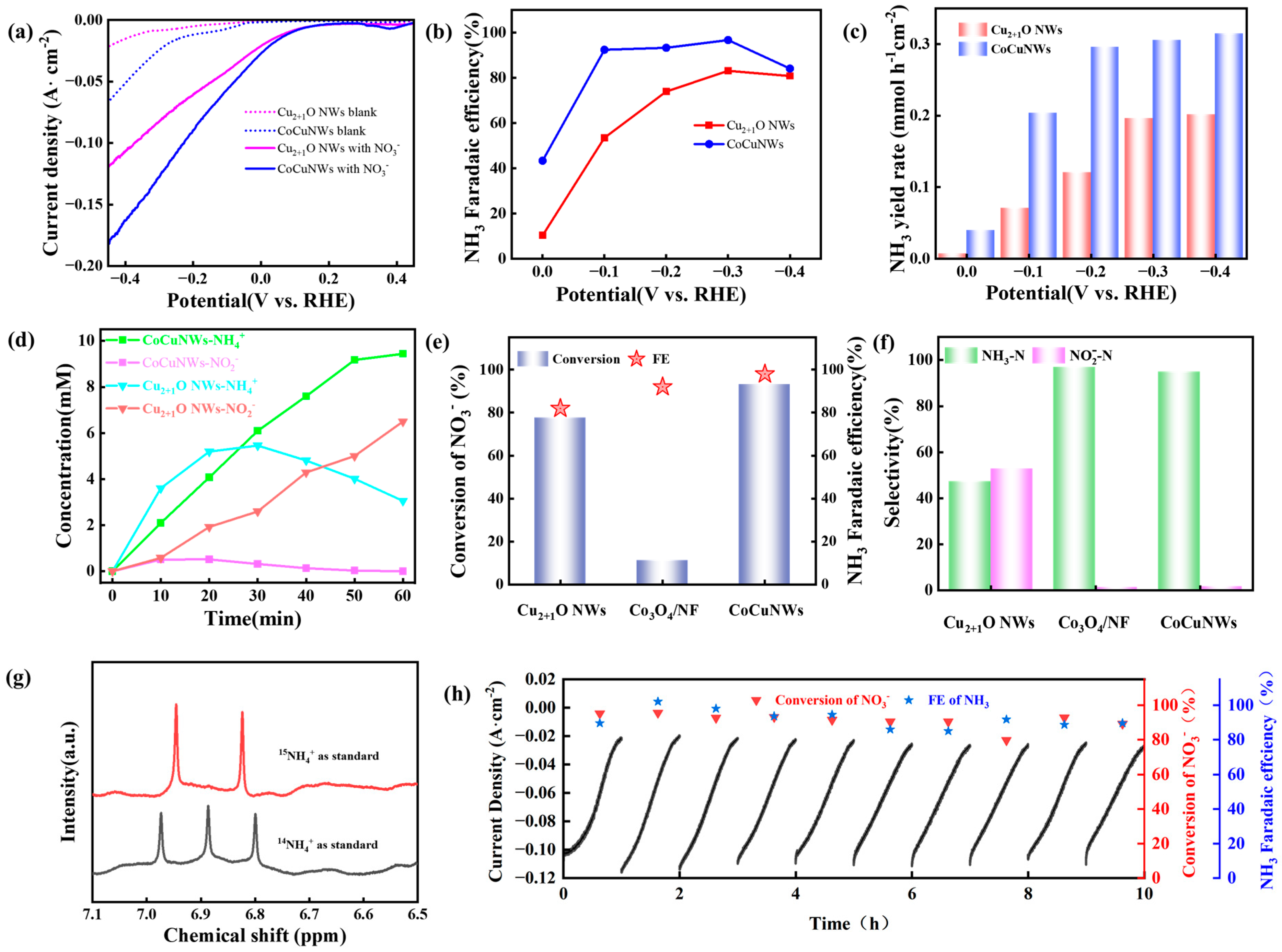
2.2. Electrocatalytic Performance in the Reduction of NO3− to NH3
2.3. Mechanism Study of CoCuNWs in NO3−RR
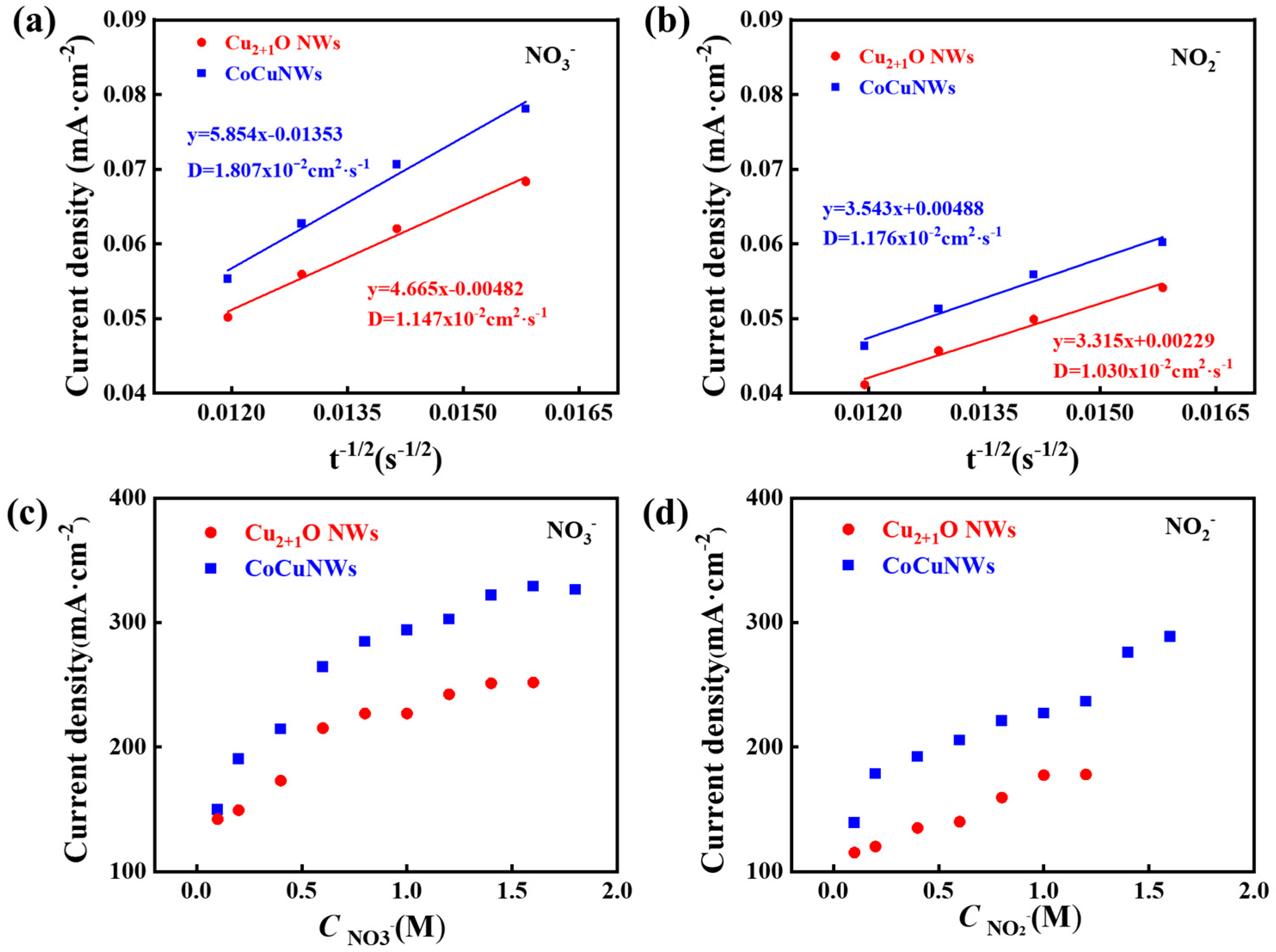
2.3.1. Kinetics Study: Diffusion and Adsorption Behavior on CoCuNWs and Cu2+1O NWs Catalysts
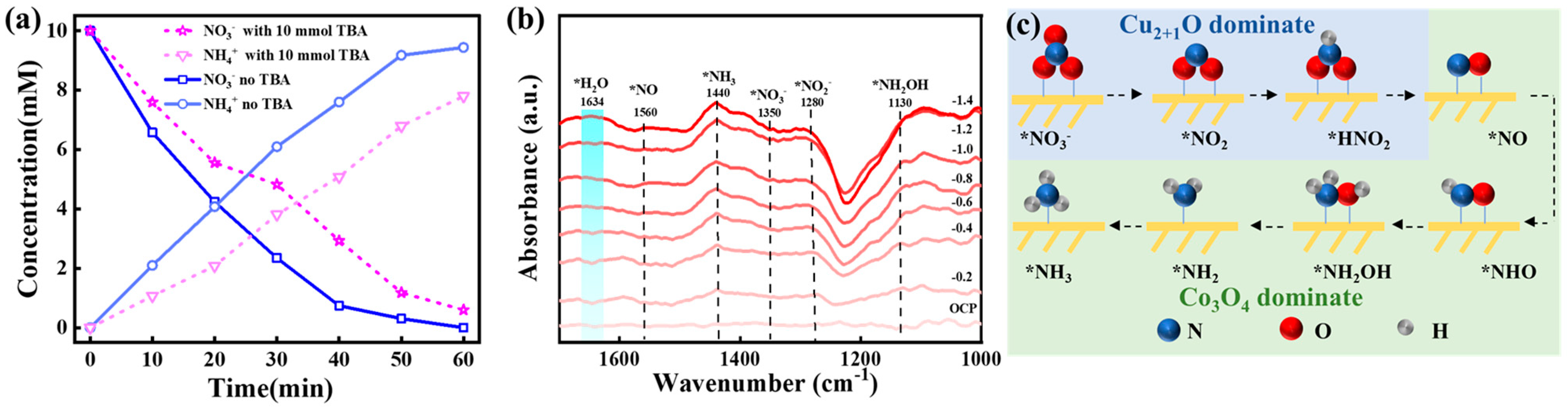
2.3.2. Reaction Pathway on CoCuNWs
3. Materials and Methods
3.1. Reagent and Chemicals
3.2. Material Synthesis
3.2.1. Synthesis of Cu(OH)2 NWs
3.2.2. Synthesis of Co(OH)2/Cu(OH)2 NWs
3.2.3. Synthesis of CoCuNWs, Cu2+1O NWs and Co3O4/NF
3.3. Materials Characterization
3.4. Electrochemical Measurements
3.5. ATR–SEIRAS Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, H.; Peramaiah, K.; Huang, K.-W. Rethinking nitrate reduction: Redirecting electrochemical efforts from ammonia to nitrogen for realistic environmental impacts. Energy Environ. Sci. 2024, 17, 2682–2685. [Google Scholar] [CrossRef]
- Gruber, N.; Galloway, J.N. An Earth-system perspective of the global nitrogen cycle. Nature 2008, 451, 293–296. [Google Scholar] [CrossRef]
- Su, W.; Cheng, X.; Shang, S.; Pan, R.; Qi, M.; Sang, Q.; Xie, Z.; Zhang, H.; Wang, K.; Liu, Y. Advances in In Situ Investigations of Heterogeneous Catalytic Ammonia Synthesis. Catalysts 2025, 15, 160. [Google Scholar] [CrossRef]
- Asif, M.; Sidra Bibi, S.; Ahmed, S.; Irshad, M.; Shakir Hussain, M.; Zeb, H.; Kashif Khan, M.; Kim, J. Recent advances in green hydrogen production, storage and commercial-scale use via catalytic ammonia cracking. Chem. Eng. J. 2023, 473, 145381. [Google Scholar] [CrossRef]
- Lan, R.; Irvine, J.T.; Tao, S. Synthesis of ammonia directly from air and water at ambient temperature and pressure. Sci. Rep. 2013, 3, 1145. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ma, Y.; Chen, J.; Zhang, W.X.; Yang, J. Electrocatalytic reduction of nitrate-a step towards a sustainable nitrogen cycle. Chem. Soc. Rev. 2022, 51, 2710–2758. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, S.; Hu, X.; Zhang, C.; Ostrikov, K.; Shao, T. Recent advances in plasma-enabled ammonia synthesis: State-of-the-art, challenges, and outlook. Faraday Discuss. 2023, 243, 473–491. [Google Scholar] [CrossRef]
- Zeng, Q.; An, W.; Peng, D.; Liu, Q.; Zhang, X.; Ge, H.; Liu, H. Research Progress in Photocatalytic-Coupled Microbial Electrochemical Technology in Wastewater Treatment. Catalysts 2025, 15, 81. [Google Scholar] [CrossRef]
- Chen, S.; Liu, D.; Peng, T. Fundamentals and Recent Progress of Photocatalytic Nitrogen-Fixation Reaction over Semiconductors. Sol. RRL 2021, 5, 2000487. [Google Scholar] [CrossRef]
- Liang, J.; Li, Z.; Zhang, L.; He, X.; Luo, Y.; Zheng, D.; Wang, Y.; Li, T.; Yan, H.; Ying, B.; et al. Advances in ammonia electrosynthesis from ambient nitrate/nitrite reduction. Chem 2023, 9, 1768–1827. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, Y.; Zhou, J.; Liu, F.; Hao, F.; Fan, Z. Electrochemical Nitrate Reduction: Ammonia Synthesis and the Beyond. Adv. Mater. 2024, 36, e2304021. [Google Scholar] [CrossRef]
- Kim, K.-H.; Lim, J. Recent Advances in Membrane Electrode Assembly Based Nitrate Reduction Electrolyzers for Sustainable Ammonia Synthesis. Catalysts 2025, 15, 172. [Google Scholar] [CrossRef]
- Yu, J.; Du, Y.; Liu, S.; Liu, Y.; Li, Y.; Cheng, Y.; Cao, P.; Zhang, X.; Yuan, X.; Liu, N.; et al. Co-Carbonization of Straw and ZIF-67 to the Co/Biomass Carbon for Electrocatalytic Nitrate Reduction. Catalysts 2024, 14, 817. [Google Scholar] [CrossRef]
- Zhang, R.; Hong, H.; Liu, X.; Zhang, S.; Li, C.; Cui, H.; Wang, Y.; Liu, J.; Hou, Y.; Li, P.; et al. Molecular Engineering of a Metal-Organic Polymer for Enhanced Electrochemical Nitrate-to-Ammonia Conversion and Zinc Nitrate Batteries. Angew. Chem. Int. Ed. 2023, 62, e202309930. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, Y.; Lin, H.; Lu, X.; Zhou, C.; Li, Y.Y. Copper-based electro-catalytic nitrate reduction to ammonia from water: Mechanism, preparation, and research directions. Environ. Sci. Ecotechnol. 2024, 20, 100383. [Google Scholar] [CrossRef]
- Hu, T.; Wang, C.; Wang, M.; Li, C.M.; Guo, C. Theoretical Insights into Superior Nitrate Reduction to Ammonia Performance of Copper Catalysts. ACS Catal. 2021, 11, 14417–14427. [Google Scholar] [CrossRef]
- Wei, M.; Li, S.; Wang, X.; Zuo, G.; Wang, H.; Meng, X.; Wang, J. A Perspective on Cu-Based Electrocatalysts for Nitrate Reduction for Ammonia Synthesis. Adv. Energy Sustain. Res. 2023, 5, 2300173. [Google Scholar] [CrossRef]
- Ren, Z.; Shi, K.; Feng, X. Elucidating the Intrinsic Activity and Selectivity of Cu for Nitrate Electroreduction. ACS Energy Lett. 2023, 8, 3658–3665. [Google Scholar] [CrossRef]
- Ren, T.; Sheng, Y.; Wang, M.; Ren, K.; Wang, L.; Xu, Y. Recent Advances of Cu-Based Materials for Electrochemical Nitrate Reduction to Ammonia. Chin. J. Struct. Chem. 2022, 41, 2212089–2212106. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, S.; Hu, G. Recent Progress and Perspectives on Transition Metal-Based Electrocatalysts for Efficient Nitrate Reduction. Energy Fuels 2024, 38, 6701–6722. [Google Scholar] [CrossRef]
- Chen, F.; Chen, C.; Hu, Q.; Xiang, B.; Song, T.; Zou, X.; Li, W.; Xiong, B.; Deng, M. Synthesis of CuO@CoNi LDH on Cu foam for high-performance supercapacitors. Chem. Eng. J. 2020, 401, 126145. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, T.; Li, X.; Hu, G. Highly selective and efficient electrocatalytic removal of low-concentration nitrate ions using self-supported porous Ni0.8Cu0.2O@CuO heterostructure arrays. Sep. Purif. Technol. 2023, 325, 124579. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, A.; Wang, Z.; Huang, L.; Li, J.; Li, F.; Wicks, J.; Luo, M.; Nam, D.H.; Tan, C.S.; et al. Enhanced Nitrate-to-Ammonia Activity on Copper-Nickel Alloys via Tuning of Intermediate Adsorption. J. Am. Chem. Soc. 2020, 142, 5702–5708. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, J.; Zheng, M.; Jin, X.; Shen, Z.; Li, Z.; Wang, Y.; Wang, Q.; Wang, X.; Wei, H.; et al. Fe/Cu diatomic catalysts for electrochemical nitrate reduction to ammonia. Nat. Commun. 2023, 14, 3634. [Google Scholar] [CrossRef]
- Hao, R.; Fang, S.; Tian, L.; Xia, R.; Guan, Q.; Jiao, L.; Liu, Y.; Li, W. Elucidation of the electrocatalytic activity origin of Fe3C species and application in the NOx full conversion to valuable ammonia. Chem. Eng. J. 2023, 467, 143371. [Google Scholar] [CrossRef]
- Chen, L.; Hao, Y.; Chu, J.; Liu, S.; Bai, F.; Luo, W. Electrocatalytic nitrate reduction to ammonia: A perspective on Fe/Cu-containing catalysts. Chin. J. Catal. 2024, 58, 25–36. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Z.; Huang, Z.; Zheng, L.; Zhao, X.; Hu, J.; Cao, H.; Li, Y.; Ren, X.; Ouyang, X.; et al. Modulating the valence electronic structure of Co3O4 to improve catalytic activity of electrochemical nitrate-to-ammonia conversion. Sci. China Mater. 2023, 66, 3901–3911. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Dong, L.; Du, F.; Li, J.; Chen, C.; Ma, R.; Li, C.; Guo, C. Bimetallic CuCo nanocrystals to tailor absorption energy of intermediators for efficient electrochemical nitrate conversion to ammonia in neutral electrolyte. J. Power Sources 2023, 556, 232523. [Google Scholar] [CrossRef]
- Ren, Z.; Shi, K.; Meng, Z.; Willis, M.D.; Feng, X. Complete Single-Pass Conversion of Dilute Nitrate to Ammonia Using Cu/Co(OH)2 Tandem Electrocatalyst. ACS Energy Lett. 2024, 9, 3849–3858. [Google Scholar] [CrossRef]
- Li, S.; Jin, H.; Wang, Y. Recent progress on the synthesis of metal alloy nanowires as electrocatalysts. Nanoscale 2023, 15, 2488–2515. [Google Scholar] [CrossRef]
- Fang, J.Y.; Zheng, Q.Z.; Lou, Y.Y.; Zhao, K.M.; Hu, S.N.; Li, G.; Akdim, O.; Huang, X.Y.; Sun, S.G. Ampere-level current density ammonia electrochemical synthesis using CuCo nanosheets simulating nitrite reductase bifunctional nature. Nat. Commun. 2022, 13, 7899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Sun, P.; Huang, Y.; Tang, M.; Zou, X.; Pan, Z.; Huo, X.; Wu, J.; Lin, C.; Sun, Z.; et al. Electrochemical Nitrate Reduction to Ammonia on CuCo Nanowires at Practical Level. Adv. Funct. Mater. 2024, 34, 2405179. [Google Scholar] [CrossRef]
- Liu, X.; Xiang, T.; Liang, Y.; Zhou, X.; Wang, Z.; Liu, J.; Cheng, M.; Zhou, C.; Yang, Y. When electrocatalytic nitrate reduction meets copper-based atomic site catalysts. J. Mater. Chem. A 2024, 12, 26367–26389. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Li, Y.; Yu, L.; Chen, F.; Li, L. Conductive polymer protection strategy to promote electrochemical nitrate reduction to ammonia in highly acidic condition over Cu-based catalyst. Chem. Eng. J. 2024, 481, 148596. [Google Scholar] [CrossRef]
- Groß, A. Challenges in the modeling of elementary steps in electrocatalysis. Curr. Opin. Electrochem. 2023, 37, 101170. [Google Scholar] [CrossRef]
- Han, J.; Duan, L.; He, L.; Li, X. Template-assisted synthesis of porous Co3O4 nanosheet with excellent electrochemical performance for rechargeable lithium-ion batteries. J. Nanopart. Res. 2021, 23, 241. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, D.; Lu, D.; Wang, H. The synergistic tandem effect of Cu2+1O and Co3O4 enhances the activity and selectivity of nitrate reduction to ammonia in neutral solution. Appl. Catal. A Gen. 2024, 677, 119695. [Google Scholar] [CrossRef]
- Xiang, S.; Dong, L.; Wang, Z.-Q.; Han, X.; Daemen, L.L.; Li, J.; Cheng, Y.; Guo, Y.; Liu, X.; Hu, Y.; et al. A unique Co@CoO catalyst for hydrogenolysis of biomass-derived 5-hydroxymethylfurfural to 2,5-dimethylfuran. Nat. Commun. 2022, 13, 3657. [Google Scholar] [CrossRef]
- Gao, J.; Yang, L.; Wang, D.; Cao, D. Hollow Nanotube Ru/Cu2+1O Supported on Copper Foam as a Bifunctional Catalyst for Overall Water Splitting. Chem.–A Eur. J. 2020, 26, 4112–4119. [Google Scholar] [CrossRef]
- Sun, R.; Wei, C.; Huang, Z.; Niu, S.; Han, X.; Chen, C.; Wang, H.; Song, J.; Yi, J.-D.; Wu, G.; et al. Cu2+1O/CuOx heterostructures promote the electrosynthesis of C2+ products from CO2. Nano Res. 2023, 16, 4698–4705. [Google Scholar] [CrossRef]
- Weng, Y.; You, H.; Liu, D.; Chen, H.; Lin, H.; Fu, G.; Si, F.; Zhang, J.; Fu, X.-Z.; Luo, J.-L. In-situ fabrication of defect rich Cu2+1O on electrodeposited Cu cone arrays for promoting electrocatalytic anode hydrogen production. Chem. Eng. J. 2024, 499, 155921. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Jia, R.; Yu, Y.; Zhang, B. Unveiling the Activity Origin of a Copper-based Electrocatalyst for Selective Nitrate Reduction to Ammonia. Angew. Chem. Int. Ed. 2020, 59, 5350–5354. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, J.; Du, F.; Yang, L.; Huang, S.; Gao, J.; Li, C.; Guo, C. A core–shell copper oxides-cobalt oxides heterostructure nanowire arrays for nitrate reduction to ammonia with high yield rate. Green Energy Environ. 2023, 8, 1619–1629. [Google Scholar] [CrossRef]
- Gao, H.; Lv, X.; Zhang, M.; Li, Q.; Chen, J.; Hu, Z.; Jia, H. Copper-cobalt strong interaction to improve photothermocatalytic performance of cobalt-copper oxides supported on copper foam for toluene oxidation. Chem. Eng. J. 2022, 434, 134618. [Google Scholar] [CrossRef]
- Anantharaj, S.; Noda, S. Appropriate Use of Electrochemical Impedance Spectroscopy in Water Splitting Electrocatalysis. Chemelectrochem 2020, 7, 2297–2308. [Google Scholar] [CrossRef]
- Mohamed, I.M.A.; Kanagaraj, P.; Yasin, A.S.; Iqbal, W.; Liu, C. Electrochemical impedance investigation of urea oxidation in alkaline media based on electrospun nanofibers towards the technology of direct-urea fuel cells. J. Alloys Compd. 2020, 816, 152513. [Google Scholar] [CrossRef]
- Wang, J.; Feng, J.; Soyol-Erdene, T.-O.; Wei, Z.; Tang, W. Electrodeposited NiCoP on nickel foam as a self-supported cathode for highly selective electrochemical reduction of nitrate to ammonia. Sep. Purif. Technol. 2023, 320, 124155. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, X.M.; Liu, X.; Liu, Z.; Wang, Y.Q. Cu(2+1)O/Ag Heterostructure for Boosting the Electrocatalytic Nitrate Reduction to Ammonia Performance. Inorg. Chem. 2023, 62, 7525–7532. [Google Scholar] [CrossRef]
- Shafiq, F.; Yang, L.; Zhu, W. Recent progress in the advanced strategies, rational design, and engineering of electrocatalysts for nitrate reduction toward ammonia. Phys. Chem. Chem. Phys. 2024, 26, 11208–11216. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, X.; Eikerling, M. The rate-determining term of electrocatalytic reactions with first-order kinetics. Electrochim. Acta 2021, 393, 139019. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [PubMed]
- Aouina, N.; Cachet, H.; Debiemme-chouvy, C.; Tran, T.T.M. Insight into the electroreduction of nitrate ions at a copper electrode, in neutral solution, after determination of their diffusion coefficient by electrochemical impedance spectroscopy. Electrochim. Acta 2010, 55, 7341–7345. [Google Scholar] [CrossRef]
- Panda, S.; Maity, T.; Sarkar, S.; Manna, A.K.; Mondal, J.; Haldar, R. Diffusion-programmed catalysis in nanoporous material. Nat. Commun. 2025, 16, 1231. [Google Scholar] [CrossRef]
- Grebenkov, D.S. Diffusion-Controlled Reactions: An Overview. Molecules 2023, 28, 7570. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhao, C.; Sha, Y.; Li, Z.; Liu, T.; Ling, M.; Zhou, S.; Liang, C. Electrochemical redox behavior of organic quinone compounds in aqueous metal ion electrolytes. Nano Energy 2020, 73, 104766. [Google Scholar] [CrossRef]
- Suárez, I.J.; Otero, T.F.; Márquez, M. Diffusion Coefficients in Swelling Polypyrrole: ESCR and Cottrell Models. J. Phys. Chem. B 2005, 109, 1723–1729. [Google Scholar] [CrossRef]
- Yeh, H.S.J.; Wills, G.B.J.J.o.C.; Data, E. Diffusion coefficient of sodium nitrate in aqueous solution at 25.deg. as a function of concentration from 0.1 to 1.0M. J. Chem. Eng. Data 1970, 15, 187–189. [Google Scholar] [CrossRef]
- Christophe, J.; Tsakova, V.; Buess-Herman, C. Electroreduction of Nitrate at Copper Electrodes and Copper-PANI Composite Layers. Z. Fur Phys. Chem. -Int. J. Res. Phys. Chem. Chem. Phys. 2007, 221, 1123–1136. [Google Scholar] [CrossRef]
- Hao, L.; Ren, Q.; Yang, J.; Luo, L.; Ren, Y.; Guo, X.; Zhou, H.; Xu, M.; Kong, X.; Li, Z.; et al. Promoting Electrocatalytic Hydrogenation of Oxalic Acid to Glycolic Acid via an Al3+ Ion Adsorption Strategy Coupled with Ethylene Glycol Oxidation. ACS Appl. Mater. Interfaces 2023, 15, 13176–13185. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Seri-Levy, A.; Avnir, D. Effects of heterogeneous surface geometry on adsorption. Langmuir 1993, 9, 3067–3076. [Google Scholar] [CrossRef]
- Ritter, H.; Ramm, J.H.; Brühwiler, D. Influence of the Structural Properties of Mesoporous Silica on the Adsorption of Guest Molecules. Materials 2010, 3, 4500–4509. [Google Scholar] [CrossRef]
- Monteiro, M.K.S.; Cardozo, J.C.; Araújo, A.M.d.M.; Gondim, A.D.; Feijoó, T.N.; Loor-Urgilés, L.D.; Martínez-Huitle, C.A.; Quiroz, M.A.; dos Santos, E.V. Electrochemical Looping Green Hydrogen Production by Using Water Electrochemically Treated as a Raw Material for the Electrolyzer. Catalysts 2025, 15, 447. [Google Scholar] [CrossRef]
- Shah, A.H.; Wan, C.; Huang, Y.; Duan, X. Toward Molecular Level Understandings of Hydrogen Evolution Reaction on Platinum Surface. J. Phys. Chem. C 2023, 127, 12841–12848. [Google Scholar] [CrossRef]
- Zheng, S.; Yang, X.; Shi, Z.Z.; Ding, H.; Pan, F.; Li, J.F. The Loss of Interfacial Water-Adsorbate Hydrogen Bond Connectivity Position Surface-Active Hydrogen as a Crucial Intermediate to Enhance Nitrate Reduction Reaction. J. Am. Chem. Soc. 2024, 146, 26965–26974. [Google Scholar] [CrossRef]
- Gan, G.; Hong, G.; Zhang, W. Active Hydrogen for Electrochemical Ammonia Synthesis. Adv. Funct. Mater. 2024, 2401472. [Google Scholar] [CrossRef]
- Yan, S.; Gong, S.; Zhang, S.; Sun, H.; Yu, H.; Chen, L.; Han, J.; Wang, H. In Situ/Operando Insights into the Selectivity of CH4/C2H4 in CO2 Electroreduction by Fine-Tuning the Composition of Cu/SiO2 Catalysts. ChemSusChem 2025, e2402461. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I. Identification of Neutral and Charged NxOy Surface Species by IR Spectroscopy. Catal. Rev. 2000, 42, 71–144. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Fu, Y.; Sang, J.; Wei, P.; Li, R.; Gao, D.; Wang, G.; Bao, X. Ammonia electrosynthesis from nitrate using a stable amorphous/crystalline dual-phase Cu catalyst. Nat. Commun. 2025, 16, 897. [Google Scholar] [CrossRef]
- Yu, X.-H.; Yi, J.-L.; Zhang, R.-L.; Wang, F.-Y.; Liu, L. Hollow carbon spheres and their noble metal-free hybrids in catalysis. Front. Chem. Sci. Eng. 2021, 15, 1380–1407. [Google Scholar] [CrossRef]
- Dou, F.; Guo, F.; Li, B.; Zhang, K.; Graham, N.; Yu, W. Pulsed electro-catalysis enables effective conversion of low-concentration nitrate to ammonia over Cu2O@Pd tandem catalyst. J. Hazard. Mater. 2024, 472, 134522. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Willis, M.D.; Ren, Z.; Feng, X. Efficient Recycling of Dilute Nitrate to Ammonia Using Cu Nanowire Electrocatalyst. J. Phys. Chem. C 2023, 127, 20710–20717. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, C.; Bai, X.; Wang, Z.; Yu, X.; Tong, X.; Wang, Z.; Zhang, H.; Pang, H.; Zhou, L.; et al. Facile Synthesis of Carbon Nanobelts Decorated with Cu and Pd for Nitrate Electroreduction to Ammonia. ACS Appl. Mater. Interfaces 2022, 14, 30969–30978. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Rong, H.; Zhang, J.; Wang, D.; Li, Y. Modulating the local coordination environment of single-atom catalysts for enhanced catalytic performance. Nano Res. 2020, 13, 1842–1855. [Google Scholar] [CrossRef]
- Zhao, F.; Li, G.; Hua, Q.; Cao, J.; Song, J.; Gao, L.; Ma, T.; Ren, X.; Liu, A. A two-dimensional MXene-supported CuRu catalyst for efficient electrochemical nitrate reduction to ammonia. Catal. Sci. Technol. 2023, 13, 5543–5548. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Yan, S.; Zhang, J.; Wang, H. Kinetic Understanding of the Enhanced Electroreduction of Nitrate to Ammonia for Co3O4–Modified Cu2+1O Nanowire Electrocatalyst. Catalysts 2025, 15, 491. https://doi.org/10.3390/catal15050491
Yu H, Yan S, Zhang J, Wang H. Kinetic Understanding of the Enhanced Electroreduction of Nitrate to Ammonia for Co3O4–Modified Cu2+1O Nanowire Electrocatalyst. Catalysts. 2025; 15(5):491. https://doi.org/10.3390/catal15050491
Chicago/Turabian StyleYu, Hao, Shen Yan, Jiahua Zhang, and Hua Wang. 2025. "Kinetic Understanding of the Enhanced Electroreduction of Nitrate to Ammonia for Co3O4–Modified Cu2+1O Nanowire Electrocatalyst" Catalysts 15, no. 5: 491. https://doi.org/10.3390/catal15050491
APA StyleYu, H., Yan, S., Zhang, J., & Wang, H. (2025). Kinetic Understanding of the Enhanced Electroreduction of Nitrate to Ammonia for Co3O4–Modified Cu2+1O Nanowire Electrocatalyst. Catalysts, 15(5), 491. https://doi.org/10.3390/catal15050491






