Self-Supported Ir-FeOOH on Iron Foam for Efficient Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Results and Discussion
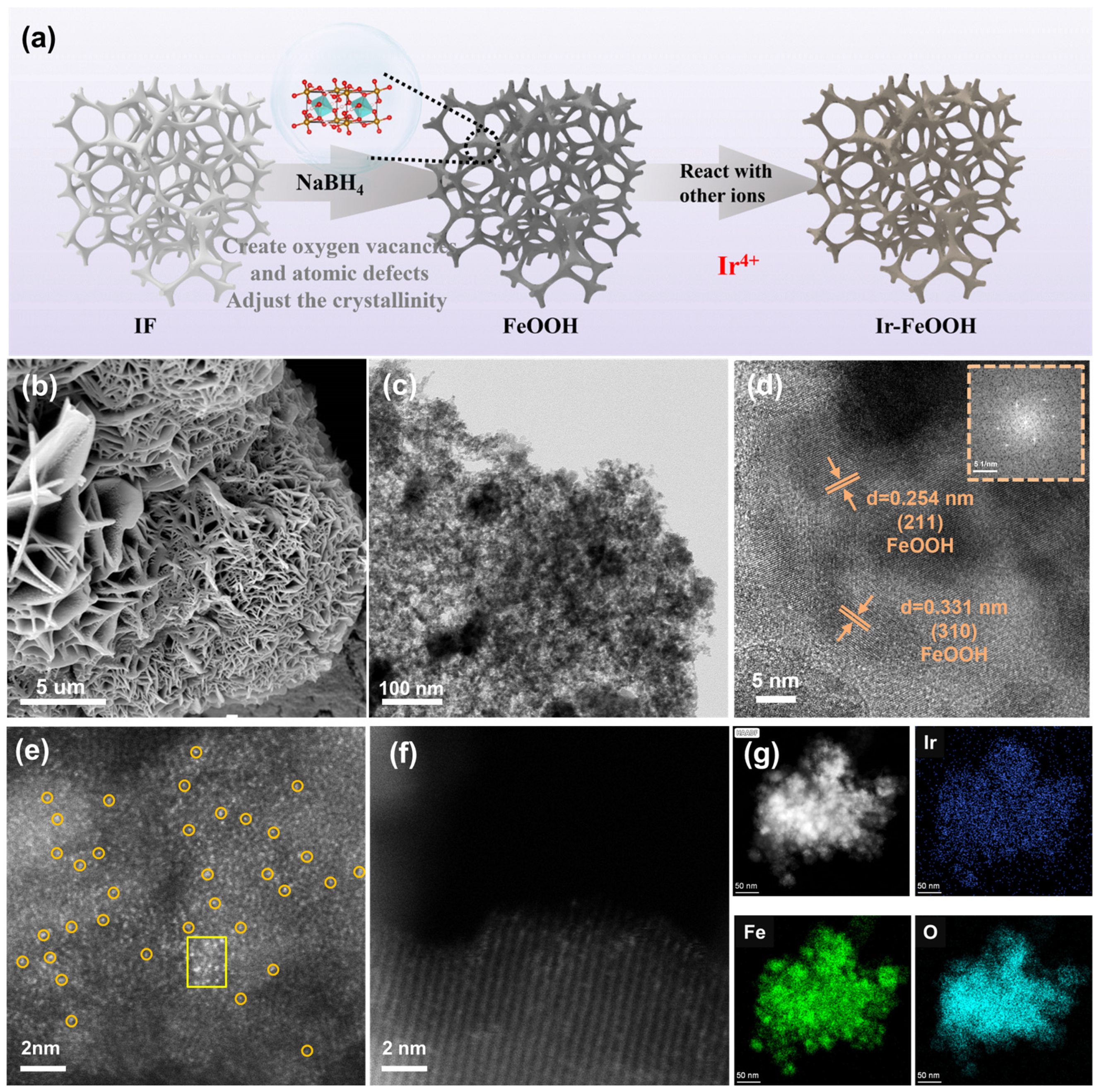
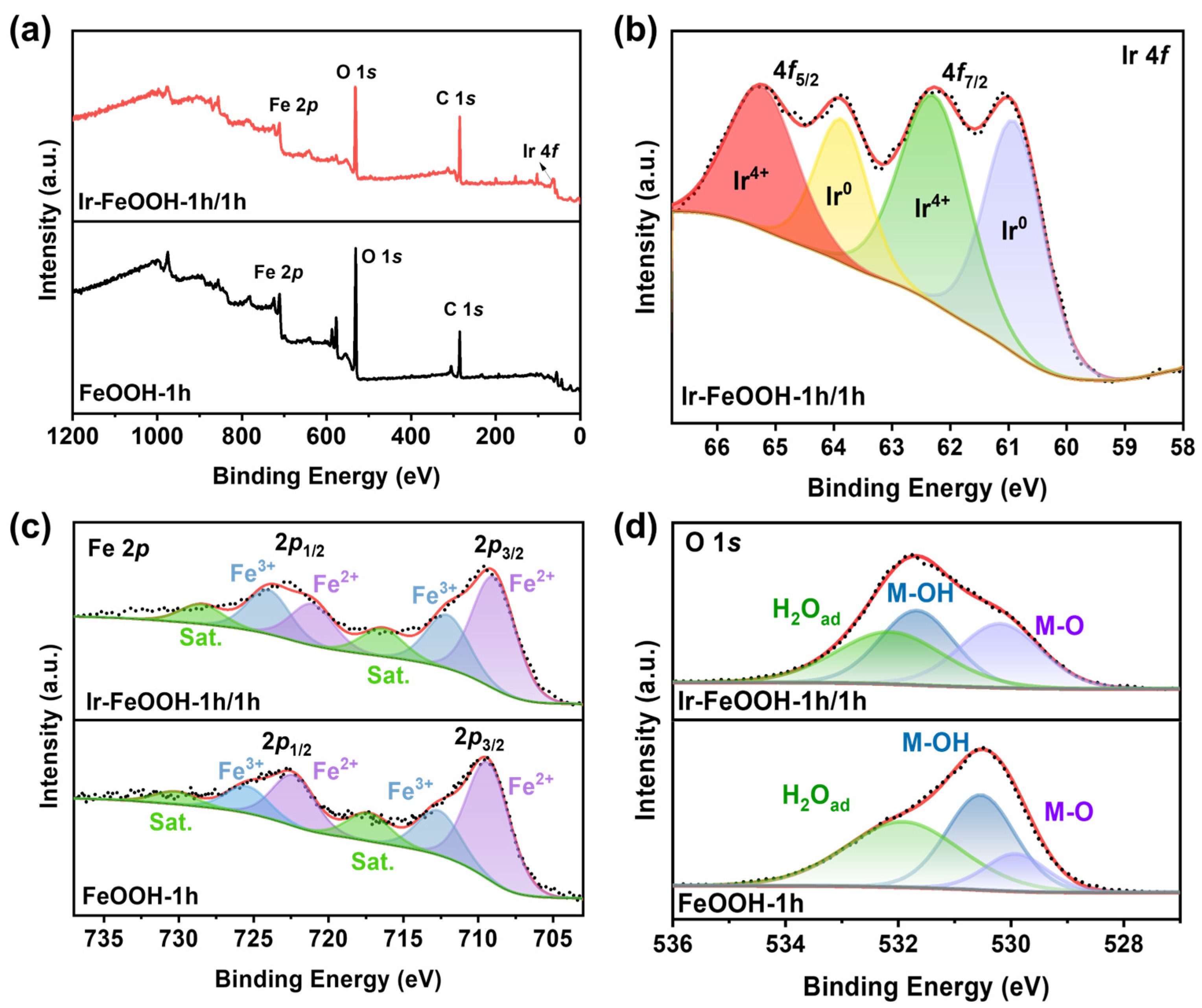
2.1. Structural Characterization of Catalysts
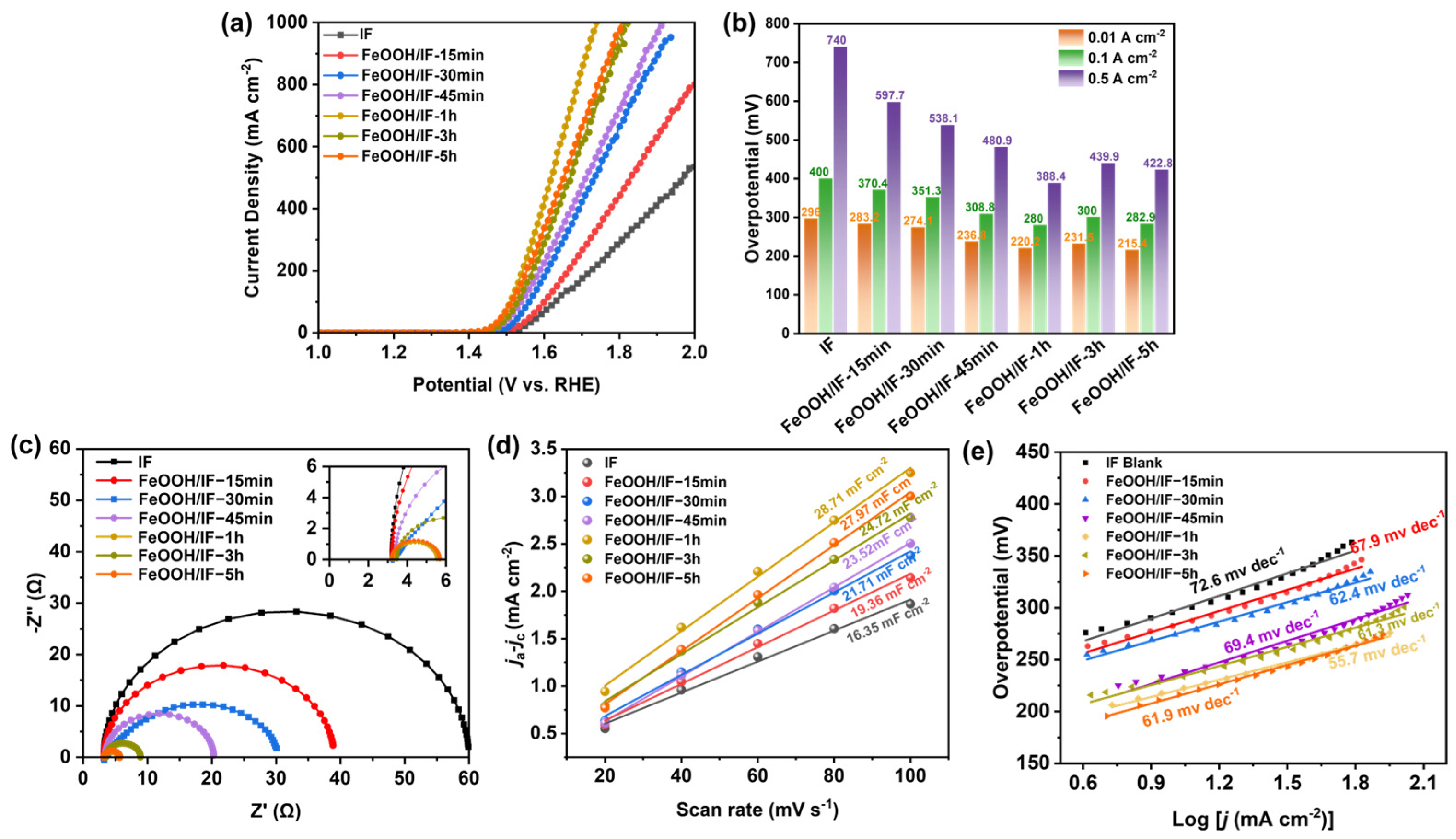
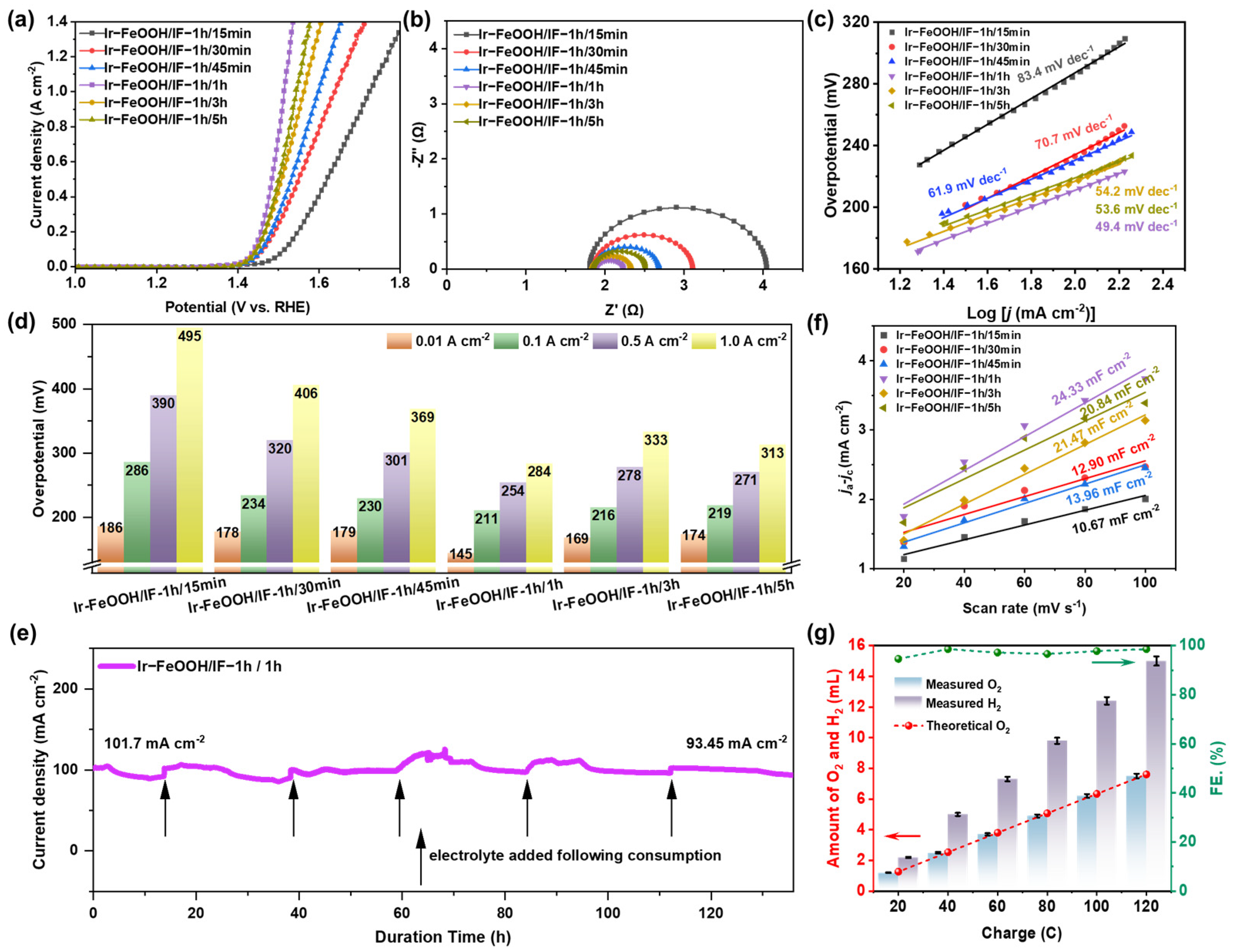
2.2. Electrocatalytic Performances of Ir-FeOOH/IF
3. Materials and Methods
3.1. Materials
3.2. Synthesis of FeOOH/IF
3.3. Synthesis of Ir-FeOOH/IF
3.4. Instruments and Characterization
3.5. Catalyst Characterization and Electrochemical Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lv, L.; Yang, Z.; Chen, K.; Wang, C.; Xiong, Y. 2D Layered Double Hydroxides for Oxygen Evolution Reaction: From Fundamental Design to Application. Adv. Energy Mater. 2019, 9, 1803358. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, Q.; Zhong, Y.; Tahini, H.A.; Shao, Z.; Wang, H. Metal Oxide-Based Materials as an Emerging Family of Hydrogen Evolution Electrocatalysts. Energy Environ. Sci. 2020, 13, 3361–3392. [Google Scholar] [CrossRef]
- Joo, J.; Kim, T.; Lee, J.; Choi, S.-I.; Lee, K. Morphology-Controlled Metal Sulfides and Phosphides for Electrochemical Water Splitting. Adv. Mater. 2019, 31, 1806682. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, M.; Yu, Y.; Zhang, B. Recent Advances in Nanostructured Transition Metal Phosphides: Synthesis and Energy-Related Applications. Energy Environ. Sci. 2020, 13, 4564–4582. [Google Scholar] [CrossRef]
- Wan, R.; Yuan, T.; Wang, L.; Li, B.; Liu, M.; Zhao, B. Earth-Abundant Electrocatalysts for Acidic Oxygen Evolution. Nat. Catal. 2024, 7, 1288–1304. [Google Scholar] [CrossRef]
- Boakye, F.O.; Harrath, K.; Zhang, D.; You, Y.; Zhang, W.; Wang, Z.; Zhang, H.; Zhu, J.; Long, J.; Zhu, J.; et al. Synergistic Engineering of Dopant and Support of Ru Oxide Catalyst Enables Ultrahigh Performance for Acidic Oxygen Evolution. Adv. Funct. Mater. 2024, 34, 2408714. [Google Scholar] [CrossRef]
- Li, Y.; Hu, L.; Zheng, W.; Peng, X.; Liu, M.; Chu, P.K.; Lee, L.Y.S. Ni/Co-Based Nanosheet Arrays for Efficient Oxygen Evolution Reaction. Nano Energy 2018, 52, 360–368. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, R.; Zhou, X.; Huang, G.; Wu, X.; Shen, M. Coating of Ni on Fe (Oxy) Hydroxide: Superior Catalytic Activity for Oxygen-Involved Reaction During Water Splitting. ACS Sustain. Chem. Eng. 2019, 7, 19832–19838. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, C.; Geng, F.; Zhuo, S.; Zhang, B. Nanoporous Hollow Transition Metal Chalcogenide Nanosheets Synthesized via the Anion-Exchange Reaction of Metal Hydroxides with Chalcogenide Ions. ACS Nano 2014, 8, 10909–10919. [Google Scholar] [CrossRef]
- Zheng, X.; Han, X.; Cao, Y.; Zhang, Y.; Nordlund, D.; Wang, J.; Chou, S.; Liu, H.; Li, L.; Zhong, C.; et al. Identifying Dense NiSe2/CoSe2 Heterointerfaces Coupled with Surface High-Valence Bimetallic Sites for Synergistically Enhanced Oxygen Electrocatalysis. Adv. Mater. 2020, 32, 2000607. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Cao, L.; Dai, P.; Gu, X.; Liu, D.; Li, L.; Wang, Y.; Zhao, X. Metal-Organic Frameworks Derived Nanotube of Nickel–Cobalt Bimetal Phosphides as Highly Efficient Electrocatalysts for Overall Water Splitting. Adv. Funct. Mater. 2017, 27, 1703455. [Google Scholar] [CrossRef]
- Mijowska, E.; Dymerska, A.; Leniec, G.; Maślana, K.; Aleksandrzak, M.; Zairov, R.; Nazmutdinov, R.; Chen, X. Ni-Based Compounds in Multiwalled Graphitic Shell for Electrocatalytic Oxygen Evolution Reactions. Adv. Compos. Hybrid Mater. 2024, 7, 172. [Google Scholar] [CrossRef]
- Ajmal, M.; Guo, X.; Memon, M.A.; Asim, M.; Shi, C.; Gao, R.; Pan, L.; Zhang, X.; Huang, Z.-F.; Zou, J.-J. Ligand-Regulated Ni-Based Coordination Compounds to Promote Self-Reconstruction for Improved Oxygen Evolution Reaction. J. Mater. Chem. A 2024, 12, 18294–18303. [Google Scholar] [CrossRef]
- Amin, H.M.A.; Attia, M.; Tetzlaff, D.; Apfel, U.-P. Tailoring the Electrocatalytic Activity of Pentlandite FexNi9-XS8 Nanoparticles via Variation of the Fe: Ni Ratio for Enhanced Water Oxidation. ChemElectroChem 2021, 8, 3863–3874. [Google Scholar] [CrossRef]
- Wu, L.; Ning, M.; Xing, X.; Wang, Y.; Zhang, F.; Gao, G.; Song, S.; Wang, D.; Yuan, C.; Yu, L.; et al. Boosting Oxygen Evolution Reaction of (Fe,Ni)OOH via Defect Engineering for Anion Exchange Membrane Water Electrolysis Under Industrial Conditions. Adv. Mater. 2023, 35, 2306097. [Google Scholar] [CrossRef]
- Liu, X.; Gong, M.; Xiao, D.; Deng, S.; Liang, J.; Zhao, T.; Lu, Y.; Shen, T.; Zhang, J.; Wang, D. Turning Waste into Treasure: Regulating the Oxygen Corrosion on Fe Foam for Efficient Electrocatalysis. Small 2020, 16, 2000663. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Q.; Cheng, Y.; Xing, H.; Li, J.; Zhu, X.; Ma, L.; Li, Y.; Liu, D. S-Doping Triggers Redox Reactivities of Both Iron and Lattice Oxygen in FeOOH for Low-Cost and High-Performance Water Oxidation. Adv. Funct. Mater. 2022, 32, 2112674. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, H.; Luan, H.; Chen, N.; Gong, P.; Yao, K.; Shen, Y.; Shao, Y. NiFe Layered Double Hydroxides Grown on a Corrosion-Cell Cathode for Oxygen Evolution Electrocatalysis. Adv. Energy Mater. 2022, 12, 210237. [Google Scholar] [CrossRef]
- Hao, Y.; Li, Y.; Wu, J.; Meng, L.; Wang, J.; Jia, C.; Liu, T.; Yang, X.; Liu, Z.-P.; Gong, M. Recognition of Surface Oxygen Intermediates on NiFe Oxyhydroxide Oxygen-Evolving Catalysts by Homogeneous Oxidation Reactivity. J. Am. Chem. Soc. 2021, 143, 1493–1502. [Google Scholar] [CrossRef]
- Kovalskii, V.; Shubin, A.; Chen, Y.; Ovchinnikov, D.; Ruzankin, S.P.; Hasegawa, J.; Zilberberg, I.; Parmon, V.N. Hidden Radical Reactivity of the [FeO]2+ Group in the H-Abstraction from Methane: DFT and CASPT2 Supported Mechanism by the Example of Model Iron (Hydro) Oxide Species. Chem. Phys. Lett. 2017, 679, 193–199. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, X.; Voznyy, O.; Comin, R.; Bajdich, M.; García-Melchor, M.; Han, L.; Xu, J.; Liu, M.; Zheng, L.; et al. Homogeneously Dispersed Multimetal Oxygen-Evolving Catalysts. Science 2016, 352, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Amin, H.M.A.; Cebe, A.; Ayata, S.; Baltruschat, H. Metal-Supported Perovskite as an Efficient Bifunctional Electrocatalyst for Oxygen Reduction and Evolution: Substrate Effect. J. Electrochem. Soc. 2021, 168, 34504. [Google Scholar] [CrossRef]
- Amin, H.M.A.; Zan, L.; Baltruschat, H. Boosting the Bifunctional Catalytic Activity of Co3O4 on Silver and Nickel Substrates for the Alkaline Oxygen Evolution and Reduction Reactions. Surf. Interfaces 2024, 54, 105218. [Google Scholar] [CrossRef]
- Amin, H.M.A.; Apfel, U.-P. Metal-Rich Chalcogenides as Sustainable Electrocatalysts for Oxygen Evolution and Reduction: State of the Art and Future Perspectives. Eur. J. Inorg. Chem. 2020, 2020, 2679–2690. [Google Scholar] [CrossRef]
- Niu, S.; Jiang, W.-J.; Wei, Z.; Tang, T.; Ma, J.; Hu, J.-S.; Wan, L.-J. Se-Doping Activates FeOOH for Cost-Effective and Efficient Electrochemical Water Oxidation. J. Am. Chem. Soc. 2019, 141, 7005–7013. [Google Scholar] [CrossRef]
- Yu, L.; Wu, L.; McElhenny, B.; Song, S.; Luo, D.; Zhang, F.; Yu, Y.; Chen, S.; Ren, Z. Ultrafast Room-Temperature Synthesis of Porous S-Doped Ni/Fe (Oxy)Hydroxide Electrodes for Oxygen Evolution Catalysis in Seawater Splitting. Energy Environ. Sci. 2020, 13, 3439–3446. [Google Scholar] [CrossRef]
- Jung, S.Y.; Kang, S.; Kim, K.M.; Mhin, S.; Kim, J.C.; Kim, S.J.; Enkhtuvshin, E.; Choi, S.; Han, H. Sulfur-Incorporated Nickel-Iron Layered Double Hydroxides for Effective Oxygen Evolution Reaction in Seawater. Appl. Surf. Sci. 2021, 568, 150965. [Google Scholar] [CrossRef]
- Zhong, B.; Kuang, P.; Wang, L.; Yu, J. Hierarchical Porous Nickel Supported NiFeOxHy Nanosheets for Efficient and Robust Oxygen Evolution Electrocatalyst under Industrial Condition. Appl. Catal. B Environ. 2021, 299, 120668. [Google Scholar] [CrossRef]
- Sun, X.; Shen, W.; Liu, H.; Xi, P.; Jaroniec, M.; Zheng, Y.; Qiao, S.-Z. Corrosion-Resistant NiFe Anode towards Kilowatt-Scale Alkaline Seawater Electrolysis. Nat. Commun. 2024, 15, 10351. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Designing Single-Atom Catalysts toward Improved Alkaline Hydrogen Evolution Reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Perovskite for Electrocatalytic Oxygen Evolution at Elevated Temperatures. ChemSusChem 2024, 17, e202301534. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Guo, Y.; Zhang, Z.; Zhang, X.; Ji, Q.; Zhang, H.; Song, Z.; Liu, D.; Zeng, J.; Chuang, C.; et al. Out-of-Plane Coordination of Iridium Single Atoms with Organic Molecules and Cobalt–Iron Hydroxides to Boost Oxygen Evolution Reaction. Nat. Nanotechnol. 2025, 20, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.-H.; Huang, Y.; Song, K.; Li, T.-T.; Cui, J.-Y.; Meng, C.; Zhang, H.; Wang, J.-J. Ir Single Atoms Boost Metal–Oxygen Covalency on Selenide-Derived NiOOH for Direct Intramolecular Oxygen Coupling. J. Am. Chem. Soc. 2024, 146, 6846–6855. [Google Scholar] [CrossRef]
- Zhao, L.; Yan, J.; Huang, H.; Du, X.; Chen, H.; He, X.; Li, W.; Fang, W.; Wang, D.; Zeng, X.; et al. Regulating Electronic Structure of Bimetallic NiFe-THQ Conductive Metal–Organic Frameworks to Boost Catalytic Activity for Oxygen Evolution Reaction. Adv. Funct. Mater. 2024, 34, 2310902. [Google Scholar] [CrossRef]
- Amin, H.M.A.; Bondue, C.J.; Eswara, S.; Kaiser, U.; Baltruschat, H. A Carbon-Free Ag–Co3O4 Composite as a Bifunctional Catalyst for Oxygen Reduction and Evolution: Spectroscopic, Microscopic and Electrochemical Characterization. Electrocatalysis 2017, 8, 540–553. [Google Scholar] [CrossRef]
- Yang, Z.; Lai, F.; Mao, Q.; Liu, C.; Peng, S.; Liu, X.; Zhang, T. Breaking the Mutual-Constraint of Bifunctional Oxygen Electrocatalysis via Direct O─O Coupling on High-Valence Ir Single-Atom on MnO. Adv. Mater. 2025, 37, 2412950. [Google Scholar] [CrossRef]
- Ko, W.; Shim, J.; Ahn, H.; Kwon, H.J.; Lee, K.; Jung, Y.; Antink, W.H.; Lee, C.W.; Heo, S.; Lee, S.; et al. Controlled Structural Activation of Iridium Single Atom Catalyst for High-Performance Proton Exchange Membrane Water Electrolysis. J. Am. Chem. Soc. 2025, 147, 2369–2379. [Google Scholar] [CrossRef]
- Abd-Elrahim, A.G.; Ali, M.A.; Chun, D.-M. Enhanced Oxygen Evolution Using Sulfate-Intercalated Amorphous FeNiS@FeS Layered Double Hydroxide Nanoflowers for Advanced Water-Splitting Performance. J. Power Sources 2025, 635, 236472. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Q.; Xia, J.; Yang, C.; Tao, Y.; Xie, J.; Wang, H.; Li, H.; Fan, J. Self-Supported Ir-FeOOH on Iron Foam for Efficient Oxygen Evolution Reaction. Catalysts 2025, 15, 464. https://doi.org/10.3390/catal15050464
Ren Q, Xia J, Yang C, Tao Y, Xie J, Wang H, Li H, Fan J. Self-Supported Ir-FeOOH on Iron Foam for Efficient Oxygen Evolution Reaction. Catalysts. 2025; 15(5):464. https://doi.org/10.3390/catal15050464
Chicago/Turabian StyleRen, Qinglin, Jinshan Xia, Chengcheng Yang, Yinghao Tao, Jiawei Xie, Hui Wang, Hong Li, and Jinchen Fan. 2025. "Self-Supported Ir-FeOOH on Iron Foam for Efficient Oxygen Evolution Reaction" Catalysts 15, no. 5: 464. https://doi.org/10.3390/catal15050464
APA StyleRen, Q., Xia, J., Yang, C., Tao, Y., Xie, J., Wang, H., Li, H., & Fan, J. (2025). Self-Supported Ir-FeOOH on Iron Foam for Efficient Oxygen Evolution Reaction. Catalysts, 15(5), 464. https://doi.org/10.3390/catal15050464






