Recent Advances in the Synthesis of Quinolines: A Focus on Oxidative Annulation Strategies
Abstract
1. Introduction
2. Oxidative Synthesis of Quinolines via C–H Transformations
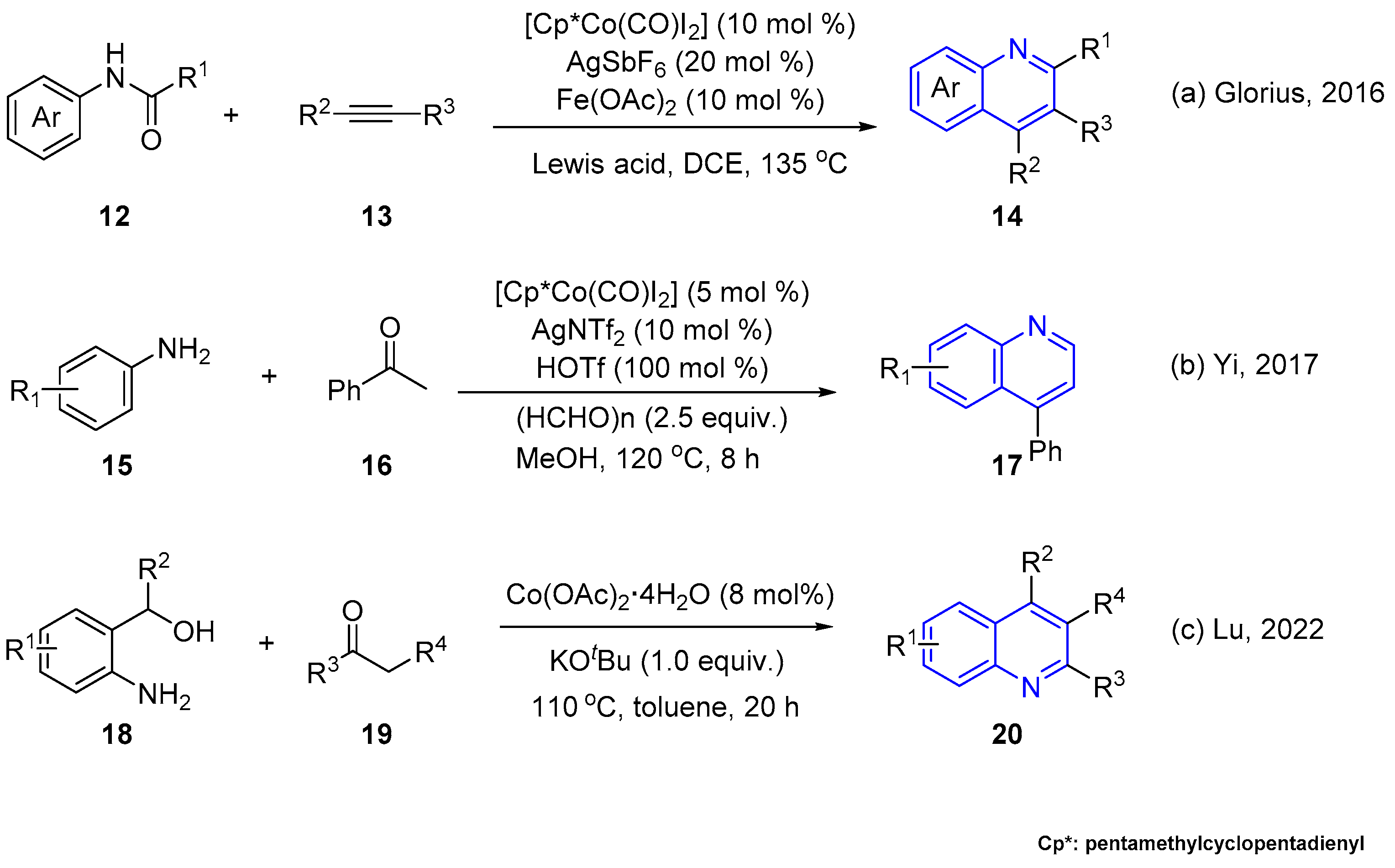
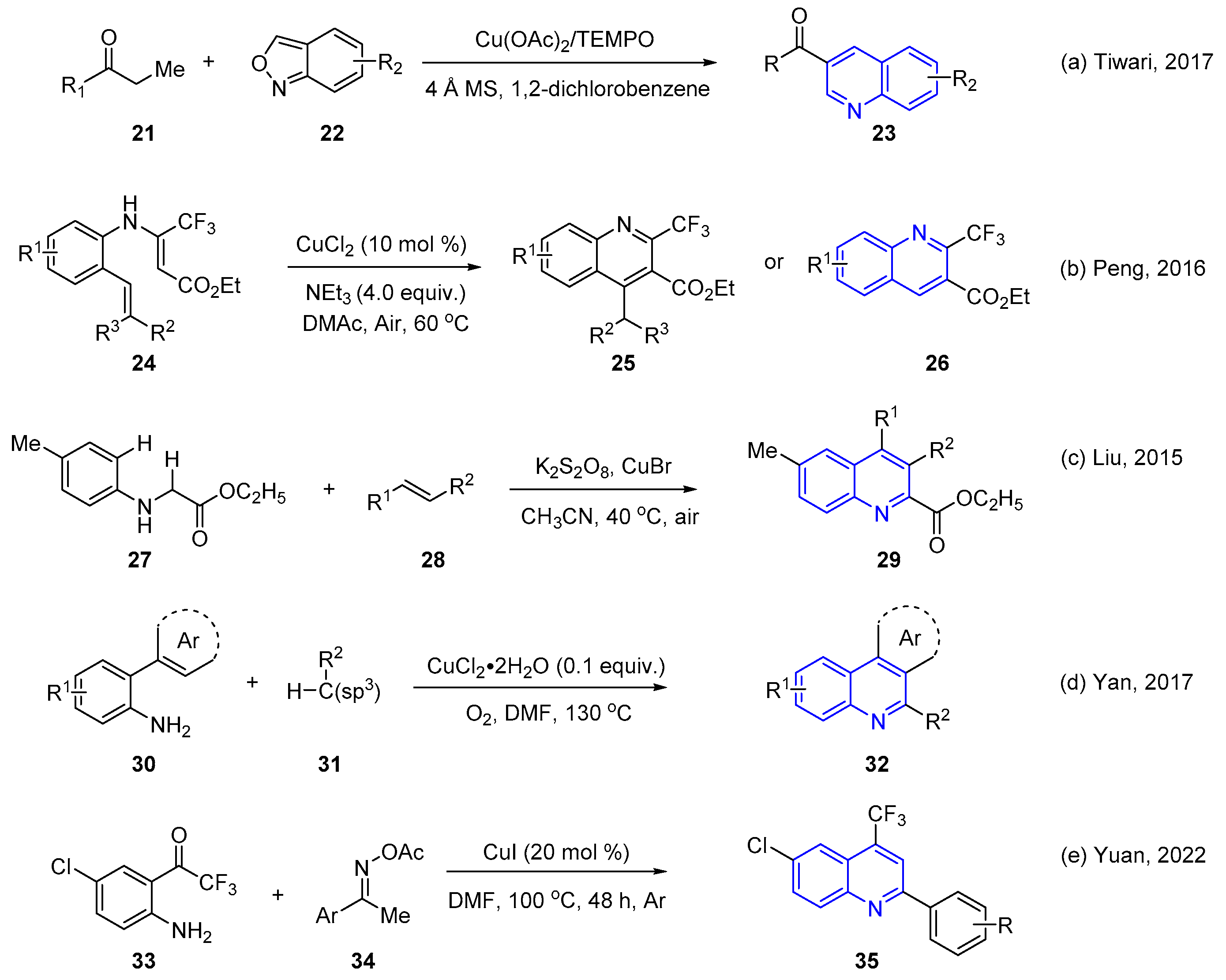
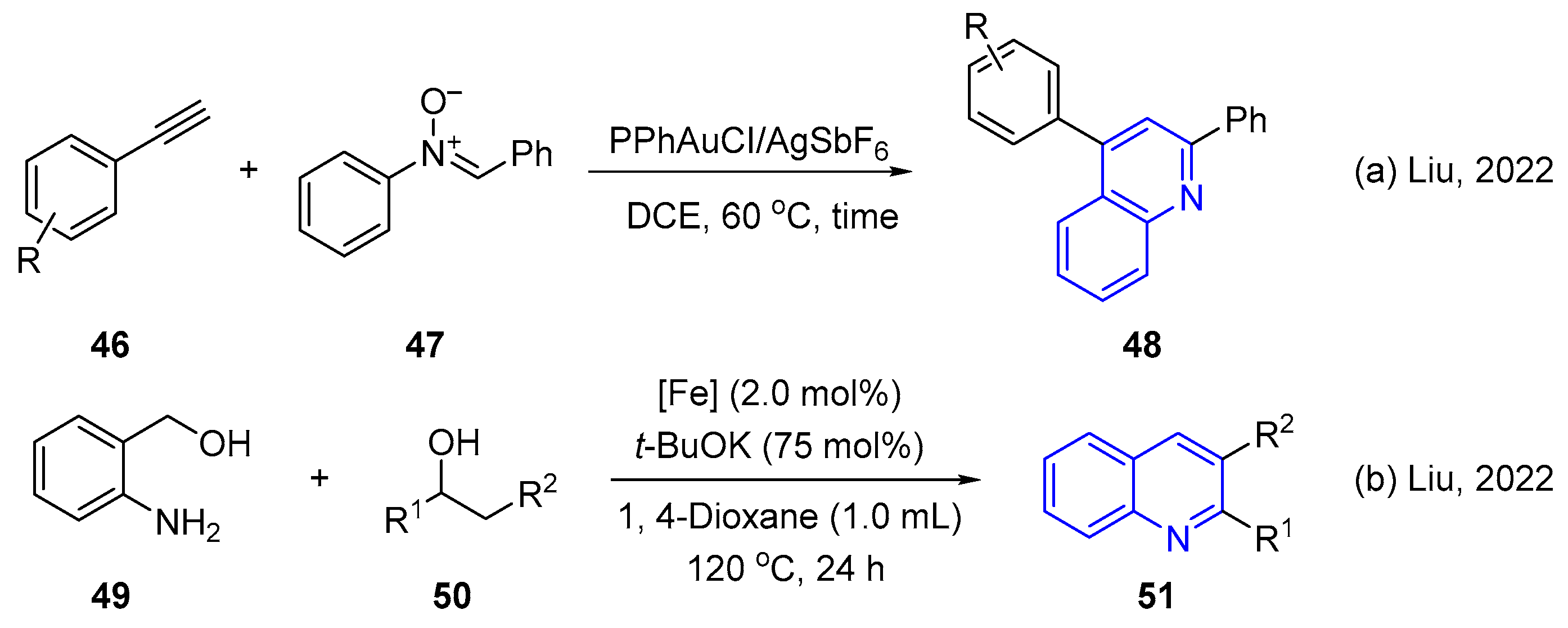
2.1. Transition Metal-Catalyzed C–H Activation Pathways
2.2. Oxidative Synthesis of Quinolines via Transition-Metal-Free C–H Transformations
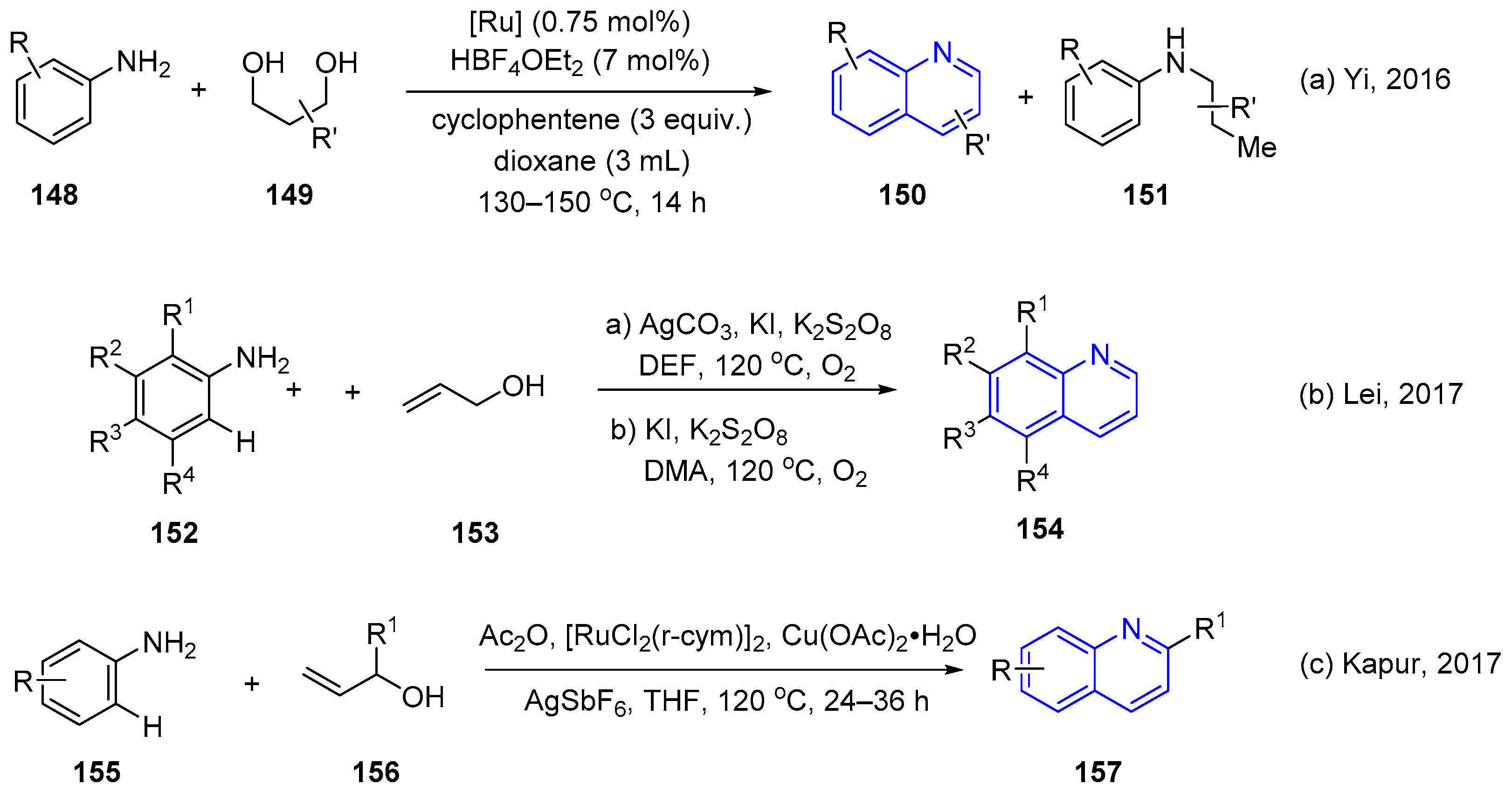
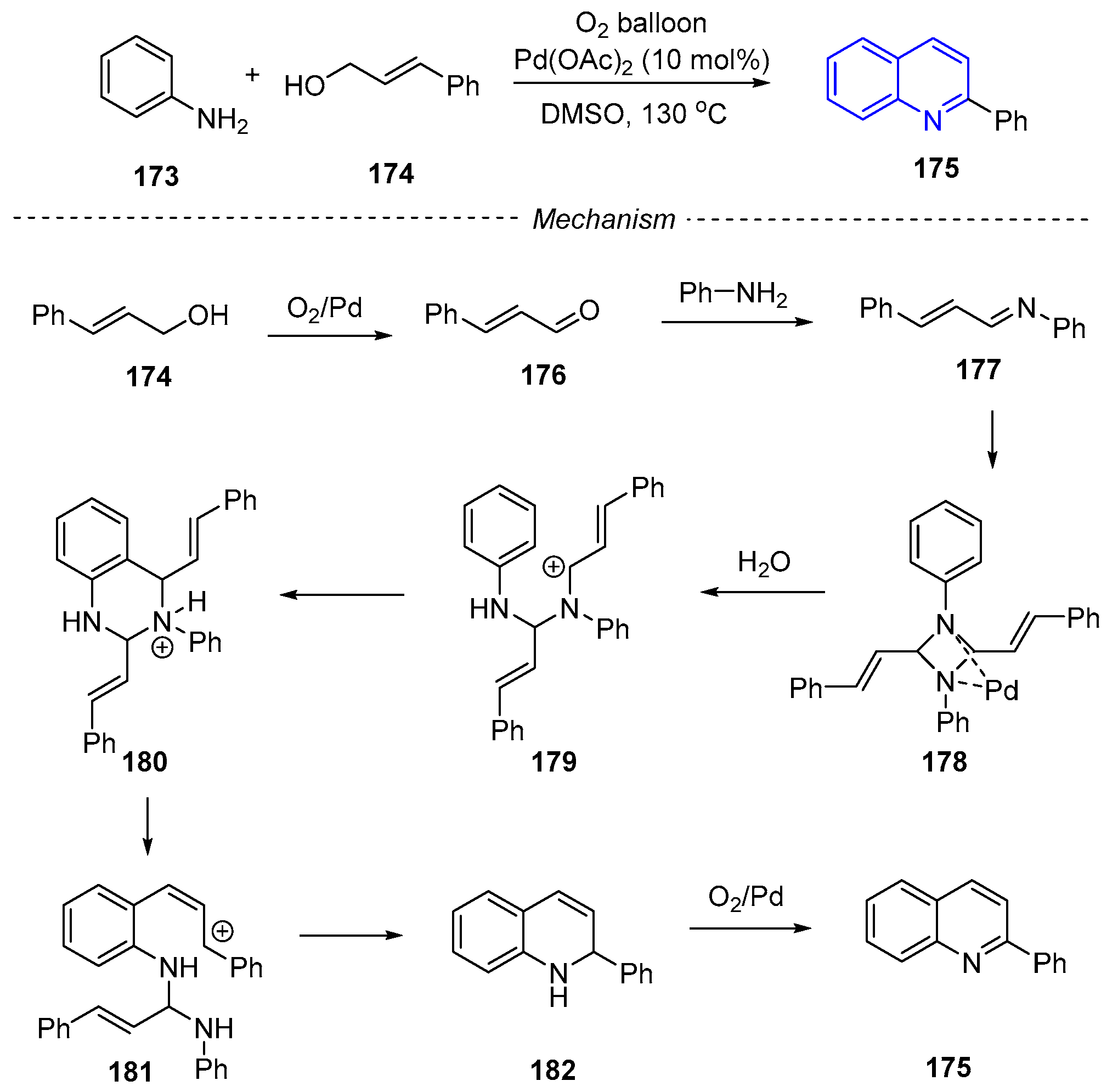
3. Oxidative Synthesis of Quinolines via Coupling Reaction of Aromatic Amine with Alcohol or Olefin
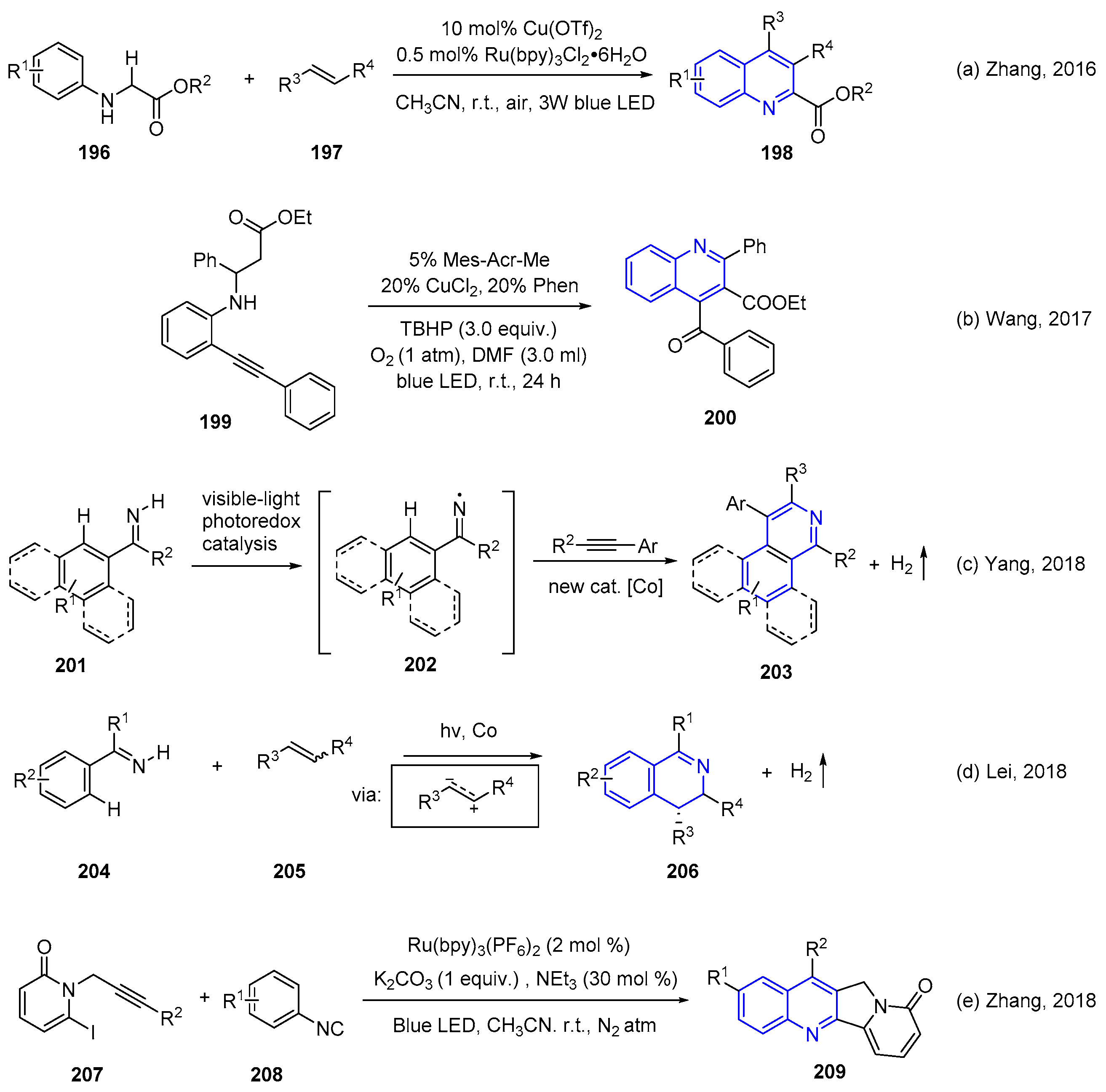
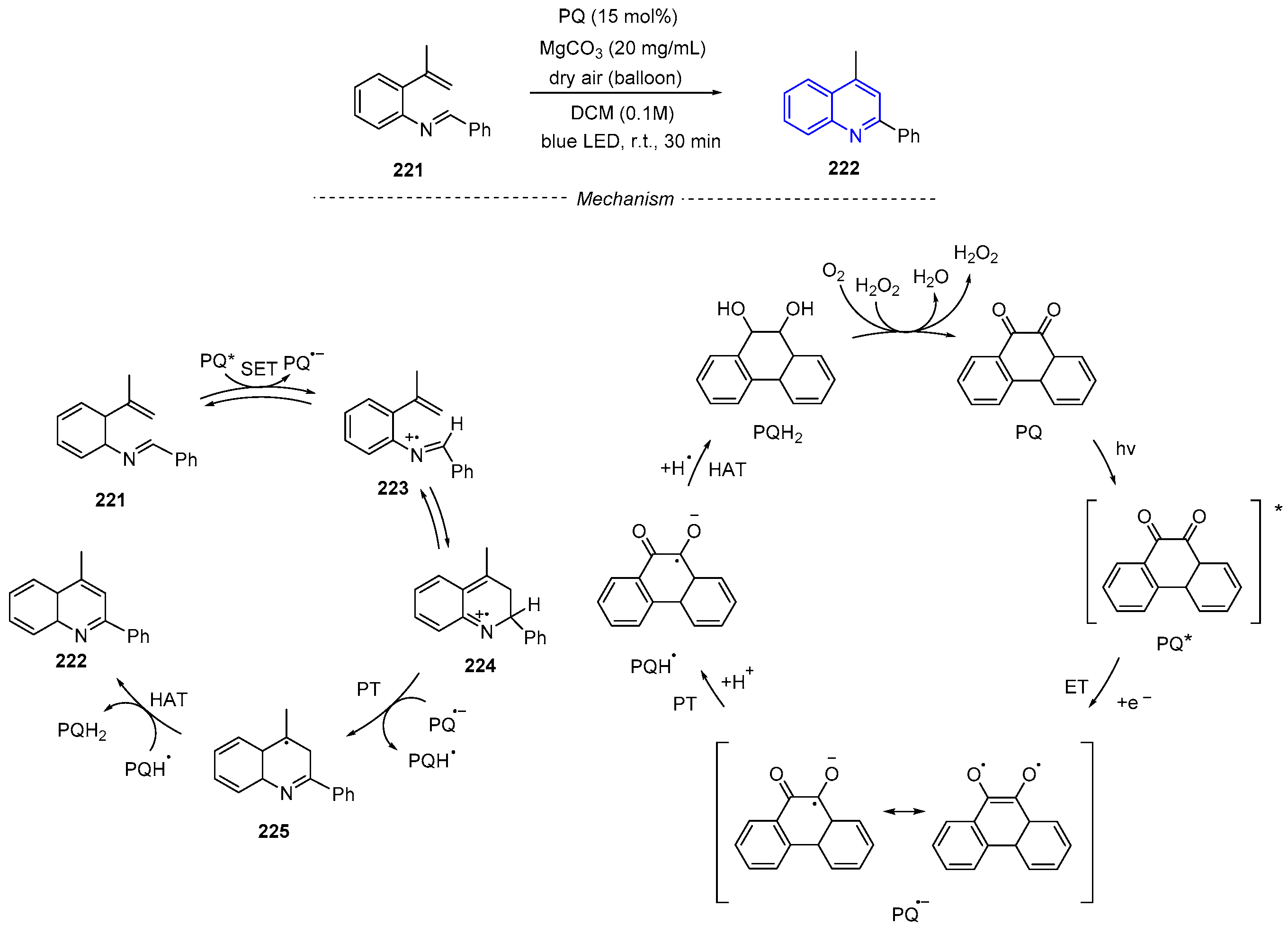
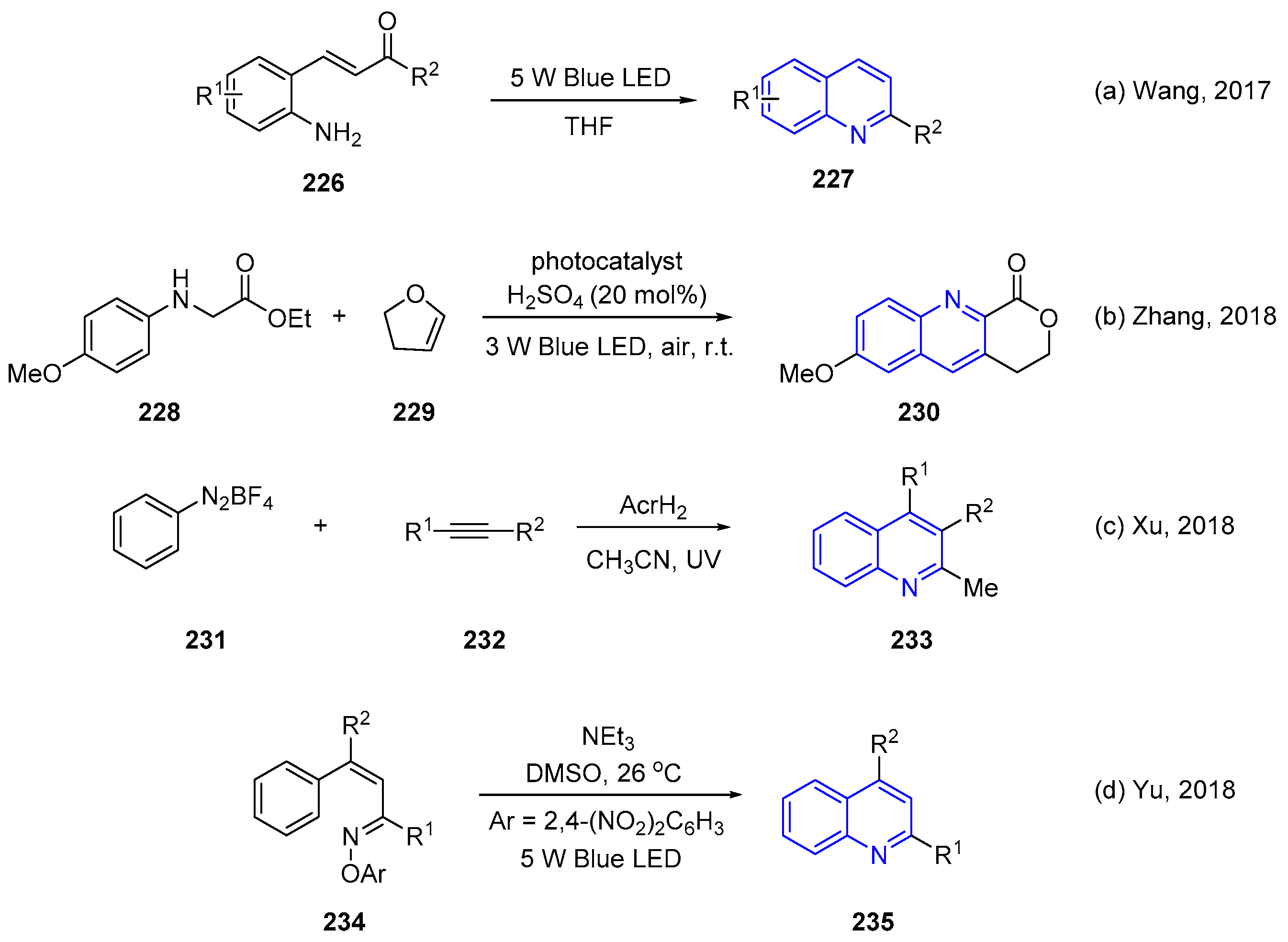
4. Oxidative Synthesis of Quinolines via Photo-Induced Oxidative Annulation Reactions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, B.; Studer, A. Recent Advances in the Synthesis of Nitrogen Heterocycles via Radical Cascade Reactions using Isonitriles as Radical Acceptors. Chem. Soc. Rev. 2015, 44, 3505–3521. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.M.; Patel, K.D.; Vekariya, R.H.; Panchal, S.N.; Patel, H.D. Recent Advances in the Synthesis of Quinolines: A Review. RSC Adv. 2014, 4, 24463–24476. [Google Scholar] [CrossRef]
- Ramann, G.A.; Cowen, B.J. Recent Advances in Metal-Free Quinoline Synthesis. Molecules 2016, 21, 986. [Google Scholar] [CrossRef]
- Hu, Y.-Q.; Gao, C.; Zhang, S.; Xu, L.; Xu, Z.; Feng, L.-S.; Wu, X.; Zhao, F. Quinoline Hybrids and Their Antiplasmodial and Antimalarial Activities. Eur. J. Med. Chem. 2017, 139, 22–47. [Google Scholar] [CrossRef]
- Chu, X.-M.; Wang, C.; Liu, W.; Liang, L.-L.; Gong, K.-K.; Zhao, C.-Y.; Sun, K.-L. Quinoline and Quinolone Dimers and Their Biological Activities: An overview. Eur. J. Med. Chem. 2019, 161, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Gadakh, S.K.; Dey, S.; Sudalai, A. Rhodium-catalyzed ortho-C–H bond Activation of Arylamines for the Synthesis of Quinoline Carboxylates. Org. Biomol. Chem. 2016, 14, 2969–2977. [Google Scholar] [CrossRef]
- Yan, K.; Liu, M.; Wen, J.; Liu, X.; Wang, X.; Sui, X.; Shang, W.; Wang, X. Synthesis of 3-Substituted Quinolines by Ruthenium-catalyzed aza-Michael Addition and Intramolecular Annulation of Enaminones with Anthranils. New J. Chem. 2022, 46, 7329–7333. [Google Scholar] [CrossRef]
- Lu, Q.; Vásquez-Céspedes, S.; Gensch, T.; Glorius, F. Control over Organometallic Intermediate Enables Cp*Co(III) Catalyzed Switchable Cyclization to Quinolines and Indoles. ACS Catal. 2016, 6, 2352–2356. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Y.; Chen, X.; Zhang, X.; Yi, W. The One-pot Synthesis of Quinolines via Co(III)-catalyzed C–H Activation/carbonylation/cyclization of Anilines. Org. Biomol. Chem. 2017, 15, 9061–9065. [Google Scholar] [CrossRef]
- Hao, Z.; Zhou, X.; Ma, Z.; Zhang, C.; Han, Z.; Lin, J.; Lu, G.-L. Dehydrogenative Synthesis of Quinolines and Quinazolines via Ligand-Free Cobalt-Catalyzed Cyclization of 2-Aminoaryl Alcohols with Ketones or Nitriles. J. Org. Chem. 2022, 87, 12596–12607. [Google Scholar] [CrossRef]
- Tiwari, D.K.; Phanindrudu, M.; Wakade, S.B.; Nanubolu, J.B.; Tiwari, D.K. α,β-Functionalization of Saturated Ketones with Anthranils via Cu-catalyzed Sequential Dehydrogenation/aza-Michael addition/annulation Cascade Reactions in One-pot. Chem. Commun. 2017, 53, 5302–5305. [Google Scholar] [CrossRef]
- Chen, W.; Ding, Q.; Nie, Z.; Peng, Y. Synthesis of 2-Trifluoromethylquinolines via Copper-mediated Intramolecular Oxidative Cyclization of N-(2-alkenylaryl) Enamines. RSC Adv. 2016, 6, 48767–48773. [Google Scholar] [CrossRef]
- Liu, G.; Qian, J.; Hua, J.; Cai, F.; Li, X.; Liu, L. An Economical Synthesis of Substituted Quinoline-2-carboxylates through the Potassium Persulfate-mediated Cross-dehydrogenative Coupling of N-aryl Glycine Derivatives with Olefins. Org. Biomol. Chem. 2016, 14, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Wu, M.; Ni, J.; Zhang, F.; Lan, J.; Chen, B.; Yan, R. Copper-Catalyzed Tandem Aerobic Oxidative Cyclization for the Synthesis of Polysubstituted Quinolines via C(sp3)/C(sp2)–H Bond Functionalization. J. Org. Chem. 2017, 82, 10110–10120. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-H.; Shen, L.-W.; Yang, P.; You, Y.; Zhao, J.-Q.; Yuan, W.-C. Access to 4-Trifluoromethyl Quinolines via Cu-Catalyzed Annulation Reaction of Ketone Oxime Acetates with ortho-Trifluoroacetyl Anilines under Redox-Neutral Conditions. J. Org. Chem. 2022, 87, 5804–5816. [Google Scholar] [CrossRef]
- Cai, W.; Wang, S.; Jalani, H.B.; Wu, J.; Lu, H.; Li, G. Oxidative Cascade Reaction of N-Aryl-3-alkylideneazetidines and Carboxylic Acids: Access to Fused Pyridines. Org. Lett. 2018, 20, 3833–3837. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X. Silver-catalyzed Oxidative Coupling of Aniline and Ene Carbonyl/acetylenic Carbonyl Compounds: An Efficient Route for the Synthesis of Quinolines. Chem. Asian J. 2014, 9, 3089–3093. [Google Scholar] [CrossRef]
- Barik, D.; Liu, R.S. Gold(I)-Catalyzed [4+2] Annulation between Arylynes and C,N-Diaryl Nitrones for Chemoselective Synthesis of Quinoline Scaffolds via Gold Acetylide Intermediates. J. Org. Chem. 2022, 87, 7097–7105. [Google Scholar] [CrossRef]
- Yu, K.; Chen, Q.; Liu, W. Iron-catalysed Quinoline Synthesis via Acceptorless Dehydrogenative Coupling. Org. Chem. Front. 2022, 9, 6573–6578. [Google Scholar] [CrossRef]
- Liu, L.; Lin, J.; Pang, M.; Jin, H.; Yu, X.; Wang, S. Photo-Thermo-Mechanochemical Approach to Synthesize Quinolines via Addition/Cyclization of Sulfoxonium Ylides with 2-Vinylanilines Catalyzed by Iron(II) Phthalocyanine. Org. Lett. 2022, 24, 1146–1151. [Google Scholar] [CrossRef]
- Shi, K.; Zhu, H.; Ren, F.; Liu, S.; Song, Y.; Li, W.; Zou, L.-H. Copper-catalyzed [3+2+1] Annulation of Anthranils with Phenylacetaldehydes: Synthesis of 8-Acylquinolines. Eur. J. Org. Chem. 2020, 2021, 1003–1006. [Google Scholar] [CrossRef]
- Zhu, S.; Shi, K.; Zhu, H.; Jia, Z.-K.; Xia, X.-F.; Wang, D.; Zou, L.-H. Copper-Catalyzed Annulation or Homocoupling of Sulfoxonium Ylides: Synthesis of 2,3-Diaroylquinolines or α,α,β-Tricarbonyl Sulfoxonium Ylides. Org. Lett. 2020, 22, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
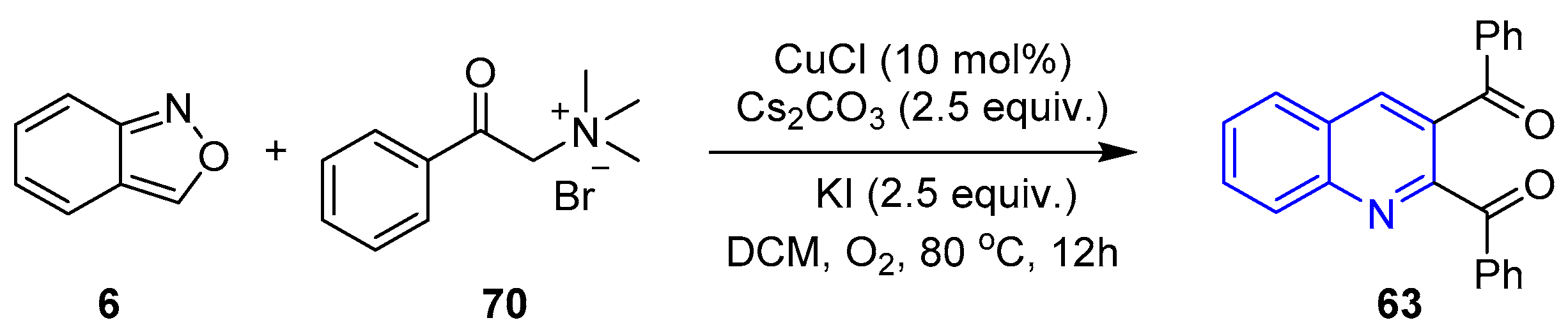
- Yang, J.-C.; Ma, C.-W.; Liao, M.-L.; Li, P.-G.; Zou, L.-H. Copper-Catalyzed [4+1+1] Annulation of Ammonium Salts and Anthranils: Synthesis of 2,3-Diaroylquinolines. J. Org. Chem. 2024, 89, 7446–7454. [Google Scholar] [CrossRef]
- Deng, G.-B.; Li, H.-B.; Yang, X.-H.; Song, R.-J.; Hu, M.; Li, J.-H. Dehydrogenative [2+2+1] Heteroannulation Using a Methyl Group as a One-Carbon Unit: Access to Pyrazolo[3,4-c]quinolines. Org. Lett. 2016, 18, 2012–2015. [Google Scholar] [CrossRef]
- Wu, X.; Geng, X.; Zhao, P.; Zhang, J.; Gong, X.; Wu, Y.-D.; Wu, A.-X. I(2)-Promoted Povarov-Type Reaction Using 1,4-Dithane-2,5-diol as an Ethylene Surrogate: Formal [4+2] Synthesis of Quinolines. Org. Lett. 2017, 19, 1550–1553. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.-C.; Wang, Z.-X.; Cheng, Y.; Xia, S.-Q.; Wang, M.; Tang, B.-C.; Wu, Y.-D.; Wu, A.-X. Divergent Synthesis of Functionalized Quinolines from Aniline and Two Distinct Amino Acids. J. Org. Chem. 2017, 82, 9210–9216. [Google Scholar] [CrossRef]
- Wakade, S.B.; Tiwari, D.K.; Ganesh, P.; Phanindrudu, M.; Likhar, P.R.; Tiwari, D.K. Transition-Metal-Free Quinoline Synthesis from Acetophenones and Anthranils via Sequential One-Carbon Homologation/Conjugate Addition/Annulation Cascade. Org. Lett. 2017, 19, 4948–4951. [Google Scholar] [CrossRef]
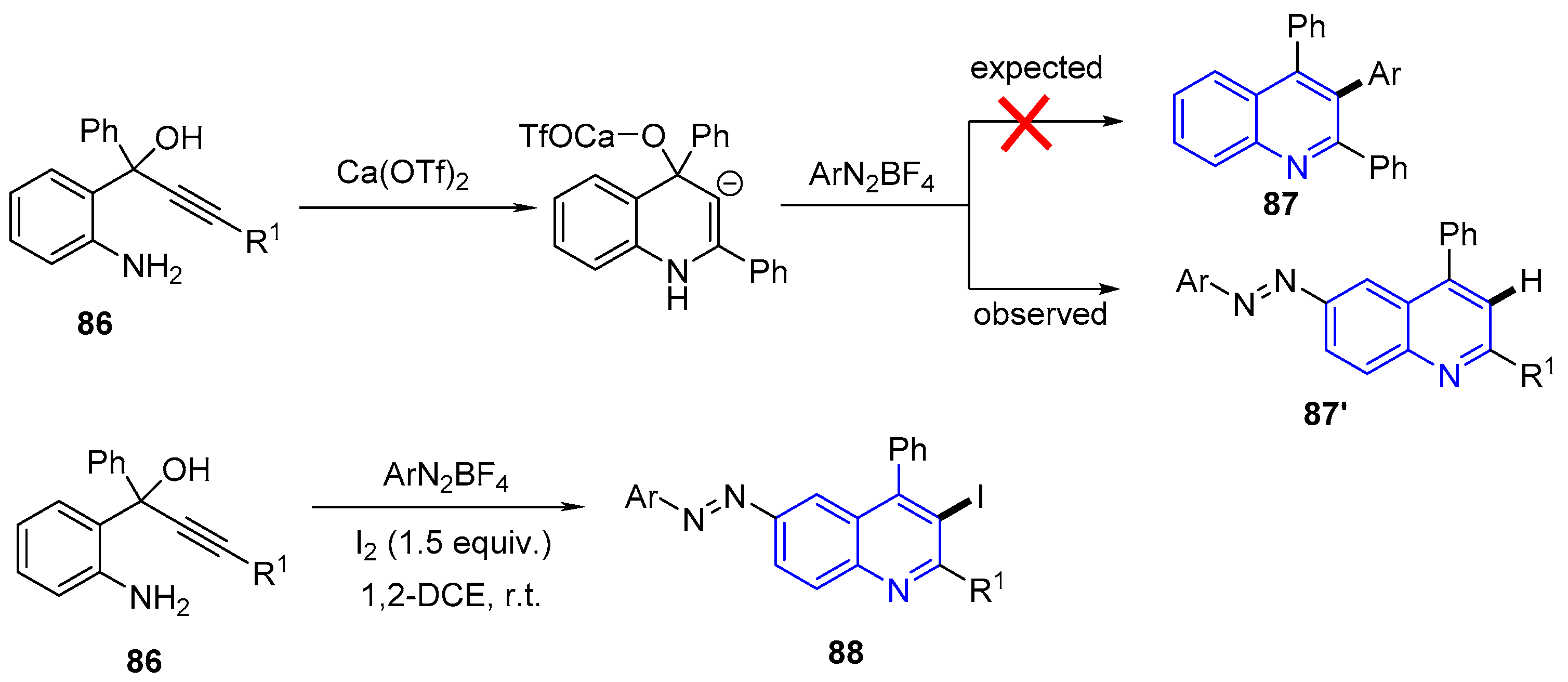
- Yaragorla, S.; Pareek, A. Electrophilic Cyclization of 2-Aminophenylprop-1-yn-3-ols into 3-Iodo-6-(aryldiazenyl)quinolines by Using a One-Pot Azo-Coupling and Iodocyclization Sequence. Eur. J. Org. Chem. 2018, 2018, 1863–1871. [Google Scholar] [CrossRef]
- Ye, J.-L.; Zhu, Y.-N.; Geng, H.; Huang, P.-Q. Metal-free Synthesis of Quinolines by Direct Condensation of Amides with Alkynes: Revelation of N-Aryl Nitrilium Intermediates by 2D NMR Techniques. Sci. China Chem. 2017, 61, 687–694. [Google Scholar] [CrossRef]
- Jiang, T.-S.; Wang, X.; Zhang, X. Synthesis of Quinolines from Anilines, Acetophenones and DMSO under Air. Tetrahedron Lett. 2018, 59, 2979–2982. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, W.; Luo, D.; Min, L.; Wang, X.; Hu, Y. A Tf2O-Promoted Synthesis of Functionalized Quinolines from Ketoximes and Alkynes. Adv. Synth. Catal. 2019, 361, 1995–1999. [Google Scholar] [CrossRef]
- Beesu, M.; Mehta, G. Synthesis of Quinolines and Isoquinolines via Site-Selective, Domino Benzannulation of 2- and 3-Chloropyridyl Ynones with Nitromethane. J. Org. Chem. 2019, 84, 8731–8742. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.; Stentzel, M.R.; Klumpp, D.A. Superacid-promoted Synthesis of Quinoline Derivatives. Tetrahedron Lett. 2020, 61, 151630. [Google Scholar] [CrossRef]
- Gerosa, G.G.; Schwengers, S.A.; Maji, R.; De, C.K.; List, B. Homologation of the Fischer Indolization: A Quinoline Synthesis via Homo-Diaza-Cope Rearrangement. Angew. Chem. Int. Ed. 2020, 59, 20485–20488. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yu, F.; Huang, R.; Weng, M.; Chen, H.; Zhang, Z. Synthesis of Polysubstituted Quinolines through Promoter-regulated Selective Annulation and C–C bond Cleavage from 2-Styrylanilines and β-Keto Esters. Org. Chem. Front. 2020, 7, 3368–3373. [Google Scholar] [CrossRef]
- Wang, C.-C.; Chan, C.-K.; Lai, C.-Y. Environmentally Friendly Nafion-Mediated Friedländer Quinoline Synthesis under Microwave Irradiation: Application to One-Pot Synthesis of Substituted Quinolinyl Chalcones. Synthesis 2020, 52, 1779–1794. [Google Scholar] [CrossRef]
- Mäkelä, M.K.; Bulatov, E.; Malinen, K.; Talvitie, J.; Nieger, M.; Melchionna, M.; Lenarda, A.; Hu, T.; Wirtanen, T.; Helaja, J. Carbocatalytic Cascade Synthesis of Polysubstituted Quinolines from Aldehydes and 2-Vinyl Anilines. Adv. Synth. Catal. 2021, 363, 3775–3782. [Google Scholar] [CrossRef]
- Mathuri, A.; Pramanik, M.; Mal, P. 3-Arylsulfonylquinolines from N-Propargylamines via Cascaded Oxidative Sulfonylation Using DABSO. J. Org. Chem. 2022, 87, 6812–6823. [Google Scholar] [CrossRef]
- Zhang, K.-L.; Yang, J.-C.; Guo, Q.; Zou, L.-H. Transition Metal-Free Synthesis of 3-Acylquinolines through Formal [4+2] Annulation of Anthranils and Enaminones. Catalysts 2023, 13, 778. [Google Scholar] [CrossRef]
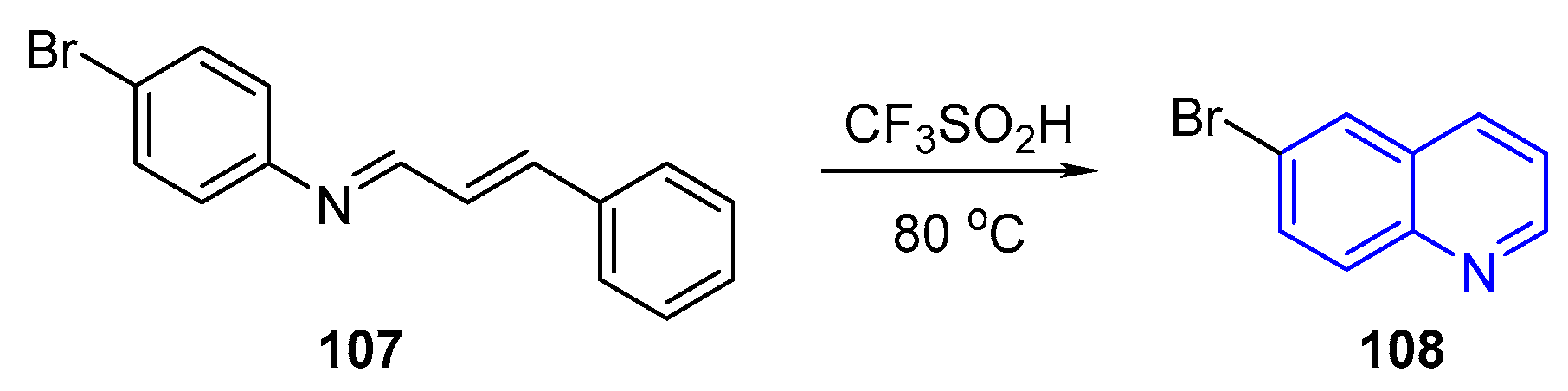
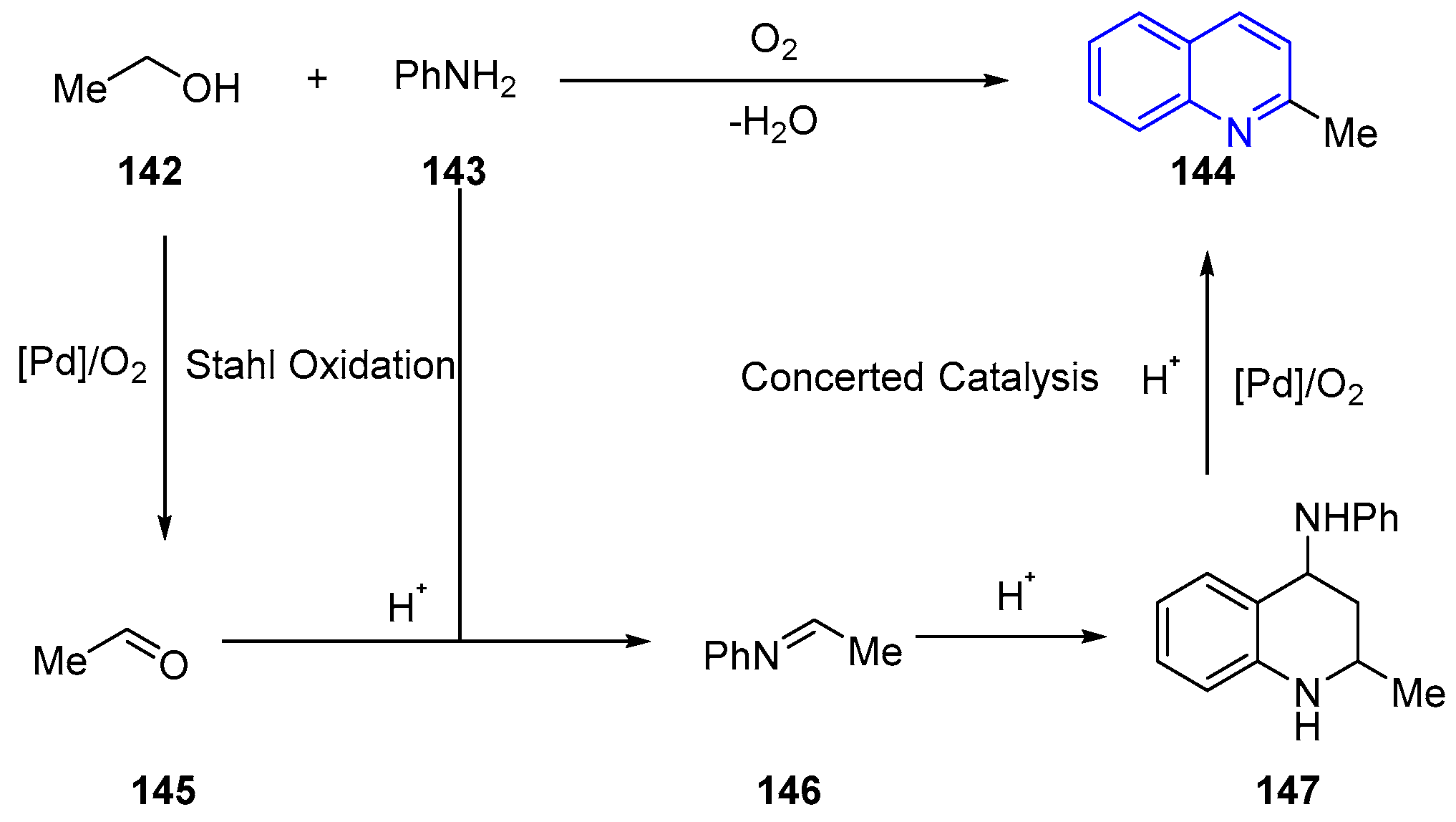
- Li, J.; Zhang, J.; Yang, H.; Jiang, G. Assembly of Diversely Substituted Quinolines via Aerobic Oxidative Aromatization from Simple Alcohols and Anilines. J. Org. Chem. 2017, 82, 3284–3290. [Google Scholar] [CrossRef]
- Lee, H.; Yi, C.S. Catalytic Synthesis of Substituted Indoles and Quinolines from the Dehydrative C–H Coupling of Arylamines with 1,2- and 1,3-Diols. Organometallics 2016, 35, 1973–1977. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liao, Z.; Liu, D.; Chiang, C.W.; Li, Z.; Zhou, Z.; Yi, H.; Zhang, X.; Deng, Z.; Lei, A. From Anilines to Quinolines: Iodide- and Silver-Mediated Aerobic Double C–H Oxidative Annulation-Aromatization. Chemistry 2017, 23, 15874–15878. [Google Scholar] [CrossRef]
- Kumar, G.S.; Kumar, P.; Kapur, M. Traceless Directing-Group Strategy in the Ru-Catalyzed, Formal [3+3] Annulation of Anilines with Allyl Alcohols: A One-Pot, Domino Approach for the Synthesis of Quinolines. Org. Lett. 2017, 19, 2494–2497. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; An, Y.; Peng, J.; Wu, W.; Jiang, H. Palladium-Catalyzed Allylic C–H Oxidative Annulation for Assembly of Functionalized 2-Substituted Quinoline Derivatives. J. Org. Chem. 2016, 81, 12189–12196. [Google Scholar] [CrossRef]
- Xu, J.; Sun, J.; Zhao, J.; Huang, B.; Li, X.; Sun, Y. Palladium-catalyzed Synthesis of Quinolines from Allyl Alcohols and Anilines. RSC Adv. 2017, 7, 36242–36245. [Google Scholar] [CrossRef]
- Saunthwal, R.K.; Patel, M.; Verma, A.K. Regioselective Synthesis of C-3-Functionalized Quinolines via Hetero-Diels-Alder Cycloaddition of Azadienes with Terminal Alkynes. J. Org. Chem. 2016, 81, 6563–6572. [Google Scholar] [PubMed]
- Jadhav, S.D.; Singh, A. Oxidative Annulations Involving DMSO and Formamide: K2S2O8 Mediated Syntheses of Quinolines and Pyrimidines. Org. Lett. 2017, 19, 5673–5676. [Google Scholar]
- Wan, J.-P.; Jing, Y.; Wei, L. Branched C=C and C−N Bond Cleavage on Enaminones Toward the Synthesis of 3-Acyl Quinolines. Asian J. Org. Chem. 2017, 6, 666–668. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; Li, Y.; Zhang, Y. Visible-Light-Induced Photocatalytic Aerobic Oxidative C(sp3)–H Functionalization of Glycine Derivatives: Synthesis of Substituted Quinolines. J. Org. Chem. 2016, 81, 12433–12442. [Google Scholar] [CrossRef]
- Xia, X.-F.; Zhang, G.-W.; Wang, D.; Zhu, S.-L. Visible-Light Induced and Oxygen-Promoted Oxidative Cyclization of Aromatic Enamines for the Synthesis of Quinolines Derivatives. J. Org. Chem. 2017, 82, 8455–8463. [Google Scholar]
- Tian, W.-F.; Wang, D.-P.; Wang, S.-F.; He, K.-H.; Cao, X.-P.; Li, Y. Visible-Light Photoredox-Catalyzed Iminyl Radical Formation by N–H Cleavage with Hydrogen Release and Its Application in Synthesis of Isoquinolines. Org. Lett. 2018, 20, 1421–1425. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, G.; Bu, F.; Lei, A. Selective Oxidative [4+2] Imine/Alkene Annulation with H2 Liberation Induced by Photo-Oxidation. Angew. Chem. Int. Ed. 2018, 57, 1286–1290. [Google Scholar] [CrossRef]
- Yuan, Y.; Dong, W.; Gao, X.; Gao, H.; Xie, X.; Zhang, Z. Visible-Light-Induced Radical Cascade Cyclization: Synthesis of the ABCD Ring Cores of Camptothecins. J. Org. Chem. 2018, 83, 2840–2846. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, X.; Niu, X.; Zhu, L.; Yao, X. Visible-light-induced Photoxidation-Povarov Cascade Reaction: Synthesis of 2-Arylquinoline through Alcohol and N-Benzylanilines under Mild Conditions via Ag/g-C3N4 Nanometric Semiconductor Catalyst. Chem. Commun. 2020, 56, 4840–4843. [Google Scholar] [CrossRef]
- Talvitie, J.; Alanko, I.; Bulatov, E.; Koivula, J.; Pollanen, T.; Helaja, J. Phenanthrenequinone-Sensitized Photocatalytic Synthesis of Polysubstituted Quinolines from 2-Vinylarylimines. Org. Lett. 2022, 24, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qiu, S.; Wang, S.; Wang, H.; Zhai, H. Blue-light-promoted Carbon-carbon Double Bond Isomerization and Its Application in the Syntheses of Quinolines. Org. Biomol. Chem. 2017, 15, 6349–6352. [Google Scholar] [CrossRef]
- He, Y.; Yan, B.; Tao, H.; Zhang, Y.; Li, Y. Metal-free Photocatalyzed Aerobic Oxidative C(sp3)–H Functionalization of Glycine Derivatives: One-step Generation of Quinoline-fused Lactones. Org. Biomol. Chem. 2018, 16, 3816–3823. [Google Scholar] [CrossRef]
- Zhou, X.-F.; Sun, Y.-Y.; Dai, J.-J.; Xu, J.; Xu, H.-J. Photoinduced Synthesis of Quinoline Derivatives Catalyzed by Organic Photocatalyst at Room Temperature. J. Photochem. Photobio. A Chem. 2018, 355, 186–193. [Google Scholar] [CrossRef]
- Sun, J.; He, Y.; An, X.-D.; Zhang, X.; Yu, L.; Yu, S. Visible-light-induced Iminyl Radical Formation via Electron-donor-acceptor Complexes: A Photocatalyst-free Approach to Phenanthridines and Quinolines. Org. Chem. Front. 2018, 5, 977–981. [Google Scholar] [CrossRef]
- Yuan, J.-M.; Li, J.; Zhou, H.; Xu, J.; Zhu, F.; Liang, Q.; Liu, Z.; Huang, G.; Huang, J. Synthesis of 3-Sulfonylquinolines by Visible-light Promoted Metal-free Cascade Cycloaddition Involving N-Propargylanilines and Sodium Sulfinates. New J. Chem. 2020, 44, 3189–3193. [Google Scholar] [CrossRef]






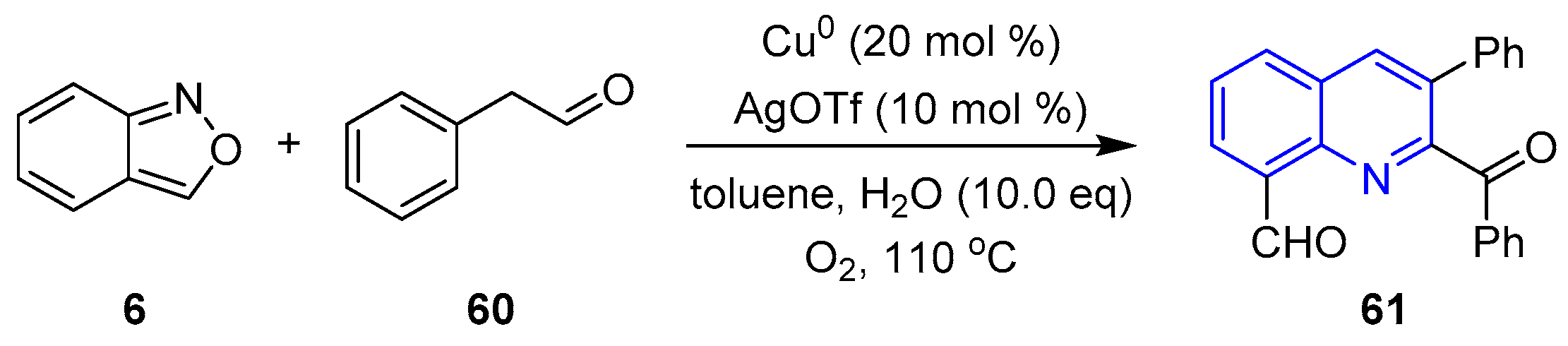




















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, M.-L.; Liu, P.-P.; Yang, J.-C.; Li, P.-G.; Zou, L.-H. Recent Advances in the Synthesis of Quinolines: A Focus on Oxidative Annulation Strategies. Catalysts 2025, 15, 441. https://doi.org/10.3390/catal15050441
Liao M-L, Liu P-P, Yang J-C, Li P-G, Zou L-H. Recent Advances in the Synthesis of Quinolines: A Focus on Oxidative Annulation Strategies. Catalysts. 2025; 15(5):441. https://doi.org/10.3390/catal15050441
Chicago/Turabian StyleLiao, Mao-Lin, Peng-Peng Liu, Jia-Cheng Yang, Ping-Gui Li, and Liang-Hua Zou. 2025. "Recent Advances in the Synthesis of Quinolines: A Focus on Oxidative Annulation Strategies" Catalysts 15, no. 5: 441. https://doi.org/10.3390/catal15050441
APA StyleLiao, M.-L., Liu, P.-P., Yang, J.-C., Li, P.-G., & Zou, L.-H. (2025). Recent Advances in the Synthesis of Quinolines: A Focus on Oxidative Annulation Strategies. Catalysts, 15(5), 441. https://doi.org/10.3390/catal15050441






