Growing Nanocrystalline Ru on Amorphous/Crystalline Heterostructure for Efficient and Durable Hydrogen Evolution Reaction
Abstract
:1. Introduction
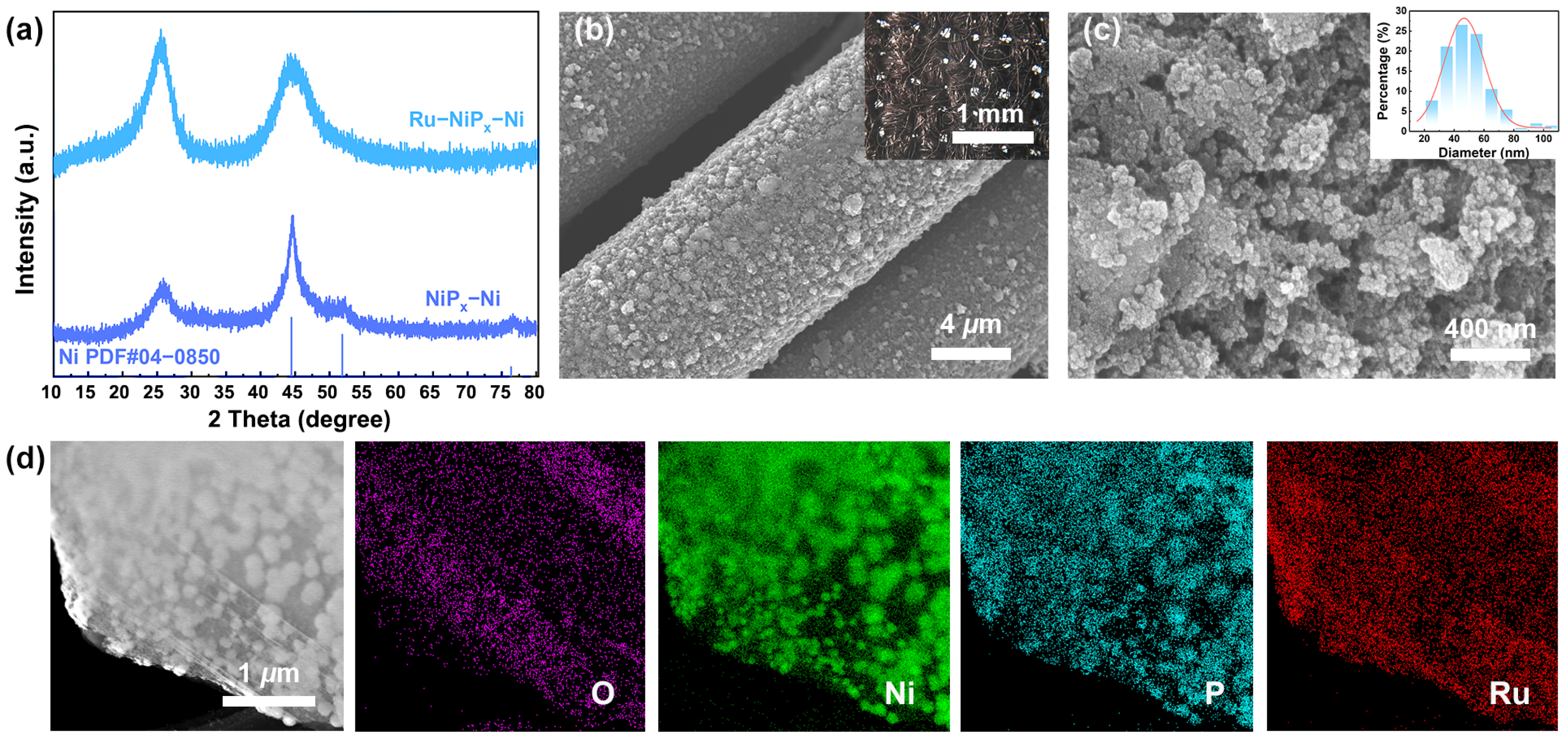
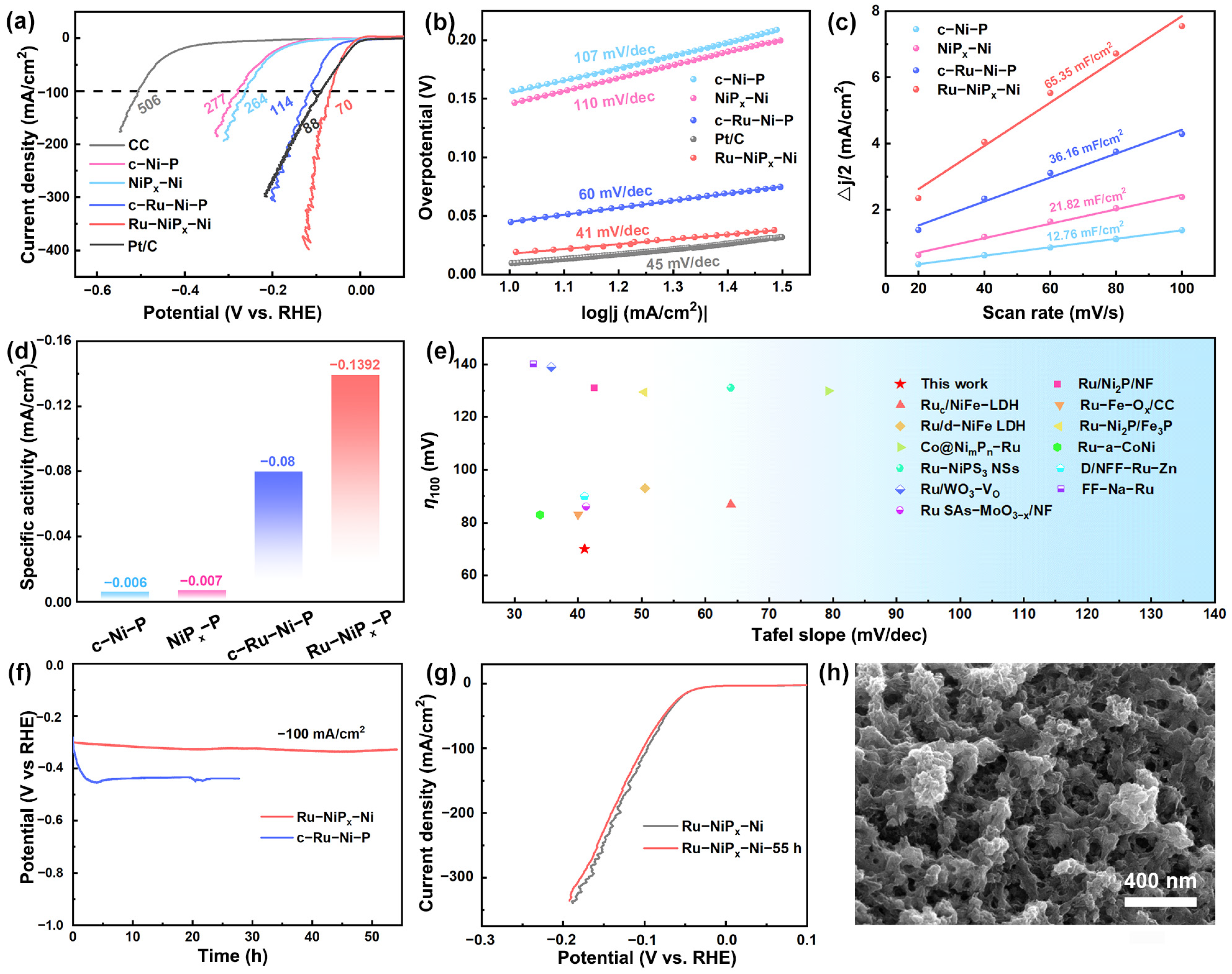
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Surface Hydrophilization of CC
3.3. Preparation of NiPx-Ni and Ru-NiPx-Ni
3.4. Preparation of C-Ni-P and C-Ru-Ni-P
3.5. Preparation of Pt/C, A-RuNi, and Ru@CC
3.6. Characterization
3.7. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, J.; Li, Z.; Liu, T.; Zhao, S.; Guan, D.; Chen, D.; Shao, Z.; Ni, M. Morphology control and electronic tailoring of CoxAy (A= P, S, Se) electrocatalysts for water splitting. Chem. Eng. J. 2023, 460, 141674. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.; Kim, S. Recent advances in amorphous electrocatalysts for oxygen evolution reaction. Front. Chem. 2022, 10, 1030803. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Song, S.Z.; Wu, W.T.; Deng, Z.F.; Tang, C. Bridging laboratory electrocatalysts with industrially relevant alkaline water electrolyzers. Adv. Energy Mater. 2024, 14, 2303451. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, J.; Huang, K.; Zhang, B.; Wu, F.; Liang, Y.; Lu, S.; Huang, Y.; Wu, J. In situ growth of an active catalytic layer on commercial stainless steel via a hydrothermal-assisted corrosion process for efficient oxygen evolution reaction. J. Mater. Chem. A 2024, 12, 19008–19017. [Google Scholar] [CrossRef]
- Zhao, W.; Xu, H.; Luan, H.; Chen, N.; Gong, P.; Yao, K.; Shen, Y.; Shao, Y. NiFe layered double hydroxides grown on a corrosion-cell cathode for oxygen evolution electrocatalysis. Adv. Energy Mater. 2021, 12, 2102372. [Google Scholar] [CrossRef]
- Feidenhans’l, A.A.; Regmi, Y.N.; Wei, C.; Xia, D.; Kibsgaard, J.; King, L.A. Precious Metal Free Hydrogen Evolution Catalyst Design and Application. Chem. Rev. 2024, 124, 5617–5667. [Google Scholar] [CrossRef]
- Wu, H.; Huang, Q.X.; Shi, Y.Y.; Chang, J.W.; Lu, S.Y. Electrocatalytic water splitting: Mechanism and electrocatalyst design. Nano Res. 2023, 16, 9142–9157. [Google Scholar] [CrossRef]
- Liu, X.P.; Gong, M.X.; Deng, S.F.; Zhao, T.H.; Shen, T.; Zhang, J.; Wang, D.L. Transforming damage into benefit: Corrosion engineering enabled electrocatalysts for water splitting. Adv. Funct. Mater. 2021, 31, 2009032. [Google Scholar] [CrossRef]
- Shi, X.; Ye, Q.; Huang, Q.; Ma, J.; Liu, Y.; Lin, S. Engineering Amorphous CoNiRuOx Nanoparticles Grown on Nickel Foam for Boosted Electrocatalytic Hydrogen Evolution. Catalysts 2025, 15, 211. [Google Scholar] [CrossRef]
- Shang, M.; Zhou, B.; Qiu, H.; Gong, Y.; Xin, L.; Xiao, W.; Xu, G.; Dai, C.; Zhang, H.; Wu, Z.; et al. Self-supported Ru-Fe-Ox nanospheres as efficient electrocatalyst to boost overall water-splitting in acid and alkaline media. J. Colloid Interface Sci. 2024, 669, 856–863. [Google Scholar] [CrossRef]
- Chade, D.; Berlouis, L.; Infield, D.; Cruden, A.; Nielsen, P.T.; Mathiesen, T. Evaluation of Raney nickel electrodes prepared by atmospheric plasma spraying for alkaline water electrolysers. Int. J. Hydrogen Energy 2013, 38, 14380–14390. [Google Scholar] [CrossRef]
- Yang, H.; Guo, P.; Wang, R.; Chen, Z.; Xu, H.; Pan, H.; Sun, D.; Fang, F.; Wu, R. Sequential Phase Conversion-Induced Phosphides Heteronanorod Arrays for Superior Hydrogen Evolution Performance to Pt in Wide pH Media. Adv. Mater. 2022, 34, 2107548. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Feng, C.; Mi, W.; Guo, M.; Guan, Z.; Li, M.; Chen, H.C.; Liu, Y.; Pan, Y. Defect-induced electron redistribution between Pt-N3S1 single atomic sites and Pt clusters for synergistic electrocatalytic hydrogen production with ultra-high mass activity. Adv. Funct. Mater. 2023, 34, 2309474. [Google Scholar] [CrossRef]
- Hu, Y.D.; Luo, G.; Wang, L.G.; Liu, X.K.; Qu, Y.T.; Zhou, Y.S.; Zhou, F.Y.; Li, Z.J.; Li, Y.F.; Yao, T.; et al. Single Ru Atoms Stabilized by Hybrid Amorphous/Crystalline FeCoNi Layered Double Hydroxide for Ultraefficient Oxygen Evolution. Adv. Energy Mater. 2021, 11, 2002816. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, X.; Lin, S.; Liu, Y.; Zou, X.; Chen, H. Corrosion engineering for electrode fabrication toward alkaline water electrolysis. Chem. Synth. 2025, 5. [Google Scholar] [CrossRef]
- Feng, D.; Wang, P.; Qin, R.; Shi, W.; Gong, L.; Zhu, J.; Ma, Q.; Chen, L.; Yu, J.; Liu, S.; et al. Flower-Like Amorphous MoO3−x Stabilized Ru Single Atoms for Efficient Overall Water/Seawater Splitting. Adv. Sci. 2023, 10, 2300342. [Google Scholar] [CrossRef]
- Niu, Z.; Lu, Z.; Qiao, Z.; Wang, S.; Cao, X.; Chen, X.; Yun, J.; Zheng, L.; Cao, D. Robust Ru-VO2 Bifunctional Catalysts for All-pH Overall Water Splitting. Adv. Mater. 2023, 36, 2310690. [Google Scholar] [CrossRef]
- Geng, S.; Tian, F.Y.; Li, M.G.; Liu, Y.Q.; Sheng, J.; Yang, W.W.; Yu, Y.S.; Hou, Y.L. Activating interfacial S sites of MoS2 boosts hydrogen evolution electrocatalysis. Nano Res. 2022, 15, 1809–1816. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Q.; Sun, W.; Sun, K.; Shen, Y.; An, W.; Zhang, L.; Chen, H.; Zou, X. Nanostructured intermetallics: From rational synthesis to energy electrocatalysis. Chem. Synth. 2023, 3, 28. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Y.; Feng, Y.; Meng, X.; Xia, J.; Zhang, G. Constructing Ru-O-TM bridge in NiFe-LDH enables high current hydrazine-assisted H2 production. Adv. Mater. 2024, 36, 2401694. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Zhai, W.; Liu, H.; Sakthivel, T.; Guo, S.; Dai, Z. Metastabilizing the Ruthenium Clusters by Interfacial Oxygen Vacancies for Boosted Water Splitting Electrocatalysis. Adv. Energy Mater. 2024, 14, 2400059. [Google Scholar] [CrossRef]
- Gou, W.; Wang, Y.; Zhang, M.; Tan, X.; Ma, Y.; Qu, Y. A review on fundamentals for designing stable ruthenium-based catalysts for the hydrogen and oxygen evolution reactions. Chin. J. Catal. 2024, 60, 68–106. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, J.; Zheng, Y.; Xiao, M.; Gao, R.; Zhang, Z.; Wen, G.; Dou, H.; Deng, Y.P.; Yu, A. Materials engineering toward durable electrocatalysts for proton exchange membrane fuel cells. Adv. Energy Mater. 2022, 12, 2102665. [Google Scholar] [CrossRef]
- Liu, R.; Sun, M.; Liu, X.; Lv, Z.; Yu, X.; Wang, J.; Liu, Y.; Li, L.; Feng, X.; Yang, W.; et al. Enhanced Metal-Support Interactions Boost the Electrocatalytic Water Splitting of Supported Ruthenium Nanoparticles on a Ni3N/NiO Heterojunction at Industrial Current Density. Angew. Chem. Int. Ed. 2023, 62, e202312644. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Jadhav, A.R.; Yang, T.; Hwang, Y.; Wang, H.; Wang, L.; Luo, Y.; Kumar, A.; Lee, J.; et al. Unraveling the Function of Metal-Amorphous Support Interactions in Single-Atom Electrocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2022, 61, e202114160. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, M.; Zhang, C.; Chang, H.H.; Zhang, Y.; Li, L.; Chen, H.Y.; Peng, S. Manipulating the microenvironment of single atoms by switching support crystallinity for industrial hydrogen evolution. Angew. Chem. Int. Ed. 2023, 63, e202317220. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Zhang, C.; Wang, R.; Wei, G.; Yang, T.; Zhang, J.; Chen, Y.; Gao, S. A corrosion-etching strategy for fabricating RuO2 coupled with defective NiFeZn(OH)x for a highly efficient hydrogen evolution reaction. J. Mater. Chem. A 2022, 10, 20453–20463. [Google Scholar] [CrossRef]
- Chen, M.; Li, W.; Lu, Y.; Qi, P.; Wu, H.; Liu, G.; Zhao, Y.; Tang, Y. Corrosion engineering approach to rapidly prepare Ni(Fe)OOH/Ni(Fe)Sx nanosheet arrays for efficient water oxidation. J. Mater. Chem. A 2023, 11, 4608–4618. [Google Scholar] [CrossRef]
- Li, X.G.; Liu, C.; Fang, Z.T.; Xu, L.; Lu, C.L.; Hou, W.H. Ultrafast room-temperature synthesis of self-supported NiFe-layered double hydroxide aslLarge-current-density oxygen evolution electrocatalyst. Small 2022, 18, 2104354. [Google Scholar] [CrossRef]
- Chen, H.Y.; Gao, R.T.; Chen, H.J.; Yang, Y.; Wu, L.M.; Wang, L. Ruthenium And Silver Synergetic Regulation NiFe LDH Boosting Long-Duration Industrial Seawater Electrolysis. Adv. Funct. Mater. 2024, 34, 2315674. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L.; Tan, L.; Liu, X.; Wen, Y.; Hou, W.; Zhan, T. Electrodeposition of NiFe-layered double hydroxide layer on sulfur-modified nickel molybdate nanorods for highly efficient seawater splitting. J. Colloid Interface Sci. 2022, 613, 349–358. [Google Scholar] [CrossRef]
- Lee, J.; Jung, H.; Park, Y.S.; Woo, S.; Kwon, N.; Xing, Y.; Oh, S.H.; Choi, S.M.; Han, J.W.; Lim, B. Corrosion-engineered bimetallic oxide electrode as anode for high-efficiency anion exchange membrane water electrolyzer. Chem. Eng. J. 2021, 420, 127670. [Google Scholar] [CrossRef]
- Cullity, B.D.; Smoluchowski, R. Elements of X-ray Diffraction. Phys. Today 1957, 10, 50. [Google Scholar] [CrossRef]
- Gui, Y.; Liu, Z.; Feng, X.; Jia, Y.; Zhang, Y.; Zhang, Y.; Yang, H.; Zhang, Y.; Li, M.; Liang, L.; et al. One-step electrodeposition synthesis of NiFePS on carbon cloth as self-supported electrodes for electrochemical overall water splitting. J. Colloid Interface Sci. 2024, 673, 444–452. [Google Scholar] [CrossRef]
- Fu, Q.; Wong, L.W.; Zheng, F.; Zheng, X.; Tsang, C.S.; Lai, K.H.; Shen, W.; Ly, T.H.; Deng, Q.; Zhao, J. Unraveling and leveraging in situ surface amorphization for enhanced hydrogen evolution reaction in alkaline media. Nat. Commun. 2023, 14, 6462. [Google Scholar] [CrossRef]
- Nie, J.; Shi, J.; Huang, T.; Xie, M.Y.; Ouyang, Z.Y.; Xian, M.H.; Huang, G.F.; Wan, H.; Hu, W.; Huang, W.Q. Cation-Induced Deep Reconstruction and Self-Optimization of NiFe Phosphide Precatalysts for Hydrogen Evolution and Overall Water Splitting. Adv. Funct. Mater. 2024, 34, 2314172. [Google Scholar] [CrossRef]
- Fu, C.; Hao, W.; Fan, J.; Zhang, Q.; Guo, Y.; Fan, J.; Chen, Z.; Li, G. Fabrication of Ultra-Durable and Flexible NiPx -Based Electrode toward High-Efficient Alkaline Seawater Splitting at Industrial Grade Current Density. Small 2023, 19, 2205689. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, C.; Luo, W.; Yu, X.; Chang, Z.; Kong, F.; Zhu, L.; Huang, Y.; Tian, H.; Cui, X.; et al. In Situ Active Site Refreshing of Electro-Catalytic Materials for Ultra-Durable Hydrogen Evolution at Elevated Current Density. Adv. Energy Mater. 2024, 14, 2304099. [Google Scholar] [CrossRef]
- Zhang, X.; Tong, L.; Shi, X.H.; Li, Z.S.; Xiao, Z.H.; Liu, Y.P.; Zhang, T.; Lin, S.W. Tailoring atomically local electric field of NiFe layered double hydroxides with Ag dopants to boost oxygen evolution kinetics. J. Colloid Interface Sci. 2024, 668, 502–511. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Zhang, Z.; Chen, P.; Zhang, Y.; Dong, X. Dual-metal hydroxide@oxide heterojunction catalyst constructed via corrosion engineering for large-current oxygen evolution reaction. Appl. Catal. B-Environ. 2023, 325, 122311. [Google Scholar] [CrossRef]
- Zou, D.; Yi, Y.; Song, Y.; Guan, D.; Xu, M.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. The BaCe0.16Y0.04Fe0.8O3−δ nanocomposite: A new high-performance cobalt-free triple-conducting cathode for protonic ceramic fuel cells operating at reduced temperatures. J. Mater. Chem. A 2022, 10, 5381–5390. [Google Scholar] [CrossRef]
- Yan, L.; Du, Z.; Lai, X.; Lan, J.; Liu, X.; Liao, J.; Feng, Y.; Li, H. Synergistically modulating the electronic structure of Cr-doped FeNi LDH nanoarrays by O-vacancy and coupling of MXene for enhanced oxygen evolution reaction. Int. J. Hydrogen Energy 2023, 48, 1892–1903. [Google Scholar] [CrossRef]
- Qiu, Z.; Dai, Y.; Yang, F.; Zhang, R.; Guo, W.; Xiao, X.; Tong, Y.; Yao, L.; Yang, Z. Optimizing the activity of hydrogen evolution reaction through controllable synthesis of single crystal or polycrystalline NixPy. J. Alloys Compd. 2024, 976, 173029. [Google Scholar] [CrossRef]
- Singha Roy, S.; Madhu, R.; Karmakar, A.; Kundu, S. From Theory to Practice: A Critical and Comparative Assessment of Tafel Slope Analysis Techniques in Electrocatalytic Water Splitting. ACS Mater. Lett. 2024, 6, 3112–3123. [Google Scholar] [CrossRef]
- Chen, W.; Wu, B.; Wang, Y.; Zhou, W.; Li, Y.; Liu, T.; Xie, C.; Xu, L.; Du, S.; Song, M.; et al. Deciphering the alternating synergy between interlayer Pt single-atom and NiFe layered double hydroxide for overall water splitting. Energy Environ. Sci. 2021, 14, 6428–6440. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, M.; Jia, F.; Huang, J.; Amini, A.; Song, S.; Yi, H.; Cheng, C. Noble-Metal-Free oxygen evolution reaction electrocatalysts working at high current densities over 1000 mA cm−2: From fundamental understanding to design principles. Energy Environ. Mater. 2023, 6, e12457. [Google Scholar] [CrossRef]
- Jiang, B.; Zhu, J.; Xia, Z.; Lyu, J.; Li, X.; Zheng, L.; Chen, C.; Chaemchuen, S.; Bu, T.; Verpoort, F.; et al. Correlating Single-Atomic Ruthenium Interdistance with Long-Range Interaction Boosts Hydrogen Evolution Reaction Kinetics. Adv. Mater. 2024, 36, 2310699. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, T.; Hao, Y.; Wu, J.; Lei, C.; Chen, Z.; Du, W.; Gong, M. Unveiling the Cation Dependence in Alkaline Hydrogen Evolution by Differently-Charged Ruthenium/Molybdenum Sulfide Hybrids. Adv. Mater. 2024, 36, 2410422. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Wang, X.; Ning, H.; Yang, T.; Yu, J.; Kumar, A.; Luo, Y.; Wang, H.; Wang, L. Unraveling the synergy of chemical hydroxylation and the physical heterointerface upon improving the hydrogen evolution kinetics. ACS Nano 2021, 15, 15017–15026. [Google Scholar] [CrossRef]
- Fu, L.; Wang, S.; Cai, J.; Huang, H.; Yang, F.; Xie, S. Recent advances in platinum-group-metal based electrocatalysts for alkaline hydrogen oxidation reaction. Chem. Synth. 2024, 4, 8. [Google Scholar] [CrossRef]
- Wang, H.Y.; Hung, S.F.; Chen, H.Y.; Chan, T.S.; Chen, H.M.; Liu, B. In Operando Identification of Geometrical-Site-Dependent Water Oxidation Activity of Spinel Co3O4. J. Am. Chem. Soc. 2016, 138, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Harrington, D.; Conway, B. Behavior of overpotential—Deposited species in Faradaic reactions—II. ac Impedance measurements on H2 evolution kinetics at activated and unactivated Pt cathodes. Electrochim. Acta 1987, 32, 1713–1731. [Google Scholar] [CrossRef]
- He, S.; Tu, Y.; Zhang, J.; Zhang, L.; Ke, J.; Wang, L.; Du, L.; Cui, Z.; Song, H. Ammonia-Induced FCC Ru Nanocrystals for Efficient Alkaline Hydrogen Electrocatalysis. Small 2024, 20, 2308053. [Google Scholar] [CrossRef]
- Hao, Y.; Hung, S.F.; Tian, C.; Wang, L.; Chen, Y.Y.; Zhao, S.; Peng, K.S.; Zhang, C.; Zhang, Y.; Kuo, C.H.; et al. Polarized Ultrathin BN Induced Dynamic Electron Interactions for Enhancing Acidic Oxygen Evolution. Angew. Chem. Int. Ed. 2024, 63, e202402018. [Google Scholar] [CrossRef]
- Malhotra, D.; Nguyen, T.H.; Tran, D.T.; Dinh, V.A.; Kim, N.H.; Lee, J.H. Triphasic Ni2P-Ni12P5 -Ru with Amorphous Interface Engineering Promoted by Co Nano-Surface for Efficient Water Splitting. Small 2024, 20, 2309122. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.P.; Wang, X.H.; Gong, M.X.; Wang, R.; Hao, X.F.; Chen, Y. Corrosion strategy for synthesizing Ru-decorated FeOOH nanoneedles as advanced hydrogen evolution reaction catalysts. J. Alloys Compd. 2023, 958, 170430. [Google Scholar] [CrossRef]
- Feng, X.Y.; Sun, T.T.; Feng, X.F.; Yu, H.; Yang, Y.; Chen, L.M.; Zhang, F.Q. Single-Atomic-Site Platinum Steers Middle Hydroxyl Selective Oxidation on Amorphous/Crystalline Homojunction for Photoelectrochemical Glycerol Oxidation Coupled with Hydrogen Generation. Adv. Funct. Mater. 2024, 34, 2316238. [Google Scholar] [CrossRef]
- Ma, G.; Yang, N.; Xue, Y.; Zhou, G.; Wang, X. Ethylene glycol electrochemical reforming using ruthenium nanoparticle-decorated nickel phosphide ultrathin nanosheets. ACS Appl. Mater. Interfaces 2021, 13, 42763–42772. [Google Scholar] [CrossRef]
- Li, X.; Niu, Z.; Niu, M.; Wang, J.; Cao, D.; Zeng, X. Single atom Ru doped Ni2P/Fe3P heterostructure for boosting hydrogen evolution for water splitting. Small 2024, 20, 2311335. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, W.; Liu, Y.; Shen, R.; Xie, X.; Tian, W.; Zhang, X.; Ding, J.; Liu, Y.; Li, B. Interfacial synergistic effect of Ru nanoparticles embedded onto amorphous/crystalline WO3 nanorods on boosting the pH-universal hydrogen evolution reaction. Appl. Catal. B Environ. 2023, 343, 123502. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, Y.; Wu, H.; Gao, Y.; Chen, Z.; Jin, W.; Wang, J.; Ma, T.; Wang, L. Corrosion Engineering on Iron Foam toward Efficiently Electrocatalytic Overall Water Splitting Powered by Sustainable Energy. Adv. Funct. Mater. 2021, 31, 2010437. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Zhang, X.; Tong, L.; Liu, Y.; Lin, S. Growing Nanocrystalline Ru on Amorphous/Crystalline Heterostructure for Efficient and Durable Hydrogen Evolution Reaction. Catalysts 2025, 15, 434. https://doi.org/10.3390/catal15050434
Huang Q, Zhang X, Tong L, Liu Y, Lin S. Growing Nanocrystalline Ru on Amorphous/Crystalline Heterostructure for Efficient and Durable Hydrogen Evolution Reaction. Catalysts. 2025; 15(5):434. https://doi.org/10.3390/catal15050434
Chicago/Turabian StyleHuang, Quanbin, Xu Zhang, Li Tong, Yipu Liu, and Shiwei Lin. 2025. "Growing Nanocrystalline Ru on Amorphous/Crystalline Heterostructure for Efficient and Durable Hydrogen Evolution Reaction" Catalysts 15, no. 5: 434. https://doi.org/10.3390/catal15050434
APA StyleHuang, Q., Zhang, X., Tong, L., Liu, Y., & Lin, S. (2025). Growing Nanocrystalline Ru on Amorphous/Crystalline Heterostructure for Efficient and Durable Hydrogen Evolution Reaction. Catalysts, 15(5), 434. https://doi.org/10.3390/catal15050434






