An Overview of Solid Acid Catalysts in Lignocellulose Biorefineries
Abstract
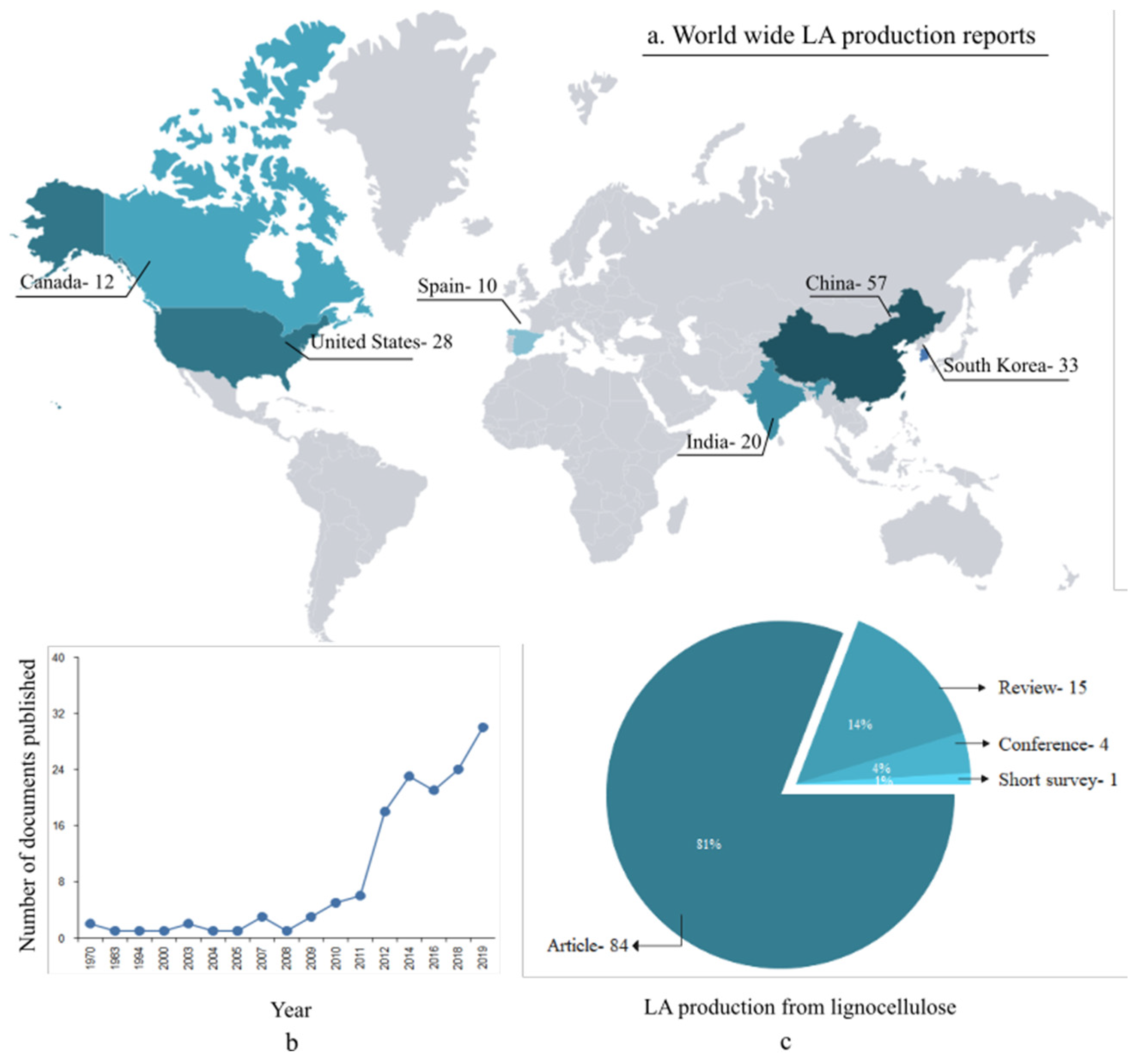
1. Introduction
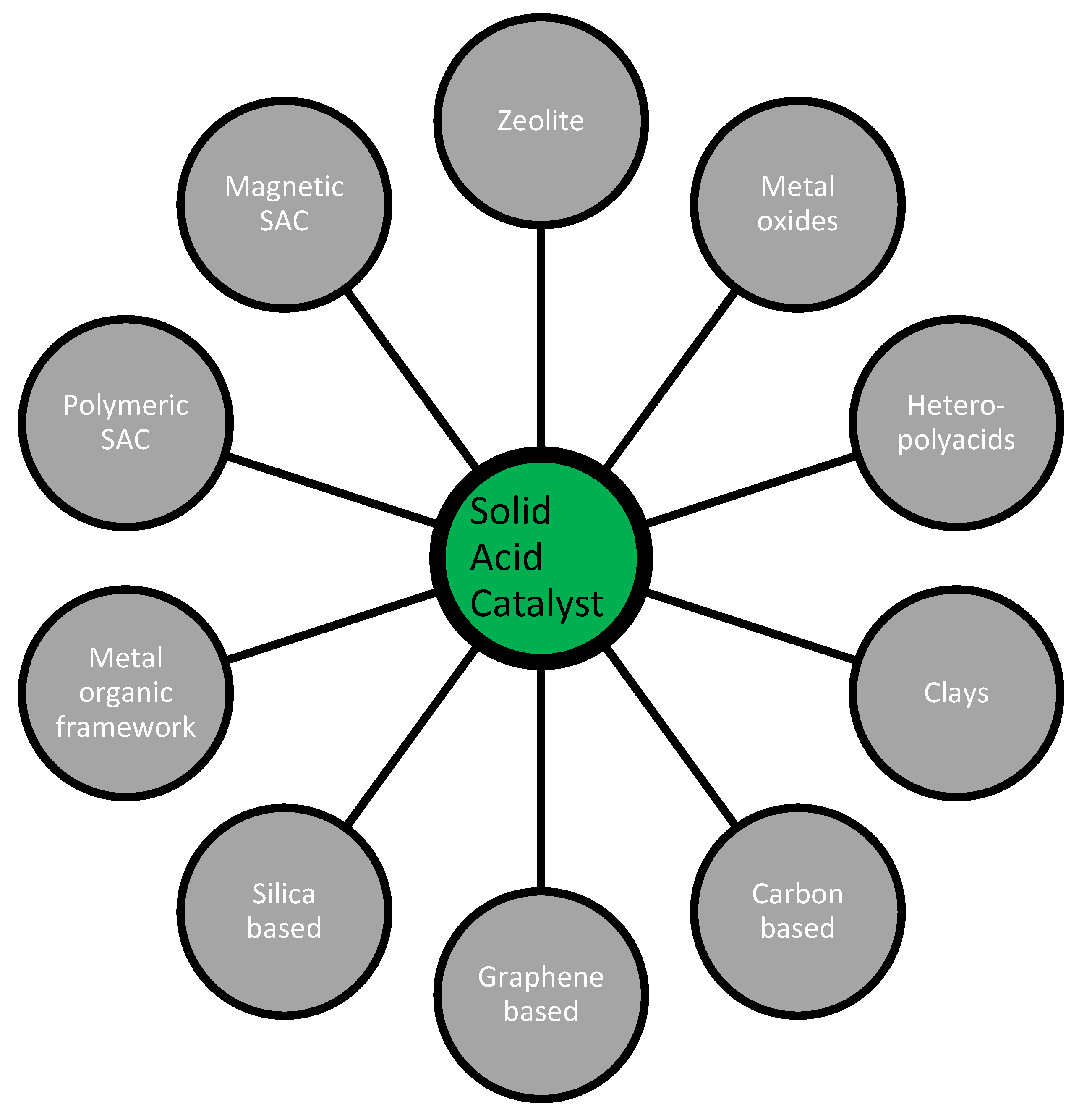
2. Solid Acid Catalysts
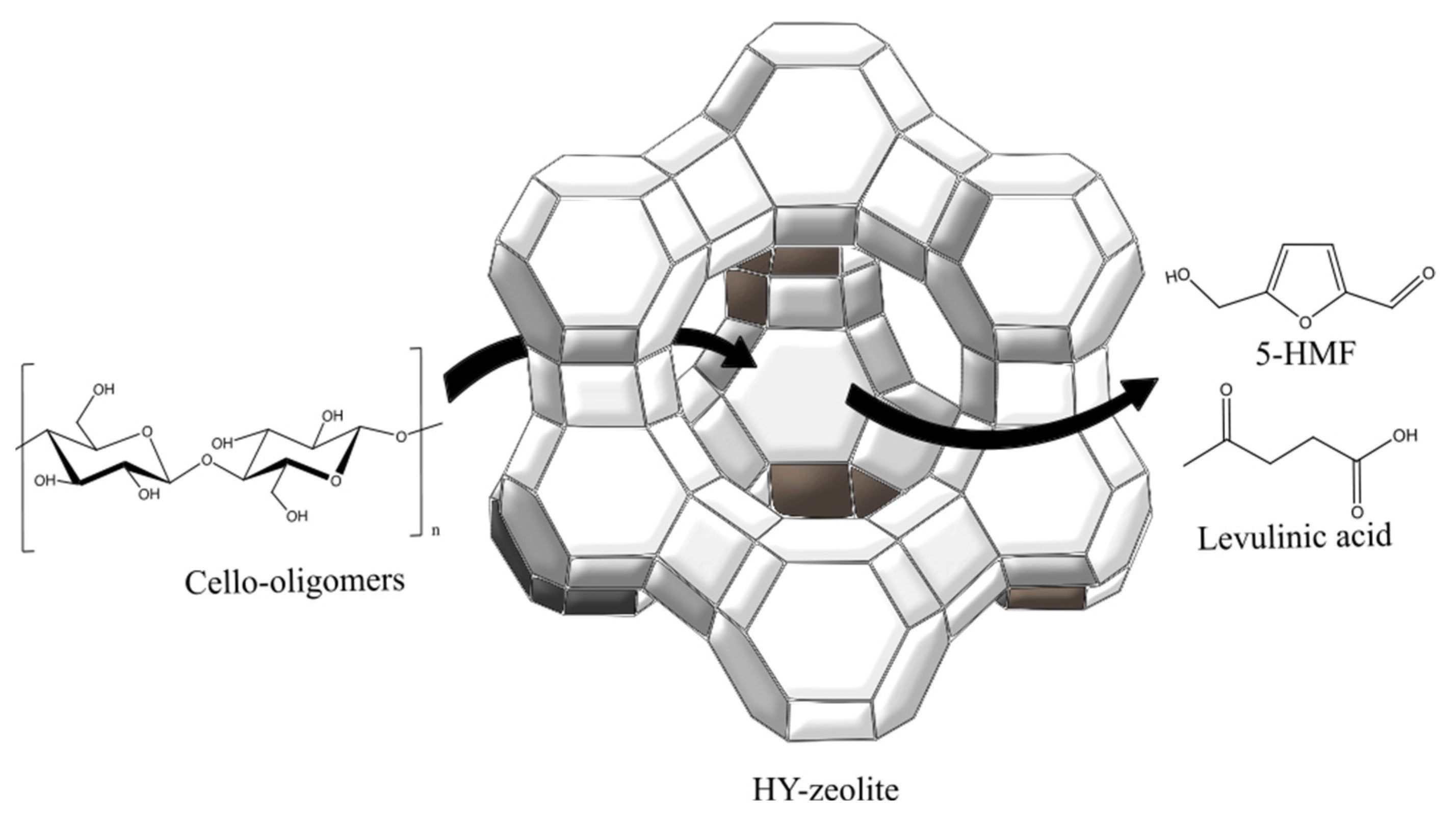
2.1. Zeolite
2.2. Metal Oxides
2.3. Heteropolyacids (HPAs)
2.4. Clays
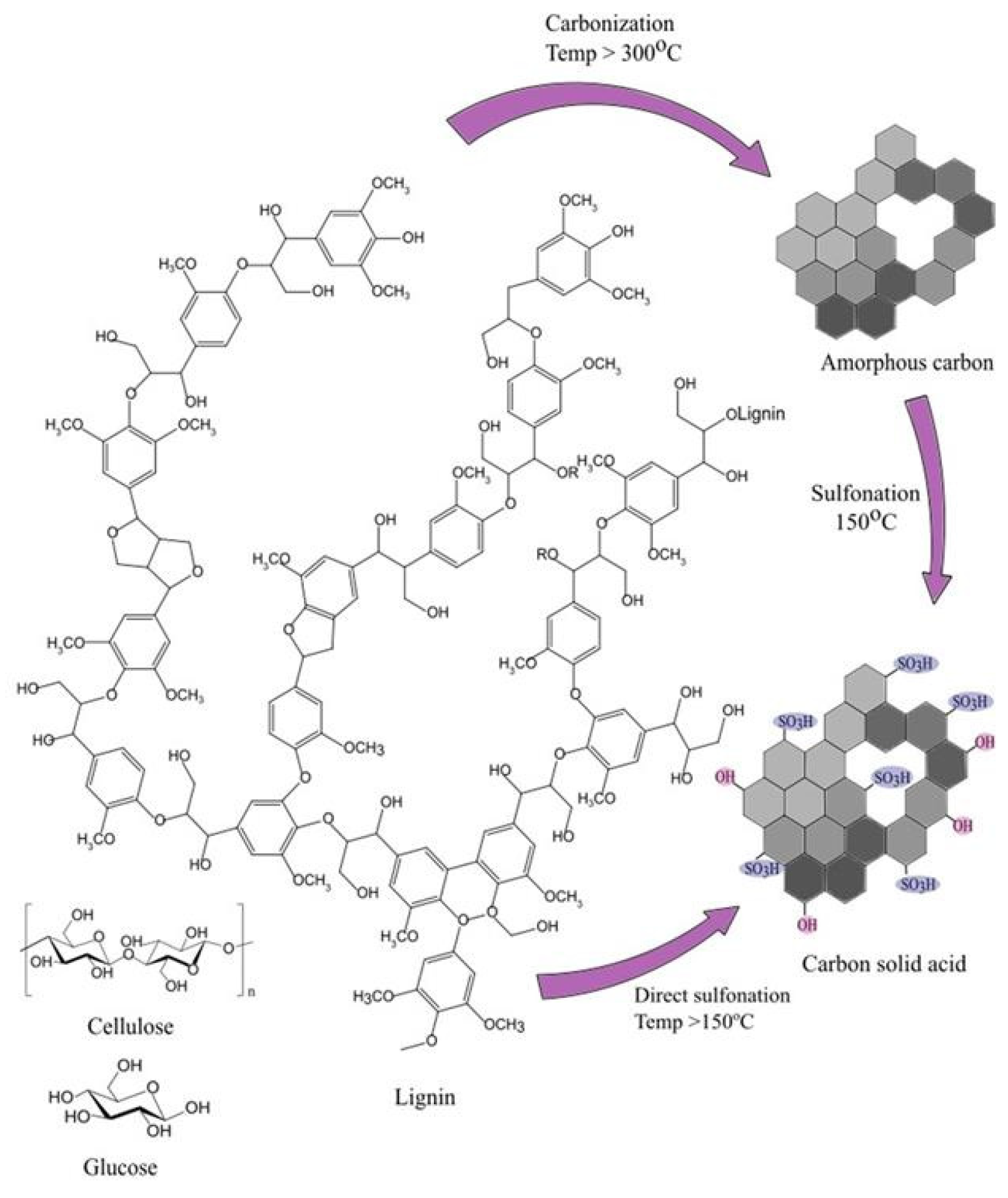
2.5. Carbon-Based SAC
2.6. Graphene/Graphene Oxide
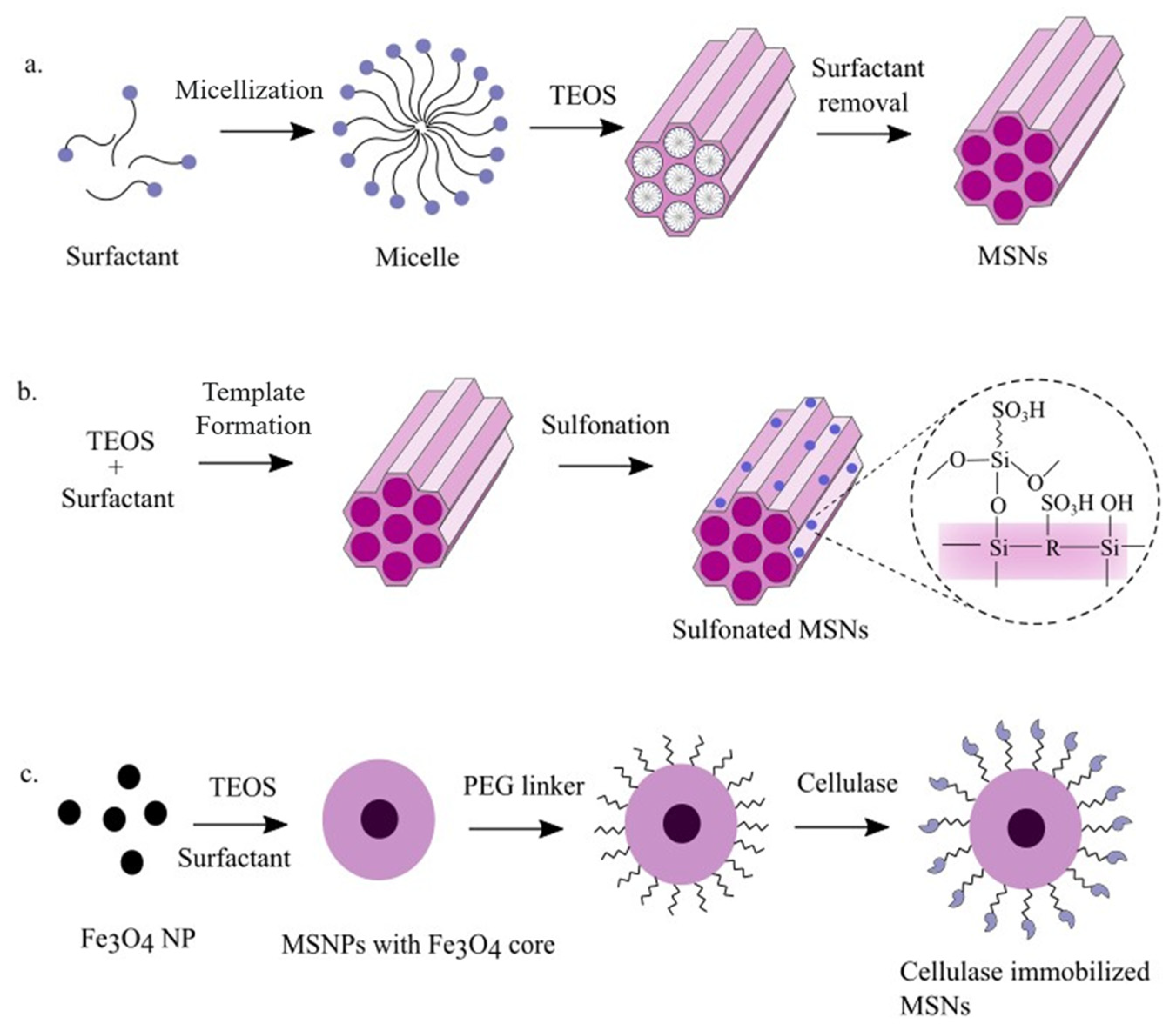
2.7. Mesoporous Silica Nanoparticles (MSNs)
2.8. Metal-Organic Frameworks (MOFs)
2.9. Polymeric Catalysts
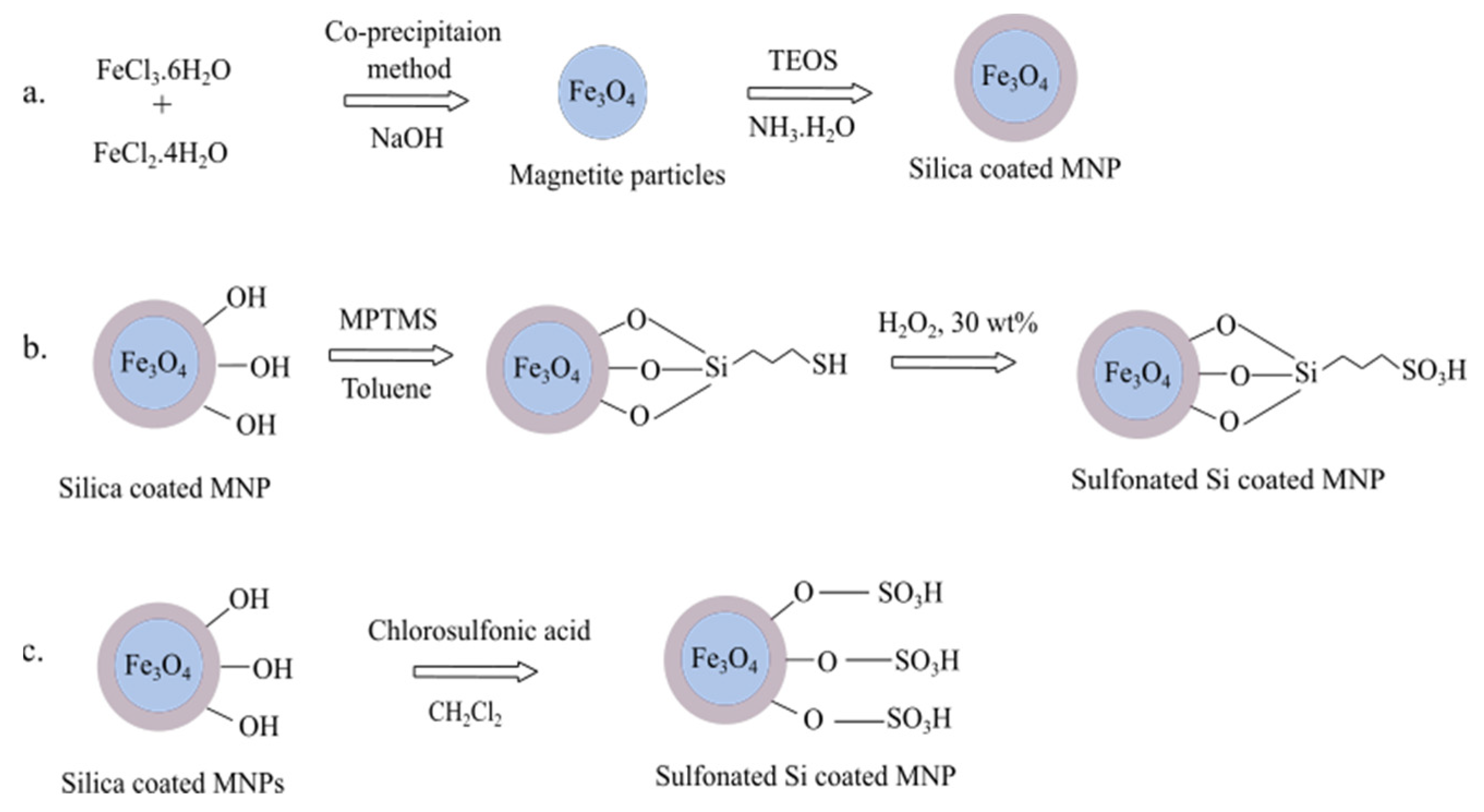
2.10. Magnetic Solid Acid Catalyst
3. Characteristics of Solid Acids
4. Application of Solid Acids in Biorefinery Processes
4.1. Lignin Depolymerization
4.2. Carbohydrate Hydrolysis
4.3. Esterification and Transesterification
4.4. Platform Chemicals
4.4.1. HMF
4.4.2. Furfural
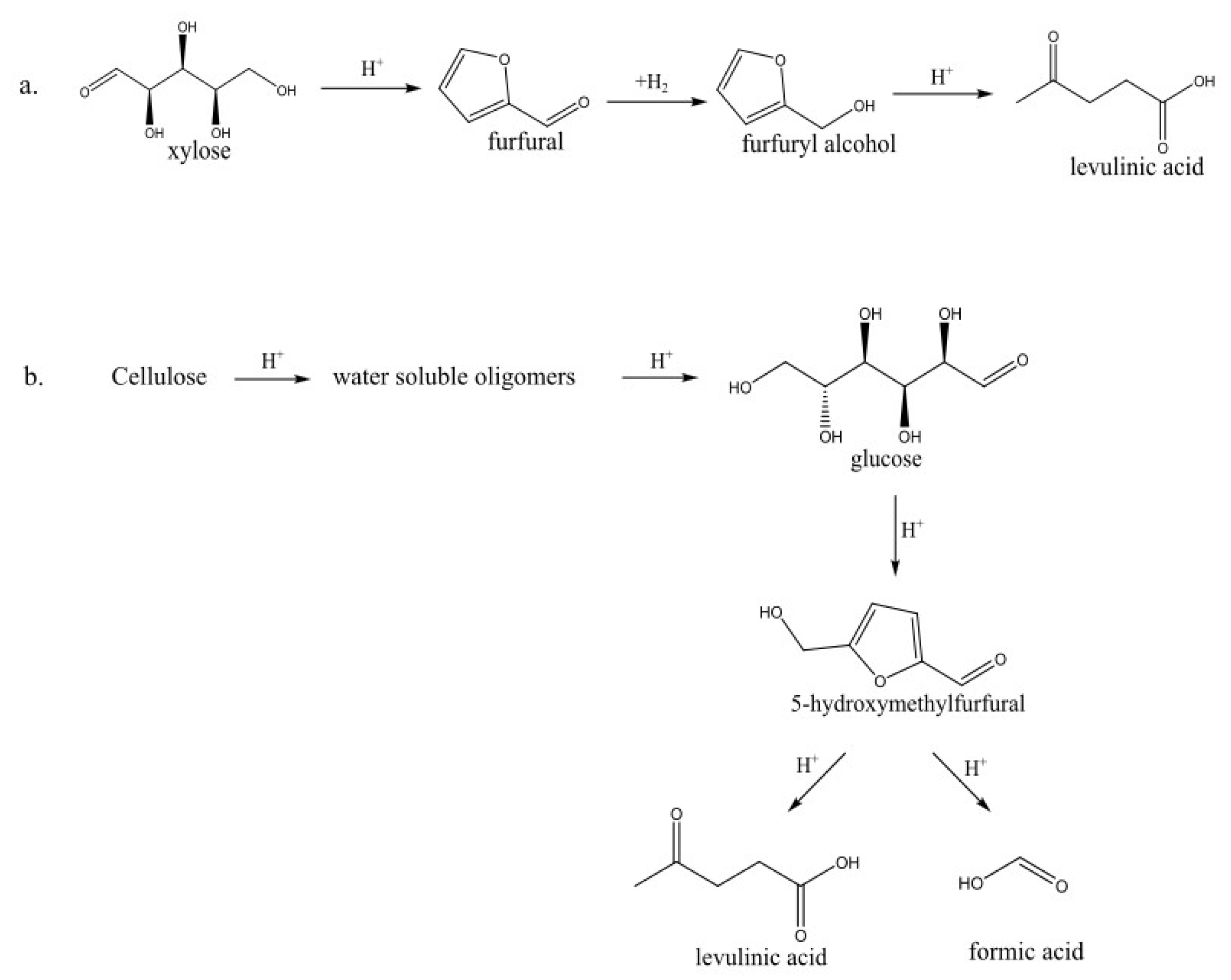
4.4.3. Levulinic Acid
4.4.4. γ-Valerolactone (GVL)
4.4.5. Lignin Monomers
5. Summary and Outlook
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| AL | Alkali lignin |
| CP | Chloromethyl polystyrene |
| CTAB | Cetyl trimethyl ammonium bromide |
| FAME | Fatty acid methyl ester |
| FFA | Free fatty acids |
| GO | Graphene oxide |
| GVL | γ-valerolactone |
| HCSS | Hydrothermal carbonized sulfuric acid |
| HMF | 5-hydroxy methyl furfural |
| HPA(s) | Heteropoly acid(s) |
| LA | Levulinic acid |
| LPF | Lignin based phenolic foam |
| MCM | Mobil Composition of Matter |
| MNP | Magnetic nanoparticle |
| MOF(s) | Metal organic framework(s) |
| MPTMS | 3-(mercaptopropyl) trimethyl silane |
| MSN(s) | Mesoporous silica nanoparticle(s) |
| OMC | Ordered mesoporous carbon |
| PAL | Phenolated alkali lignin |
| PC | Phosphoric carbons |
| POM | Polyoxometalates |
| POPS | Porous polymer organic frameworks |
| SAC(s) | Solid acid catalyst(s) |
| SAPO | Silico aluminophosphates |
| SBA | Santa Barbara Amorphous |
| SDG | Sustainable development goal |
| TEOS | Tetraethylorthosilicate |
| TPA | Tungstophosphoric acid |
| TPD | Temperature programmed desorption |
| TRS | Total reducing sugar |
| USY zeolite | Ultra stable Y zeolite |
References
- Arhin, S.G.; Cesaro, A.; Capua, F.D.; Esposito, G. Recent progress and challenges in biotechnological valorization of lignocellulosic materials: Towards sustainable biofuels and platform chemicals synthesis. Sci. Total Environ. 2023, 857, 159333. [Google Scholar] [CrossRef]
- Xu, H.; Wu, P. New progress in zeolite synthesis and catalysis. Natl. Sci. Rev. 2022, 9, nwac045. [Google Scholar] [CrossRef]
- Chu, S.; Guo, X.; Dong, L.; Chen, X.; Li, Y.; Mu, X. The influence of pore structure and Si/Al ratio of HZSM-5 zeolites on the product distributions of α-cellulose hydrolysis. Mol. Catal. 2018, 445, 240–247. [Google Scholar] [CrossRef]
- Corma, A. Solid acid catalysts. Curr. Opin. Solid State Mater. Sci. 1997, 2, 63–75. [Google Scholar] [CrossRef]
- Zakharova, M.V.; Kleitz, F.; Fontaine, F.-G. Lewis acidity quantification and catalytic activity of Ti, Zr and Al-supported mesoporous silica. Dalton Trans. 2017, 46, 3864–3876. [Google Scholar] [CrossRef] [PubMed]
- Songtawee, S.; Rungtaweevoranit, B.; Klaysom, C.; Faungnawakij, K. Tuning Brønsted and Lewis acidity on phosphated titanium dioxides for efficient conversion of glucose to 5-hydroxymethylfurfural. RSC Adv. 2021, 11, 29196–29206. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Liu, J.; Wang, T.; Shen, Y.; Zheng, K.; Jiang, F.; Xu, Y.; Liu, X. Tuning the Lewis acidity of ZrO2 for efficient conversion of CH4 and CO2 into acetic acid. New J. Chem. 2021, 45, 8978–8985. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Y.; Baiker, A.; Huang, J. Pentacoordinated Aluminum Species: New Frontier for Tailoring Acidity-Enhanced Silica–Alumina Catalysts. Acc. Chem. Res. 2020, 53, 2648–2658. [Google Scholar] [CrossRef]
- Aihara, T.; Asazuma, K.; Miura, H.; Shishido, T. Highly active and durable WO3/Al2O3 catalysts for gas-phase dehydration of polyols. RSC Adv. 2020, 10, 37538–37544. [Google Scholar] [CrossRef]
- Zeidan, H.; Erünal, E.; Marti, M.E. The relation between surface acidity and MoO3:Al2O3 ratio on the ternary mixed oxide catalysts for the conversion of propan-2-ol. Turk. J. Chem. 2022, 46, 2090–2101. [Google Scholar] [CrossRef]
- Takagaki, A. Rational design of metal oxide solid acids for sugar conversion. Catalysts 2019, 9, 907. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Z.; Wang, X.; Dong, C.; Liu, Y. Catalytic Upgrading of Biomass Fast Pyrolysis Vapors Using Ordered Mesoporous ZrO2, TiO2 and SiO2. Energy Procedia 2014, 61, 1937–1941. [Google Scholar] [CrossRef]
- Guan, W.; Tsang, C.-W.; Lin, C.S.K.; Len, C.; Hu, H.; Liang, C. A review on high catalytic efficiency of solid acid catalysts for lignin valorization. Bioresour. Technol. 2020, 298, 122432. [Google Scholar] [CrossRef] [PubMed]
- Arata, K.; Matsuhashi, H.; Hino, M.; Nakamura, H. Synthesis of solid superacids and their activities for reactions of alkanes. Catal. Today 2003, 81, 17–30. [Google Scholar] [CrossRef]
- Gupta, P.; Paul, S. Solid acids: Green alternatives for acid catalysis. Catal. Today 2014, 236, 153–170. [Google Scholar] [CrossRef]
- Krueger, J.H.; Poller, M.J.; Lyall, C.; Lowe, J.; Hintermair, U.; Albert, J. In-Situ Investigations of Polyoxometalate-Catalysed Biomass Oxidation to Formic Acid by Using Multinuclear High Resolution Flow NMR Spectroscopy. ChemCatChem 2024, 16, e202400402. [Google Scholar] [CrossRef]
- Raabe, J.; Poller, M.J.; Voß, D.; Albert, J. H8[PV5Mo7O40]—A Unique Polyoxometalate for Acid and RedOx Catalysis: Synthesis, Characterization, Modern Applications in Green Chemical Processes. ChemSusChem 2023, 16, e202300072. [Google Scholar] [CrossRef]
- He, Z.; Hou, Y.; Li, H.; Wei, J.; Ren, S.; Wu, W. Novel chemical looping oxidation of biomass-derived carbohydrates to super-high-yield formic acid using heteropolyacids as oxygen carrier. Renew. Energy 2023, 207, 461–470. [Google Scholar] [CrossRef]
- Rafiee, E.; Eavani, S. Polyoxometalates as Heterogeneous Catalysts for Organic Reactions. Curr. Org. Chem. 2017, 21, 752–778. [Google Scholar] [CrossRef]
- Guo, H.; Qi, X.; Li, L.; Smith, R.L., Jr. Hydrolysis of cellulose over functionalized glucose-derived carbon catalyst in ionic liquid. Bioresour. Technol. 2012, 116, 355–359. [Google Scholar] [CrossRef]
- Mateo, W.; Lei, H.; Villota, E.; Qian, M.; Zhao, Y.; Huo, E.; Zhang, Q.; Lin, X.; Wang, C. One-step synthesis of biomass-based sulfonated carbon catalyst by direct carbonization-sulfonation for organosolv delignification. Bioresour. Technol. 2021, 319, 124194. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Imamura, K.; Onda, A. Hydrolysis of oligosaccharides and polysaccharides on sulfonated solid acid catalysts: Relations between adsorption properties and catalytic activities. ACS Omega 2020, 5, 24964–24972. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, M.; Liu, Q.; Wang, X.; Lv, T.; Jia, L. Graphene oxide mediated cellulose-derived carbon as a highly selective catalyst for the hydrolysis of cellulose to glucose. Appl. Catal. A Gen. 2017, 543, 218–224. [Google Scholar] [CrossRef]
- Huh, S.; Wiench, J.W.; Trewyn, B.G.; Song, S.; Pruski, M.; Lin, V.S.-Y. Tuning of particle morphology and pore properties in mesoporous silicas with multiple organic functional groups. Chem. Commun. 2003, 18, 2364–2365. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Collanton, R.P.; Monroe, J.I.; Casey, T.M.; Shell, M.S.; Han, S.; Scott, S.L. Evidence for Entropically Controlled Interfacial Hydration in Mesoporous Organosilicas. J. Am. Chem. Soc. 2022, 144, 1766–1777. [Google Scholar] [CrossRef]
- Torres, C.; Potts, D.S.; Flaherty, D.W. Solvent Mediated Interactions on Alkene Epoxidations in Ti-MFI: Effects of Solvent Identity and Silanol Density. ACS Catal. 2023, 13, 8925–8942. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Shen, K.; Vohs, J.M.; Gorte, R.J. Modification of SBA-15 for stabilizing supported oxides. J. Porous Mater. 2025, 32, 97–106. [Google Scholar] [CrossRef]
- Serrano, D.P.; Calleja, G.; Botas, J.A.; Gutierrez, F.J. Adsorption and hydrophobic properties of mesostructured MCM-41 and SBA-15 materials for volatile organic compound removal. Ind. Eng. Chem. Res. 2004, 43, 7010–7018. [Google Scholar] [CrossRef]
- Chaudhary, V.; Sharma, S. An overview of ordered mesoporous material SBA-15: Synthesis, functionalization and application in oxidation reactions. J. Porous Mater. 2017, 24, 741–749. [Google Scholar] [CrossRef]
- Sharmin, E.; Zafar, F. Introductory chapter: Metal organic frameworks (MOFs). In Metal-Organic Frameworks; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Wang, L.; Zhi, W.; Wan, J.; Han, J.; Li, C.; Wang, Y. Recyclable β-glucosidase by one-pot encapsulation with Cu-MOFs for enhanced hydrolysis of cellulose to glucose. ACS Sustain. Chem. Eng. 2019, 7, 3339–3348. [Google Scholar] [CrossRef]
- Ahmed, I.N.; Yang, X.-L.; Dubale, A.A.; Li, R.-F.; Ma, Y.-M.; Wang, L.-M.; Hou, G.-H.; Guan, R.-F.; Xie, M.-H. Hydrolysis of cellulose using cellulase physically immobilized on highly stable zirconium based metal-organic frameworks. Bioresour. Technol. 2018, 270, 377–382. [Google Scholar] [CrossRef]
- Martín, N.; Dusselier, M.; Vos, D.E.; Cirujano, F.G. Metal-Organic framework derived metal oxide clusters in porous aluminosilicates: A catalyst design for the synthesis of bioactive aza-heterocycles. ACS Catal. 2018, 9, 44–48. [Google Scholar] [CrossRef]
- Villandiera, N.; Corma, A. One pot catalytic conversion of cellulose into biodegradable surfactants. Chem. Commun. 2010, 46, 4408–4410. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Gong, J.; Yang, C.; Cheng, Y.; Lu, J.; Yang, Q.; Wang, H. A recyclable and regenerable solid acid for efficient hydrolysis of cellulose to glucose. Biomass Bioenergy 2020, 138, 105611. [Google Scholar] [CrossRef]
- Takagaki, A.; Nishimura, M.; Nishimura, S.; Ebitani, K. Hydrolysis of sugars using magnetic silica nanoparticles with sulfonic acid groups. Chem. Lett. 2011, 40, 1195–1197. [Google Scholar] [CrossRef]
- Lai, D.M.; Deng, L.; Li, J.; Liao, B.; Guo, Q.; Fu, Y. Hydrolysis of cellulose into glucose by magnetic solid acid. ChemSusChem 2011, 4, 55–58. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, Z.; Wang, X.; Liu, B.; Lin, J. Hydrolysis of cellulose in ionic liquids catalyzed by a magnetically-recoverable solid acid catalyst. Chem. Eng. J. 2014, 235, 349–355. [Google Scholar] [CrossRef]
- Xuan, S.; Hao, L.; Jiang, W.; Gong, X.; Hu, Y.; Chen, Z. A facile method to fabricate carbon-encapsulated Fe3O4 core/shell composites. Nanotechnology 2007, 18, 035602. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Liu, F.; Wang, L.; He, H. Magnetic core–shell Fe3O@ C-SO3 H nanoparticle catalyst for hydrolysis of cellulose. Cellulose 2013, 20, 127–134. [Google Scholar] [CrossRef]
- Hu, L.; Li, Z.; Wu, Z.; Lin, L.; Zhou, S. Catalytic hydrolysis of microcrystalline and rice straw-derived cellulose over a chlorine-doped magnetic carbonaceous solid acid. Ind. Crops Prod. 2016, 84, 408–417. [Google Scholar] [CrossRef]
- Chung, P.-W.; Charmot, A.; Olatunji-Ojo, O.A.; Durkin, K.A.; Katz, A. Hydrolysis catalysis of miscanthus xylan to xylose using weak-acid surface sites. ACS Catal. 2013, 4, 302–310. [Google Scholar] [CrossRef]
- Gorte, R.J. What do we know about the acidity of solid acids? Catal. Lett. 1999, 62, 1–13. [Google Scholar] [CrossRef]
- Gorte, R.J. Temperature-programmed desorption of oxide catalysts. Catal. Today 1996, 28, 405–414. [Google Scholar] [CrossRef]
- Azzouz, A.; Nistor, D.; Miron, D.; Ursu, A.; Sajin, T.; Monette, F.; Niquette, P.; Hausler, R. Assessment of acid–base strength distribution of ion-exchanged montmorillonites through NH3 and CO2-TPD measurements. Thermochim. Acta 2006, 449, 27–34. [Google Scholar] [CrossRef]
- Fan, M.; Wang, C.-Y.; Gorte, R.J.; Vohs, J.M. Synthesis of Lewis Acid Sites in SBA-15 and Amorphous Silica Using Vapor Phase Infiltration of Zr and Sn. Catal. Lett. 2024, 154, 2725–2734. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Kwon, O.; Gorte, R.J.; Vohs, J.M. Synthesis of high-surface area tungstated zirconia by atomic layer deposition on mesoporous silica. Microporous Mesoporous Mater. 2022, 335, 111821. [Google Scholar] [CrossRef]
- Damont, A.; Legrand, A.; Cao, C.; Fenaille, F.; Tabet, J.-C. Hydrogen/deuterium exchange mass spectrometry in the world of small molecules. Mass Spectrom. Rev. 2023, 42, 1300–1331. [Google Scholar] [CrossRef]
- Fu, J.; Liu, S.; Zheng, W.; Huang, R.; Wang, C.; Lawal, A.; Alexopoulos, K.; Liu, S.; Wang, Y.; Yu, K.; et al. Modulating the dynamics of Brønsted acid sites on PtWOx inverse catalyst. Nat. Catal. 2022, 5, 144–153. [Google Scholar] [CrossRef]
- Carà, P.D.; Pagliaro, M.; Elmekawy, A.; Brown, D.R.; Verschuren, P.; Shiju, N.R.; Rothenberg, G. Hemicellulose hydrolysis catalysed by solid acids. Catal. Sci. Technol. 2013, 3, 2057–2061. [Google Scholar] [CrossRef]
- Gazit, O.M.; Charmot, A.; Katz, A. Grafted cellulose strands on the surface of silica: Effect of environment on reactivity. Chem. Commun. 2011, 47, 376–378. [Google Scholar] [CrossRef]
- Capon, B. Intramolecular catalysis in glucoside hydrolysis. Tetrahedron Lett. 1963, 4, 911–913. [Google Scholar] [CrossRef]
- Shen, F.; Smith, R.L., Jr.; Li, L.; Yan, L.; Qi, X. Eco-friendly method for efficient conversion of cellulose into levulinic acid in pure water with cellulase-mimetic solid acid catalyst. ACS Sustain. Chem. Eng. 2017, 5, 2421–2427. [Google Scholar] [CrossRef]
- Vu, A.; Wickramasinghe, S.R.; Qian, X. Polymeric solid acid catalysts for lignocellulosic biomass fractionation. Ind. Eng. Chem. Res. 2018, 57, 4514–4525. [Google Scholar] [CrossRef]
- Tan, I.S.; Lee, K.T. Solid acid catalysts pretreatment and enzymatic hydrolysis of macroalgae cellulosic residue for the production of bioethanol. Carbohydr. Polym. 2015, 124, 311–321. [Google Scholar] [CrossRef]
- Amarasekara, A. Acid Hydrolysis of Cellulose and Hemicellulose. In Handbook of Cellulosic Ethanol; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 247–281. [Google Scholar]
- Zhou, L.; Liu, Z.; Shi, M.; Du, S.; Su, Y.; Yang, X.; Xu, J. Sulfonated hierarchical H-USY zeolite for efficient hydrolysis of hemicellulose/cellulose. Carbohydr. Polym. 2013, 98, 146–151. [Google Scholar] [CrossRef]
- Freitas, C.; Barrow, N.S.; Zholobenko, V. Accessibility and Location of Acid Sites in Zeolites as Probed by Fourier Transform Infrared Spectroscopy and Magic Angle Spinning Nuclear Magnetic Resonance. Johns. Matthey Technol. Rev. 2018, 62, 279–290. [Google Scholar] [CrossRef]
- Mansir, N.; Taufiq-Yap, Y.H.; Rashid, U.; Lokman, I.M. Investigation of heterogeneous solid acid catalyst performance on low grade feedstocks for biodiesel production: A review. Energy Convers. Manag. 2017, 141, 171–182. [Google Scholar] [CrossRef]
- Jow, J.; Rorrer, G.L.; Hawley, M.C.; Lamport, D.T. Dehydration of D-fructose to levulinic acid over LZY zeolite catalyst. Biomass 1987, 14, 185–194. [Google Scholar] [CrossRef]
- Ishida, K.; Matsuda, S.; Watanabe, M.; Kitajima, H.; Kato, A.; Iguchi, M.; Aida, T.M.; Smith, R.L.; Qi, X.; Tago, T.; et al. Hydrolysis of cellulose to produce glucose with solid acid catalysts in 1-butyl-3-methyl-imidazolium chloride ([bmIm][Cl]) with sequential water addition. Biomass Convers. Biorefinery 2014, 4, 323–331. [Google Scholar] [CrossRef]
- Cai, H.; Li, C.; Wang, A.; Xu, G.; Zhang, T. Zeolite-promoted hydrolysis of cellulose in ionic liquid, insight into the mutual behavior of zeolite, cellulose and ionic liquid. Appl. Catal. B Environ. 2012, 123, 333–338. [Google Scholar] [CrossRef]
- Ramli, N.A.S.; Amin, N.A.S. Fe/HY zeolite as an effective catalyst for levulinic acid production from glucose: Characterization and catalytic performance. Appl. Catal. B Environ. 2015, 163, 487–498. [Google Scholar] [CrossRef]
- Degirmenci, V.; Uner, D.; Cinlar, B.; Shanks, B.H.; Yilmaz, A.; van Santen, R.A.; Hensen, E.J.M. Sulfated zirconia modified SBA-15 catalysts for cellobiose hydrolysis. Catal. Lett. 2011, 141, 33–42. [Google Scholar] [CrossRef]
- Porrang, S.; Davaran, S.; Rahemi, N.; Allahyari, S.; Mostafavi, E. How Advancing are Mesoporous Silica Nanoparticles? A Comprehensive Review of the Literature. Int. J. Nanomed. 2022, 17, 1803–1827. [Google Scholar] [CrossRef]
- Lanzafame, P.; Temi, D.; Perathoner, S.; Spadaro, A.; Centi, G. Direct conversion of cellulose to glucose and valuable intermediates in mild reaction conditions over solid acid catalysts. Catal. Today 2012, 179, 178–184. [Google Scholar] [CrossRef]
- Rizzi, F.; Castaldo, R.; Latronico, T.; Lasala, P.; Gentile, G.; Lavorgna, M.; Striccoli, M.; Agostiano, A.; Comparelli, R.; Depalo, N.; et al. High Surface Area Mesoporous Silica Nanoparticles with Tunable Size in the Sub-Micrometer Regime: Insights on the Size and Porosity Control Mechanisms. Molecules 2021, 26, 4247. [Google Scholar] [CrossRef]
- Fan, J.; Yu, C.; Lei, J.; Zhang, Q.; Li, T.; Tu, B.; Zhou, W.; Zhao, D. Low-temperature strategy to synthesize highly ordered mesoporous silicas with very large pores. J. Am. Chem. Soc. 2005, 127, 10794–10795. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, G.; Zhang, D.; Zhao, H.; Guo, M.; Shi, W.; Qiu, S. Novel mesoporous silica spheres with ultra-large pore sizes and their application in protein separation. J. Mater. Chem. 2009, 19, 2013–2017. [Google Scholar] [CrossRef]
- Peng, W.-H.; Lee, Y.-Y.; Wu, C.; Wu, K.C.-W. Acid–base bi-functionalized, large-pored mesoporous silica nanoparticles for cooperative catalysis of one-pot cellulose-to-HMF conversion. J. Mater. Chem. 2012, 22, 23181–23185. [Google Scholar] [CrossRef]
- Samykutty, A.; Grizzle, W.E.; Fouts, B.L.; McNally, M.W.; Chuong, P.; Thomas, A.; Chiba, A.; Otali, D.; Woloszynska, A.; Said, N.; et al. Optoacoustic imaging identifies ovarian cancer using a microenvironment targeted theranostic wormhole mesoporous silica nanoparticle. Biomaterials 2018, 182, 114–126. [Google Scholar] [CrossRef]
- Zhao, Y.-X.; Gao, C.-G.; Li, Y.-X.; Zhang, T.-M. Wormhole-like mesoporous silica templated by double-chained cationic surfactant. Microporous Mesoporous Mater. 2009, 124, 42–44. [Google Scholar] [CrossRef]
- Ke, W.; Liu, Y.; Wang, X.; Qin, X.; Chen, L.; Palomino, R.M.; Simonovis, J.P.; Lee, I.; Waluyo, I.; Rodriguez, J.A.; et al. Nucleation and Initial Stages of Growth during the Atomic Layer Deposition of Titanium Oxide on Mesoporous Silica. Nano Lett. 2020, 20, 6884–6890. [Google Scholar] [CrossRef] [PubMed]
- Pagán-Torres, Y.J.; Gallo, J.M.R.; Wang, D.; Pham, H.N.; Libera, J.A.; Marshall, C.L.; Elam, J.W.; Datye, A.K.; Dumesic, J.A. Synthesis of Highly Ordered Hydrothermally Stable Mesoporous Niobia Catalysts by Atomic Layer Deposition. ACS Catal. 2011, 1, 1234–1245. [Google Scholar] [CrossRef]
- Majumder, A.; Radzanowski, A.N.; Wang, C.-Y.; Mu, Y.; Coughlin, E.B.; Gorte, R.J.; Vohs, J.M.; Lee, D. Modulating the Contact Angle between Nonpolar Polymers and SiO2 Nanoparticles. Macromolecules 2024, 57, 8554–8561. [Google Scholar] [CrossRef]
- Charmot, A.; Chung, P.-W.; Katz, A. Catalytic hydrolysis of cellulose to glucose using weak-acid surface sites on postsynthetically modified carbon. ACS Sustain. Chem. Eng. 2014, 2, 2866–2872. [Google Scholar] [CrossRef]
- Hu, L.; Lin, L.; Wu, Z.; Zhou, S.; Liu, S. Chemocatalytic hydrolysis of cellulose into glucose over solid acid catalysts. Appl. Catal. B Environ. 2015, 174, 225–243. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top value added chemicals from biomass: Volume I. In Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Lab: Golden, CO, USA, 2004. [Google Scholar]
- Pooja, S.; Anbarasan, B.; Ponnusami, V.; Arumugam, A. Efficient production and optimization of biodiesel from kapok (Ceiba pentandra) oil by lipase transesterification process: Addressing positive environmental impact. Renew. Energy 2021, 165, 619–631. [Google Scholar] [CrossRef]
- Deepa, A.K.; Dhepe, P.L. Solid acid catalyzed depolymerization of lignin into value added aromatic monomers. RSC Adv. 2014, 4, 12625–12629. [Google Scholar] [CrossRef]
- Deepa, A.K.; Dhepe, P.L. Lignin Depolymerization into Aromatic Monomers over Solid Acid Catalysts. ACS Catal. 2015, 5, 365–379. [Google Scholar] [CrossRef]
- Adhikari, S.; Srinivasan, V.; Fasina, O. Catalytic Pyrolysis of Raw and Thermally Treated Lignin Using Different Acidic Zeolites. Energy Fuels 2014, 28, 4532–4538. [Google Scholar] [CrossRef]
- Asawaworarit, P.; Daorattanachai, P.; Laosiripojana, W.; Sakdaronnarong, C.; Shotipruk, A.; Laosiripojana, N. Catalytic depolymerization of organosolv lignin from bagasse by carbonaceous solid acids derived from hydrothermal of lignocellulosic compounds. Chem. Eng. J. 2019, 356, 461–471. [Google Scholar] [CrossRef]
- Li, Z.; Bai, X.; Wei, X.-Y.; Dilixiati, Y.; Fan, Z.-C.; Kong, Q.-Q.; Li, L.; Li, J.-H.; Lu, K.-L.; Zhao, J.; et al. A solid acid-catalyzed depolymerization of pine lignin to obtain guaiacol using a hydrogen-free strategy. Fuel Process. Technol. 2023, 249, 107843. [Google Scholar] [CrossRef]
- Zhou, M.; Shi, H.-Q.; Li, C.; Sheng, X.; Sun, Y.; Hou, M.; Niu, M.; Pan, X. Depolymerization and Activation of Alkali Lignin by Solid Acid-Catalyzed Phenolation for Preparation of Lignin-Based Phenolic Foams. Ind. Eng. Chem. Res. 2020, 59, 14296–14305. [Google Scholar] [CrossRef]
- Wang, G.; Liu, X.; Zhang, J.; Sui, W.; Jang, J.; Si, C. One-pot lignin depolymerization and activation by solid acid catalytic phenolation for lightweight phenolic foam preparation. Ind. Crops Prod. 2018, 124, 216–225. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, Y.; Tong, D.; Yang, M.; Fang, K.; Zhou, C.; Yu, W. Catalytic conversion of cellulose to reducing sugars over clay-based solid acid catalyst supported nanosized SO42− ZrO2. Appl. Clay Sci. 2020, 185, 105376. [Google Scholar] [CrossRef]
- Qi, W.; Liu, G.; He, C.; Liu, S.; Lu, S.; Yue, J.; Wang, Q.; Wang, Z.; Yuan, Z.; Hu, J. An efficient magnetic carbon-based solid acid treatment for corncob saccharification with high selectivity for xylose and enhanced enzymatic digestibility. Green Chem. 2019, 21, 1292–1304. [Google Scholar] [CrossRef]
- Nata, I.F.; Irawan, C.; Mardina, P.; Lee, C.-K. Carbon-based strong solid acid for cornstarch hydrolysis. J. Solid State Chem. 2015, 230, 163–168. [Google Scholar] [CrossRef]
- Rinaldi, R.; Schüth, F. Design of solid catalysts for the conversion of biomass. Energy Environ. Sci. 2009, 2, 610–626. [Google Scholar] [CrossRef]
- Geboers, J.; Vyver, S.; Carpentier, K.; Jacobs, P.; Sels, B. Efficient hydrolytic hydrogenation of cellulose in the presence of Ru-loaded zeolites and trace amounts of mineral acid. Chem. Commun. 2011, 47, 5590–5592. [Google Scholar] [CrossRef]
- Bai, C.; Zhu, L.; Shen, F.; Qi, X. Black liquor-derived carbonaceous solid acid catalyst for the hydrolysis of pretreated rice straw in ionic liquid. Bioresour. Technol. 2016, 220, 656–660. [Google Scholar] [CrossRef]
- Chang, K.-L.; Chen, X.-M.; Han, Y.-J.; Wang, X.-Q.; Potprommanee, L.; Ning, X.-A.; Liu, J.-Y.; Sun, J.; Peng, Y.-P.; Sun, S.-Y.; et al. Synergistic effects of surfactant-assisted ionic liquid pretreatment rice straw. Bioresour. Technol. 2016, 214, 371–375. [Google Scholar] [CrossRef]
- Si, W.; Li, Y.; Zheng, J.; Wang, D. Enhanced hydrolysis of bamboo biomass by chitosan based solid acid catalyst with surfactant addition in ionic liquid. Carbohydr. Polym. 2017, 174, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.-T.; Kim, S.-K.; Park, D.-H. Application of solid-acid catalyst and marine macro-algae Gracilaria verrucosa to production of fermentable sugars. Bioresour. Technol. 2015, 181, 1–6. [Google Scholar] [CrossRef]
- Jeong, G.-T.; Kim, S.-K.; Oh, B.-R. Production of fermentable sugars from Chlorella sp. by solid-acid catalyst. Algal Res. 2020, 51, 102044. [Google Scholar] [CrossRef]
- Li, H.X.; Shi, W.J.; Zhang, X.; Liu, P.; Cao, Q.; Jin, L. Catalytic hydrolysis of cellulose to total reducing sugars with superior recyclable magnetic multifunctional MCMB-based solid acid as a catalyst. J. Chem. Technol. Biotechnol. 2020, 95, 770–780. [Google Scholar] [CrossRef]
- Su, T.; Zeng, J.; Gao, H.; Jiang, L.; Bai, X.; Zhou, H.; Xu, F. One-pot synthesis of a chemically functional magnetic carbonaceous acid catalyst for fermentable sugars production from sugarcane bagasse. Fuel 2020, 262, 116512. [Google Scholar] [CrossRef]
- Li, X.; Shu, F.; He, C.; Liu, S.; Leksawasdi, N.; Wang, Q.; Qi, W.; Alam, A.; Yuan, Z.; Gao, Y. Preparation and investigation of highly selective solid acid catalysts with sodium lignosulfonate for hydrolysis of hemicellulose in corncob. RSC Adv. 2018, 8, 10922–10929. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, C.; Huang, F.; Wang, F.; Jia, H.; Zhou, H.; Wei, P. Selective hydrolysis of hemicellulose from wheat straw by a nanoscale solid acid catalyst. Carbohydr. Polym. 2015, 131, 384–391. [Google Scholar] [CrossRef]
- Arumugam, A.; Ponnusami, V. Biodiesel production from Calophyllum inophyllum oil using lipase producing Rhizopus oryzae cells immobilized within reticulated foams. Renew. Energy 2014, 64, 276–282. [Google Scholar] [CrossRef]
- Melero, J.A.; Iglesias, J.; Morales, G. Heterogeneous acid catalysts for biodiesel production: Current status and future challenges. Green Chem. 2009, 11, 1285–1308. [Google Scholar] [CrossRef]
- Thuijl, E.; Roos, C.; Beurskens, L. An overview of biofuel technologies, markets and policies in Europe. In ECN Policy Studies Report; No. ECN-C–03-008; Energy Research Centre of the Netherlands: Petten, The Netherlands, 2003. [Google Scholar]
- Saka, S.; Kusdiana, D. Biodiesel fuel from rapeseed oil as prepared in supercritical methanol. Fuel 2001, 80, 225–231. [Google Scholar] [CrossRef]
- Kiss, A.A.; Dimian, A.C.; Rothenberg, G. Solid acid catalysts for biodiesel production—Towards sustainable energy. Adv. Synth. Catal. 2006, 348, 75–81. [Google Scholar] [CrossRef]
- Okuhara, T. Water-Tolerant Solid Acid Catalysts. Chem. Rev. 2002, 102, 3641–3666. [Google Scholar] [CrossRef]
- Sherrington, D.C.; Kybett, A.P. (Eds.) Supported Catalysts and Their Applications; Royal Society of Chemistry: Cambridge, UK, 2007. [Google Scholar]
- Kulkarni, M.G.; Gopinath, R.; Meher, L.C.; Dalai, A.K. Solid acid catalyzed biodiesel production by simultaneous esterification and transesterification. Green Chem. 2006, 8, 1056–1062. [Google Scholar] [CrossRef]
- Andrijanto, E.; Dawson, E.; Brown, D. Hypercrosslinked polystyrene sulphonic acid catalysts for the esterification of free fatty acids in biodiesel synthesis. Appl. Catal. B Environ. 2012, 115, 261–268. [Google Scholar] [CrossRef]
- Lou, W.-Y.; Zong, M.-H.; Duan, Z.-Q. Efficient production of biodiesel from high free fatty acid-containing waste oils using various carbohydrate-derived solid acid catalysts. Bioresour. Technol. 2008, 99, 8752–8758. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.; Dey, B.; Balasubramanian, P. Algal biodiesel production with engineered biochar as a heterogeneous solid acid catalyst. Bioresour. Technol. 2020, 310, 123392. [Google Scholar] [CrossRef] [PubMed]
- Hara, M. Biomass conversion by a solid acid catalyst. Energy Environ. Sci. 2014, 3, 601–607. [Google Scholar] [CrossRef]
- Zong, M.-H.; Duan, Z.-Q.; Lou, W.-Y.; Smith, T.J.; Wu, H. Preparation of a sugar catalyst and its use for highly efficient production of biodiesel. Green Chem. 2007, 9, 434–437. [Google Scholar] [CrossRef]
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.; Lim, S.; Pang, Y.L. Investigation of carbon-based solid acid catalyst from Jatropha curcas biomass in biodiesel production. Energy Convers. Manag. 2017, 144, 10–17. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X.; Zhao, X.; Feng, P. Sulfonated ordered mesoporous carbon for catalytic preparation of biodiesel. Carbon 2008, 46, 1664–1669. [Google Scholar] [CrossRef]
- Chang, B.; Fu, J.; Tian, Y.; Dong, X. Multifunctionalized ordered mesoporous carbon as an efficient and stable solid acid catalyst for biodiesel preparation. J. Phys. Chem. C 2013, 117, 6252–6258. [Google Scholar] [CrossRef]
- Lee, J.; Jung, J.-M.; Kim, H.J.; Kim, T.-W.; Kim, K.-H.; Kwon, E.E. Methylation of volatile fatty acids with ordered mesoporous carbon and carbon nanotube for renewable energy application. ACS Sustain. Chem. Eng. 2017, 5, 7433–7438. [Google Scholar] [CrossRef]
- Gardy, J.; Osatiashtiani, A.; Céspedes, O.; Hassanpour, A.; Lai, X.; Lee, A.F.; Wilson, K.; Rehan, M. A magnetically separable SO4/Fe-Al-TiO2 solid acid catalyst for biodiesel production from waste cooking oil. Appl. Catal. B Environ. 2018, 234, 268–278. [Google Scholar] [CrossRef]
- Graaf, W.D.; Lange, J.-P. Process for the Conversion of Furfuryl Alcohol into Levulinic Acid or Alkyl Levulinate. US20070049771A1, 4 September 2007. Available online: https://patents.google.com/patent/US20070049771A1/en (accessed on 15 April 2025).
- Cui, X.; Zheng, L.; Li, Q.; Guo, Y. A remarkable bifunctional carbon-based solid acid catalyst derived from waste bio-tar for efficient synthesis of 5-hydroxymethylfurfural from glucose. Chem. Eng. J. 2023, 474, 146006. [Google Scholar] [CrossRef]
- Di Bitonto, L.; Scelsi, E.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Hájek, M.; Mustafa, A.; Pastore, C. A closed-loop biorefinery approach for the valorization of winery waste: The production of iron-sulfonated magnetic biochar catalysts and 5-hydroxymethyl furfural from grape pomace and stalks. Catalysts 2024, 14, 185. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Q.; Bai, X.; Du, Y. Conversion of biomass into 5-hydroxymethylfurfural using solid acid catalyst. Bioresour. Technol. 2011, 102, 3424–3429. [Google Scholar] [CrossRef]
- Bhaumik, P.; Deepa, A.K.; Kane, T.; Dhepe, P.L. Value addition to lignocellulosics and biomass-derived sugars: An insight into solid acid-based catalytic methods. J. Chem. Sci. 2014, 126, 373–385. [Google Scholar] [CrossRef]
- Mondal, S.; Mondal, J.; Bhaumik, A. Sulfonated porous polymeric nanofibers as an efficient solid acid catalyst for the production of 5-hydroxymethylfurfural from biomass. ChemCatChem 2015, 7, 3570–3578. [Google Scholar] [CrossRef]
- Karimi, B.; Mirzaei, H.M. The influence of hydrophobic/hydrophilic balance of the mesoporous solid acid catalysts in the selective dehydration of fructose into HMF. RSC Adv. 2013, 3, 20655. [Google Scholar] [CrossRef]
- Yan, L.; Liu, N.; Wang, Y.; Machida, H.; Qi, X. Production of 5-hydroxymethylfurfural from corn stalk catalyzed by corn stalk-derived carbonaceous solid acid catalyst. Bioresour. Technol. 2014, 173, 462–466. [Google Scholar] [CrossRef]
- Modak, A.; Mankar, A.R.; Pant, K.K.; Bhaumik, A. Mesoporous porphyrin-silica nanocomposite as solid acid catalyst for high yield synthesis of HMF in water. Molecules 2021, 26, 2519. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Tong, X.; Xia, F.; Zheng, C.; Qin, L.; Jiang, X. Efficient hydroxymethylfurfural production over phosphoric carbon solid acids. Catal. Lett. 2018, 148, 1848–1855. [Google Scholar] [CrossRef]
- Perez, G.P.; Dumont, M.-J. Production of HMF in high yield using a low cost and recyclable carbonaceous catalyst. Chem. Eng. J. 2020, 382, 122766. [Google Scholar] [CrossRef]
- Wang, S.; Eberhardt, T.L.; Guo, D.; Feng, J.; Pan, H. Efficient conversion of glucose into 5-HMF catalyzed by lignin-derived mesoporous carbon solid acid in a biphasic system. Renew. Energy 2022, 190, 1–10. [Google Scholar] [CrossRef]
- Tzeng, T.-W.; Bhaumik, P.; Chung, P.-W. Understanding the production of 5-hydroxymethylfurfural (HMF) from chitosan using solid acids. Mol. Catal. 2019, 479, 110627. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Z.; Huang, K. Cellulose sulfuric acid as a bio-supported and recyclable solid acid catalyst for the synthesis of 5-hydroxymethylfurfural and 5-ethoxymethylfurfural from fructose. Cellulose 2013, 20, 2081–2089. [Google Scholar] [CrossRef]
- Yang, X.; Guo, H.; Cao, X.; Ma, Y.; Wang, W.; Guo, N. Solid Acid Catalyst Derived from Cotton for Conversion of Xylose and Corn Cob to Furfural. ChemistrySelect 2022, 7, e202203762. [Google Scholar] [CrossRef]
- Wang, W.; Cao, X.; Guo, H.; Yang, X.; Guo, N.; Ma, Y. Carbon-based solid acid derived from lignin and polyvinyl chloride for conversion of xylose and crop wastes to furfural. Mol. Catal. 2022, 524, 112329. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Hoang, S.A.; Pham, K.D.; Nguyen, T.Q. Preparation of carbon-based solid acid catalyst from rice straw for furfural production in aqueous media. BioResources 2024, 19, 7856–7869. [Google Scholar] [CrossRef]
- Gao, J.; Wang, H.; Cao, X.; Li, Z.; Guo, H.; Yang, X.; Wang, W.; Guo, N.; Ma, Y. Nitrogen doped carbon solid acid for improving its catalytic transformation of xylose and agricultural biomass residues to furfural. Mol. Catal. 2023, 535, 112890. [Google Scholar] [CrossRef]
- Li, H.; Deng, A.; Ren, J.; Liu, C.; Lu, Q.; Zhong, L.; Peng, F.; Sun, R. Catalytic hydrothermal pretreatment of corncob into xylose and furfural via solid acid catalyst. Bioresour. Technol. 2014, 158, 313–320. [Google Scholar] [CrossRef]
- Antonetti, C.; Licursi, D.; Fulignati, S.; Valentini, G.; Galletti, A.M.R. New frontiers in the catalytic synthesis of levulinic acid: From sugars to raw and waste biomass as starting feedstock. Catalysts 2016, 6, 196. [Google Scholar] [CrossRef]
- Dutta, S.; Yu, I.K.; Tsang, D.C.; Ng, Y.H.; Ok, Y.S.; Sherwood, J.; Clark, J.H. Green synthesis of γ-valerolactone (GVL) through hydrogenation of biomass-derived levulinic acid using non-noble metal catalysts: A critical review. Chem. Eng. J. 2019, 372, 992–1006. [Google Scholar] [CrossRef]
- Csaba, A.; ZoltAn, M.; Matthew, Y.L.; Laszlo, T.M. The Chemistry of Levulinic Acid: Its Potential in the Production of Biomass-Based Chemicals. Adv. Synth.Catal. 2024, 366, 4846–4888. [Google Scholar] [CrossRef]
- Bevilaqua, D.B.; Rambo, M.K.; Rizzetti, T.M.; Cardoso, A.L.; Martins, A.F. Cleaner production: Levulinic acid from rice husks. J. Clean. Prod. 2013, 47, 96–101. [Google Scholar] [CrossRef]
- Dutta, S.; Yu, I.K.; Tsang, D.C.; Su, Z.; Hu, C.; Wu, K.C.; Yip, A.C.; Ok, Y.S.; Poon, C.S. Influence of green solvent on levulinic acid production from lignocellulosic paper waste. Bioresour. Technol. 2020, 298, 122544. [Google Scholar] [CrossRef]
- Chen, S.S.; Yu, I.K.; Tsang, D.C.; Yip, A.C.; Khan, E.; Wang, L.; Ok, Y.S.; Poon, C.S. Valorization of cellulosic food waste into levulinic acid catalyzed by heterogeneous Brønsted acids: Temperature and solvent effects. Chem. Eng. J. 2017, 327, 328–335. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; Antonetti, C.; Luise, V.; Licursi, D.; Nassi, N. Levulinic acid production from waste biomass. BioResources 2012, 7, 1824–1835. [Google Scholar]
- Chen, S.S.; Wang, L.; Yu, I.K.; Tsang, D.C.; Hunt, A.J.; Jérôme, F.; Zhang, S.; Ok, Y.S.; Poon, C.S. Valorization of lignocellulosic fibres of paper waste into levulinic acid using solid and aqueous Brønsted acid. Bioresour. Technol. 2018, 247, 387–394. [Google Scholar] [CrossRef]
- Yan, L.; Yang, N.; Pang, H.; Liao, B. Production of levulinic acid from bagasse and paddy straw by liquefaction in the presence of hydrochloride acid. CLEAN–Soil Air Water 2008, 36, 158–163. [Google Scholar] [CrossRef]
- Chang, C.; Ma, X.; Cen, P. Kinetics of levulinic acid formation from glucose decomposition at high temperature. Chin. J. Chem. Eng. 2006, 14, 708–712. [Google Scholar] [CrossRef]
- Weingarten, R.; Cho, J.; Xing, R.; Conner, W.C., Jr.; Huber, G.W. Kinetics and reaction engineering of levulinic acid production from aqueous glucose solutions. ChemSusChem 2012, 5, 1280–1290. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Fu, J.; Mo, J.; Lu, X. Synergy of Lewis and Brønsted acids on catalytic hydrothermal decomposition of hexose to levulinic acid. Energy Fuels 2013, 27, 6973–6978. [Google Scholar] [CrossRef]
- Beh, G.K.; Wang, C.T.; Kim, K.; Qu, J.; Cairney, J.; Ng, Y.H.; An, A.K.; Ryoo, R.; Urakawa, A.; Teoh, W.Y. Flame-made amorphous solid acids with tunable acidity for the aqueous conversion of glucose to levulinic acid. Green Chem. 2020, 22, 688–698. [Google Scholar] [CrossRef]
- Kumar, V.B.; Pulidindi, I.N.; Mishra, R.K.; Gedanken, A. Development of Ga salt of molybdophosphoric acid for biomass conversion to levulinic acid. Energy Fuels 2016, 30, 10583–10591. [Google Scholar] [CrossRef]
- Acharjee, T.C.; Lee, Y.Y. Production of levulinic acid from glucose by dual solid-acid catalysts. Environ. Prog. Sustain. Energy 2018, 37, 471–480. [Google Scholar] [CrossRef]
- Alonso, D.M.; Gallo, J.M.R.; Mellmer, M.A.; Wettstein, S.G.; Dumesic, J.A. Direct conversion of cellulose to levulinic acid and γ-valerolactone using solid acid catalysts. Catal. Sci. Technol. 2013, 3, 927–931. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, Y.; Fu, Y. Catalytic conversion of cellulose into levulinic acid by a sulfonated chloromethyl polystyrene solid acid catalyst. ChemCatChem 2014, 6, 753–757. [Google Scholar] [CrossRef]
- Van de Vyver, S.; Thomas, J.; Geboers, J.; Keyzer, S.; Smet, M.; Dehaen, W.; Jacobs, P.A.; Sels, B.F. Catalytic production of levulinic acid from cellulose and other biomass-derived carbohydrates with sulfonated hyperbranched poly(arylene oxindole)s. Energy Environ. Sci. 2011, 4, 3601–3610. [Google Scholar] [CrossRef]
- Han, Y.; Ye, L.; Gu, X.; Zhu, P.; Lu, X. Lignin-based solid acid catalyst for the conversion of cellulose to levulinic acid using γ-valerolactone as solvent. Ind. Crops Prod. 2019, 127, 88–93. [Google Scholar] [CrossRef]
- Ding, D.; Wang, J.; Xi, J.; Liu, X.; Lu, G.; Wang, Y. High-yield production of levulinic acid from cellulose and its upgrading to γ-valerolactone. Green Chem. 2014, 16, 3846–3853. [Google Scholar] [CrossRef]
- Wei, W.; Yang, H.; Wu, S. Efficient conversion of carbohydrates into levulinic acid over chromium modified niobium phosphate catalyst. Fuel 2019, 256, 115940. [Google Scholar] [CrossRef]
- Xu, X.; Liang, B.; Zhu, Y.; Chen, J.; Gan, T.; Hu, H.; Zhang, Y.; Huang, Z.; Qin, Y. Direct and efficient conversion of cellulose to levulinic acid catalyzed by carbon foam-supported heteropolyacid with Brønsted–Lewis dual-acidic sites. Bioresour. Technol. 2023, 387, 129600. [Google Scholar] [CrossRef] [PubMed]
- Ya’aini, N.; Amin, N.A.S.; Asmadi, M. Optimization of levulinic acid from lignocellulosic biomass using a new hybrid catalyst. Bioresour. Technol. 2012, 116, 58–65. [Google Scholar] [CrossRef]
- Chen, H.; Yu, B.; Jin, S. Production of levulinic acid from steam exploded rice straw via solid superacid, S2O82−/ZrO2–SiO2–Sm2O3. Bioresour. Technol. 2011, 102, 3568–3570. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Lin, Q.; Cheng, B.; Kong, F.; Li, H.; Ren, J. Solid acid-induced hydrothermal treatment of bagasse for production of furfural and levulinic acid by a two-step process. Ind. Crops Prod. 2018, 123, 118–127. [Google Scholar] [CrossRef]
- Chen, G.; Fang, B. Preparation of solid acid catalyst from glucose–starch mixture for biodiesel production. Bioresour. Technol. 2011, 102, 2635–2640. [Google Scholar] [CrossRef]
- Li, X.; Lei, T.; Wang, Z.; Li, X.; Wen, M.; Yang, M.; Chen, G.; He, X.; Guan, Q.; Li, Z. Catalytic pyrolysis of corn straw with magnetic solid acid catalyst to prepare levulinic acid by response surface methodology. Ind. Crops Prod. 2018, 116, 73–80. [Google Scholar] [CrossRef]
- Joshi, S.S.; Zodge, A.D.; Pandare, K.V.; Kulkarni, B.D. Efficient conversion of cellulose to levulinic acid by hydrothermal treatment using zirconium dioxide as a recyclable solid acid catalyst. Ind. Eng. Chem. Res. 2014, 53, 18796–18805. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Fu, R.; Xu, J.; Lu, J.; Wen, Z.; Xue, B. One-Pot Conversion of Furfural to Gamma-Valerolactone over Zr-SBA-15: Cooperation of Lewis and Brønsted Acidic Sites. ACS Appl. Nano Mater. 2023, 6, 13196–13207. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Zhou, Y.; Wang, Z.; Du, M.; Wen, Z.; Yan, B.; Ma, Q.; Liu, N.; Xue, B. Phosphotungstic acid supported on Zr-SBA-15 as an efficient catalyst for one-pot conversion of furfural to γ-valerolactone. Fuel 2024, 356, 129631. [Google Scholar] [CrossRef]
- Lai, J.; Zhou, S.; Liu, X.; Yang, Y.; Lei, J.; Xu, Q.; Yin, D. Catalytic transfer hydrogenation of biomass-derived ethyl levulinate into gamma-valerolactone over graphene oxide-supported zirconia catalysts. Catal. Lett. 2019, 149, 2749–2757. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Lu, Y.-M.; Liu, Y.-X.; Wu, Z.-B.; Hu, D.-Y.; Yang, S. Cascade catalytic transfer hydrogenation–cyclization of ethyl levulinate to γ-valerolactone with Al–Zr mixed oxides. Appl. Catal., A 2016, 510, 11–19. [Google Scholar] [CrossRef]
- Jayakumari, M.T.; Krishnan, C.K. Tuning Al sites in Y-zeolite for selective production of ϒ-valerolactone from levulinic acid. Appl. Catal. A 2023, 663, 119318. [Google Scholar] [CrossRef]
- Yu, X.; Liu, J.; Ru, C.; Cai, S.; Wang, J.; Liu, M.; Zhang, D.; Shen, J.; Jin, X.; Yang, C. Lattice expansion and electronic reconfiguration of MnCu oxide catalysts for enhanced transfer hydrogenation of levulinate. ACS Sustain. Chem. Eng. 2022, 10, 13402–13414. [Google Scholar] [CrossRef]
- Yang, T.; Li, H.; He, J.; Liu, Y.; Zhao, W.; Wang, Z.; Ji, X.; Yang, S. Porous Ti/Zr microspheres for efficient transfer hydrogenation of biobased ethyl levulinate to γ-valerolactone. ACS Omega 2017, 2, 1047–1054. [Google Scholar] [CrossRef]
- Zhang, C.; Huo, Z.; Ren, D.; Song, Z.; Liu, Y.; Jin, F.; Zhou, W. Catalytic transfer hydrogenation of levulinate ester into γ-valerolactone over ternary Cu/ZnO/Al2O3 catalyst. J. Energy Chem. 2019, 32, 189–197. [Google Scholar] [CrossRef]
- Tabanelli, T.; Paone, E.; Vásquez, P.B.; Pietropaolo, R.; Cavani, F.; Mauriello, F. Transfer hydrogenation of methyl and ethyl levulinate promoted by a ZrO2 catalyst: Comparison of batch vs. continuous gas-flow conditions. ACS Sustain. Chem. Eng. 2019, 7, 9937–9947. [Google Scholar] [CrossRef]
- Xu, S.; Yu, D.; Ye, T.; Tian, P. Catalytic transfer hydrogenation of levulinic acid to γ-valerolactone over a bifunctional tin catalyst. RSC Adv. 2017, 7, 1026–1031. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Q.; Shu, R.; Xu, Y.; Ma, L.; Wang, T. Catalytic depolymerization of the hydrolyzed lignin over mesoporous catalysts. Bioresour. Technol. 2017, 226, 125–131. [Google Scholar] [CrossRef]
- Kong, L.; Liu, C.; Gao, J.; Wang, Y.; Dai, L. Efficient and controllable alcoholysis of Kraft lignin catalyzed by porous zeolite-supported nickel-copper catalyst. Bioresour. Technol. 2019, 276, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ruan, H.; Feng, M.; Qin, Y.; Job, H.; Luo, L.; Wang, C.; Engelhard, M.H.; Kuhn, E.; Chen, X.; et al. One-Pot Process for Hydrodeoxygenation of Lignin to Alkanes Using Ru-Based Bimetallic and Bifunctional Catalysts Supported on Zeolite Y. ChemSusChem 2017, 10, 1846–1856. [Google Scholar] [CrossRef]
- Singh, S.K.; Ekhe, J.D. Cu–Mo doped zeolite ZSM-5 catalyzed conversion of lignin to alkyl phenols with high selectivity. Catal. Sci. Technol. 2015, 5, 2117–2124. [Google Scholar] [CrossRef]
- Nenasheva, M.V.; Gorbunov, D.N. Recent Progress and Strategies on the Design of Zeolite-Based Catalysts for Hydroformylation of Olefins. Catalysts 2024, 14, 942. [Google Scholar] [CrossRef]
- Chu, D.; Ma, J.; Liu, Q.; Fu, J.; Yin, H. Effects of zeolite porosity and acidity on catalytic conversion of carbohydrates to bio-based chemicals: A review. Catal. Sci. Technol. 2024, 14, 6980–7001. [Google Scholar] [CrossRef]
- Elías, V.R.; Ferrero, G.O.; Idriceanu, M.G.; Eimer, G.A.; Domine, M.E. From biomass-derived furans to aromatic compounds: Design of Al–Nb-SBA-15 mesoporous structures and study of their acid properties on catalytic performance. Catal. Sci. Technol. 2024, 14, 1488–1500. [Google Scholar] [CrossRef]
- Matthews, T.; Seroka, N.S.; Khotseng, L. Potential application of green synthesised SBA-15 from biomass wastes as a catalyst for sustainable biodiesel production. Discov. Appl. Sci. 2024, 6, 525. [Google Scholar] [CrossRef]
- Cao, M.; Meng, Y.; Tan, Z.; Wang, Q.; Yan, W.; Liu, C.; Jiang, X.; Song, H.; Huang, H.; Liu, G.; et al. High-Performance Nickel Phosphide Confined in SBA-15 for Low-Temperature Hydrogenation of Biomass-Derived Compounds. ACS Catal. 2025, 15, 4880–4891. [Google Scholar] [CrossRef]
- Akhtar, M.; Naseem, M.; Ali, S.; Zaman, W. Metal-Based Catalysts in Biomass Transformation: From Plant Feedstocks to Renewable Fuels and Chemicals. Catalysts 2025, 15, 40. [Google Scholar] [CrossRef]
- Fitria; Kumar, A.; Dewa, M.; Liu, J.; Ha, S.; Yang, B. Development of sulfonated carbon-based solid-acid catalysts derived from biorefinery residues and biomass ash for xylan hydrolysis. Bioresour. Technol. Rep. 2023, 24, 101607. [Google Scholar] [CrossRef]
- Dias, C.N.; Santos-Vieira, I.C.M.S.; Gomes, C.R.; Mirante, F.; Balula, S.S. Heteropolyacids@Silica Heterogeneous Catalysts to Produce Solketal from Glycerol Acetalization. Nanomaterials 2024, 14, 733. [Google Scholar] [CrossRef] [PubMed]
- Esmi, F.; Borugadda, V.B.; Dalai, A.K. Heteropoly acids as supported solid acid catalysts for sustainable biodiesel production using vegetable oils: A review. Catal. Today 2022, 404, 19–34. [Google Scholar] [CrossRef]
- Laeim, H.; Molahalli, V.; Prajongthat, P.; Pattanaporkratana, A.; Pathak, G.; Phettong, B.; Hongkarnjanakul, N.; Chattham, N. Porosity Tunable Metal-Organic Framework (MOF)-Based Composites for Energy Storage Applications: Recent Progress. Polymers 2025, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.; Sharma, P.; Le, H.S.; Le, H.C.; Le, D.T.N.; Cao, D.N.; Truong, T.H.; Tran, V.D. Metal-organic frameworks as potential catalysts for biodiesel production and biomass conversion: Mechanism and characteristics. Ind. Crops Prod. 2024, 211, 118232. [Google Scholar] [CrossRef]
- Srivastava, V.; Lappalainen, K.; Rusanen, A.; Morales, G.; Lassi, U. Current Status and Challenges for Metal-Organic-Framework-Assisted Conversion of Biomass into Value-Added Chemicals. ChemPlusChem 2023, 88, e202300309. [Google Scholar] [CrossRef]
- Yu, H.; Wu, J.; Wei, W.; Zhang, X.; Ren, C.; Dong, Y.; Cheng, S. Synthesis of magnetic carbonaceous acid derived from waste garlic peel for biodiesel production via esterification. Front. Energy 2023, 17, 176–187. [Google Scholar] [CrossRef]
- Delshad, Y.; Rafipour, D.; Kargar, P.G.; Maleki, B.; Ghani, M. Carboxylic acids dendrimer functionalized magnetic nanoparticles for carbohydrates conversion to 5-hydroxymethylfurfural. Sci. Rep. 2025, 15, 10969. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Li, J. Magnetic solid catalysts for sustainable and cleaner biodiesel production: A comprehensive review. Renew. Sustain. Energy Rev. 2023, 171, 113017. [Google Scholar] [CrossRef]
- Zeng, M.; Pan, X. Insights into solid acid catalysts for efficient cellulose hydrolysis to glucose: Progress, challenges, and future opportunities. Catal. Rev. 2022, 64, 445–490. [Google Scholar] [CrossRef]
| Catalyst | Catalyst Preparation | Substrate | Substrate:Catalyst Ratio | Solvent | Temp (°C) | Time, min | HMF Yield% | Ref. |
|---|---|---|---|---|---|---|---|---|
| Carbon-based Al-Ti-loaded bifunctional catalyst | Bio-tar-derived porous carbon from pyrolysis was activated using H2O2 and acetic acid; bifunctionalization using Al-Ti metal loading followed by sulfonation and calcination. | Glucose | 1:1 | 20 wt% NaCl in DMSO: Water (4:1) | 140 | 240 | 74.6 | [120] |
| Iron-sulfonated magnetic biochar | Biochar obtained from pyrolysis was subjected to sulfonation at 150 °C, and then iron was deposited using the wet impregnation method. | Fructose | 0.36:1 | GVL:Water (2:1) | 130 | 360 | 40.9 | [121] |
| Nb2O5·nH2O | Mixing niobic acid with H3PO4, stirring for 52 h, aging for 12 h, washing, drying at 333 K and 383 K, and calcining at 573 K for 3 h. | Fructose | 1.2:0.1 | Water and 2-butanol | 160 | 50 | 89 | [122] |
| Silicoalumino-phosphates (SAPO) | Mixing aging gels (pseudoboehmite and phosphoric acid) and cyclohexylamine and silica, followed by stirring, aging, filtration, washing, drying, and calcining at 550 °C. | Fructose | 1:0.286 | Water and methyl iso-butyl ketone (1:5 v/v) | 175 | 60 | 78 | [123] |
| Sulfonated polymer polytriphenylamine (SPPTPA-1) | Triphenylamine was polymerized using FeCl3 in dichloroethane to create PPTPA-1; sulfonation of PPTPA-1 with chlorosulfonic acid yielded SPPTPA-1. | Fructose | 1:0.2 | DMSO | 140 | 20 | 94.6 | [124] |
| SBA15-PrSO3H | SBA15PrSO3H (2a) is synthesized by combining TEOS, MPTMS, PTES, and CSPTMS. | Fructose | 1:0.02 | Water/nitromethane | 140 | 30 | 69.8 | [125] |
| HCSS | Treating corn stalks with water under pressure at 250 °C, centrifuging, and then drying the solid. Then, it is heated with concentrated sulfuric acid at 200 °C, cooled, washed, and dried. | Corn stalks | 1:1 | [BMIM][Cl] | 150 | 30 | 44.1 | [126] |
| PorPOPS | Terephthalaldehyde, pyrrole, and FeCl3 are stirred with colloidal silica and then heated in a Teflon autoclave at 180 °C for 48 h. The precipitate is filtered, washed, and dried to get PorPOP. PorPOP is treated with chlorosulfonic acid, washed, and dried. | Fructose | 1:0.5 | Water | 160 | 120 | 85 | [127] |
| Phosphoric carbons (PC) | 1 g glucose in 56% phosphoric acid is heated at 180 °C for 12 h and then cooled. The water is evaporated and the mixture is calcined at 300 °C for 4 h in nitrogen. | Fructose | 1:0.2 | DMSO | 160 | 180 | 93.7 | [128] |
| HTC 24-140 | Hydrothermal carbonization of freeze-dried softwood pulp at 200–240 °C, followed by filtration, washing, and drying. The material is then sulfonated with concentrated H2SO4 at 140 °C, filtered, washed until neutral pH, and dried. | Fructose | 1:0.05 | [BMIM][Cl] and methyl iso-butyl ketone | 112 | 24 | 98.6 | [129] |
| LDMCS-700 | Alkali lignin and KCl are mixed and carbonized in a tube furnace at 700 °C. The carbonized product is washed, dried, and sulfonated with sulfuric acid at 180 °C. The resulting sulfonated product is washed to neutral pH and dried. | Glucose | 1:1 | Aq. NaCl-THF | 160 | 150 | 57.8 | [130] |
| Nafion®50 resin (NR50) | Commercially purchased. | Chitosan | 1:0.55 | Methyl isobutyl ketone and DI water | 180 | 120 | 32.6 | [131] |
| Cellulose sulfuric acid | Cellulose (5 g) is mixed with CHCl3 and chlorosulfonic acid and stirred at 0 °C for 2 h. The mixture is stirred, filtered, washed with CHCl3, and vacuum-dried at room temperature for 6 h. | Fructose | 1:0.28 | DMSO | 100 | 45 | 93.6 | [132] |
| Catalyst | Catalyst Preparation | Substrate | Substrate:Catalyst Ratio | Solvent | Temp (°C) | Time, min | Furfural Yield% | Ref. |
|---|---|---|---|---|---|---|---|---|
| Polyaniline carbon-based solid acid catalyst (PAC-S) | Prepared polyaniline precursor was carbonized at 800 °C in nitrogen atmosphere for 2 h and sulfonated using conc. H2SO4 at 180 °C for 3 h to obtain PAC-S. | Xylose | 4:1 | GVL | 170 | 45 | 88.2 * | [136] |
| Polyaniline carbon-based solid acid catalyst (PAC-S) | Prepared polyaniline precursor was carbonized at 800 °C in nitrogen atmosphere for 2 h and sulfonated using conc. H2SO4 at 180 °C for 3 h to obtain PAC-S. | Corn cob, rice husk, corn stalk, rice straw, wheat straw | 1:1 | GVL:Water (4:1) | 180 | 30 | 95, 77.4, >40, <20, ~20 * | [136] |
| Sulfonated carbon derived from lignin, polyvinyl chloride | Synthesized using the calcination-sulfonation method using lignin, KOH, polyvinyl chloride-in mass-ratio of 1:1:1 calcined at 750 °C in N2 atmosphere for 4 h, and sulfonated using p-aminobenzenesulfonic acid. | Xylose | 2:1 | GVL | 160 | 60 | 84.3 * | [134] |
| Sulfonated carbon derived from lignin, polyvinyl chloride | Synthesized using the calcination-sulfonation method using lignin, KOH, polyvinyl chloride-in mass-ratio of 1:1:1 calcined at 750 °C in N2 atmosphere for 4 h, and sulfonated using p-aminobenzenesulfonic acid. | Corn cob, rice husk, corn stalk, rice straw, wheat straw | 2:1 | GVL:Water (4:1) | 160 | 60 | 76.4, 66.3, 16.1, 13.5, 5.3 * | [134] |
| Sulfonated carbon derived from cotton | Using the one-step carbonization-sulfonation method, cotton in sulfuric acid is stirred at different mass ratios and carbonized subsequently at various temperatures. | Xylose | 2:1 | GVL:Water (5.67:1) | 180 | 90 | 87.3 * | [133] |
| Sulfonated carbon derived from cotton | Using the one-step carbonization-sulfonation method, cotton in sulfuric acid is stirred at different mass ratios and carbonized subsequently at various temperatures. | Corn cob | 1:1 | GVL:Water (4:1) | 190 | 90 | 63.2 * | [133] |
| SO42−/TiO2−ZrO2/La3+ Solid acid catalyst | Using the coprecipitation and impregnation method. Ti, La, and Zr salts are used to form TiO2–ZrO2/La3+, impregnated in 1M H2SO4, dried, and calcined at 550 °C for 4 h. | Corn cob | 10:1 | Water | 180 | 120 | 6.18 ** | [137] |
| Rice-straw-derived sulfonated SAC | Two-step procedure consists of rice straw carbonization at 300 °C for 2 h in N2 atmosphere, followed by sulfonation using conc. H2SO4, ultrasonication at 150 °C. | Rice straw | 10:1 | Water | 160 | 300 | 6.83 ** | [135] |
| Catalyst | Catalyst Preparation | Substrate and Solvent | Substrate: Catalyst Ratio | Solvent | Temp, °C | Time, min | Levulinic Acid Yield | Ref. |
|---|---|---|---|---|---|---|---|---|
| Amberlyst 70 | Commercial catalyst. | Corn stover | 0.18:0.2 | Water | 160 | 120 | 54 wt% | [153] |
| Hybrid catalyst chromium chloride (CrCl3) and HY zeolite | The hybrid catalyst was synthesized using the wet impregnation method. Commercial HY zeolite and aqueous CrCl3 (10 wt/v%) were mixed and dried at 120 °C followed by calcination at 400 °C. | Empty fruit bunch (41.1% cellulose) | 1:12 | Water | 145.2 | 146 | 15.5 wt% | [161] |
| Kenaf (32% cellulose) | 1:12 | Water | 145.2 | 146 | 15 wt% | |||
| S2O82−/ZrO2–SiO2−Sm2O3 (solid super acid) | The catalyst was synthesized using the precipitation method with ammonia persulfate as the promoter instead of H2SO4. | Steam-exploded rice straw (superfine grinded) | 1:2 | Water | 200 | 10 | 22.8 wt% | [163] |
| Sn-MMT/SO42− | Two wt% montmorillonite (MMT) was mixed in distilled water, followed by the slow addition of SnCl4. The solution was heated at 85 °C for 2 h through microwave irradiation. The resulting Sn-MMT 5 wt% was mixed with 1 mol/L H2SO4 at 30 °C for 6 h to obtain Sn-MMT/SO42−. | Sugarcane bagasse | 0.2:0.15 | DCM-water | 170 | 144 | 62 mol% | [162] |
| Biomass-based magnetic ferric oxide/SO42− | Corn straw was carbonized at 549 °C for 13 h, and the resulting carbon was sulfonated with H2SO4 at 121 °C for 6 h to obtain the biomass-based precursor. Magnetic iron oxide particles were mixed with the precursor (1:2 ratio) in 1 mol/L H2SO4 for 24 h. They were calcined at 500 °C for 3 h to obtain magnetic ferric oxide/SO42−. | Corn straw | 2:1 | Water | 250 | 67 | 23.17 wt% | [164] |
| Amberlyst 36 | Commercial catalyst. | Paper towel | 1:1 | Water | 150 | 60 | 40 mol% | [145] |
| Amberlyst 36 | Commercial catalyst. | Pennisetum alopecurmoides | 1:1 | 30:70 (wt%) GVL:water | 180 | 60 | 20 mol% | [13] |
| Gallium salt of molydophosphoric (Ga@HPMo) | Gallium (Ga) metal was dissolved in molybdophosphoric acid ethanol solution at 50 °C and sonicated for 12 min. The precipitated Ga@HPMo was separated and dried under an N2 atmosphere. | Glucose | 1:5 | Water | 150 | 600 | 56 wt% | [151] |
| Dual acid catalyst (Amberlyst 15 and Sn-Beta) | Zeolite beta 5 wt% was mixed with 13 M HNO3 at 90 °C for 20 h (dealumination). The recovered zeolite was ground with tin(II) acetate for 15 min, followed by calcination at 550 °C for 6 h. The catalyst was mixed with Amberlyst 15 at a 1:10 ratio. | glucose | 1:2.2 | Water | 140 | 120 | 45 mol% | [152] |
| Amberlyst 70 | Commercial catalyst. | Cellulose | 0.3:1 | 90:10 (wt%) GVL:water | 160 | 120 | 69 wt% (based on theoretical LA yield) | [153] |
| Chloromethyl polystyrene resin | Chloromethyl polystyrene (CP) resin with a -Cl group was partially substituted with a -SO3H group using thiourea as sulfonic acid as a precursor. Thiourea-substituted resin was added to 1N NaOH resin, followed by washing with water and protonation by 2N H2SO4. | Cellulose | 1:3 | 90:10 (wt%) GVL:water | 170 | 600 | 65 wt% | [154] |
| Sulfonated hyperbranched poly(arylene oxindole) | A2 monomer isatin and B3 monomer (0.11:0.5) were used for hyperbranched polymer synthesis through polycondensation. The monomers were mixed with methanesulfonic acid (0.05M), and the polymerization was carried out at 35 °C for 48 h. The precipitated powder was dissolved in DCM and reprecipitated to remove impurities, resulting in sulfonated hyperbranched poly(arylene oxindole). | Ball-milled cellulose | 1:1 | Water | 170 | 180 | 25 wt% | [155] |
| Zirconium di oxide | Commercial catalyst. | Cellulose | 1:1 | Water | 180 | 180 | 53.9 mol% | [165] |
| Lignin-based solid acid | Alkali lignin was carbonized at 200 °C for 5 h. The resulting 1 g of carbon was mixed with ferrous sulfide solution (25 g/L) and heated to 105 °C for 10 h. The recovered solid acid was washed with diethyl ether and dried. | Microcrystalline cellulose | 1:1 | 10:1 (wt%) GVL:water | 185 | 120 | 35.64 wt% | [156] |
| Aluminum-modified mesoporous niobium phosphate | (NH4)2HPO4 0.01 M was mixed in 20 mL of water with pH adjusted to 2 with H3PO4. Al precursor, prepared by dissolving Al(OH3) in 1.5 M oxalic acid solution, was added. Niobium tartrate, 20 mL, was added to the mixture and poured into CTAB (1 g in 13 mL water). The precipitated niobium phosphorous was aged at 160 °C for 24 h. After drying, the catalyst was calcined at 500 °C for 5 h. | Cellulose | 0.5:0.4 | Water | 180 | 720 | 52.9 wt% | [157] |
| Chromium-modified niobium phosphate (Cr/NbP) | Niobium tartrate solution, 0.5 mol/L, was added to 3.3 g (NH4)2HPO4 and chromium precursor (CrCl3). The mixture was added to CTAB solution with pH adjusted to 2 using H3PO4. The mixture was aged at 60 °C for 24 h followed by calcination at 500 °C for 5 h. | Cellulose | 1:0.3 | Water | 180 | 180 | 62.4 mol% | [158] |
| Catalyst | Catalyst Preparation | Substrate | Substrate:Catalyst Ratio | Solvent | Temp (°C) | Time, min | γ-Valerolactone Yield% | Ref. |
|---|---|---|---|---|---|---|---|---|
| Zr-SBA-15_x (x-molar ratio of Si/Zr) | In situ synthesized zirconia-incorporated SBA-15 was calcined at 550 °C for 3 h with different molar ratios of Si/Zr. | Furfural | 1.92:1 | 2-propanol | 190 | 1440 | 93.3 | [166] |
| Phosphotungstic-acid-supported Zr-SBA-15 | Prepared by an impregnation method using SBA-15, ZrOCl2⋅8H2O, calcined at 300 °C for 3 h, and obtained HPW/Zr-SBA-15 (x:y:z). [x:y:z-mass ratio of HPW: ZrOCl2⋅8H2O: SBA-15] | Furfural | 1.92:1 | 2-propanol | 190 | 1440 | 83 | [167] |
| Graphene-oxide-supported Zirconia catalyst (GO/ZrO2) | GO prepared using the modified Hummer’s method. Further, preparation of ZrO2/GO was carried out using the hydrothermal method using ZrOCl⋅8H2O at 160 °C for 12 h. | Ethyl levulinate | 7.2:1 | 2-propanol | 180 | 180 | 58.6 | [168] |
| Al-Zr-mixed oxide | ZrOCl2·8H2O (18 mmol) and Al(NO3)3·9H2O (42 mmol) employed using co-precipitation method to obtain Al-Zr-mixed oxides at different calcination temperatures. | Ethyl levulinate | 2:1 | 2-propanol | 220 | 240 | 83.2 | [169] |
| Al sites tuned Y zeolite | Y zeolite-treated samples treated at different temperatures either in water vapor saturated air or in air atmosphere. | Levulinic acid | 1:1 | 2-propanol | 175 | 720 | 94 | [170] |
| Mn Cu-mixed oxide | Simple co-precipitation method employed utilizing Cu(NO3)2·3H2O and Mn(NO3)2, calcined at 400 °C in air for 8 h. | Ethyl levulinate | 8.64:1 | 2-propanol | 200 | 180 | 67.5 | [171] |
| Ti/Zr porous oxides | By using the sol-gel method, titanium isopropoxide and zirconium propoxide as precursors at different molar ratios, and calcined at 500 °C for 2 h. | Ethyl levulinate | 2:1 | 2-propanol | 180 | 360 | 90.1 | [172] |
| Cu/ZnO/Al2O3 | Cu/Zn/Al molar ratio of 6/3/1 utilized to prepare ternary catalyst using the co-precipitation method and calcined at 300 °C for 3 h in 5% H2/Ar mixture. | Ethyl levulinate | 0.96:1 | 2-propanol | 140 | 120 | 99 | [173] |
| ZrO2 | ZrO2 prepared using zirconium(IV)nitrate dihydrate calcined at 500 °C for 12 h. | Ethyl levulinate | - | 2-propanol | 250 | 1440 | 64 | [174] |
| SnO2/SBA-15 (Sn-modified silica catalyst) | Using dimethyldichlorostannane as Sn precursor and SBA-15, a bifunctional catalyst was prepared, calcined at 600 °C for 2 h. | Levulinic acid | 2.49:1 | 2-propanol | 110 | 480 | 81 | [175] |
| Catalyst | Catalyst Preparation | Substrate | Substrate:Catalyst Ratio | Solvent | Pressure Bar | Atmosphere | Temp (°C) | Time, min | Product | Yield% | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PP@M-[PS][TFMS-] | The sulfonated polypyrrole (PP)@M complex was sulfonated by 1,3-propanesultone (PS), Tri-floromethanesulphonic anion (TFMS-) was attached to the sulfonated PP to generate LASs. | Lignin model (Cβ-O and Car-Cα bonds) | 1:0.1 | Methanol | 10 | N2 | 250 | 60 | Guaiacol | 96.6 | [84] |
| Zeolites (HUSY) | Commercial. | Dealkaline lignin | 1:1 | Water-methanol (1:5 v/v) | 7 | N2 | 250 | 30 | Lignin oil | 60 | [80] |
| Carbonaceous solid acids | Prepared by hydrothermally carbonizing carbon precursor in water at 220 °C for 3 h. Then, filtered, washed to neutral pH, dried, then treated with concentrated H2SO4 at 150 °C for 15 h. | Organosolv lignin from bagasse | 1:0.1 | Methyl isobutyl ketone | 20 | N2 | 300 | 60 | Lignin oil | 32.8 | [83] |
| Ni/Al-SBA-15 (20) | Synthesized using P123, hydrothermally aged at 110 °C, dried, and calcined at 550 °C. Nickel nitrate solution (20 wt.% Ni) was impregnated on Al-SBA-15, dried at 100 °C, and calcined at 550 °C. | Hydrolyzed lignin | 1:0.4 | Ethanol | 10 | H2 | 300 | 240 | Lignin oil | 17.83 | [176] |
| Ni-Cu/H-Beta | Ni and Cu precursor solutions were mixed, supports added, and urea introduced. The mixture was stirred, filtered, washed, dried, calcined at 400 °C, and reduced at 550 °C in H2/N2 flow for final activation. | Kraft lignin | 1:0.4 | Isopropanol | - | N2 | 350 | 300 | Cycloalkanes | 40.39 | [177] |
| Ru-Cu/HY | Prepared by incipient wetness impregnation with metal salt solutions, stirred, dried, calcined at 550 °C, and reduced at 250 °C under hydrogen pressure before use. | Diphenyl ether | 1:1 | Water | 40 | H2 | 250 | 120 | Cyclohexane | 81.6 | [178] |
| Benzyloxy benzene | Cyclohexane | 56.1 | |||||||||
| Benzofuran | octahydrobenzofuran | 85.1 | |||||||||
| Cu/Mo-ZSM-5 | Prepared by dissolving ammonium heptamolybdate and copper sulfate in water, mixing with HZSM-5, and adding NaBH4. The mixture was centrifuged, washed, dried, and calcined at 500 °C. | Kraft lignin | 1:0.25 | Water-methanol (1:1 v/v) | - | Ar | 220 | 420 | Alkyl phenols | 20.6 | [179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balasubramanian, S.; Shanmugam, R.; Basha, A.C.; Sriariyanun, M.; Shanmugam, S.R.; Venkatachalam, P. An Overview of Solid Acid Catalysts in Lignocellulose Biorefineries. Catalysts 2025, 15, 432. https://doi.org/10.3390/catal15050432
Balasubramanian S, Shanmugam R, Basha AC, Sriariyanun M, Shanmugam SR, Venkatachalam P. An Overview of Solid Acid Catalysts in Lignocellulose Biorefineries. Catalysts. 2025; 15(5):432. https://doi.org/10.3390/catal15050432
Chicago/Turabian StyleBalasubramanian, Sujithra, Ratheeshkumar Shanmugam, Arul Chan Basha, Malinee Sriariyanun, Saravanan Ramiah Shanmugam, and Ponnusami Venkatachalam. 2025. "An Overview of Solid Acid Catalysts in Lignocellulose Biorefineries" Catalysts 15, no. 5: 432. https://doi.org/10.3390/catal15050432
APA StyleBalasubramanian, S., Shanmugam, R., Basha, A. C., Sriariyanun, M., Shanmugam, S. R., & Venkatachalam, P. (2025). An Overview of Solid Acid Catalysts in Lignocellulose Biorefineries. Catalysts, 15(5), 432. https://doi.org/10.3390/catal15050432











