Magnetic Iron Oxide Nanomaterials for Lipase Immobilization: Promising Industrial Catalysts for Biodiesel Production
Abstract
1. Introduction
2. Main Factors Influencing Biodiesel Bio-Production Using Immobilized Lipases
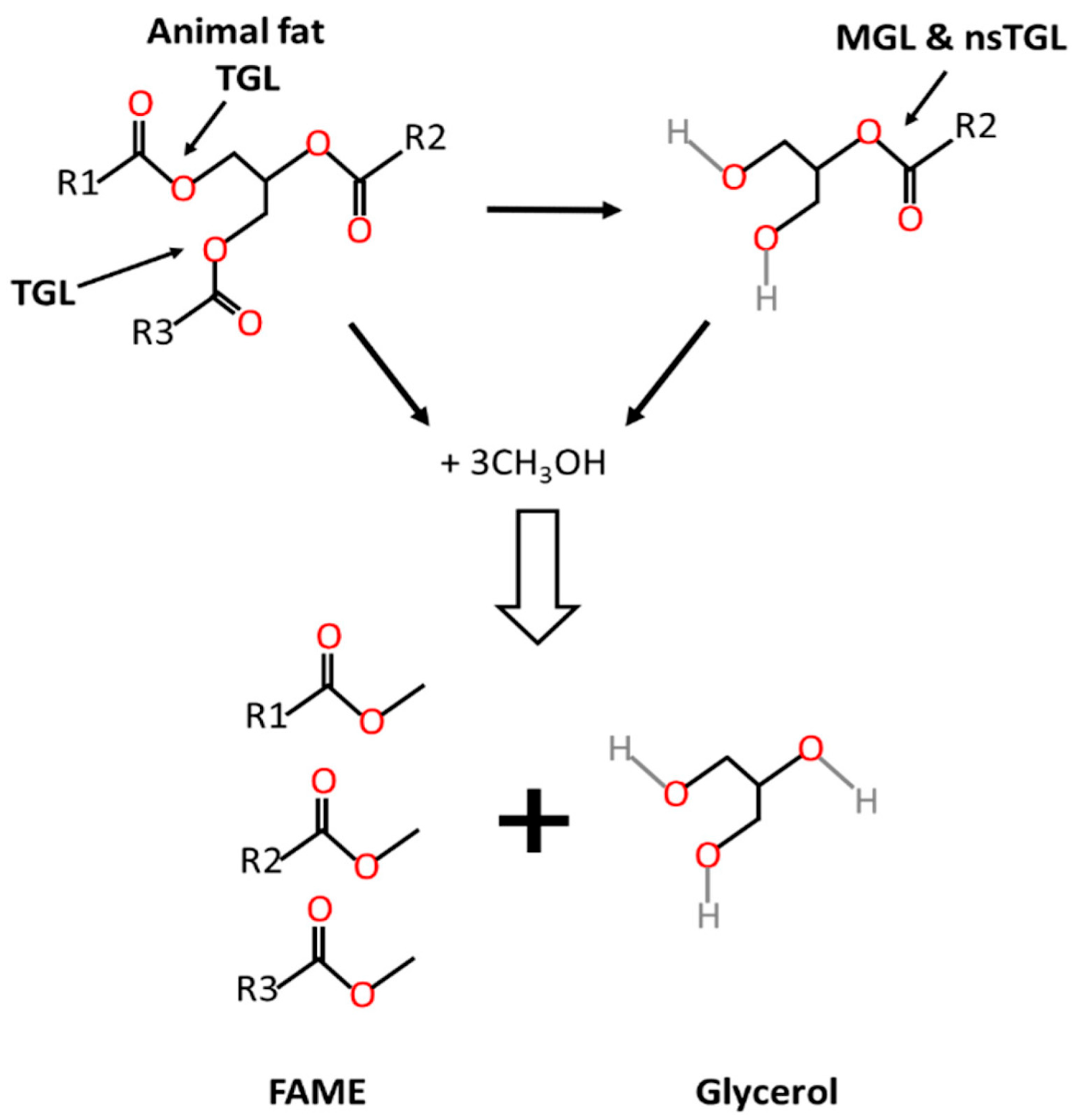
2.1. Feedstocks
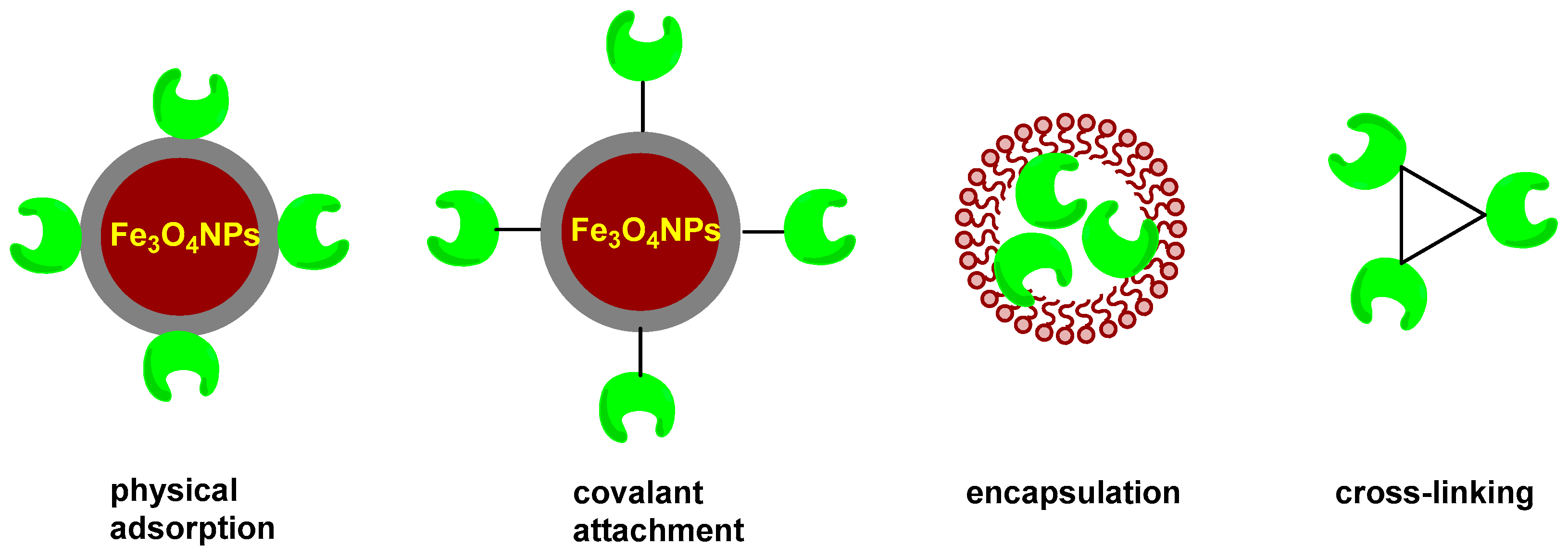
2.2. Immobilization Methods
2.3. Lipase Specificity
2.4. Lipase Source
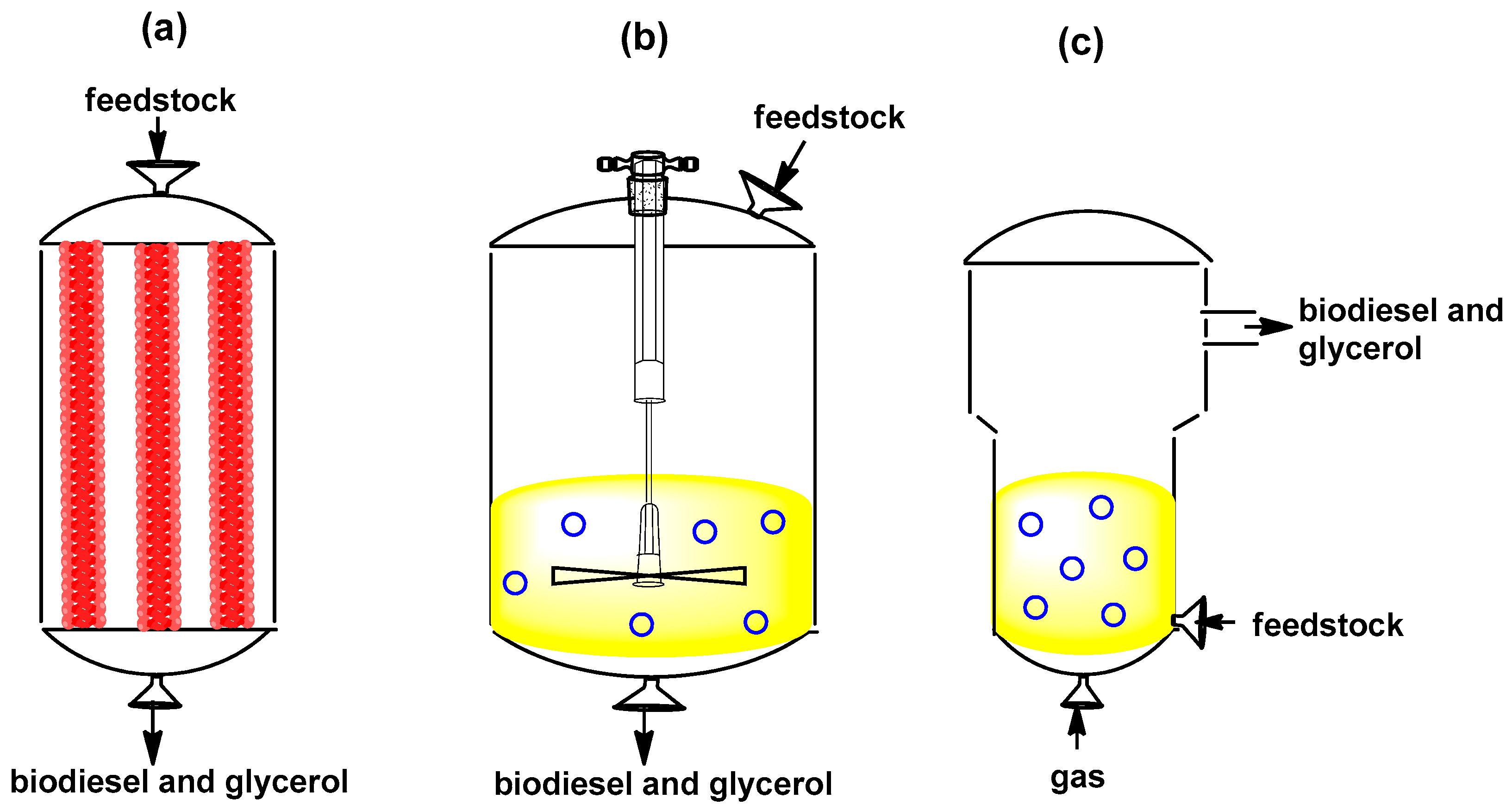
2.5. Operation Mode
| Nano-Support | Reactor | Process | Ref. |
|---|---|---|---|
| magnetic nanoparticles | STR | batch | [122] |
| snowman-like Fe3O4/Au nanoparticles | STR | batch | [123] |
| Fe3O4 coated with poly(styrene-methacrylic acid) | STR | batch | [124] |
| electrospun poly(acrylonitrile) | PBR | continuous | [125] |
| nano silicon | FBR | batch | [126] |
| nano silicon | reverse fluidized-bed reactor | continuous | [127] |
| nano silicon | PBR | continuous | [128] |
| Fe3O4 nanoparticles | PBR | continuous | [129] |
| Fe3O4 nanoparticles coated with glutaraldehyde | PBR | continuous | [130] |
2.6. Glycerol
3. Inorganic Nanocarriers Used to Develop Lipase-Based Nano-Biocatalysts
3.1. Magnetic Iron Oxide Nanoparticles
3.2. Importance of Surface Modification of Magnetic Nanoparticles
3.3. Silane-Functionalized Magnetic Nanocarriers
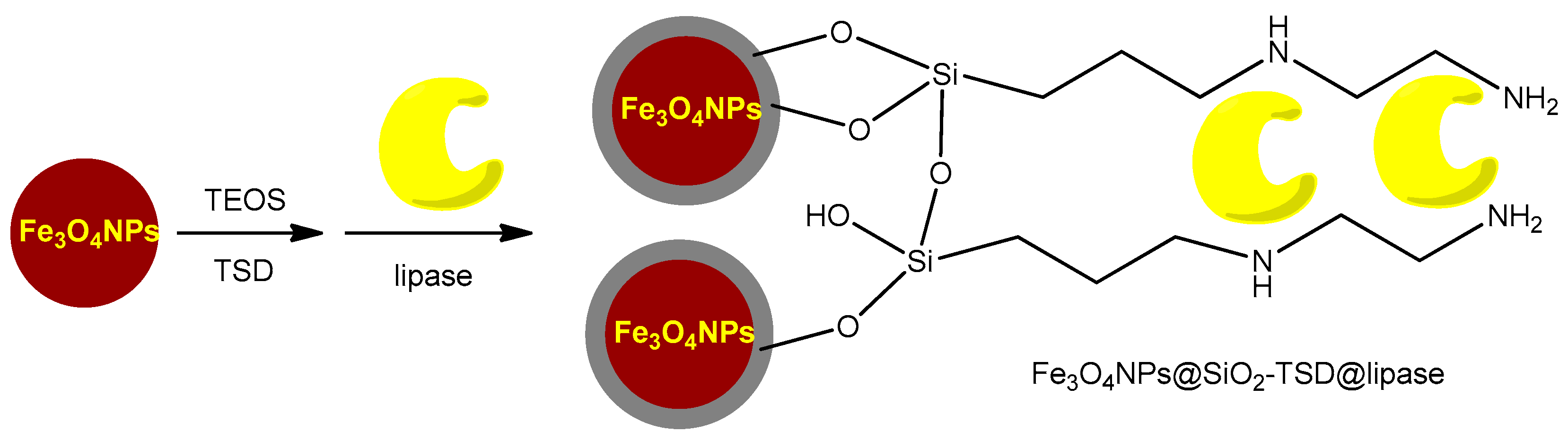
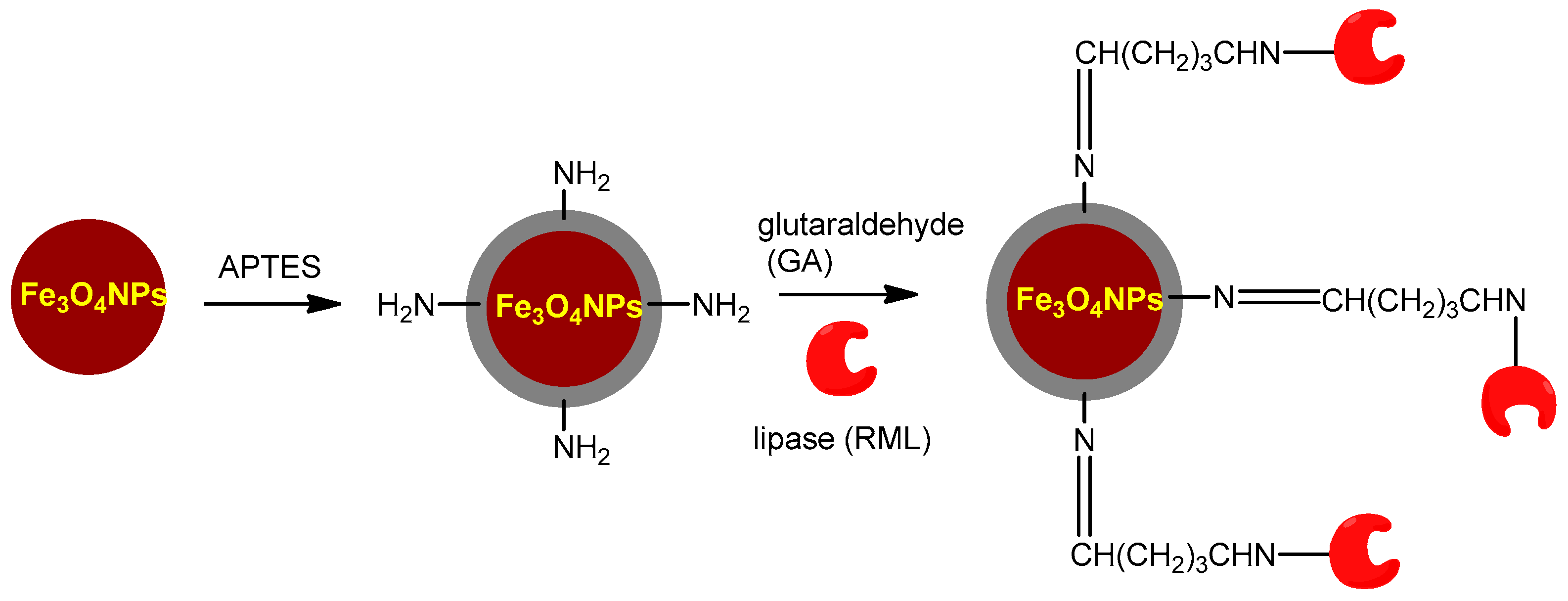
3.3.1. Silane Functionalization Providing –NH2 Groups
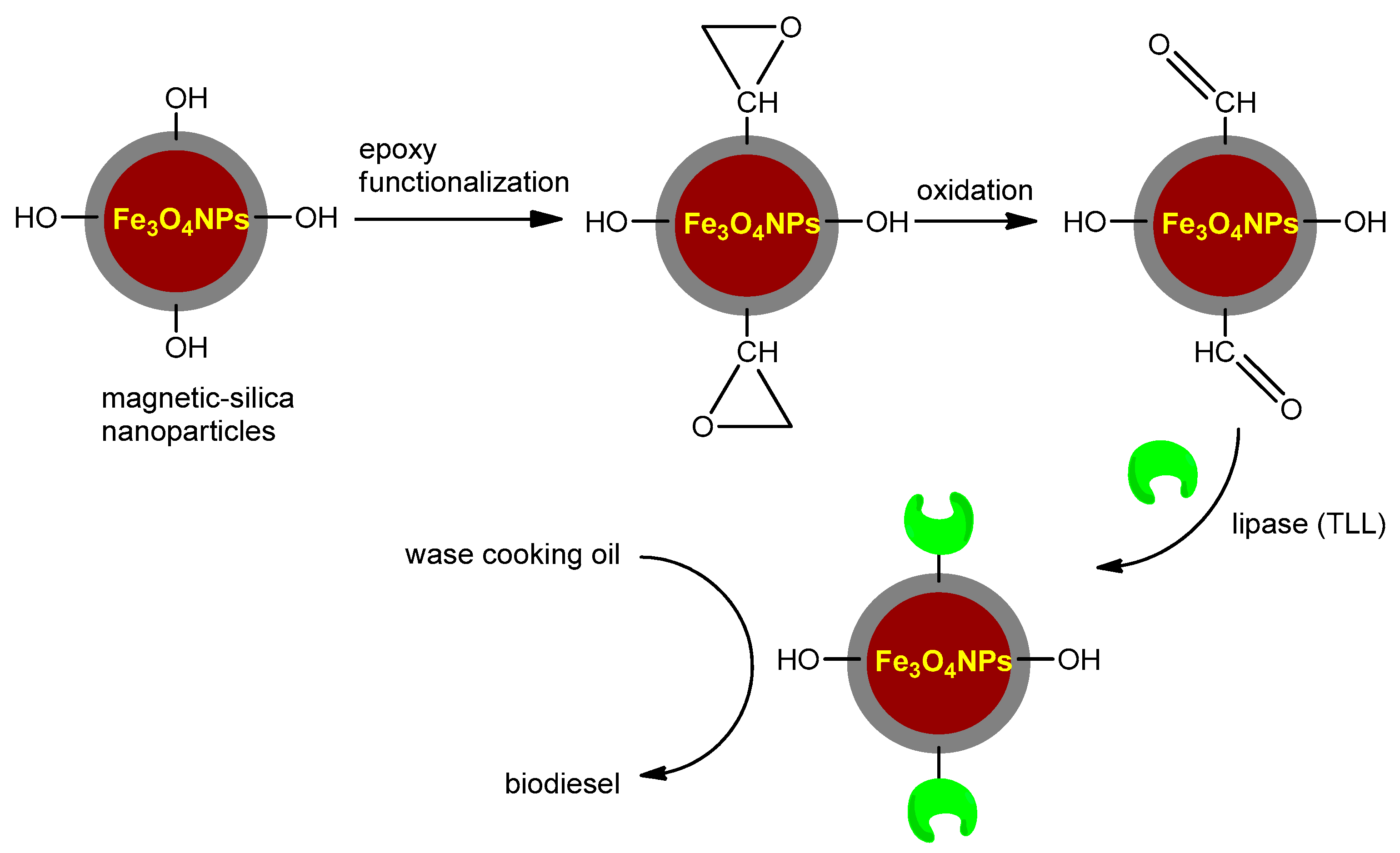
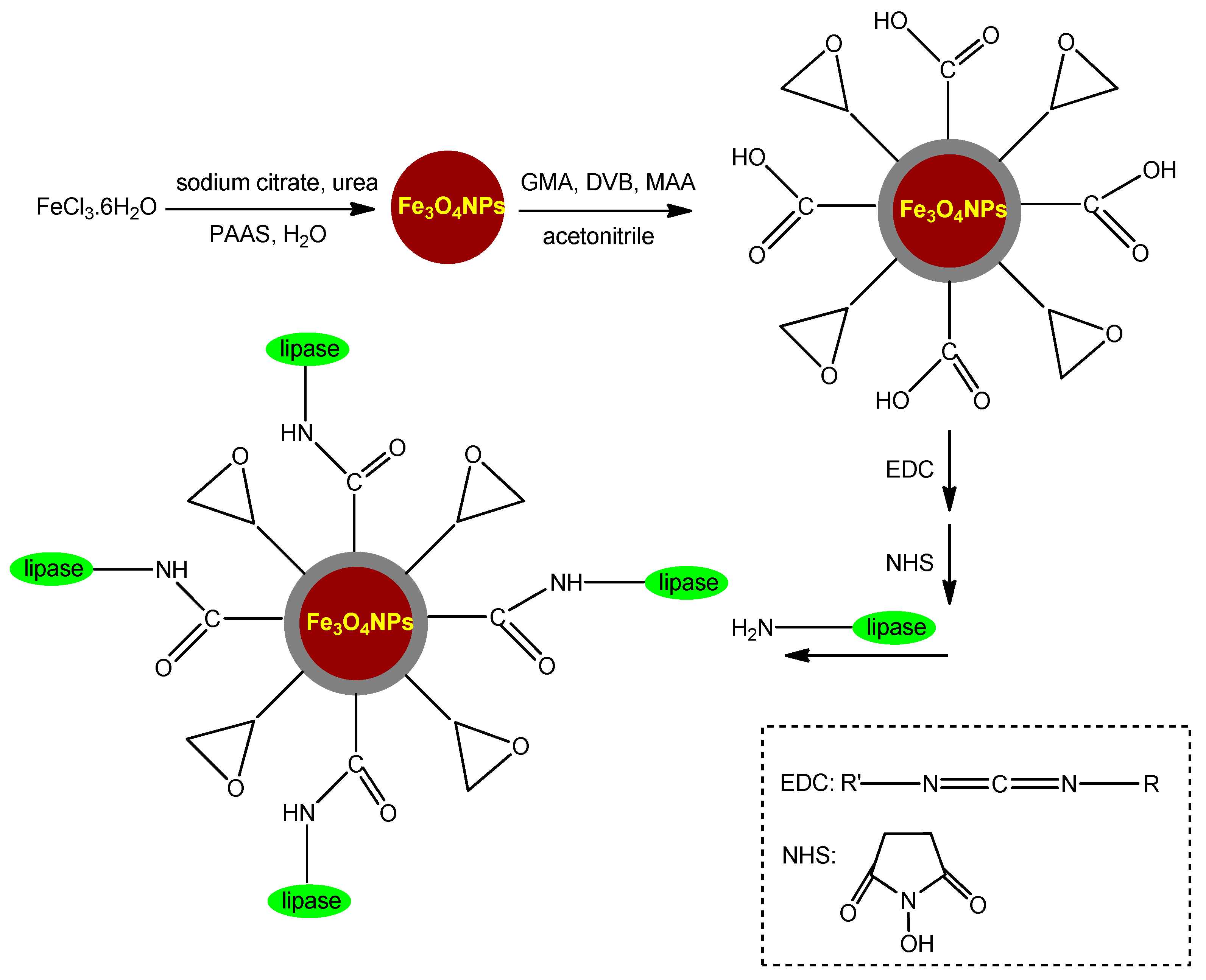
3.3.2. Silane Functionalizion Providing Epoxy Groups
3.4. Magnetic Nanocarriers Functionalized with Non-Silane Linkers
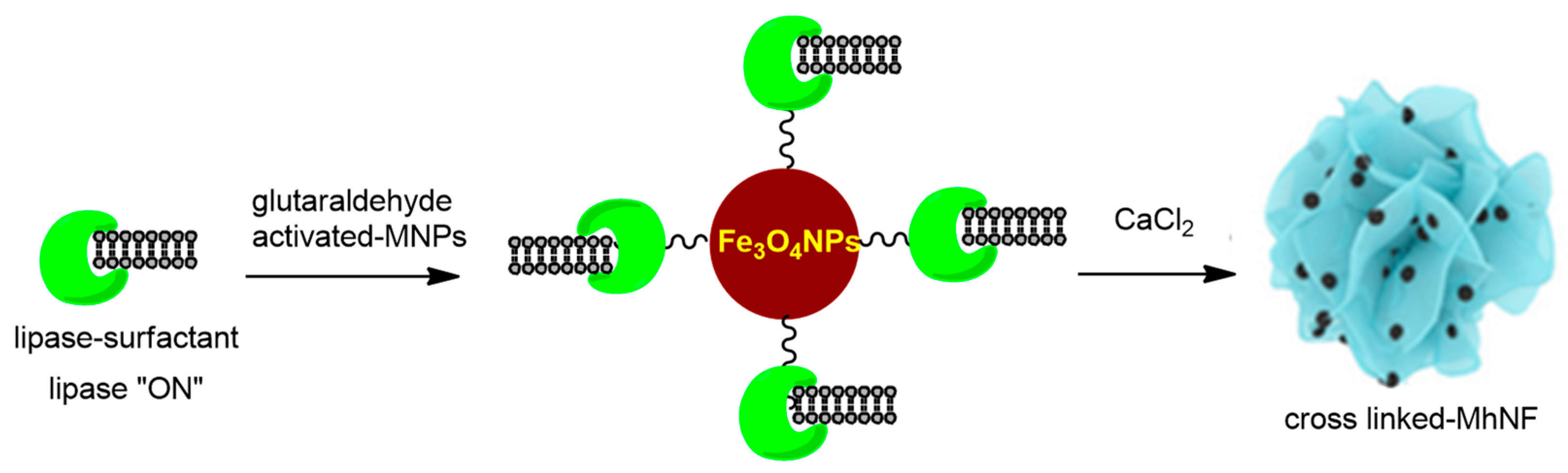
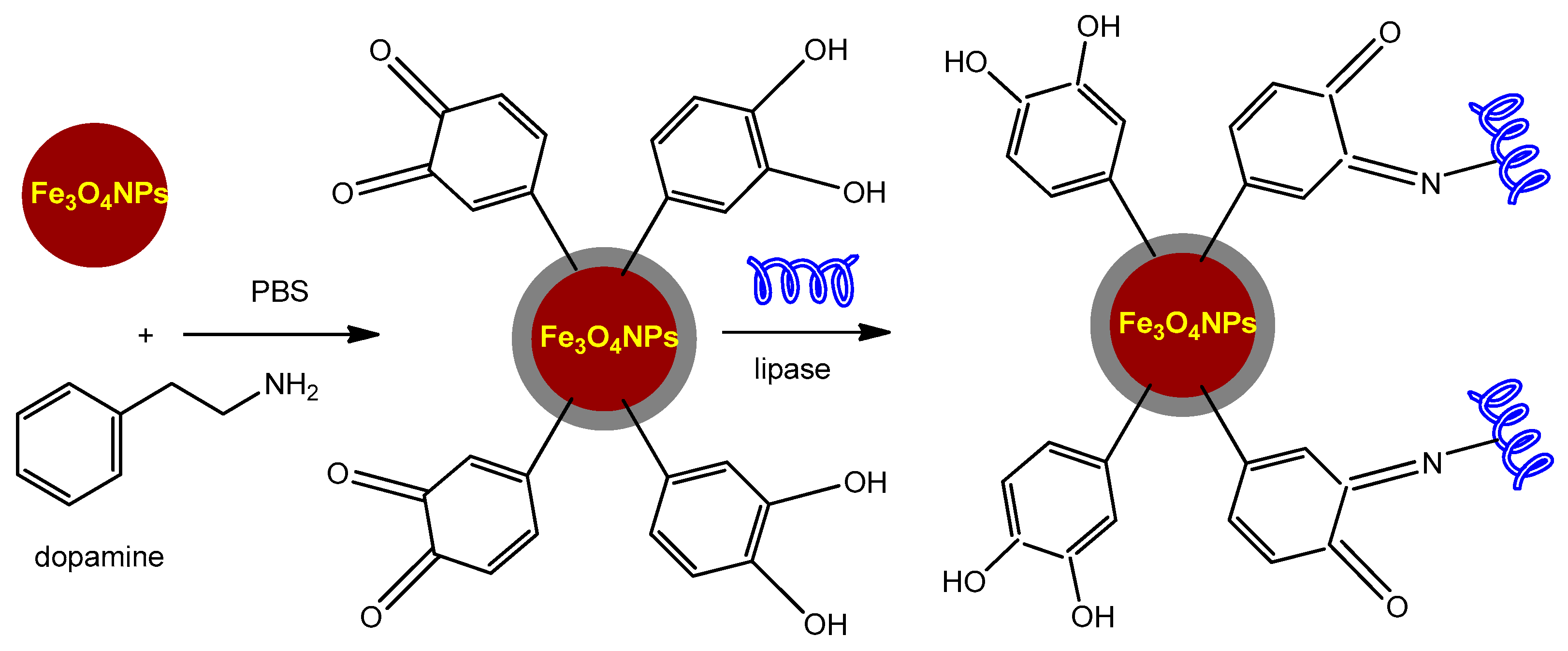
3.4.1. Magnetic Nanocarriers Functionalized with Small Molecules and Other Functionalizing Agents
3.4.2. Magnetic Nanocarriers Functionalized with Polymers
4. Conclusions: Current Challenges and Future Trends
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rade, L.L.; Da Silva, M.N.P.; Vieira, P.S.; Milan, N.; De Souza, C.M.; De Melo, R.R.; Klein, B.C.; Bonomi, A.; De Castro, H.F.; Murakami, M.T.; et al. A Novel Fungal Lipase with Methanol Tolerance and Preference for Macaw Palm Oil. Front. Bioeng. Biotechnol. 2020, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Faisal, S.; Ahmed, I.A.; Munir, M.; Cipolatti, E.P.; Manoel, E.A.; Pastore, C.; Di Bitonto, L.; Hanelt, D.; Nitbani, F.O.; et al. Has the Time Finally Come for Green Oleochemicals and Biodiesel Production Using Large-Scale Enzyme Technologies? Current Status and New Developments. Biotechnol. Adv. 2023, 69, 108275. [Google Scholar] [CrossRef]
- Ahmad, T.; Zhang, D. A Critical Review of Comparative Global Historical Energy Consumption and Future Demand: The Story Told so Far. Energy Rep. 2020, 6, 1973–1991. [Google Scholar] [CrossRef]
- Oltulular, S. Circular Behavior of Oil Consumption and the Impact of Oil Prices with the Fourier Approach. Energy Strat. Rev. 2024, 53, 101386. [Google Scholar] [CrossRef]
- Cronshaw, I. World Energy Outlook 2014 Projections to 2040: Natural Gas and Coal Trade, and the Role of China. Aust. J. Agric. Resour. Econ. 2015, 59, 571–585. [Google Scholar] [CrossRef]
- Arguelles-Arguelles, A.; Amezcua-Allieri, M.A.; Ramírez-Verduzco, L.F. Life Cycle Assessment of Green Diesel Production by Hydrodeoxygenation of Palm Oil. Front. Energy Res. 2021, 9, 690725. [Google Scholar] [CrossRef]
- Monika; Banga, S.; Pathak, V.V. Biodiesel Production from Waste Cooking Oil: A Comprehensive Review on the Application of Heterogeneous Catalysts. Energy Nexus 2023, 10, 100209. [Google Scholar] [CrossRef]
- Neupane, D. Biofuels from Renewable Sources, a Potential Option for Biodiesel Production. Bioengineering 2022, 10, 29. [Google Scholar] [CrossRef]
- Melo, R.L.F.; Sales, M.B.; De Castro Bizerra, V.; De Sousa Junior, P.G.; Cavalcante, A.L.G.; Freire, T.M.; Neto, F.S.; Bilal, M.; Jesionowski, T.; Soares, J.M.; et al. Recent Applications and Future Prospects of Magnetic Biocatalysts. Int. J. Biol. Macromol. 2023, 253, 126709. [Google Scholar] [CrossRef] [PubMed]
- Thanh, L.T.; Okitsu, K.; Boi, L.V.; Maeda, Y. Catalytic Technologies for Biodiesel Fuel Production and Utilization of Glycerol: A Review. Catalysts 2012, 2, 191–222. [Google Scholar] [CrossRef]
- Sarin, A.; Singh, M.; Sharma, N.; Singh, N.P. Prospects of Tectona Grandis as a Feedstock for Biodiesel. Front. Energy Res. 2017, 5, 28. [Google Scholar] [CrossRef]
- Klein, T. FAO-OECD: The Global Biofuels Market Is Still Growing. Transport Energy Strategies. 2019. Available online: https://www.transportenergystrategies.com/2016/07/05/global-biofuels-market-still-growing (accessed on 5 May 2024).
- Ogunkunle, O.; Ahmed, N.A. A Review of Global Current Scenario of Biodiesel Adoption and Combustion in Vehicular Diesel Engines. Energy Rep. 2019, 5, 1560–1579. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Inda, C.S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A Comprehensive Review of Biodiesel Production from Waste Cooking Oil and Its Use as Fuel in Compression Ignition Engines: 3rd Generation Cleaner Feedstock. J. Clean. Prod. 2021, 307, 127299. [Google Scholar] [CrossRef]
- Wang, B.; Wang, B.; Shukla, S.K.; Wang, R. Enabling Catalysts for Biodiesel Production via Transesterification. Catalysts 2023, 13, 740. [Google Scholar] [CrossRef]
- Helwani, Z.; Othman, M.R.; Aziz, N.; Fernando, W.J.N.; Kim, J. Technologies for Production of Biodiesel Focusing on Green Catalytic Techniques: A Review. Fuel Process Technol. 2009, 90, 1502–1514. [Google Scholar] [CrossRef]
- Avhad, M.R.; Marchetti, J.M. Innovation in Solid Heterogeneous Catalysis for the Generation of Economically Viable and Ecofriendly Biodiesel: A Review. Catal. Rev. 2016, 58, 157–208. [Google Scholar] [CrossRef]
- Zhong, L.; Feng, Y.; Wang, G.; Wang, Z.; Bilal, M.; Lv, H.; Jia, S.; Cui, J. Production and Use of Immobilized Lipases in/on Nanomaterials: A Review from the Waste to Biodiesel Production. Int. J. Biol. Macromol. 2020, 152, 207–222. [Google Scholar] [CrossRef]
- Suzihaque, M.U.H.; Alwi, H.; Kalthum Ibrahim, U.; Abdullah, S.; Haron, N. Biodiesel Production from Waste Cooking Oil: A Brief Review. Mater. Today Proc. 2022, 63, S490–S495. [Google Scholar] [CrossRef]
- Mustafa, A. Chapter 11-Lipase Catalyzed Reactions: A Promising Approach for Clean Synthesis of Oleochemicals. In Sustainable Solutions for Environmental Pollution; El-Gendy, N.S., Ed.; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, Heterogeneous and Enzymatic Catalysis for Transesterification of High Free Fatty Acid Oil (Waste Cooking Oil) to Biodiesel: A Review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef]
- Kalak, T. Potential Use of Industrial Biomass Waste as a Sustainable Energy Source in the Future. Energies 2023, 16, 1783. [Google Scholar] [CrossRef]
- Amini, Z.; Ilham, Z.; Ong, H.C.; Mazaheri, H.; Chen, W.-H. State of the Art and Prospective of Lipase-Catalyzed Transesterification Reaction for Biodiesel Production. Energy Convers. Manag. 2017, 141, 339–353. [Google Scholar] [CrossRef]
- Baadhe, R.R.; Ptumarthi, R.; Gupta, V.K. Chapter 8—Lipase Catalyzed Biodiesel Production: Technical Challenges. In Bioenergy Research: Advances and Application; Gupta, V.K., Tuohy, M.G., Kubicek, C.P., Saddler, J., Xu, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 119–129. [Google Scholar]
- Lotti, M.; Pleiss, J.; Valero, F.; Ferrer, P. Effects of Methanol on Lipases: Molecular, Kinetic and Process Issues in the Production of Biodiesel. Biotechnol. J. 2014, 10, 22–30. [Google Scholar] [CrossRef]
- Avhad, M.R.; Marcheffi, J.M. Chapter 7—Uses of Enzymes for Biodiesel Production. In Advanced Bioprocessing for Alternative Fuels, Biobased Chemicals and Bioproducts; Hosseini, M., Ed.; Woodhead Publishing Series in Energy; Woodhead Publishing; Elsevier: Sawston, UK, 2019; pp. 135–152. [Google Scholar]
- Shimada, Y.; Watanabe, Y.; Samukawa, T.; Sugihara, A.; Noda, H.; Fukuda, H.; Tominaga, Y. Conversion of Vegetable Oil to Biodiesel Using Immobilized Candida antarctica Lipase. J. Am. Oil. Chem. Soc. 1999, 76, 789–793. [Google Scholar] [CrossRef]
- Watanabe, Y.; Shimada, Y.; Sugihara, A.; Tominaga, Y. Stepwise Ethanolysis of Tuna Oil Using Immobilized Candida antarctica Lipase. J. Biosci. Bioeng. 1999, 88, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Bilal, M.; Li, X.; Ju, F.; Teng, Y.; Iqbal, H.M.N. Nanomaterial-Immobilized Lipases for Sustainable Recovery of Biodiesel—A Review. Fuel 2022, 316, 123429. [Google Scholar] [CrossRef]
- De Andrade Silva, T.; Keijok, W.J.; Guimarães, M.C.C.; Cassini, S.T.A.; De Oliveira, J.P. Impact of Immobilization Strategies on the Activity and Recyclability of Lipases in Nanomagnetic Supports. Sci. Rep. 2022, 12, 6815. [Google Scholar] [CrossRef] [PubMed]
- Cipolatti, E.P.; Valério, A.; Henriques, R.O.; Moritz, D.E.; Ninow, J.L.; Freire, D.M.G.; Manoel, E.A.; Fernandez-Lafuente, R.; De Oliveira, D. Nanomaterials for Biocatalyst Immobilization-State of the Art and Future Trends. RSC Adv. 2016, 6, 104675–104692. [Google Scholar] [CrossRef]
- Girelli, A.M.; Chiappini, V. Renewable, Sustainable, and Natural Lignocellulosic Carriers for Lipase Immobilization: A Review. J. Biotechnol. 2023, 365, 29–47. [Google Scholar] [CrossRef]
- Costantini, A.; Califano, V. Lipase Immobilization in Mesoporous Silica Nanoparticles for Biofuel Production. Catalysts 2021, 11, 629. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.; Jesionowski, T.; Pinelo, M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts 2018, 8, 92. [Google Scholar] [CrossRef]
- Bartha-Vári, J.-H.; Moisă, M.E.; Bencze, L.C.; Irimie, F.-D.; Paizs, C.; Toșa, M.I. Efficient Biodiesel Production Catalyzed by Nanobioconjugate of Lipase from Pseudomonas fluorescens. Molecules 2020, 25, 651. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ke, C.; Su, F.; Li, K.; Yan, Y. Various Types of Lipases Immobilized on Dendrimer-Functionalized Magnetic Nanocomposite and Application in Biodiesel Preparation. Energy Fuels 2017, 31, 4372–4381. [Google Scholar] [CrossRef]
- Ravi, B.; Mehrotra, S.; Mehrotra, R. Advances in Heterogeneous and Enzymatic Catalysis for the Industrial Production of Biodiesel by Transesterification: An Overview. Curr. Chem. Biol. 2013, 7, 104–113. [Google Scholar] [CrossRef]
- Singh, N.; Dhanya, B.S.; Verma, M.L. Nano-Immobilized Biocatalysts and Their Potential Biotechnological Applications in Bioenergy Production. Mater. Sci. Energy Technol. 2020, 3, 808–824. [Google Scholar] [CrossRef]
- Mohammadi-Mahani, H.; Badoei-dalfard, A.; Karami, Z. Synthesis and Characterization of Cross-Linked Lipase-Metal Hybrid Nanoflowers on Graphene Oxide with Increasing the Enzymatic Stability and Reusability. Biochem. Eng. J. 2021, 172, 108038. [Google Scholar] [CrossRef]
- Nematian, T.; Shakeri, A.; Salehi, Z.; Saboury, A.A.; Dalai, A.K. The Effect of 3D Structure Preparation Method on Lipase/3DMGO Biocatalytic Parameters and Catalytic Performance in Transesterification of Microalgae Bio-Oil. Catal. Today 2022, 404, 105–116. [Google Scholar] [CrossRef]
- Nuraliyah, A.; Perdani, M.S.; Putri, D.N.; Sahlan, M.; Wijanarko, A.; Hermansyah, H. Effect of Additional Amino Group to Improve the Performance of Immobilized Lipase from Aspergillus niger by Adsorption-Crosslinking Method. Front. Energy Res. 2021, 9, 616945. [Google Scholar] [CrossRef]
- Wang, S.; Li, S.; Liu, R.; Zhang, W.; Xu, H.; Hu, Y. Immobilization of Interfacial Activated Candida rugosa Lipase Onto Magnetic Chitosan Using Dialdehyde Cellulose as Cross-Linking Agent. Front. Bioeng. Biotechnol. 2022, 10, 946117. [Google Scholar] [CrossRef] [PubMed]
- Mendonca Ferreira, R.D.; Brackmann, R.; Pereira, E.B.; Costa Da Rocha, R.D. Immobilization of Lipase from Candida rugosa onto Niobium Oxide. Biocatal. Agric. Biotechnol. 2020, 30, 101812. [Google Scholar] [CrossRef]
- Moreira, K.D.S.; De Oliveira, A.L.B.; Júnior, L.S.D.M.; Monteiro, R.R.C.; Da Rocha, T.N.; Menezes, F.L.; Fechine, L.M.U.D.; Denardin, J.C.; Michea, S.; Freire, R.M.; et al. Lipase From Rhizomucor miehei Immobilized on Magnetic Nanoparticles: Performance in Fatty Acid Ethyl Ester (FAEE) Optimized Production by the Taguchi Method. Front. Bioeng. Biotechnol. 2020, 8, 693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jun, S.-H.; Wee, Y.; Kim, H.S.; Hwang, E.T.; Shim, J.; Hwang, S.Y.; Lee, J.; Kim, J. Activation of Crosslinked Lipases in Mesoporous Silica via Lid Opening for Recyclable Biodiesel Production. Int. J. Biol. Macromol. 2022, 222, 2368–2374. [Google Scholar] [CrossRef] [PubMed]
- Arana-Peña, S.; Rios, N.S.; Carballares, D.; Mendez-Sanchez, C.; Lokha, Y.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Effects of Enzyme Loading and Immobilization Conditions on the Catalytic Features of Lipase from Pseudomonas fluorescens Immobilized on Octyl-Agarose Beads. Front. Bioeng. Biotechnol. 2020, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Dumri, K.; Hung Anh, D. Immobilization of Lipase on Silver Nanoparticles via Adhesive Polydopamine for Biodiesel Production. Enzyme Res. 2014, 2014, 389739. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Lin, Z.; Tan, T.; Svec, F. Preparation of Reusable Bioreactors Using Reversible Immobilization of Enzyme on Monolithic Porous Polymer Support with Attached Gold Nanoparticles. Biotechnol. Bioeng. 2014, 111, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, C.; Degennaro, L.; Luisi, R. Titanium Dioxide as a Catalyst in Biodiesel Production. Catalysts 2019, 9, 75. [Google Scholar] [CrossRef]
- Gkantzou, E.; Patila, M.; Stamatis, H. Magnetic Microreactors with Immobilized Enzymes-From Assemblage to Contemporary Applications. Catalysts 2018, 8, 282. [Google Scholar] [CrossRef]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E.-S. Enzyme Immobilization Technologies and Industrial Applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef] [PubMed]
- Seenuvasan, M.; Vinodhini, G.; Malar, C.G.; Balaji, N.; Kumar, K.S. Magnetic Nanoparticles: A Versatile Carrier for Enzymes in Bio-processing Sectors. IET Nanobiotechnol. 2018, 12, 535–548. [Google Scholar] [CrossRef]
- Mariño, M.A.; Fulaz, S.; Tasic, L. Magnetic Nanomaterials as Biocatalyst Carriers for Biomass Processing: Immobilization Strategies, Reusability, and Applications. Magnetochemistry 2021, 7, 133. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.; Akram, M.; Udego, I.; Li, H.; Niu, X. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.G.; Dalai, A.K. Waste Cooking Oil-An Economical Source for Biodiesel: A Review. Ind. Eng. Chem. Res. 2006, 45, 2901–2913. [Google Scholar] [CrossRef]
- Demirbas, A. Production of Biodiesel Fuels from Linseed Oil Using Methanol and Ethanol in Non-Catalytic SCF Conditions. Biomass Bioenergy 2009, 33, 113–118. [Google Scholar] [CrossRef]
- Bukhari, S.A.Z.; Ullah, K.; Ahmad, M.; Zafar, M.; Ullah, A.; Sultana, S.; Ibrahim, M.M.; Hessien, M.M.; Mersal, G.A.M.; Ghoneim, S.S.M. Biodiesel from Dodonaea Plant Oil: Synthesis and Characterization-A Promising Nonedible Oil Source for Bioenergy Industry. Front. Bioeng. Biotechnol. 2022, 10, 864415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, S. A Review on Biodiesel Production Using Basic Ionic Liquids as Catalysts. Ind. Crop. Prod. 2023, 202, 117099. [Google Scholar] [CrossRef]
- Knothe, G.; Razon, L.F. Biodiesel Fuels. Prog. Energ. Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Banković-Ilić, I.B.; Stamenković, O.S.; Veljković, V.B. Biodiesel Production from Non-Edible Plant Oils. Renew. Sustain. Energy Rev. 2012, 16, 3621–3647. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Ojumu, T.V.; Oyekola, O.O.; Madzimbamuto, T.F.; Ikhu-Omoregbe, D.I.O. A Review of Current Technology for Biodiesel Production: State of the Art. Biomass Bioenergy 2014, 61, 276–297. [Google Scholar] [CrossRef]
- Azócar, L.; Ciudad, G.; Heipieper, H.J.; Navia, R. Biotechnological Processes for Biodiesel Production Using Alternative Oils. Appl. Microbiol. Biotechnol. 2010, 88, 621–636. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Demirbas, A. Biodiesel: Hopes and Dreads. Biofuel. Res. J. 2016, 3, 379. [Google Scholar] [CrossRef]
- Mu, H.; Song, Z.; Wang, X.; Wang, D.; Zheng, X.; Li, X. Microencapsulation of Algae Oil by Complex Coacervation of Chitosan and Modified Starch: Characterization and Oxidative Stability. Int. J. Biol. Macromol. 2022, 194, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Biodiesel Production from Waste Cooking Oil: 1. Process Design and Technological Assessment. Bioresour. Technol. 2003, 89, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Soares Dias, A.P.; Pedra, I.; Salvador, É.; Rijo, B.; Costa Pereira, M.F.; Serralha, F.; Nogueira, I. Biodiesel Production over Banana Peel Biochar as a Sustainable Catalyst. Catalysts 2024, 14, 266. [Google Scholar] [CrossRef]
- Hsu, A.; Jones, K.C.; Foglia, T.A.; Marmer, W.N. Continuous Production of Ethyl Esters of Grease Using an Immobilized Lipase. J. Am. Oil Chem. Soc. 2004, 81, 749–752. [Google Scholar] [CrossRef]
- Arumugam, A.; Thulasidharan, D.; Jegadeesan, G.B. Process Optimization of Biodiesel Production from Hevea brasiliensis Oil Using Lipase Immobilized on Spherical Silica Aerogel. Renew. Energy 2018, 116, 755–761. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R.; Armisén, P.; Sabuquillo, P.; Fernández-Lorente, G.; Guisán, J.M. Immobilization of Lipases by Selective Adsorption on Hydrophobic Supports. Chem. Phys. Lipids 1998, 93, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; Dos Santos, J.C.S.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of Lipases on Hydrophobic Supports: Immobilization Mechanism, Advantages, Problems, and Solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef] [PubMed]
- Manoel, E.A.; Dos Santos, J.C.S.; Freire, D.M.G.; Rueda, N.; Fernandez-Lafuente, R. Immobilization of Lipases on Hydrophobic Supports Involves the Open Form of the Enzyme. Enzyme Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, K.; Fernandez-Lafuente, R. Lipase B from Candida antarctica Immobilized on Octadecyl Sepabeads: A Very Stable Biocatalyst in the Presence of Hydrogen Peroxide. Process Biochem. 2011, 46, 873–878. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Jiang, Y.; Zhou, L.; Ma, L.; He, Y.; Gao, J. Biocatalytic Pickering Emulsions Stabilized by Lipase-Immobilized Carbon Nanotubes for Biodiesel Production. Catalysts 2018, 8, 587. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Zare, A.; Bordbar, A.-K.; Jafarian, F.; Tangestaninejad, S. Candida rugosa Lipase Immobilization on Various Chemically Modified Chromium Terephthalate MIL-101. J. Mol. Liq. 2018, 254, 137–144. [Google Scholar] [CrossRef]
- Mendes, A.A.; Giordano, R.C.; Giordano, R.D.L.C.; De Castro, H.F. Immobilization and Stabilization of Microbial Lipases by Multipoint Covalent Attachment on Aldehyde-Resin Affinity: Application of the Biocatalysts in Biodiesel Synthesis. J. Mol. Catal. B Enzym. 2011, 68, 109–115. [Google Scholar] [CrossRef]
- Jambulingam, R.; Shalma, M.; Shankar, V. Biodiesel Production Using Lipase Immobilised Functionalized Magnetic Nanocatalyst from Oleaginous Fungal Lipid. J. Clean. Prod. 2019, 215, 245–258. [Google Scholar] [CrossRef]
- Xie, W.; Huang, M. Immobilization of Candida rugosa Lipase onto Graphene Oxide Fe3O4 Nanocomposite: Characterization and Application for Biodiesel Production. Energy Convers. Manag. 2018, 159, 42–53. [Google Scholar] [CrossRef]
- Shahedi, M.; Yousefi, M.; Habibi, Z.; Mohammadi, M.; As’habi, M.A. Co-Immobilization of Rhizomucor miehei Lipase and Candida antarctica Lipase B and Optimization of Biocatalytic Biodiesel Production from Palm Oil Using Response Surface Methodology. Renew. Energy 2019, 141, 847–857. [Google Scholar] [CrossRef]
- Langellotti, V.; Melchiorre, M.; Cucciolito, M.E.; Esposito, R.; Grieco, D.; Pinto, G.; Ruffo, F. Biodiesel from Waste Cooking Oil: Highly Efficient Homogeneous Iron(III) Molecular Catalysts. Catalysts 2023, 13, 1496. [Google Scholar] [CrossRef]
- Adlercreutz, P. Immobilisation and Application of Lipases in Organic Media. Chem. Soc. Rev. 2013, 42, 6406. [Google Scholar] [CrossRef] [PubMed]
- Gkaniatsou, E.; Sicard, C.; Ricoux, R.; Mahy, J.-P.; Steunou, N.; Serre, C. Metal-Organic Frameworks: A Novel Host Platform for Enzymatic Catalysis and Detection. Mater. Horiz. 2017, 4, 55–63. [Google Scholar] [CrossRef]
- Duan, Y.; Zou, T.; Wu, S.; Cheng, H. Immobilization of Lipases on Modified Silica Clay for Bio-Diesel Production: The Effect of Surface Hydrophobicity on Performance. Catalysts 2022, 12, 242. [Google Scholar] [CrossRef]
- Alzuhair, S.; Ling, F.W.; Limsong, J. Proposed Kinetic Mechanism of the Production of Biodiesel from Palm Oil Using Lipase. Process Biochem. 2007, 42, 951–960. [Google Scholar] [CrossRef]
- Toldrá-Reig, F.; Mora, L.; Toldrá, F. Developments in the Use of Lipase Transesterification for Biodiesel Production from Animal Fat Waste. Appl. Sci. 2020, 10, 5085. [Google Scholar] [CrossRef]
- Tsurumura, T.; Tsuge, H. Substrate Selectivity of Bacterial Monoacylglycerol Lipase Based on Crystal Structure. J. Struct. Funct. Genom. 2014, 15, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-Y.; Zhang, Y.-Q.; Zhang, Y.; Jiang, W.-X.; Wang, Y.-J.; Zhang, Y.-S.; Sun, Z.-Z.; Li, C.-Y.; Zhang, Y.-Z.; Shi, M.; et al. Study on a Novel Cold-Active and Halotolerant Monoacylglycerol Lipase Widespread in Marine Bacteria Reveals a New Group of Bacterial Monoacylglycerol Lipases Containing Unusual C(A/S)HSMG Catalytic Motifs. Front. Microbiol. 2020, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Davranov, K. Microbial Lipases in Biotechnology (Review). Appl. Biochem. Microbiol. 1994, 30, 527–534. [Google Scholar]
- Jaeger, K.-E.; Ransac, S.; Dijkstra, B.W.; Colson, C.; Heuvel, M.; van Misset, O. Bacterial Lipases. FEMS Microbiol. Rev. 1994, 15, 29–63. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, R.W.; Qiang, L.; Wei, D.; Liu, D.H. Acyl Migration and Kinetics Study of 1(3)-Positional Specific Lipase of Rhizopus oryzae-Catalyzed Methanolysis of Triglyceride for Biodiesel Production. Process Biochem. 2010, 45, 1888–1893. [Google Scholar]
- Toldrá-Reig, F.; Mora, L.; Toldrá, F. Trends in Biodiesel Production from Animal Fat Waste. Appl. Sci. 2020, 10, 3644. [Google Scholar] [CrossRef]
- Schreck, S.D.; Grunden, A.M. Biotechnological Applications of Halophilic Lipases and Thioesterases. Appl. Microbiol. Biotechnol. 2014, 98, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Chandra Kishore, S.; Perumal, S.; Atchudan, R.; Sundramoorthy, A.K.; Alagan, M.; Sangaraju, S.; Lee, Y.R. A Review of Biomass-Derived Heterogeneous Catalysts for Biodiesel Production. Catalysts 2022, 12, 1501. [Google Scholar] [CrossRef]
- Najeeb, J.; Akram, S.; Mumtaz, M.W.; Danish, M.; Irfan, A.; Touqeer, T.; Rashid, U.; Ghani, W.A.W.A.K.; Choong, T.S.Y. Nanobiocatalysts for Biodiesel Synthesis through Transesterification-A Review. Catalysts 2021, 11, 171. [Google Scholar] [CrossRef]
- Houde, A.; Kademi, A.; Leblanc, D. Lipases and Their Industrial Applications: An Overview. Appl. Abiochem. Biotechnol. 2004, 118, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Schmid, R.D.; Verger, R. Lipases: Interfacial Enzymes with Attractive Applications. Angew. Chem. Int. Ed. Engl. 1998, 37, 1608–1633. [Google Scholar] [CrossRef]
- Bordes, F.; Barbe, S.; Escalier, P.; Mourey, L.; André, I.; Marty, A.; Tranier, S. Exploring the Conformational States and Rearrangements of Yarrowia lipolytica Lipase. Biophys. J. 2010, 99, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.C.; Nguyen, M.-L.; Su, C.-H.; Ong, H.C.; Juan, H.-Y.; Wu, S.-J. Bio-Derived Catalysts: A Current Trend of Catalysts Used in Biodiesel Production. Catalysts 2021, 11, 812. [Google Scholar] [CrossRef]
- Fjerbaek, L.; Christensen, K.V.; Norddahl, B. A Review of the Current State of Biodiesel Production Using Enzymatic Transesterification. Biotechnol. Bioeng. 2009, 102, 1298–1315. [Google Scholar] [CrossRef]
- Alves, J.S.; Vieira, N.S.; Cunha, A.S.; Silva, A.M.; Záchia Ayub, M.A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Combi-Lipase for Heterogeneous Substrates: A New Approach for Hydrolysis of Soybean Oil Using Mixtures of Biocatalysts. RSC Adv. 2014, 4, 6863–6868. [Google Scholar] [CrossRef]
- Schoffelen, S.; Van Hest, J.C. Chemical Approaches for the Construction of Multi-Enzyme Reaction Systems. Curr. Opin. Struct. Biol. 2013, 23, 613–621. [Google Scholar] [CrossRef]
- Babaki, M.; Yousefi, M.; Habibi, Z.; Mohammadi, M. Process Optimization for Biodiesel Production from Waste Cooking Oil Using Multi-Enzyme Systems through Response Surface Methodology. Renew. Energy 2017, 105, 465–472. [Google Scholar] [CrossRef]
- Filho, D.G.; Silva, A.G.; Guidini, C.Z. Lipases: Sources, Immobilization Methods, and Industrial Applications. Appl Microbiol. Biotechnol. 2019, 103, 7399–7423. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.-H.; Lee, J.; Kim, B.C.; Lee, J.E.; Joo, J.; Park, H.; Lee, J.H.; Lee, S.-M.; Lee, D.; Kim, S.; et al. Highly Efficient Enzyme Immobilization and Stabilization within Meso-Structured Onion-Like Silica for Biodiesel Production. Chem. Mater. 2012, 24, 924–929. [Google Scholar] [CrossRef]
- Li, S.-F.; Fan, Y.-H.; Hu, R.-F.; Wu, W.-T. Pseudomonas Cepacia Lipase Immobilized onto the Electrospun PAN Nanofibrous Membranes for Biodiesel Production from Soybean Oil. J. Mol. Catal. B Enzym. 2011, 72, 40–45. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, H.; Lu, H.; Wu, M.; Lin, M.; Zhang, C.; Zhao, Z.; Li, W.; Zhang, C.; Li, X.; et al. Characterization of an Aspergillus niger for Efficient Fatty Acid Ethyl Ester Synthesis in Aqueous Phase and the Molecular Mechanism. Front. Microbiol. 2022, 12, 820380. [Google Scholar] [CrossRef] [PubMed]
- Bencze, L.C.; Bartha-Vári, J.H.; Katona, G.; Toşa, M.I.; Paizs, C.; Irimie, F.-D. Nanobioconjugates of Candida antarctica Lipase B and Single-Walled Carbon Nanotubes in Biodiesel Production. Bioresour. Technol. 2016, 200, 853–860. [Google Scholar] [CrossRef]
- Cubides-Roman, D.C.; Pérez, V.H.; De Castro, H.F.; Orrego, C.E.; Giraldo, O.H.; Silveira, E.G.; David, G.F. Ethyl Esters (Biodiesel) Production by Pseudomonas fluorescens Lipase Immobilized on Chitosan with Magnetic Properties in a Bioreactor Assisted by Electromagnetic Field. Fuel 2017, 196, 481–487. [Google Scholar] [CrossRef]
- Liu, L.; Shih, Y.; Liu, W.; Lin, C.; Huang, H. Enzyme Immobilized on Nanoporous Carbon Derived from Metal-Organic Framework: A New Support for Biodiesel Synthesis. ChemSusChem 2017, 10, 1364–1369. [Google Scholar] [CrossRef]
- Rafiei, S.; Tangestaninejad, S.; Horcajada, P.; Moghadam, M.; Mirkhani, V.; Mohammadpoor-Baltork, I.; Kardanpour, R.; Zadehahmadi, F. Efficient Biodiesel Production Using a lipase@ZIF-67 Nanobioreactor. Chem. Eng. J. 2018, 334, 1233–1241. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, V.; Dubey, V.K.; Srivastava, A.; Garg, S.K.; Singh, V.P.; Arora, P.K. Industrial Applications of Fungal Lipases: A Review. Front. Microbiol. 2023, 14, 1142536. [Google Scholar] [CrossRef] [PubMed]
- Qu, P.; Li, D.; Lazim, R.; Xu, R.; Xiao, D.; Wang, F.; Li, X.; Zhang, Y. Improved Thermostability of Thermomyces lanuginosus Lipase by Molecular Dynamics Simulation and in Silico Mutation Prediction and Its Application in Biodiesel Production. Fuel 2022, 327, 125039. [Google Scholar] [CrossRef]
- Sarmah, N.; Revathi, D.; Sheelu, G.; Yamuna Rani, K.; Sridhar, S.; Mehtab, V.; Sumana, C. Recent Advances on Sources and Industrial Applications of Lipases. Biotechnol. Prog. 2018, 34, 5–28. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Accelerating Transesterification Reaction with Biodiesel as Co-Solvent: A Case Study for Solid Acid Sulfated Tin Oxide Catalyst. Fuel 2010, 89, 3866–3870. [Google Scholar] [CrossRef]
- Farobie, O.; Matsumura, Y. State of the Art of Biodiesel Production under Supercritical Conditions. Prog. Energy Combust. Sci. 2017, 63, 173–203. [Google Scholar] [CrossRef]
- Balcão, V.M.; Paiva, A.L.; Xavier Malcata, F. Bioreactors with Immobilized Lipases: State of the Art. Enzyme Microb. Technol. 1996, 18, 392–416. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, M.; Aghbashlo, M.; Dehhaghi, M.; Panahi, H.K.S.; Mollahosseini, A.; Hosseini, M.; Soufiyan, M.M. Reactor Technologies for Biodiesel Production and Processing: A Review. Prog. Energy Combust. Sci. 2019, 74, 239–303. [Google Scholar] [CrossRef]
- Poppe, J.K.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A.Z. Enzymatic Reactors for Biodiesel Synthesis: Present Status and Future Prospects. Biotechnol. Adv. 2015, 33, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Singh, D. Biodiesel Production through the Use of Different Sources and Characterization of Oils and Their Esters as the Substitute of Diesel: A Review. Renew. Sustain. Energy Rev. 2010, 14, 200–216. [Google Scholar] [CrossRef]
- Budžaki, S.; Miljić, G.; Sundaram, S.; Tišma, M.; Hessel, V. Cost Analysis of Enzymatic Biodiesel Production in Small-Scaled Packed-Bed Reactors. Appl. Energ. 2018, 210, 268–278. [Google Scholar] [CrossRef]
- Fernandes, P. Enzymes in Food Processing: A Condensed Overview on Strategies for Better Biocatalysts. Enzyme Res. 2010, 2010, 862537. [Google Scholar] [CrossRef] [PubMed]
- Ambat, I.; Srivastava, V.; Sillanpää, M. Recent Advancement in Biodiesel Production Methodologies Using Various Feedstock: A Review. Renew. Sustain. Energy Rev. 2018, 90, 356–369. [Google Scholar] [CrossRef]
- Sarno, M.; Iuliano, M. Highly Active and Stable Fe3O4/Au Nanoparticles Supporting Lipase Catalyst for Biodiesel Production from Waste Tomato. Appl. Surf. Sci. 2019, 474, 135–146. [Google Scholar] [CrossRef]
- Xie, W.; Wang, J. Enzymatic Production of Biodiesel from Soybean Oil by Using Immobilized Lipase on Fe3O4/Poly(Styrene-Methacrylic Acid) Magnetic Microsphere as a Biocatalyst. Energ. Fuels 2014, 28, 2624–2631. [Google Scholar] [CrossRef]
- Carlucci, C. An Overview on the Production of Biodiesel Enabled by Continuous Flow Methodologies. Catalysts 2022, 12, 717. [Google Scholar] [CrossRef]
- Narayanan, C. Performance Characteristics of Circulating Fluidized Bed Bioreactors Employing Immobilized Enzyme Nanoparticles. IUP J. Chem. Eng. 2011, 3, 1–10. [Google Scholar]
- Narayanan, C.M.; Pandey, A. Studies on Biodiesel Synthesis Using Nanosilica Immobilised Lipase in Inverse Fluidized Bed Bioreactors. J. Adv. Chem. 2018, 15, 6072–6086. [Google Scholar] [CrossRef]
- Ramos, L.; Martin, L.S.; Santos, J.C.; De Castro, H.F. Combined Use of a Two-Stage Packed Bed Reactor with a Glycerol Extraction Column for Enzymatic Biodiesel Synthesis from Macaw Palm Oil. Ind. Eng. Chem. Res. 2017, 56, 1–7. [Google Scholar] [CrossRef]
- Wang, X.; Dou, P.; Zhao, P.; Zhao, C.; Ding, Y.; Xu, P. Immobilization of Lipases onto Magnetic Fe3O4 Nanoparticles for Application in Biodiesel Production. ChemSusChem 2009, 2, 947–950. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Zhao, C.; Ding, Y.; Xu, P. Biodiesel Production in Packed-Bed Reactors Using Lipase-Nanoparticle Biocomposite. Bioresour. Technol. 2011, 102, 6352–6355. [Google Scholar] [CrossRef]
- Narayanan, C.M.; Monangi, T.; Prasad, H.; Chakraborty, N. Studies on Performance Analysis and Computer Aided Design of Inverse Fluidized Bed Bioreactors with Nanosupport Particles. Int. J. Chem. React. Eng. 2014, 12, 303–315. [Google Scholar] [CrossRef]
- Zhou, G.; Chen, G.; Yan, B. Biodiesel Production in a Magnetically-Stabilized, Fluidized Bed Reactor with an Immobilized Lipase in Magnetic Chitosan Microspheres. Biotechnol. Lett. 2014, 36, 63–68. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Karemore, A.; Das, S.; Deysarkar, A.; Sen, R. Biocatalytic Production of Biodiesel from Cottonseed Oil: Standardization of Process Parameters and Comparison of Fuel Characteristics. Appl. Energy 2011, 88, 1251–1256. [Google Scholar] [CrossRef]
- Li, L.; Du, W.; Liu, D.; Wang, L.; Li, Z. Lipase-Catalyzed Transesterification of Rapeseed Oils for Biodiesel Production with a Novel Organic Solvent as the Reaction Medium. J. Mol. Catal. B Enzym. 2006, 43, 58–62. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.B.; Kang, S.W.; Song, Y.S.; Park, C.; Han, S.O.; Kim, S.W. Biodiesel Production by a Mixture of Candida rugosa and Rhizopus Oryzae Lipases Using a Supercritical Carbon Dioxide Process. Bioresour. Technol. 2011, 102, 2105–2108. [Google Scholar] [CrossRef] [PubMed]
- Szczęsna Antczak, M.; Kubiak, A.; Antczak, T.; Bielecki, S. Enzymatic Biodiesel Synthesis-Key Factors Affecting Efficiency of the Process. Renew. Energy 2009, 34, 1185–1194. [Google Scholar] [CrossRef]
- Gamba, M.; Lapis, A.A.M.; Dupont, J. Supported Ionic Liquid Enzymatic Catalysis for the Production of Biodiesel. Adv. Synth. Catal. 2008, 350, 160–164. [Google Scholar] [CrossRef]
- Lozano, P.; Bernal, J.M.; Vaultier, M. Towards Continuous Sustainable Processes for Enzymatic Synthesis of Biodiesel in Hydrophobic Ionic Liquids/Supercritical Carbon Dioxide Biphasic Systems. Fuel 2011, 90, 3461–3467. [Google Scholar] [CrossRef]
- Pandit, C.; Banerjee, S.; Pandit, S.; Lahiri, D.; Kumar, V.; Chaubey, K.K.; Al-Balushi, R.; Al-Bahry, S.; Joshi, S.J. Recent Advances and Challenges in the Utilization of Nanomaterials in Transesterification for Biodiesel Production. Heliyon 2023, 9, e15475. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, A.; Ivars, F.; Luque, R. Advances in Nanocatalyst Design for Biofuel Production. ChemCatChem 2018, 10, 1968–1981. [Google Scholar] [CrossRef]
- Hama, S.; Kondo, A. Enzymatic Biodiesel Production: An Overview of Potential Feedstocks and Process Development. Bioresour. Technol. 2013, 135, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Fernandes, C.D.; Mehmood, T.; Nadeem, F.; Tabassam, Q.; Ferreira, L.F.R. Immobilized Lipases-Based Nano-Biocatalytic Systems—A Versatile Platform with Incredible Biotechnological Potential. Int. J. Biol. Macromol. 2021, 175, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Matveeva, V.G.; Bronstein, L.M. Magnetic Nanoparticle-Containing Supports as Carriers of Immobilized Enzymes: Key Factors Influencing the Biocatalyst Performance. Nanomaterials 2021, 11, 2257. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-S.; Zhu, J.-P.; Wang, Y.; Yu, Y.; Zhao, Z. Recent Progress in Magnetic Nanoparticles and Mesoporous Materials for Enzyme Immobilization: An Update. Braz. J. Biol. 2022, 82, e244496. [Google Scholar] [CrossRef] [PubMed]
- Vasić, K.; Hojnik Podrepšek, G.; Knez, Ž.; Leitgeb, M. Biodiesel Production Using Solid Acid Catalysts Based on Metal Oxides. Catalysts 2020, 10, 237. [Google Scholar] [CrossRef]
- Krishnan, S.; Diagaradjane, P.; Cho, S.H. Nanoparticle-Mediated Thermal Therapy: Evolving Strategies for Prostate Cancer Therapy. Int. J. Hyperthermia 2010, 26, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Aliru, M.L.; Chadha, A.S.; Asea, A.; Krishnan, S. Hyperthermia Using Nanoparticles-Promises and Pitfalls. Int. J. Hyperthermia 2016, 32, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Zhao, Y.; Rasheed, T.; Iqbal, H.M.N. Magnetic Nanoparticles as Versatile Carriers for Enzymes Immobilization: A Review. Int. J. Biol. Macromol. 2018, 120, 2530–2544. [Google Scholar] [CrossRef]
- Liu, D.-M.; Chen, J.; Shi, Y.-P. Advances on Methods and Easy Separated Support Materials for Enzymes Immobilization. TrAC Trends Anal. Chem. 2018, 102, 332–342. [Google Scholar] [CrossRef]
- Xu, J.; Sun, J.; Wang, Y.; Sheng, J.; Wang, F.; Sun, M. Application of Iron Magnetic Nanoparticles in Protein Immobilization. Molecules 2014, 19, 11465–11486. [Google Scholar] [CrossRef] [PubMed]
- Hajareh Haghighi, F.; Binaymotlagh, R.; Chronopoulou, L.; Cerra, S.; Marrani, A.G.; Amato, F.; Palocci, C.; Fratoddi, I. Self-Assembling Peptide-Based Magnetogels for the Removal of Heavy Metals from Water. Gels 2023, 9, 621. [Google Scholar] [CrossRef] [PubMed]
- Hajareh Haghighi, F.; Mercurio, M.; Cerra, S.; Salamone, T.A.; Binaymotlagh, R.; Palocci, C.; Romano Spica, V.; Fratoddi, I. Surface Modification of TiO2 Nanoparticles with Organic Molecules and Their Biological Applications. J. Mater. Chem. B 2023, 11, 2334–2366. [Google Scholar] [CrossRef] [PubMed]
- Brandão Júnior, J.; Andrade Do Nascimento, J.G.; França Silva, M.P.; Lima Brandão, E.D.A.; De Castro Bizerra, V.; Dos Santos, K.M.; Serpa, J.D.F.; Santos, J.C.S.D.; Da Fonseca, A.M.; Vasconcelos De Oliveira, D.L.; et al. Performance of Eversa Transform 2.0 Lipase in Ester Production Using Babassu Oil (Orbignya Sp.) and Tucuman Oil (Astrocaryum vulgar): A Comparative Study between Liquid and Immobilized Forms in Fe3O4 Nanoparticles. Catalysts 2023, 13, 571. [Google Scholar] [CrossRef]
- Bauer, L.C.; Santos, L.S.; Sampaio, K.A.; Ferrão, S.P.B.; Fontan, R.D.C.I.; Minim, L.A.; Veloso, C.M.; Bonomo, R.C.F. Physicochemical and Thermal Characterization of Babassu Oils (Orbignya phalerata Mart.) Obtained by Different Extraction Methods. Food Res. Int. 2020, 137, 109474. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.Y.; Chan, E.-S.; Song, C.P. Biodiesel Production Catalysed by Low-Cost Liquid Enzyme Eversa® Transform 2.0: Effect of Free Fatty Acid Content on Lipase Methanol Tolerance and Kinetic Model. Fuel 2021, 283, 119266. [Google Scholar] [CrossRef]
- Parandi, E.; Safaripour, M.; Abdellattif, M.H.; Saidi, M.; Bozorgian, A.; Rashidi Nodeh, H.; Rezania, S. Biodiesel Production from Waste Cooking Oil Using a Novel Biocatalyst of Lipase Enzyme Immobilized Magnetic Nanocomposite. Fuel 2022, 313, 123057. [Google Scholar] [CrossRef]
- Lozano, P.; Bernal, J.M.; Sánchez-Gómez, G.; López-López, G.; Vaultier, M. How to Produce Biodiesel Easily Using a Green Biocatalytic Approach in Sponge-like Ionic Liquids. Energy Environ. Sci. 2013, 6, 1328. [Google Scholar] [CrossRef]
- Ciftci, O.N.; Temelli, F. Continuous Production of Fatty Acid Methyl Esters from Corn Oil in a Supercritical Carbon Dioxide Bioreactor. J. Supercrit. Fluids 2011, 58, 79–87. [Google Scholar] [CrossRef]
- Wu, X.; Xiong, J.; Huang, Z.; Cao, S.; Zong, M.; Lou, W. Improving Biocatalysis of Cefaclor with Penicillin acylase Immobilized on Magnetic Nanocrystalline Cellulose in Deep Eutectic Solvent Based Co-Solvent. Bioresour. Technol. 2019, 288, 121548. [Google Scholar] [CrossRef] [PubMed]
- Elgharbawy, A.A.; Riyadi, F.A.; Alam, M.Z.; Moniruzzaman, M. Ionic Liquids as a Potential Solvent for Lipase-Catalysed Reactions: A Review. J. Mol. Liq. 2018, 251, 150–166. [Google Scholar] [CrossRef]
- Itoh, T.; Takagi, Y. Laccase-Catalyzed Reactions in Ionic Liquids for Green Sustainable Chemistry. ACS Sustain. Chem. Eng. 2021, 9, 1443–1458. [Google Scholar] [CrossRef]
- Taher, H.; Al-Zuhair, S. The Use of Alternative Solvents in Enzymatic Biodiesel Production: A Review. Biofuels Bioprod. Bioref. 2017, 11, 168–194. [Google Scholar] [CrossRef]
- Patel, A.; Arora, N.; Sartaj, K.; Pruthi, V.; Pruthi, P.A. Sustainable Biodiesel Production from Oleaginous Yeasts Utilizing Hydrolysates of Various Non-Edible Lignocellulosic Biomasses. Renew. Sustain. Energy Rev. 2016, 62, 836–855. [Google Scholar] [CrossRef]
- Xing, X.; Zhou, Z.-W.; Wei, Y.-J.; Liu, Y.-H.; Li, K.; Yu, X.-Q.; Wang, N. An Efficient and Stable Magnetic Nano-Biocatalyst for Biodiesel Synthesis in Recyclable Ionic Liquids. Biomass Conv. Bioref. 2023, 13, 11947–11957. [Google Scholar] [CrossRef]
- Lv, L.; Dai, L.; Du, W.; Liu, D. Progress in Enzymatic Biodiesel Production and Commercialization. Processes 2021, 9, 355. [Google Scholar] [CrossRef]
- Zhang, C.; Xing, X.-H. Enzyme Bioreactors. In Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 319–329. [Google Scholar] [CrossRef]
- Allain, J. Biosafty and the Environmental Uses of Micro-Organisms; OECD: Paris, France, 2015. [Google Scholar] [CrossRef]
- Bento, H.B.S.; Reis, C.E.R.; Pinto, P.A.; Cortez, D.V.; Vilas Bôas, R.N.; Costa-Silva, T.A.; Carvalho, A.K.F.; De Castro, H.F. Continuous Synthesis of Biodiesel from Outstanding Kernel Oil in a Packed Bed Reactor Using Burkholderia cepacia Lipase Immobilized on Magnetic Nanosupport. Catal. Lett. 2022, 152, 2434–2444. [Google Scholar] [CrossRef]
- Cao, X.; Xu, H.; Li, F.; Zou, Y.; Ran, Y.; Ma, X.; Cao, Y.; Xu, Q.; Qiao, D.; Cao, Y. One-Step Direct Transesterification of Wet Yeast for Biodiesel Production Catalyzed by Magnetic Nanoparticle-Immobilized Lipase. Renew. Energy 2021, 171, 11–21. [Google Scholar] [CrossRef]
- Asmat, S.; Husain, Q. Exquisite Stability and Catalytic Performance of Immobilized Lipase on Novel Fabricated Nanocellulose Fused Polypyrrole/Graphene Oxide Nanocomposite: Characterization and Application. Int. J. Biol. Macromol. 2018, 117, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Clohessy, J.; Kwapinski, W. Carbon-Based Catalysts for Biodiesel Production-A Review. Appl. Sci. 2020, 10, 918. [Google Scholar] [CrossRef]
- Nematian, T.; Shakeri, A.; Salehi, Z.; Saboury, A.A. Lipase Immobilized on Functionalized Superparamagnetic Few-Layer Graphene Oxide as an Efficient Nanobiocatalyst for Biodiesel Production from Chlorella Vulgaris Bio-Oil. Biotechnol. Biofuels 2020, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Nematian, T.; Salehi, Z.; Shakeri, A. Conversion of Bio-Oil Extracted from Chlorella Vulgaris Microalgae to Biodiesel via Modified Superparamagnetic Nano-Biocatalyst. Renew. Energy 2020, 146, 1796–1804. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, T.; Zhu, Y.; Hong, L.; Li, T.; Wang, X.; Fu, Y. Lipases Immobilized on the Modified Polyporous Magnetic Cellulose Support as an Efficient and Recyclable Catalyst for Biodiesel Production from Yellow Horn Seed Oil. Renew. Energy 2020, 145, 1246–1254. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, J.-K.; Cho, H.-J.; Yeo, Y.-K. Techno-Economic Study of a Biodiesel Production from Palm Fatty Acid Distillate. Ind. Eng. Chem. Res. 2013, 52, 462–468. [Google Scholar] [CrossRef]
- Hidayat, A.; Rochmadi; Wijaya, K.; Budiman, A. Esterification of Free Fatty Acid on Palm Fatty Acid Distillate Using Activated Carbon Catalysts Generated from Coconut Shell. Procedia Chem. 2015, 16, 365–371. [Google Scholar] [CrossRef]
- Buchori, L.; Widayat, W.; Hadiyanto, H.; Satriadi, H.; Chasanah, N.; Kurniawan, M.R. Modification of Magnetic Nanoparticle Lipase Catalyst with Impregnation of Activated Carbon Oxide (ACO) in Biodiesel Production from PFAD (Palm Fatty Acid Distillate). Bioresour. Technol. Rep. 2022, 19, 101137. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, R.; Liu, M.; Ma, L.; Zhang, W. Co-Immobilization of Lipases with Different Specificities for Efficient and Recyclable Biodiesel Production from Waste Oils: Optimization Using Response Surface Methodology. Int. J. Mol. Sci. 2023, 24, 4726. [Google Scholar] [CrossRef]
- Ashjari, M.; Garmroodi, M.; Amiri Asl, F.; Emampour, M.; Yousefi, M.; Pourmohammadi Lish, M.; Habibi, Z.; Mohammadi, M. Application of Multi-Component Reaction for Covalent Immobilization of Two Lipases on Aldehyde-Functionalized Magnetic Nanoparticles; Production of Biodiesel from Waste Cooking Oil. Process Biochem. 2020, 90, 156–167. [Google Scholar] [CrossRef]
- Parandi, E.; Safaripour, M.; Mosleh, N.; Saidi, M.; Rashidi Nodeh, H.; Oryani, B.; Rezania, S. Lipase Enzyme Immobilized over Magnetic Titanium Graphene Oxide as Catalyst for Biodiesel Synthesis from Waste Cooking Oil. Biomass Bioenergy 2023, 173, 106794. [Google Scholar] [CrossRef]
- Soltani, S.; Khanian, N.; Shean Yaw Choong, T.; Asim, N.; Zhao, Y. Microwave-Assisted Hydrothermal Synthesis of Sulfonated TiO2-GO Core–Shell Solid Spheres as Heterogeneous Esterification Mesoporous Catalyst for Biodiesel Production. Energy Convers. Manag. 2021, 238, 114165. [Google Scholar] [CrossRef]
- Borah, M.J.; Devi, A.; Saikia, R.A.; Deka, D. Biodiesel Production from Waste Cooking Oil Catalyzed by In-Situ Decorated TiO2 on Reduced Graphene Oxide Nanocomposite. Energy 2018, 158, 881–889. [Google Scholar] [CrossRef]
- Jume, B.H.; Valizadeh Dana, N.; Rastin, M.; Parandi, E.; Darajeh, N.; Rezania, S. Sulfur-Doped Binary Layered Metal Oxides Incorporated on Pomegranate Peel-Derived Activated Carbon for Removal of Heavy Metal Ions. Molecules 2022, 27, 8841. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Jiao, X.; Hu, H.; Shen, X.; Zhao, J.; Feng, Y.; Li, C.; Du, Y.; Cui, J.; Jia, S. Activated Magnetic Lipase-Inorganic Hybrid Nanoflowers: A Highly Active and Recyclable Nanobiocatalyst for Biodiesel Production. Renew. Energy 2021, 171, 825–832. [Google Scholar] [CrossRef]
- Sarnoab, M.; Iulianoc, M. Enzymatic Production of Biodiesel from Grapeseed Oil. Chem. Eng. Trans. 2020, 80, 301–306. [Google Scholar] [CrossRef]
- EN 14214:2012+A2:2019; Liquid Petroleum Products. Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications. Requirements and Test Methods. European Standards: Plzen, Czech Republic, 2021. Available online: https://www.en-standard.eu/bs-en-14214-2012-a2-2019-liquid-petroleum-products-fatty-acid-methyl-esters-fame-for-use-in-diesel-engines-and-heating-applications-requirements-and-test-methods/ (accessed on 19 May 2024).
- Sarnoab, M.; Iuliano, M. Biodiesel Production from Dairy Waste Scum by Using an Efficient Nano-Biocatalyst. Chem. Eng. Trans. 2020, 79, 181–186. [Google Scholar] [CrossRef]
- Sarno, M.; Iuliano, M. Biodiesel Production from Waste Cooking Oil. Green Process. Synth. 2019, 8, 828–836. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Shen, S.; Zhang, B.; Yu, W.W. Magnetic Responsive Thermomyces lanuginosus Lipase for Biodiesel Synthesis. Mater. Today Commun. 2020, 24, 101197. [Google Scholar] [CrossRef] [PubMed]
- Mannina, L.; Luchinat, C.; Emanuele, M.C.; Segre, A. Acyl Positional Distribution of Glycerol Tri-Esters in Vegetable Oils: A 13C NMR Study. Chem. Phys. Lipids 1999, 103, 47–55. [Google Scholar] [CrossRef]
- Xu, Y.; Du, W.; Zeng, J.; Liu, D. Conversion of Soybean Oil to Biodiesel Fuel Using Lipozyme TL IM in a Solvent-Free Medium. Biocatal. Biotransfor. 2004, 22, 45–48. [Google Scholar] [CrossRef]
- Aggarwal, S.; Ikram, S. Zinc Oxide Nanoparticles-Impregnated Chitosan Surfaces for Covalent Immobilization of Trypsin: Stability & Kinetic Studies. Int. J. Biol. Macromol. 2022, 207, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.L.; Kumar, S.; Das, A.; Randhawa, J.S.; Chamundeeswari, M. Chitin and Chitosan-Based Support Materials for Enzyme Immobilization and Biotechnological Applications. Environ. Chem. Lett. 2020, 18, 315–323. [Google Scholar] [CrossRef]
- Wang, X.; Shi, J.; Li, Z.; Zhang, S.; Wu, H.; Jiang, Z.; Yang, C.; Tian, C. Facile One-Pot Preparation of Chitosan/Calcium Pyrophosphate Hybrid Microflowers. ACS Appl. Mater. Interfaces 2014, 6, 14522–14532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, C.; Yin, C. Galactosylated Trimethyl Chitosan-Cysteine Nanoparticles Loaded with Map4k4 siRNA for Targeting Activated Macrophages. Biomaterials 2013, 34, 3667–3677. [Google Scholar] [CrossRef] [PubMed]
- Bagaria, H.G.; Wong, M.S. Polyamine-Salt Aggregate Assembly of Capsules as Responsive Drug Delivery Vehicles. J. Mater. Chem. 2011, 21, 9454. [Google Scholar] [CrossRef]
- Wei, H.; Wang, Q.; Zhang, R.; Liu, M.; Zhang, W. Efficient Biodiesel Production from Waste Cooking Oil by Fast Co-Immobilization of Lipases from Aspergillus oryzae and Rhizomucor miehei in Magnetic Chitosan Microcapsules. Process Biochem. 2023, 125, 171–180. [Google Scholar] [CrossRef]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile Conjugation of Biomolecules onto Surfaces via Mussel Adhesive Protein Inspired Coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Rivera, J.G.; He, L.; Kulkarni, H.; Lee, D.-K.; Messersmith, P.B. Facile, High Efficiency Immobilization of Lipase Enzyme on Magnetic Iron Oxide Nanoparticles via a Biomimetic Coating. BMC Biotechnol. 2011, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Touqeer, T.; Mumtaz, M.W.; Mukhtar, H.; Irfan, A.; Akram, S.; Shabbir, A.; Rashid, U.; Nehdi, I.A.; Choong, T.S.Y. Fe3O4-PDA-Lipase as Surface Functionalized Nano Biocatalyst for the Production of Biodiesel Using Waste Cooking Oil as Feedstock: Characterization and Process Optimization. Energies 2019, 13, 177. [Google Scholar] [CrossRef]
- Chiu, M.C.; Gioielli, L.A. Conteúdo de Gordura Sólida Da Gordura Abdominal de Frango, de Suas Estearinas e de Suas Misturas Binárias Com Toucinho. Ciênc. Tecnol. Aliment. 2002, 22, 151–157. [Google Scholar] [CrossRef]
- Shafiq, F.; Mumtaz, M.W.; Mukhtar, H.; Touqeer, T.; Raza, S.A.; Rashid, U.; Nehdi, I.A.; Choong, T.S.Y. Response Surface Methodology Approach for Optimized Biodiesel Production from Waste Chicken Fat Oil. Catalysts 2020, 10, 633. [Google Scholar] [CrossRef]
- Xie, W.; Huang, M. Fabrication of Immobilized Candida rugosa Lipase on Magnetic Fe3O4-Poly(Glycidyl Methacrylate-Co-Methacrylic Acid) Composite as an Efficient and Recyclable Biocatalyst for Enzymatic Production of Biodiesel. Renew. Energy 2020, 158, 474–486. [Google Scholar] [CrossRef]
- Tang, A.; Zhang, Y.; Wei, T.; Wu, J.; Li, Q.; Liu, Y. Immobilization of Candida cylindracea Lipase by Covalent Attachment on Glu-Modified Bentonite. Appl. Biochem. Biotechnol. 2019, 187, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Christopher, L.P.; Kumar, H.; Zambare, V. Enzymatic Biodiesel: Challenges and Opportunities. Appl. Energy 2014, 119, 497–520. [Google Scholar] [CrossRef]
- Zhang, D.-H.; Peng, L.-J.; Wang, Y.; Li, Y.-Q. Lipase Immobilization on Epoxy-Activated Poly(Vinyl Acetate-Acrylamide) Microspheres. Colloids Surf. B Biointerfaces 2015, 129, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Ma, N. Immobilized Lipase on Fe3O4 Nanoparticles as Biocatalyst for Biodiesel Production. Energ. Fuels 2009, 23, 1347–1353. [Google Scholar] [CrossRef]
- Peffi Ferreira, L.F.; Mazzi De Oliveira, T.; Toma, S.H.; Toyama, M.M.; Araki, K.; Avanzi, L.H. Superparamagnetic Iron Oxide Nanoparticles (SPIONs) Conjugated with Lipase Candida antarctica A for Biodiesel Synthesis. RSC Adv. 2020, 10, 38490–38496. [Google Scholar] [CrossRef] [PubMed]
- Esmi, F.; Nematian, T.; Salehi, Z.; Khodadadi, A.A.; Dalai, A.K. Amine and Aldehyde Functionalized Mesoporous Silica on Magnetic Nanoparticles for Enhanced Lipase Immobilization, Biodiesel Production, and Facile Separation. Fuel 2021, 291, 120126. [Google Scholar] [CrossRef]
- Adnan, M.; Li, K.; Xu, L.; Yan, Y. X-Shaped ZIF-8 for Immobilization Rhizomucor miehei Lipase via Encapsulation and Its Application toward Biodiesel Production. Catalysts 2018, 8, 96. [Google Scholar] [CrossRef]
- Babaki, M.; Yousefi, M.; Habibi, Z.; Mohammadi, M.; Yousefi, P.; Mohammadi, J.; Brask, J. Enzymatic Production of Biodiesel Using Lipases Immobilized on Silica Nanoparticles as Highly Reusable Biocatalysts: Effect of Water, t-Butanol and Blue Silica Gel Contents. Renew. Energy 2016, 91, 196–206. [Google Scholar] [CrossRef]
- Miao, C.; Yang, L.; Wang, Z.; Luo, W.; Li, H.; Lv, P.; Yuan, Z. Lipase Immobilization on Amino-Silane Modified Superparamagnetic Fe3O4 Nanoparticles as Biocatalyst for Biodiesel Production. Fuel 2018, 224, 774–782. [Google Scholar] [CrossRef]
- Thangaraj, B.; Jia, Z.; Dai, L.; Liu, D.; Du, W. Effect of Silica Coating on Fe3O4 Magnetic Nanoparticles for Lipase Immobilization and Their Application for Biodiesel Production. Arab. J. Chem. 2019, 12, 4694–4706. [Google Scholar] [CrossRef]
- Wan, D.; Yan, C.; Zhang, Q. Facile and Rapid Synthesis of Hollow Magnetic Mesoporous Polydopamine Nanoflowers with Tunable Pore Structures for Lipase Immobilization: Green Production of Biodiesel. Ind. Eng. Chem. Res. 2019, 58, 16358–16369. [Google Scholar] [CrossRef]
- Badoei-dalfard, A.; Malekabadi, S.; Karami, Z.; Sargazi, G. Magnetic Cross-Linked Enzyme Aggregates of Km12 Lipase: A Stable Nanobiocatalyst for Biodiesel Synthesis from Waste Cooking Oil. Renew. Energy 2019, 141, 874–882. [Google Scholar] [CrossRef]
- Cruz-Izquierdo, Á.; Picó, E.A.; López, C.; Serra, J.L.; Llama, M.J. Magnetic Cross-Linked Enzyme Aggregates (mCLEAs) of Candida antarctica Lipase: An Efficient and Stable Biocatalyst for Biodiesel Synthesis. PLoS ONE 2014, 9, e115202. [Google Scholar] [CrossRef]
- Mehde, A.A.; Mehdi, W.A.; Severgün, O.; Çakar, S.; Özacar, M. Lipase-Based on Starch Material as a Development Matrix with Magnetite Cross-Linked Enzyme Aggregates and Its Application. Int. J. Biol. Macromol. 2018, 120, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Badoei-dalfard, A.; Karami, Z.; Malekabadi, S. Construction of CLEAs-Lipase on Magnetic Graphene Oxide Nanocomposite: An Efficient Nanobiocatalyst for Biodiesel Production. Bioresour. Technol. 2019, 278, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Oke, M.A.; Ojo, S.A.; Fasiku, S.A.; Adebayo, E.A. Nanotechnology and Enzyme Immobilization: A review. Nanotechnology 2023, 34, 385101. [Google Scholar] [CrossRef] [PubMed]






| Country | 2022 Share | 2012–2022 Annual Growth Rate |
|---|---|---|
| USA | 19.7% | 0.9% |
| China | 14.7% | 3.6% |
| European Union | 11.1% | −0.4% |
| India | 5.3% | 3.5% |
| Saudi Arabia | 4.0% | 1.1% |
| Russia | 3.7% | 1.2% |
| Japan | 3.4% | −3.3% |
| South Korea | 2.9% | 1.5% |
| Brazil | 2.6% | −0.3% |
| Canada | 2.4% | −0.6% |
| Turkey | 1.1% | −0.6% |
| Mexico | 2.2% | −0.6% |
| New Zealand | 0.2% | −0.1% |
| Luxembourg | 0.1% | −1.6% |
| Estonia | Less than 0.05% | −2.5% |
| OECD * countries | 46.4% | Less than 0.05% |
| Non-OECD countries | 53.6% | 1.8% |
| Global Total | 100% | 0.9% |
| Method | Advantage | Disadvantage |
|---|---|---|
| physical methods (gas-phase deposition) | easy to perform | difficult to control particle size |
| electron beam lithography | well controlled inter-particle spacing | requirement of expensive and highly complex machines |
| wet chemical methods (sol-gel synthesis) | precisely controlled in size, aspect ratio, and internal structure | weak bonding, low wear-resistance, high permeability |
| oxidation method | uniform size and narrow size distribution | small-sized ferrite colloids |
| chemical co-precipitation | simple and efficient | not suitable for the preparation of highly pure, accurate stoichiometric phase |
| hydrothermal reactions | easy to control particle size and shape | high reaction temperature, high pressure |
| flow injection synthesis | good reproducibility and high mixing homogeneity together with a precise control of the process | segmented mixing of reagents under a laminar the flow regime in a capillary reactor |
| electrochemical method | easy to control particle size | reproducibility |
| aerosol/vapour phase method | high yields | extremely high temperatures |
| sonochemical decomposition reactions | narrow particle size distribution | mechanism not yet understood |
| supercritical fluid method | efficient control of the particle size, no organic solvents involved | critical pressure and temperature |
| synthesis using nanoreactors | possibility to precisely control NP size | complex conditions |
| microbial methods (microbial incubation) | high yield, good reproducibility, good scalability, low cost | time-consuming |
| Lipase Source | Nano-Support | Immobilization Methodology | Feedstock | Solvent | Biodiesel Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| Thermomyces lanuginosa | APTES-modified Fe3O4 | covalent attachment | soybean oil | solvent-free | 92.8 | [207] |
| Candida antarctica | APC-modified Fe3O4 (APC: (3,4-dihydroxyaldehyde or protocatechuic aldehyde) | covalent attachment | soybean oil | anhydrous methanol | 4.6 | [208] |
| Rhizopus oryzae | ROL-MNPs@MS-AP-GA | covalent attachment and physical adsorption | olive oil | anhydrous methanol | 88 | [209] |
| Rhizomucor miehei | CRL/MNP@ZIF-8 | encapsulation | soybean oil | - | 84 | [210] |
| Thermomyces lanuginosus Rhizomucor miehei | 3-glycidyloxypropyl trimethoxysilane modified mesoporous silicon | covalent attachment | canola oil | solvent-free | 98 | [211] |
| Candida antarctica | APTES modified Fe3O4 | covalent attachment | rapeseed oil | solvent-free | 89 | [212] |
| Aspergillus niger | Fe3O4 coated with APTES/MPTMS modified mesoporous silicon | covalent attachment | soybean oil | solvent-free | >90 | [213] |
| Candida rugosa | hollow Fe3O4 coated with mesoporous dopamine | physical adsorption | oleic acid | solvent-free | 87 | [214] |
| APTES modified Fe3O4 | cross-link and covalent attachment | waste cooking oil | solvent-free | 71 | [215] | |
| Candida antarctica | APTES modified Fe3O4 | cross-link and covalent attachment | waste frying oils Unrefined soybean oil | solvent-free | 80–92 | [216] |
| Candida antarctica | tannic acid-modified Fe3O4 | cross-link and physical adsorption | sunflower oil | solvent-free | 67 | [217] |
| Enterobacter MG10 | graphene oxide with APTES-modified Fe3O4 | cross-link and covalent attachment | Ricinus communis oil | solvent-free | 78 | [218] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajareh Haghighi, F.; Binaymotlagh, R.; Palocci, C.; Chronopoulou, L. Magnetic Iron Oxide Nanomaterials for Lipase Immobilization: Promising Industrial Catalysts for Biodiesel Production. Catalysts 2024, 14, 336. https://doi.org/10.3390/catal14060336
Hajareh Haghighi F, Binaymotlagh R, Palocci C, Chronopoulou L. Magnetic Iron Oxide Nanomaterials for Lipase Immobilization: Promising Industrial Catalysts for Biodiesel Production. Catalysts. 2024; 14(6):336. https://doi.org/10.3390/catal14060336
Chicago/Turabian StyleHajareh Haghighi, Farid, Roya Binaymotlagh, Cleofe Palocci, and Laura Chronopoulou. 2024. "Magnetic Iron Oxide Nanomaterials for Lipase Immobilization: Promising Industrial Catalysts for Biodiesel Production" Catalysts 14, no. 6: 336. https://doi.org/10.3390/catal14060336
APA StyleHajareh Haghighi, F., Binaymotlagh, R., Palocci, C., & Chronopoulou, L. (2024). Magnetic Iron Oxide Nanomaterials for Lipase Immobilization: Promising Industrial Catalysts for Biodiesel Production. Catalysts, 14(6), 336. https://doi.org/10.3390/catal14060336











