The Effect of Surfactant P123 on the KCuLaZrO2 Catalysts in the Direct Conversion of Syngas to Higher Alcohols
Abstract
:1. Introduction
2. Results
2.1. Catalytic Performance
2.2. Physicochemical Properties of Catalysts
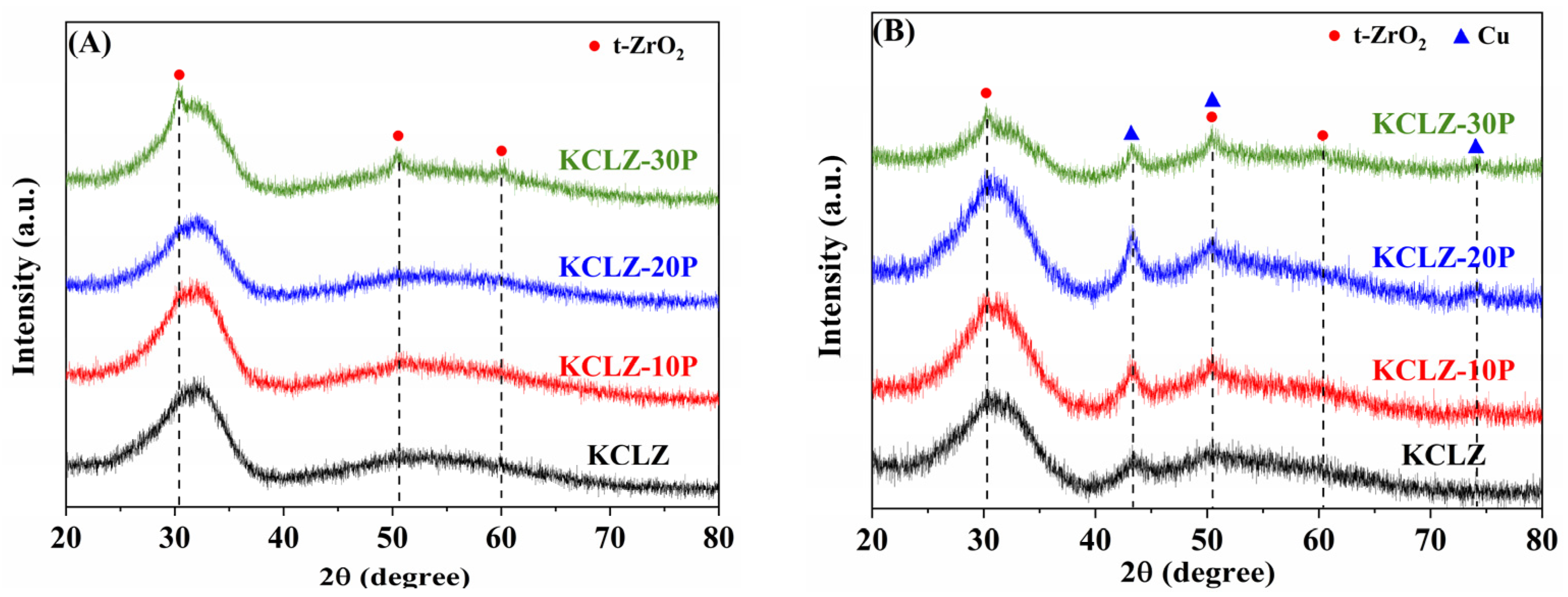
2.3. XRD
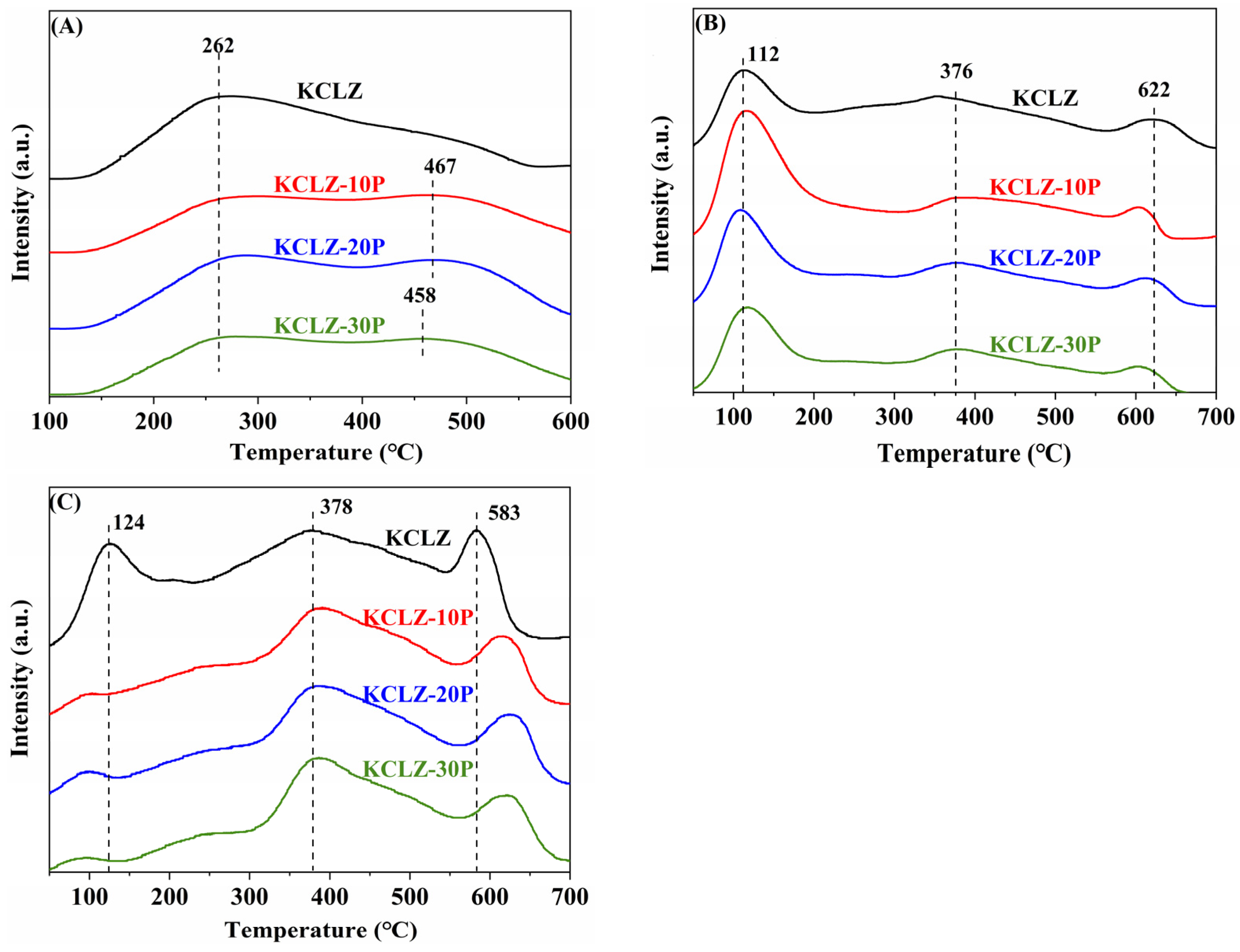
2.4. H2-TPR
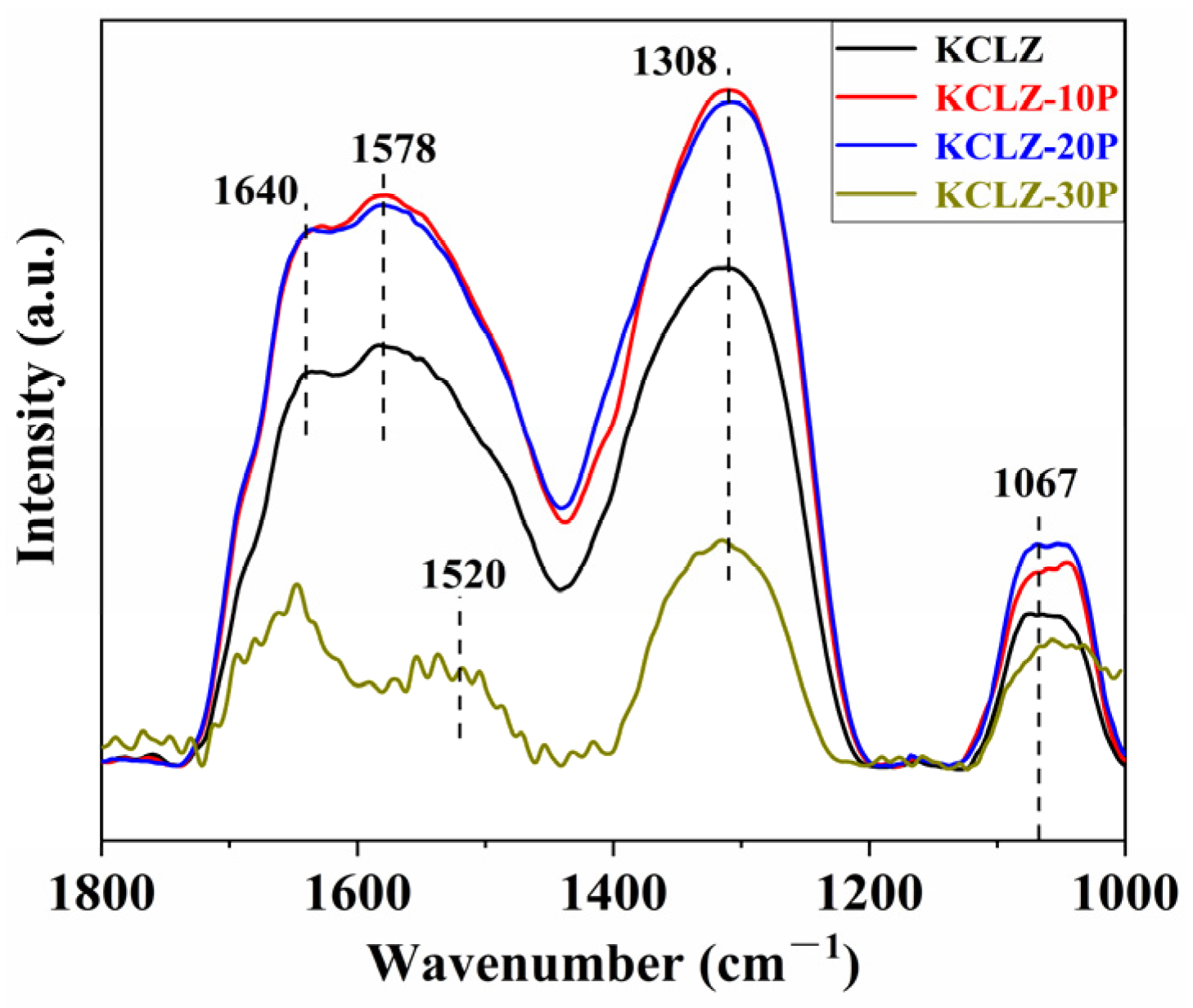
2.5. CO, CO2, NH3-TPD
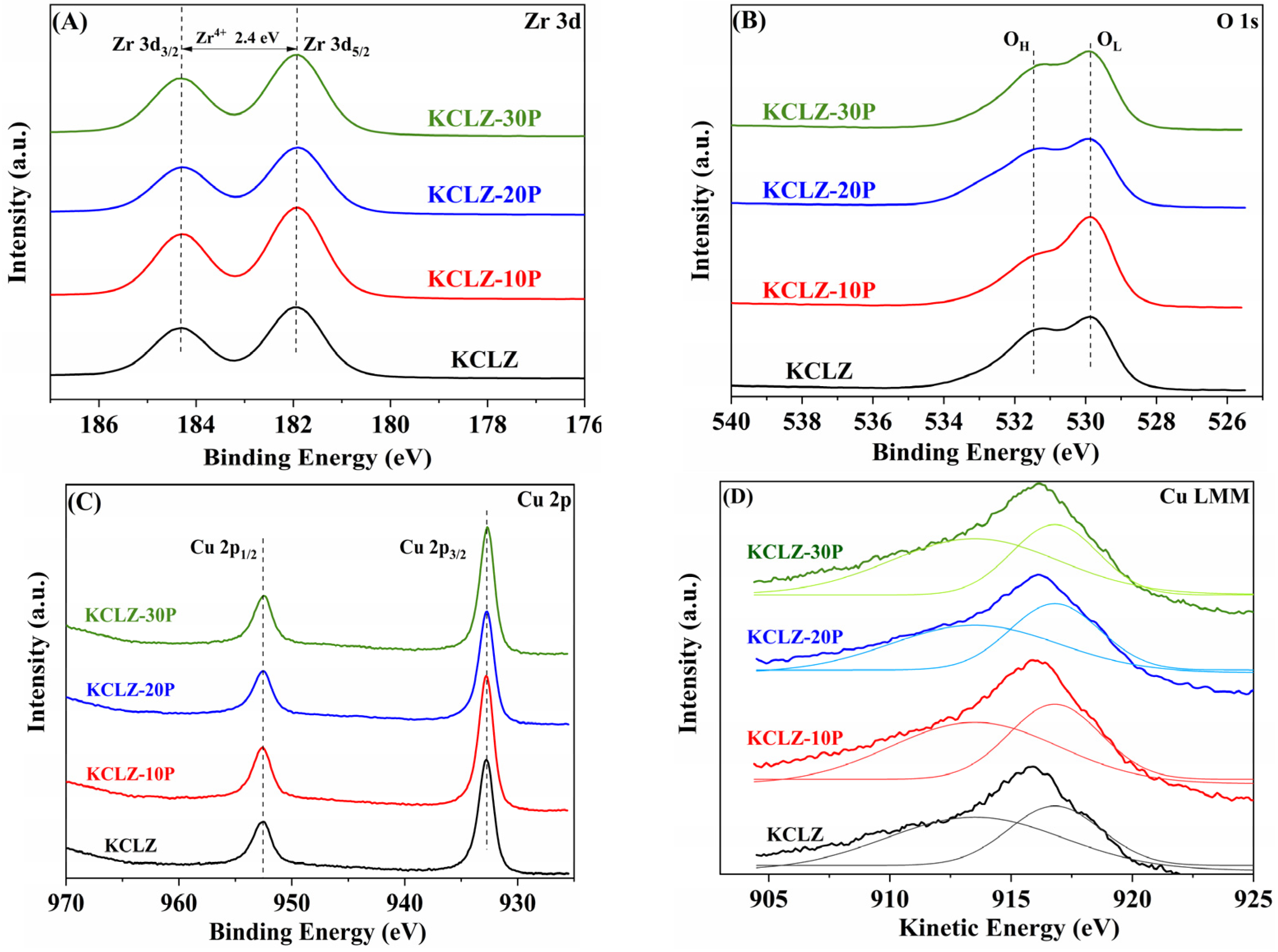
2.6. XPS
2.7. In Situ CO-DRIFTS
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Catalyst Characterization
4.3. Catalytic Performance Evaluation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, G.; Yang, G.; Peng, X.; Wu, J.; Tsubaki, N. Recent advances in the routes and catalysts for ethanol synthesis from syngas. Chem. Soc. Rev. 2022, 51, 5606–5659. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, D.; Zhao, Y.; Liang, Y.; Zhang, L.; Sun, J.; Lin, J.; Wang, S.; Yao, Y.; Wan, S.; et al. Recent advances and perspectives in catalyst design for converting syngas to higher alcohols. Energy Fuels 2024, 38, 14769–14796. [Google Scholar] [CrossRef]
- Gong, N.; Wu, Y.; Ma, Q.; Tan, Y. A simple strategy stabilizing for a CuFe/SiO2 catalyst and boosting higher alcohols’ synthesis from syngas. Catalysts 2023, 13, 237. [Google Scholar] [CrossRef]
- Wang, C.; Lin, T.; Qi, X.; Yu, F.; Lu, Y.; Zhong, L.; Sun, Y. Direct conversion of syngas to higher alcohols over multifunctional catalyst: The role of copper-based component and catalytic mechanism. J. Phys. Chem. C 2021, 125, 6137–6146. [Google Scholar] [CrossRef]
- Shui, M.; Huang, C.; Ma, P.; Li, W.; He, Q.; Wu, W.; Tan, Y.; Bao, J. Accelerating C2+ alcohols synthesis from syngas by simultaneous optimizations of CO dissociation and chain growth over CuCo alloy catalyst. Chin. Chem. Lett. 2021, 32, 2203–2206. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, K.; Fang, Y.; Lv, Q.; Jiang, K.; Zhao, H.; Chen, Y.; Wang, P.; Yang, H.; Wu, L.; et al. Synergetic effect of Ce/Zr for ethanol synthesis from syngas over Rh-based catalyst. Fuel 2023, 334, 126770. [Google Scholar] [CrossRef]
- Gupta, M.; Smith, M.; Spivey, J. Heterogeneous catalytic conversion of dry syngas to ethanol and higher alcohols on Cu-based catalysts. ACS Catal. 2011, 1, 641–656. [Google Scholar] [CrossRef]
- Spivey, J.; Egbebi, A. Heterogeneous catalytic synthesis of ethanol from biomass-derived syngas. Chem. Soc. Rev. 2007, 36, 1514–1528. [Google Scholar] [CrossRef]
- Suárez París, R.; Lopez, L.; Barrientos, J.; Pardo, F.; Boutonnet, M.; Järås, S. Catalytic conversion of biomass-derived synthesis gas to fuels. Catalysis 2015, 27, 62–143. [Google Scholar]
- Yang, J.; Gong, N.; Wang, L.; Wu, Y.; Zhang, T.; Xie, H.; Yang, G.; Tan, Y. Tuning the Cu+ species of Cu-based catalysts for direct synthesis of ethanol from syngas. New. J. Chem. 2021, 45, 20832–20839. [Google Scholar] [CrossRef]
- Yang, J.; Gong, N.; Wang, L.; Wu, Y.; Zhang, T.; Zeng, C.; Tan, Y. Insights into the one-step ethanol synthesis through CO hydrogenation over surfactant-assisted preparation of CuCo/SiO2 catalyst. Fuel 2022, 327, 125078. [Google Scholar] [CrossRef]
- Gong, N.; Zhang, T.; Tan, M.; Wang, L.; Yang, J.; Tan, L.; Yang, G.; Wu, P.; Wu, Y.; Tan, Y. Realizing and revealing complex isobutyl alcohol production over a simple Cu-ZrO2 catalyst. ACS Catal. 2023, 13, 3563–3574. [Google Scholar] [CrossRef]
- Shi, G.; Zhang, X.; Li, X.; Hou, C.; Guo, Y.; Jiao, W. Facile synthesis of super-microporous mesoporous MgO-ZrO2 composites and their adsorption properties for CO2. Chem. Phys. 2024, 583, 112319. [Google Scholar] [CrossRef]
- Fisher, I.; Bell, A. In-Situ infrared study of methanol synthesis from H2/CO2 over Cu/SiO2 and Cu/ZrO2/SiO2. J. Catal. 1997, 172, 222–237. [Google Scholar] [CrossRef]
- Fisher, I.; Woo, H.C.; Bell, A. Effects of zirconia promotion on the activity of Cu/SiO2 for methanol synthesis from CO/H2 and CO2/H2. Catal. Lett. 1997, 44, 11–17. [Google Scholar] [CrossRef]
- Scotti, N.; Zaccheria, F.; Evangelisti, C.; Psaro, R.; Ravasio, N. Dehydrogenative coupling promoted by copper catalysts: A way to optimise and upgrade bio-alcohols. Catal. Sci. Technol. 2017, 7, 1386–1393. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Wang, L.; Gong, N.; Yang, J.; Zhang, T.; Xie, H.; Tan, Y. Effect of iron on ZrO2-based catalysts for direct synthesis of isobutanol from syngas. Fuel 2021, 304, 121342. [Google Scholar] [CrossRef]
- Wu, Y.; Xie, H.; Tian, S.; Tsubaki, N.; Han, Y.; Tan, Y. Isobutanol synthesis from syngas over K-Cu/ZrO2-La2O3(x) catalysts: Effect of La-loading. J. Mol. Catal. A Chem. 2015, 396, 254–260. [Google Scholar] [CrossRef]
- Yang, J.; Gong, N.; Wang, L.; Wu, Y.; Zhang, T.; Tan, Y. Effect of La2O3-decorated SiO2 on the performance of CuCo catalyst for direct conversion of syngas to ethanol. Fuel 2022, 319, 123811. [Google Scholar] [CrossRef]
- Brown Bourzutschky, J.; Homs, N.; Bell, A. Conversion of synthesis gas over LaMn1-xCuxO3+λ perovskites and related copper catalysts. J. Catal. 1990, 124, 52–72. [Google Scholar] [CrossRef]
- Chu, Z.; Chen, H.; Yu, Y.; Wang, Q.; Fang, D. Surfactant-assisted preparation of Cu/ZnO/Al2O3 catalyst for methanol synthesis from syngas. J. Mol. Catal. A Chem. 2013, 366, 48–53. [Google Scholar] [CrossRef]
- Shen, J.; Bai, Y.; Tai, X.; Wang, W.; Wang, G. Surface activity, spreading, and aggregation behavior of ecofriendly perfluoropolyether amide propyl betaine in aqueous solution. ACS Sustain. Chem. Eng. 2018, 6, 6183–6191. [Google Scholar] [CrossRef]
- Marcos, F.; Lin, L.; Betancourt, L.; Senanayake, S.; Rodriguez, J.; Assaf, J.; Giudici, R.; Assaf, E. Insights into the methanol synthesis mechanism via CO2 hydrogenation over Cu-ZnO-ZrO2 catalysts: Effects of surfactant/Cu-Zn-Zr molar ratio. J. CO2 Util. 2020, 41, 101215. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Yin, C.; Wu, Y.; Chai, Y.; Dong, D.; Li, X.; Liu, C. One-pot synthesis of ordered mesoporous NiMo-Al2O3 catalysts for dibenzothiophene hydrodesulfurization. Appl. Catal. B-Environ. 2016, 198, 493–507. [Google Scholar] [CrossRef]
- Ganiyu, S.; Alhooshani, K.; Ali, S. Single-pot synthesis of Ti-SBA-15-NiMo hydrodesulfurization catalysts: Role of calcination temperature on dispersion and activity. Appl. Catal. B-Environ. 2017, 203, 428–441. [Google Scholar] [CrossRef]
- Han, P.; Liu, T.; Ji, X.; Tang, S. Morphology-controlled synthesis of mesoporous silica with co-template of surfactant P123 and ionic liquid [Dmim]Cl. Chin. Chem. Lett. 2018, 29, 1305–1309. [Google Scholar] [CrossRef]
- Qin, T.; Lin, T.; Qi, X.; Wang, C.; Li, L.; Tang, Z.; Zhong, L.; Sun, Y. Tuning chemical environment and synergistic relay reaction to promote higher alcohols synthesis via syngas conversion. Appl. Catal. B-Environ. 2021, 285, 119840. [Google Scholar] [CrossRef]
- Huang, C.; Zhu, C.; Zhang, M.; Chen, J.; Fang, K. Design of efficient ZnO/ZrO2 modified CuCoAl catalysts for boosting higher alcohol synthesis in syngas conversion. Appl. Catal. B-Environ. 2022, 300, 120739. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, R.; Ma, S.; Xu, K.; Chen, Y.; Jiang, K.; Fang, Y.; Zhu, C.; Liu, X.; Tang, Y.; et al. The role of Cu1-O3 species in single-atom Cu/ZrO2 catalyst for CO2 hydrogenation. Nat. Catal. 2022, 5, 818–831. [Google Scholar] [CrossRef]
- Wu, C.; Lin, L.; Liu, J.; Zhang, J.; Zhang, F.; Zhou, T.; Rui, N.; Yao, S.; Deng, Y.; Yang, F.; et al. Inverse ZrO2/Cu as a highly efficient methanol synthesis catalyst from CO2 hydrogenation. Nat. Commun. 2020, 11, 5767. [Google Scholar] [CrossRef]
- Tada, S.; Kayamori, S.; Honma, T.; Kamei, H.; Nariyuki, A.; Kon, K.; Toyao, T.; Shimizu, T.; Satokawa, S. Design of interfacial sites between Cu and amorphous ZrO2 dedicated to CO2-to-methanol hydrogenation. ACS Catal. 2018, 8, 7809–7819. [Google Scholar] [CrossRef]
- Sun, K.; Geng, S.; Yang, J.; Song, F.; Gu, Y.; Feng, H.; Tsubaki, N.; Zhang, Q.; Tan, Y. Potassium-mediated control of adsorbed intermediates on CuCoAl layered nanoplates for ethanol synthesis from syngas. Fuel 2024, 373, 132404. [Google Scholar] [CrossRef]
- Hong, Z.; Wang, J.; Gao, Z.; Huang, W. Tuning CuO crystallite size by different solvents for higher alcohols synthesis from syngas over CuZnAl catalyst. Int. J. Hydrogen Energy 2024, 56, 1032–1037. [Google Scholar] [CrossRef]
- Śliwa, M.; Samson, K. Influence of synthesis parameters on physicochemical properties of CuO/ZrO2 catalysts. Chem. Pap. 2019, 73, 2793–2802. [Google Scholar] [CrossRef]
- Zhan, H.; Li, F.; Gao, P.; Zhao, N.; Xiao, F.; Wei, W.; Zhong, L.; Sun, Y. Methanol synthesis from CO2 hydrogenation over La-M-Cu-Zn-O (M = Y, Ce, Mg, Zr) catalysts derived from perovskite-type precursors. J. Power Sources 2014, 251, 113–121. [Google Scholar] [CrossRef]
- Yu, Q.; Yao, X.; Zhang, H.; Gao, F.; Dong, L. Effect of ZrO2 addition method on the activity of Al2O3-supported CuO for NO reduction with CO: Impregnation vs. coprecipitation. Appl. Catal. A-Gen. 2012, 423–424, 42–51. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, L.; Dong, L.; Li, D.; Liu, B.; Gao, F.; Sun, K.; Dong, L.; Chen, Y. Effects of Ce/Zr ratio on the reducibility, adsorption and catalytic activity of CuO/CexZr1-xO2/γ-Al2O3 catalysts for NO reduction by CO. Appl. Catal. B-Environ. 2010, 96, 350–360. [Google Scholar] [CrossRef]
- Gao, B.; Lim, T.; Subagio, D.; Lim, T. Zr-doped TiO2 for enhanced photocatalytic degradation of bisphenol A. Appl. Catal. A 2010, 375, 107–115. [Google Scholar] [CrossRef]
- Sato, A.; Volanti, D.; Meira, D.; Damyanova, S.; Longo, E.; Bueno, J. Effect of the ZrO2 phase on the structure and behavior of supported Cu catalysts for ethanol conversion. J. Catal. 2013, 307, 1–17. [Google Scholar] [CrossRef]
- Zhang, Z.; Jing, M.; Chen, H.; Okejiri, F.; Liu, J.; Leng, Y.; Liu, H.; Song, W.; Hou, Z.; Lu, X.; et al. Transfer hydrogenation of fatty acids on Cu/ZrO2: Demystifying the role of carrier structure and metal-support interface. ACS Catal. 2020, 10, 9098–9108. [Google Scholar] [CrossRef]
- Song, X.; Yang, C.; Li, X.; Wang, Z.; Pei, C.; Zhao, Z.; Gong, J. On the role of hydroxyl groups on Cu/Al2O3 in CO2 hydrogenation. ACS Catal. 2022, 12, 14162–14172. [Google Scholar] [CrossRef]
- Huang, H.; Wang, B.; Wang, Y.; Zhao, Y.; Wang, S.; Ma, X. Partial hydrogenation of dimethyl oxalate on Cu/SiO2 catalyst modified by sodium silicate. Catal. Today 2020, 358, 68–73. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Zhao, Y.; Lv, J.; Wang, S.; Ma, X. Insight into the balancing effect of active Cu species for hydrogenation of carbon-oxygen bonds. ACS Catal. 2015, 5, 6200–6208. [Google Scholar] [CrossRef]
- Sun, K.; Wu, Y.; Tan, M.; Wang, L.; Yang, G.; Zhang, M.; Zhang, W.; Tan, Y. Ethanol and higher alcohols synthesis from syngas over CuCoM (M = Fe, Cr, Ga and Al) nanoplates derived from hydrotalcite-like precursors. ChemCatChem 2019, 11, 2695–2706. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, S.; Cheng, D.; Li, L.; Zhu, W.; Zhong, D.; Zhao, Z.; Li, J.; Wang, T.; Gong, J. Controllable Cu0-Cu+ sites for electrocatalytic reduction of carbon dioxide. Angew. Chem. Int. Ed. 2021, 28, 15344–15347. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, G.; Li, Z.; Tang, C.; Feng, Z.; An, H.; Liu, H.; Liu, T.; Li, C. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol. Sci. Adv. 2017, 3, e1701290. [Google Scholar] [CrossRef]
- Kattel, S.; Yan, B.; Yang, Y.; Chen, J.; Liu, P. Optimizing binding energies of key intermediates for CO2 hydrogenation to methanol over oxide-supported copper. J. Am. Chem. Soc. 2016, 138, 12440–12450. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, C.; Wei, W.; Li, W.; Sun, Y. Catalytic performance of copper supported on zirconia polymorphs for CO hydrogenation. J. Mol. Catal. A Chem. 2005, 231, 75–81. [Google Scholar] [CrossRef]
- He, D.; Ding, Y.; Luo, H.; Li, C. Effects of zirconia phase on the synthesis of higher alcohols over zirconia and modified zirconia. J. Mol. Catal. A Chem. 2004, 208, 267–271. [Google Scholar] [CrossRef]
- Wang, L.; Gao, X.; Bai, Y.; Tan, M.; Sun, K.; Zhang, T.; Wu, Y.; Pan, J.; Xie, H.; Tan, Y. The synergistic effect between ZnO and ZnCr2O4 on the catalytic performance for isobutanol synthesis from syngas. Fuel 2019, 253, 1570–1577. [Google Scholar] [CrossRef]
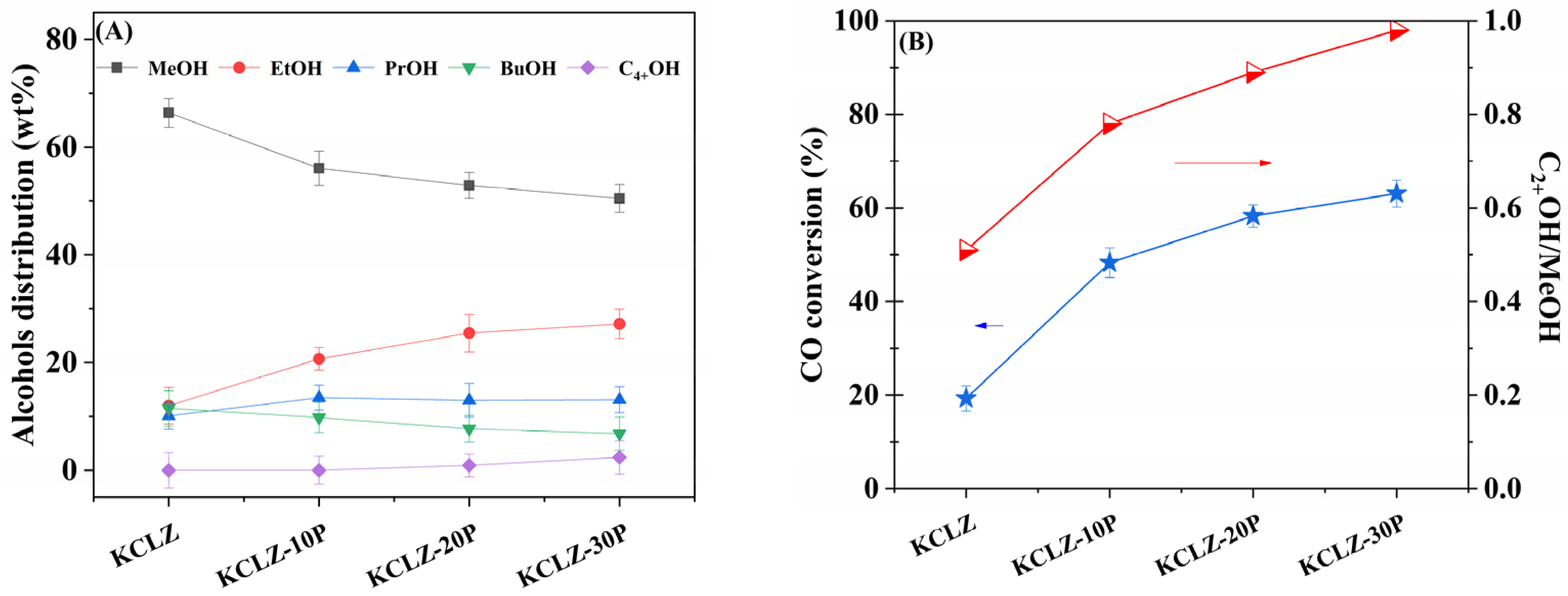
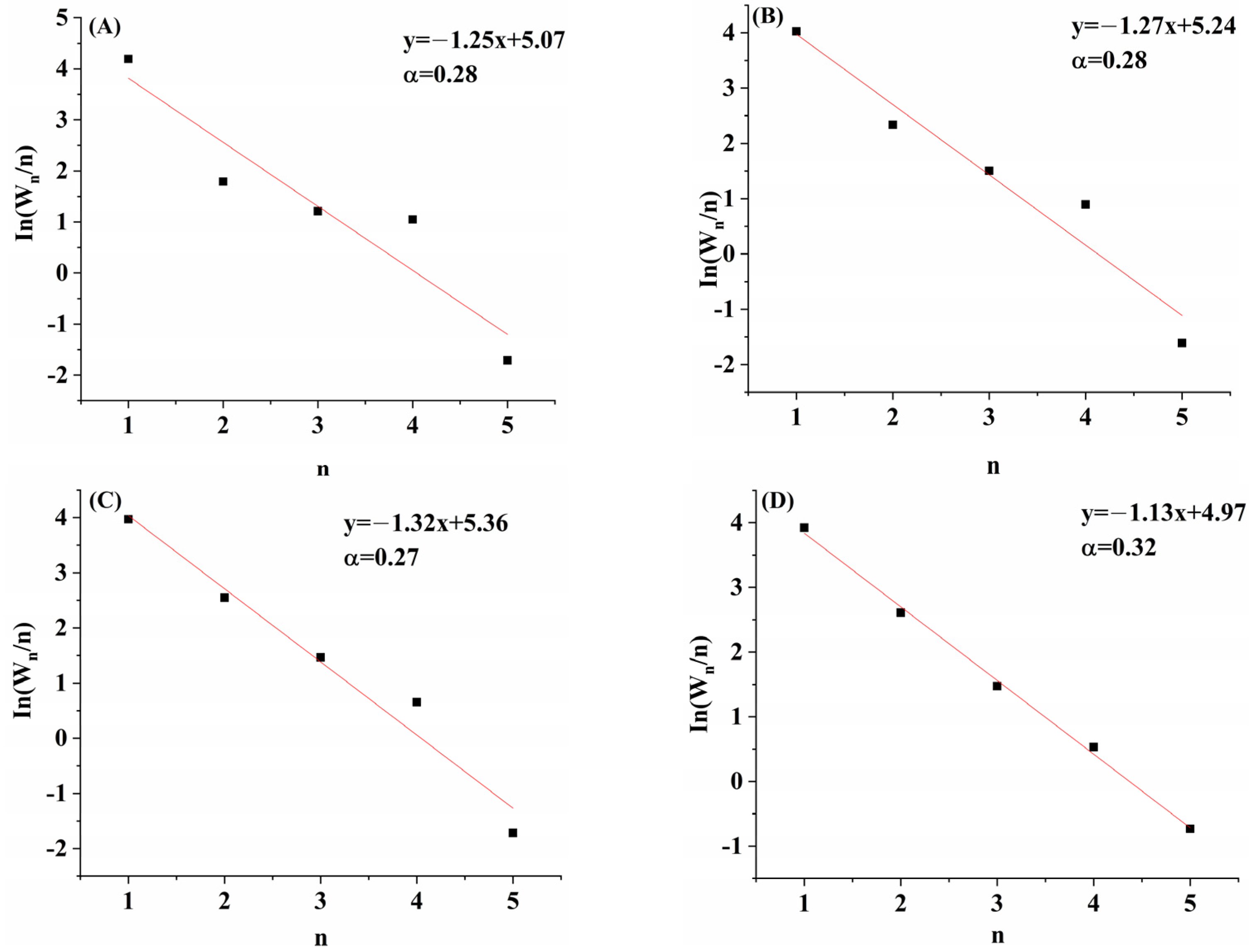



| Catalyst | XCO/% | Selectivity/% | STYROH g/(L·h) | STYgas g/(L·h) | C2+OH/MeOH | Distribution of Alcohols/(wt)% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROH | CO2 | CHx | CH4 | C1 | C2 | C3 | C4 | i-C4 | C4+ | |||||
| KCLZ | 19.3 | 37.2 | 36.3 | 12.9 | 12.1 | 40 | 77 | 0.51 | 66.4 | 12.0 | 10.1 | 2.2 | 9.3 | 0 |
| KCLZ-10P | 48.3 | 7.7 | 49.5 | 18.9 | 23.7 | 30 | 411 | 0.78 | 56.1 | 20.7 | 13.5 | 3.0 | 6.8 | 0 |
| KCLZ-20P | 58.3 | 7.6 | 47 | 20.6 | 24.5 | 38 | 522 | 0.89 | 52.9 | 25.5 | 13.0 | 3.3 | 4.4 | 0.9 |
| KCLZ-30P | 63.1 | 6.5 | 45.6 | 24.6 | 23.0 | 33 | 528 | 0.98 | 50.5 | 27.2 | 13.1 | 3.7 | 3.1 | 2.4 |
| Catalyst | Surface Area SBET (m2/g) | Pore Volume Vp (cm3/g) | Pore Size Dp (nm) | XCu+ (%) |
|---|---|---|---|---|
| KCLZ | 82.58 | 0.10 | 3.15 | 63 |
| KCLZ-10P | 88.53 | 0.11 | 3.65 | 60 |
| KCLZ-20P | 91.65 | 0.11 | 3.57 | 57 |
| KCLZ-30P | 72.75 | 0.10 | 3.79 | 60 |
| Catalyst | CO Adsorption (mmol/g) | Basic Amount (mmol/g) | Acid Amount (mmol/g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Weak | Strong | Total | Weak | Medium | High | Total | Weak | Medium | High | Total | |
| KCLZ | 0.081 (70%) | 0.035 (30%) | 0.116 | 0.142 | 0.288 | 0.056 | 0.486 | 0.092 | 0.341 | 0.09 | 0.553 |
| KCLZ-10P | 0.056 (53%) | 0.050 (47%) | 0.106 | 0.273 | 0.180 | 0.039 | 0.492 | 0.010 | 0.242 | 0.08 | 0.400 |
| KCLZ-20P | 0.072 (56%) | 0.057 (44%) | 0.129 | 0.193 | 0.191 | 0.046 | 0.430 | 0.012 | 0.237 | 0.079 | 0.386 |
| KCLZ-30P | 0.059 (56%) | 0.047 (44%) | 0.106 | 0.181 | 0.193 | 0.041 | 0.415 | 0.007 | 0.241 | 0.076 | 0.367 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Jin, J.; Xue, D.; Zhi, L.; Wang, Z.; Sun, K. The Effect of Surfactant P123 on the KCuLaZrO2 Catalysts in the Direct Conversion of Syngas to Higher Alcohols. Catalysts 2025, 15, 431. https://doi.org/10.3390/catal15050431
Yang J, Jin J, Xue D, Zhi L, Wang Z, Sun K. The Effect of Surfactant P123 on the KCuLaZrO2 Catalysts in the Direct Conversion of Syngas to Higher Alcohols. Catalysts. 2025; 15(5):431. https://doi.org/10.3390/catal15050431
Chicago/Turabian StyleYang, Jiaqian, Jiayu Jin, Duomei Xue, Lifei Zhi, Zhongqiang Wang, and Kai Sun. 2025. "The Effect of Surfactant P123 on the KCuLaZrO2 Catalysts in the Direct Conversion of Syngas to Higher Alcohols" Catalysts 15, no. 5: 431. https://doi.org/10.3390/catal15050431
APA StyleYang, J., Jin, J., Xue, D., Zhi, L., Wang, Z., & Sun, K. (2025). The Effect of Surfactant P123 on the KCuLaZrO2 Catalysts in the Direct Conversion of Syngas to Higher Alcohols. Catalysts, 15(5), 431. https://doi.org/10.3390/catal15050431






