Cu1Ni2/Al2O3 Catalyst from Its Hydrotalcite Precusor with Highly Active Sites for Efficient Hydrogenation of Levulinic Acid Toward 2-Methyltetrahydrofuran
Abstract
1. Introduction
2. Results
2.1. Characterization of CuNiAl Hydrotalcites
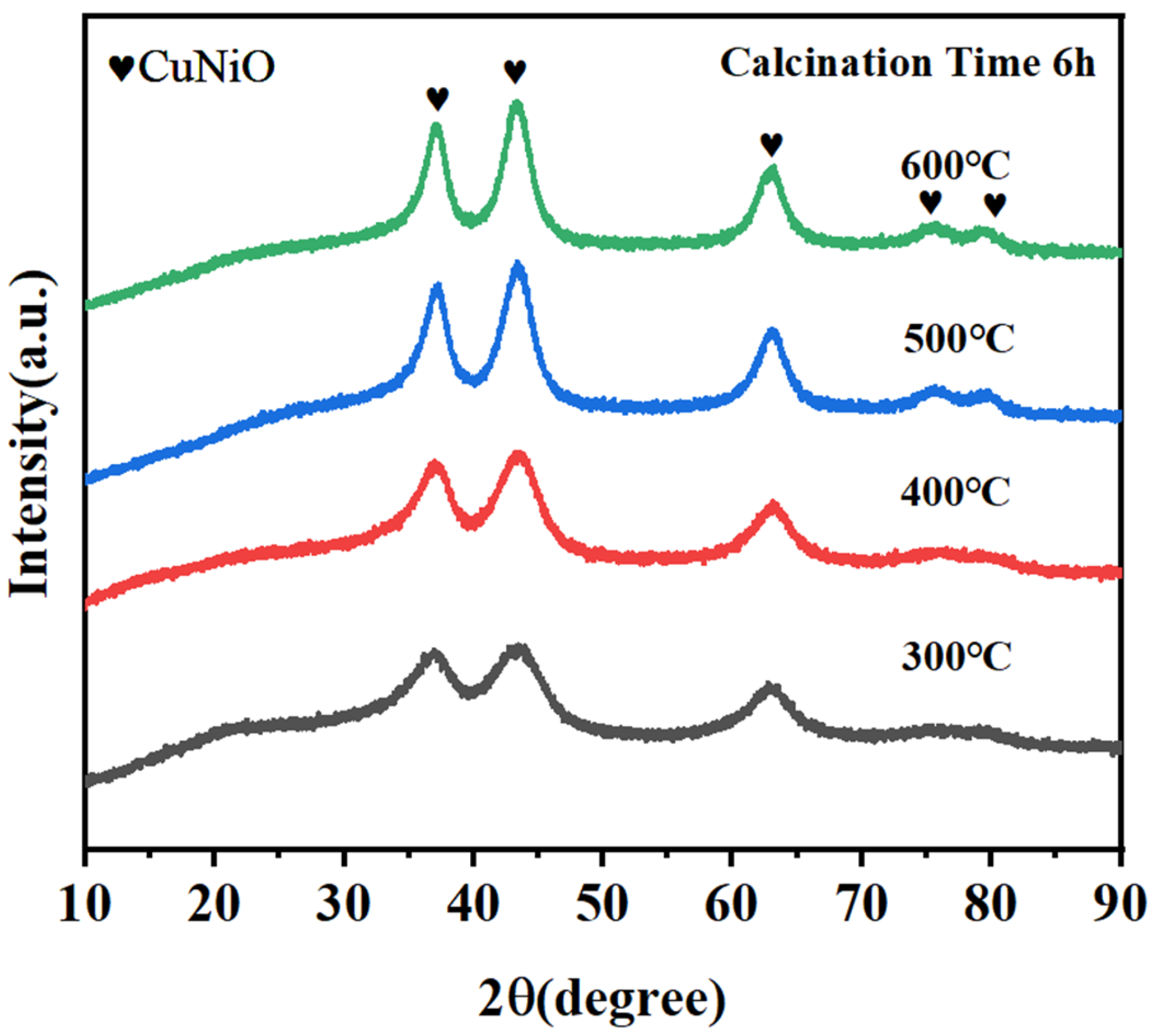
2.1.1. CuNiAl Hydrotalcites XRD Analysis
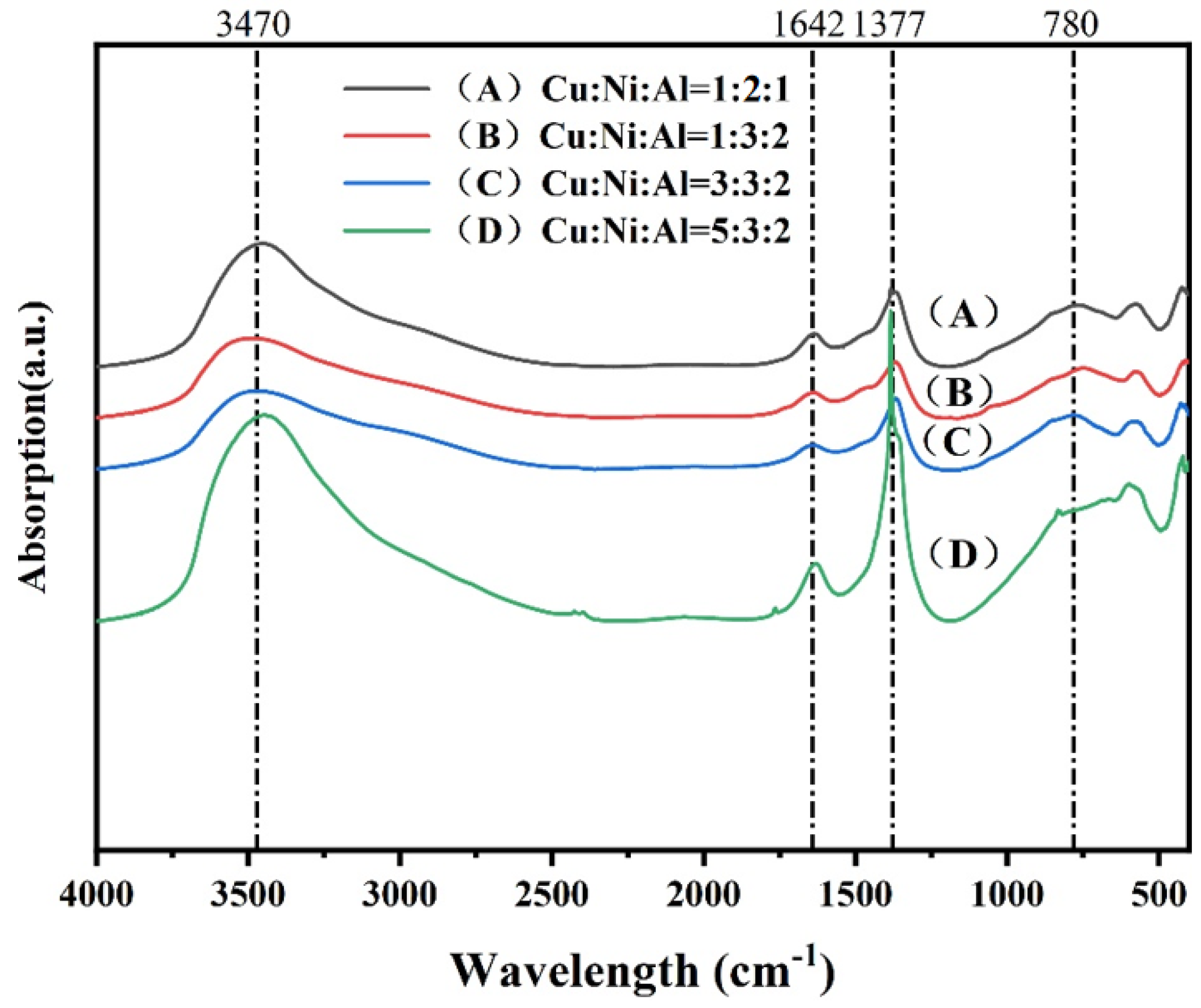
2.1.2. CuNiAl Hydrotalcites FT-IR Analysis
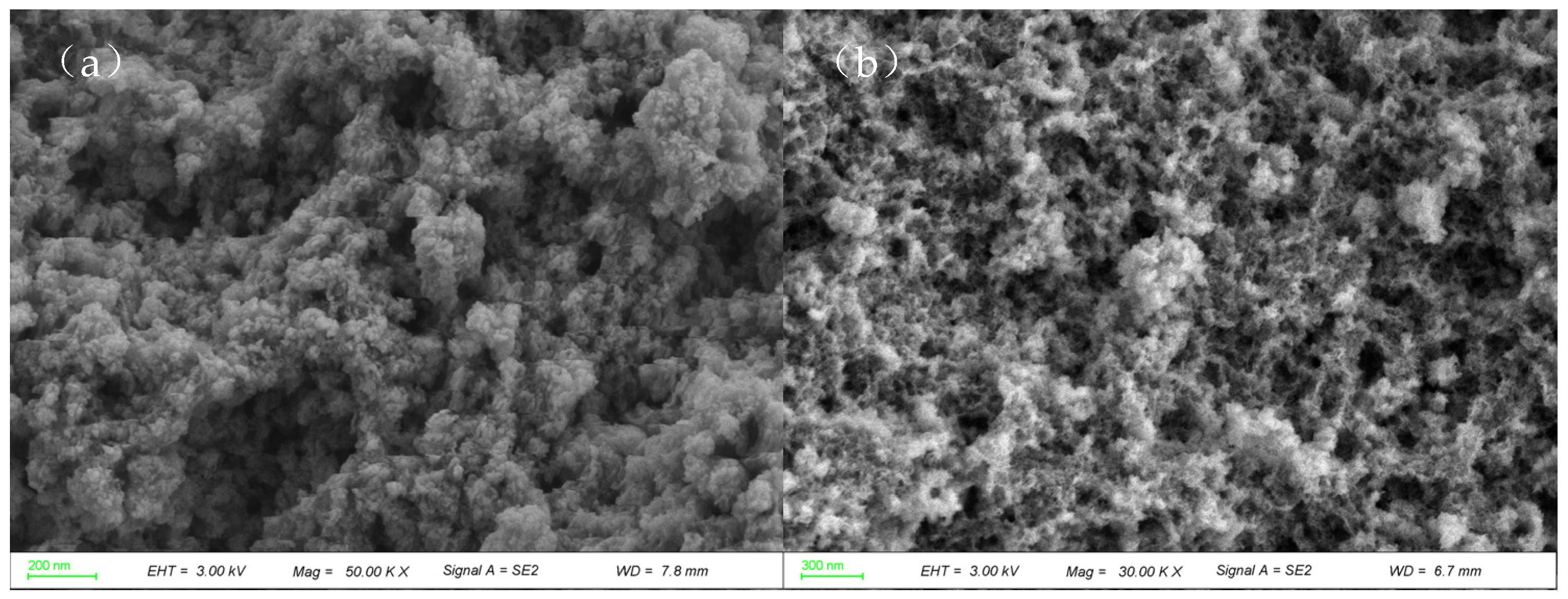
2.1.3. CuNiAl Hydrotalcites SEM Analysis
2.2. Characterization of Cu1Ni2/Al2O3 Catalyst
2.2.1. Cu1Ni2/Al2O3 Catalyst XRD Analysis
2.2.2. Cu1Ni2/Al2O3 Catalyst SEM Analysis
2.2.3. Cu1Ni2/Al2O3 Catalyst BET Analysis
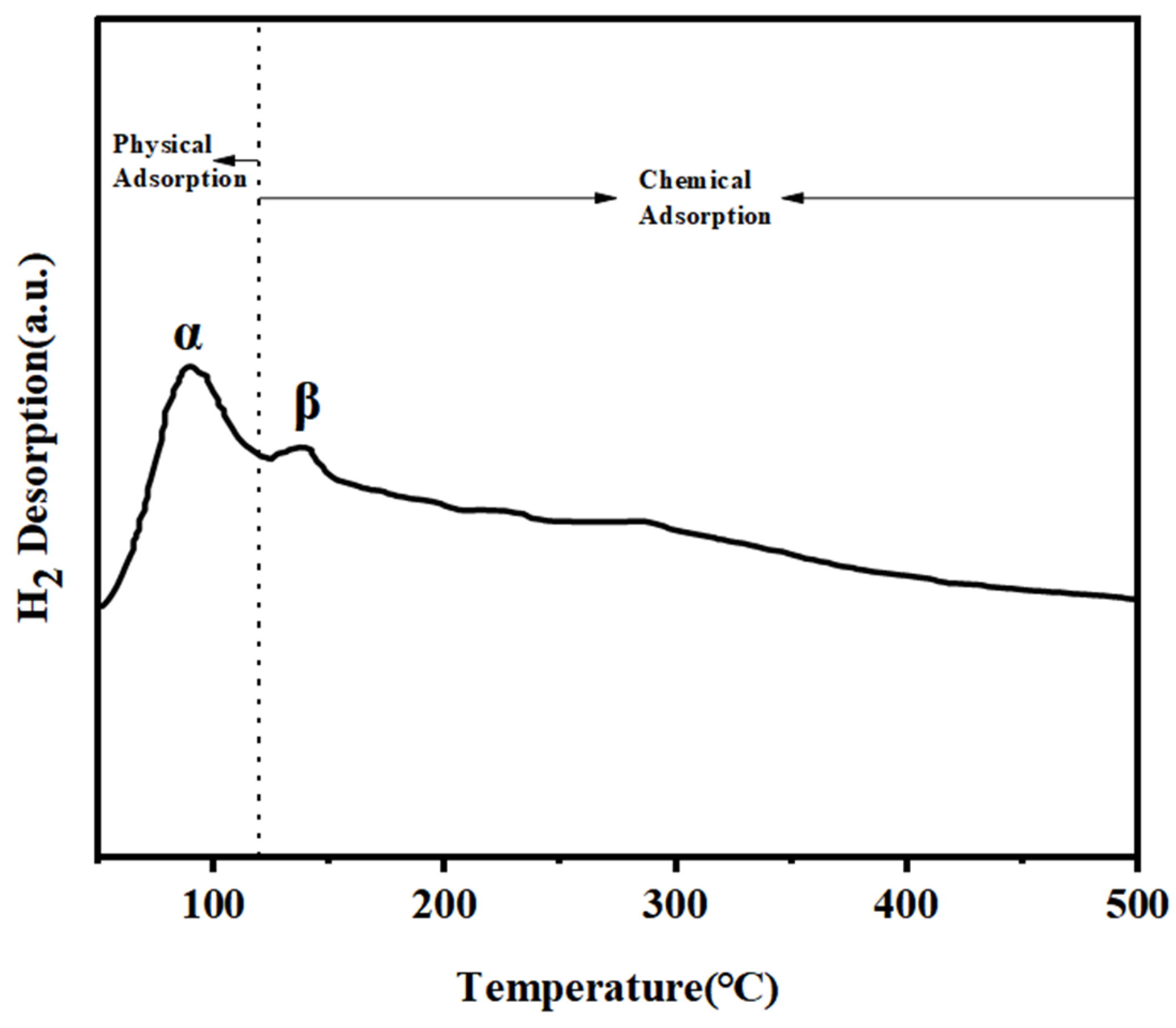
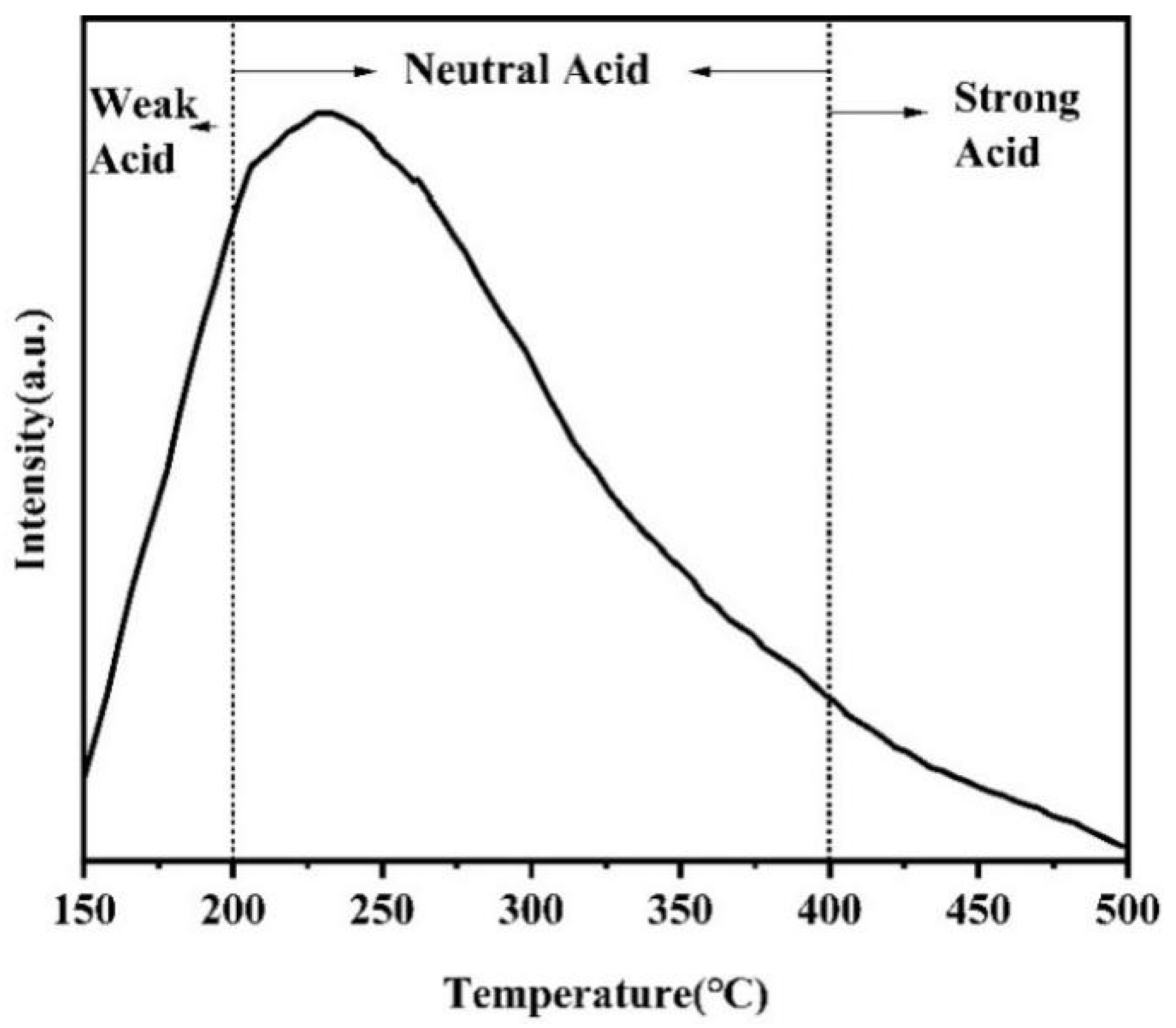
2.2.4. Cu1Ni2/Al2O3 Catalyst TPD Analysis
2.3. Analysis of Influencing Factors
2.3.1. Effect of Reaction Temperature
2.3.2. Effect of Reaction Time
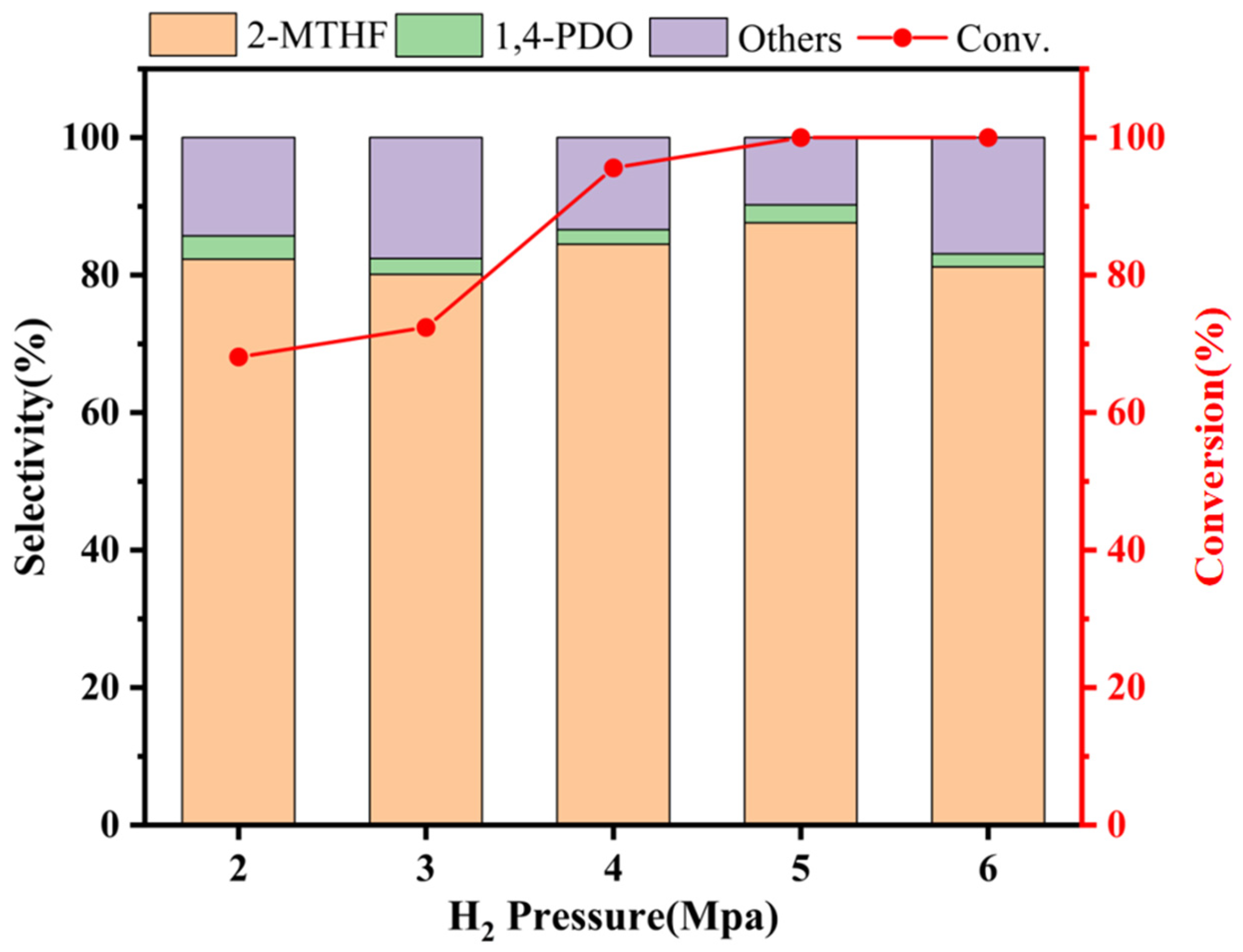
2.3.3. Effect of Reaction Hydrogen Pressure
2.3.4. Research on Recycling Effect
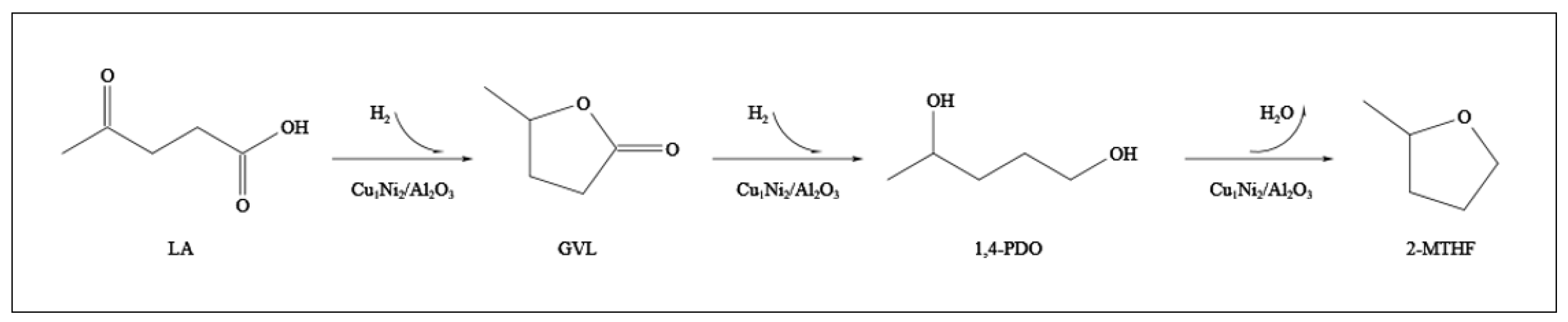
2.3.5. Investigation of Reaction Mechanism
3. Materials and Methods
3.1. Raw Materials
3.2. Methods of Catalyst Preparation
3.2.1. Synthesis of CuNiAl Hydrotalcite
3.2.2. Preparation of Cu1Ni2/Al2O3 Catalyst
3.3. Catalyst Testing Methods
3.3.1. Characterization
3.3.2. Catalyst Activity Test
3.3.3. Analysis of Reaction Products
4. Conclusions
- (1)
- Through the comprehensive use of characterization techniques such as XRD, FT-IR, and SEM, high-quality hydrotalcites with high crystallinity, structural stability, and expected functional groups can be obtained under well-controlled synthesis conditions and an optimal Cu:Ni:Al = 1:2:1 molar ratio of 1:2:1;
- (2)
- XRD analysis indicated that CuNiAl hydrotalcite gradually transformed into a homogeneous composite tri-metal oxide with increasing calcination temperature; however, excessively high temperatures led to catalyst structural degradation. SEM analysis highlighted morphological changes of the catalysts under different calcination conditions, demonstrating that appropriate calcination temperature and time fostered an optimal pore structure. The ideal preparation conditions were established as a calcination temperature of 500 °C, a calcination time of 6 h, and a reduction temperature of 400 °C. BET results confirmed that catalysts prepared under these optimal conditions exhibited a high specific surface area, facilitating catalytic reactions. Additionally, TPD experiments validated that the catalysts had strong hydrogen adsorption capacity and suitable acid–base properties, supporting their high catalytic performance;
- (3)
- The systematic investigation of reaction temperature, time, and hydrogen pressure revealed that conducting the reaction at 190 °C and 5 MPa H2 for 5 h resulted in the complete conversion of LA, achieving a 2-MTHF yield of 87.6%. This demonstrated a highly efficient one-step conversion of LA to 2-MTHF;
- (4)
- The recycling performance tests of the bimetallic Cu1Ni2/Al2O3 catalyst were conducted to assess its stability and economic viability during reuse. The results indicated that the catalyst exhibited excellent reusability and stability, with only a slight decrease in activity after six cycles. This demonstrates its feasibility and reliability for industrial applications.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, Y.; Xu, H.; Ma, X.; Bao, X.; Wang, B. Laminar burning characteristics of 2-MTHF compared with ethanol and isooctane. Fuel 2017, 190, 10–20. [Google Scholar] [CrossRef]
- Leitner, W.; Klankermayer, J.; Pischinger, S.; Pitsch, H.; Kohse-Höinghaus, K. Advanced Biofuels and Beyond: Chemistry Solutions for Propulsion and Production. Angew. Chem. Int. Ed. 2017, 56, 5412–5452. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Qiaoling, L.; Jinfang, W.; Mu, T. Valorization of Levulinic Acid over Non-Noble Metal Catalysts: Challenges and Opportunities. Green Chem. 2018, 20, 4391–4408. [Google Scholar] [CrossRef]
- Rodiansono; Astuti, M.D.; Mustikasari, K.; Husain, S.; Ansyah, F.R.; Hara, T.; Shimazu, S. Unravelling the one-pot conversion of biomass-derived furfural and levulinic acid to 1,4-pentanediol catalysed by supported RANEY® Ni–Sn alloy catalysts. RSC Adv. 2021, 12, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Al Shaal, M.; Dzierbinski, A.; Palkovits, R. Solvent-free γ-valerolactone hydrogenation to 2-methyltetrahydrofuran catalysed by Ru/C: A reaction network analysis. Green Chem. 2013, 16, 1358–1364. [Google Scholar] [CrossRef]
- Gundekari; Srinivasan, K. Hydrous ruthenium oxide: A new generation remarkable catalyst precursor for energy efficient and sustainable production of γ-valerolactone from levulinic acid in aqueous medium. Appl. Catal. A Gen. 2018, 569, 117–125. [Google Scholar] [CrossRef]
- Manzer, L.E. Catalytic synthesis of α-methylene-γ-valerolactone: A biomass-derived acrylic monomer. Appl. Catal. A Gen. 2004, 272, 249–256. [Google Scholar] [CrossRef]
- Upare, P.P.; Lee, J.-M.; Hwang, D.W.; Halligudi, S.B.; Hwang, Y.K.; Chang, J.-S. Selective hydrogenation of levulinic acid to γ-valerolactone over carbon-supported noble metal catalysts. J. Ind. Eng. Chem. 2011, 17, 287–292. [Google Scholar] [CrossRef]
- Al-Shaal, M.G.; Wright, W.R.H.; Palkovits, R. Exploring the ruthenium catalysed synthesis of γ-valerolactone in alcohols and utilisation of mild solvent-free reaction conditions. Green Chem. 2012, 14, 1260–1263. [Google Scholar] [CrossRef]
- Du, X.; He, L.; Zhao, S.; Liu, Y.; Cao, Y.; He, H.; Fan, K. Hydrogen-independent reductive transformation of carbohydrate biomass into γ-valerolactone and pyrrolidone derivatives with supported gold catalysts. Angew. Chem. 2011, 50, 7815–7819. [Google Scholar] [CrossRef]
- Yan, K.; Lafleur, T.; Wu, G.; Liao, J.; Ceng, C.; Xie, X. Highly selective production of value-added γ-valerolactone from biomass-derived levulinic acid using the robust Pd nanoparticles. Appl. Catal. A Gen. 2013, 468, 52–58. [Google Scholar] [CrossRef]
- Bangalore Ashok, R.P.; Oinas, P.; Forssell, S. Techno-economic evaluation of a biorefinery to produce γ-valerolactone (GVL), 2-methyltetrahydrofuran (2-MTHF) and 5-hydroxymethylfurfural (5-HMF) from spruce. Renew. Energy 2022, 190, 396–407. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Tong, X.; Wang, Y.; Jin, G.; Guo, X. Highly active Ir/SiC catalyst for aqueous hydrogenation of levulinic acid to γ-valerolactone. Catal. Commun. 2020, 139, 105971. [Google Scholar] [CrossRef]
- Ferlin, F.; Anastasiou, I.; Carpisassi, L.; Vaccaro, L. Aerobic waste-minimized Pd-catalysed C–H alkenylation in GVL using a tube-in-tube heterogeneous flow reactor. Green Chem. 2021, 23, 6576–6582. [Google Scholar] [CrossRef]
- Siddiqui, N.; Pendem, C.; Goyal, R.; Khatun, R.; Khan, T.S.; Samanta, C.; Chiang, K.; Shah, K.; Ali Haider, M.; Bal, R. Study of γ-valerolactone production from hydrogenation of levulinic acid over nanostructured Pt-hydrotalcite catalysts at low temperature. Fuel 2022, 323, 124272. [Google Scholar] [CrossRef]
- Maumela, M.; Marx, S.; Meijboom, R. Heterogeneous Ru Catalysts as the Emerging Potential Superior Catalysts in the Selective Hydrogenation of Bio-Derived Levulinic Acid to γ-Valerolactone: Effect of Particle Size, Solvent, and Support on Activity, Stability, and Selectivity. Catalysts 2021, 11, 292. [Google Scholar] [CrossRef]
- Date, N.S.; Hengne, A.M.; Huang, K.W.; Chikate, R.C.; Rode, C.V. One Pot Hydrogenation of Furfural to 2-Methyl Tetrahydrofuran over Supported Mono- and Bi-metallic Catalysts. ChemistrySelect 2020, 5, 9590–9600. [Google Scholar] [CrossRef]
- Obregón, I.; Gandarias, I.; Al-Shaal, M.G.; Mevissen, C.; Arias, P.L.; Palkovits, R. The Role of the Hydrogen Source on the Selective Production of γ-Valerolactone and 2-Methyltetrahydrofuran from Levulinic Acid. ChemSusChem 2016, 9, 2488–2495. [Google Scholar] [CrossRef]
- Shesterkina, A.; Vikanova, K.; Kostyukhin, E.; Strekalova, A.; Shuvalova, E.; Kapustin, G.; Salmi, T. Microwave Synthesis of Copper Phyllosilicates as Effective Catalysts for Hydrogenation of C≡C Bonds. Molecules 2022, 27, 988. [Google Scholar] [CrossRef]
- Shesterkina, A.A.; Vikanova, K.V.; Zhuravleva, V.S.; Kustov, A.L.; Davshan, N.A.; Mishin, I.V.; Strekalova, A.A.; Kustov, L.M. A novel catalyst based on nickel phyllosilicate for the selective hydrogenation of unsaturated compounds. Mol. Catal. 2023, 547, 113341. [Google Scholar] [CrossRef]
- Cui, G.; Zhang, X.; Wang, H.; Li, Z.; Wang, W.; Yu, Q.; Zheng, L.; Wang, Y.; Zhu, J.; Wei, M. ZrO2-x modified Cu nanocatalysts with synergistic catalysis towards carbon-oxygen bond hydrogenation. Appl. Catal. B Environ. 2021, 280, 119406. [Google Scholar] [CrossRef]
- Bermudez Menendez, J.; Menéndez, J.A.; Romero, A.; Serrano, E.; García-Martínez, J.; Luque, R. Continuous flow nanocatalysis: Reaction pathways in the conversion of levulinic acid to valuable chemicals. Green Chem. 2013, 15, 2786–2792. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Liu, A.-F.; Zhang, Q.; Li, K.-M.; Porterfield, W.; Li, L.-C.; Wang, F. Mechanistic Insights into the Solvent-Driven Adsorptive Hydrodeoxygenation of Biomass Derived Levulinate Acid/Ester to 2-Methyltetrahydrofuran over Bimetallic Cu–Ni Catalysts. ACS Sustain. Chem. Eng. 2020, 8, 11477–11490. [Google Scholar] [CrossRef]
- Zhang, C.; Huo, Z.; Ren, D.; Song, Z.; Liu, Y.; Jin, F.; Zhou, W. Catalytic transfer hydrogenation of levulinate ester into γ-valerolactone over ternary Cu/ZnO/Al2O3 catalyst. J. Energy Chem. 2019, 32, 189–197. [Google Scholar] [CrossRef]
- Gu, C.; Chen, L.; Liu, Y.; Zhang, X.; Liu, J.; Zhang, q.; Wang, C.; Ma, L. One-pot conversion of biomass-derived levulinic acid to furanic biofuel 2-methyltetrahydrofuran over bimetallic NiCo/γ-Al2O3 catalysts. Mol. Catal. 2022, 524, 112317. [Google Scholar] [CrossRef]
- Obregón, I.; Gandarias, I.; Miletić, N.; Ocio, A.; Arias, P.L. One-Pot 2-Methyltetrahydrofuran Production from Levulinic Acid in Green Solvents Using Ni-Cu/Al2O3 Catalysts. ChemSusChem 2015, 8, 3483–3488. [Google Scholar] [CrossRef]
- Xie, Z.; Bingfeng, C.; Wu, H.; Liu, M.; Liu, H.; Zhang, J.; Yang, G.; Han, B. Highly efficient hydrogenation of levulinic acid to 2-methyltetrahydro-furan over Ni-Cu/Al2O3-ZrO2 bifunctional catalysts. Green Chem. 2019, 21, 606–613. [Google Scholar] [CrossRef]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef]
- Figueiredo, R.T.; Martínez-Arias, A.; Granados, M.L.; Fierro, J.L.G. Spectroscopic Evidence of Cu–Al Interactions in Cu–Zn–Al Mixed Oxide Catalysts Used in CO Hydrogenation. J. Catal. 1998, 178, 146–152. [Google Scholar] [CrossRef]
- Shishido, T.; Yamamoto, M.; Li, D.; Tian, Y.; Morioka, H.; Honda, M.; Sano, T.; Takehira, K. Water-gas shift reaction over Cu/ZnO and Cu/ZnO/Al2O3 catalysts prepared by homogeneous precipitation. Appl. Catal. A Gen. 2006, 303, 62–71. [Google Scholar] [CrossRef]
- Wettstein, S.G.; Bond, J.Q.; Alonso, D.M.; Pham, H.N.; Datye, A.K.; Dumesic, J.A. RuSn bimetallic catalysts for selective hydrogenation of levulinic acid to γ-valerolactone. Appl. Catal. B Environ. 2012, 117–118, 321–329. [Google Scholar] [CrossRef]
- He, L.; Li, X.; Lin, W.; Li, W.; Cheng, H.; Yu, Y.; Fujita, S.-I.; Arai, M.; Zhao, F. The selective hydrogenation of ethyl stearate to stearyl alcohol over Cu/Fe bimetallic catalysts. J. Mol. Catal. A Chem. 2014, 392, 143–149. [Google Scholar] [CrossRef]
- Tichit, D.; Lutic, D.; Coq, B.; Durand, R.; Teissier, R. The aldol condensation of acetaldehyde and heptanal on hydrotalcite-type catalysts. J. Catal. 2003, 219, 167–175. [Google Scholar] [CrossRef]
- Xu, Z.P.; Zhang, J.; Adebajo, M.O.; Zhang, H.; Zhou, C. Catalytic applications of layered double hydroxides and derivatives. Appl. Clay Sci. 2011, 53, 139–150. [Google Scholar] [CrossRef]
- Chaves, L.H.; Curry, J.; Stone, D.; Carducci, M.; Chorover, J. Nickel incorporation in FE(II, III) hydroxysulfate green Rust: Effect on crystal lattice spacing and oxidation products. Rev. Brasi. Ciênc. Solo 2009, 33, 1115–1123. [Google Scholar] [CrossRef]
- Sideris, P.; Nielsen, U.G.; Gan, Z.; Grey, C.P. Mg/Al Ordering in Layered Double Hydroxides Revealed by Multinuclear NMR Spectroscopy. Science 2008, 321, 113–117. [Google Scholar] [CrossRef]
- Li, C.; Wei, M.; Evans, D.G.; Duan, X. Layered double hydroxide-based nanomaterials as highly efficient catalysts and adsorbents. Small 2014, 10, 4469–4486. [Google Scholar] [CrossRef]
- Xianjun, D.; Zhang, D.; Gao, R.; Huang, L.; Shi, L.; Zhang, J. Design of modular catalysts derived from NiMgAl-LDH@m-SiO2 with dual confinement effects for dry reforming of methane. Chem. Commun. 2013, 49, 6770–6772. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Shang, N.; Gao, S.; Wang, C.; Wang, Z. Conversion of biomass-derived levulinate esters to γ-valerolactone with robust CuNi bimetallic catalyst. New J. Chem. 2020, 44, 15671–15676. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Z.; Ye, L.; Zhang, M.; Xiong, J.; Zhang, R.; Li, X.; Ji, N.; Lu, X. Layered Double Hydroxide-Derived Bimetallic Ni−Cu Catalysts Prompted the Efficient Conversion of γ-Valerolactone to 2-Methyltetrahydrofuran. ChemCatChem 2022, 14, e202101441. [Google Scholar] [CrossRef]
- Wu, X.; Ci, C.; Du, Y.; Liu, X.; Li, X.; Xie, X. Facile synthesis of NiAl-LDHs with tunable establishment of acid-base activity sites. Mater. Chem. Phys. 2018, 211, 72–78. [Google Scholar] [CrossRef]
- Kong, X.; Zheng, R.; Zhu, Y.; Ding, G.; Zhu, Y.; Li, Y.-W. Rational design of Ni-based catalysts derived from hydrotalcite for selective hydrogenation of 5-hydroxymethylfurfural. Green Chem. 2015, 17, 2504–2514. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Fan, G.; Yang, L.; Li, F. Hierarchical Flower-like Bimetallic NiCu catalysts for Catalytic Transfer Hydrogenation of Ethyl Levulinate into γ-Valerolactone. Ind. Eng. Chem. Res. 2019, 58, 10317–10327. [Google Scholar] [CrossRef]
- Liu, M.; Yuan, L.; Fan, G.; Zheng, L.; Yang, L.; Li, F. NiCu Nanoparticles for Catalytic Hydrogenation of Biomass-Derived Carbonyl Compounds. ACS Appl. Nano Mater. 2020, 3, 9226–9237. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Feng, J.; He, Y.; Du, Y.; Li, D. Layered double hydroxide-derived Ni-Cu nanoalloy catalysts for semi-hydrogenation of alkynes: Improvement of selectivity and anti-coking ability via alloying of Ni and Cu. J. Catal. 2018, 359, 251–260. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, L.; Wang, J.; Xia, S.; Chen, P.; Hou, Z.; Zheng, X. Hydrogenolysis of glycerol over homogenously dispersed copper on solid base catalysts. Appl. Catal. B Environ. 2011, 101, 431–440. [Google Scholar] [CrossRef]
- Xia, S.; Nie, R.; Lu, X.; Wang, L.; Chen, P.; Hou, Z. Hydrogenolysis of glycerol over Cu0.4/Zn5.6−xMgxAl2O8.6 catalysts: The role of basicity and hydrogen spillover. J. Catal. 2012, 296, 1–11. [Google Scholar] [CrossRef]
- Wu, J.; Gao, G.; Li, J.; Sun, P.; Long, X.; Li, F. Efficient and versatile CuNi alloy nanocatalysts for the highly selective hydrogenation of furfural. Appl. Catal. B Environ. 2017, 203, 227–236. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, X.; Bai, H.; Xiong, J.; Feng, W.; Ji, N. Effects of Solid Acid Supports on the Bifunctional Catalysis of Levulinic Acid to γ-Valerolactone: Catalytic Activity and Stability. Chem.—Asian J. 2020, 15, 1182–1201. [Google Scholar] [CrossRef]
- Obregón, I.; Gandarias, I.; Ocio, A.; García-García, I.; Alvarez de Eulate, N.; Arias, P.L. Structure-activity relationships of Ni-Cu/Al2O3 catalysts for γ-valerolactone conversion to 2-methyltetrahydrofuran. Appl. Catal. B Environ. 2017, 210, 328–341. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Z.; Arai, M.; Zhang, C.; Liu, K.L.; Shi, R.; Wu, P.; Wang, Z.; Lin, W.; Cheng, H.; et al. Transformation of γ-valerolactone into 1,4-pentanediol and 2-methyltetrahydrofuran over Zn-promoted Cu/Al2O3 catalysts. Catal. Sci. Technol. 2020, 10, 4412–4423. [Google Scholar] [CrossRef]
- Cavuoto, D.; Ardemani, L.; Ravasio, N.; Zaccheria, F.; Scotti, N. Some Insights into the Use of Heterogeneous Copper Catalysts in the Hydroprocessing of Levulinic Acid. Catalysts 2023, 13, 697. [Google Scholar] [CrossRef]
- Zhang, G.; Li, W.; Fan, G.; Yang, L.; Li, F. Controlling product selectivity by surface defects over MoOx-decorated Ni-based nanocatalysts for γ-valerolactone hydrogenolysis. J. Catal. 2019, 379, 100–111. [Google Scholar] [CrossRef]









| Hydrotalcite | Cu:Ni:Al | MCu (mmol) | MNi (mmol) | MAl (mmol) |
|---|---|---|---|---|
| CuNiAl | 5:3:2 | 50 | 30 | 20 |
| CuNiAl | 3:3:2 | 30 | 30 | 20 |
| CuNiAl | 1:3:2 | 10 | 30 | 20 |
| CuNiAl | 1:2:1 | 10 | 20 | 10 |
| Catalysts | SBET/m2·g−1 |
|---|---|
| Cu1Ni2/Al2O3(300) | 127.43 |
| Cu1Ni2/Al2O3(400) | 121.53 |
| Cu1Ni2/Al2O3(500) | 113.34 |
| Cu1Ni2/Al2O3(600) | 98.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, J.; Chen, G.; Zheng, K.; Wu, J.; Wang, F.; Liu, X.; Weng, R. Cu1Ni2/Al2O3 Catalyst from Its Hydrotalcite Precusor with Highly Active Sites for Efficient Hydrogenation of Levulinic Acid Toward 2-Methyltetrahydrofuran. Catalysts 2025, 15, 416. https://doi.org/10.3390/catal15050416
Qin J, Chen G, Zheng K, Wu J, Wang F, Liu X, Weng R. Cu1Ni2/Al2O3 Catalyst from Its Hydrotalcite Precusor with Highly Active Sites for Efficient Hydrogenation of Levulinic Acid Toward 2-Methyltetrahydrofuran. Catalysts. 2025; 15(5):416. https://doi.org/10.3390/catal15050416
Chicago/Turabian StyleQin, Jie, Guohong Chen, Kaiqi Zheng, Jiajun Wu, Fanan Wang, Xueping Liu, and Rengui Weng. 2025. "Cu1Ni2/Al2O3 Catalyst from Its Hydrotalcite Precusor with Highly Active Sites for Efficient Hydrogenation of Levulinic Acid Toward 2-Methyltetrahydrofuran" Catalysts 15, no. 5: 416. https://doi.org/10.3390/catal15050416
APA StyleQin, J., Chen, G., Zheng, K., Wu, J., Wang, F., Liu, X., & Weng, R. (2025). Cu1Ni2/Al2O3 Catalyst from Its Hydrotalcite Precusor with Highly Active Sites for Efficient Hydrogenation of Levulinic Acid Toward 2-Methyltetrahydrofuran. Catalysts, 15(5), 416. https://doi.org/10.3390/catal15050416







