Waste Incineration Fly Ash-Based Bifunctional Catalyst for Upgrading Glucose to Levulinic Acid
Abstract
1. Introduction
2. Results and Discussion
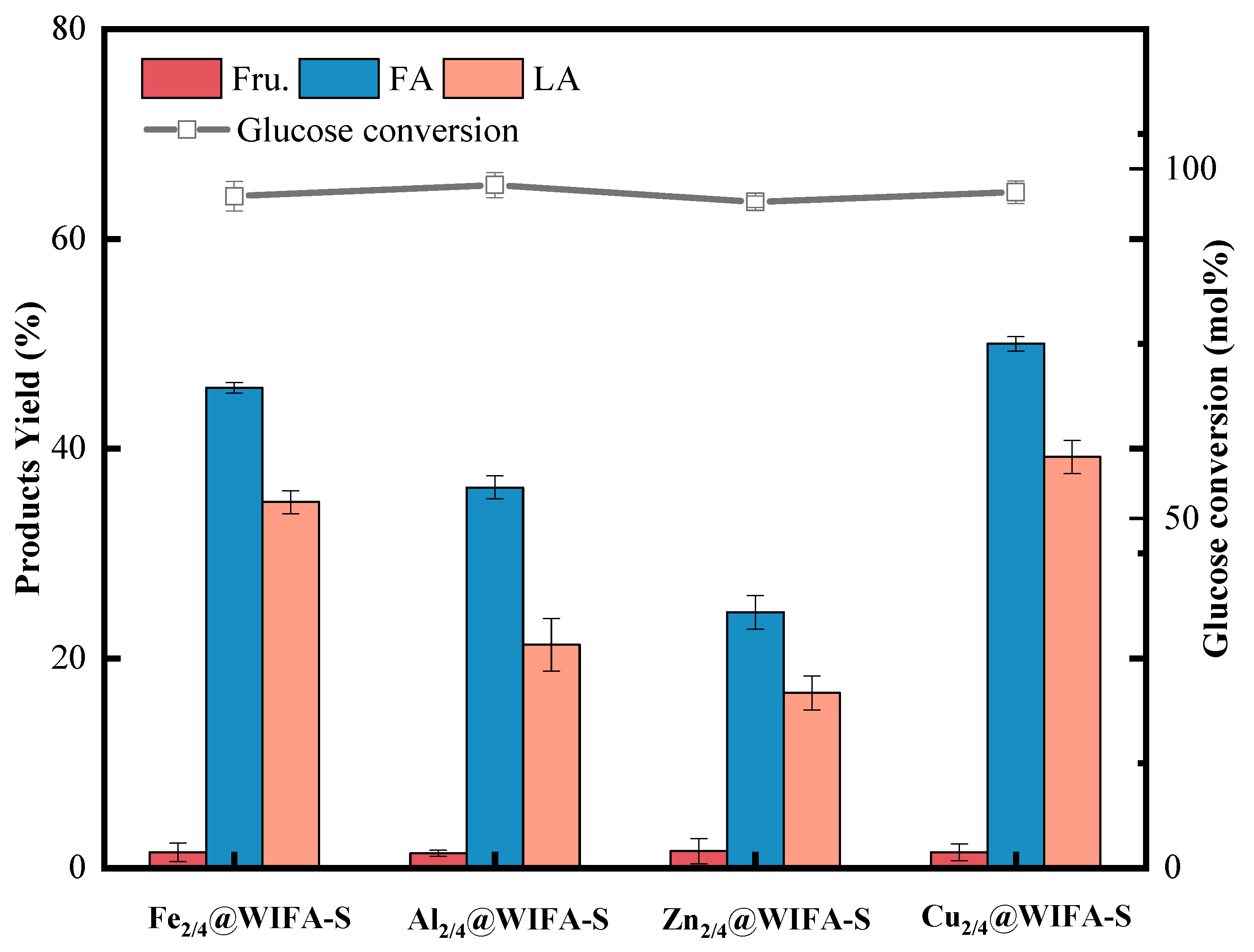
2.1. Effect of Different Metals and Loading Rates on the Preparation of LA from Glucose
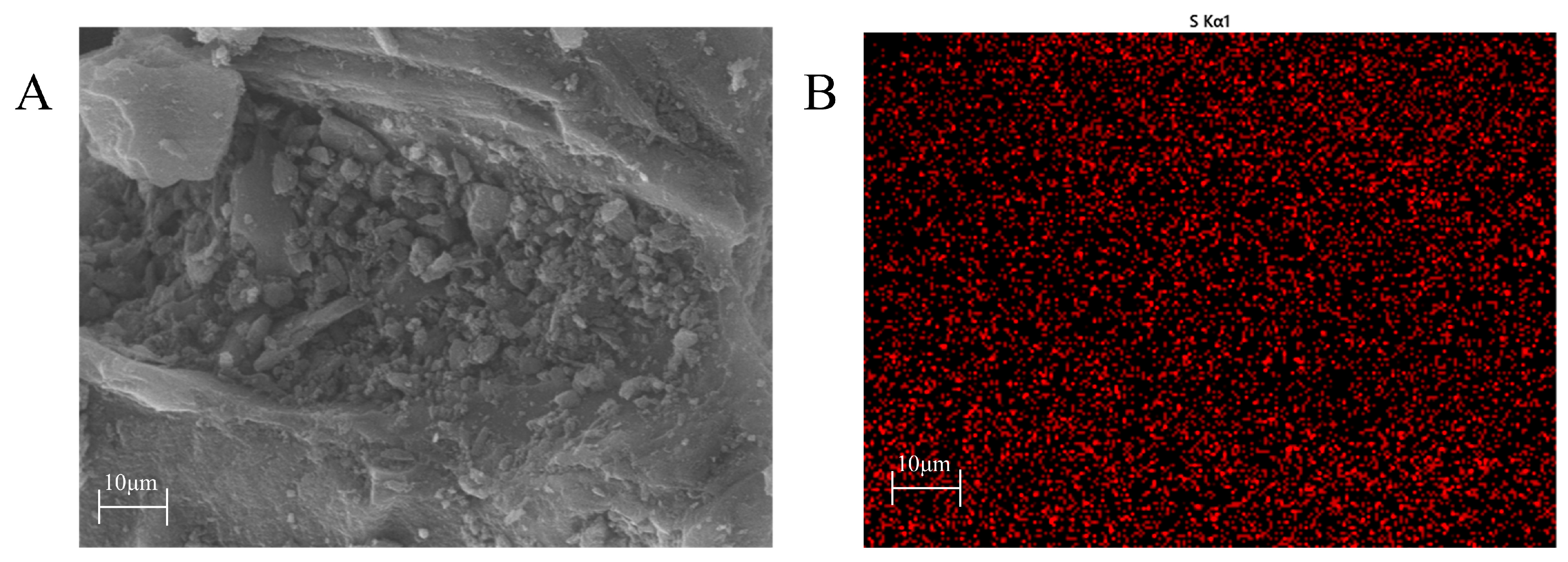
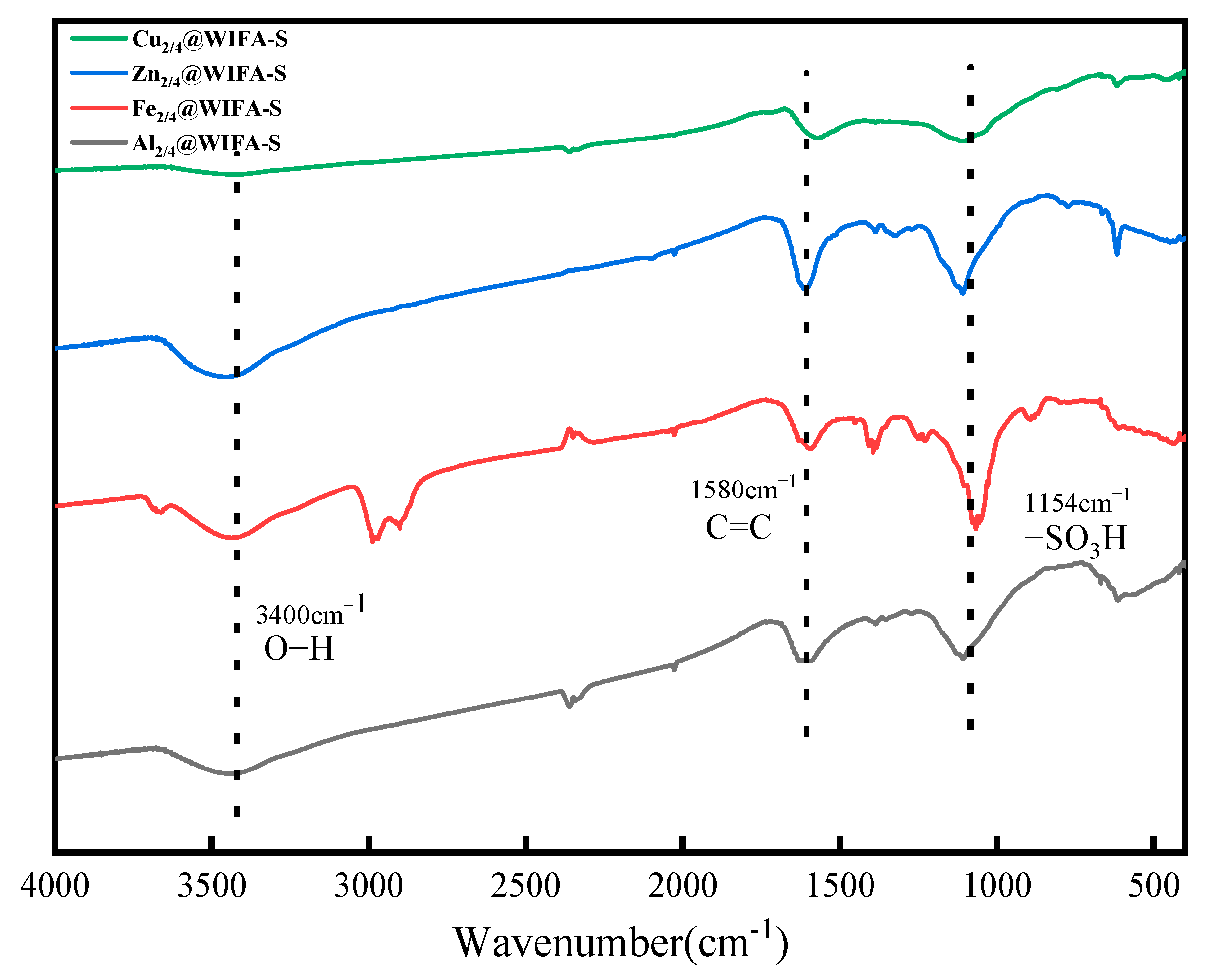
2.2. Catalyst Characterization
2.3. Catalyst Performance Evaluation
2.4. Optimization and Kinetic Analysis of Solvent Systems for LA Production
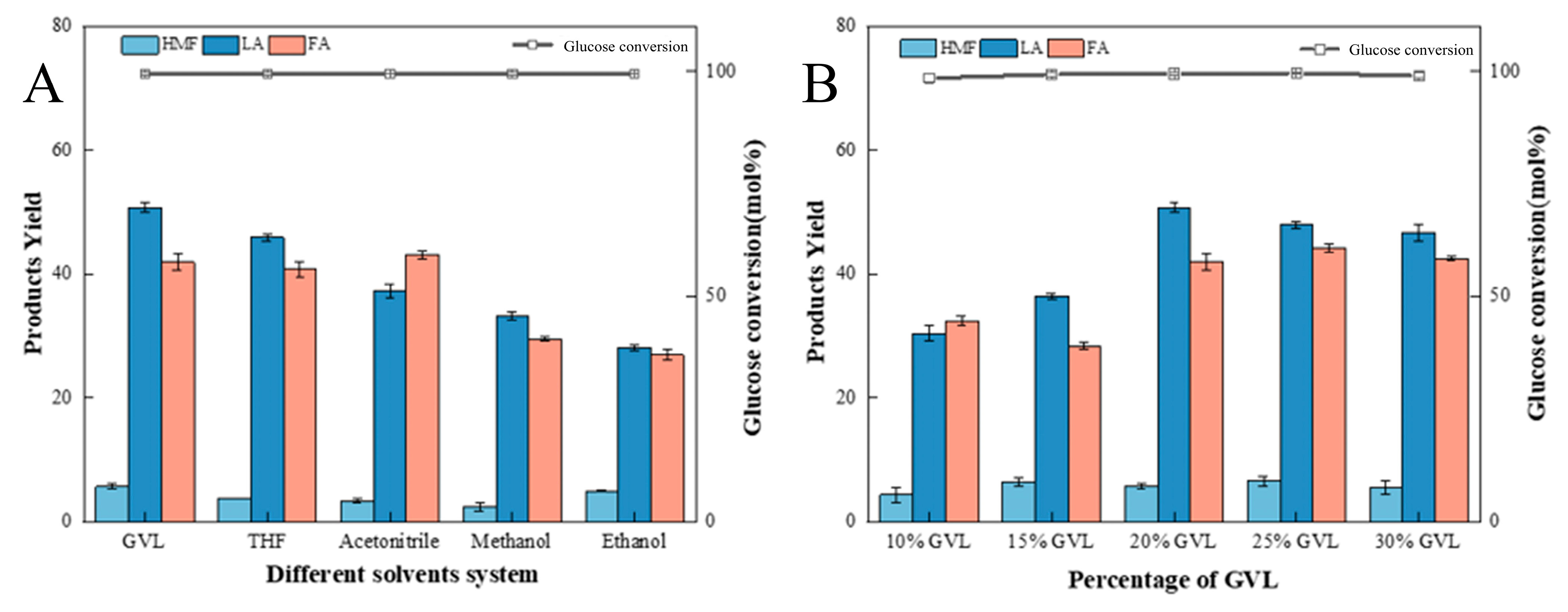
2.4.1. Effect of Different Solvent Systems and Percentages of GVL on LA Preparation from Glucose
2.4.2. Reaction Kinetics of Glucose to LA
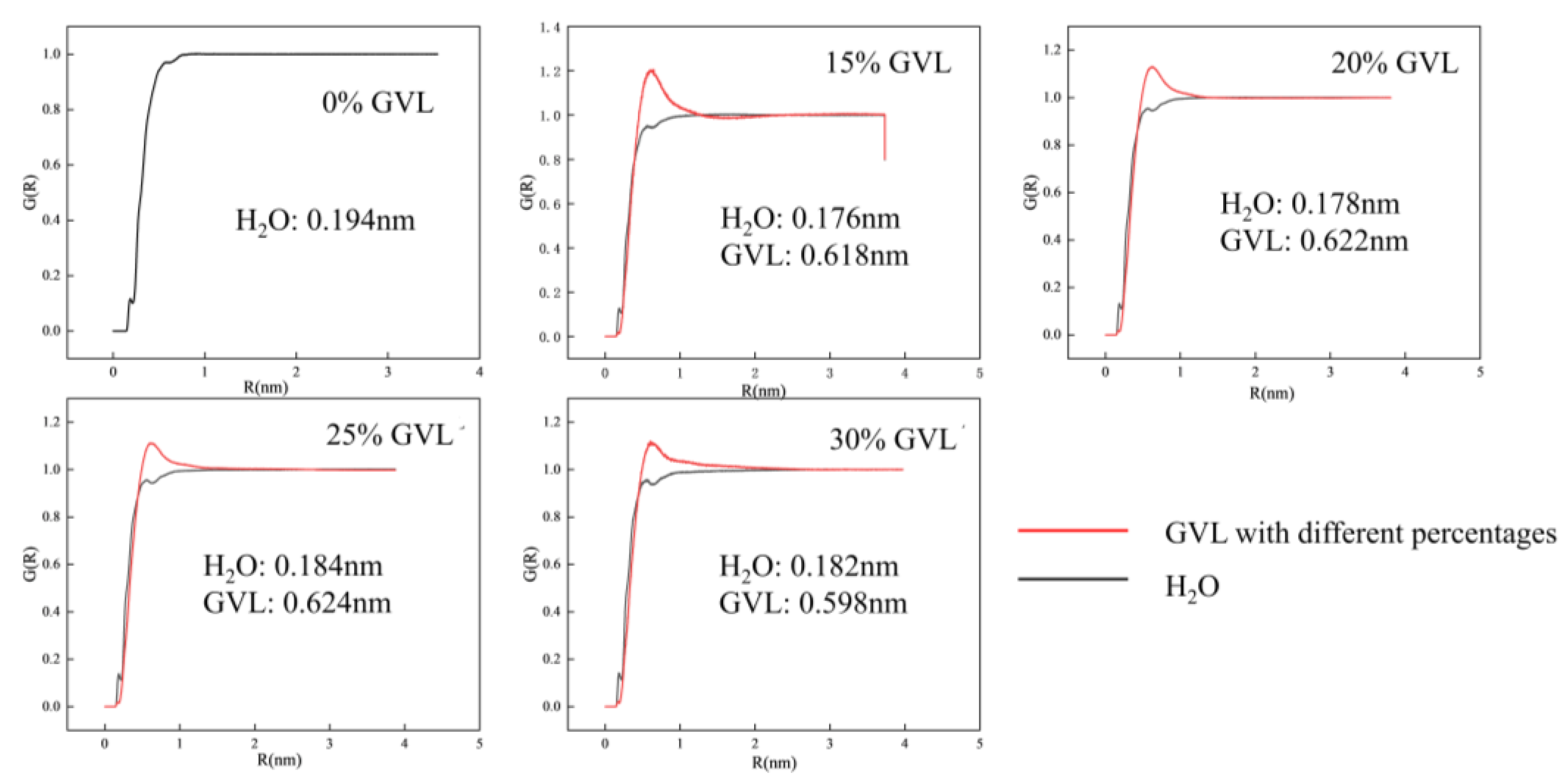
2.4.3. Molecular Dynamics of Glucose to LA
2.5. Catalyst Environmental Risk Assessment
2.5.1. Analysis of Dioxin Compositions in WIFA and WIFA-S
2.5.2. Evaluation of the Risk of Leaching Heavy Metals from Reaction Solutions
3. Materials and Experiments
3.1. Materials
3.2. Preparation and Characterization of Catalysts
3.2.1. Preparation of WIFA-S
3.2.2. Preparation of Metalsx/4@WIFA-S
3.2.3. Metalsx/4@WIFA-S Characterization
3.3. Catalytic Reactions of Metalsx/4@WIFA-S
3.4. Molecular Dynamics Simulation
4. Conclusions
- After screening, Cu2/4@WIFA-S was identified as the best catalyst, and under the pure water system—when the reaction temperature was 180 °C, the reaction time was 4 h, the catalyst dosage was 1 g, and the concentration of glucose was 20 g/L—the glucose conversion rate was 99%, and the highest LA yield was 42.3%.
- It was shown that GVL has a facilitating effect on the reaction that turns glucose into LA. When 10 mL of GVL was added to 50 mL of solvent system, glucose conversion was maintained at 99% and LA yield was 50.7%, which is an increase of 8.4% in LA yield as compared to water as the solvent.
- The reaction kinetic analysis showed that the reaction energy barrier of glucose to LA was 51.33 kJ/mol when 20% GVL was added in the solvent system and 62.35 kJ/mol in the pure water system. The addition of appropriate GVL in the solvent system was favorable for the generation of LA from glucose. Molecular dynamics simulations showed that the addition of GVL provided a protective shell for LA, which reduced the possibility of side reactions of LA and thus improved the yield of LA.
- The dioxin decomposition rate reached 99.87% following the preparation processes of WIFA, effectively achieving catalyst detoxification. The concentrations of all detected heavy metals in the reaction solution were below the standard limits set by GB 8978-1996 for the leaching toxicity of hazardous wastes. That is, it should be a promising approach with low environmental risk for WIFA to prepare value-added bifunctional catalysts for upgrading glucose to levulinic acid.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, Y.; Wu, Z.; Zhou, J.; Zhao, J.; Ruan, X.; Liu, J.; Qian, G. Chemical characteristics and risk assessment of typical municipal solid waste incineration (MSWI) fly ash in China. J. Hazard. Mater. 2013, 261, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, Y.; Wang, X.; Zhu, X.; Liu, X. Chlorine removal technologies for resource utilization of municipal solid waste incineration fly ash. Environ. Res. 2025, 268, 120784. [Google Scholar] [CrossRef]
- Ma, X.D.; Da, Y.; He, T.S.; Su, F.Y.; Wan, Z.M. Improvement of harmlessness and resource utilization of incineration fly ash by high temperature sintering. J. Build. Eng. 2024, 84, 108589. [Google Scholar] [CrossRef]
- Xiao, Y.X.; Huang, Y.J.; Cheng, H.Q.; Zhu, Z.C.; Yu, M.Z.; Xu, W.T.; Li, Z.Y.; Zuo, W.; Zhou, H.Y.; Jin, B.S. Advances and Outlook of Heavy Metal Treatment Technology from Municipal Solid Waste Incineration Fly Ash: A Review. Energy Fuels 2023, 38, 895–918. [Google Scholar] [CrossRef]
- Lawagon, C.P.; Faungnawakij, K.; Srinives, S.; Thongratkaew, S.; Chaipojjana, K.; Smuthkochorn, A.; Srisrattha, P.; Charinpanitkul, T. Sulfonated graphene oxide from petrochemical waste oil for efficient conversion of fructose into levulinic acid. Cat. Today 2021, 375, 197–203. [Google Scholar] [CrossRef]
- Pan, X.; Yan, J.; Xie, Z. Detoxifying PCDD/Fs and heavy metals in fly ash from medical waste incinerators with a DC double arc plasma torch. J. Environ. Sci. 2013, 25, 1362–1367. [Google Scholar] [CrossRef]
- Wu, H.-L.; Lu, S.-Y.; Yan, J.-H.; Li, X.-D.; Chen, T. Thermal removal of PCDD/Fs from medical waste incineration fly ash–effect of temperature and nitrogen flow rate. Chemosphere 2011, 84, 361–367. [Google Scholar] [CrossRef]
- De Greef, J.; Villani, K.; Goethals, J.; Van Belle, H.; Van Caneghem, J.; Vandecasteele, C. Optimising energy recovery and use of chemicals, resources and materials in modern waste-to-energy plants. Waste Manag. 2013, 33, 2416–2424. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Yang, H.; Wang, Y. Overview of resources reuse technologies and corresponding products for municipal solid waste incineration fly ash. Environ. Chem. 2023, 42, 2669–2687. [Google Scholar]
- Ma, W.; Chen, D.; Pan, M.; Gu, T.; Zhong, L.; Chen, G.; Yan, B.; Cheng, Z. Performance of chemical chelating agent stabilization and cement solidification on heavy metals in MSWI fly ash: A comparative study. J. Environ. Manag. 2019, 247, 169–177. [Google Scholar] [CrossRef]
- Yu, D.; Li, Z.; Li, J.; He, J.; Li, B.; Wang, Y. Enhancement of H2 and light oil production and CO2 emission mitigation during co-pyrolysis of oily sludge and incineration fly ash. J. Hazard. Mater. 2024, 462, 132618. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Yang, Y.; Ren, T.; Pan, L.; Fang, P.; Chen, D.; Cen, C. Modified fly ash from municipal solid waste incineration as catalyst support for Mn-Ce composite oxides. IOP Conf. Ser. Earth Environ. Sci. 2017, 18, 012146. [Google Scholar] [CrossRef]
- Feng, W.; Lu, X.; Xiong, J.; Yu, Z.; Wang, Y.; Cui, J.; Zhang, R.; Weng, R. Solid–Waste–Derived Geopolymer–Type Zeolite–like High Functional Catalytic Materials Catalyze Efficient Hydrogenation of Levulinic Acid. Catalysts 2022, 12, 1361. [Google Scholar] [CrossRef]
- de Guzman, D.; de Leon, R. Preliminary Optimization and Kinetics of SnCl2-HCl Catalyzed Hydrothermal Conversion of Microcrystalline Cellulose to Levulinic Acid. J. Renew. Mater. 2021, 9, 145–162. [Google Scholar] [CrossRef]
- Iris, K.; Hanif, A.; Tsang, D.C.; Yip, A.C.; Lin, K.-Y.A.; Gao, B.; Ok, Y.S.; Poon, C.S.; Shang, J. Tailoring acidity and porosity of alumina catalysts via transition metal doping for glucose conversion in biorefinery. Sci. Total Environ. 2020, 704, 135414. [Google Scholar]
- Zhang, X.; Zhang, X.; Sun, N.; Wang, S.; Wang, X.; Jiang, Z. High production of levulinic acid from cellulosic feedstocks being catalyzed by temperature-responsive transition metal substituted heteropolyacids. Renew. Energy 2019, 141, 802–813. [Google Scholar] [CrossRef]
- Taghavi, S.; Ghedini, E.; Menegazzo, F.; Giordana, A.; Cerrato, G.; Cruciani, G.; Di Michele, A.; Zendehdel, M.; Signoretto, M. Balanced acidity by microwave-assisted ion-exchange of ZSM-5 zeolite as a catalyst for transformation of glucose to levulinic acid. Biomass Convers. Biorefin. 2022, 14, 8251–8269. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, G.Y.; Gao, M.M.; Cao, S.S.; Li, L.; Liu, S.W.; Xie, C.X.; Huang, L.; Yu, S.T.; Ragauskas, A.J. Clean production of 5-hydroxymethylfurfural from cellulose using a hydrothermal/biomass-based carbon catalyst. J. Clean. Prod. 2019, 213, 1096–1102. [Google Scholar] [CrossRef]
- Zeng, D.L.; Zhang, Q.; Chen, S.Y.; Liu, S.L.; Wang, G.H. Synthesis porous carbon-based solid acid from rice husk for esterification of fatty acids. Microporous Mesoporous Mater. 2016, 219, 54–58. [Google Scholar] [CrossRef]
- Qi, L.; Mui, Y.F.; Lo, S.W.; Lui, M.Y.; Akien, G.R.; Horváth, I.T. Catalytic Conversion of Fructose, Glucose, and Sucrose to 5-(Hydroxymethyl)furfural and Levulinic and Formic Acids in γ-Valerolactone As a Green Solvent. ACS Catal. 2014, 4, 1470–1477. [Google Scholar] [CrossRef]
- Thiyam, D.S.; Nongmeikapam, A.C.; Nandeibam, A.D.; Heikham, F.D.; Henam, P.S. Biosynthesized Quantum Dot Size Cu Nanocatalyst: Peroxidase Mimetic and Aqueous Phase Conversion of Fructose. Chem. Sel. 2018, 3, 12183–12191. [Google Scholar] [CrossRef]
- Hu, L.; Wu, Z.; Xu, J.; Sun, Y.; Lin, L.; Liu, S. Zeolite-promoted transformation of glucose into 5-hydroxymethylfurfural in ionic liquid. Chem. Eng. J. 2014, 244, 137–144. [Google Scholar] [CrossRef]
- Tyagi, U.; Anand, N.; Kumar, D. Synergistic effect of modified activated carbon and ionic liquid in the conversion of microcrystalline cellulose to 5-Hydroxymethyl Furfural. Bioresour. Technol. 2018, 267, 326–332. [Google Scholar] [CrossRef]
- Shi, N.; Liu, Q.; Ju, R.; He, X.; Zhang, Y.; Tang, S.; Ma, L. Condensation of α-carbonyl aldehydes leads to the formation of solid humins during the hydrothermal degradation of carbohydrates. ACS Omega 2019, 4, 7330–7343. [Google Scholar] [CrossRef]
- Kumar, K.; Parveen, F.; Patra, T.; Upadhyayula, S. Hydrothermal Conversion of Glucose to Levulinic acid using Multifunctional Ionic Liquids: Effect of metal ion co-catalysts on Product Yield. New J. Chem. 2017, 42, 228–236. [Google Scholar] [CrossRef]
- Wu, Y.; Song, X.; Cai, F.; Xiao, G. Synthesis of glycerol carbonate from glycerol and diethyl carbonate over Ce-NiO catalyst: The role of multiphase Ni. J. Alloys Compd. 2017, 720, 360–368. [Google Scholar] [CrossRef]
- Ramli, N.A.S.; Amin, N.A.S. A new functionalized ionic liquid for efficient glucose conversion to 5-hydroxymethyl furfural and levulinic acid. J. Mol. Catal. A Chem. 2015, 407, 113–121. [Google Scholar] [CrossRef]
- Shan, H.; Li, L.; Bai, W.; Liu, L. Evolution Process of Humins Derived from Glucose. Chem. Sel. 2022, 7, e202201237. [Google Scholar] [CrossRef]
- Guo, H.; Duereh, A.; Su, Y.; Hensen, E.J.; Qi, X.; Smith, R.L., Jr. Mechanistic role of protonated polar additives in ethanol for selective transformation of biomass-related compounds. Appl. Catal. B Environ. 2020, 264, 118509. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, Y.; Guo, S.; Cao, X.; Pan, A.; Fang, G.; Zhou, J.; Liang, S. Fundamentals and perspectives in developing zinc-ion battery electrolytes: A comprehensive review. Energy Environ. Sci. 2020, 13, 4625–4665. [Google Scholar] [CrossRef]
- Yu, P.; Liu, X.; Lei, Y.; Gao, Y.; Peng, H. Study on Restart Safety of Waxy Crude Pipelines Based on Reliability Principle under Constant Flow. ACS Omega 2022, 7, 10687–10694. [Google Scholar] [CrossRef]
- Mellmer, M.A.; Sanpitakseree, C.; Demir, B.; Ma, K.; Elliott, W.A.; Bai, P.; Johnson, R.L.; Walker, T.W.; Shanks, B.H.; Rioux, R.M.; et al. Effects of chloride ions in acid-catalyzed biomass dehydration reactions in polar aprotic solvents. Nat. Commun. 2019, 10, 1132. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, C.; Fulignati, S.; Licursi, D.; Raspolli Galletti, A.M. Turning point toward the sustainable production of 5-hydroxymethyl-2-furaldehyde in water: Metal salts for its synthesis from fructose and inulin. ACS Sustain. Chem. Eng. 2019, 7, 6830–6838. [Google Scholar] [CrossRef]
- Wei, W.; Wu, S. Experimental and kinetic study of glucose conversion to levulinic acid in aqueous medium over Cr/HZSM-5 catalyst. Fuel 2018, 225, 311–321. [Google Scholar] [CrossRef]
- Mushrif, S.H.; Caratzoulas, S.; Vlachos, D.G. Understanding solvent effects in the selective conversion of fructose to 5-hydroxymethyl-furfural: A molecular dynamics investigation. Phys. Chem. Chem. Phys. 2012, 14, 2637–2644. [Google Scholar] [CrossRef]
- Vasudevan, V.; Mushrif, S.H. Insights into the solvation of glucose in water, dimethyl sulfoxide (DMSO), tetrahydrofuran (THF) and N,N-dimethylformamide (DMF) and its possible implications on the conversion of glucose to platform chemicals. R. Soc. Chem. 2015, 5, 20756–20763. [Google Scholar] [CrossRef]
- Lin, Y.; Wei, G.; Liu, H.; Liu, Z.; Li, Q. Innovative Technology for Secondary Fly Ash Full Resource Utilization: Industrial Testing and Life Cycle Assessment Research. ACS Sustain. Chem. Eng. 2024, 12, 17770–17782. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecularsimulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]






| No. | Catalyst | Specific Surface Area (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) | Amount of Weak Acid (mmol/g) | Amount of Strong Acid (mmol/g) | Total Acid Quantity (mmol/g) |
|---|---|---|---|---|---|---|---|
| 1 | Zn2/4@WIFA-S | 238 | 3.03 | 4.38 | 0.621 | 1.681 | 2.302 |
| 2 | Fe2/4@WIFA-S | 267 | 3.07 | 5.61 | 0.158 | 2.166 | 2.324 |
| 3 | Al2/4@WIFA-S | 84 | 6.75 | 0.16 | 0.15 | 2.117 | 2.267 |
| 4 | Cu2/4@WIFA-S | 190 | 3.78 | 0.50 | 8.273 | 16.069 | 24.342 |
| 5 | WIFA-S | 399 | 5.15 | 8.33 | — | — | — |
| Solvent Systems | Tem. (°C) | Glucose Dehydration KG | Ea (kJ/mol) | HMF Rehydration KH | Ea (kJ/mol) |
|---|---|---|---|---|---|
| H2O | 160 | 0.06769 | 54.95 | 0.11013 | 7.4 |
| 180 | 0.0881 | 0.12427 | |||
| 200 | 0.24956 | 0.13086 | |||
| 15% GVL | 160 | 0.10209 | 18.37 | 0.3408 | 25.05 |
| 180 | 0.12709 | 0.3985 | |||
| 200 | 0.15727 | 0.617 | |||
| 20% GVL | 160 | 0.3829 | 30.38 | 0.08151 | 20.95 |
| 180 | 0.46388 | 0.14557 | |||
| 200 | 0.62801 | 0.16549 | |||
| 25% GVL | 160 | 0.08961 | 44.45 | 0.3932 | 50.84 |
| 180 | 0.16211 | 0.7683 | |||
| 200 | 0.25451 | 1.2985 |
| Test Elements | Reaction Solution (mg/L) | Standard Limit Value (Class I Standard of GB8978–1996) (mg/L) |
|---|---|---|
| Cu | 0.021 | ≤0.5 |
| Zn | N.A. | ≤2 |
| Pb | 0.442 | ≤1 |
| Cd | 0.100 | ≤0.1 |
| Name | CaO | SO3 | SiO2 | Cl | Fe2O3 | MgO | Al2O3 | K2O | TiO2 | ZaO | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WIFA | 57.27 | 10.73 | 5.55 | 4.5 | 3.57 | 2.45 | 1.47 | 2.93 | 1.1 | 1.72 | 19.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Wu, H.; Li, J.; Chen, D.; Li, S.; Chen, J.; Li, X.; Xiong, J.; Yu, Z.; Lu, X. Waste Incineration Fly Ash-Based Bifunctional Catalyst for Upgrading Glucose to Levulinic Acid. Catalysts 2025, 15, 402. https://doi.org/10.3390/catal15040402
Zhang R, Wu H, Li J, Chen D, Li S, Chen J, Li X, Xiong J, Yu Z, Lu X. Waste Incineration Fly Ash-Based Bifunctional Catalyst for Upgrading Glucose to Levulinic Acid. Catalysts. 2025; 15(4):402. https://doi.org/10.3390/catal15040402
Chicago/Turabian StyleZhang, Rui, Han Wu, Jiantao Li, Dezhi Chen, Shimin Li, Jiale Chen, Xiaoyun Li, Jian Xiong, Zhihao Yu, and Xuebin Lu. 2025. "Waste Incineration Fly Ash-Based Bifunctional Catalyst for Upgrading Glucose to Levulinic Acid" Catalysts 15, no. 4: 402. https://doi.org/10.3390/catal15040402
APA StyleZhang, R., Wu, H., Li, J., Chen, D., Li, S., Chen, J., Li, X., Xiong, J., Yu, Z., & Lu, X. (2025). Waste Incineration Fly Ash-Based Bifunctional Catalyst for Upgrading Glucose to Levulinic Acid. Catalysts, 15(4), 402. https://doi.org/10.3390/catal15040402








