Recent Advances in Enantioselective Transition Metal Catalysis Mediated by Ligand–Substrate Noncovalent Interactions
Abstract
1. Introduction
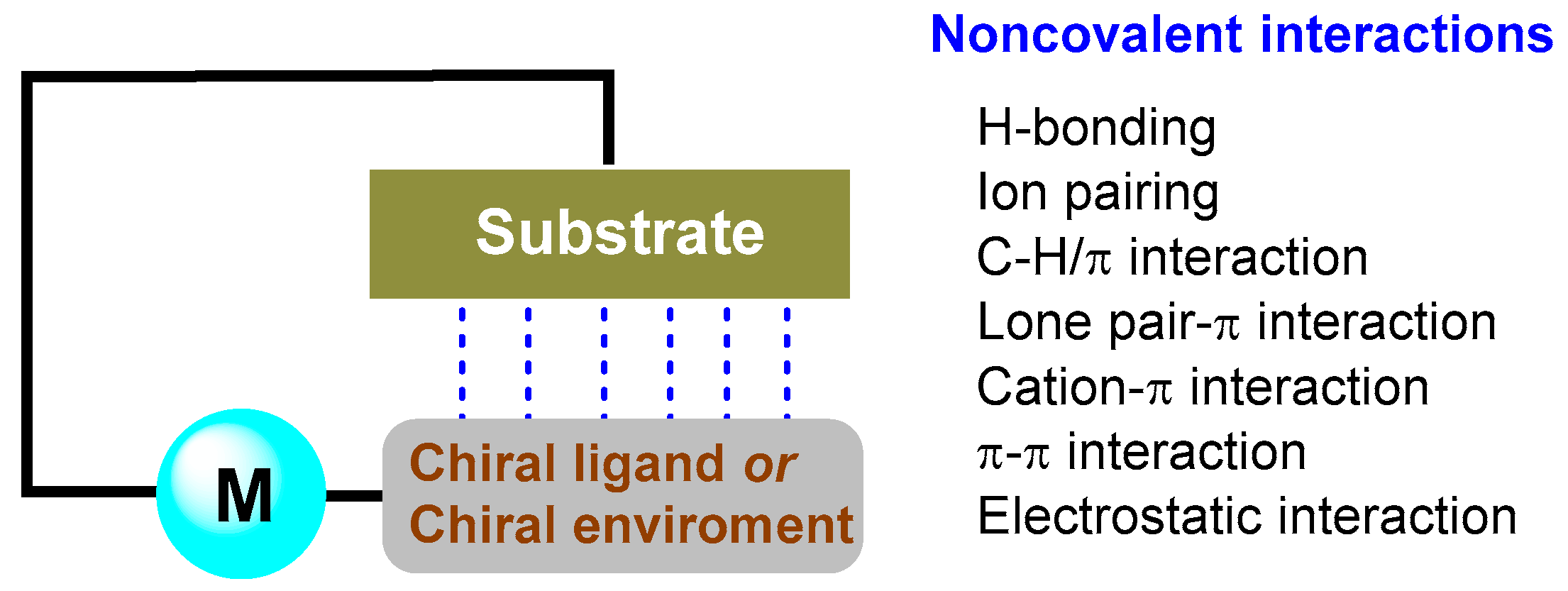
2. Ligand–Substrate Noncovalent Interactions
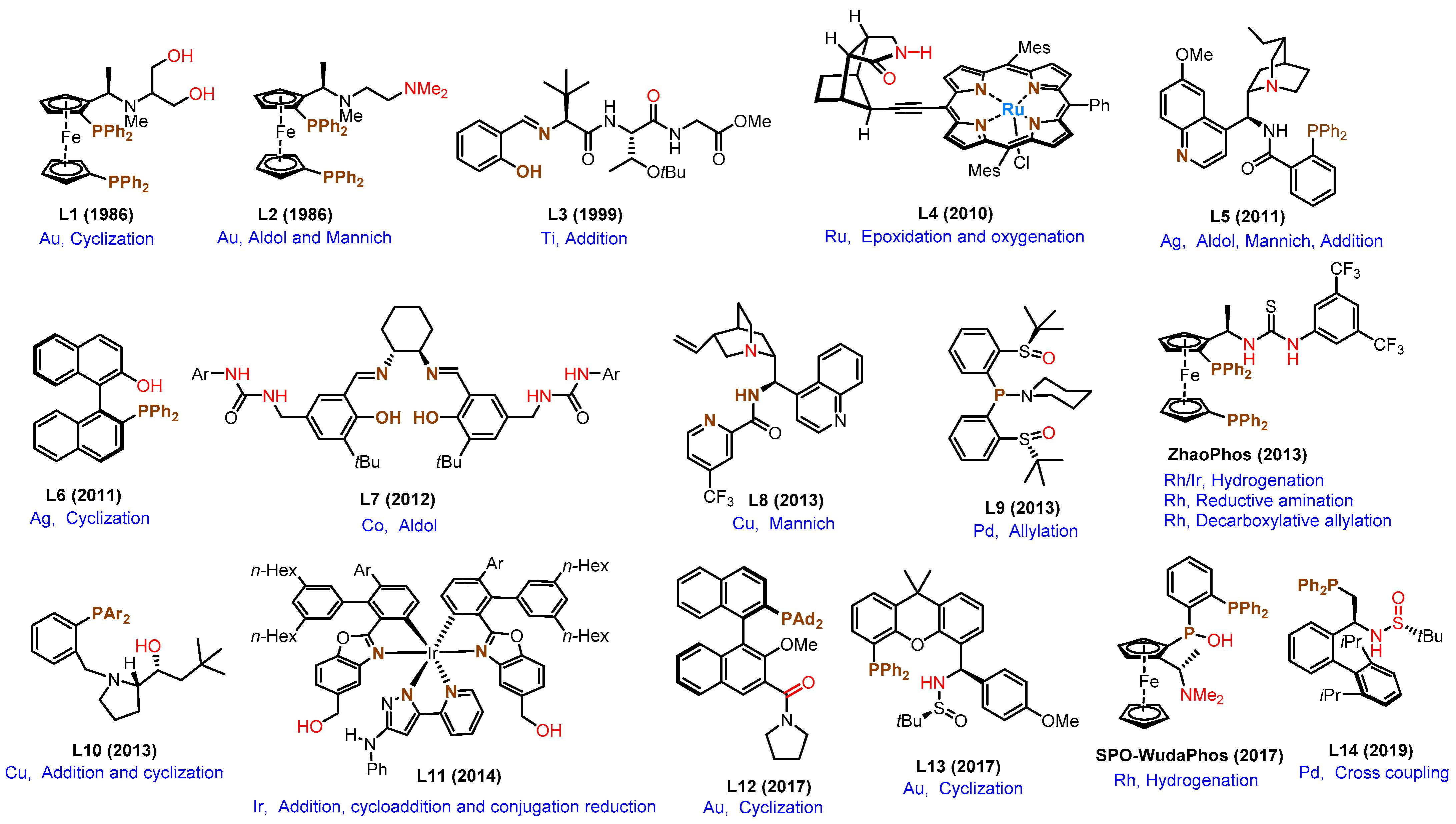
2.1. Representative Examples Involve NCIs Before 2020
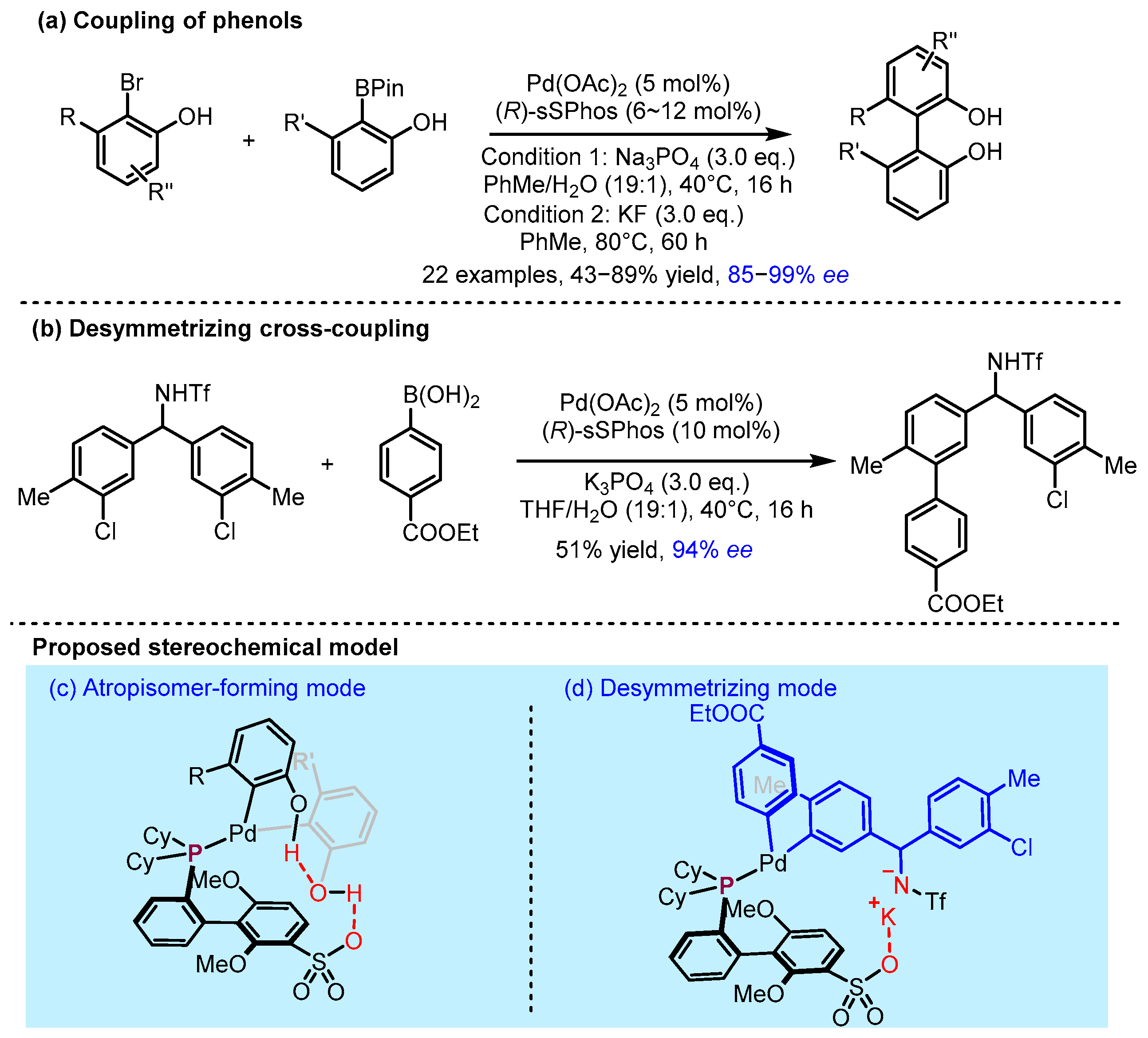
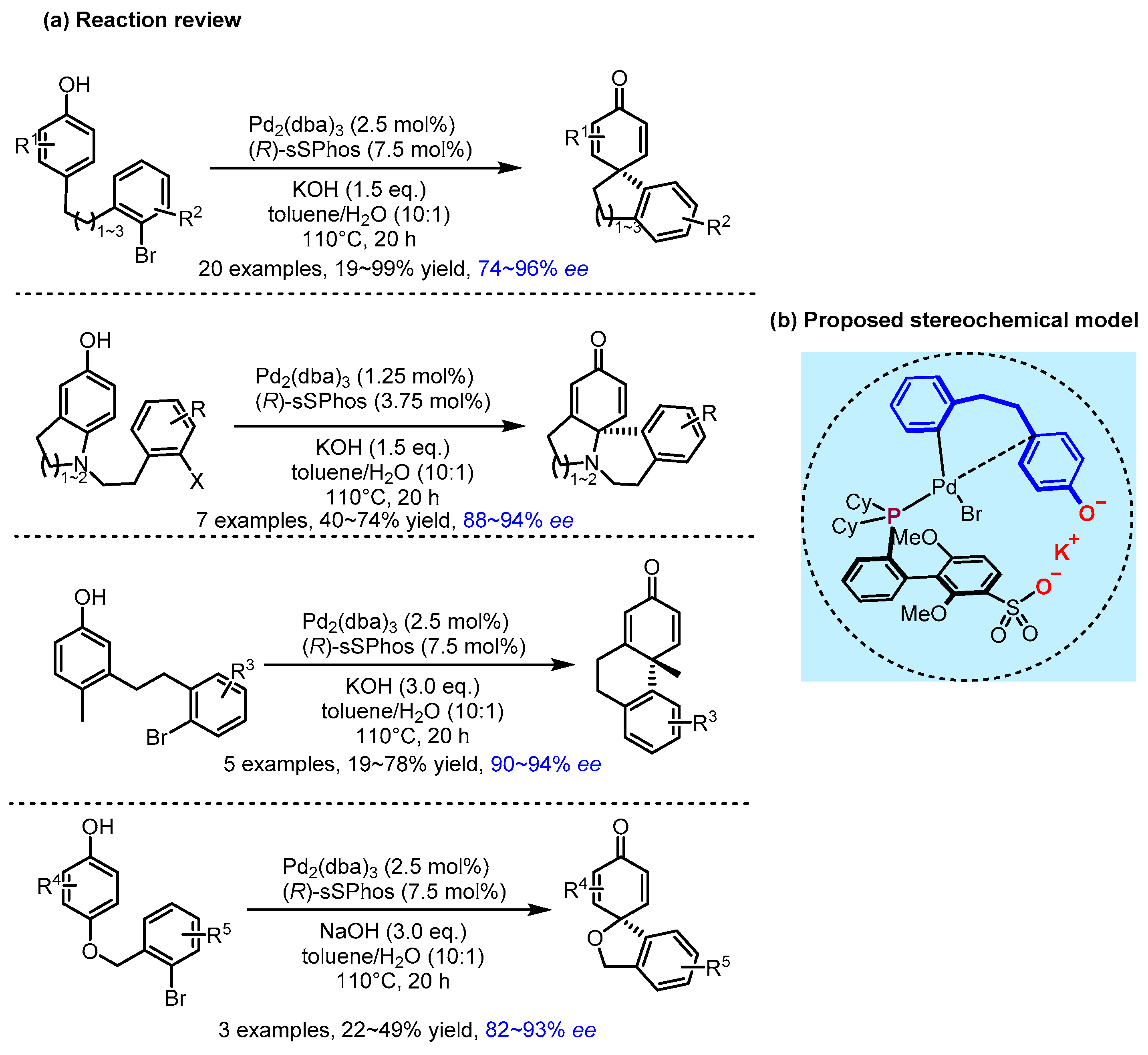
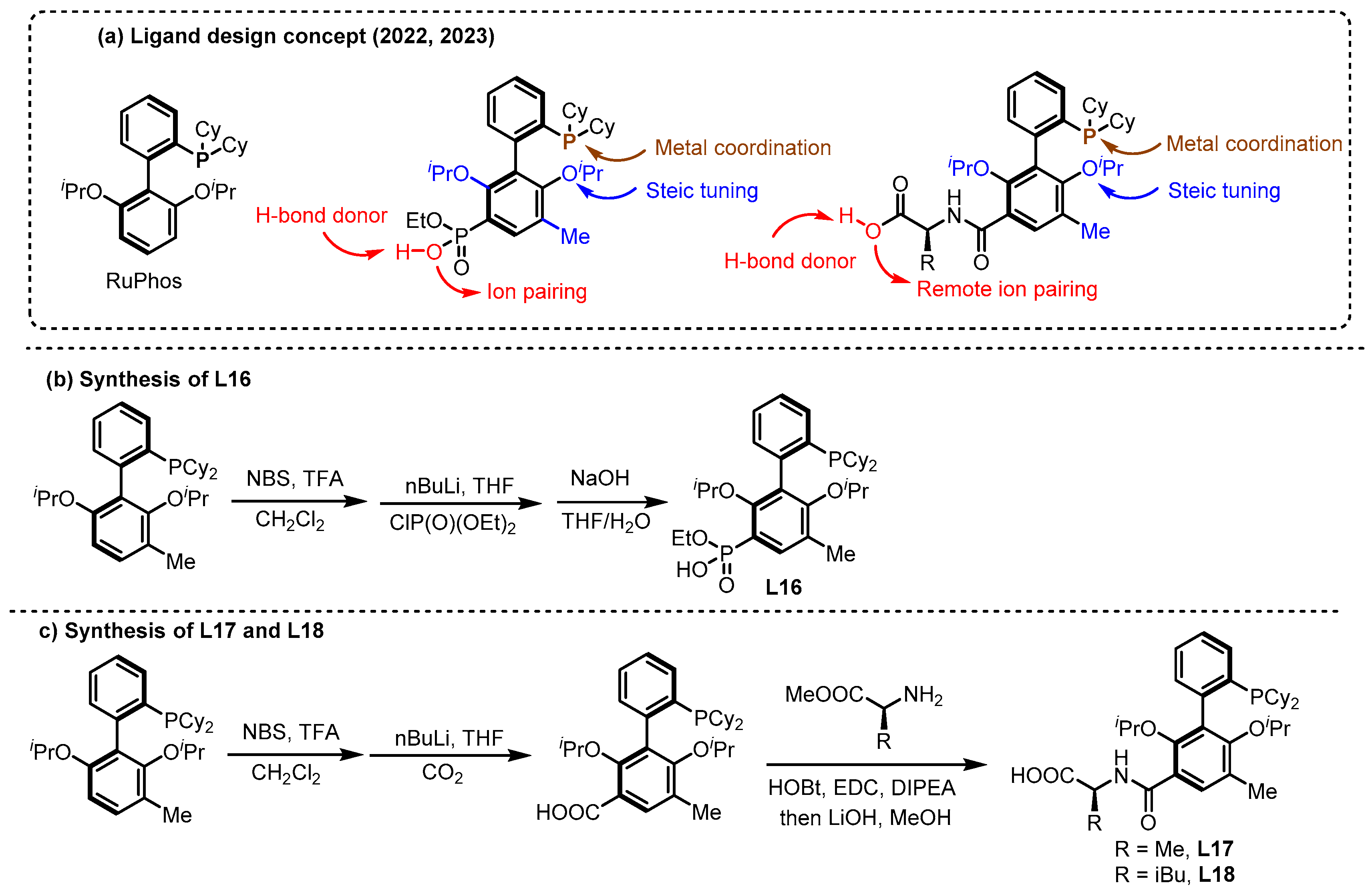
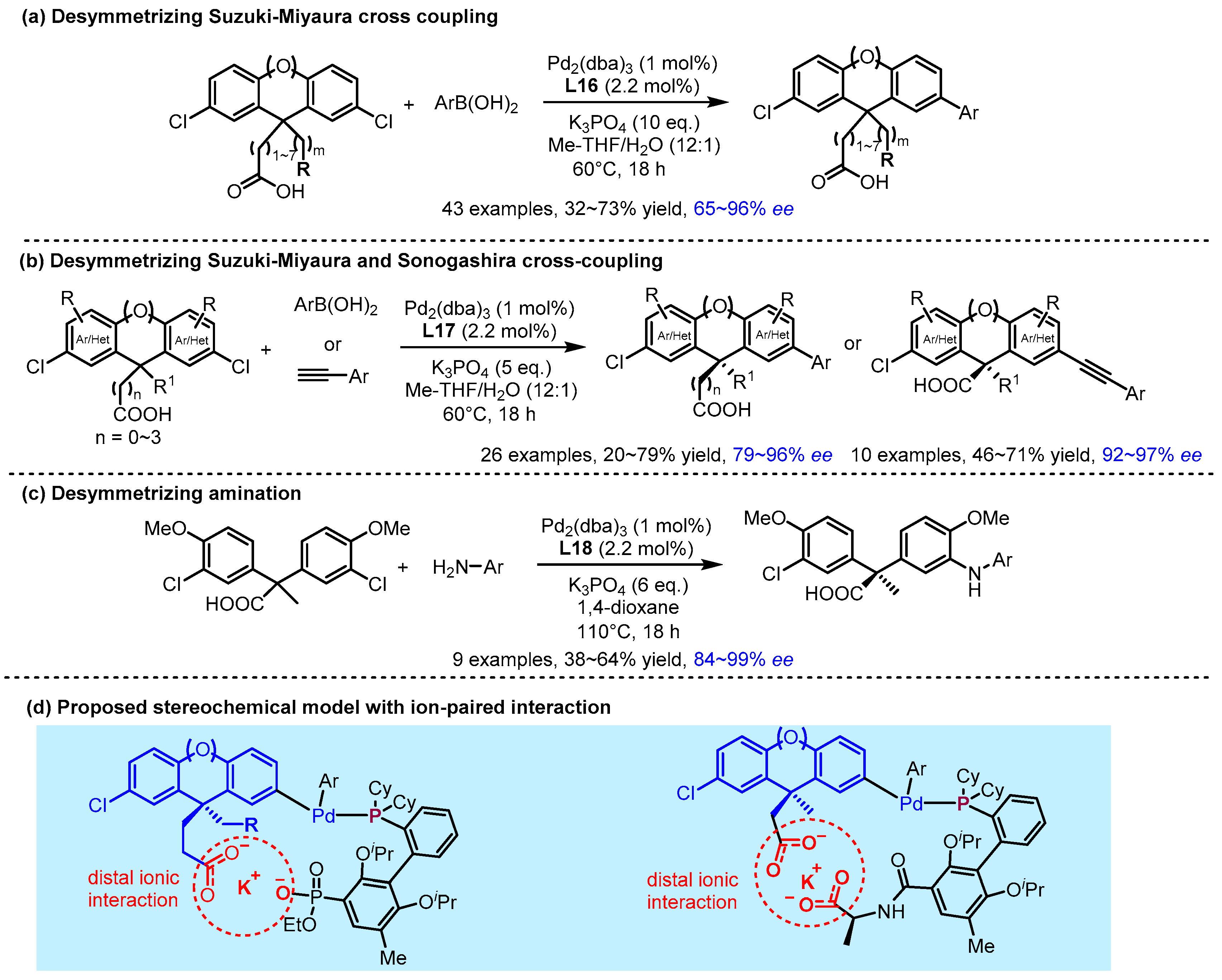
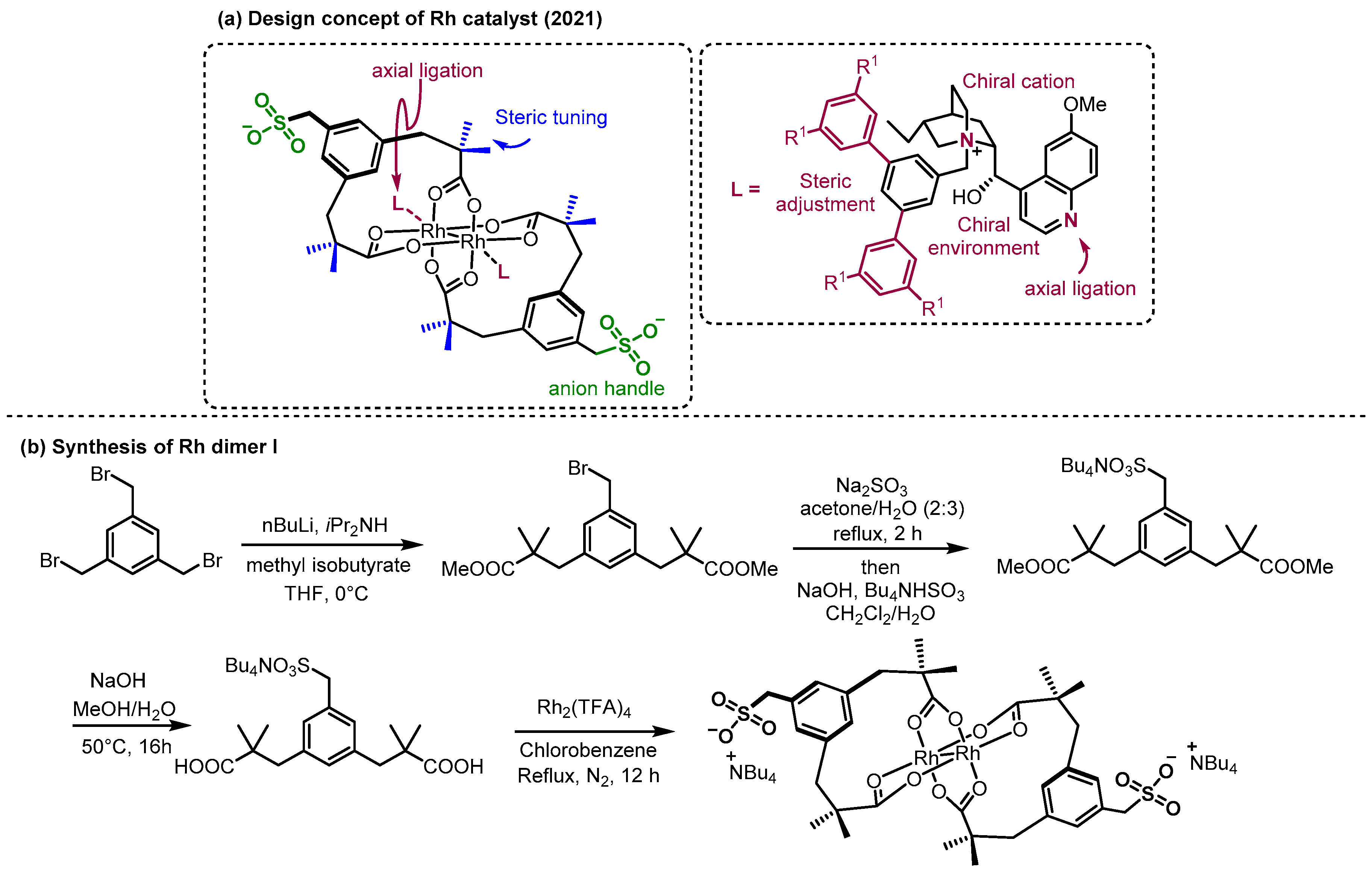
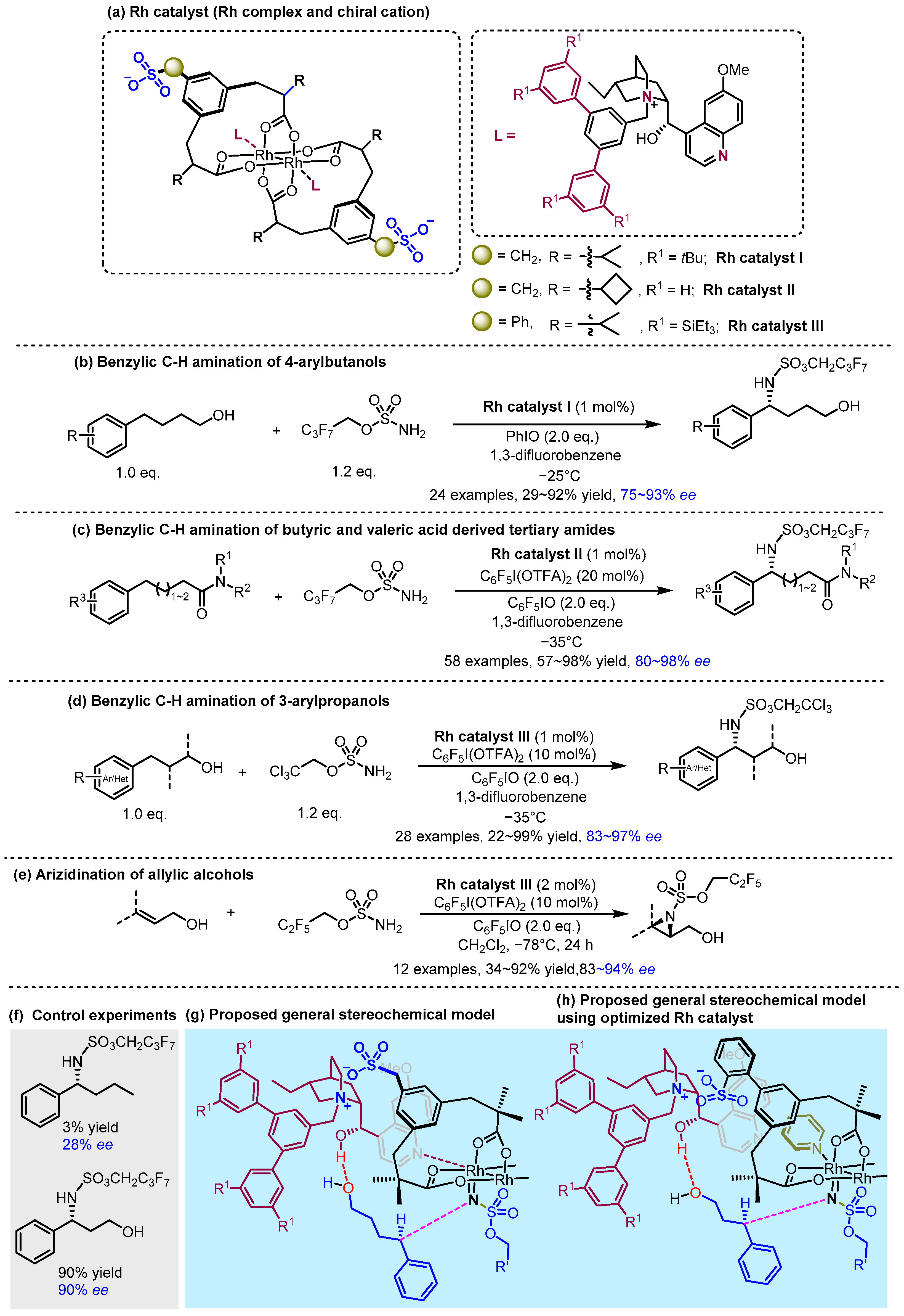
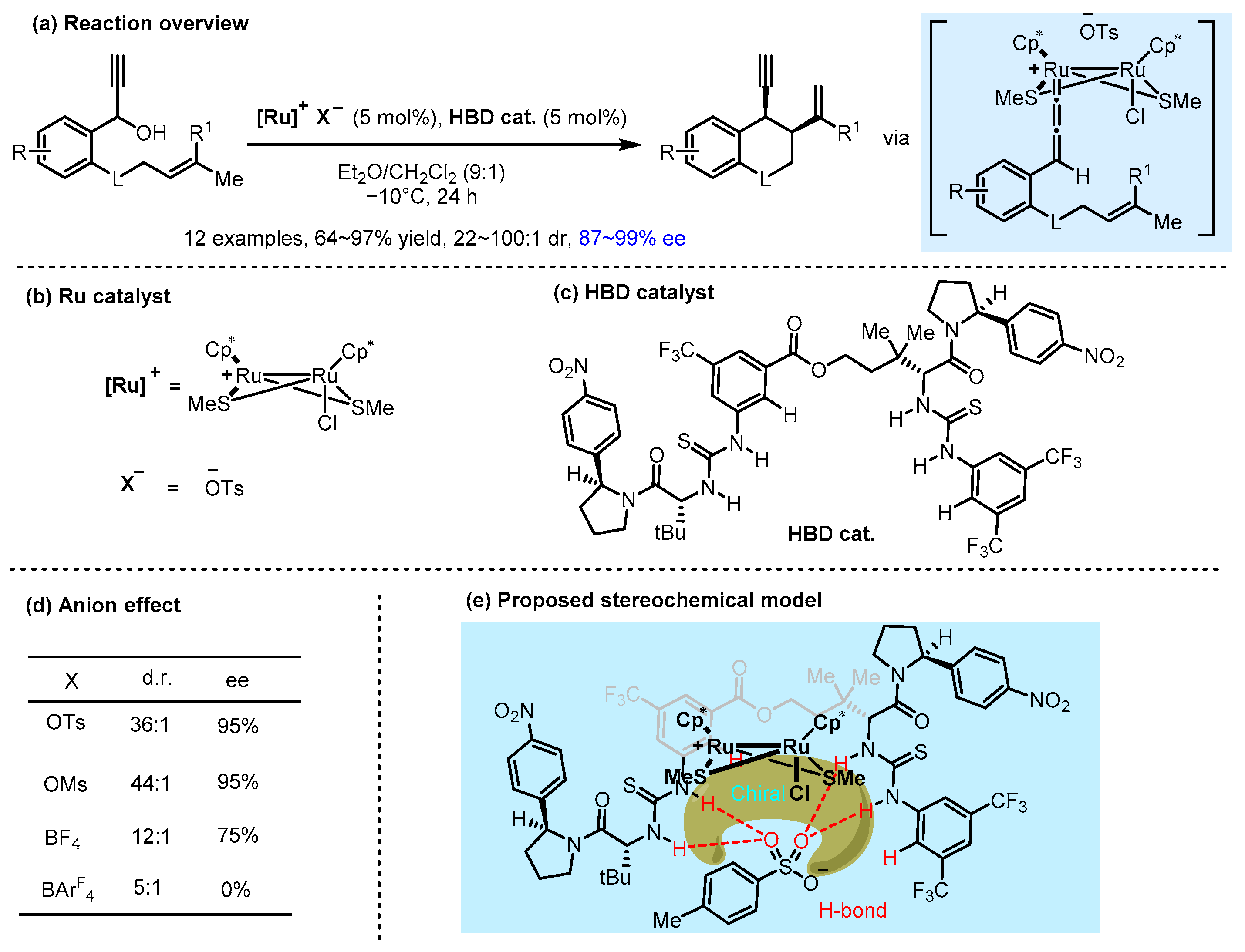
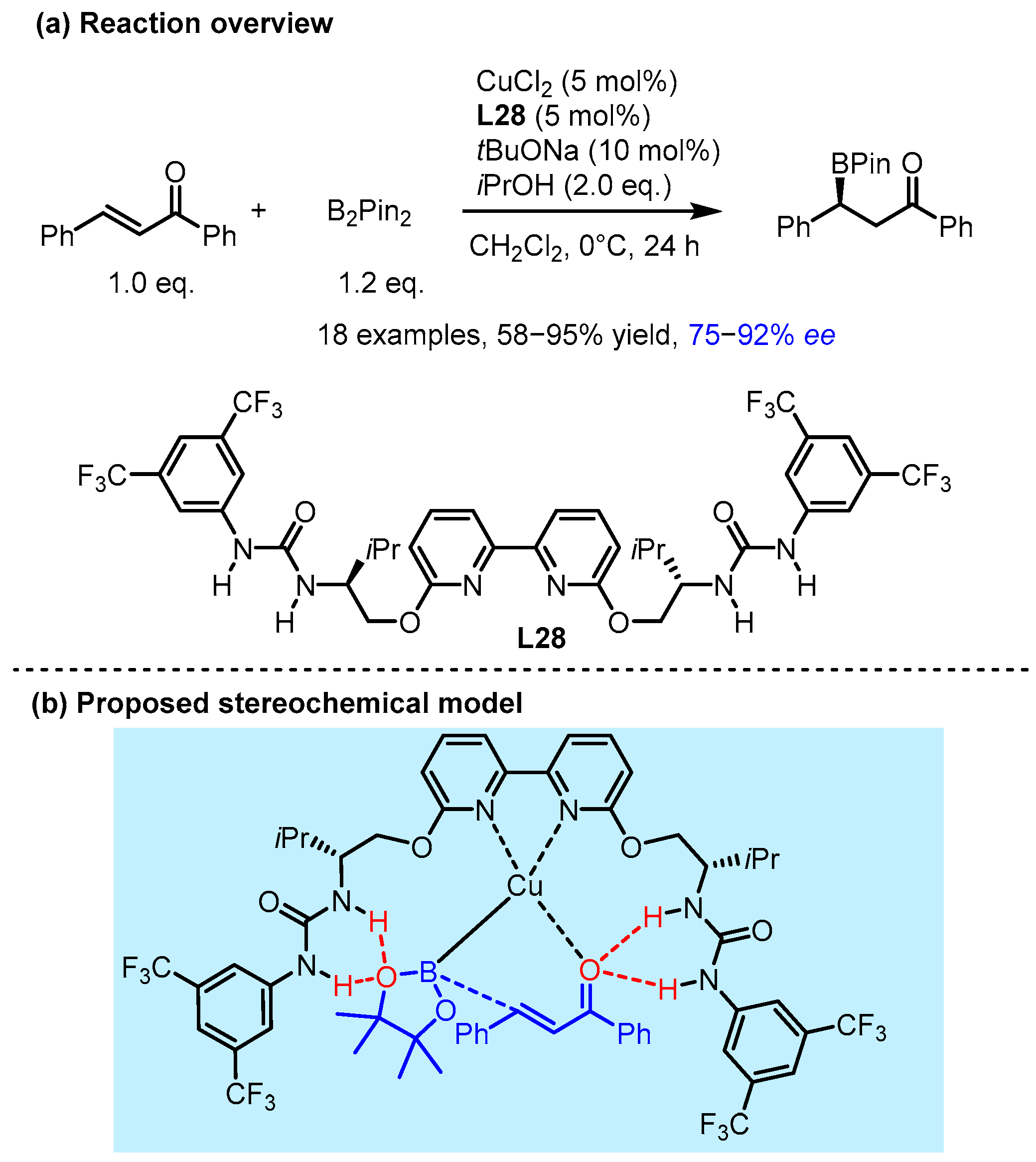
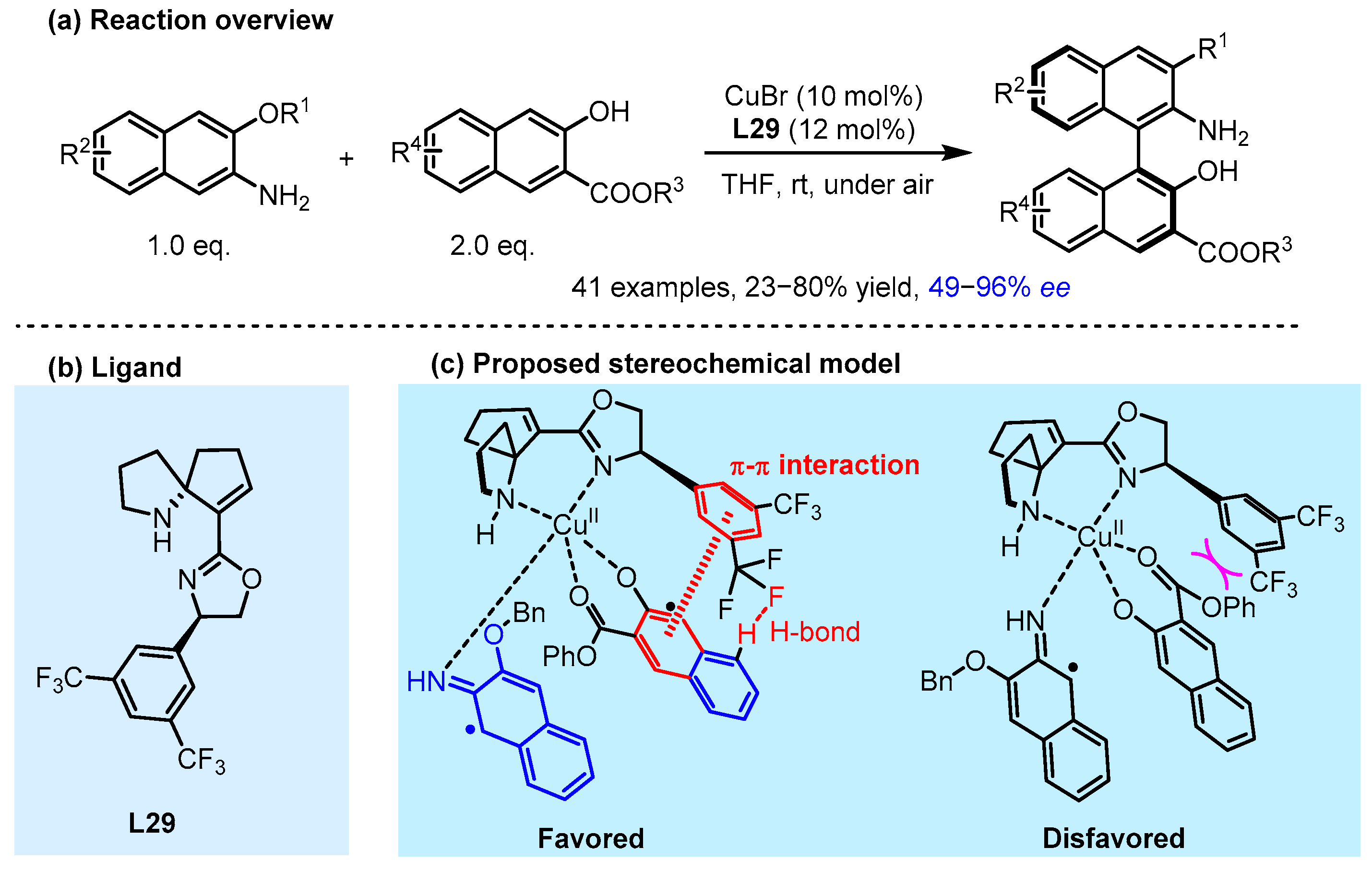
2.2. Hydrogen Bonding and Ion Pairing Involved Enantioselective Transition Metal Catalysis
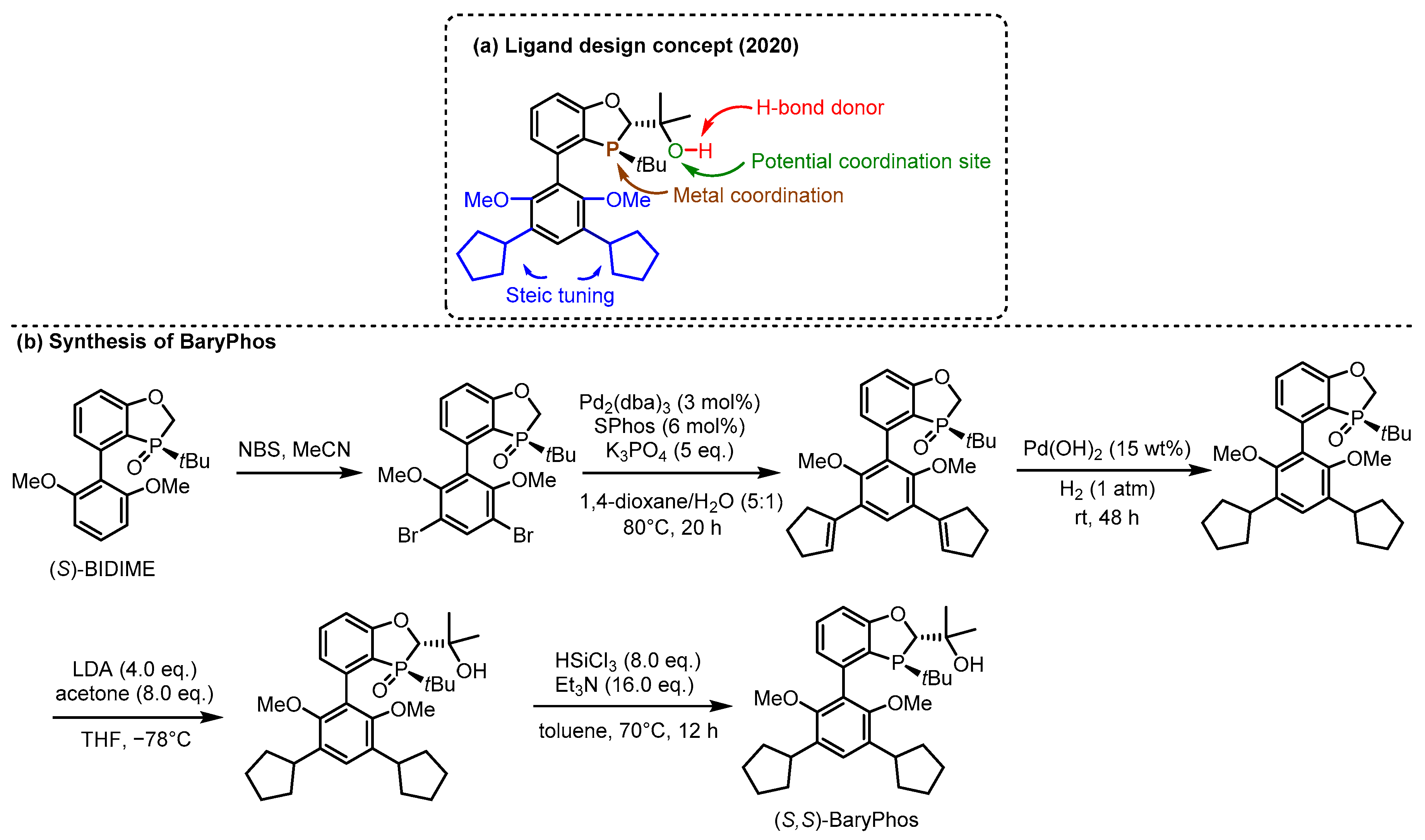
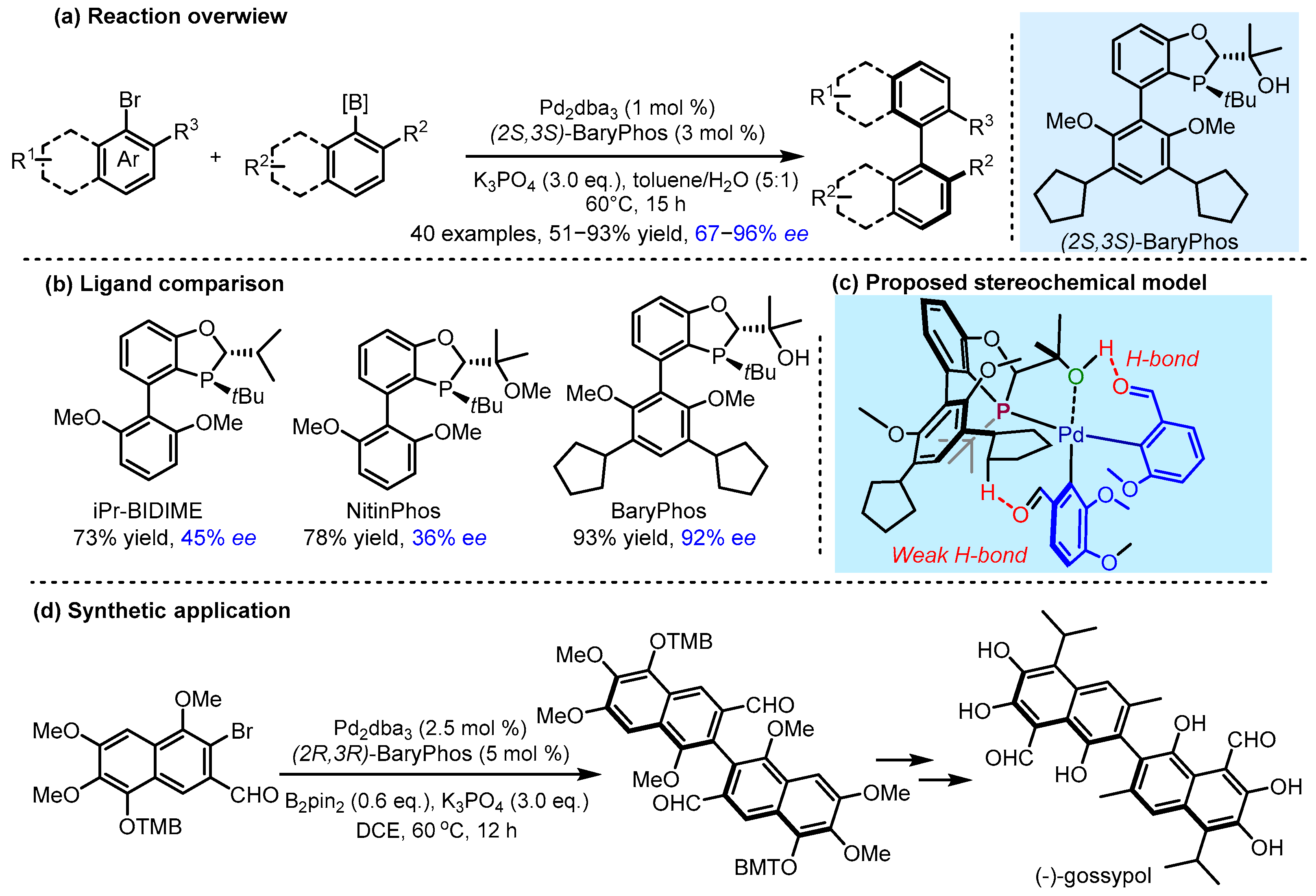
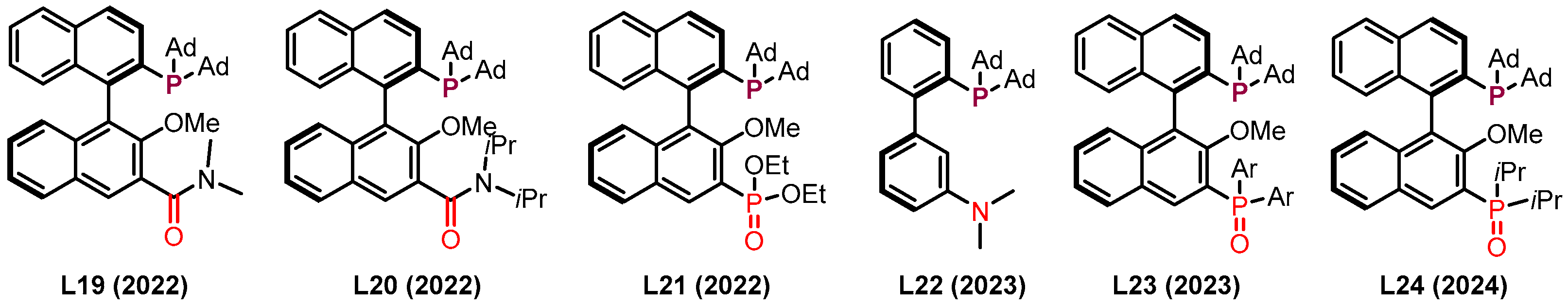
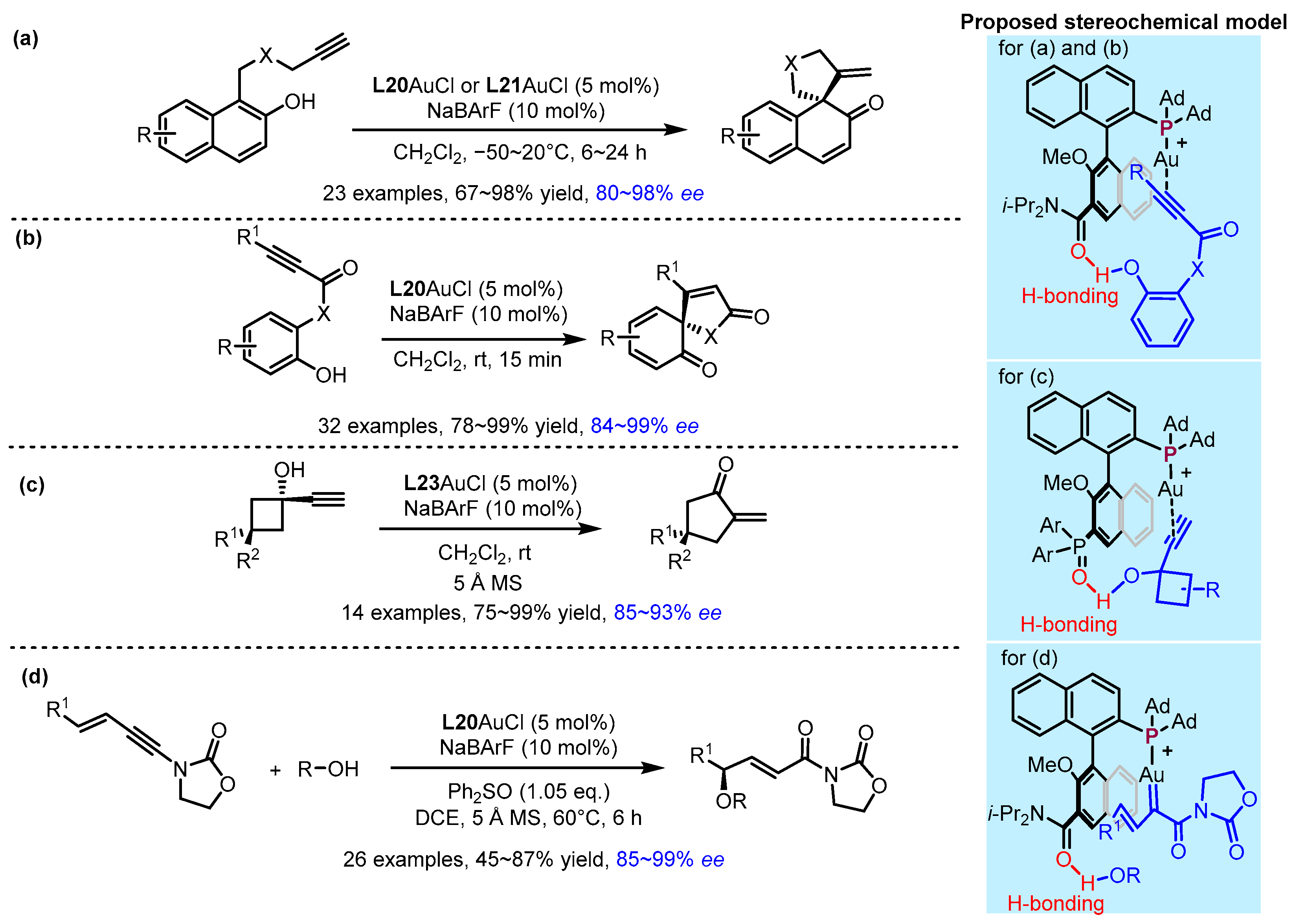
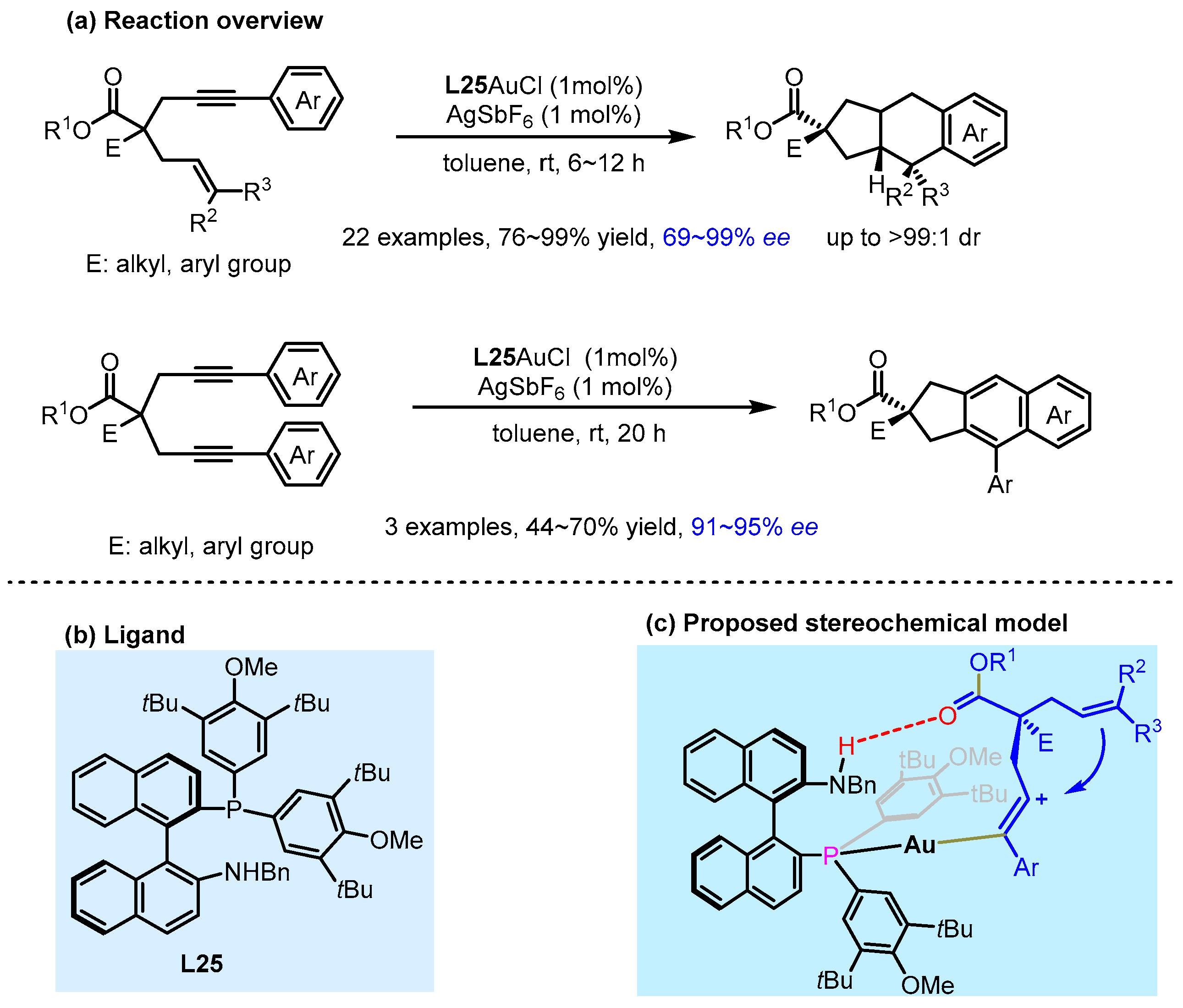
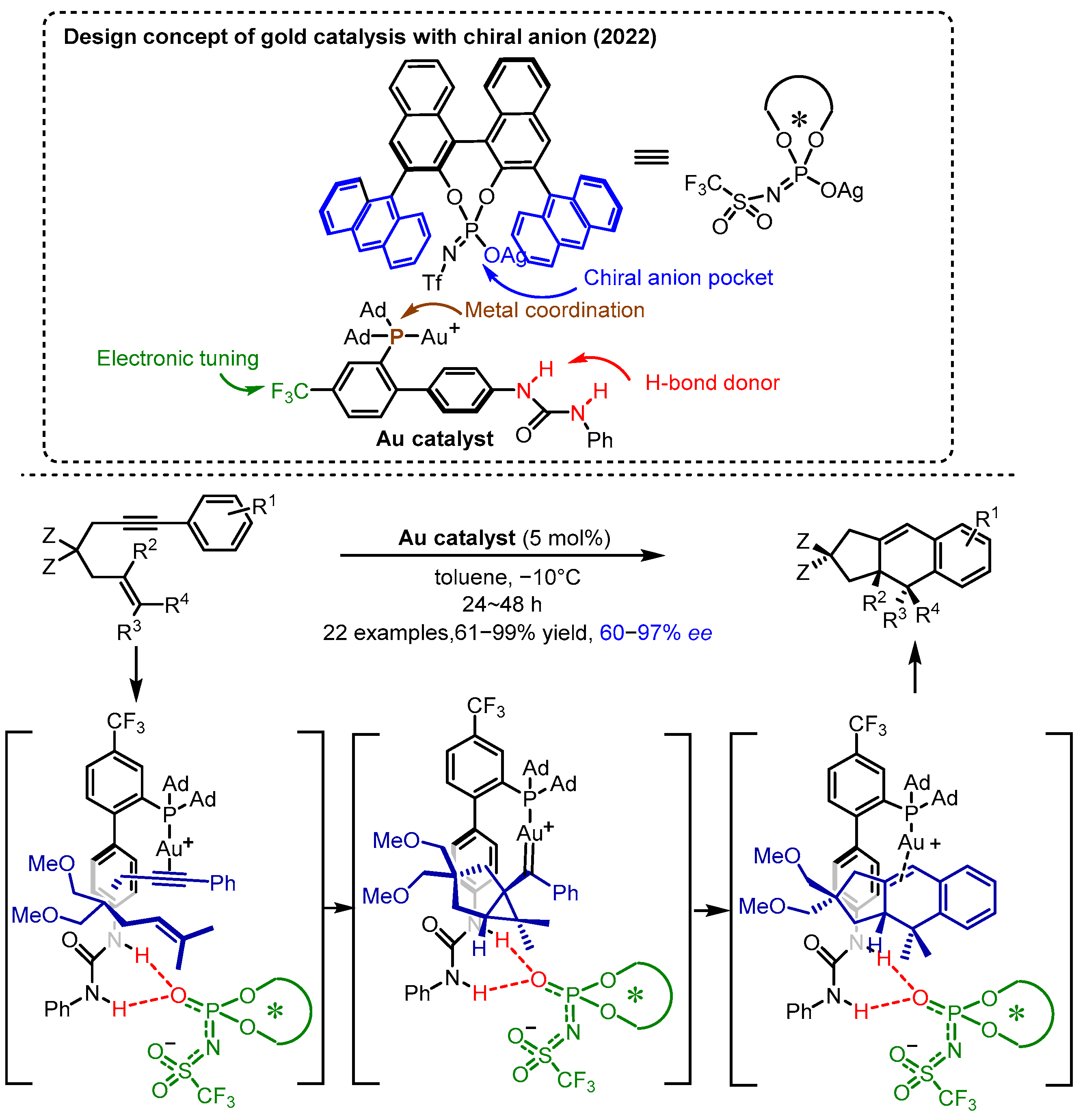
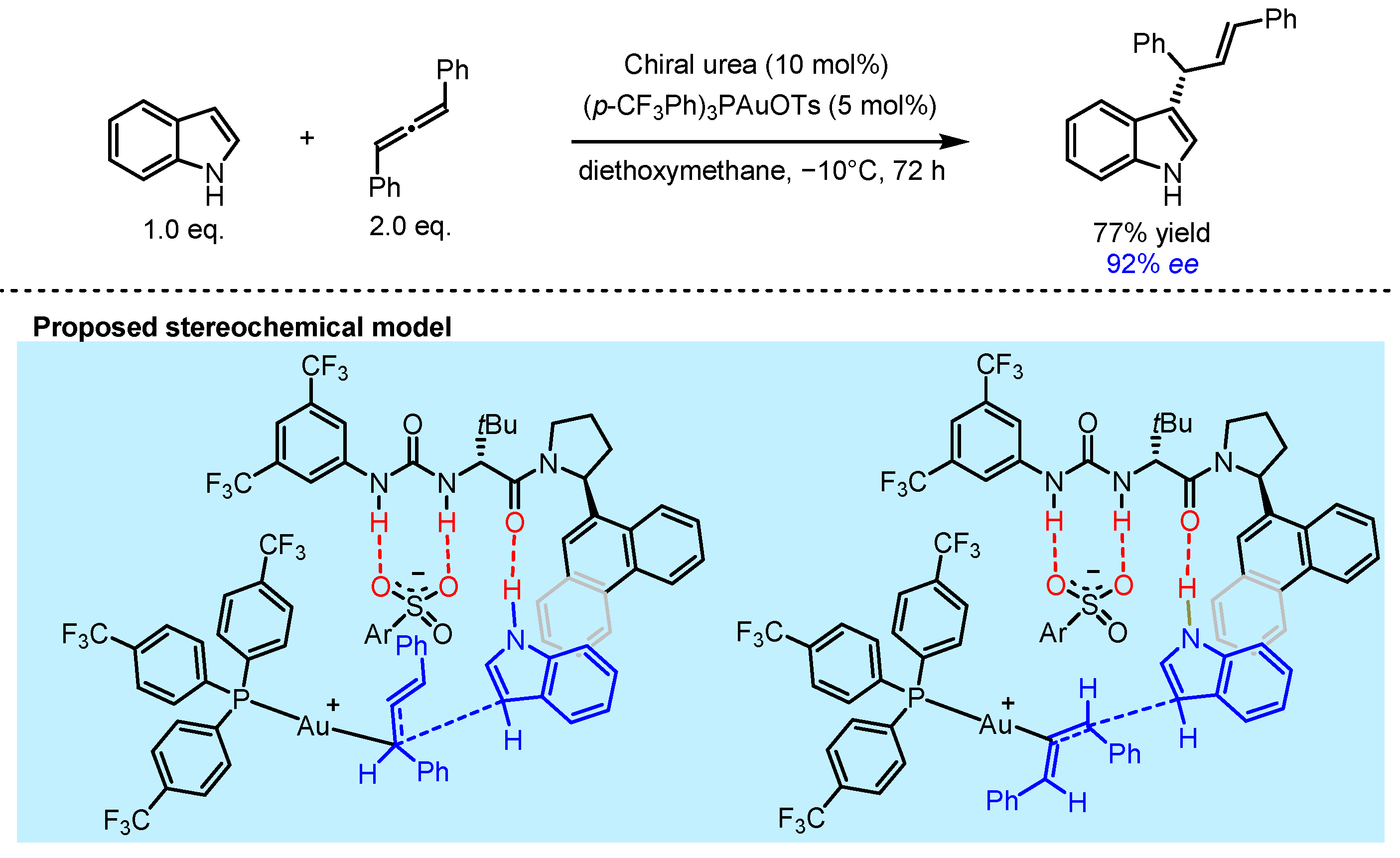
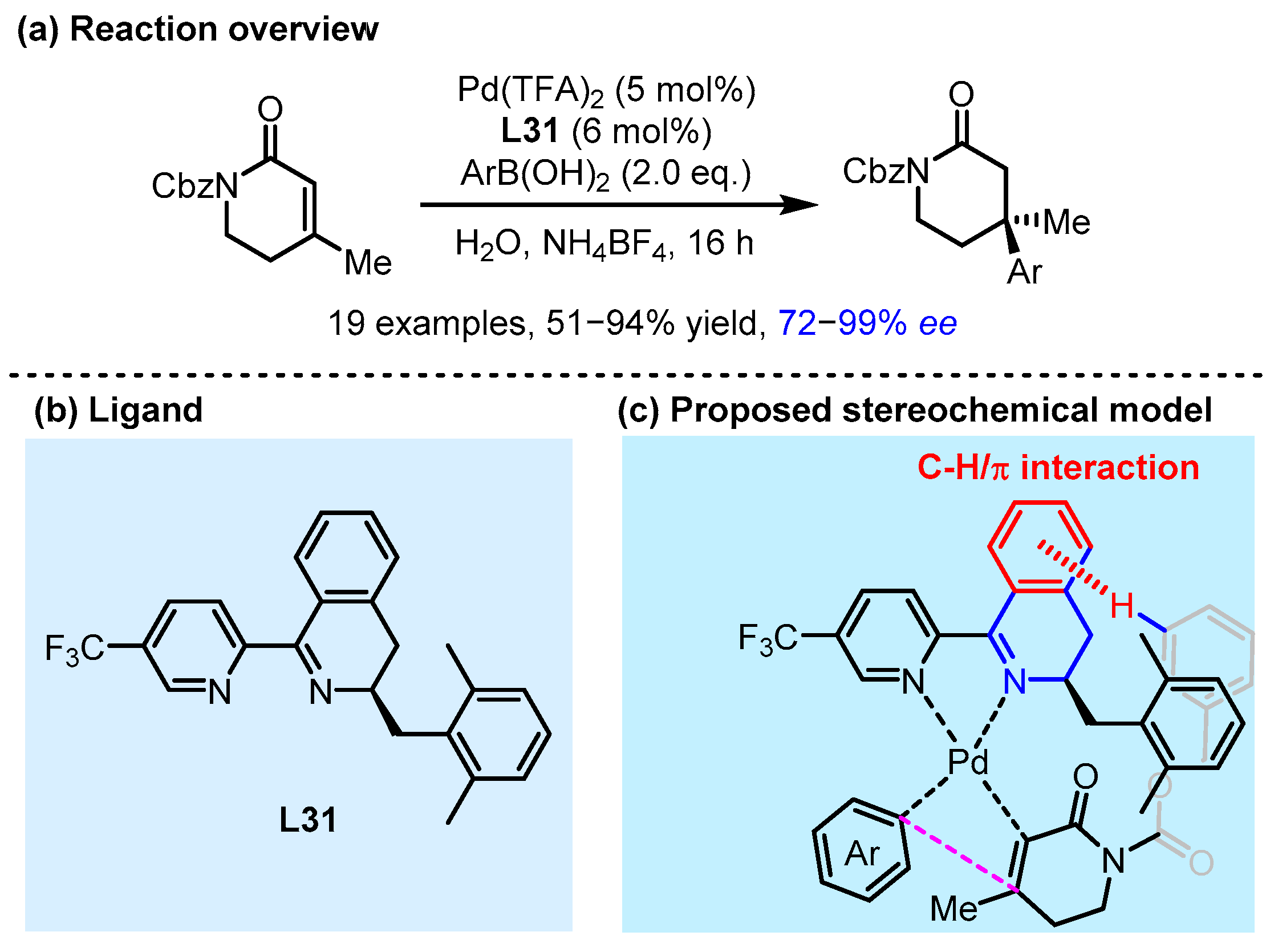
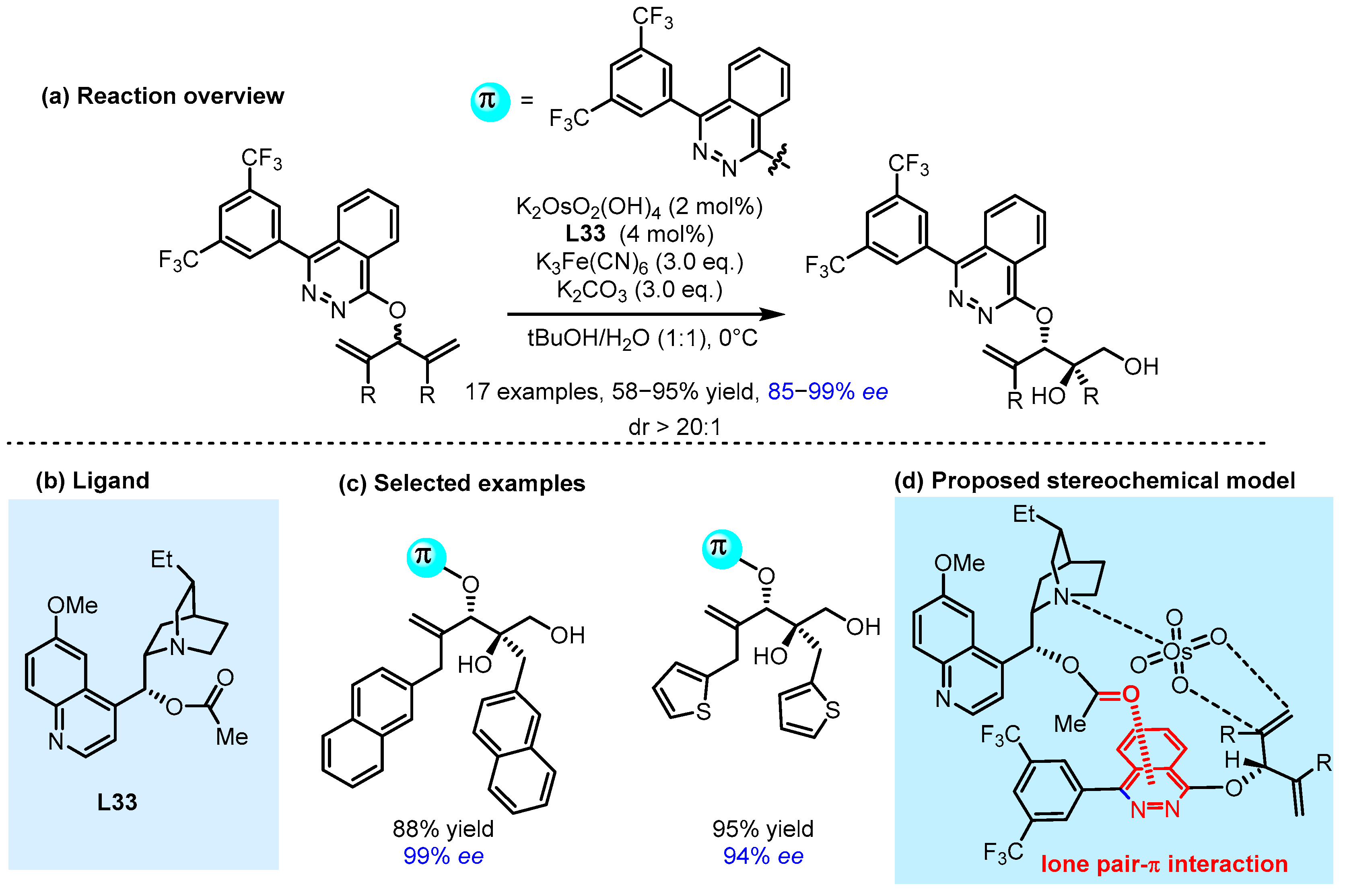
2.3. π Interactions Involved Enantioselective Transition Metal Catalysis
3. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Seki, M. A New Catalytic System for Ru-Catalyzed C–H Arylation Reactions and Its Application in the Practical Syntheses of Pharmaceutical Agents. Org. Process Res. Dev. 2016, 20, 867–877. [Google Scholar] [CrossRef]
- Mercs, L.; Albrecht, M. Beyond Catalysis: N-Heterocyclic Carbene Complexes as Components for Medicinal, Luminescent, and Functional Materials Applications. Chem. Soc. Rev. 2010, 39, 1903–1912. [Google Scholar] [CrossRef]
- Jenck, J.F.; Agterberg, F.; Droescher, M.J. Products and Processes for a Sustainable Chemical Industry: A Review of Achievements and Prospects. Green. Chem. 2004, 6, 544–556. [Google Scholar] [CrossRef]
- He, Y.-M.; Cheng, Y.-Z.; Duan, Y.; Zhang, Y.-D.; Fan, Q.-H.; You, S.-L.; Luo, S.; Zhu, S.-F.; Fu, X.-F.; Zhou, Q.-L. Recent Progress of Asymmetric Catalysis from a Chinese Perspective. CCS Chem. 2023, 5, 2685–2716. [Google Scholar] [CrossRef]
- Piou, T.; Rovis, T. Electronic and Steric Tuning of a Prototypical Piano Stool Complex: Rh(III) Catalysis for C–H Functionalization. Acc. Chem. Res. 2018, 51, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-M.; Lackner, A.D.; Toste, F.D. Development of Catalysts and Ligands for Enantioselective Gold Catalysis. Acc. Chem. Res. 2014, 47, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Reek, J.N.H.; de Bruin, B.; Pullen, S.; Mooibroek, T.J.; Kluwer, A.M.; Caumes, X. Transition Metal Catalysis Controlled by Hydrogen Bonding in the Second Coordination Sphere. Chem. Rev. 2022, 122, 12308–12369. [Google Scholar] [CrossRef]
- Peluso, P.; Chankvetadze, B. Recognition in the Domain of Molecular Chirality: From Noncovalent Interactions to Separation of Enantiomers. Chem. Rev. 2022, 122, 13235–13400. [Google Scholar] [CrossRef]
- Sawamura, M.; Ito, Y. Catalytic Asymmetric Synthesis by Means of Secondary Interaction between Chiral Ligands and Substrates. Chem. Rev. 1992, 92, 857–871. [Google Scholar] [CrossRef]
- Pachisia, S.; Gupta, R. Supramolecular Catalysis: The Role of H-Bonding Interactions in Substrate Orientation and Activation. Dalton Trans. 2021, 50, 14951–14966. [Google Scholar] [CrossRef]
- Fanourakis, A.; Docherty, P.J.; Chuentragool, P.; Phipps, R.J. Recent Developments in Enantioselective Transition Metal Catalysis Featuring Attractive Noncovalent Interactions between Ligand and Substrate. ACS Catal. 2020, 10, 10672–10714. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Sawamura, M.; Hayashi, T. Catalytic Asymmetric Aldol Reaction: Reaction of Aldehydes with Isocyanoacetate Catalyzed by a Chiral Ferrocenylphosphine-Gold(I) Complex. J. Am. Chem. Soc. 1986, 108, 6405–6406. [Google Scholar] [CrossRef]
- Carboni, S.; Gennari, C.; Pignataro, L.; Piarulli, U. Supramolecular Ligand–Ligand and Ligand–Substrate Interactions for Highly Selective Transition Metal Catalysis. Dalton Trans. 2011, 40, 4355–4373. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, C.; Wen, J.; Dong, X.-Q.; Zhang, X. Noncovalent Interaction-Assisted Ferrocenyl Phosphine Ligands in Asymmetric Catalysis. Acc. Chem. Res. 2020, 53, 1905–1921. [Google Scholar] [CrossRef]
- Yao, Q.-J.; Shi, B.-F. Cobalt(III)-Catalyzed Enantioselective C–H Functionalization: Ligand Innovation and Reaction Development. Acc. Chem. Res. 2025, 26, 1895–1899. [Google Scholar] [CrossRef] [PubMed]
- Krueger, C.A.; Kuntz, K.W.; Dzierba, C.D.; Wirschun, W.G.; Gleason, J.D.; Snapper, M.L.; Hoveyda, A.H. Ti-Catalyzed Enantioselective Addition of Cyanide to Imines. A Practical Synthesis of Optically Pure α-Amino Acids. J. Am. Chem. Soc. 1999, 121, 4284–4285. [Google Scholar] [CrossRef]
- Yang, T.; Sun, Y.; Wang, H.; Lin, Z.; Wen, J.; Zhang, X. Iridium-Catalyzed Enantioselective Hydrogenation of Oxocarbenium Ions: A Case of Ionic Hydrogenation. Angew. Chem. Int. Ed. 2020, 59, 6108–6114. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Z.; Jin, S.; Fan, X.; Geng, M.; Zhou, Y.; Wen, S.; Wang, X.; Chung, L.W.; Dong, X.-Q.; et al. Enzyme-Inspired Chiral Secondary-Phosphine-Oxide Ligand with Dual Noncovalent Interactions for Asymmetric Hydrogenation. Angew. Chem. Int. Ed. 2017, 56, 6808–6812. [Google Scholar] [CrossRef]
- Wen, J.; Tan, R.; Liu, S.; Zhao, Q.; Zhang, X. Strong Brønsted Acid Promoted Asymmetric Hydrogenation of Isoquinolines and Quinolines Catalyzed by a Rh–Thiourea Chiral Phosphine Complex Via Anion Binding. Chem. Sci. 2016, 7, 3047–3051. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, S.; Huang, K.; Wang, R.; Zhang, X. A Novel Chiral Bisphosphine-Thiourea Ligand for Asymmetric Hydrogenation of Β,Β-Disubstituted Nitroalkenes. Org. Lett. 2013, 15, 4014–4017. [Google Scholar] [CrossRef]
- Du, L.; Cao, P.; Xing, J.; Lou, Y.; Jiang, L.; Li, L.; Liao, J. Hydrogen-Bond-Promoted Palladium Catalysis: Allylic Alkylation of Indoles with Unsymmetrical 1,3-Disubstituted Allyl Acetates Using Chiral Bis(Sulfoxide) Phosphine Ligands. Angew. Chem. Int. Ed. 2013, 52, 4207–4211. [Google Scholar] [CrossRef]
- Wang, Z.; Nicolini, C.; Hervieu, C.; Wong, Y.-F.; Zanoni, G.; Zhang, L. Remote Cooperative Group Strategy Enables Ligands for Accelerative Asymmetric Gold Catalysis. J. Am. Chem. Soc. 2017, 139, 16064–16067. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Li, W.; Li, Z.; Zhang, J. P-Chiral Phosphines Enabled by Palladium/Xiao-Phos-Catalyzed Asymmetric P–C Cross–Coupling of Secondary Phosphine Oxides and Aryl Bromides. J. Am. Chem. Soc. 2019, 141, 20556–20564. [Google Scholar] [CrossRef] [PubMed]
- Fackler, P.; Berthold, C.; Voss, F.; Bach, T. Hydrogen-Bond-Mediated Enantio- and Regioselectivity in a Ru-Catalyzed Epoxidation Reaction. J. Am. Chem. Soc. 2010, 132, 15911–15913. [Google Scholar] [CrossRef] [PubMed]
- Sladojevich, F.; Trabocchi, A.; Guarna, A.; Dixon, D.J. A New Family of Cinchona-Derived Amino Phosphine Precatalysts: Application to the Highly Enantio- and Diastereoselective Silver-Catalyzed Isocyanoacetate Aldol Reaction. J. Am. Chem. Soc. 2011, 133, 1710–1713. [Google Scholar] [CrossRef]
- Lang, K.; Park, J.; Hong, S. Urea/Transition-Metal Cooperative Catalyst for Anti-Selective Asymmetric Nitroaldol Reactions. Angew. Chem. Int. Ed. 2012, 51, 1620–1624. [Google Scholar] [CrossRef]
- Hayashi, M.; Iwanaga, M.; Shiomi, N.; Nakane, D.; Masuda, H.; Nakamura, S. Direct Asymmetric Mannich-Type Reaction of A-Isocyanoacetates with Ketimines Using Cinchona Alkaloid/Copper(II) Catalysts. Angew. Chem. Int. Ed. 2014, 53, 8411–8415. [Google Scholar] [CrossRef]
- Ma, J.; Ding, X.; Hu, Y.; Huang, Y.; Gong, L.; Meggers, E. Metal-Templated Chiral Brønsted Base Organocatalysis. Nat. Commun. 2014, 5, 4531. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Di, X.; Dai, Q.; Zhang, Z.-M.; Zhang, J. Gold-Catalyzed Asymmetric Intramolecular Cyclization of N-Allenamides for the Synthesis of Chiral Tetrahydrocarbolines. Angew. Chem. Int. Ed. 2017, 56, 15905–15909. [Google Scholar] [CrossRef]
- Yang, H.; Sun, J.; Gu, W.; Tang, W. Enantioselective Cross-Coupling for Axially Chiral Tetra-Ortho-Substituted Biaryls and Asymmetric Synthesis of Gossypol. J. Am. Chem. Soc. 2020, 142, 8036–8043. [Google Scholar] [CrossRef]
- Tang, W.; Capacci, A.G.; Wei, X.; Li, W.; White, A.; Patel, N.D.; Savoie, J.; Gao, J.J.; Rodriguez, S.; Qu, B.; et al. A General and Special Catalyst for Suzuki–Miyaura Coupling Processes. Angew. Chem. Int. Ed. 2010, 49, 5879–5883. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tang, W. Enantioselective Construction of Ortho-Sulfur- or Nitrogen-Substituted Axially Chiral Biaryls and Asymmetric Synthesis of Isoplagiochin D. Nat. Commun. 2022, 13, 4577. [Google Scholar] [CrossRef]
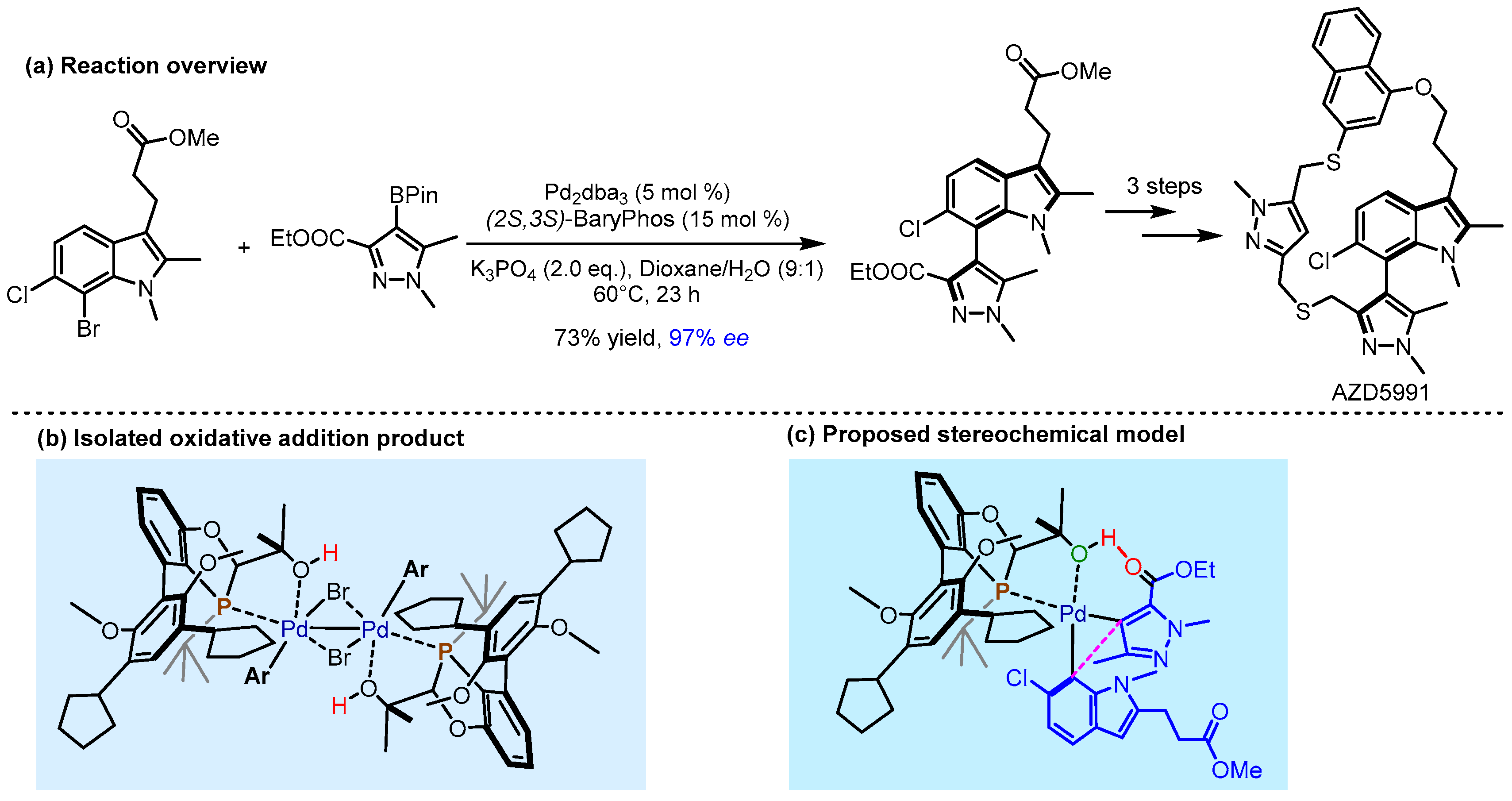
- Howell, G.P.; Agnew, L.R.; Bauer, C.; Bell, F.J.; Campbell, A.D.; Dai, K.; Dave, D.; Ellis, S.R.; Foulkes, M.J.; Gall, M.A.Y.; et al. Comprehensive Synthetic Route Redesign of AZD5991: A High-Complexity Atropisomeric Macrocycle. Org. Process Res. Dev. 2025, 29, 804–827. [Google Scholar] [CrossRef]
- Das, S.; Incarvito, C.D.; Crabtree, R.H.; Brudvig, G.W. Molecular Recognition in the Selective Oxygenation of Saturated C–H Bonds by a Dimanganese Catalyst. Science 2006, 312, 1941–1943. [Google Scholar] [CrossRef] [PubMed]
- Burg, F.; Buchelt, C.; Kreienborg, N.M.; Merten, C.; Bach, T. Enantioselective Synthesis of Diaryl Sulfoxides Enabled by Molecular Recognition. Org. Lett. 2021, 23, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
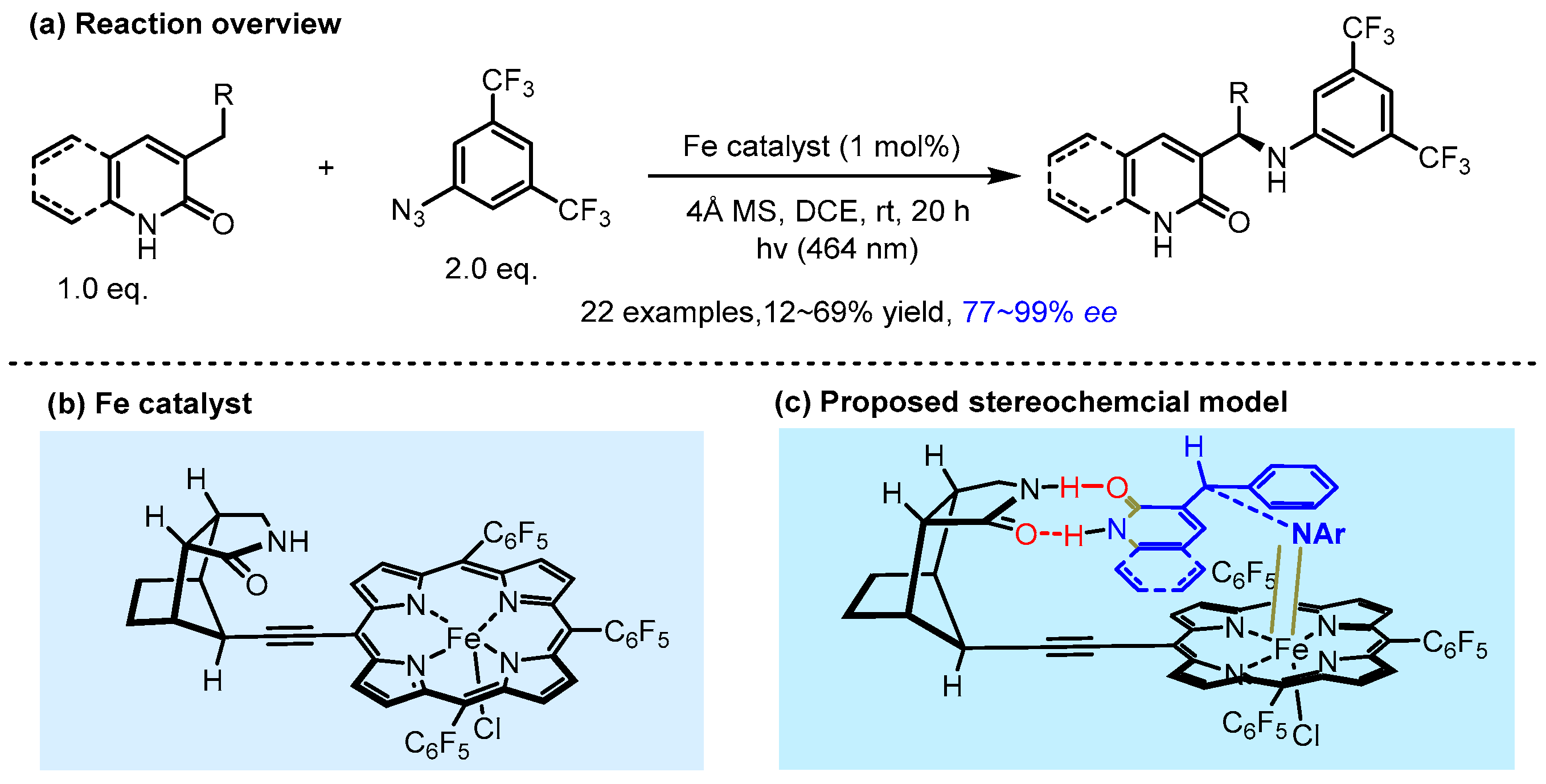
- Ahmed, H.; Ghosh, B.; Breitenlechner, S.; Feßner, M.; Merten, C.; Bach, T. Intermolecular Enantioselective Amination Reactions Mediated by Visible Light and a Chiral Iron Porphyrin Complex. Angew. Chem. Int. Ed. 2024, 63, e202407003. [Google Scholar] [CrossRef]
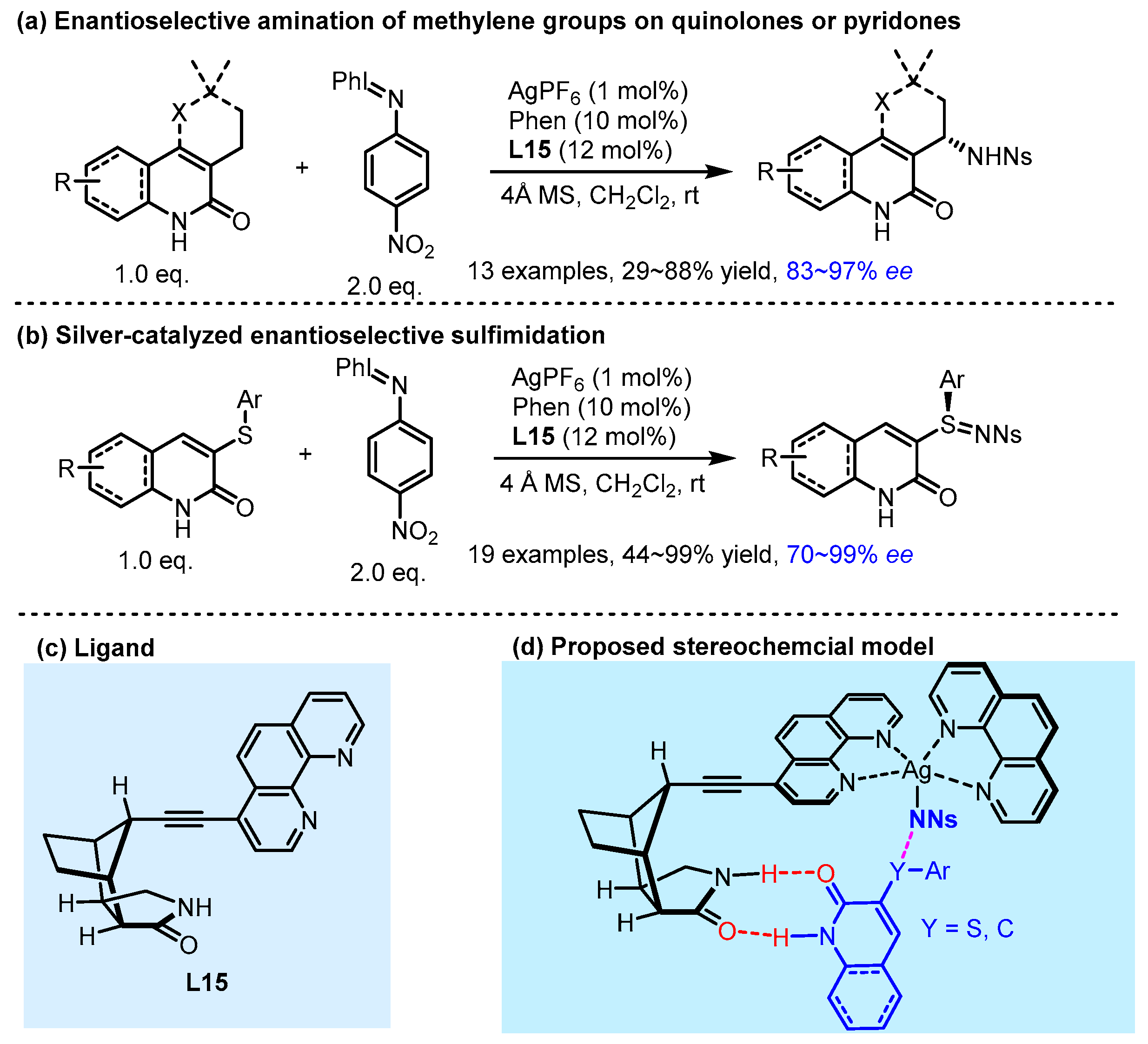
- Annapureddy, R.R.; Jandl, C.; Bach, T. A Chiral Phenanthroline Ligand with a Hydrogen-Bonding Site: Application to the Enantioselective Amination of Methylene Groups. J. Am. Chem. Soc. 2020, 142, 7374–7378. [Google Scholar] [CrossRef]
- Annapureddy, R.R.; Burg, F.; Gramüller, J.; Golub, T.P.; Merten, C.; Huber, S.M.; Bach, T. Silver-Catalyzed Enantioselective Sulfimidation Mediated by Hydrogen Bonding Interactions. Angew. Chem. Int. Ed. 2021, 60, 7920–7926. [Google Scholar] [CrossRef]
- Burg, F.; Breitenlechner, S.; Jandl, C.; Bach, T. Enantioselective Oxygenation of Exocyclic Methylene Groups by a Manganese Porphyrin Catalyst with a Chiral Recognition Site. Chem. Sci. 2020, 11, 2121–2129. [Google Scholar] [CrossRef]
- Anderson, K.W.; Buchwald, S.L. General Catalysts for the Suzuki–Miyaura and Sonogashira Coupling Reactions of Aryl Chlorides and for the Coupling of Challenging Substrate Combinations in Water. Angew. Chem. Int. Ed. 2005, 44, 6173–6177. [Google Scholar] [CrossRef]
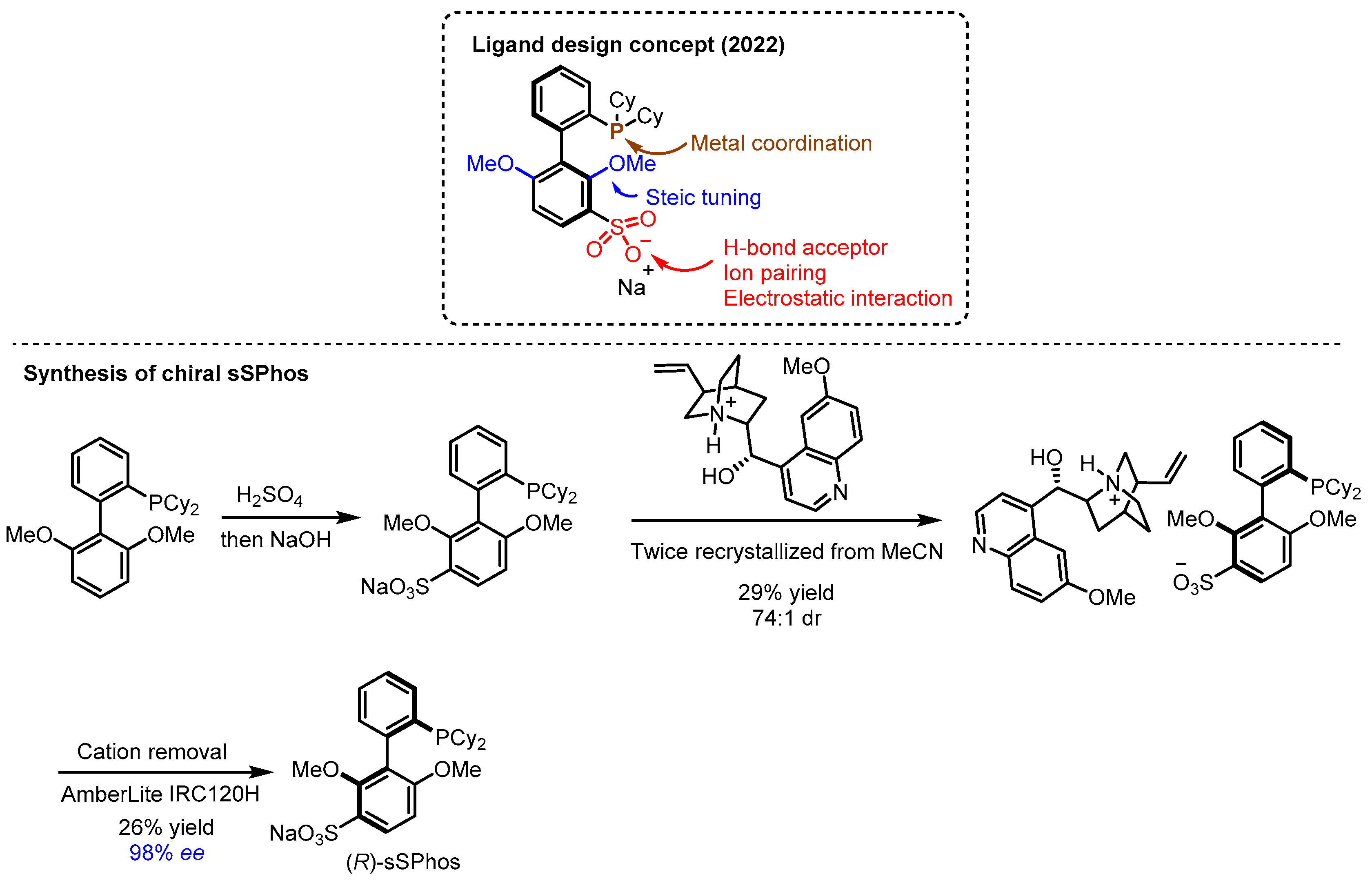
- Pearce-Higgins, R.; Hogenhout, L.N.; Docherty, P.J.; Whalley, D.M.; Chuentragool, P.; Lee, N.; Lam, N.Y.S.; McGuire, T.M.; Valette, D.; Phipps, R.J. An Enantioselective Suzuki–Miyaura Coupling to Form Axially Chiral Biphenols. J. Am. Chem. Soc. 2022, 144, 15026–15032. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, S.; García-Fortanet, J.; Del Aguila Sanchez, M.A.; Buchwald, S.L. Palladium(0)-Catalyzed Arylative Dearomatization of Phenols. J. Am. Chem. Soc. 2011, 133, 9282–9285. [Google Scholar] [CrossRef] [PubMed]
- Kadarauch, M.; Whalley, D.M.; Phipps, R.J. Ssphos: A General Ligand for Enantioselective Arylative Phenol Dearomatization Via Electrostatically-Directed Palladium Catalysis. J. Am. Chem. Soc. 2023, 145, 25553–25558. [Google Scholar] [CrossRef]
- Lou, Y.; Wei, J.; Li, M.; Zhu, Y. Distal Ionic Substrate–Catalyst Interactions Enable Long-Range Stereocontrol: Access to Remote Quaternary Stereocenters through a Desymmetrizing Suzuki–Miyaura Reaction. J. Am. Chem. Soc. 2022, 144, 123–129. [Google Scholar] [CrossRef]
- Wei, J.; Gandon, V.; Zhu, Y. Amino Acid-Derived Ionic Chiral Catalysts Enable Desymmetrizing Cross-Coupling to Remote Acyclic Quaternary Stereocenters. J. Am. Chem. Soc. 2023, 145, 16796–16811. [Google Scholar] [CrossRef] [PubMed]
- Roizen, J.L.; Harvey, M.E.; Du Bois, J. Metal-Catalyzed Nitrogen-Atom Transfer Methods for the Oxidation of Aliphatic C–H Bonds. Acc. Chem. Res. 2012, 45, 911–922. [Google Scholar] [CrossRef]
- Fanourakis, A.; Williams, B.D.; Paterson, K.J.; Phipps, R.J. Enantioselective Intermolecular C–H Amination Directed by a Chiral Cation. J. Am. Chem. Soc. 2021, 143, 10070–10076. [Google Scholar] [CrossRef] [PubMed]
- Paterson, K.J.; Dahiya, A.; Williams, B.D.; Phipps, R.J. Tertiary Amides as Directing Groups for Enantioselective C−H Amination Using Ion-Paired Rhodium Complexes. Angew. Chem. Int. Ed. 2024, 63, e202317489. [Google Scholar] [CrossRef]
- Hodson, N.J.; Takano, S.; Fanourakis, A.; Phipps, R.J. Enantioselective Nitrene Transfer to Hydrocinnamyl Alcohols and Allylic Alcohols Enabled by Systematic Exploration of the Structure of Ion-Paired Rhodium Catalysts. J. Am. Chem. Soc. 2024, 146, 22629–22641. [Google Scholar] [CrossRef]
- Dorel, R.; Echavarren, A.M. Gold(I)-Catalyzed Activation of Alkynes for the Construction of Molecular Complexity. Chem. Rev. 2015, 115, 9028–9072. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, L. Designed Bifunctional Ligands in Cooperative Homogeneous Gold Catalysis. CCS Chem. 2020, 3, 1989–2002. [Google Scholar] [CrossRef]
- Zhao, K.; Kohnke, P.; Yang, Z.; Cheng, X.; You, S.-L.; Zhang, L. Enantioselective Dearomative Cyclization Enabled by Asymmetric Cooperative Gold Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202207518. [Google Scholar] [CrossRef] [PubMed]
- Kohnke, P.; Zhang, L. Bifunctional Phosphine-Enabled Regioselective Cycloisomerization of Enynyl Esters En Route to Bicyclo[2.2.1]Heptenes. Org. Lett. 2023, 25, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, C.D.; Zhao, K.; Zhang, L. Gold-Catalyzed Asymmetric Transformation of Hydroxylated Propargylic Esters. ChemPlusChem 2023, 88, e202300314. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, K.; Li, X.; Quintanilla, C.D.; Zhang, L. Asymmetric Dearomatization of Phenols Via Ligand-Enabled Cooperative Gold Catalysis. Angew. Chem. Int. Ed. 2023, 62, e202309256. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Yang, Z.; Yang, J.; Li, X.; Quintanilla, C.D.; Zhang, L. Desymmetrization and Parallel Kinetic Resolution of 1-Ethynylcyclobutanols Via Asymmetric Cooperative Gold Catalysis. J. Am. Chem. Soc. 2023, 145, 27205–27210. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, L. Gold-Catalyzed Homo/Heterodimerization of Terminal Alkynes Facilitated by Metal–Ligand Cooperation. Org. Lett. 2024, 26, 5736–5740. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, K.; Quintanilla, C.D.; Zhang, L. Chiral Bifunctional Phosphine Ligand Enables Asymmetric Trapping of Catalytic Vinyl Gold Carbene Species. J. Am. Chem. Soc. 2024, 146, 2308–2312. [Google Scholar] [CrossRef]
- Zuccarello, G.; Mayans, J.G.; Escofet, I.; Scharnagel, D.; Kirillova, M.S.; Pérez-Jimeno, A.H.; Calleja, P.; Boothe, J.R.; Echavarren, A.M. Enantioselective Folding of Enynes by Gold(I) Catalysts with a Remote C2-Chiral Element. J. Am. Chem. Soc. 2019, 141, 11858–11863. [Google Scholar] [CrossRef]
- Ji, K.; Zheng, Z.; Wang, Z.; Zhang, L. Enantioselective Oxidative Gold Catalysis Enabled by a Designed Chiral P,N-Bidentate Ligand. Angew. Chem. Int. Ed. 2015, 54, 1245–1249. [Google Scholar] [CrossRef]
- Zhang, J.-Q.; Liu, Y.; Wang, X.-W.; Zhang, L. Synthesis of Chiral Bifunctional Nhc Ligands and Survey of Their Utilities in Asymmetric Gold Catalysis. Organometallics 2019, 38, 3931–3938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Smal, V.; Retailleau, P.; Voituriez, A.; Frison, G.; Marinetti, A.; Guinchard, X. Tethered Counterion-Directed Catalysis: Merging the Chiral Ion-Pairing and Bifunctional Ligand Strategies in Enantioselective Gold(I) Catalysis. J. Am. Chem. Soc. 2020, 142, 3797–3805. [Google Scholar] [CrossRef]
- Li, X.; Liao, S.; Wang, Z.; Zhang, L. Ligand-Accelerated Gold-Catalyzed Addition of in Situ Generated Hydrazoic Acid to Alkynes under Neat Conditions. Org. Lett. 2017, 19, 3687–3690. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Xiao, Y.; Yang, T.; Chen, G.-Q.; Zhang, X.; Che, C.-M. Gold-Catalyzed Highly Enantioselective Cycloadditions of 1,6-Enynes and 1,6-Diynes Assisted by Remote Hydrogen Bonding Interaction. iScience 2024, 27, 110876. [Google Scholar] [CrossRef] [PubMed]
- Franchino, A.; Martí, À.; Echavarren, A.M. H-Bonded Counterion-Directed Enantioselective Au(I) Catalysis. J. Am. Chem. Soc. 2022, 144, 3497–3509. [Google Scholar] [CrossRef]
- Huang, B.; Khanh Mai, B.; Warzok, U.; Liu, P.; Dean Toste, F. On the Gold(I)-Catalyzed Enantioselective Addition of Indole to Diphenylallene Via Anion-Binding Catalysis. Tetrahedron Lett. 2024, 149, 155247. [Google Scholar] [CrossRef]
- Ovian, J.M.; Vojáčková, P.; Jacobsen, E.N. Enantioselective Transition-Metal Catalysis Via an Anion-Binding Approach. Nature 2023, 616, 84–89. [Google Scholar] [CrossRef]
- Li, J.-Y.; Xie, P.-P.; Zhou, T.; Qian, P.-F.; Zhou, Y.-B.; Li, H.-C.; Hong, X.; Shi, B.-F. Ir(III)-Catalyzed Asymmetric C–H Activation/Annulation of Sulfoximines Assisted by the Hydrogen-Bonding Interaction. ACS Catal. 2022, 12, 9083–9091. [Google Scholar] [CrossRef]
- Zhou, T.; Qian, P.-F.; Li, J.-Y.; Zhou, Y.-B.; Li, H.-C.; Chen, H.-Y.; Shi, B.-F. Efficient Synthesis of Sulfur-Stereogenic Sulfoximines Via Ru(II)-Catalyzed Enantioselective C–H Functionalization Enabled by Chiral Carboxylic Acid. J. Am. Chem. Soc. 2021, 143, 6810–6816. [Google Scholar] [CrossRef]
- Sakai, S.; Fujioka, A.; Imai, K.; Uchiyama, K.; Shimizu, Y.; Higashida, K.; Sawamura, M. Silver-Catalyzed Asymmetric Aldol Reaction of Isocyanoacetic Acid Derivatives Enabled by Cooperative Participation of Classical and Nonclassical Hydrogen Bonds. Adv. Synth. Catal. 2022, 364, 2333–2339. [Google Scholar] [CrossRef]
- Tsutsumi, R.; Taguchi, R.; Yamanaka, M. Chiral Bipyridine Ligand with Flexible Molecular Recognition Site: Development and Application to Copper-Catalyzed Asymmetric Borylation of α,β-Unsaturated Ketones. ChemCatChem 2022, 14, e202101278. [Google Scholar] [CrossRef]
- Cardoso, F.S.P.; Abboud, K.A.; Aponick, A. Design, Preparation, and Implementation of an Imidazole-Based Chiral Biaryl P,N-Ligand for Asymmetric Catalysis. J. Am. Chem. Soc. 2013, 135, 14548–14551. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Patel, N.D.; Xu, G.; Xu, X.; Savoie, J.; Ma, S.; Hao, M.-H.; Keshipeddy, S.; Capacci, A.G.; Wei, X.; et al. Efficient Chiral Monophosphorus Ligands for Asymmetric Suzuki–Miyaura Coupling Reactions. Org. Lett. 2012, 14, 2258–2261. [Google Scholar] [CrossRef]
- Rokade, B.V.; Guiry, P.J. Axially Chiral P,N-Ligands: Some Recent Twists and Turns. ACS Catal. 2018, 8, 624–643. [Google Scholar] [CrossRef]
- Changotra, A.; Bhaskararao, B.; Hadad, C.M.; Sunoj, R.B. Insights on Absolute and Relative Stereocontrol in Stereodivergent Cooperative Catalysis. J. Am. Chem. Soc. 2020, 142, 9612–9624. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Ji, Y.; Liu, H.; Pang, Y.; Zhou, B.; Cheng, M.; Liu, Y.; Lin, B.; Liu, Y. Silver Triflate/N-Fluorobenzenesulfonimide-Catalyzed Cycloisomerization of Tryptamine-Ynamide to Spiro[Indoline-3,4’-Piperidine] Induced by Cation-π-π Interactions between Substrate and Metal Ligand. Adv. Synth. Catal. 2020, 362, 192–205. [Google Scholar] [CrossRef]
- Zhao, X.-J.; Li, Z.-H.; Ding, T.-M.; Tian, J.-M.; Tu, Y.-Q.; Wang, A.-F.; Xie, Y.-Y. Enantioselective Synthesis of 3,3’-Disubstituted 2-Amino-2’-Hydroxy-1,1’-Binaphthyls by Copper-Catalyzed Aerobic Oxidative Cross-Coupling. Angew. Chem. Int. Ed. 2021, 60, 7061–7065. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Xie, P.-P.; Liu, L.; Fan, J.; Zhang, Z.-Z.; Hong, X.; Shi, B.-F. Cp*Co(III)-Catalyzed Enantioselective Hydroarylation of Unactivated Terminal Alkenes Via C–H Activation. J. Am. Chem. Soc. 2021, 143, 19112–19120. [Google Scholar] [CrossRef]
- Baek, D.; Ryu, H.; Hahm, H.; Lee, J.; Hong, S. Palladium Catalysis Featuring Attractive Noncovalent Interactions Enabled Highly Enantioselective Access to β-Quaternary δ-Lactams. ACS Catal. 2022, 12, 5559–5564. [Google Scholar] [CrossRef]
- Jin, M.Y.; Zhen, Q.; Xiao, D.; Tao, G.; Xing, X.; Yu, P.; Xu, C. Engineered Non-Covalent π Interactions as Key Elements for Chiral Recognition. Nat. Commun. 2022, 13, 3276. [Google Scholar] [CrossRef]
- Zhen, Q.; Liu, C.; Lv, X.; Jin, M.Y.; Xu, C. Asymmetric Dihydroxylation-Based Desymmetrization Enabled by Lone-Pair−π Interaction. ACS Catal. 2023, 13, 9745–9752. [Google Scholar] [CrossRef]
- Zhu, C.-L.; Yan, X.; Bin, H.-Y.; Wu, X.; Huang, Z.-Y.; Yan, P.-C.; Huang, G.; Xie, J.-H.; Zhou, Q.-L. Enantioselective Synthesis of Chiral 1,4-Dihydroquinolines Via Iridium-Catalyzed Asymmetric Partial Hydrogenation of Quinolines. J. Am. Chem. Soc. 2025, 147, 5725–5735. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; He, D.; Luo, L.; Tang, W. Recent Advances in Enantioselective Transition Metal Catalysis Mediated by Ligand–Substrate Noncovalent Interactions. Catalysts 2025, 15, 395. https://doi.org/10.3390/catal15040395
Cao Z, He D, Luo L, Tang W. Recent Advances in Enantioselective Transition Metal Catalysis Mediated by Ligand–Substrate Noncovalent Interactions. Catalysts. 2025; 15(4):395. https://doi.org/10.3390/catal15040395
Chicago/Turabian StyleCao, Zhen, Dongyang He, Lin Luo, and Wenjun Tang. 2025. "Recent Advances in Enantioselective Transition Metal Catalysis Mediated by Ligand–Substrate Noncovalent Interactions" Catalysts 15, no. 4: 395. https://doi.org/10.3390/catal15040395
APA StyleCao, Z., He, D., Luo, L., & Tang, W. (2025). Recent Advances in Enantioselective Transition Metal Catalysis Mediated by Ligand–Substrate Noncovalent Interactions. Catalysts, 15(4), 395. https://doi.org/10.3390/catal15040395








