Abstract
An asymmetric [2 + 2 + 1] cycloaddition reaction between three-component 3-hydroxy-1H-pyrrole-2,5-diones, ethyl diazoacetate, and nitrosobenzene was successfully developed. A new series of chiral polysubstituted chiral isoxazolidinopyrrolidinediones with three consecutive stereocentres were obtained in up to 87% yield with up to >20:1 dr and 78% ee. In addition, a scaled-up synthesis was carried out, and a possible reaction mechanism was also proposed.
1. Introduction
Pyrrolidone is an important five-membered heterocyclic unit containing nitrogen, which exists widely in drug molecules and natural products [1,2,3]. Fused-ring compounds also play a very important role in drug molecules [4,5,6]. The development of an asymmetric synthetic method for a pyrrolidone-fused structure is of great importance to the development of pharmaceutical chemistry. 3-Hydroxy-1H-pyrrole-2,5-dione is a class of highly active pyrrolidone derivatives. The synthesis of optically active pyrrolidinediones with 3-hydroxy-1H-pyrrole-2,5-diones as substrates is worthy of further study. Asymmetric reactions catalyzed by organic small molecules have attracted widespread attention [7,8,9].
Isoxazolidines are a class of heterocyclic skeletons containing both nitrogen and oxygen atoms and often exist in a number of biologically active natural products and drug molecules [10,11] (Figure 1). Dactylicapnosinine has analgesic, antiemetic, and antihypertensive properties, and pyrinodemine has antiseptic and disinfectant properties. Flueggine A is a potential hypolipidemic drug. In addition, a number of molecules containing the isoxazolidine backbone have attracted much attention as nucleoside analogues [12]. In organic chemistry, isoxazolines are a very important class of intermediates with a wide range of uses because the nitrogen–oxygen bond in isoxazolines is easily broken. Accordingly, researchers can synthesize compounds such as β-lactams, β-amino acids, and 3-amino alcohols via reductive cleavage of the nitrogen–oxygen bond of isoxazolines thereby [13,14,15]. On this basis, some researchers have focused on the development of novel, green, and efficient synthetic methods for isoxazolidines. The current methods for the synthesis of isoxazolidines include the cycloaddition of hydroxylamine with olefins, the cycloaddition of oxaziridines with olefins or aromatics, the cycloaddition of cyclopropanes with nitroimines, and the cycloaddition of 2-nitropyridines with allylstannane, among others [16,17,18,19,20]. Although these methods are more helpful in the synthesis of isoxazolidines, the most commonly used method nowadays is the [3 + 2] cycloaddition reaction of nitrones with olefins.
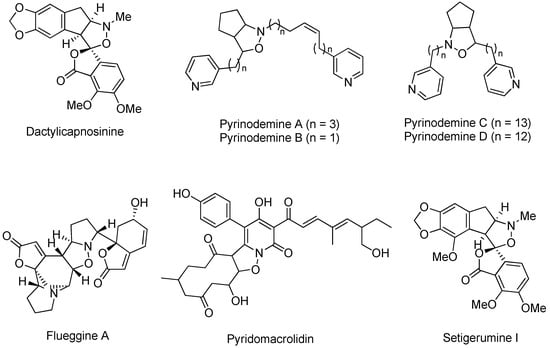
Figure 1.
Bioactive and pharmaceutical molecules containing isoxazolidine.
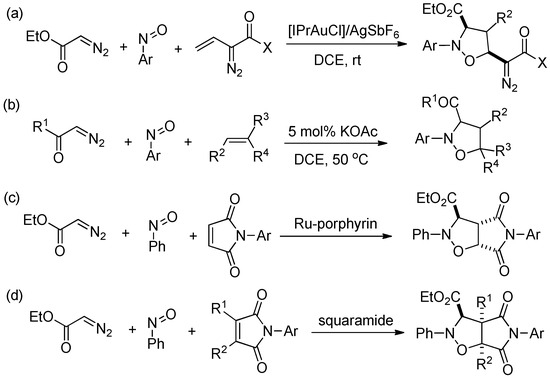
Seidl and co-worker [21] reported an efficient one-pot three-component synthesis of 2-pheny-3-n-proylisoxazolidine-4,5-cis-dicarboxylic acid N-phenylimide from N-phenylhydroxylamine, n-butyraldehyde, and N-phenylmaleimide. Chakraborty and coworker reported [22] the synthesis of some new scaffolds of amino isoxazolidine derivatives using α-amino nitrones with four different maleimides and methyl acrylate, methyl vinyl ketone, and styrene, respectively, in water. Rueping and co-worker [23] developed a novel [3 + 2] cycloaddition of nitrones generated in situ from N-substituted hydroxylamines with alkenes. This new protocol using visible-light photoredox-catalyzed synthesis of nitrones and provides a rapid and efficient access to valuable isoxazolidine heterocycles. In recent years, the synthesis of a series of isoxazolidines through the generation of nitrones from nitrosobenzene and ethyl diazoacetate analogues has received extensive attention from researchers. For example, in 2015, Liu’s group [24] successfully synthesized polysubstituted isoxazolines from a three-component process consisting of ethyl diazoacetate, nitrosoarenes, nitrosobenzenes, and vinyldiazo carbonyl species using a complex of the transition metal gold as a catalyst (Scheme 1a). This provides an efficient and convenient method for the synthesis of isoxazolidine derivatives. With the development of their research, the researchers successfully eliminated of the limitation of transition metal catalysis and realized the three-component synthesis of isoxazolidines without the action of a metal catalyst. In 2019, Li’s group [25] reported a direct and efficient KOAc-catalyzed three-component one-pot process to prepare various functionalized isoxazolidines by the 1,3-dipolar cycloaddition reaction of readily available diazo compounds, nitrosobenzene, and olefins (Scheme 1b). Che and co-worker [26] reported a ruthenium porphyrin-catalyzed tandem nitrone formation/1,3-dipolar cycloaddition of diazo compounds, nitrosoarenes, and alkenes to form isoxazolidines in good-to-high yields and with excellent regio-, chemo- and diastereo-selectivities. A series of alkenes, including malinoimides, is applicable to this protocol, and various functional groups are compatible with the reaction conditions (Scheme 1c). A series of functionalized isoxazolidines were synthesized using cheap and readily available catalysts and starting materials. The wide range of substrates, excellent yields, and good chemo-, regio-, and diastereo-selectivity make it a popular route for the synthesis of isoxazolidine derivatives.
Scheme 1.
Previous representative reports and our work. (a) Liu’s work [24], (b) Li’s work [25], (c) Che’s work [26], (d) our current work.
The present article intends to combine the above synthetic studies of isoxazolidines with the asymmetric synthesis catalysed by organic small-molecule catalysts studied by our group and to explore the reaction progression of this reaction in the presence of organocatalysts, such as squaramide (Scheme 1d), thiourea, and phosphoric acid, with the aim of synthesizing a series of chiral isoxazolidine derivatives to fill the gap of the three-component synthesis of chiral isoxazolidines and to contribute to the synthesis of drug candidates and the development of chiral chemistry.
2. Results
2.1. Optimization of Reaction Conditions
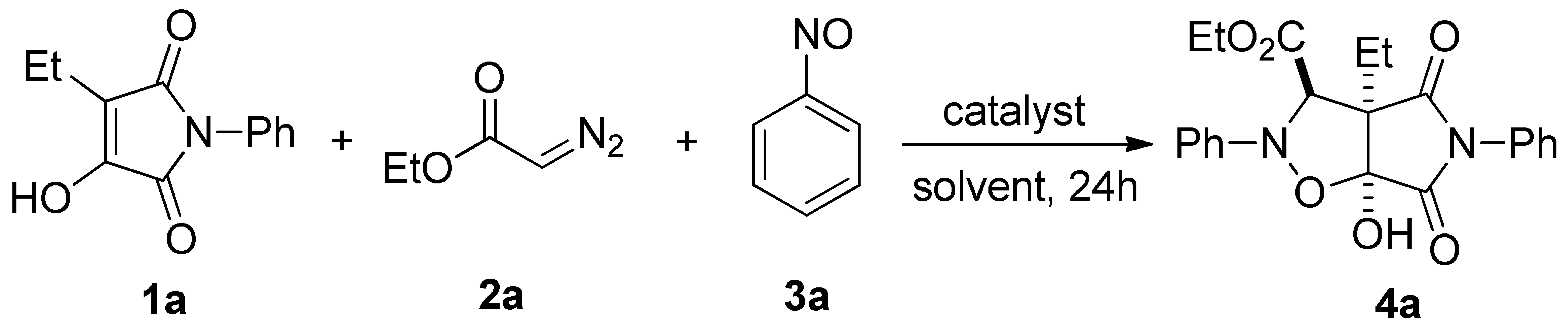
The feasibility of the reaction was attempted using 3-hydroxy-4-ethyl-1H-pyrrole-2,5-dione 1a, ethyl diazoacetate 2a, and nitrosobenzene 3a as substrates and bis(trifluoromethyl)-substituted squaramide C1 derived from cinchona alkaloid as a catalyst. Mimicking the substrate ratios available for this type of reaction, it was determined that the experiments were carried out with 3-hydroxy-4-ethyl-1H-pyrrole-2,5-dione, nitrosobenzene, and ethyl diazoacetate according to a molar ratio of 1:1.5:1.5, with CH2Cl2 serving as the solvent and 10 mol% catalyst loading. After 24 h of reaction at room temperature, the formation of a new substance was detected by TLC. Subsequently, the product, 4a, was isolated and purified by column chromatography, and the structure was verified by a series of assays to determine that it was the target product, i.e., an isoxazolidine derivative. The yield of 4a was 86% with 64% ee and >20:1 dr. Although the enatioselectivity of the reaction was not as expected, the experimental results verified that the reaction can be properly catalyzed by a squaramide catalyst, and it is expected that the stereoselectivity will be improved through the screening of a series of conditions such as the type and loading of the catalysts, the type of solvents, the concentration, and the temperature. The results are outlined in Table 1.

Table 1.
Optimization of the reaction conditions.
The structure of the catalyst plays a crucial role in the asymmetric catalytic reaction, so a series of different organocatalysts were screened first, and the selected organocatalysts are shown in Figure 2. The catalytic effects of squaramide and thiourea catalysts with the same substituent group are first compared, and it was found that thiourea catalyst C2 (79% yield, >20:1 dr, and 56% ee) was not as effective as squaramide C1 (86% yield, >20:1 dr, 64% ee) with the same substituent group. Therefore, we screened a series of squaramide catalysts. Among these catalysts, C3, C4, and C6 showed good stereoselectivity, all of which afforded the product with 67% ee, but the yield using C6 was lower than that of using other two catalysts, which was 80%. The yield using catalyst C7 was high (89%), but its enantioselectivity was the worst among the selected catalysts (53% ee). When phosphoric acid C9 was used, no formation of the target product was observed. Ultimately, C4 (Table 1, entry 4) was selected as the best catalyst to ensure both good yield and good stereoselectivity (87% yield, >20:1 dr, 67% ee). The catalytic effects of different catalysts are shown in Table 1, entries 1–9.
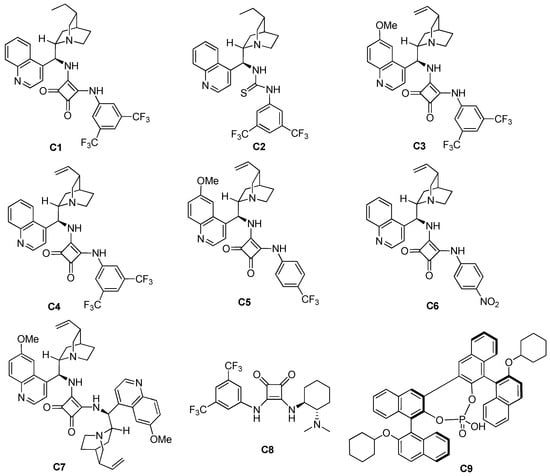
Figure 2.
Selection of organocatalysts.
After the selection of the optimal catalyst, some common organic solvents, such as 1,2-dichloroethane (DCE), CH3CN, EtOAc, THF, MeOH, CHCl3, PhCH3, and 1,4-dioxane were screened (Table 1, entries 10–17). It is easy to see that the reaction becomes less effective in MeOH, THF, EtOAc, PhCH3, 1,4-dioxane or CHCl3 solvents, compared to CH2Cl2, and better in DCE or CH3CN solvents, with the best results being identified with CH3CN solvents (86% yield, >20:1 dr, 75% ee). So, CH3CN was found to be the best solvent for this reaction.
To further explore the effect of concentration on the reaction, the amount of solvent was screened (Table 1, entries 18 and 19). Surprisingly, it was found that when the amount of solvent was increased, the enantioselectivity of the reaction decreased very significantly, i.e., from the original 75% ee to 53% ee (entry 19). At the same time, reducing the amount of solvent to increase the concentration had no significant effect on the reaction (entry 18). Therefore, 1.0 mL of CH3CN was still chosen as the optimal amount of solvent for this reaction.
The catalyst loading also has an important effect on the asymmetric catalytic reaction, so attempts were made to vary the catalyst loading to improve the stereoselectivity of the reaction (Table 1, entries 20 and 21). When the catalyst loading was reduced from the original 10 mol% to 5 mol%, the reaction was slightly improved (87% yield, >20:1 dr, 77% ee). However, when continuing to reduce the catalyst loading to 2.5 mol%, the catalytic effect for the reaction decreased dramatically (75% yield, >20:1 dr, 58% ee). As a result, the optimum catalyst loading was determined to be 5 mol%.
Finally, the effect of temperature on the reaction was explored (Table 1, entry 22). When the reaction temperature was reduced to 0 °C (67% yield, >20:1 dr, 60% ee), the reaction was not as effective as at room temperature.
At this point, the screening of a series of conditions, such as the type and amount of catalyst, the solvent, the concentration, and the temperature, was completed, and the optimal reaction conditions for the reaction were determined to be 3-hydroxy-4-ethyl-1H-pyrrole-2,5-dione 1a (0.10 mmol), ethyl diazoacetate 2a (0.15 mmol), nitrosobenzene 3a (0.15 mmol), and catalyst C4 (5 mol%), reacted in 1.0 mL of CH3CN with stirring at room temperature for 24 h.
2.2. Substrate Scope
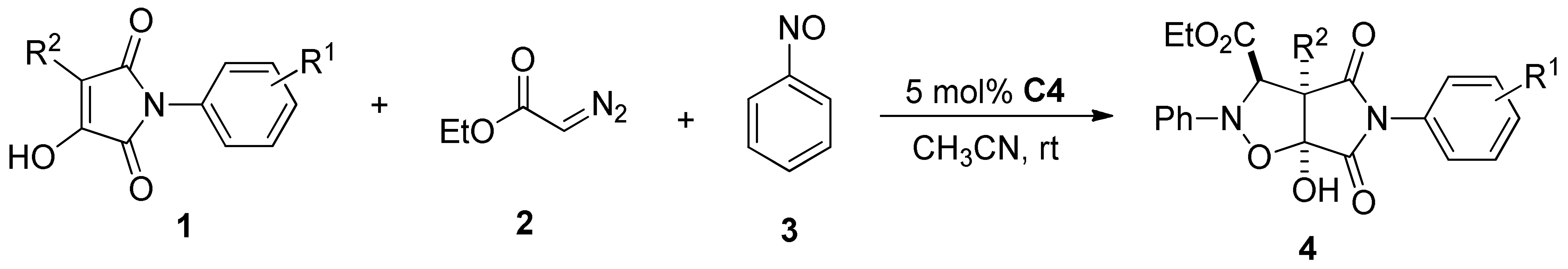
After screening the optimal reaction conditions for this reaction, a series of reaction substrate expansions were carried out to explore the universality of the reaction, and a series of polysubstituted chiral isoxazolidinopyrrolidinedione compounds were also synthesized. The results are summarized in Table 2.

Table 2.
Substrate scope of the 1,3-dipolar cycloaddition reaction.
The reactions were carried out with 1 (0.10 mmol), 2 (0.15 mmol), 3 (0.15 mmol), and catalyst C4 (5 mol%) in CH3CN solvent (1.0 mL), with stirring at room temperature for 24 h. The yields were isolated after column chromatography. The dr values were determined by 1H NMR, and the ee values were determined by HPLC analysis.
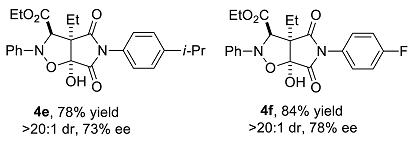
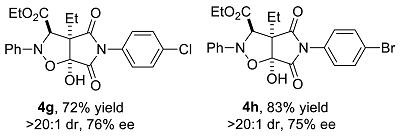
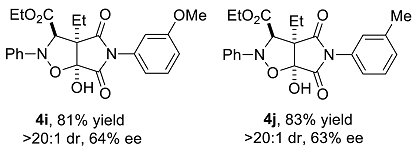
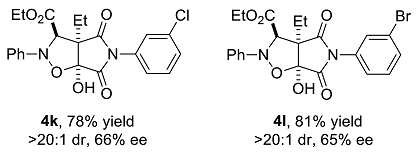
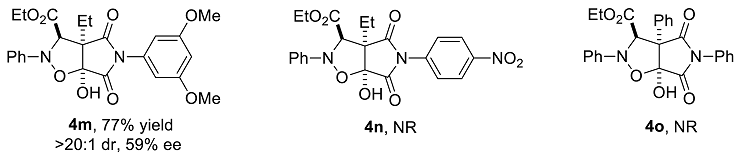
We have performed a series of substrate expansions, mainly of 3-hydroxy-1H-pyrrole-2,5-diones, including the expansion of substituent R1 on the benzene ring and substituent R2 on the 4-position. para- and meta-substitution of the benzene ring, including eight para-substituents, namely, p-methoxy 1b, p-methyl 1c, p-ethyl 1d, p-isopropyl 1e, p-fluoro 1f, p-chloro 1g, p-bromo 1h, and p-nitro 1n; and five meta-substituents, namely, m-methoxy 1i, m-methyl 1j, m-chloro 1k, m-bromo 1l, and 3,5-dimethoxy 1m, were evaluated. Overall, para-substitution on the benzene ring was superior to meta-substitution. Among the para-substitutions, the most effective was the p-fluoro-substituted substrate (84% yield, >20:1 dr, 78% ee), and the least stereoselective was the p-ethyl substituted substrate, which showed an enantioselectivity of 71% ee, and the para-substituted substrates showed enantioselectivities ranging from 71% to 78% ee. The reaction of p-nitro-substituted substrate 1n with ethyl diazoacetate and nitrosobenzene was messy, 4n could not be obtained. Furthermore, among the meta-substitutions, the most effective was the m-chloro-substituted substrate (78% yield, >20:1 dr, 66% ee), and the least effective was the 3,5-dimethoxy-substituted substrate (77% yield, >20:1 dr, 59% ee).
In the next step, the 4-position of 3-hydroxy-1H-pyrrole-2,5-dione was substituted with a phenyl group. The results showed that 3-hydroxy-4-phenyl-1H-pyrrole-2,5-dione 1o did not react with ethyl diazoacetate and nitrosobenzene. Based on its structure, it was surmised that the reason the reaction did not proceed properly may have been due to the large steric impulsion of the benzene ring substituent at the 4-position, which prevented the reaction from occurring.
The above is the substrate expansions undertaken. As can be seen, most of the reactions proceeded normally, except they showed differences in stereoselectivity. Therefore, we believe that this reaction has good generality, and a series of multi-substituted chiral isoxazolidinopyrrolidinedione compounds can be synthesized with the help of this method.
2.3. Scaled-Up Synthesis
In order to fully investigate this three-component asymmetric [2 + 2 + 1] cycloaddition reaction, we explored the effect of the reaction under optimal conditions for a tenfold amplification of the reaction, using 4h as an example (Scheme 2). It was found that a tenfold amplification of the reaction substrate in equal proportions resulted in a slight decrease in reaction yield and stereoselectivity, i.e., from the original 83% yield, 75% ee, and >20:1 dr to 77% yield, 69% ee, and >20:1 dr. Although there is a slight decrease in the reaction effect, this result fully demonstrates the reliability of the reaction and also provides a strong support for the synthetic application of chiral isoxazolidinopyrrolidinediones.
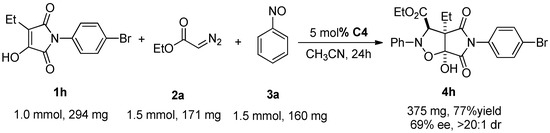
Scheme 2.
Scaled-up synthesis of 4 h.
2.4. Proposed Mechanism
Owing to 4 products being oily liquids, we cannot obtain the single 4 products crystal for x-ray diffraction analysis. We also tried to obtain the single crystal of 4 products with other compounds using the co-crystallization method, but all attempts failed. Based on the above experimental results and the corresponding literature [25], a plausible mechanism is proposed in Scheme 3. First, in the presence of 5 mol% C4, the starting material 2a reacts with nitrosylbenzene 3a to produce intermediate A, which is followed by dinitrogen to form nitrone B. This in situ-generated nitrone then undergoes a 1,3-dipolar cycloaddition reaction with 1a to produce the final product isoxazolidine 4a. Thus, catalyst C4 may first activate the reaction of the diazonium ester with the nitrosylbenzene and promote the subsequent cleavage of the diazonium moiety to produce the nitrone intermediate. At the same time, the squaramide hydrogens in catalyst C4 are bonded with the carbonyl group of 1a, and the tertiary amine N atom activates the hydroxyl group in 1a via hydrogen bonding. The squaramide hydrogen in catalyst C4 also bonds with the nitrone group and synergistically promotes a dipolar cycloaddition reaction that leads to the formation of the cycloadduct 4a. The trans-configuration of substituents was tentatively assigned according to the plausible mechanism.
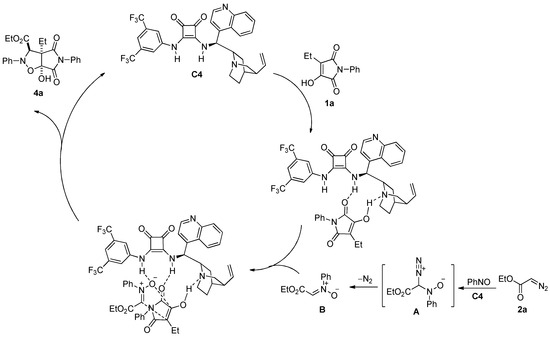
Scheme 3.
Proposed mechanism.
3. Materials and Methods
3.1. General Information
Commercially available compounds were used without further purification. Solvents were dried according to standard procedures. Column chromatography was performed with silica gel (200–300 mesh). The melting points were determined with an XT-4 melting point apparatus and were not corrected. 1H NMR spectra were measured with a Bruker Ascend 400 MHz spectrometer (Bruker, Karlsurhe, Germany), and the chemical shifts were reported in δ (ppm) relative to tetramethylsilane (TMS) as the internal standard. 13C NMR spectra were measured at 100 MHz with a 400 MHz spectrometer, and the chemical shifts were reported in ppm relative to tetramethylsilane and referenced to the solvent peak (CDCl3, δC = 77.00 ppm). High-resolution mass spectra were measured with an Agilent 6520 Accurate-Mass Q-TOF MS system (Agilent, Santa Clara, CA, USA), equipped with an electrospray ionization (ESI) source. Optical rotations were measured with a Krüss P8000 polarimeter (Beijing, China) at the indicated concentration with units of g/100 mL. Enantiomeric excesses were determined by chiral HPLC analysis using an Agilent 1200 LC instrument (Agilent, Santa Clara, CA, USA) with a Daicel Chiralpak IA, IB, IC, or AD-H column.
3.2. Materials
The 3-hydroxy-1H-pyrrole-2,5-dione 1 was synthesized with reference to [27], the synthesis of chiral squaramide catalysts was undertaken with reference to [28,29,30,31], the synthesis of chiral thiourea catalysts was undertaken with reference to [31,32], and nitrosobenzene and ethyl diazoacetate are commercially available.
3.3. Procedure for the Asymmetric Synthesis of Compounds 4
In a small, dried vial, 1 (0.10 mmol), 2 (0.15 mmol), 3 (0.15 mmol), chiral catalyst C4 (5 mol%), and CH3CN (1.0 mL) were added. The mixture was stirred at room temperature for 24 h. After completing the reaction, the residue was purified by flash column chromatography on silica gel to obtain the pure product 4 (petroleum ether: EtOAc = 6:1). The racemates were prepared by simply replacing the above chiral catalyst with 5 mol% K2CO3.
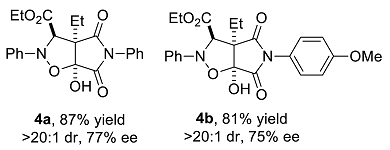
Ethyl 3a-ethyl-6a-hydroxy-4,6-dioxo-2,5-diphenyl-2H-pyrrolo[3,4-d]isoxazolidine-3-carboxylate (4a). Yellow oily liquid 4a obtained from the general procedure (21.6 mg, 87% yield). HPLC (Daicel Chiralpak IC, n-hexane–ethanol = 85:15; flow rate: 1.0 mL/min; detection at 254 nm): tR = 16.9 min (minor), tR = 18.1 min (major); 77% ee. [α]D25 = −15.2 (c = 3.44, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.41–7.35 (m, 3H, ArH), 7.30–7.25 (m, 2H, ArH), 7.06–7.01 (m, 3H, ArH), 6.93–6.91 (m, 2H, ArH), 5.09 (s, 1H, CH), 4.24 (q, J = 7.2 Hz, 2H, CH2), 2.14–2.04 (m, 1H, CH2), 2.00–1.91 (m, 1H, CH2), 1.23 (t, J = 7.2 Hz, 3H, CH3), 1.07 (t, J = 7.2 Hz, 3H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ 173.6, 169.5, 168.4, 147.2, 130.6, 129.2, 129.1, 126.0, 123.5, 114.8, 102.4, 70.4, 63.0, 62.5, 21.6, 13.9, and 8.7 ppm. HRMS (ESI): m/z calculated for C22H23N2O6 [M + H]+ 411.1551, found 411.1546.
Ethyl 3a-ethyl-6a-hydroxy-4,6-dioxo-2-phenyl-5-p-methoxyphenyl-2H-pyrrolo[3,4-d]isoxazolidine-3-carboxylate (4b). Yellow oily liquid 4b obtained from the general procedure (35.6 mg, 81% yield). HPLC (Daicel Chiralpak AD-H, n-hexane–2-propanol = 85:15; flow rate: 1.0 mL/min; detection at 254 nm): tR = 23.8 min (minor), tR = 26.3 min (major); 75% ee. [α]D25 = 10.5 (c = 3.21, CH2Cl2). 1H NMR (400 MHz, CDCl3): 7.26 (t, J = 7.8 Hz, 2H, ArH), 7.06–7.01 (m, 3H, ArH), 6.88 (d, J = 9.2 Hz, 2H, ArH), 6.82 (d, J = 8.8 Hz, 2H, ArH), 5.08 (s, 1H, CH), 4.96 (s, 1H, OH), 4.24 (q, J = 7.2 Hz, 2H, CH2), 3.79 (s, 3H, OCH3), 2.13–2.04 (m, 1H, CH2), 1.99–1.90 (m, 1H, CH2), 1.23 (t, J = 7.2 Hz, 3H, CH3), 1.05 (t, J = 7.6 Hz, 3H, CH3) ppm. 13C NMR (100 MHz, CDCl3): 173.8, 169.7, 168.4, 159.8, 147.3, 129.2, 127.3, 123.4, 123.1, 114.8, 114.3, 102.4, 70.4, 62.9, 62.4, 55.4, 21.6, 13.9, and 8.7 ppm. HRMS (ESI): m/z calculated for C23H25N2O7 [M + H]+ 441.1656, found 441.1668.
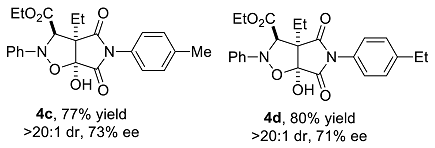
Ethyl 3a-ethyl-6a-hydroxy-4,6-dioxo-2-phenyl-5-tosyl-2H-pyrrolo[3,4-d]isoxazolidine-3-carboxylate (4c). Yellow oily liquid 4c obtained from the general procedure (32.6 mg, 77% yield). HPLC (Daicel Chiralpak IC, n-hexane–ethanol = 95:5; flow rate: 1.0 mL/min; detection at 254 nm): tR = 12.0 min (minor), tR = 13.0 min (major); 73% ee. [α]D25 = 14.9 (c = 3.56, CH2Cl2). 1H NMR (400 MHz, CDCl3): 7.26 (s, 2H, ArH), 7.18 (d, J = 5.6 Hz, 2H, ArH), 7.04 (d, J = 6.0 Hz, 3H, ArH), 6.79 (d, J = 5.6 Hz, 2H, ArH), 5.081 (s, 1H, CH), 5.075 (s, 1H, OH), 4.24–4.22 (m, 2H, CH2), 2.34 (s, 3H, CH3), 2.11–2.06 (m, 1H, CH2), 1.98–1.92 (m, 1H, CH2), 1.25–1.21 (m, 3H, CH3), 1.05–1.04 (m, 3H, CH3) ppm. 13C NMR (100 MHz, CDCl3): 173.7, 169.6, 168.4, 147.3, 139.2, 129.7, 129.2, 127.9, 125.8, 123.4, 114.8, 102.3, 70.4, 63.0, 62.4, 21.6, 21.1, 13.9, and 8.7 ppm. HRMS (ESI): m/z calculated for C23H25N2O6 [M + H]+ 425.1707, found 425.1718.
Ethyl 3a-ethyl-6a-hydroxy-4,6-dioxo-2-phenyl-5-p-ethylphenyl-2H-pyrrolo[3,4-d]isoxazolidine-3-carboxylate (4d). Yellow oily liquid 4d obtained from the general procedure (35.1 mg, 80% yield). HPLC (Daicel Chiralpak IC, n-hexane–ethanol = 90:10; flow rate: 1.0 mL/min; detection at 254 nm): tR = 11.0 min (minor), tR = 12.0 min (major); 71% ee. [α]D25 = 24.1 (c = 4.06, CH2Cl2). 1H NMR (400 MHz, CDCl3): 7.27 (t, J = 7.8 Hz, 2H, ArH), 7.20 (d, J = 8.0 Hz, 2H, ArH), 7.06–7.00 (m, 3H, ArH), 6.82 (d, J = 8.4 Hz, 2H, ArH), 5.08 (s, 1H, CH), 4.99 (s, 1H, OH), 4.24 (q, J = 7.2 Hz, 2H, CH2), 2.64 (q, J = 7.6 Hz, 2H, CH2), 2.13–2.04 (m, 1H, CH2), 2.00–1.91 (m, 1H, CH2), 1.23 (t, J = 7.4 Hz, 3H, CH3), 1.21 (t, J = 7.6 Hz, 3H, CH3), 1.06 (t, J = 7.4 Hz, 3H, CH3) ppm. 13C NMR (100 MHz, CDCl3): 173.7, 169.6, 168.4, 147.3, 145.4, 129.2, 128.5, 128.1, 125.8, 123.4, 114.8, 102.4, 70.4, 63.0, 62.4, 28.5, 21.6, 15.2, 13.9, and 8.7 ppm. HRMS (ESI): m/z calculated for C24H27N2O6 [M + H]+ 439.1864, found 439.1882.
Ethyl 3a-ethyl-6a-hydroxy-4,6-dioxo-2-phenyl-5-p-isopropylphenyl-2H-pyrrolo[3,4-d]isoxazolidine-3-carboxylate (4e). Yellow oily liquid 4e obtained from the general procedure (35.3 mg, 78% yield). HPLC (Daicel Chiralpak IC, n-hexane–ethanol = 90:10; flow rate: 1.0 mL/min; detection at 254 nm): tR = 10.0 min (minor), tR = 11.1 min (major); 73% ee. [α]D25 = 22.5 (c = 2.57, CH2Cl2). 1H NMR (400 MHz, CDCl3): 7.29–7.22 (m, 4H, ArH), 7.06–7.01 (m, 3H, ArH), 6.83 (d, J = 8.0 Hz, 2H, ArH), 5.08 (s, 1H, CH), 4.96 (s, 1H, OH), 4.24 (q, J = 7.2 Hz, 2H, CH2), 2.93–2.86 (m, 1H, CH), 2.13–2.04 (m, 1H, CH2), 2.00–1.91 (m, 1H, CH2), 1.25–1.21 (m, 9H, CH3), 1.06 (t, J = 7.4 Hz, 3H, CH3) ppm. 13C NMR (100 MHz, CDCl3): 173.7, 169.6, 168.4, 149.9, 147.3, 129.2, 128.1, 127.1, 125.8, 123.4, 114.8, 102.4, 70.4, 63.0, 62.4, 33.9, 23.8, 23.7, 21.6, 13.9, and 8.7 ppm. HRMS (ESI): m/z calculated for C25H29N2O6 [M + H]+ 453.2020, found 453.2037.
Ethyl 3a-ethyl-6a-hydroxy-4,6-dioxo-2-phenyl-5-p-fluorophenyl-2H-pyrrolo[3,4-d]isoxazolidine-3-carboxylate (4f). Yellow oily liquid 4f obtained from the general procedure (36.0 mg, 84% yield). HPLC (Daicel Chiralpak IC, n-hexane–ethanol = 90:10; flow rate: 1.0 mL/min; detection at 254 nm): tR = 9.3 min (minor), tR = 10.5 min (major); 78% ee. [α]D25 = 18.6 (c = 3.59, CH2Cl2). 1H NMR (400 MHz, CDCl3): 7.27 (t, J = 7.6 Hz, 2H, ArH), 7.09–7.01 (m, 5H, ArH), 6.89 (dd, J1= 8.8 Hz, J2 = 4.8 Hz, 2H, ArH), 5.08 (s, 1H, CH), 5.02 (br s, 1H, OH), 4.24 (q, J = 7.2 Hz, 2H, CH2), 2.12–2.04 (m, 1H, CH2), 2.00–1.91 (m, 1H, CH2), 1.24 (t, J = 7.2 Hz, 3H, CH3), 1.05 (t, J = 7.4 Hz, 3H, CH3) ppm. 13C NMR (100 MHz, CDCl3): 173.6, 169.3, 168.3, 162.4 (d, 1JCF = 248.0 Hz), 147.2, 129.2, 128.0 (d, 3JCF = 8.8 Hz), 123.5, 116.1 (d, 2JCF = 22.9 Hz), 114.8, 102.4, 70.4, 63.0, 62.5, 21.6, 13.9, and 8.7 ppm. HRMS (ESI): m/z calculated for C22H22FN2O6 [M + H]+ 429.1456, found 429.1471.
Ethyl 3a-ethyl-6a-hydroxy-4,6-dioxo-2-phenyl-5-p-chlorophenyl-2H-pyrrolo[3,4-d]isoxazolidine-3-carboxylate (4g). Yellow oily liquid 4g obtained from the general procedure (32.0 mg, 72% yield). HPLC (Daicel Chiralpak IC, n-hexane–ethanol = 90:10; flow rate: 1.0 mL/min; detection at 254 nm): tR = 9.0 min (minor), tR = 10.5 min (major); 76% ee. [α]D25 = 40.1 (c = 3.28, CH2Cl2). 1H NMR (400 MHz, CDCl3): 7.37–7.33 (m, 2H, ArH), 7.28–7.24 (m, 2H, ArH), 7.05–7.01 (m, 3H, ArH), 6.88–6.85 (m, 2H, ArH), 5.08 (s, 1H, CH), 5.02 (br s, 1H, OH), 4.24 (q, J = 7.2 Hz, 2H, CH2), 2.11–2.04 (m, 1H, CH2), 1.98–1.92 (m, 1H, CH2), 1.23 (t, J = 7.0 Hz, 3H, CH3), 1.05 (t, J = 7.6 Hz, 3H, CH3) ppm. 13C NMR (100 MHz, CDCl3): 173.4, 169.1, 168.3, 147.2, 134.9, 129.3, 129.2, 129.0, 127.3, 123.5, 114.8, 102.4, 70.4, 63.0, 62.5, 21.6, 13.9, and 8.7 ppm. HRMS (ESI): m/z calculated for C22H22ClN2O6 [M + H]+ 445.1161, found 445.1169.
Ethyl 3a-ethyl-6a-hydroxy-4,6-dioxo-2-phenyl-5-p-bromophenyl-2H-pyrrolo[3,4-d]isoxazolidine-3-carboxylate (4h). Yellow oily liquid 4h obtained from the general procedure (40.7 mg, 83% yield). HPLC (Daicel Chiralpak IC, n-hexane–ethanol = 90:10; flow rate: 1.0 mL/min; detection at 254 nm): tR = 9.3 min (minor), tR = 10.9 min (major); 75% ee. [α]D25 = +1.1 (c = 2.92, CH2Cl2). 1H NMR (400 MHz, CDCl3): 7.53–7.49 (m, 2H, ArH), 7.28–7.24 (m, 2H, ArH), 7.05–7.01 (m, 3H, ArH), 6.82–6.79 (m, 2H, ArH), 5.08 (s, 1H, CH), 4.98 (s, 1H, OH), 4.24 (q, J = 7.2 Hz, 2H, CH2), 2.13–2.04 (m, 1H, CH2), 1.99–1.90 (m, 1H, CH2), 1.24 (t, J = 7.2 Hz, 3H, CH3), 1.05 (t, J = 7.6 Hz, 3H, CH3) ppm. 13C NMR (100 MHz, CDCl3): 173.3, 169.0, 168.4, 147.2, 132.3, 129.5, 129.2, 127.5, 123.6, 123.0, 114.8, 102.4, 70.4, 63.0, 62.6, 21.6, 13.9, and 8.7 ppm. HRMS (ESI): m/z calculated for C22H2279BrN2O6 [M + H]+ 489.0656, found 489.0662; calculated for C22H2281BrN2O6 [M + H]+ 491.0635, found 491.0644.
Ethyl 3a-ethyl-6a-hydroxy-4,6-dioxo-2-phenyl-5-methoxyphenyl-2H-pyrrolo[3,4-d]isoxazolidine-3-carboxylate (4i). Yellow oily liquid 4i obtained from the general procedure (35.7 mg, 81% yield). HPLC (Daicel Chiralpak IC, n-hexane–ethanol = 90:10; flow rate: 1.0 mL/min; detection at 254 nm): tR = 13.3 min (minor), tR = 15.0 min (major); 64% ee. [α]D25 = +0.6 (c = 2.81, CH2Cl2). 1H NMR (400 MHz, CDCl3): 7.30–7.25 (m, 3H, ArH), 7.07–7.00 (m, 3H, ArH), 6.90 (dd, J1 = 8.2 Hz, J2 = 2.2 Hz, 1H, ArH), 6.53 (dd, J1 = 7.8 Hz, J2 = 1.0 Hz, 1H, ArH), 6.41 (t, J = 2.0 Hz, 1H, ArH), 5.09 (s, 1H, CH), 5.00 (s, 1H, OH), 4.24 (q, J = 7.2 Hz, 2H, CH2), 3.74 (s, 3H, OCH3), 2.12–2.04 (m, 1H, CH2), 1.98–1.93 (m, 1H, CH2), 1.24 (t, J = 7.2 Hz, 3H, CH3), 1.06 (t, J = 7.4 Hz, 3H, CH3) ppm. 13C NMR (100 MHz, CDCl3): 173.5, 169.3, 168.4, 160.0, 147.3, 131.5, 129.8, 129.2, 123.4, 118.3, 115.2, 114.8, 111.6, 102.4, 70.4, 63.0, 62.5, 55.5, 21.6, 13.9, and 8.7 ppm. HRMS (ESI): m/z calculated for C23H25N2O7 [M + H]+ 441.1656, found 441.1669.
Ethyl 3a-ethyl-6a-hydroxy-4,6-dioxo-2-phenyl-5-methylphenyl-2H-pyrrolo[3,4-d]isoxazolidine-3-carboxylate (4j). Yellow oily liquid 4j obtained from the general procedure (35.2 mg, 83% yield). HPLC (Daicel Chiralpak IC, n-hexane–ethanol = 90:10; flow rate: 1.0 mL/min; detection at 254 nm): tR = 10.8 min (minor), tR = 12.2min (major); 63% ee. [α]D25 = 2.9 (c = 1.92, CH2Cl2). 1H NMR (400 MHz, CDCl3): 7.29–7.24 (m, 3H, ArH), 7.16 (d, J = 7.6 Hz, 1H, ArH), 7.07–7.01 (m, 3H, ArH), 6.72 (d, J = 8.0 Hz, 1H, ArH), 6.62(s, 1H, ArH), 5.09 (s, 1H, CH), 5.02 (s, 1H, OH), 4.24 (q, J = 7.2 Hz, 2H, CH2), 2.31 (s, 3H, CH3), 2.12–2.04 (m, 1H, CH2), 2.00–1.93 (m, 1H, CH2), 1.23 (t, J = 7.2 Hz, 3H, CH3), 1.06 (t, J = 7.6 Hz, 3H, CH3) ppm. 13C NMR (100 MHz, CDCl3): 173.7, 169.6, 168.4, 147.3, 139.2, 130.4, 129.9, 129.2, 128.8, 126.6, 123.4, 123.1, 114.8, 102.4, 70.4, 63.0, 62.4, 21.6, 21.2, 13.9, and 8.7 ppm. HRMS (ESI): m/z calculated for C23H25N2O6 [M + H]+ 425.1707, found 425.1716.
Ethyl 3a-ethyl-6a-hydroxy-4,6-dioxo-2-phenyl-5-m-chlorophenyl-2H-pyrrolo[3,4-d]isoxazolidine-3-carboxylate (4k). Yellow oily liquid 4k obtained from the general procedure (34.6 mg, 78% yield). HPLC (Daicel Chiralpak IC, n-hexane–ethanol = 90:10; flow rate: 1.0 mL/min; detection at 254 nm): tR = 8.3 min (minor), tR = 9.5 min (major); 66% ee. [α]D25 = +1.6 (c = 3.43, CH2Cl2). 1H NMR (400 MHz, CDCl3): 7.37–7.28 (m, 4H, ArH), 7.05 (d, J = 6.8 Hz, 3H, ArH), 6.87–6.84 (m, 2H, ArH), 5.09 (s, 1H, CH), 5.05 (br s, 1H, OH), 4.25 (q, J = 7.2 Hz, 2H, CH2), 2.12–2.05 (m, 1H, CH2), 2.00–1.93 (m, 1H, CH2), 1.24 (t, J = 7.2 Hz, 3H, CH3), 1.05 (t, J = 7.6 Hz, 3H, CH3) ppm. 13C NMR (100 MHz, CDCl3): 173.3, 169.1, 168.4, 147.2, 134.7, 131.6, 130.0, 129.3, 126.4, 124.3, 123.7, 114.8, 102.5, 70.4, 63.1, 62.6, 21.7, 14.0, and 8.8 ppm. HRMS (ESI): m/z calculated for C22H22ClN2O6 [M + H]+ 445.1161, found 445.1166.
Ethyl 3a-ethyl-6a-hydroxy-4,6-dioxo-2-phenyl-5-m-bromophenyl-2H-pyrrolo[3,4-d]isoxazolidine-3-carboxylate (4l). Yellow oily liquid 4l obtained from the general procedure (39.6 mg, 81% yield). HPLC (Daicel Chiralpak IC, n-hexane–ethanol = 90:10; flow rate: 1.0 mL/min; detection at 254 nm): tR = 8.8 min (minor), tR = 10.2 min (major); 65% ee. [α]D25 = +1.4 (c = 3.09, CH2Cl2). 1H NMR (400 MHz, CDCl3): 7.50–7.48 (m, 1H, ArH), 7.30–7.23 (m, 3H, ArH), 7.07–7.04 (m, 3H, ArH), 6.96 (t, J = 1.8 Hz, 1H, ArH), 6.92–6.90 (m, 1H, ArH), 5.09 (s, 1H, CH), 5.04 (br s, 1H, OH), 4.25 (q, J = 7.2 Hz, 2H, CH2), 2.14–2.04 (m, 1H, CH2), 2.00–1.91 (m, 1H, CH2), 1.24 (t, J = 7.2 Hz, 3H, CH3), 1.05 (t, J = 7.6 Hz, 3H, CH3) ppm. 13C NMR (100 MHz, CDCl3): 173.3, 169.0, 168.3, 147.2, 132.2, 131.6, 130.2, 129.3, 129.1, 124.8, 123.7, 122.3, 114.8, 102.4, 70.4, 63.0, 62.6, 21.6, 13.9, and 8.7 ppm. HRMS (ESI): m/z calculated for C22H2279BrN2O6 [M + H]+ 489.0656, found 489.0665; calculated for C22H2281BrN2O6 [M + H]+ 491.0635, found 491.0648.
Ethyl 3a-ethyl-6a-hydroxy-4,6-dioxo-2-phenyl-5-(3,5-dimethoxyphenyl)-2H-pyrrolo[3,4-d]isoxazolidine-3-carboxylate (4m). Yellow oily liquid 4m obtained from the general procedure (36.2 mg, 77% yield). HPLC (Daicel Chiralpak IC, n-hexane–ethanol = 90:10; flow rate: 1.0 mL/min; detection at 254 nm): tR = 15.6 min (minor), tR = 17.7 min (major); 59% ee. [α]D25 = 17.2 (c = 2.93, CH2Cl2). 1H NMR (400 MHz, CDCl3): 7.30–7.26 (m, 2H, ArH), 7.07–7.00 (m, 3H, ArH), 6.45 (t, J = 2.4 Hz, 1H, ArH), 6.03 (d, J = 2.4 Hz, 2H, ArH), 5.09 (s, 1H, CH), 4.95 (s, 1H, OH), 4.25 (q, J = 7.2 Hz, 2H, CH2), 3.72 (s, 6H, OCH3), 2.11–2.04 (m, 1H, CH2), 2.00–1.92 (m, 1H, CH2), 1.24 (t, J = 7.2 Hz, 3H, CH3), 1.06 (t, J = 7.6 Hz, 3H, CH3) ppm. 13C NMR (100 MHz, CDCl3): 173.4, 169.3, 168.4, 160.9, 147.3, 132.0, 129.2, 123.4, 114.8, 104.4, 102.4, 101.6, 70.3, 63.1, 62.5, 55.6, 21.7, 13.9, and 8.7 ppm. HRMS (ESI): m/z calculated for C24H27N2O8 [M + H]+ 471.1762, found 471.1776.
4. Conclusions
In this paper, we have successfully developed an asymmetric [2 + 2 + 1] cycloaddition reaction between three-component 3-hydroxy-1H-pyrrole-2,5-diones, ethyl diazoacetate, and nitrosobenzene to synthesize a series of polysubstituted chiral isoxazolidinopyrrolidinediones. The reaction employed cinchona-derived squaramide as a chiral bifunctional catalyst, and the optimal reaction conditions for the reaction were determined by screening a series of reaction conditions, such as the temperature and the catalyst and its loading, solvent, concentration, and temperature. A series of substrates were evaluated under the optimal reaction conditions to verify the generality of the reaction, in which most of the substrates could be carried out successfully, with some differences in the reaction effects, such as yield and stereoselectivity. Meanwhile, in order to verify the reliability of the reaction, a tenfold amplification of the reaction was also performed. Although the yield and stereoselectivity slightly decreased after the tenfold amplification, we can still prove that the reaction has the potential for synthetic applications, which provides an idea for the synthesis of multi-substituted chiral isoxazolidinopyrrolidinediones.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/catal15040393/s1: Spectroscopic data (1H and 13C NMR) and chiral HPLC chromatograms for all new compounds 4.
Author Contributions
D.-H.X. performed the experiments and acquired and analyzed the original data. Y.D. wrote the preliminary manuscript and supplemented relevant experimental data. D.-M.D. conceived and designed the experiments, revised all figures and schemes, analyzed the data, and reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within this article and Supplementary Materials.
Acknowledgments
We thank the Analysis and Testing Center of the Beijing Institute of Technology for the measurement of NMR and mass spectrometry.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Taylor, R.D.; MacCoss, M.; Lawson, A.D.G. Rings in drugs. J. Med. Chem. 2014, 57, 5845–5859. [Google Scholar] [CrossRef] [PubMed]
- Vo, C.V.T.; Bode, J.W. Synthesis of saturated N-heterocycles. J. Org. Chem. 2014, 79, 2809–2815. [Google Scholar] [CrossRef] [PubMed]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yue, J.; Ji, X.; Nian, M.; Kang, K.; Qiao, H.; Zheng, X. Research progress in biological activities of succinimide derivatives. Bioorg. Chem. 2021, 108, 104557. [Google Scholar] [CrossRef]
- Silva, T.S.; Rodrigues, M.T.; Santos, H.; Zeoly, L.A.; Almeida, W.P.; Barcelos, R.C.; Gomes, R.C.; Fernandes, F.S.; Coelho, F. Recent advances in indoline synthesis. Tetrahedron 2019, 75, 2063–2097. [Google Scholar] [CrossRef]
- Li, L.M.; Shi, S.D.; Liu, Y.; Zou, Q. Bioactivity-guided isolation and identification of new and immunosuppressive monoterpenoid indole alkaloids from Rauvolfia yunnanensis Tsiang. Molecules 2019, 24, 4574. [Google Scholar] [CrossRef]
- Bertelsen, S.; Jørgensen, K.A. Organocatalysis—After the gold rush. Chem. Soc. Rev. 2009, 38, 2178–2189. [Google Scholar] [CrossRef]
- Zhao, B.L.; Li, J.H.; Du, D.M. Squaramide-catalyzed asymmetric reactions. Chem. Record 2017, 17, 994–1018. [Google Scholar] [CrossRef]
- Wang, Y.; Du, D.M. Recent advances in organocatalytic asymmetric oxa-Michael addition triggered cascade reactions. Org. Chem. Front. 2020, 7, 3266–3283. [Google Scholar] [CrossRef]
- Berthet, M.; Cheviet, T.; Dujardin, G.; Parrot, I.; Martinez, J. Isoxazolidine: A privileged scaffold for organic and medicinal chemistry. Chem. Rev. 2016, 116, 15235–15283. [Google Scholar] [CrossRef]
- Ellis, B.D.; Vanderwal, C.D. Hughes and Gleason’s virosaine A-appreciating the art in synthesis. Angew. Chem. Int. Ed. 2017, 56, 13940–13942. [Google Scholar] [CrossRef] [PubMed]
- Chiacchio, U.; Corsaro, A.; Iannazzo, D.; Piperno, A.; Pistarà, V.; Rescifina, A.; Romeo, R.; Valveri, V.; Mastino, A.; Rom, G. Enantioselective syntheses and cytotoxicity of N,O-nucleosides. J. Med. Chem. 2003, 46, 3696–3702. [Google Scholar] [CrossRef] [PubMed]
- Vincent, G.; Guillot, R.; Kouklovsky, C. Stereodivergent synthesis of substituted N,O-containing bicyclic compounds by sequential addition of nucleophiles to N-alkoxybicyclolactams. Angew. Chem. Int. Ed. 2011, 50, 1350–1353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cividino, P.; Poisson, J.F.; Pavlo Shpak-Kraievskyi, P.; Laurent, M.Y.; Martel, A.; Dujardin, G.; Py, S. Asymmetric synthesis of α, α-disubstituted amino acids by cycloaddition of (E)-ketonitrones with vinyl ethers. Org. Lett. 2014, 16, 1936–1939. [Google Scholar] [CrossRef]
- Takeda, N.; Futaki, E.; Kobori, Y.; Ueda, M.; Miyata, O. Nucleophilic arylation of N,O-ketene acetals with triaryl aluminum reagents: Access to α-aryl amides through an umpolung process. Angew. Chem. Int. Ed. 2017, 56, 16342–16346. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Taniguchi, Y.; Hayama, N.; Inokuma, T.; Takemoto, Y. A powerful hydrogen bond-donating organocatalyst for the enantioselective intramolecular oxa-Michael reaction of α,β-unsaturated amides and esters. Angew. Chem. Int. Ed. 2013, 52, 11114–11118. [Google Scholar] [CrossRef]
- Partridge, K.M.; Anzovino, M.E.; Yoon, T.P. Cycloadditions of N-sulfonyl nitrones generated by Lewis acid catalyzed rearrangement of oxaziridines. J. Am. Chem. Soc. 2008, 130, 2920–2921. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Chatterjee, I.; Wibbeling, B.; Daniliuc, C.G.; Studer, A. Stereospecific formal [3 + 2] dipolar cycloaddition of cyclopropanes with nitrosoarenes: An approach to isoxazolidines. Angew. Chem. Int. Ed. 2014, 53, 5964–5968. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, J.; Wu, J.; Liu, C.; Sioson, K.; Devany, M.; Hu, C.; Zheng, S. Double hetero-Michael addition of N-substituted hydroxylamines to quinone monoketals: Synthesis of bridged isoxazolidines. Org. Lett. 2013, 15, 3534–3537. [Google Scholar] [CrossRef]
- Ramakrishna, I.; Ramaraju, P.; Baidya, M. Synthesis of chiral 1,2-oxazinanes and isoxazolidines via nitroso aldol reaction of distal dialdehydes. Org. Lett. 2018, 20, 1023–1026. [Google Scholar] [CrossRef]
- Brüning, I.; Grashey, R.; Hauck, H.; Huisgen, R.; Seidl, H. 2-Phenyl-3-n-propylisoxazolidine-4,5-cis-dicarboxylic acid N-phenylimide. Org. Synth. 1996, 46, 96–97. [Google Scholar]
- Chakraborty, B.; Rai, N. Synthesis ovf some new scaffolds of amino isoxazolidines and their further functionalization into new class of peptides: A new approach. J. Heterocyclic Chem. 2018, 55, 1053–1060. [Google Scholar] [CrossRef]
- Hou, H.; Zhu, S.; Pan, F.; Rueping, M. Visible-light photoredox-catalyzed synthesis of nitrones: Unexpected rate acceleration by water in the synthesis of ioxazolidines. Org. Lett. 2014, 16, 2872–2875. [Google Scholar] [CrossRef] [PubMed]
- Pagar, V.V.; Liu, R.S. Gold-catalyzed cycloaddition reactions of ethyl diazoacetate, nitrosoarenes, and vinyldiazo carbonyl compounds: Synthesis of isoxazolidine and benzo[b]azepine derivatives. Angew. Chem. Int. Ed. 2015, 54, 4923–4926. [Google Scholar] [CrossRef]
- Li, X.; Feng, T.; Li, D.; Chang, H.; Gao, W.; Wei, W. KOAc-catalyzed one-pot three-component 1,3-dipolar cycloaddition of α-diazo compounds, nitrosoarenes, and alkenes: An approach to functionalized isoxazolidines. J. Org. Chem. 2019, 84, 4402–4412. [Google Scholar] [CrossRef]
- Reddy, A.R.; Guo, Z.; Siu, F.M.; Lok, C.N.; Liu, F.; Yeung, K.C.; Zhou, C.Y.; Che, C.M. Diastereoselective ruthenium porphyrin-catalyzed tandem nitrone formation/1,3-dipolar cycloaddition for isoxazolidines. Synthesis, in silico docking study and in vitro biological activities. Org. Biomol. Chem. 2012, 10, 9165–9174. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, H.X.; Chen, F.; Zhang, Z.B.; Zou, Y.; Chen, C.; Song, X.J.; Tian, F.; Peng, L.; Wang, L.X. Organocatalytic asymmetric annulation between hydroxymaleimides and nitrosoarenes: Stereoselective preparation of chiral quaternary N-hydroxyindolines. Org. Lett. 2017, 19, 2805–2808. [Google Scholar] [CrossRef]
- Zhu, Y.; Malerich, J.P.; Rawal, V.H. Squaramide-catalyzed enantioselective Michael addition of diphenyl phosphite to nitroalkenes. Angew. Chem. Int. Ed. 2010, 49, 153–156. [Google Scholar] [CrossRef]
- Yang, W.; Du, D.M. Highly enantioselective Michael addition of nitroalkanes to chalcones using chiral squaramides as hydrogen bonding organocatalysts. Org. Lett. 2010, 12, 5450–5453. [Google Scholar] [CrossRef]
- Yang, W.; Du, D.M. Chiral squaramide-catalyzed highly enantioselective Michael addition of 2-hydroxy-1,4-naphthoquinones to nitroalkenes. Adv. Synth. Catal. 2011, 353, 1241–1246. [Google Scholar] [CrossRef]
- Yoneda, N.; Fukata, Y.; Asano, K.; Matsubara, S. Asymmetric synthesis of spiroketals with aminothiourea catalysts. Angew. Chem. Int. Ed. 2015, 54, 15497–15500. [Google Scholar] [CrossRef]
- Vakulya, B.; Varga, S.; Csámpai, A.; Soós, T. Highly enantioselective conjugate addition of nitromethane to chalcones using bifunctional cinchona organocatalysts. Org. Lett. 2005, 7, 1967–1969. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).