Abstract
An organocatalytic asymmetric Michael addition reaction of α-azidoindanones with azadienes was developed. A series of optically active benzofuran derivatives containing an azido group was obtained in 32–82% yields with 66:34–>95:5 dr and 53:47–90:10 er. These products demonstrate excellent stability. Furthermore, when the template reaction was scaled up, the reaction efficiency remained consistent. To further assess the practicality of this catalytic asymmetric reaction, two derivative reactions were successfully conducted, affording the corresponding derivative products in good yields and with high stereoselectivities. In addition, a plausible reaction mechanism was also proposed.
1. Introduction
Azides are widely used in organic chemistry as precursors to amines, potential sources of nitro compounds, dipoles in 1,3-diapolar cycloadditions, and starting materials for iminophosphines. The azido group exhibits widespread reactivity in many chemical transformations and is a powerful tool for synthesizing nitrogen-containing heterocycles [1,2,3,4,5]. Chiral azides are a special subclass of chiral amines that are widely present in biologically active compounds (Figure 1). For example, azido nucleosides have been used to treat AIDS, R-1479 is a hepatitis C inhibitor, azido glutathione derivatives are potent inhibitors of the aldehyde oxidase enzyme in Staphylococcus aureus, and the compound also has the potential to induce apoptosis in Malleya holdei, Turbella florida, and Mycobacterium tuberculosis. Chiral azides play an important structural role in the synthesis of various derivatives of chiral products, and the catalytic asymmetric synthesis of chiral azides is a growing research topic that is increasingly being recognized [6,7,8,9,10,11].
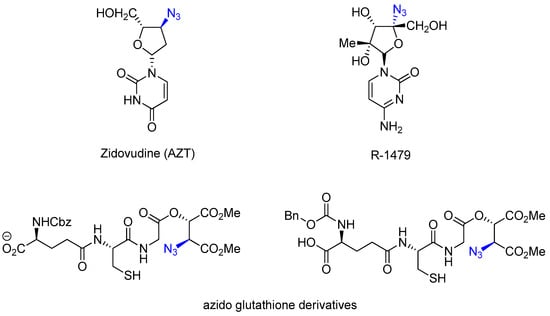
Figure 1.
Chiral azides with biological activity.
In recent years, the deprotonation of α-azide carbonyl compounds has been an effective method for the catalytic asymmetric synthesis of α-chiral azides [12,13]. However, the methods of catalytic enantioselective synthesis of chiral tertiary azides are still limited, and catalytic asymmetric synthesis of chiral tertiary azides is still rarely reported. α-Azidoindanone is a class of highly reactive substrates containing the azido group. By participating in the Michael addition reaction of α-azidoindanones, multifunctional chiral tertiary azide compounds with high values can be efficiently and simply synthesized, which has attracted the attention of many organic chemists in recent years.
In 2020, Ding and co-workers [12] used a bifunctional phosphoramide catalyst and squaramide catalyst to conduct a Michael addition reaction between α-azidoindanones and nitroolefins (Scheme 1). In this reaction, hydrogen bond donor played a key role in controlling diastereoselectivity, and a bifunctional phosphoramide catalyst can selectively provide cis addition products. The squaramide catalyst selectively provided the trans-addition products. In the same year, they also synthesized α-chiral tertiary azides using α-azidoindanones by Michael addition reaction with β,γ-unsaturated α-keto esters (Scheme 2) [13]. Syn-addition products were provided by the bifunctional phosphoramide catalyst and hexafluoroisopropyl alcohol. Thiourea catalyst provided anti-addition products in the absence of the additive hexafluoroisopropyl alcohol, and the additive hexafluoroisopropyl alcohol has also been shown to be a key control factor in achieving diastereoselective reversal when using a phosphoramide catalyst, and the products were easily converted into enantiomeric enriched nitrogenous heterocyclic rings. In our previous work, a ditrifluoromethyl aniline and quinine-derived squaramide catalyst was used to catalyze a Michael addition reaction between α-thiocyanindones and azadienes, in which α-thiocyanoindanone was enolized and deprotonated by quinine unit of chiral squaramide C1. At the same time, benzofuran-derived azadiene was activated by catalyst C1 by forming hydrogen bonding [14].
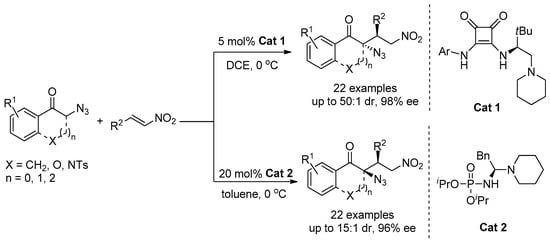
Scheme 1.
Asymmetric Michael addition reaction of α-azidoindanones with nitroolefins.
Scheme 2.
Asymmetric Michael addition reaction of α-azidoindanones with β,γ-unsaturated α-ketoesters.
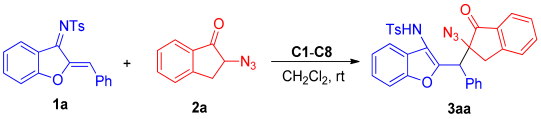
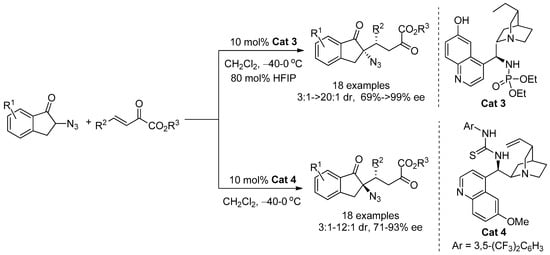
However, the synthesis of chiral azides is still a challenge due to the challenge of the formation of quaternary carbon stereocenters and the easy decomposition of α-azido carbonyl compounds. Therefore, in this paper, the catalytic asymmetric Michael addition reaction of α-azidoindanones with azadienes was developed, and optically active benzofuran derivatives containing α-tertiary azide can be efficiently and easily synthesized through this protocol.
2. Results
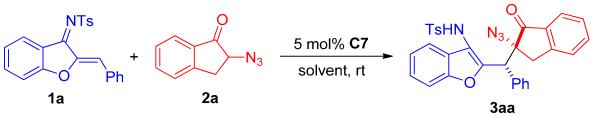
First, a series of squaramide and thiourea organocatalysts (C1–C8) was screened (Figure 2). By comparing the experimental results of C1, C2, and C3 (Table 1, entry 1–3), it can be found that the enantioselectivity of using quinine alkaloids-derived squaramide catalyst for this Michael addition reaction is better than that of thiourea or cyclohexanediamine-derived catalyst. Therefore, different squaramide catalysts are further screened. Finally, it was found that the enantioselectivity of the product catalyzed by the quinidine-derived mono-trifluoromethyl-substituted squaramide catalyst C7 (Table 1, entry 7) increased to a certain extent, reaching 14:86 er, and the diastereoselectivity also improved compared with C1. Therefore, C7 was chosen as the optimal catalyst.
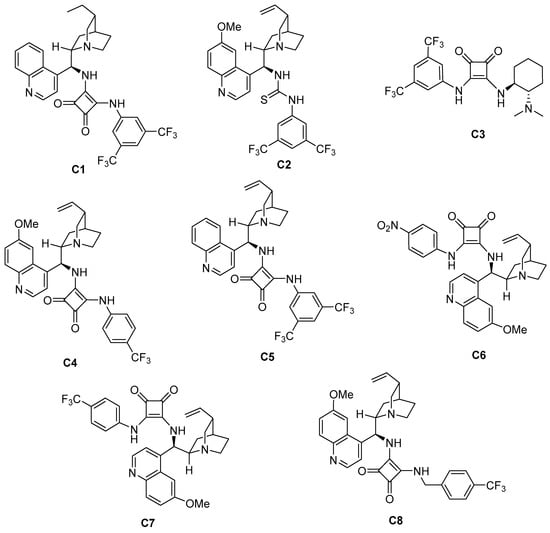
Figure 2.
Catalysts used for reaction condition screening.

Table 1.
Evaluation of catalysts.
Then, the reaction solvent, catalyst loading, and reaction temperature were further examined. First, the reaction effect of this Michael addition reaction in different solvents was evaluated. Similar enantioselectivities and diastereoselectivities were observed in CH2Cl2, AcOEt, MTBE, CHCl3, and ClCH2CH2Cl solvents, while in PhCH3, THF, CCl4, and PhCF3 solvents, reduced enantioselectivities were obtained. When acetone or acetonitrile were used as the solvent (Table 2, entries 12 and 13), the enantioselectivity, yield, and diastereoselectivity were improved compared with dichloromethane. Through comparison of the data, it was found that the enantioselectivity can be increased to 90:10 er when using acetonitrile as the solvent, and the stereoselectivity was slightly better than acetone. Therefore, acetonitrile was chosen as the optimal solvent.

Table 2.
Evaluation of solvent, catalyst loading, and temperature.
After the optimal solvent was determined, the catalyst loading was further investigated. It was found that the catalyst loading had no effect on the enantioselectivity and diastereoselectivity of the product, and the effect on the yield of the product was not particularly significant (Table 2, entries 14 and 15). However, the use of catalyst loading has a certain impact on the reaction time. When the catalyst loading was increased to 10 mol% (Table 2, entry 14), the enantioselectivity, diastereoselectivity, and yield were not effectively improved, and the reaction time was only shortened compared with the lower catalyst loading. Next, we tried to reduce the temperature to improve the enantioselectivity of the reaction. Although the enantioselectivity of the reaction was further improved, the reaction time was greatly extended and the reaction was incomplete (Table 2, entry 16). Therefore, in order to obtain the reaction product in the principle of high efficiency and economy, the substrate expansion reaction after completion at room temperature is still selected with the 5 mol% loading of catalyst. Therefore, at room temperature, adding 0.05 mmol of 1a, 0.10 mmol 2a, and 5 mol% C7 to 0.5 mL of CH3CN can obtain the best reaction effect (Table 2, entry 13).
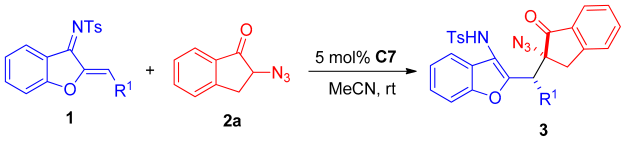
After the optimum reaction conditions were determined, the effect of different substrates on the reaction was further investigated. First, the R1 group of azadienes was varied, and the results of the reaction are shown in Table 3. The Michael addition of different substituted azadienes 1 with α-azidoindanone 2a afforded a series of chiral α-tertiary azidoindanones in 44–82% yields with moderate to good diastereoselectivities (66:34–>95:5 dr) and enantioselectivities (56:44–90:10 er). Compared with the template reaction, most of the azadiene substrates reacted less effectively with α-azidoindanone 2a. In the process of reaction, it can be found that different substituted azadienes were not well dissolved in the acetonitrile solution, which directly led to a decrease in the reaction efficiency between various substituted azadienes and α-azidoindanone 2a. Among them, when R1 in azadienes were the para-substitutents, the product obtained by the reaction of para-fluorinated azadiene 1d can maintain the same yield (82%), enantioselectivity (90:10 er) and diastereoselectivity (90:10 er) as the template reaction (Table 3, entry 4). The para-chloro- or para-methoxy-substituted azadienes 1e and 1g also maintained excellent diastereoselectivities (Table 3, entries 5 and 7), while the other para-substituted azadienes (1b, 1c, and 1f) showed lower yield, enantioselectivity, and diastereoselectivity (Table 3, entries 2, 3, and 6) than the template reaction. When R1 in azadienes were meta-substitutents (Table 3, entries 8 and 9), it could be found that when the meta-position was a Br group, the reaction effect was not much different from that of para-Bromo substrate 1b. But when the meta-position was an electron-donating MeO group (1h), the reaction effect was obviously inferior to that of the para-MeO substrate 1g. In addition, it can be found that ortho-substituted azadiene 1m (Table 3, entry 13) was more enantioselective than para-substituted substrate 1e, although the yield of the reaction was lower than that of 1e. When R1 in azadiene was 2-naphthyl group (Table 3, entry 10), although the reaction can maintain excellent diastereoselectivity, it only gave unsatisfactory enantioselectivity (57:43 er). However, the 2-thienyl-substituted azadiene 1k showed a decrease in both enantioselectivity and diastereoselectivity (Table 3, entry 11).

Table 3.
Scope of azadiene substrates.
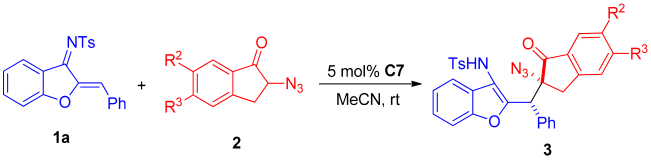
Next, the scope of α-azidoindanones was evaluated, and the reaction results are shown in Table 4. The Michael addition reaction of azadiene 1a with different substituted α-azidoindanone 2 afforded a series of chiral α-tertiary azides in 32–75% yields with moderate to good diastereoselectivities (68:32–>95:5 dr) and enantioselectivities (53:47–83:17 er). It can be found that the enantioselectivity of the reaction of 6-position electron-donating substituted azidoindanone 2c with azadiene 1a was better than that of 6-position electron-withdrawing substituted azidoindanone 2b with azadiene 1a (Table 4, entry 1–2). When the reaction using 5-substituted azidoindanones (Table 4, entry 4 and 6), the enantioselectivity using 5-position electron-drawing substituted azidoindanone 2f was better than that using 5-position electron-donating substituted azidoindanone 2g. The reaction yield of 5,6-dimethoxy-substituted azidoindanone 2d with azadiene 1a was not much different from that of the template reaction (Table 4, entry 3), but the enantioselectivity and diastereoselectivity were decreased. In general, the reaction effect of the expanded azidoindanone substrates with azadiene 1a was not as good as that of the template reaction.

Table 4.
Scope of α-azidoindanone substrates.
To illustrate the synthetic practicality of this asymmetric Michael addition reaction, an amplification of template reaction for tenfold was performed. Azadiene 1a (0.1875 g, 0.5 mmol), azidoindanone 2a (0.1744 g, 1.0 mmol), and 5 mol% C7 in 5 mL acetonitrile solvent was stirred at room temperature for 18 h (Scheme 3); the reaction provided 3aa in 83% yield with 87:13 dr and 90:10 er. It can be observed that after tenfold amplification, except for a slight decrease in diastereoselectivity, both enantioselectivity and yield can effectively maintain the small-scale effect of the template reaction.
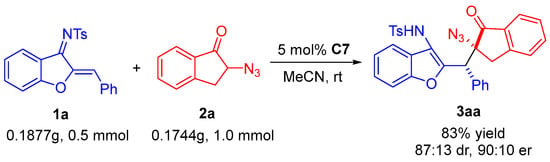
Scheme 3.
The amplification reaction.
To further explore the practicability of this asymmetric reaction, a derivatization reaction was performed on compound 3aa (Scheme 4). Compound 3aa was dissolved in anhydrous THF, and triphenylphosphine was added under vigorous stirring at 0 °C. The reduction product 4 was obtained in 74% yield with >95:5 dr and 90:10 er. In addition, the compound 3aa, phenylacetylene, and tert-butyl 2,2,2-trichloroacetimidate (TBTA) were dissolved in t-BuOH, and sodium ascorbate dissolved in water and copper sulfate pentahydrate were added under vigorous stirring. The reaction succeeded in obtaining the triazole-containing benzofuran derivative 5 in 65% yield with 87:13 dr and 87:13 er. These derivatization reactions demonstrate the application value of this strategy in the synthesis of bioactive molecules.
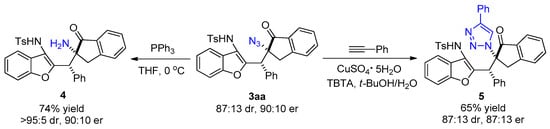
Scheme 4.
Derivatization of the product.
We have tried to culture the single crystal of products 3 or derivatives 4 and 5, but all trials failed to obtain enantiopure single crystals for x-ray diffraction analysis. Based on the similar reaction substrates with our previous report on the Michael addition reaction of α-thiocyanoindanones with azadienes [14], the relative configurations were assigned for products 3 based on a similar reaction pathway. We proposed a plausible reaction mechanism for this squaramide-catalyzed asymmetric Michael addition (Scheme 5). α-Azidoindanone 2a is enolized and deprotonated by quinidine of chiral quadramide C7. At the same time, benzofuran-derived azadiene 1a is activated by catalyst C7 through hydrogen bonding. This dual activation pattern of chiral quadramide C7 on two substrates (transition state A) leads to stereoselective Michael addition to give the intermediate B. The intermediate B then undergoes intramolecular protonation to complete the Michael addition to give the product 3aa.
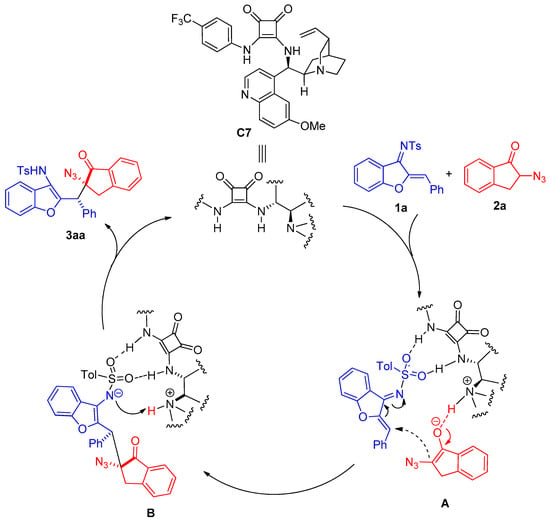
Scheme 5.
The proposed reaction mechanism.
3. Materials and Methods
3.1. General Information
Commercially available compounds were used without further purification, and solvents were dried following commonly used drying methods. Silica gel (200–300 mesh) was used for column chromatography. Melting points was determined using XT-4 melting point apparatus and without correction. 1H NMR was measured by a Bruker Ascend 400 MHz spectrometer (Bruker, Karlsurhe, Germany), and chemical shifts were reported in δ (ppm) units relative to tetramethylsilane (TMS) as the internal standard. 13C NMR spectra were measured at 100 MHz with a 400 MHz spectrometer, and chemical shifts were reported in ppm relative to tetramethylsilane and referenced to the solvent peak (CDCl3, δC = 77.00). High-resolution mass spectra (electron spray ionization) were measured with an Agilent 6520 Accurate-Mass Q-TOF MS system (Agilent, Santa Clara, CA, USA) equipped with an electrospray ionization (ESI) source. Optical rotations were measured with a Krüss P8000 polarimeter at the indicated concentration with the units of g/100 mL. Enantiomeric excesses were determined by chiral HPLC analysis using an Agilent 1200 LC instrument (Agilent, Santa Clara, CA, USA) with a Daicel Chiralpak IA, IC, or ADH column.
3.2. Materials
The synthesis of the squaramide catalyst was conducted according to the method reported in the literature [15,16,17,18]. The azadienes were synthesized according to the literature report [19,20]. α-azidoindanones were synthesized according to the method reported in the literature [21,22].
3.3. Procedure for the Synthesis of Racemates of 3
To a dried small bottle were added 1 (0.04 mmol), 2 (0.03 mmol), Et3N (1.0 mg, 0.01 mmol, 0.25 equiv), and CH3CN (0.5 mL). After stirring at room temperature for 12 h, the reaction mixture was concentrated and the residue was directly purified by silica gel column chromatography using eluent (ethyl acetate/petroleum ether = 1:5) to afford the racemates of 3.
3.4. Procedure for the Synthesis of Chiral Compounds 3
To a dried small bottle were added 1 (0.1 mmol), 2 (0.2 mmol), chiral organocatalyst C7 (5 mol%), and CH3CN (1.0 mL). The mixture was stirred at room temperature, and after completion, the reaction mixture was concentrated and the crude product was directly purified by silica gel column chromatography using eluent (ethyl acetate/petroleum ether = 1:5) to afford the desired product 3.
N-(2-((2-azido-1-oxo-2,3-dihydro-1H-inden-2-yl(phenyl)methyl)benzofuran-3-yl)-4-methyl-benzenesulfonamide (3aa). White solid, 43.4 mg (79% yield), m.p. 94–96 °C. HPLC (Daicel Chiralpak IC, n-hexane/2-propanol = 85:15, flow rate 1.0 mL/min, detection at 254 nm): tR = 54.7 min (minor), tR = 59.5 min (major); 90:10 er. [α]D20 = −74.5 (c 1.1, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.70 (d, J = 7.2 Hz, 1H, ArH), 7.54–7.49 (m, 4H, ArH), 7.42 (d, J = 8.0 Hz, 1H, ArH), 7.34–7.28 (m, 3H, ArH), 7.21 (t, J = 7.2 Hz, 1H, ArH), 7.14–7.11 (m, 2H, ArH), 7.08–7.06 (m, 3H, ArH), 6.97 (d, J = 8.0 Hz, 2H, ArH), 6.49 (s, 1H, NH), 4.86 (s, 1H, CH), 3.75 (d, J = 18.0 Hz, 1H, CH2), 3.11 (d, J = 18.0 Hz, 1H, CH2), 2.26 (s, 3H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ 200.8, 153.5, 152.4, 151.9, 143.7, 136.1, 136.0, 134.7, 134.0, 129.5, 129.4, 128.2, 128.0, 127.4, 127.3, 126.2, 125.7, 125.0, 124.9, 123.5, 120.0, 116.2, 111.5, 71.4, 44.9, 36.5, 21.5 ppm. HRMS (ESI): m/z calculated for C31H28N5O4S [M + NH4]+ 566.1857, found 566.1852.
N-(2-((2-azido-1-oxo-2,3-dihydro-1H-inden-2-yl(4-bromophenyl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3ba). White solid, 31.2 mg (50% yield), m.p. 103–105 °C. HPLC (Daicel Chiralpak IA, n-hexane/2-propanol = 70:30, flow rate 1.0 mL/min, detection at 254 nm): tR = 10.2 min (major), tR = 12.6 min (minor); 68:32 er. [α]D20 = −21.5 (c 1.1, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.72 (d, J = 7.6 Hz, 1H, ArH), 7.56 (td, J1 = 0.8 Hz, J2 = 7.2 Hz, 1H, ArH), 7.52 (d, J = 8.0 Hz, 1H, ArH), 7.48 (d, J = 8.0 Hz, 2H, ArH), 7.36−7.31 (m, 4H, ArH), 7.22–7.18 (m, 3H, ArH), 7.05 (d, J = 8.4 Hz, 2H, ArH), 6.98 (d, J = 8.4 Hz, 2H, ArH), 6.38 (s, 1H, NH), 4.89 (s, 1H, CH), 3.67 (d, J = 18.0 Hz, 1H, CH2), 3.10 (d, J = 18.4 Hz, 1H, CH2), 2.30 (s, 3H, CH3) ppm. 13C NMR (100 MHz, CDCl3): δ 200.5, 153.5, 152.0, 151.7, 144.0, 136.4, 135.9, 133.9, 133.8, 131.4, 131.2, 129.5, 128.2, 127.3, 126.3, 125.5, 125.2, 125.1, 123.6, 121.8, 119.9, 116.4, 111.5, 71.1, 44.1, 36.4, 21.6 ppm. HRMS (ESI): m/z calculated for C31H2379BrN4NaO4S [M + Na]+ 649.0516, found 649.0519; calculated for C31H2381BrN4NaO4S [M + Na]+ 651.0496, found 651.0482.
N-(2-((2-azido-1-oxo-2,3-dihydro-1H-inden-2-yl(p-tolyl)methyl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3ca). White solid, 37.6 mg (67% yield), m.p. 91–93 °C. HPLC (Daicel Chiralpak IC, n-hexane/ethyl acetate = 80:20, flow rate 1.0 mL/min, detection at 254 nm): tR = 10.5 min (minor), tR = 13.3 min (major); 76:24 er. [α]D20 = −37.9 (c 1.4, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.70 (d, J = 7.6 Hz, 1H, ArH), 7.54–7.49 (m, 4H, ArH), 7.40 (d, J = 7.6 Hz, 1H, ArH), 7.33–7.29 (m, 3H, ArH), 7.19 (t, J = 7.6 Hz, 1H, ArH), 7.02–6.97 (m, 4H, ArH), 6.87 (d, J = 8.0 Hz, 2H, ArH), 6.39 (s, 1H, NH), 4.81 (s, 1H, CH), 3.73 (d, J = 18.0 Hz, 1H, CH2), 3.09 (d, J = 18.0 Hz, 1H, CH2), 2.27 (s, 3H, CH3), 2.19 (s, 3H, CH3) ppm; 13C NMR (100 MHz, CDCl3): δ 200.7, 153.5, 152.7, 151.9, 143.8, 137.1, 136.04, 135.96, 133.9, 131.6, 129.5, 129.3, 129.0, 127.9, 127.3, 126.2, 125.8, 125.0, 124.9, 123.4, 119.9, 116.0, 111.5, 71.4, 44.4, 36.5, 21.5, 21.0 ppm. HRMS (ESI): m/z calculated for C32H30N5O4S [M + NH4]+ 580.2013, found 580.2017.
N-(2-((2-azido-1-oxo-2,3-dihydro-1H-inden-2-yl(4-fluorophenyl)methyl)benzo-furan-3-yl)-4-methylbenzenesulfonamide (3da). White solid, 46.6 mg (82% yield), m.p. 95–97 °C. HPLC (Daicel Chiralpak IC, n-hexane/2-propanol = 85:15, flow rate 1.0 mL/min, detection at 254 nm): tR = 28.3 min (major), tR = 37.8 min (minor); 90:10 er. [α]D20 = −72.9 (c 0.83, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.71 (d, J = 7.6 Hz, 1H, ArH), 7.57–7.48 (m, 4H, ArH), 7.35–7.31(m, 4H, ArH), 7.21–7.14 (m, 3H, ArH), 7.00 (d, J = 8.0 Hz, 2H, ArH), 6.79–6.75 (m, 2H, ArH), 6.41 (s, 1H, NH), 4.90 (s, 1H, CH), 3.72 (d, J = 18.0 Hz, 1H, CH2), 3.10 (d, J = 18.0 Hz, 1H, CH2), 2.28 (s, 3H, CH3) ppm; 13C NMR (100 MHz, CDCl3): δ 200.7, 161.9 (d, 1JC–F = 245.0 Hz), 153.5, 152.3, 151.8, 143.9, 136.3, 136.0, 133.9, 131.3 (d, 3JC–F = 8.0 Hz), 130.6 (d, 4JC–F = 3.1 Hz), 129.5, 128.1, 127.3, 126.2, 125.6, 125.1, 125.0, 123.6, 119.9, 116.2, 115.2 (d, 2JC–F = 21.2 Hz), 111.5, 71.3, 44.1, 36.4, 21.5 ppm; HRMS (ESI): m/z calculated for C31H23FN4NaO4S [M + Na]+ 589.1316, found 589.1303.
N-(2-((2-azido-1-oxo-2,3-dihydro-1H-inden-2-yl(4-chlorophenyl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3ea). White solid, 38.3 mg (66% yield), m.p. 93–95 °C. HPLC (Daicel Chiralpak IC, n-hexane/ethyl acetate = 70:30, flow rate 1.0 mL/min, detection at 254 nm): tR = 6.0 min (major), tR = 7.7 min (minor); 56:44 er. [α]D20 = −6.4 (c 1.36, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.72 (d, J = 7.6 Hz, 1H, ArH), 7.56 (td, J1 = 0.8 Hz, J2 = 7.6 Hz, 1H, ArH), 7.52 (d, J = 8.4 Hz, 1H, ArH), 7.47 (d, J = 8.4 Hz, 2H, ArH), 7.37–7.31 (m, 4H, ArH), 7.19 (t, J = 7.6 Hz, 1H, ArH), 7.12 (d, J = 8.8 Hz, 2H, ArH), 7.05 (d, J = 8.8 Hz, 2H, ArH), 6.97 (d, J = 8.0 Hz, 2H, ArH), 6.53 (s, 1H, NH), 4.93 (s, 1H, CH), 3.69 (d, J = 18.0 Hz, 1H, CH2), 3.11 (d, J = 18.0 Hz, 1H, CH2), 2.28 (s, 3H, CH3) ppm; 13C NMR (100 MHz, CDCl3): δ 200.6, 153.5, 152.0, 151.8, 143.9, 136.4, 135.9, 133.8, 133.5, 133.4, 130.8, 129.4, 128.4, 128.2, 127.3, 126.3, 125.5, 125.15, 125.05, 123.6, 119.9, 116.4, 111.5, 71.2, 44.1, 36.4, 21.5 ppm. HRMS (ESI): m/z calculated for C31H27ClN5O4S [M + NH4]+ 600.1467, found 600.1480.
N-(2-((2-azido-1-oxo-2,3-dihydro-1H-inden-2-yl(4-(trifluoromethyl)phenyl)methyl)benzo-furan-3-yl)-4-methylbenzenesulfonamide (3fa). White solid, 40.4 mg (66% yield), m.p. 109–111 °C. HPLC (Daicel Chiralpak IA, n-hexane/ethyl acetate = 70:30, flow rate 1.0 mL/min, detection at 254 nm): tR = 5.6 min (major), tR = 6.8 min (minor); 85:15 er. [α]D20 = −56.3 (c 1.06, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.73 (d, J = 7.6 Hz, 1H, ArH), 7.56 (td, J1 = 1.2 Hz, J2 = 7.6 Hz, 1H, ArH), 7.52 (d, J = 8.4 Hz, 1H, ArH), 7.48 (d, J = 8.0 Hz, 2H, ArH), 7.37–7.30 (m, 7H, ArH), 7.27–7.24 (m, 1H, ArH), 7.19–7.15 (m, 1H, ArH), 6.98 (d, J = 8.4 Hz, 2H, ArH), 6.50 (s, 1H, NH), 5.03 (s, 1H, CH), 3.65 (d, J = 18.0 Hz, 1H, CH2), 3.12 (d, J = 18.0 Hz, 1H, CH2), 2.27 (s, 3H, CH3) ppm; 13C NMR (100 MHz, CDCl3): δ 200.3, 153.6, 151.7, 151.5, 143.9, 139.0, 136.4, 136.0, 133.7, 129.9, 129.5, 128.3, 127.3, 126.3, 125.4, 125.3, 125.14 (d, JC–F = 3.6 Hz), 125.13, 123.6, 122.5, 119.7, 116.6, 111.6, 71.2, 44.6, 36.7, 21.4 ppm. HRMS (ESI): m/z calculated for C32H23F3N4NaO4S [M + Na]+ 639.1284, found 639.1272.
N-(2-((2-azido-1-oxo-2,3-dihydro-1H-inden-2-yl(4-(oxo-l6-methyl)phenyl)methyl)benzo-furan-3-yl)-4-methylbenzenesulfonamide (3ga). White solid, 30.6 mg (53% yield), m.p. 91–93 °C. HPLC (Daicel Chiralpak IC, n-hexane/2-propanol = 77:23, flow rate 1.0 mL/min, detection at 254 nm): tR = 30.5 min (major), tR = 35.0 min (minor); 82:18 er. [α]D20 = −34.6 (c 0.34, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.70 (d, J = 7.6 Hz, 1H, ArH), 7.55–7.50 (m, 4H, ArH), 7.41 (d, J = 7.6 Hz, 1H, ArH), 7.34–7.30 (m, 3H, ArH), 7.23–7.19 (m, 1H, ArH), 7.05 (d, J = 8.8 Hz, 2H, ArH), 7.01 (d, J = 8.0 Hz, 2H, ArH), 6.62–6.58 (m, 2H, ArH), 6.31 (s, 1H, NH), 4.80 (s, 1H, CH), 3.75 (d, J = 18.0 Hz, 1H, CH2), 3.69 (s, 3H, CH3), 3.09 (d, J = 18.0 Hz, 1H, CH2), 2.28 (s, 3H, CH3) ppm; 13C NMR (100 MHz, CDCl3): δ 200.8, 158.7, 153.5, 152.8, 152.0, 143.8, 136.1, 136.0, 134.0, 130.6, 129.5, 128.0, 127.3, 126.6, 126.2, 125.8, 124.9, 123.5, 119.9, 115.9, 113.6, 111.5, 71.4, 55.0, 44.0, 36.3, 21.5 ppm. HRMS (ESI): m/z calculated for C32H30N5O5S [M + NH4]+ 596.1962, found 596.1940.
N-(2-((2-azido-1-oxo-2,3-dihydro-1H-inden-2-yl(m-tolyl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3ha). White solid, 29.8 mg (53% yield), m.p. 123–125 °C. HPLC (Daicel Chiralpak IA, n-hexane/ethyl acetate = 70:30, flow rate 1.0 mL/min, detection at 254 nm): tR = 6.6 min (major), tR = 8.0 min (minor); 58:42 er. [α]D20 = −22.2 (c 1.12, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.71 (d, J = 8.0 Hz, 1H, ArH), 7.53–7.50 (m, 4H, ArH), 7.44 (d, J = 7.6 Hz, 1H, ArH), 7.34–7.29 (m, 3H, ArH), 7.21 (t, J = 7.6 Hz, 1H, ArH), 7.00 (d, J = 8.0 Hz, 2H, ArH), 6.95–6.87 (m, 4H, ArH), 6.36 (s, 1H, NH), 4.81 (s, 1H, CH), 3.73 (d, J = 18.0 Hz, 1H, CH2), 3.10 (d, J = 18.0 Hz, 1H, CH2), 2.27 (s, 3H, CH3), 2.17 (s, 3H, CH3) ppm; 13C NMR (100 MHz, CDCl3): δ 200.7, 153.5, 152.5, 151.9, 143.7, 137.8, 136.1, 136.0, 134.6, 134.0, 130.0, 129.5, 128.2, 128.1, 128.0, 127.3, 126.5, 126.2, 125.8, 124.93, 124.90, 123.5, 120.0, 116.1, 111.5, 71.4, 44.8, 36.5, 21.5, 21.4 ppm. HRMS (ESI): m/z calculated forC32H30N5O4S [M + NH4]+ 580.2013, found 580.2015.
N-(2-((2-azido-1-oxo-2,3-dihydro-1H-inden-2-yl(3-bromophenyl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3ia). White solid, 27.7 mg (44% yield), m.p. 133–135 °C. HPLC (Daicel Chiralpak IC, n-hexane/ethyl acetate = 85:15, flow rate 1.0 mL/min, detection at 254 nm): tR = 14.0 min (major), tR = 17.3 min (minor); 68:32 er. [α]D20 = −30.8 (c 0.50, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.74 (d, J = 7.6 Hz, 1H, ArH), 7.58–7.48 (m, 4H, ArH), 7.40–7.32 (m, 4H, ArH), 7.28 (d, J = 1.6 Hz, 1H, ArH), 7.24–7.19 (m, 2H, ArH), 7.13 (d, J = 7.6 Hz, 1H, ArH), 7.01 (d, J = 8.0 Hz, 2H, ArH), 6.96 (t, J = 8.0 Hz, 1H, ArH), 6.38 (s, 1H, NH), 4.85 (s, 1H, CH), 3.67 (d, J = 18.0 Hz, 1H, CH2), 3.10 (d, J = 18.0 Hz, 1H, CH2), 2.28 (s, 3H, CH3) ppm; 13C NMR (100 MHz, CDCl3): δ 200.5, 153.6, 151.7, 151.6, 143.9, 137.0, 136.3, 135.8, 133.9, 132.2, 130.7, 129.8, 129.5, 128.24, 128.19, 127.3, 126.3, 125.5, 125.2, 125.1, 123.6, 122.3, 120.0, 116.6, 111.6, 71.3, 44.4, 36.5, 21.6 ppm. HRMS (ESI): m/z calculated for C31H2779BrN5O4S [M + NH4]+ 644.0962, found 644.0983; calculated for C31H2781BrN5O4S [M + NH4]+ 646.0941, found 646.0965.
N-(2-((2-azido-1-oxo-2,3-dihydro-1H-inden-2-yl(naphthalen-2-yl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3ja). White solid, 27.3 mg (46% yield), m.p. 109–111 °C. HPLC (Daicel Chiralpak IC, n-hexane/2-propanol = 80:20, flow rate 1.0 mL/min, detection at 254 nm): tR = 28.9 min (major), tR = 33.8 min (minor); 57:43 er. [α]D20 = −111.3 (c 0.68, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.72–7.65 (m, 3H, ArH), 7.59–7.53 (m, 4H, ArH), 7.49–7.35 (m, 6H, ArH), 7.28–7.24 (m, 4H, ArH), 6.75 (d, J = 8.4 Hz, 2H, ArH), 6.37 (s, 1H, NH), 5.06 (s, 1H, CH), 3.75 (d, J = 18.0 Hz, 1H, CH2), 3.14 (d, J = 18.0 Hz, 1H, CH2), 1.91 (s, 3H, CH3) ppm; 13C NMR (100 MHz, CDCl3): δ 200.7, 153.7, 152.4, 152.0, 143.8, 136.2, 135.6, 133.8, 133.0, 132.4, 132.3, 129.3, 128.8, 128.1, 128.0, 127.9, 127.4, 127.2, 126. 9, 126.3, 126.2, 125.8, 125.1, 125.0, 123.6, 120.3, 116.4, 111. 6, 71.4, 44.5, 36.4, 21.1 ppm. HRMS (ESI): m/z calculated for C35H30N5O4S [M + NH4]+ 616.2013, found 616.2026.
N-(2-((2-azido-1-oxo-2,3-dihydro-1H-inden-2-yl(thiophen-2-yl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3ka). White solid, 36.7 mg (66% yield), m.p. 119–121 °C. HPLC (Daicel Chiralpak IC, n-hexane/2-propanol = 70:30, flow rate 1.0 mL/min, detection at 254 nm): tR = 18.4 min (major), tR = 28.0 min (minor); 61:39 er. [α]D20 = −19.4 (c 1.53, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.75 (d, J = 7.6 Hz, 1H, ArH), 7.58–7.50 (m, 4H, ArH), 7.43 (d, J = 7.6 Hz, 1H, ArH), 7.37–7.31 (m, 3H, ArH), 7.22 (t, J = 7.6 Hz, 1H, ArH), 7.03–7.01 (m, 3H, ArH), 6.72–6.70 (m, 1H, ArH), 6.63 (d, J = 3.2 Hz, 1H, ArH), 6.48 (s, 1H, NH), 5.08 (s, 1H, CH), 3.85 (d, J = 18.0 Hz, 1H, CH2), 3.07 (d, J = 18.0 Hz, 1H, CH2), 2.28 (s, 3H, CH3) ppm; 13C NMR (100 MHz, CDCl3): δ 200.3, 153.4, 152.1, 151.3, 143.9, 136.2, 135.9, 135.8, 133.9, 129.5, 128.4, 128.1, 127.3, 126.34, 126.28, 125.6, 125.5, 125.2, 125.0, 123.6, 120.0, 116.2, 111.5, 71.5, 40.7, 36.4, 21.5 ppm. HRMS (ESI): m/z calculated for C29H26N5O4S2 [M + NH4]+ 572.1421, found 572.1424.
N-(2-((2-azido-1-oxo-2,3-dihydro-1H-inden-2-yl(3,5-dichlorophenyl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3la). White solid, 41.5 mg (67% yield), m.p. 103–105 °C. HPLC (Daicel Chiralpak IA, n-hexane/ethyl acetate = 70:30, flow rate 1.0 mL/min, detection at 254 nm): tR = 9.1 min (major), tR = 10.0 min (minor); 72:28 er. [α]D20 = −12.6 (c 1.58, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.94 (d, J = 8.4 Hz, 1H, ArH), 7.77 (d, J = 7.6 Hz, 1H, ArH), 7.59–7.56 (m, 3H, ArH), 7.40–7.36 (m, 2H, ArH), 7.33 (d, J =2.4 Hz, 1H, ArH), 7.30 (d, J = 8.0 Hz, 1H, ArH), 7.24–7.19 (m, 2H, ArH), 7.11 (d, J = 8.4 Hz, 1H, ArH), 7.04–7.01 (m, 1H, ArH), 6.93–6.90 (m, 1H, ArH), 6.37 (s, 1H, NH), 5.50 (s, 1H, CH), 3.40 (d, J = 17.6 Hz, 1H, CH2), 2.99 (d, J = 17.6 Hz, 1H, CH2), 2.34 (s, 3H, CH3) ppm; 13C NMR (100 MHz, CDCl3): δ 199.3, 153.3, 151.1, 150.1, 144.0, 136.6, 136.1, 135.2, 134.4, 134.2, 132.8, 132.0, 129.6, 129.4, 128.3, 127.5, 127.0, 126.1, 125.1, 125.0, 125.0, 123.3, 119.4, 115.9, 111.6, 71.0, 41.6, 39.2, 21.5 ppm. HRMS (ESI): m/z calculated for C31H22Cl2N4NaO4S [M + Na]+ 639.0631, found 639.0638.
N-(2-((2-azido-1-oxo-2,3-dihydro-1H-inden-2-yl(2-chloropheny)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3ma). White solid, 29.2 mg (50% yield), m.p. 92–95 °C. HPLC (Daicel Chiralpak IA, n-hexane/ethyl acetate = 70:30, flow rate 1.0 mL/min, detection at 254 nm): tR = 8.8 min (major), tR = 10.7 min (minor); 71:29 er. [α]D20 = −22.4 (c 0.81, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.96 (dd, J1 = 1.8 Hz, J2 = 7.4 Hz, 1H, ArH), 7.77 (d, J = 7.6 Hz, 1H, ArH), 7.62 (d, J = 8.4 Hz, 2H, ArH), 7.57 (td, J1 = 0.8 Hz, J2 = 7.6 Hz, 1H, ArH), 7.40 (d, J = 8.4 Hz, 1H, ArH), 7.37 (t, J = 7.6 Hz, 1H, ArH), 7.32–7.29 (m, 2H, ArH), 7.24–7.17 (m, 3H, ArH), 7.12 (d, J = 8.0 Hz, 2H, ArH), 7.05 (t, J = 7.6 Hz, 1H, ArH), 7.00 (d, J = 8.0 Hz, 1H, ArH), 6.27(s, 1H, NH), 5.51 (s, 1H, CH), 3.44 (d, J = 17.6 Hz, 1H, CH2), 2.97 (d, J = 17.6 Hz, 1H, CH2), 2.33 (s, 3H, CH3) ppm; 13C NMR (100 MHz, CDCl3): δ 199.4, 153.3, 151.5, 150.2, 143.8, 136.6, 136.0, 134.4, 134.3, 133.3, 132.0, 129.7, 129.6, 129.1, 128.1, 127.5, 126.7, 126.1, 125.2, 125.1, 124.9, 123.2, 119.5, 115.7, 111.6, 71.2, 42.0, 39.1, 21.5 ppm. HRMS (ESI): m/z calculated for C31H27ClN5O4S [M + NH4]+ 600.1467, found 600.1471.
N-(2-((2-azido-6-fluoro-1-oxo-2,3-dihydro-1H-inden-2-yl(phenyl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3ab). White solid, 25.8 mg (46% yield), m.p. 85–87 °C. HPLC (Daicel Chiralpak ADH, n-hexane/2-propanol = 70:30, flow rate 1.0 mL/min, detection at 254 nm): tR = 15.5 min (major), tR =22.0 min (minor); 53:47 er. [α]D20 = −3.8 (c 0.64, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.51 (t, J = 7.8 Hz, 3H, ArH), 7.37–7.27 (m, 4H, ArH), 7.25–7.18 (m, 2H, ArH), 7.14–7.07 (m, 5H, ArH), 6.99 (d, J = 8.0 Hz, 2H, ArH), 6.28 (s, 1H, NH), 4.87 (s, 1H, CH), 3.74 (d, J = 18.0 Hz, 1H, CH2), 3.06 (d, J = 18.0 Hz, 1H, CH2), 2.28 (s, 3H, CH3) ppm; 13C NMR (100 MHz, CDCl3): δ 200.1, 162.3 (d, 1JC–F = 248.0 Hz), 153.5, 152.4, 147.4, 143.9, 135.9, 135.6 (d, 3JC–F = 7.7 Hz), 134.5, 129.6, 129.4, 128.3, 127.7 (d, 3JC–F = 8.0 Hz), 127.6, 127.3, 125.7, 125.1, 123.9 (d, 2JC–F = 23.5 Hz), 123.5, 119.9, 116.2, 111.5, 110.6 (d, 2JC–F = 22.0 Hz), 72.0, 44.9, 36.0, 21.5 ppm. HRMS (ESI): m/z calculated for C31H23FN4O4SNa [M + Na]+ 589.1316, found 589.1302.
N-(2-((2-azido-6-methyl-1-oxo-2,3-dihydro-1H-inden-2-yl(phenyl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide) (3ac). White solid, 22.2 mg (39% yield), m.p. 105–107 °C. HPLC (Daicel Chiralpak IA, n-hexane/ethyl acetate = 80:20, flow rate 1.0 mL/min, detection at 254 nm): tR = 12.2 min (major), tR = 19.4 min (minor); 73:27 er. [α]D20 = −46.8 (c 0.8, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.53–7.49 (m, 4H, ArH), 7.43 (d, J = 7.6 Hz, 1H, ArH), 7.35–7.30 (m, 2H, ArH), 7.23–7.17 (m, 2H, ArH), 7.13–7.06 (m, 5H, ArH), 6.97 (d, J = 8.0 Hz, 2H, ArH), 6.38 (s, 1H, NH), 4.83 (s, 1H, CH), 3.69 (d, J = 18.0 Hz, 1H, CH2), 3.05 (d, J = 18.0 Hz, 1H, CH2), 2.33 (s, 3H, CH3), 2.27 (s, 3H, CH3) ppm; 13C NMR (100 MHz, CDCl3): δ 200.6, 153.5, 152.5, 149.3, 143.7, 138.1, 137.5, 135.9, 134.7, 134.1, 129.5, 129.4, 128.2, 127.4, 127.3, 125.9, 125.8, 125.0, 124.8, 123.5, 120.0, 116.1, 111.5, 71.7, 44.7, 36.1, 21.5, 21.0 ppm. HRMS (ESI): m/z calculated for C32H30N5O4S [M + NH4]+ 580.2013, found 580.2007.
N-(2-((2-azido-5,6-Dimethoxy-1-oxo-2,3-dihydro-1H-inden-2-yl(phenyl)methyl)benzofu-ran-3-yl)-4-methylbenzenesulfonamide (3ad). White solid, 45.9 mg (75% yield), m.p. 107–109 °C. HPLC (Daicel Chiralpak IC, n-hexane/ethyl acetate = 80:20, flow rate 1.0 mL/min, detection at 254 nm): tR = 43.3 min (major), tR = 48.8 min (minor); 81:19 er. [α]D20 = −71.0 (c 1.6, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.52–7.47 (m, 4H, ArH), 7.33 (td, J1 = 1.2 Hz, J2 = 7.2 Hz, 1H, ArH), 7.24–7.20 (m, 1H, ArH),7.12–7.06 (m, 6H, ArH), 6.96 (d, J = 8.0 Hz, 2H, ArH), 6.69 (s, 1H, ArH), 6.46 (s, 1H, NH), 4.83 (s, 1H, CH), 3.89 (s, 3H, CH3), 3.86 (s, 3H, CH3), 3.69 (d, J = 18.0 Hz, 1H, CH2), 3.01 (d, J = 18.0 Hz, 1H, CH2), 2.26 (s, 3H, CH3) ppm; 13C NMR (100 MHz, CDCl3): δ 199.1, 156.7, 153.5, 152.4, 149.8, 147.8, 143.6, 135.9, 134.8, 129.4, 129.3, 128.1, 127.3, 127.2, 126.6, 126.4, 125.8, 124.9, 123.4, 120.1, 116.2, 111.4, 106.9, 104.9, 71.9, 56.2, 56.0, 44.8, 36.1, 21.5 ppm. HRMS (ESI): m/z calculated for C33H32N5O6S [M + NH4]+ 626.2068, found 626.2074.
N-(2-((2-azido-5-fluoro-1-oxo-2,3-dihydro-1H-inden-2-yl(phenyl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3ae) White solid, 33.6 mg (59% yield), m.p. 98–100 °C. HPLC (Daicel Chiralpak IC, n-hexane/2-propanol = 75:25, flow rate 1.0 mL/min, detection at 254 nm): tR = 24.0 min (major), tR =27.5 min (minor); 83:17 er. [α]D20 = −51.2 (c 1.3, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.71 (dd, J1 = 5.2 Hz, J2 = 8.4 Hz, 1H, ArH), 7.52 (d, J = 8.0 Hz, 1H, ArH), 7.49 (d, J= 8.0 Hz, 2H, ArH), 7.37 (d, J = 8.0 Hz, 1H, ArH), 7.34–7.30 (m, 1H, ArH), 7.20 (t, J = 7.6 Hz, 1H, ArH), 7.15–7.08 (m, 5H, ArH), 7.02–6.95 (m, 4H, ArH), 6.36 (s, 1H, NH), 4.90 (s, 1H, CH), 3.76 (d, J = 18.4 Hz, 1H, CH2), 3.08 (d, J = 18.0 Hz, 1H, CH2), 2.27 (s, 3H, CH3) ppm; 13C NMR (100 MHz, CDCl3): δ 199.0, 167.8 (d, 1JC–F = 257.5 Hz), 154.9 (d, 3JC–F = 10.4 Hz), 153.5, 152.4, 143.8, 135.9, 134.6, 130.4, 129.5, 129.4, 128.3, 127.6, 127.4 (d, 3JC-F = 10.7 Hz), 127.3, 125.7, 125.1, 123.5, 119.9, 116.5 (d, 2JC–F = 23.7 Hz), 116.2, 113.0 (d, 2JC–F = 22.5 Hz), 111.5, 71.5, 44.8, 36.5, 21.5 ppm. HRMS (ESI): m/z calculated for C31H23FN4NaO4S [M + Na]+ 589.1316, found 589.1296.
N-(2-((2-azido-5-bromo-1-oxo-2,3-dihydro-1H-inden-2-yl(phenyl)methyl)benzofuran-3-yl)-4-methylbenzenesulfonamide (3af). White solid, 20.2 mg (32% yield), m.p. 98–100 °C. HPLC (Daicel Chiralpak IA, n-hexane/ethyl acetate = 70:30, flow rate 1.0 mL/min, detection at 254 nm): tR = 6.9 min (major), tR = 8.7 min (minor); 74:26 er. [α]D20 = −63.1 (c 0.86, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.55 (d, J = 8.4 Hz, 1H, ArH), 7.52 (d, J = 8.0 Hz, 1H, ArH), 7.49 (d, J = 8.0 Hz, 3H, ArH), 7.44 (d, J = 8.0 Hz, 1H, ArH), 7.35 (d, J = 8.0 Hz, 1H, ArH), 7.31 (dd, J1 = 1.2 Hz, J2 = 8.4 Hz, 1H, ArH), 7.22–7.17 (m, 1H, ArH) 7.14–7.07 (m, 5H, ArH), 6.98 (d, J = 8.0 Hz, 2H, ArH), 6.34 (s, 1H, NH), 4.90 (s, 1H, CH), 3.75 (d, J = 18.4 Hz, 1H, CH2), 3.07 (d, J = 18.0 Hz, 1H, CH2), 2.27 (s, 3H, CH3) ppm; 13C NMR (100 MHz, CDCl3): δ 199.6, 153.5, 153.4, 152.4, 143.8, 135.9, 134.5, 132.7, 131.7, 129.5, 129.4, 128.4, 127.6, 127.3, 126.0, 125.6, 125.1, 123.5, 119.8, 116.2, 111.5, 71.3, 44.8, 36.2, 21.5 ppm. HRMS (ESI): m/z calculated for C31H2379BrN4O4SNa [M + Na]+ 649.0516, found 649.0524; calculated. for C31H2381BrN4O4SNa [M + Na]+ 651.0495, found 651.0484.
N-(2-((2-azido-5-(oxo-l6-methyl)-1-oxo-2,3-dihydro-1H-inden-2-yl(phenyl)methyl)benzofu-ran-3-yl)-4-methylbenzenesulfonamide (3ag). White solid, 23.5 mg (41% yield), m.p. 108–110 °C. HPLC (Daicel Chiralpak IA, n-hexane/ethyl acetate = 70:30, flow rate 1.0 mL/min, detection at 254 nm): tR = 7.9 min (major), tR = 11.2 min (minor); 56:44 er. [α]D20 = −3.4 (c 0.48, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.63 (d, J = 8.4 Hz, 1H, ArH), 7.52–7.47 (m, 4H, ArH), 7.35–7.31 (m, 1H, ArH), 7.23 (t, J = 7.6 Hz, 1H, ArH), 7.11–7.04 (m, 5H, ArH), 6.97 (d, J = 8.0 Hz, 2H, ArH), 6.83 (dd, J1 = 2.0 Hz, J2 = 8.4 Hz, 1H, ArH), 6.71 (d, J = 1.6 Hz, 1H, ArH), 6.36 (s, 1H, NH), 4.80 (s, 1H, CH), 3.82 (s, 3H, CH3), 3.69 (d, J = 18.0 Hz, 1H, CH2), 3.04 (d, J = 18.0 Hz, 1H, CH2), 2.26 (s, 3H, CH3) ppm; 13C NMR (100 MHz, CDCl3): δ 198.5, 166.4, 155.0, 153.6, 152.5, 143.7, 135.9, 134.8, 129.5, 129.4, 128.2, 127.4, 127.3, 127.1, 126.8, 125.9, 125.0, 123.5, 120.1, 116.2, 111.4, 109.3, 71.8, 55.7, 44.7, 36.4, 21.6 ppm; HRMS (ESI): m/z calculated for C32H30N5O5S [M + NH4]+ 596.1962, found 596.1978.
3.5. Derivatization Reaction to Prepare Compound 4
The preparation of compound 4 referred to the synthesis in reference [23]. Chiral compound 3aa (32.9 mg, 0.06 mmol) was dissolved in 3 mL anhydrous THF, and then triphenylphosphine (47.2 mg, 0.18 mmol) was added under vigorous stirring at 0 °C. The reaction was monitored by TLC and stirring was continued until the starting material was consumed. Then, the reaction was quenched with 3 mL of water and the aqueous phase was extracted with ethyl acetate. The organic phase was concentrated under vacuum, the crude product waspurified by silica gel column chromatography using petroleum ether/ethyl acetate (5:1) as the eluent to obtain white solid product 4 (23.1 mg, 74% yield), m.p. 112–114 °C. HPLC (Daicel Chiralpak IA, n-hexane/2-propanol = 70:30, flow rate 1.0 mL/min, detection at 254 nm): tR = 11.8 min (major), tR =16.6 min (minor); 90:10 er. [α]D20 = −26.4 (c 0.57, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 7.62–7.59 (m, 3H, ArH), 7.50–7.44 (m, 3H, ArH), 7.29 (d, J = 7.2 Hz, 2H, ArH), 7.25–7.18 (m, 2H, ArH), 7.14 (d, J = 8.0 Hz, 2H, ArH), 7.00 (s, 5H, ArH), 4.37 (s, 1H, CH), 3.78 (d, J = 17.6 Hz, 1H, CH2), 2.93 (d, J = 17.6 Hz, 1H, CH2), 2.35 (s, 3H, CH3), 2.04–1.43 (m, 2H, NH2) ppm; 13C NMR (100 MHz, CDCl3): δ 207.2, 153.4, 152.6, 152.0, 143.9, 136.4, 135.8, 135.5, 134.6, 129.7, 129.3, 128.0, 127.6, 127.4, 127.1, 126.2, 125.9, 124.63, 124.58, 123.3, 120.3, 116.3, 111.3, 66.3, 48.9, 38.9, 21.5 ppm. HRMS (ESI): m/z calculated for C31H27N2O4S [M + H]+ 523.1686, found 523.1675.
3.6. Derivatization Reaction to Prepare Compound 5
The preparation of compound 5 referred to the synthesis in reference [24].
Chiral compound 3aa (32.9 mg, 0.06 mmol), phenylacetylene (7.0 mg, 0.072 mmol), and TBTA (0.3 mg, 0.0006 mmol) were dissolved in 0.3 mL t-BuOH, then sodium ascorbate (4.8 mg, 0.024 mmol) and copper sulfate pentahydrate (3.0 mg, 0.012 mmol) dissolved in water were added to the reaction mixture under vigorous stirring, and the reaction was monitored by TLC. After the initial raw materials were consumed, the reaction mixture was diluted with water and extracted with dichloromethane. The combined organic layers were dried using anhydrous sodium sulfate and concentrated under vacuum to remove the solvent. The crude product was separated by a silica gel column using petroleum ether/ethyl acetate = 5:1 as eluent to give white solid product 5 (29.0 mg, 65% yield), m.p. 126–128 °C. HPLC (Daicel Chiralpak IC, n-hexane/ethyl acetate = 80:20, flow rate 1.0 mL/min, detection at 254 nm): tR = 15.6 min (major), tR = 20.3min (minor); 87:13 er. [α]D20 = −56.5 (c 0.95, CH2Cl2). 1H NMR (400 MHz, CDCl3): δ 8.39 (s, 1H, ArH), 7.82–7.76 (m, 2H, ArH), 7.68 (d, J = 7.6 Hz, 1H, ArH), 7.57–7.53 (m, 1H, ArH), 7.44–7.27 (m, 9H, ArH), 7.23–7.11 (m, 6H, ArH), 6.98–6.95 (m, 3H, ArH), 6.76 (d, J = 7.6 Hz, 1H, ArH), 6.05 (s, 1H, NH), 5.59 (s, 1H, CH), 4.80 (d, J = 18.8 Hz, 1H, CH2), 4.30 (d, J = 18.4 Hz, 1H, CH2), 2.28 (s, 3H, CH3) ppm; 13C NMR (100 MHz, CDCl3): δ 199.4, 153.2, 152.1, 151.9, 151.8, 147.5, 143.8, 136.4, 136.1, 134.0, 133.6, 130.3, 130.0, 129.6, 129.5, 129.4, 128.7, 128.2, 128.0, 127.8, 127.2, 126.4, 126.0, 125.7, 125.1, 124.8, 123.2, 119.8, 119.0, 115.9, 111.4, 73.2, 48.4, 35.7, 21.4 ppm. HRMS (ESI): m/z calculated for C39H31N4O4S [M + H]+ 651.2061, found 651.2067.
4. Conclusions
In summary, the squaramide-catalyzed asymmetric Michael addition reaction between α-azidoindanones and azadienes was developed. The optimum conditions of the reaction were determined by evaluation of solvent, temperature, catalyst, catalyst loading, and concentration. A variety of α-azidoindanone and azadiene substrates were evaluated under the optimal reaction conditions, and a series of optically active and stable benzofuran derivatives containing α-tertiary azide was successfully synthesized through this catalytic asymmetric Michael addition reaction. In addition, the amplification experiment of the template reaction was also carried out. When the amount of substrates were equally amplified, the scale-up reaction could still maintain the same reaction effect as the small-scale reaction. The derivatization reactions were also performed, which further broadened the practicability of the reaction.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal15040364/s1, Spectroscopic data (1H and 13C NMR) and chiral HPLC chromatograms for all new compounds 3.
Author Contributions
X.-Y.D. performed the experiments, acquired, and analyzed the original data, Y.L. wrote the preliminary manuscript and supplemented relevant experimental data. D.-M.D. conceived and designed the experiments, revised all figures and schemes, analyzed the data, reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within this article and Supplementary Materials.
Acknowledgments
We thank the Analysis and Testing Center of Beijing Institute of Technology for the measurement of NMR and mass spectrometry.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lutz, J.F. 1,3-Dipolar cycloadditions of azides and alkynes: A universal ligation tool in polymer and materials science. Angew. Chem. Int. Ed. 2007, 46, 1018–1025. [Google Scholar] [CrossRef]
- Fu, J.; Zanoni, G.; Anderson, E.A.; Bi, X. α-Substituted vinyl azides: An emerging functionalized alkene. Chem. Soc. Rev. 2017, 46, 7208–7228. [Google Scholar] [CrossRef]
- Pauling, L.; Brockway, L.O. The adjacent charge rule and the structure of methyl azide, methyl nitrate, and fluorine nitrate. J. Am. Chem. Soc. 1937, 59, 13–20. [Google Scholar] [CrossRef]
- Ding, P.G.; Hu, X.S.; Zhou, F.; Zhou, J. Catalytic enantioselective synthesis of α-chiral azides. Org. Chem. Front. 2018, 5, 1542–1559. [Google Scholar] [CrossRef]
- Bräse, S.; Gil, C.; Knepper, K.; Zimmermann, V. Organic azides: An exploding diversity of a unique class of compounds. Angew. Chem. Int. Ed. 2005, 44, 5188–5240. [Google Scholar] [CrossRef]
- Lin, T.S.; Prusoff, W.H. Synthesis and biological activity of several amino analogs of thymidine. J. Med. Chem. 1978, 21, 109–112. [Google Scholar] [CrossRef]
- Klumpp, K.; Lévêque, V.; Le Pogam, S.; Ma, H.; Jiang, W.R.; Kang, H.S.; Granycome, C.; Singer, M.; Laxton, C.; Hang, J.Q.; et al. The novel nucleoside analog R1479 (4′-azidocytidine) is a potent inhibitor of NS5B-dependent RNA synthesis and hepatitis C virus replication in cell culture. J. Bio. Chem. 2006, 281, 3793–3799. [Google Scholar] [CrossRef]
- Williamsa, J.T.; Corciliusa, L.; Kiefelb, M.J.; Payne, R.J. Total synthesis of native 5,7-diacetylpseudaminic acid from N-acetylneuraminic acid. J. Org. Chem. 2016, 81, 2607–2611. [Google Scholar] [CrossRef]
- Patel, J.; Clavé, G.; Renard, P.Y.; Franck, X. Straightforward access to protected syn α-amino-β-hydroxy acid derivatives. Angew. Chem. Int. Ed. 2008, 47, 4224–4297. [Google Scholar] [CrossRef]
- Fukuta, Y.; Mita, T.; Fukuda, N.; Shibasaki, M. De novo synthesis of Tamiflu via a catalytic asymmetric ring-opening of meso-aziridines with TMSN3. J. Am. Chem. Soc. 2006, 128, 6312–6313. [Google Scholar] [CrossRef]
- Alexander, J.R.; Ott, A.A.; Liu, E.C.; Topczewski, J.J. Kinetic resolution of cyclic secondary azides, using an enantioselective copper-catalyzed azide–alkyne cycloaddition. Org. Lett. 2019, 21, 4355–4358. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.G.; Zhou, F.; Wang, X.; Yu, J.S.; Zhou, J. H-bond donor-directed switching of diastereoselectivity in the Michael addition of α-azido ketones to nitroolefins. Chem. Sci. 2020, 11, 3852–3861. [Google Scholar] [CrossRef]
- Ding, P.G.; Hu, X.S.; Yu, J.S.; Zhou, J. Diastereodivergent Synthesis of α-Chiral Tertiary Azides through Catalytic Asymmetric Michael Addition. Org. Lett. 2020, 22, 8578–8583. [Google Scholar] [CrossRef]
- Dong, X.Y.; Du, D.M. Asymmetric 1,4-Michael Addition Reaction of Azadienes with α-Thiocyanoindanones Catalyzed by Bifunctional Chiral Squaramide. Molecules 2021, 26, 5146. [Google Scholar] [CrossRef]
- Ye, Z.; Malerich, J.P.; Rawal, V.H. Squaramide-catalyzed enantioselective Michael addition of diphenyl phosphite to nitroalkenes. Angew. Chem. Int. Ed. 2010, 49, 153–156. [Google Scholar]
- Yang, W.; Du, D.M. Highly enantioselective Michael addition of nitroalkanes to chalcones using chiral squaramides as hydrogen bonding organocatalysts. Org. Lett. 2015, 42, 5450–5453. [Google Scholar]
- Yang, W.; Du, D.M. Chiral squaramide-catalyzed highly enantioselective Michael addition of 2-hydroxy-1,4-naphthoquinones to nitroalkenes. Adv. Synth. Catal. 2011, 353, 1241–1246. [Google Scholar] [CrossRef]
- Vakulya, B.; Varga, S.; Csámpai, A.; Soós, T. Highly enantioselective conjugate addition of nitromethane to chalcones using bifunctional cinchona organocatalysts. Org. Lett. 2005, 7, 1967–1969. [Google Scholar] [CrossRef]
- Shi, Z.; Tong, Q.; Leong, W.W.Y.; Zhong, G. [4 + 2] Annulation of vinyl ketones initiated by a phosphine-catalyzed aza-rauhut–currier reaction: A practical cccess to densely functionalized tetrahydropyridines. Chem.–Eur. J. 2012, 18, 9802–9806. [Google Scholar] [CrossRef]
- Rong, Z.Q.; Wang, M.; Chow, C.H.E.; Zhao, Y. A catalyst-nnabled diastereodivergent aza-diels–alder reaction: Complementarity of N-Heterocyclic carbenes and chiral amines. Chem.–Eur. J. 2016, 22, 9483–9487. [Google Scholar] [CrossRef]
- More, A.A.; Pathe, G.K.; Parida, K.N.; Maksymenko, S.; Lipisa, Y.B.; Szpilman, A.M. α-N-Heteroarylation and α-azidation of ketones via enolonium species. J. Org. Chem. 2018, 83, 2442–2447. [Google Scholar] [CrossRef] [PubMed]
- Zamarija, I.; Marsh, B.J.; Magauer, T. Ring expansion of 1-indanones to 2-halo-1-naphthols as an entry point to gilvocarcin natural products. Org. Lett. 2021, 23, 9221–9226. [Google Scholar] [CrossRef]
- Paccani, R.R.; Donati, D.; Fusi, S.; Latterini, L.; Farina, G.; Zanirato, V.; Olivucci, M. Toward a stable α-cycloalkyl amino acid with a photoswitchable cationic side chain. J. Org. Chem. 2012, 77, 1738–1748. [Google Scholar] [CrossRef]
- Deng, Q.H.; Bleith, T.; Wadepohl, H.; Gade, L.H. Enantioselective iron-catalyzed azidation of β-keto esters and oxindoles. J. Am. Chem. Soc. 2013, 135, 5356–5359. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).