Low-Temperature Hydrothermal Modification with Fe/C Catalysts for Enhancing Corn Stover Anaerobic Digestion Performance and Modeling Development for Predicting Biomethane Yield
Abstract
1. Introduction
2. Results and Discussion
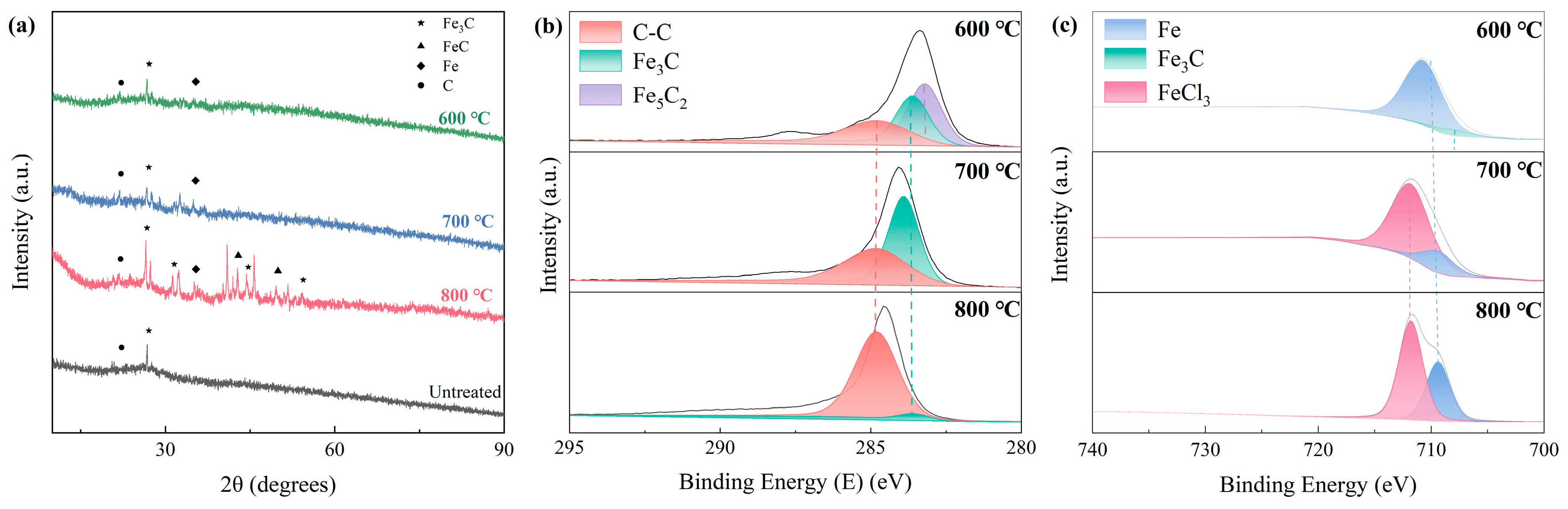
2.1. Characterization of Fe/C Catalysts
2.2. Hydrolysis Performance and Optimization of HM-Fe/C
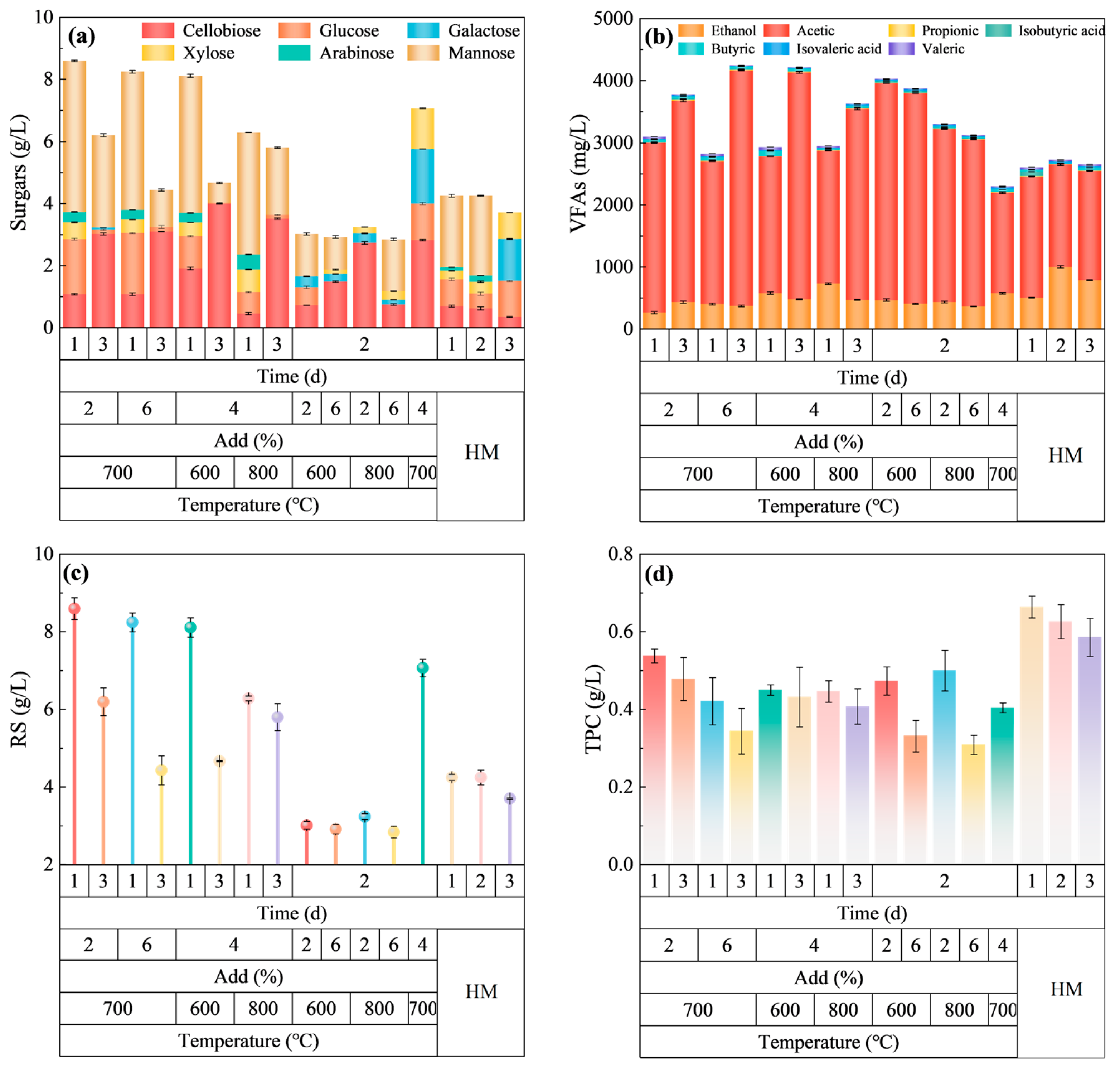
2.2.1. Hydrolysis Products After HM-Fe/C
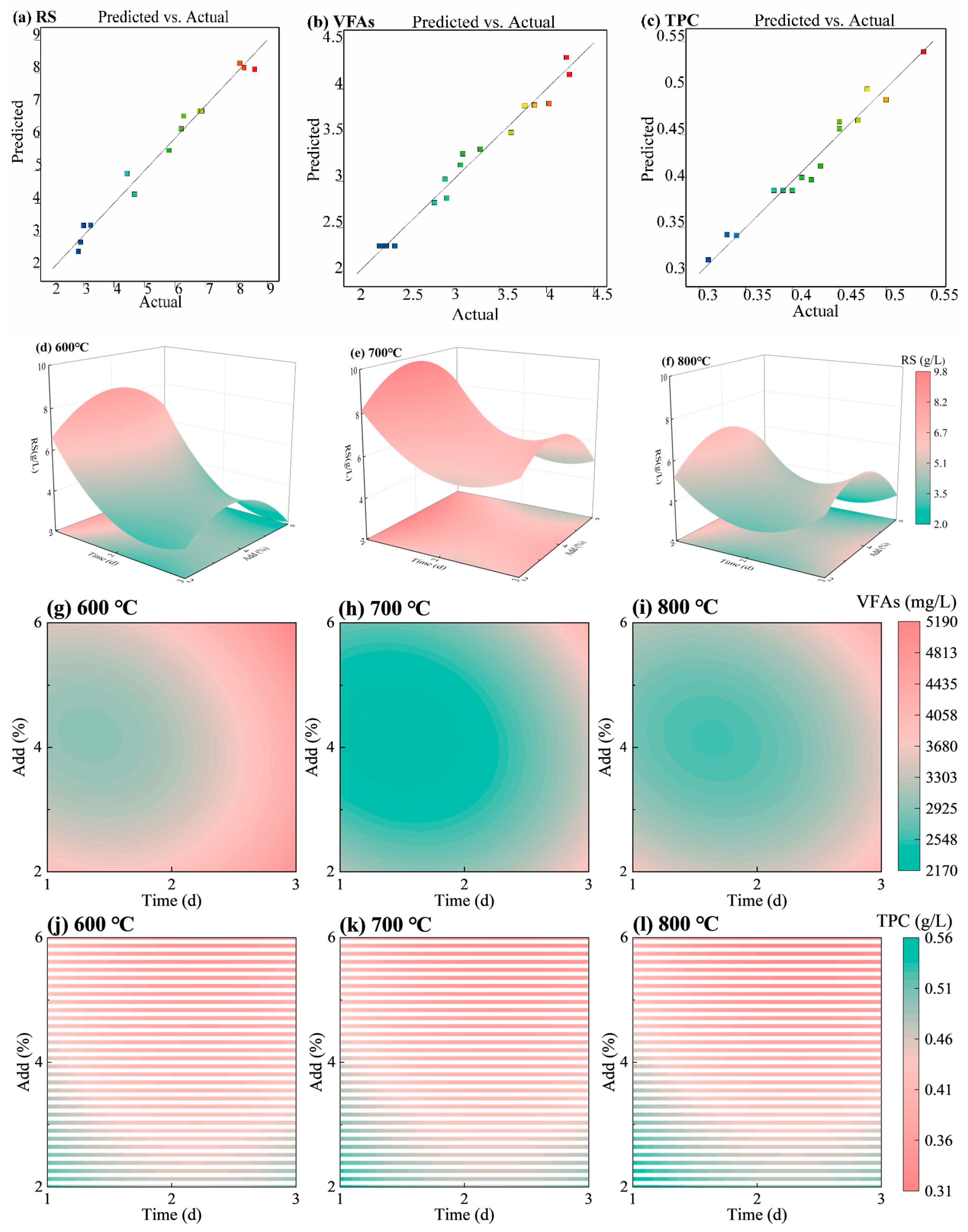
2.2.2. Optimization of HM-Fe/C Process by RSM
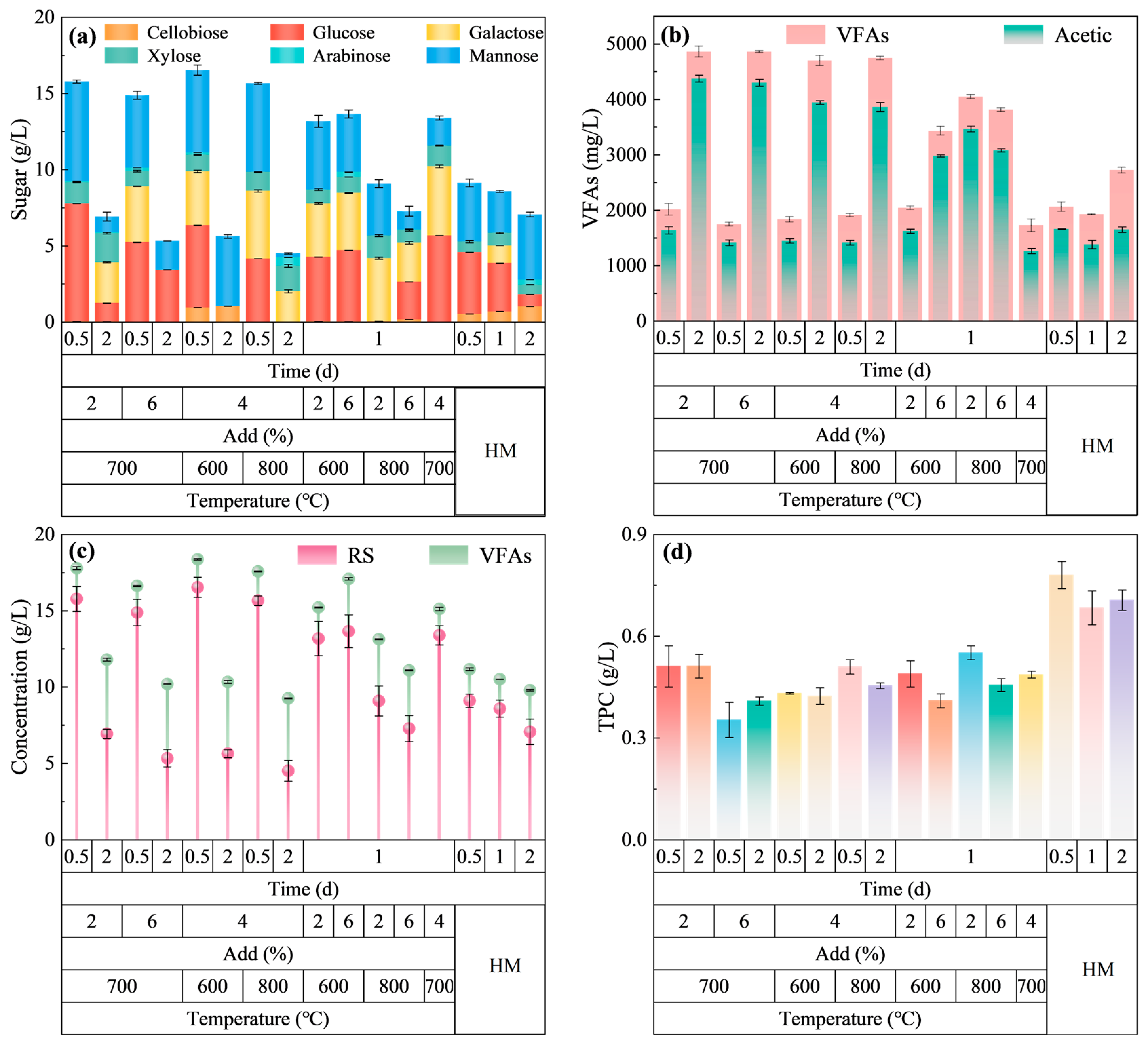
2.2.3. Hydrolysis Performance of HM-Fe/C After Optimization
2.3. Anaerobic Digestion Performances of CS by HM-Fe/C
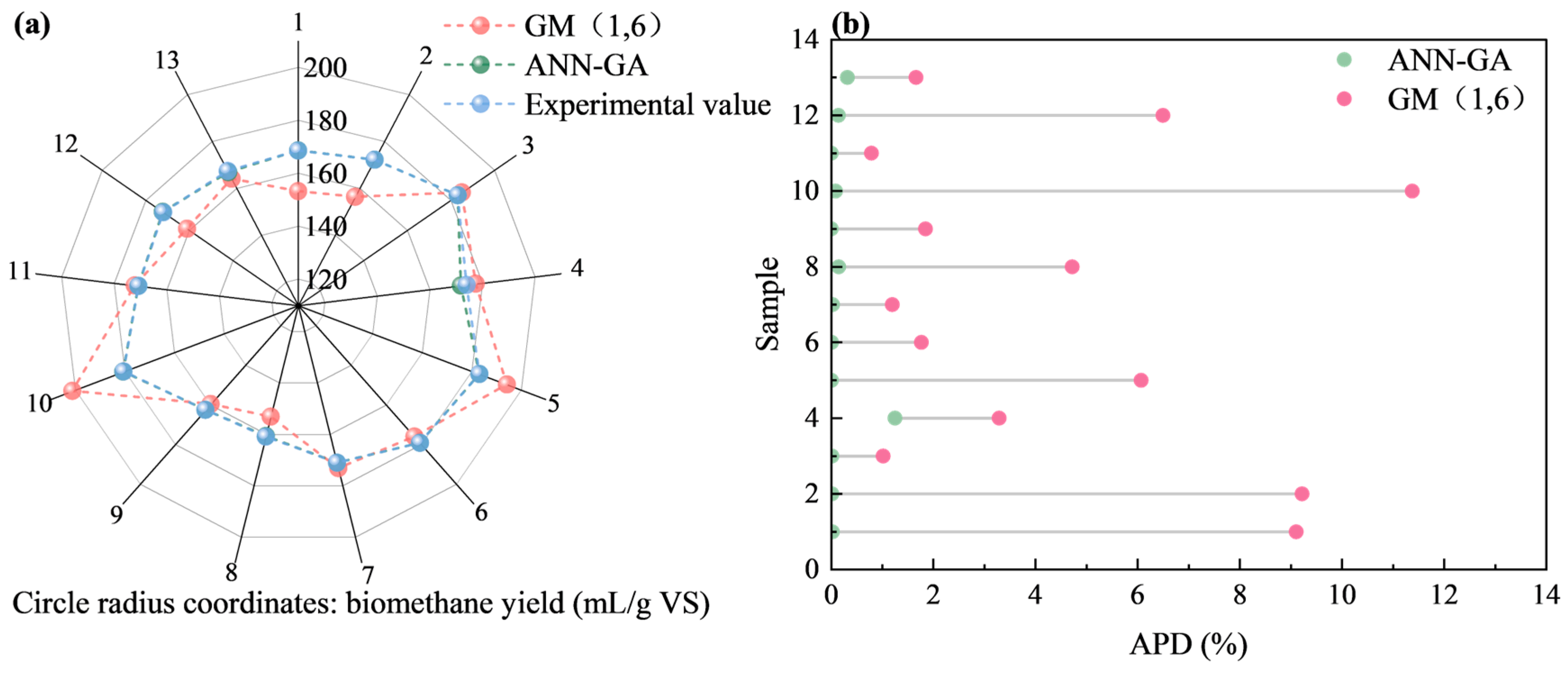
2.4. GM (1,N) and ANN-GA Modeling for BY Prediction
2.4.1. Construction of the GM (1, N) Model
2.4.2. Construction of the ANN-GA Model
2.4.3. Comparison of the GM (1, N) and ANN-GA Models
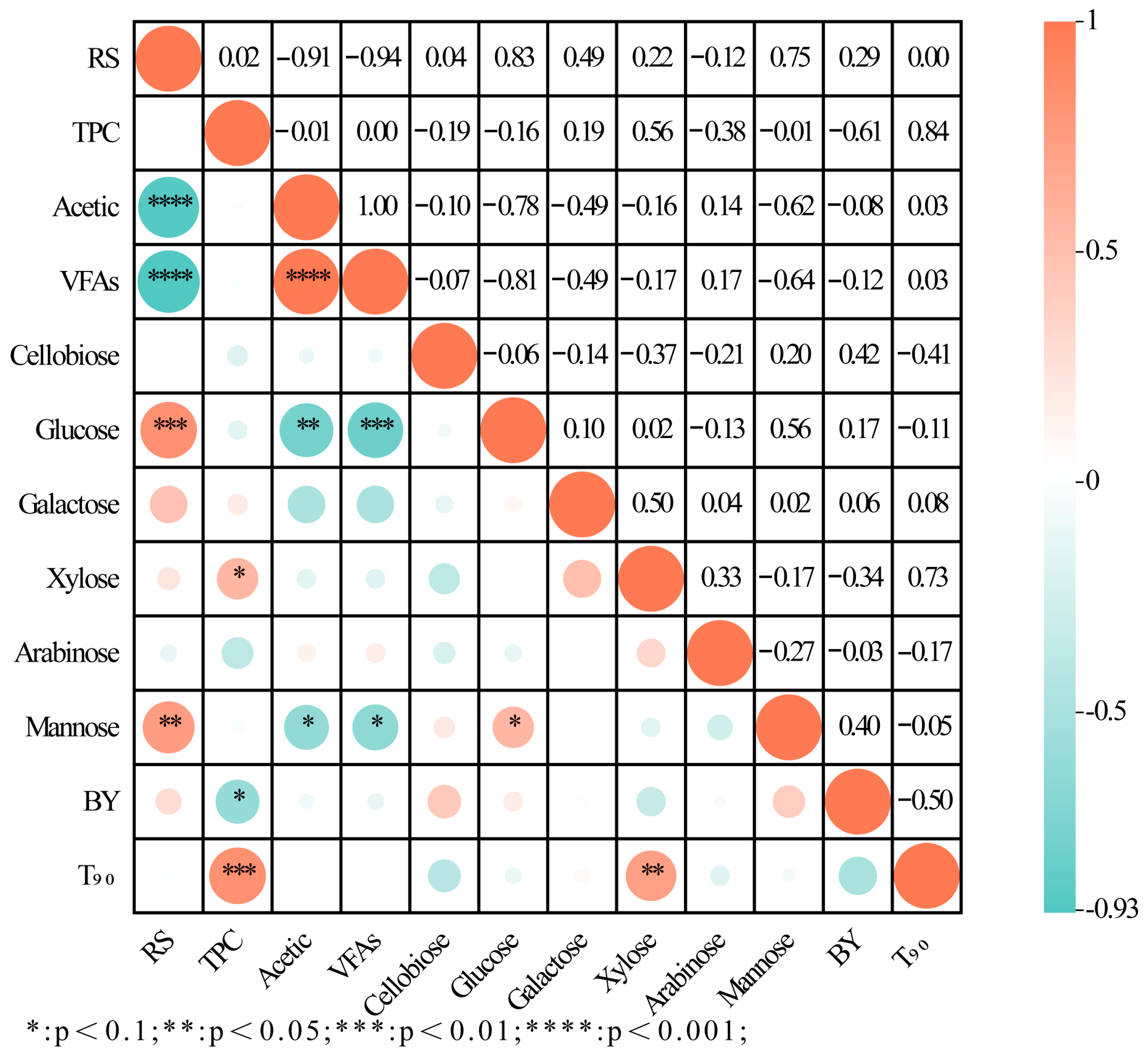
2.5. Correlation Analysis Between HM-Fe/C Parameters and BY
3. Materials and Methods
3.1. Materials
3.2. Mild Modification Methods
3.3. Batch AD Experiment
3.4. Construction of the Model
3.5. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CS | Corn stover |
| AD | Anaerobic digestion |
| HM | Hydrothermal modification |
| BY | Biomethane yield |
| T90 | The time to reach 90% of the maximum BY |
| RS | Reducing sugar |
| VFAs | Volatile fatty acids |
| TPC | Total phenolic content |
| APD | Average percentage deviation |
| RSM | Response surface methodology |
| RMSPE | Root mean square error |
| TS | Total solids |
| VS | Volatile solids |
| TC | Total carbon |
| TN | Total nitrogen |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
References
- Wang, X.; Song, X.; Yuan, H.; Li, X.; Zuo, X. Two-Step pretreatment of hydrothermal with ammonia for cow bedding: Pretreatment characteristics, anaerobic digestion performance and kinetic analysis. Waste Biomass Valoriz. 2021, 12, 5675–5687. [Google Scholar] [CrossRef]
- Kegl, T. Consideration of biological and inorganic additives in upgraded anaerobic digestion BioModel. Bioresour. Technol. 2022, 355, 127252. [Google Scholar] [CrossRef]
- Xiang, C.; Tian, D.; Hu, J.; Huang, M.; Shen, F.; Zhang, Y.; Yang, G.; Zeng, Y.; Deng, S. Why can hydrothermally pretreating lignocellulose in low severities improve anaerobic digestion performances? Sci. Total Environ. 2021, 752, 141929. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, H.; Yan, B.; Zuo, X.; Li, X. Improved performance of corn stover anaerobic digestion by low-temperature hydrothermal pretreatment with urea enhancement. Biomass Bioenerg. 2022, 164, 106553. [Google Scholar] [CrossRef]
- Deng, Z.; Xia, A.; Zhang, Z.; Zhu, X.; Huang, Y.; Zhu, X.; Liao, Q. A continuous hydrothermal pretreatment system for efficient sugar production from wheat straw. Chem. Eng. J. 2025, 508, 160867. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J. Statistical optimization of aqueous ammonia pretreatment and enzymatic hydrolysis of corn cob powder for enhancing sugars production. Biochem. Eng. J. 2021, 174, 108106. [Google Scholar] [CrossRef]
- Luo, T.; Huang, H.; Mei, Z.; Shen, F.; Ge, Y.; Hu, G.; Meng, X. Hydrothermal pretreatment of rice straw at relatively lower temperature to improve biogas production via anaerobic digestion. Chin. Chem. Lett. 2019, 30, 1219–1223. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Lee, J.-W. Catalytic effect of iron on sequential Fenton oxidation, hydrothermal treatment, and enzymatic hydrolysis to produce monosaccharide from lignocellulosic biomass. Ind. Crop Prod. 2020, 158, 112953. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Zhang, Y.; Chen, Y.; Feng, Q.; Wang, W.; Liang, C.; Hu, Y.; Qi, W. Enhanced enzymatic hydrolysis of poplar cellulosic residue fractionated by a magnetic carbon-based solid-acid catalyst in the γ-valerolactone-water system. Ind. Crop Prod. 2022, 176, 114397. [Google Scholar] [CrossRef]
- Kegl, T.; Kovač Kralj, A. An enhanced anaerobic digestion BioModel calibrated by parameters optimization based on measured biogas plant data. Fuel 2022, 312, 122984. [Google Scholar] [CrossRef]
- Karki, R.; Chuenchart, W.; Surendra, K.C.; Sung, S.; Raskin, L.; Khanal, S.K. Anaerobic co-digestion of various organic wastes: Kinetic modeling and synergistic impact evaluation. Bioresour. Technol. 2022, 343, 126063. [Google Scholar] [CrossRef]
- Long, F.; Wang, L.; Cai, W.; Lesnik, K.; Liu, H. Predicting the performance of anaerobic digestion using machine learning algorithms and genomic data. Water Res. 2021, 199, 117182. [Google Scholar] [CrossRef] [PubMed]
- Kegl, T.; Paramasivan, B.; Maharaj, B.C. Mathematical model-based optimization of trace metal dosage in anaerobic batch bioreactors. Bioengineering 2025, 12, 117. [Google Scholar] [CrossRef]
- Jacob, S.; Banerjee, R. Modeling and optimization of anaerobic codigestion of potato waste and aquatic weed by response surface methodology and artificial neural network coupled genetic algorithm. Bioresour. Technol. 2016, 214, 386–395. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhu, J. Response surface methodology and artificial neural network-genetic algorithm for modeling and optimization of bioenergy production from biochar-improved anaerobic digestion. Appl. Energ. 2024, 355, 122336. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Jing, Y.; Liu, T.; Fan, X.; Zhu, S.; Jiang, D.; Lu, C.; Zhang, H.; Yue, J.; et al. Forecasting of reducing sugar yield from corncob after ultrafine grinding pretreatment based on GM(1,N) method and evaluation of biohydrogen production potential. Bioresour. Technol. 2022, 348, 126836. [Google Scholar] [CrossRef]
- Ding, C.; Zhong, W.; Cao, Y.; Ma, T.; Ye, H.; Fang, Z.; Feng, Y.; Zhao, S.; Yang, J.; Li, Y.; et al. Fe-MOF-based catalysts for oxygen evolution reaction: Microenvironment regulated by organic ligands, metals and carbonization synergistically. Chem. Eng. Sci. 2025, 302, 120888. [Google Scholar] [CrossRef]
- Cheng, J.; Tu, X.; Zhang, L.; Han, M.; Zhou, R.; Luo, S.; Zeng, H.; Li, T.; Zou, J. A novel bifunctional particle electrode with abundant Fe/Fe3C and Fe-N-C sites to enhance the performance of electro-Fenton in degrading organic pollutant. J. Environ. Chem. Eng. 2024, 12, 112902. [Google Scholar] [CrossRef]
- Ashmath, S.; Rajeshkhanna, G.; Koyyada, G.; Pandiaraj, S.; Liu, C.; Peera, S.G.; Lee, T.-G. Biomass derived Fe3C/Fe-Nx-C as cathode catalyst for oxygen reduction reaction in dual chamber microbial fuel cells. Int. J. Hydro. Energy 2024. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, M.; Fang, Y.M.; Tan, T.W. Reductive catalytic fractionation of lignocellulose: When should the catalyst meet depolymerized lignin fragments? Sustain. Energ. Fuels 2020, 4, 5588–5594. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, C.; Yuan, H.; Li, X. Enhanced hydrothermal depolymerization with Fe/Ni Loaded C catalysts for improving anaerobic digestion performance of corn stover. Energies 2025, 18, 192. [Google Scholar] [CrossRef]
- Ding, C.; Hu, X.; Sun, W.; Hailili, R.; Liao, F.; Shu, C.; Huang, J.; Huang, L.; Wang, N. Interface of Ni-MgCr2O4 spinel promotes the autothermal reforming of acetic acid through accelerated oxidation of carbon-containing intermediate species. ACS Catal. 2023, 13, 4560–4574. [Google Scholar] [CrossRef]
- Sun, D.; Lv, Z.-W.; Rao, J.; Tian, R.; Sun, S.-N.; Peng, F. Effects of hydrothermal pretreatment on the dissolution and structural evolution of hemicelluloses and lignin: A review. Carbohydr. Polym. 2022, 281, 119050. [Google Scholar] [CrossRef]
- Sun, S.-C.; Sun, D.; Wang, H.-M.; Li, H.-Y.; Cao, X.-F.; Sun, S.-N.; Yuan, T.-Q. Effect of integrated treatment on improving the enzymatic digestibility of poplar and the structural features of isolated hemicelluloses. Carbohydr. Polym. 2021, 252, 117164. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Fan, K.; Yao, X.; Wang, M.; Wang, K. Physicochemical and morphological variation of bamboo cell wall during hydrothermal pretreatment. Pap. Biomater. 2022, 7, 1–8. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, H. Evolution of primary liquid products and evidence of in situ structural changes in cellulose with conversion during hydrolysis in hot-compressed water. Ind. Eng. Chem. Res. 2010, 49, 3919–3925. [Google Scholar] [CrossRef]
- Zha, X.; Travesset, A. Thermodynamic equilibrium of binary nanocrystal superlattices. J. Phys. Chem. C 2021, 125, 18936–18945. [Google Scholar] [CrossRef]
- Yu, H.; Xi, Z.; Zhang, Y.; Xu, R.; Zhao, C.; Wang, Y.; Guo, X.; Huang, Y.; Mi, J.; Lin, Y.; et al. High performance additional mass enhanced film structure triboelectric nanogenerator for scavenging vibration energy in broadband frequency range. Nano Energy 2023, 107, 108182. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Lu, M.; Guo, X.; Zhang, H.; Han, L. Integrated chemical and multi-scale structural analyses for the processes of acid pretreatment and enzymatic hydrolysis of corn stover. Carbohydr. Polym. 2016, 141, 1–9. [Google Scholar] [CrossRef]
- Zhang, P.; Dong, S.-J.; Ma, H.-H.; Zhang, B.-X.; Wang, Y.-F.; Hu, X.-M. Fractionation of corn stover into cellulose, hemicellulose and lignin using a series of ionic liquids. Ind. Crop Prod. 2015, 76, 688–696. [Google Scholar] [CrossRef]
- Wei, Y.; Yuan, H.; Wachemo, A.C.; Li, X. Impacts of modification of corn stover on the synergistic effect and microbial community structure of co-digestion with chicken manure. Energy Fuels 2019, 34, 401–411. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, Y.; Zhang, L.; Zuo, X.; Li, X.; Yuan, H. Substance bioconversion, hydrolases activity, and metagenomic analysis to unravel the enhanced biomethanation of corn stover with urea-hydrothermal pretreatment. J. Environ. Manag. 2023, 333, 117466. [Google Scholar] [CrossRef]
- Ahmad, F.; Mat-Isa, N.A.; Hussain, Z.; Boudville, R.; Osman, M.K. Genetic algorithm-artificial neural network (GA-ANN) hybrid intelligence for cancer diagnosis. In Proceedings of the 2010 2nd International Conference on Computational Intelligence, Communication Systems and Networks, Washington, DC, USA, 28–30 July 2010; pp. 78–83. [Google Scholar]
- Mougari, N.E.; Largeau, J.F.; Himrane, N.; Hachemi, M.; Tazerout, M. Application of artificial neural network and kinetic modeling for the prediction of biogas and methane production in anaerobic digestion of several organic wastes. Int. J. Green. Energy 2021, 18, 1584–1596. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Li, Y.; Zhang, H. Modeling and optimization of photo-fermentation biohydrogen production from co-substrates basing on response surface methodology and artificial neural network integrated genetic algorithm. Bioresour. Technol. 2023, 374, 128789. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington DC, USA, 2005. [Google Scholar]
- Zeng, Z.; Li, Y.; Yang, R.; Liu, C.; Hu, X.; Luo, S.; Gong, E.; Ye, J. The relationship between reducing sugars and phenolic retention of brown rice after enzymatic extrusion. J. Cereal Sci. 2017, 74, 244–249. [Google Scholar] [CrossRef]
- Puangbanlang, C.; Sirivibulkovit, K.; Nacapricha, D.; Sameenoi, Y. A paper-based device for simultaneous determination of antioxidant activity and total phenolic content in food samples. Talanta 2019, 198, 542–549. [Google Scholar] [CrossRef]

| Source | R2 | F Value | p Value | C.V.% | Adeq Precision |
|---|---|---|---|---|---|
| RS | 0.9819 | 42.23 | <0.0001 | 6.76 | 19.1632 |
| VFAs | 0.9805 | 39.16 | <0.0001 | 4.74 | 17.7680 |
| TPC | 0.9680 | 23.54 | 0.0002 | 3.98 | 17.5922 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Yuan, H.; Li, X. Low-Temperature Hydrothermal Modification with Fe/C Catalysts for Enhancing Corn Stover Anaerobic Digestion Performance and Modeling Development for Predicting Biomethane Yield. Catalysts 2025, 15, 362. https://doi.org/10.3390/catal15040362
Wang X, Yuan H, Li X. Low-Temperature Hydrothermal Modification with Fe/C Catalysts for Enhancing Corn Stover Anaerobic Digestion Performance and Modeling Development for Predicting Biomethane Yield. Catalysts. 2025; 15(4):362. https://doi.org/10.3390/catal15040362
Chicago/Turabian StyleWang, Xitong, Hairong Yuan, and Xiujin Li. 2025. "Low-Temperature Hydrothermal Modification with Fe/C Catalysts for Enhancing Corn Stover Anaerobic Digestion Performance and Modeling Development for Predicting Biomethane Yield" Catalysts 15, no. 4: 362. https://doi.org/10.3390/catal15040362
APA StyleWang, X., Yuan, H., & Li, X. (2025). Low-Temperature Hydrothermal Modification with Fe/C Catalysts for Enhancing Corn Stover Anaerobic Digestion Performance and Modeling Development for Predicting Biomethane Yield. Catalysts, 15(4), 362. https://doi.org/10.3390/catal15040362





