Abstract
The production of hydrocarbon-based biofuels has been the target of intense research worldwide. In this context, the core goal of the present work was to investigate the use of mesopore-rich activated carbons (ACs) as support for sulfided Mo-based catalysts intended for the hydroprocessing of lipidic feedstocks. The key motivations for the work were that, in comparison to traditional inorganic supports such as Al2O3, ACs are less propense to form coke, due to their lower acidity, and are highly resistant to hydrolysis, which is a very important aspect in the hydroprocessing of lipidic feedstocks because water is abundantly produced during the process. Furthermore, the porosity of ACs can be tailored to give rise to a high mesopore content, which is important for improving the access of bulky triglyceride molecules to metallic active sites located inside the pores network. A systematic study on the effects of the preparation conditions on the properties and performance of the obtained catalysts was carried out for the first time. The highest hydrodeoxygenation (HDO) activity was verified for the catalyst prepared through sequential deposition of Mo and Ni by wet impregnation. The prepared catalyst presented better performance for coconut oil HDO than an industrial sulfided NiMo/Al2O3 catalyst. Furthermore, it presented good stability, provided that the sulfidation degree was kept high. The obtained results evidenced that ACs have great potential to replace inorganic support in sulfided Mo-based catalysts.
1. Introduction
The production and use of biofuels have two main goals: (i) to provide alternative resources to meet the growing demand for fuels, especially in the transport sector; (ii) to make available fuels that are less harmful to the environment. Currently, bioethanol and biodiesel (fatty acid esters) are by far the most employed biofuels worldwide. Despite its lower energy density compared to gasoline, bioethanol has satisfactorily met the needs of internal combustion engines. In turn, biodiesel has been largely employed in diesel cycle engines. Nevertheless, biodiesel has features that impair some fuel properties. For example, the ester groups increase hydrophilicity and intermolecular forces. High hydrophilicity is undesirable because water promotes biological growth in storage tanks, forming sludge and slime, which can block filters and lines [1]. Furthermore, the hydrolysis of esters results in free fatty acids that increase fuel corrosivity. In turn, enhanced intermolecular forces increase viscosity and impart fuel cold flow properties. In this respect, the presence of unsaturations reduces viscosity and improves fuel cold flow properties [2]. However, unsaturations (especially conjugated polyunsaturations) markedly reduce oxidative stability, which can cause acidity and the formation of insoluble gums and sediments [3].
In the above-depicted scenario, the production of hydrocarbon biofuels has received increasing attention because they can exhibit properties and performance close to those of petrofuels, can be used without engine modification, can utilize current supply infrastructure (tanks, pumps, pipelines, etc.), and can be blended with conventional fuel in any ratio, therefore attending the “drop-in” concept. These issues are especially relevant in aviation due to the inherent risks involved in this sector [4]. Therefore, the obtention of hydrocarbon biofuels has been intensely investigated, as reported by numerous reviews covering several existing routes [4,5,6,7,8,9,10,11].
Nowadays, hydroprocessing of esters and fatty acids (HEFA) is the most mature technology available to produce hydrocarbon biofuels. The main reason is that HEFA can be easily integrated into oil refineries, thus reducing capital costs. The route has been widely employed to produce biofuels for jet turbines and diesel engines from lipidic feedstocks (vegetable, animal, and algal oils and fats) [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29].
The key step in HEFA is the deoxygenation of the fatty chains resulting from the split of acylglycerides that constitute lipidic feedstocks. This deoxygenation takes place through a process akin to the hydroprocessing of petroleum and its derivatives: thermal treatment at medium temperature (~300–400 °C) under high H2 pressure (most usually in the range of 20–100 bar) in the presence of heterogeneous catalysts. It is well known that deoxygenation of fatty acids can take place via three different pathways: decarboxylation, (hydro)decarbonylation, and hydrogenation/dehydration (Equations (1)–(3), respectively) [4,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29].
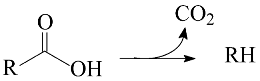
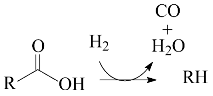
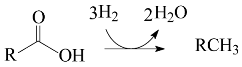
The thermal treatment of biomass derivatives in the presence of H2 aiming to remove oxygen is usually called HDO (hydrodeoxygenation). However, it is worth mentioning that some authors have used the term HDO to refer to the specific deoxygenation pathway represented by Equation (3) (see, for example, references [12,26,27,28]). In this work, to avoid misunderstandings, the term HDO is only used to refer to the hydroprocessing stage as a whole, whereas the specific pathway corresponding to Equation (3) is referred to as hydrogenation/dehydration.
HDO (not only of lipidic feedstocks, but also of lignocellulose-derived bio-oils [4,29,30,31,32] or other O-rich mixtures [4]) is most usually performed in the presence of the same catalysts employed in the hydroprocessing of petroleum and derivatives: sulfides of group 6 transition metals (Mo or W), promoted by transition metals with a higher number of electrons d (Ni or Co), deposited over oxide supports [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,33,34,35,36,37,38,39,40]. Bara et al. [41], Kibsgaard et al. [42], and Lauritsen et al. [43] published relevant works on the structure of such catalysts.
γ-Al2O3 (alumina) is the most employed support in petroleum hydroprocessing catalysts mainly because it is reasonably inexpensive and stable in this kind of application. However, γ-Al2O3 is unstable in the HDO of O-rich feedstocks such as biomass derivatives because it can be hydrolyzed to boehmite by the water abundantly formed in the process [44]. In addition, the high acidity of γ-Al2O3 favors coke deposition, with consequent catalyst loss of activity and even deactivation [26,27,45,46,47,48,49,50,51,52]. Therefore, there is great interest in the employment of new supports in catalysts intended for HDO.
In this context, activated carbons (ACs) can be pointed out as an interesting support option, mainly because they are highly resistant to chemical attack in both acidic and basic media (which includes hydrolysis). Furthermore, ACs have lower acidity than Al2O3, which contributes to reduced hydrocracking and coking [26,27,46,47,48,49,50]; present higher surfaces areas than Al2O3; have a pore size distribution and surface chemistry that can be tailored according to the application they are intended for [50,53,54,55]; are thermally stable; and can be obtained from cheap and abundant precursors (e.g., biomass residues and coals).
Despite the potential benefits, scarce works are currently available regarding the use of AC-supported sulfided Mo-based catalysts in the hydroprocessing of lipidic feedstocks. There are some works reporting the use of this kind of catalyst in the hydroprocessing of petroleum and derivatives [56,57,58,59,60,61]. Remarkably, Van Veen et al. [56] and Topsøe et al. [57] compared different supports and concluded that ACs generate weaker interactions with metal sulfides, which results in a more active phase for HDS (hydrodesulfurization). There are also some works about the use of such catalysts in the HDO of bio-oils derived from lignocellulosic feedstocks or related model compounds [50,62,63]. Nevertheless, to the best of our knowledge, there are currently only two articles reporting the use of AC-supported sulfided Mo-based catalysts in the hydroprocessing of lipidic feedstocks (or related model molecules): the papers of Tapia et al. [64] and Couman and Hensen [49]. The former reported good activity of an AC-supported sulfided NiMo catalyst in the HDO of Jatropha oil. In turn, Couman and Hensen compared the catalytic performance of sulfided NiMo deposited on different supports (AC, mesoporous alumina, and amorphous silica-alumina) in the HDO of methyl oleate. The authors reported that the AC-supported catalyst presented the highest active phase dispersion due to the weak interaction between the support and the metallic sulfides, so that it was twice as active for HDO as those catalysts having oxides as support, besides remaining active over a more prolonged reaction time. In addition, it is worth mentioning that Ferreira et al. [65] prepared a sulfided NiMo catalyst supported on carbon nanotubes, which presented similar activity in palmitic acid HDO as a commercial sulfided NiMo/Al2O3 catalyst.
In this scenario, the goal of the present work was to investigate the preparation of AC-supported sulfided Mo-based catalysts intended for the hydroprocessing of lipidic feedstocks. The support was produced from chemical activation of coconut shell (an abundant and inexpensive agrowaste) with H3PO4. This activation protocol was chosen because it has proven to permit the synthesis of ACs with high mesopore content [66], which is important to reduce diffusional restrictions and improve the access of bulky substrates (as it is the case of triglyceride molecules that constitute lipidic feedstocks) to the metallic active sites located inside the pores network.
The effects of preparation conditions on the properties and HDO activity of the prepared catalysts were systematically investigated. More specifically, we evaluated the effects of incorporating Ni as a promoter; the impregnation methodology (incipient wetness impregnation vs. wet impregnation; co-impregnation vs. sequential impregnation of Mo and Ni); and calcination temperature (400 or 500 °C).
Coconut oil was the employed feedstock, which is constituted mainly by medium-length fatty chains (primarily C12 chains) [26]. However, since the split of lipids renders a complex mixture of fatty chains, the preliminary tests aiming to establish the conditions that would render the catalyst with the highest HDO activity were carried out using dodecanoic acid (so-called lauric acid) as a model compound.
2. Results and Discussion
2.1. AC Characterization
Figure 1 shows the N2 adsorption–desorption isotherms of the bare AC P54 and some selected oxo-catalysts. The isotherm of P54 can be considered a hybrid of type I (b) and type IV according to the IUPAC classification [67], which are typical of microporous and mesoporous adsorbents, respectively. The amount of adsorbed N2 was high even for much lower equilibrium pressures, which reveals a pronounced presence of narrow micropores. Furthermore, the adsorption continued increasing up to near the saturation pressure, which indicates the existence of larger pores (wide micropores and mesopores). The occurrence of a prominent hysteresis loop confirms the presence of mesopores. The volumes of micro and mesopores of P54 were 0.72 and 0.55 cm3 g−1, respectively, which resulted in a high specific surface area (SSA) of 1643 m2 g−1 (Table 1).
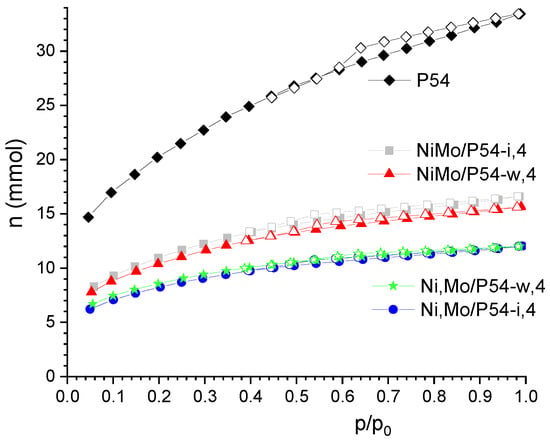
Figure 1.
N2 adsorption–desorption isotherms of P54 and some selected oxo-catalysts. (Closed and open symbols correspond to the adsorption and desorption branches, respectively).

Table 1.
Textural characterization of P54 oxo-catalysts.
The X-ray photoelectron spectroscopy (XPS) analysis of P54 (Figure 2, Table 2) indicates the presence of carbon, oxygen, phosphorus, and silicon in the proportions of 85.9, 11.9, 1.9, and 0.4 wt%, respectively. Phosphorus was incorporated during the process of activation with H3PO4, while the other elements remained from the biomass precursor.
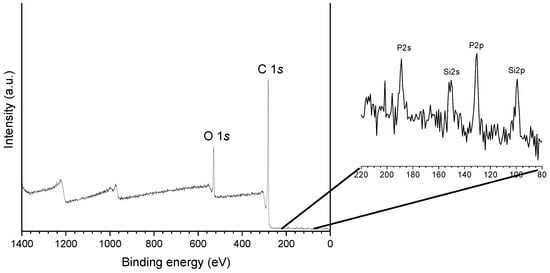
Figure 2.
XPS survey spectra of the bare P54 AC.

Table 2.
XPS elemental composition of P45 and some selected catalysts.
The high-resolution (HR) XPS C 1s core level spectrum of P54 (Figure 3a) was deconvoluted into four components, which were assigned mainly to unfunctionalized carbons (CI peak; 284.8 eV); C–O (CII; 286.2 eV); C=O (CIII; 287.4 eV); COO (CIV; 288.8 eV) [68]. In turn, the HR-XPS O 1s core level spectrum was deconvoluted into two peaks (Figure 3b) assigned mainly to O double-bounded (OI; 531.7 eV) and single-bounded to C (OII; 533.4 eV) (pertinent discussion about these assignments was presented elsewhere [55]). The relative contributions of each peak are reported in Table 3.
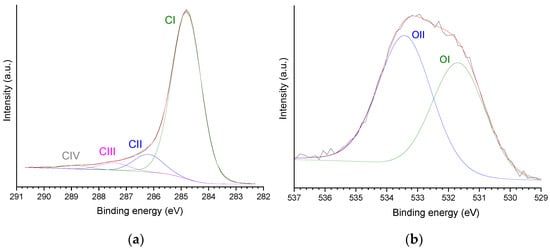
Figure 3.
HR-XPS: (a) C 1s and (b) O 1s core level spectra of P54 with respective deconvoluted peaks.

Table 3.
Relative contributions of C 1s and O 1s peaks for P54 (data obtained from the HR-XPS core level spectra considering each element separately and, in brackets, all identified elements).
In accordance with its relatively high O content, P54 gave rise to intense CO2 and CO emissions during temperature-programmed desorption/mass spectrometry (TPD/MS) analysis. The deconvolution of the CO2-TPD profile (Figure 4a) resulted in five main peaks centered at 260, 363, 500, 606, and 794 °C (CO2-I–CO2-V peaks). The first three peaks were assigned to stronger carboxylic acids, weaker carboxylic acids, and anhydrides, respectively, while the last two were attributed to different lactones. In turn, the TPD-CO profile was deconvoluted into five main components (Figure 4b), which were assigned to anhydrides, which release CO2 and CO simultaneously (CO-I; 500 °C); phenol/ether (CO-II and CO-III; 650 and 780 °C); ketones/quinones (CO-IV and CO-V; 886 and 930 °C) (see pertinent discussion about the assignments of CO2 and CO-TPD peaks in reference [55]).
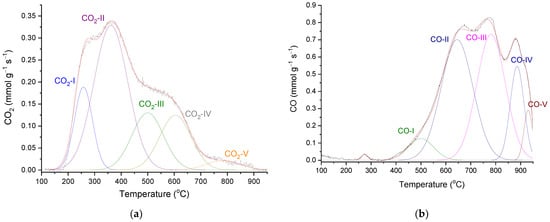
Figure 4.
(a) CO2 and (b) CO-TPD profiles of P54, with respective deconvoluted peaks.
Boehm titration (Table 4) disclosed relatively high contents of strong (0.55 mmol g−1) and weak (1.21 mmol g−1) acidic groups, besides a low content of medium acids (0.07 mmol g−1). These results are in accordance with the TPD/MS analysis because, as depicted in Section 3.4, it has been assumed that strong acids encompass carboxylic acids and anhydrides, while weak and medium strength acids correspond mainly to phenols and lactones, respectively. In turn, the content of basic groups was zero, so that it is possible to conclude that the carbonylic groups detected by TPD are neutral or have a basic character so weak that they are not detected by the employed titration protocol.

Table 4.
Data corresponding to the ash content, elemental analysis, and Boehm titration of P54.
The chemical composition of P54 was also investigated by elemental analysis (EA; Table 4). The C, H, and N contents were 84.2, 1.4, and 0.3 wt%, respectively, with a H/C atomic ratio of 0.20.
2.2. Catalysts Characterization
The inductively coupled plasma/optical emission spectrometry (ICP/OES) analyses showed that Mo and Ni were effectively deposited on the surface of P54 (Table 5). The Mo and Ni (if it was employed) contents ranged from 12.9 to 20.46 wt% and from 0.70 to 1.02 wt%, respectively. Therefore, the Ni/Mo atomic ratio was in the range of 0.07–1.12.

Table 5.
Mo and Ni contents determined by ICP/OES for the oxo-catalysts.
Images of transmission electron microscopy/energy-dispersive X-ray (TEM/EDX) elemental mapping show clusters of Mo and Ni with dimensions of a few nanometers uniformly distributed over the support surface, as illustrated in Figure 5. The respective histograms with the particle size distribution and average particle size are presented in Figure 6. The determined average values (3.6–4.6 nm and 2.9–4.2 nm for Mo and Ni, respectively) are in the same range as those usually reported in the literature for sulfided Mo-based catalysts supported on Al2O3. For example, López-Cruz et al. [69] determined average slab sizes in the range of 2.4–3.6 nm for sulfided NiMo/γ-Al2O3 catalysts. In turn, Afanasiev [70] prepared 28 different sulfided Mo/γ-Al2O3 catalysts with average metallic particle sizes ranging from 3.4 to 5.7 nm. It is worth mentioning that these authors did not use elemental mapping, so that the reported values concern the particles as a whole, rather than individual Mo and Ni particles.
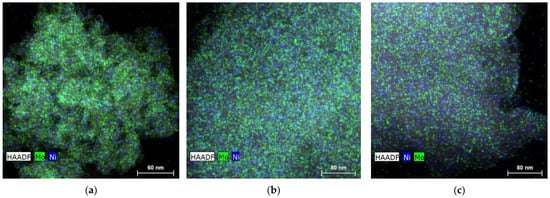
Figure 5.
TEM/EDX elemental mapping of (a) oxo-NiMo/P54-i,4, (b) oxo-NiMo/P54-w,4, and (c) oxo-Ni,Mo/P54-w,4.
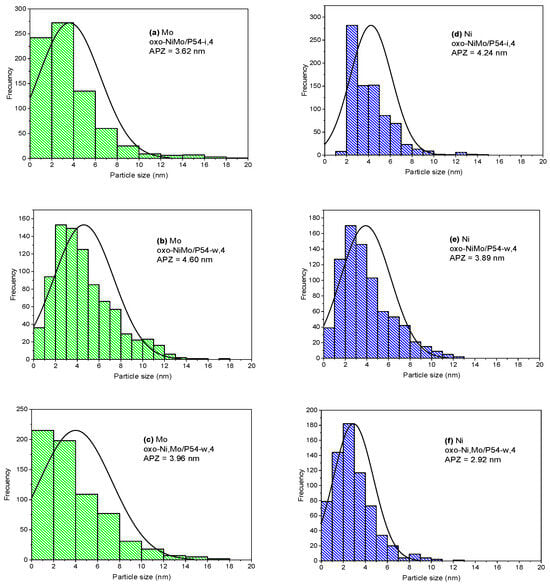
Figure 6.
Histograms of (a–c) Mo and (d–f) Ni particle size distribution and respective average particle size for the oxo-catalysts whose elemental mapping was presented in this figure.
Figure 7 presents the X-ray photoelectron spectroscopy (XPS) survey spectra of the catalyst oxo-Ni,Mo/P54-w,4 and its sulfided counterpart sulf-Ni,Mo/P54-w,4. For the oxo catalyst, the main difference in comparison to the spectrum of the bare P54 support (Figure 2) is the presence of peaks referent to molybdenum. In the case of the sulfided catalyst, besides the molybdenum peaks, there are also intense peaks due to sulfur. Furthermore, all prepared catalysts presented weak peaks due to nitrogen, with the N 1s peaks in the range of ~398–402 eV partially overlapping the Mo 3p3/2 peaks. This nitrogen was inserted due to the reaction of the support surface with the NH3 released by the decomposition of (NH4)6Mo7O24 during catalysts calcination. Concerning nickel, slight peaks could be clearly identified only by means of the HR spectra.
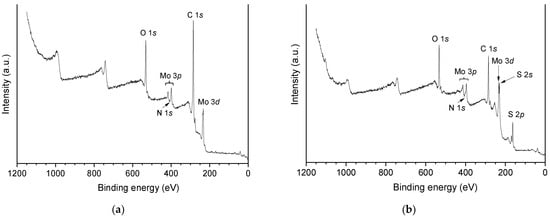
Figure 7.
XPS survey spectra of (a) oxo-Ni,Mo/P54-w,4 and (b) sulf-Ni,Mo/P54-w,4.
The contents of each element identified by XPS are reported for some selected catalysts in Table 2. However, it is valid to highlight that XPS provides an estimation of the chemical composition of the few uppermost layers of the external surface, so that the obtained results must be considered with much care.
The HR-XPS Mo 3d core level spectra of the oxo-catalysts displayed two main peaks at around 233 and 236 eV (Figure 8a–c), which roughly correspond to the binding energy (BE) of the Mo 3d5/2 and Mo 3d3/2 spin-orbit core levels of Mo6+ [33,41,62,71,72,73,74]. On the other hand, no significant peak was identified for the oxo-catalysts in the region corresponding to the Mo 3d5/2 core level of Mo4+, around 229 eV [33,41,62,71,72,73,74,75]. Furthermore, the O 1s core level spectra of the oxo-catalysts (Figure 8e–g) presented, besides the OI and OII peaks corresponding to the AC support (Section 2.1), an additional intense peak assigned to MoO3 [71,72] at 531 eV (it is valid to mention that, for MoO2, the O 1s peak is usually verified at lower BE, around 530 eV [73]). All these findings evidence that, in the oxo-catalysts, Mo is overwhelmingly present as MoO3.
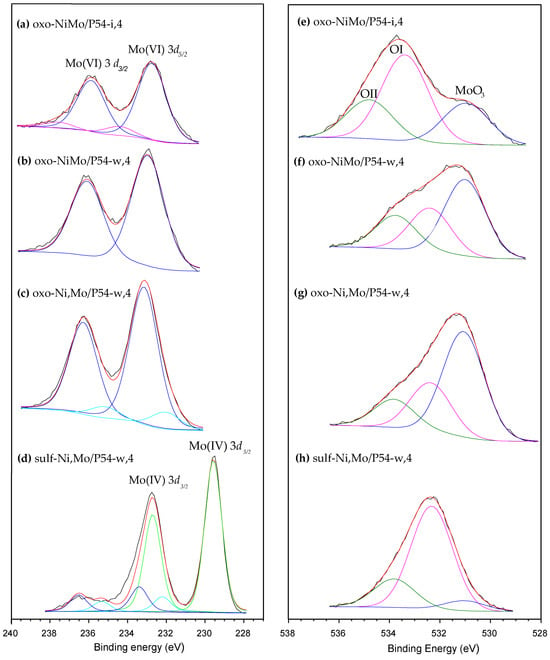
Figure 8.
HR-XPS (a–d) Mo 3d and (e–h) O 1s core level spectra of some selected catalysts.
The same way as observed for the oxo-catalysts, the HR-XPS Mo 3d core level spectra of the sulf-catalysts also presented two intense peaks due to Mo 3d5/2 and Mo 3d3/2 spin-orbit core levels; however, in this case, the peaks were centered at a lower BE, 229.5 and 232.5 eV, respectively (Figure 8d), which are typical of Mo4+ [33,41,49,62,73,74,75]. In turn, the S 2p spectra of the sulf-catalysts displayed two intense peaks at 162.4 and 163.6 eV (Figure 9), which can be assigned to the S 2p3/2 and S 2p1/2 spin-orbit core levels of MoS2, respectively [49,62,73,74,75]. In addition, there are two shallower peaks at 169.1 and 170.2 eV, which are assigned to oxidized sulfur species such as sulfonyls, sulfonates, sulfites, and sulfates [73,76]. Supposedly, the oxygen in these species (S=O) would explain the increased intensity of the OII peak [76] in Figure 8h.
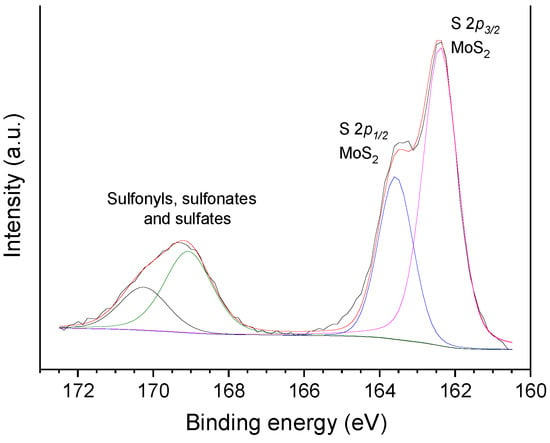
Figure 9.
HR-XPS S 2p core level spectra of sulf-Ni,Mo/P54-w,4.
The above-presented findings show that most of the MoO3 (which was predominant in the oxo-catalysts) was converted into MoS2 during the sulfidation step. Accordingly, only a slight peak was verified at ~531 eV in the O 1s spectra of the sulf-catalysts (as illustrated in Figure 8h), and no peak was observed at ~530 eV (BEs characteristics of MoO3 and MoO2, respectively).
Figure 10 presents the HR-XPS Ni 2p core level spectra of some selected catalysts, where the Ni peaks are quite shallow due to the low metal content. In general terms, two peaks can be identified, at ~856 and ~874 eV, which were assigned to the 2p3/2 and 2p1/2 spin-orbit core levels of Ni2+ species, respectively [47,49].
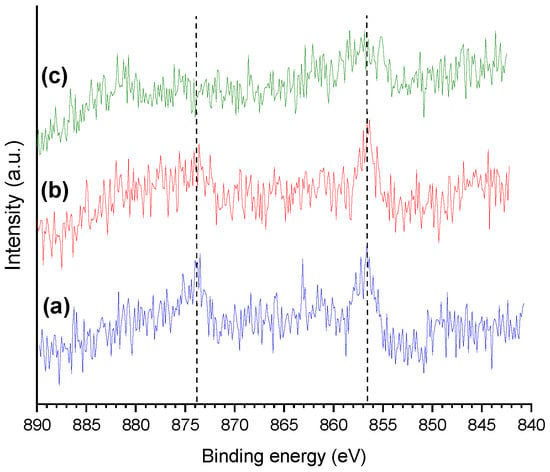
Figure 10.
HR-XPS Ni 2p core level spectra of (a) oxo-NiMo/P54-w,4, (b) oxo-Ni,Mo/P54-w,4, and (c) sulf-Ni,Mo/P54-w,4.
The synthesized catalysts were examined by X-ray diffraction (XRD). Figure 11 displays the diffractogram of the oxo-Mo/P54-i,4 catalyst (prepared by depositing only Mo over P54) and, for the sake of comparison, the XRD standard patterns of some common Mo oxides: hexagonal, orthorhombic, and monoclinic MoO3 (h-MoO3, α-MoO3, and β-MoO3, respectively), besides monoclinic MoO2. The spectrum of oxo-Mo/P54-i,4 presents intense sharp peaks, which is indicative of well-developed crystalline phases. The peaks at 2θ = 10.6, 18.4, 21.3, 26.0, 30.3, and 35.7° fit relatively well with those corresponding to the (100), (110), (200), (210), (300), and (310) diffraction planes of h-MoO3.
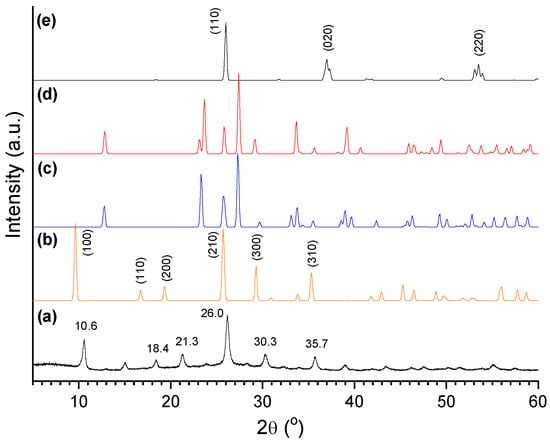
Figure 11.
(a) X-ray diffractogram of oxo-Mo/P54-i,4 catalyst and XRD standard patterns of some common crystal phases of Mo oxides: (b) h-MoO3 (JCPDS 21-0569); (c) α-MoO3 (JCPDS 05-0508); (d) β-MoO3 (JCPDS 47-1320); (e) monoclinic MoO2 (JCPDS 78-1070).
Figure 12 displays the XRD profiles of all the Mo-based oxo-catalysts prepared with the addition of Ni, which presented similar profiles to that of the Ni-free catalyst oxo-Mo/P54-i,4 (Figure 11a). This finding evidences that the Ni atoms were well dispersed over the active phase.
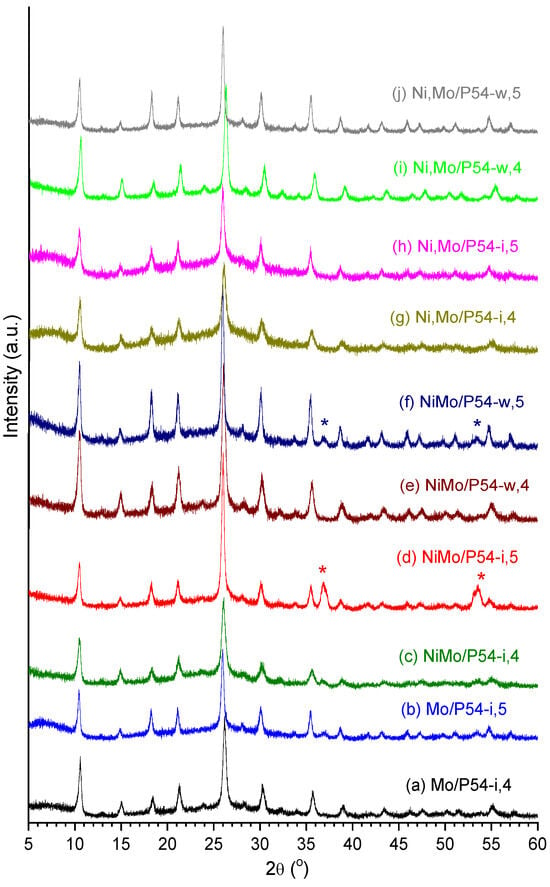
Figure 12.
X-ray diffractogram of all prepared oxo-catalysts. (The asterisks identify the peaks corresponding to the (020) and (220) diffraction planes of monoclinic MoO2 (displayed in Figure 11e)).
Figure 13 displays the XRD pattern of a sulfided catalyst (sulf-Ni,Mo/P54-w,4) and, for the sake of comparison, the XRD standard pattern of MoS2. The figure shows that, after sulfidation, the peaks characteristic of oxidic Mo compounds vanished, giving place to peaks characteristic of MoS2. Nevertheless, these peaks were quite large. Remarkably, the peaks corresponding to the (100), (101), (102), (103), and (105) diffraction planes of MoS2 were so large that they ended up overlapping to form a kind of envelope that extends from ~31 to ~55°, with two salient maxima at 34.0 and 39.0°. Furthermore, the peaks corresponding to (106), (110), and (008) diffraction planes overlapped to give rise to a unique band with a maximum at 59.0°. At this point, it is valid to mention that this DRX profile is commonly observed for synthesized MoS2 (even for unsupported MoS2) [62,75], with the peaks broadening being attributed to the presence of chemical heterogeneities and/or crystallite smallness.
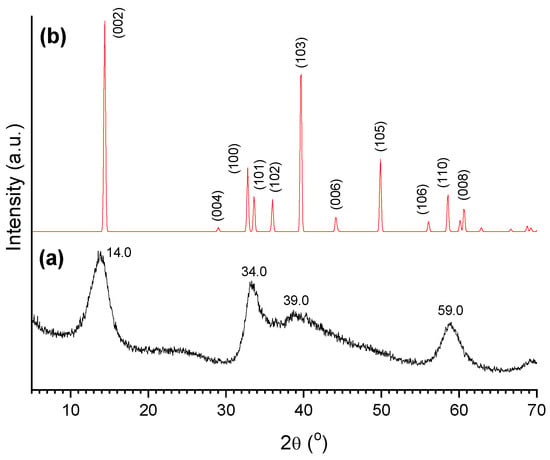
Figure 13.
(a) X-ray diffractogram of the sulf-Ni,Mo/P54-w,4 catalyst and (b) XRD standard profile of MoS2 (JCPDS 37-1492).
Additional specific issues related to the XRD analyses will be addressed throughout the text where pertinent.
As expected, the deposition of an elevated amount of metal on the surface of P54 caused pronounced reductions of porosity and SSA (Table 1). Besides reducing the volume of the pores, the deposited phase can also cause pore filling or blocking.
2.3. Searching for the Best Conditions to Prepare the Catalysts
Initially, a thorough study was carried out to search for the conditions that would result in the AC-supported Mo-based catalyst with the highest HDO activity. As already mentioned in the Introduction Section, this search was carried out using lauric acid as a model compound. The acidic index (AI) of the obtained liquid mixtures was taken as the key parameter to evaluate the catalysts activity for HDO. It is worth highlighting that the lower the AI, the higher the feedstock conversion and, therefore, the higher the catalyst activity. The AI could also be employed to evaluate the catalyst activity in the HDO of coconut oil because the triglycerides molecules that constitute the oil are first converted into the respective fatty acids [35].
2.3.1. Blank Test
A 3 h blank test with lauric acid was carried out using the bare support P54. The resulting product presented a high AI of 264.4 (Table 6, Entry 1), which was only a little lower than the value corresponding to pure lauric acid (280). This result reveals that the support, by itself, has quite a low HDO activity (if any).

Table 6.
AIs of the liquid products obtained in the tests for lauric acid HDO.
2.3.2. Catalysts Containing Mo as the Only Active Phase
Two catalysts containing Mo as the only metal in the active phase (in other words, without the addition of Ni) were prepared. Mo was deposited by incipient wetness impregnation (sometimes also called dry impregnation), and the impregnated material was calcined at 400 or 500 °C. The results of the 3 h tests of lauric acid HDO show that the catalysts presented relatively high HDO activity, with the resulting products presenting AIs of 8.1 and 7.0 in the cases of Mo/P54-i,4 and Mo/P54-i,5, respectively (Table 6, Entries 2 and 3).
Supported MoS2-based catalysts mainly consist of monolayer slabs or clusters of slabs dispersed on the surface of a porous solid. Each slab is constituted by a Mo4+ layer between two layers of S2-, with each Mo4+ coordinated to six S2- in a trigonal–prismatic geometry, as illustrated in Figure 14. Sulfur anion vacancies (also called unsaturated sites) located at the edge of the slabs are supposed to act as the active sites in the promotion of HDO reactions, whereas the basal planes would be few reactive [77]. We believe that these vacancies act as Lewis acid sites to be coordinated with the lone electron pairs of oxygen from carboxyl, carbonyl, or hydroxyl groups (in a similar way as proposed by Chorkendorff and Niemantsverdriet [77] for the HDS of ethanethiol), as illustrated in Figure 15.
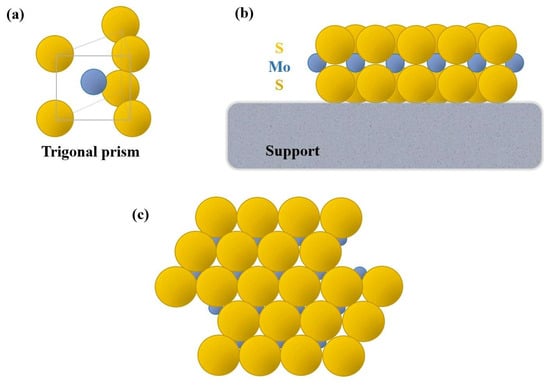
Figure 14.
Schematic picture representing (a) the unit trigonal–prismatic coordination in supported MoS2 catalysts, along with (b) a lateral, and (c) a top catalyst view (based on reference [77]).
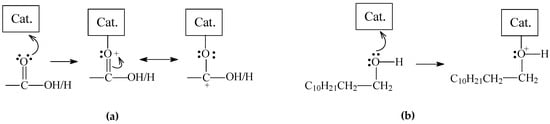
Figure 15.
Proposed coordination of (a) carboxyl/carbonyl and (b) hydroxyl groups to the anion vacancies of sulfided Mo-based catalysts.
When the double-bounded O of lauric acid coordinates with a catalyst sulfur anion vacancy (Figure 15a), the reaction could follow two distinct pathways. In the first, heterolytic cleavage of the bond between the electron-deficient carboxylic carbon and the α-carbon would give rise to a carbocation, as illustrated in Equation (4). This carbocation would be stabilized to render the corresponding alkane (n-undecane), while the catalytic active site would be regenerated by releasing CO2 (this pathway corresponds to the decarboxylation pathway (Equation (1)).
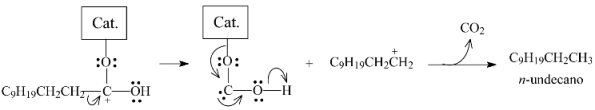
In the second pathway, the structure formed by the coordination of the carboxylic group would be hydrogenated to generate a gem-diol, which, in the sequence, would be dehydrated into dodecanal according to Equation (5).
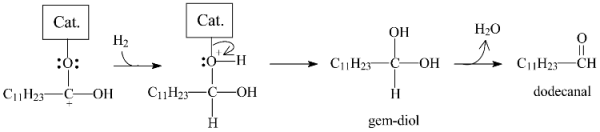
In the sequence, the dodecanal would coordinate with the catalyst, as proposed in Figure 15a, and the reaction could then also follow two different pathways. In the first, n-undecane and CO would be formed, as illustrated in Equation (6) (the combination of Equations (5) and (6) corresponds to the decarbonylation pathway (Equation (2)). In the second pathway, dodecanol would be formed, as portrayed in Equation (7).
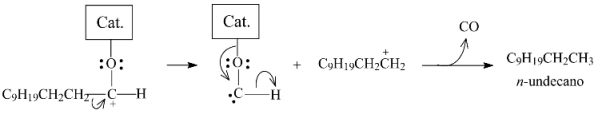
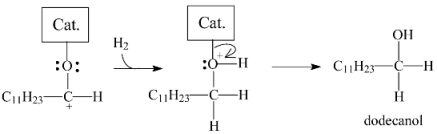
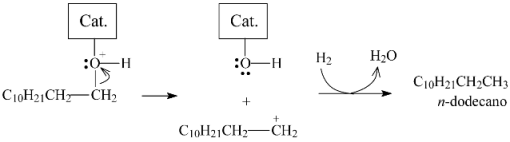
Dodecanol would then coordinate with the catalyst as illustrated in Figure 15b. The positive charge on the oxygen atom in the resulting structure would weaken the bond with the alpha carbon, favoring its heterolytic cleavage to generate a carbocation that would be stabilized to form n-dodecane, as illustrated in Equation (8) (the combination of Equations (5), (7) and (8) corresponds to the hydrogenation/dehydration pathway (Equation (3)).
2.3.3. Effects of Ni Addition
In the sequence of the work, the effect of adding Ni to the Mo-based catalysts was evaluated. Different procedures for metals deposition were investigated, as depicted in the sequence.
Co-Impregnation of Mo and Ni
Firstly, we tried to deposit Mo and Ni simultaneously; that is to say, from a unique solution (herein called the co-impregnation approach). For that, the (NH4)6Mo7O24 and Ni(NO3)2 solutions were mixed before impregnation. However, at this time, a suspension of Ni3Mo7O24 was formed. Since the employed atomic Ni/Mo ratio was nearly 0.07 (see Section 3.1), which is much inferior to the ratio in Ni3Mo7O24, it is possible to infer that the mixture was composed of nanoparticles of Ni3Mo7O24 suspended in a solution rich in Mo7O246− ions. This suspension was then used in the co-impregnation tests, which were carried out through incipient wetness impregnation or wet impregnation.
Incipient Wetness Co-Impregnation of Mo and Ni
The co-impregnation of Mo and Ni was first carried out employing the incipient wetness methodology. The obtained sulf-NiMo/P54-i,4 and sulf-NiMo/P54-i,5 catalysts were evaluated for lauric acid HDO. After 3 h, the obtained products presented AIs of 8.6 and 7.2, respectively (Table 6, Entries 4 and 5), which were similar to those verified for the Ni-free catalysts (Table 6, Entries 2 and 3). These results are in accordance with the idea that Ni was incorporated in the form of Ni3Mo7O24 nanoparticles, which would have simply rested on the support surface, while most of the Mo active phase was dispersed throughout the support surface.
Some findings seem to corroborate this hypothesis. For example, the samples oxo-NiMo/P54-i,4 and oxo-NiMo/P54-i,5 presented the highest SSA and porosity among all the prepared catalysts (Table 1). This behavior can be attributed to the fact that, due to dimensional constraints, the Ni3Mo7O24 nanoparticles would have been deposited only on the external surface and on the surface of larger pores, so that less metal remained to be located inside smaller pores (which are the main responsible for the surface area). Another interesting finding is that the oxo-catalysts prepared by co-impregnation and calcined at 500 °C (not only oxo-NiMo/P54-I,5, but also oxo-NiMo/P54-w,5) were the only ones whose DRX diffractograms presented salient peaks at around 2θ 36.9 and 53.5° (labeled with asterisks in Figure 12d,f), which correspond to the (020) and (220) diffraction planes of MoO2 (Figure 11e). Supposedly, the Mo6+ cation located in the interior of the Ni3Mo7O24 nanoparticles found conditions to be reduced to Mo4+ between 400 and 500 °C during calcination.
Wet Co-Impregnation of Mo and Ni
In the sequence of the work, the wet co-impregnation of Mo and Ni was carried out. Despite the much higher volume of the impregnating solution (~10x) employed in this procedure, compared to that employed in incipient wetness co-impregnation, the formation of a suspension of Ni3Mo7O24 nanoparticles took place even so.
The resulting catalysts presented SSA and porosity (Table 1) that were intermediate among the values verified for the samples prepared by incipient wetness co-impregnation (oxo-NiMo/P54-i,4 and oxo-NiMo/P54-i,5) and all the other prepared oxo-catalysts. Furthermore, the DRX profile of the oxo-NiMo/P54-w,5 catalyst presented the peaks typical of MoO2 at around 2θ 36.9 and 53.5° (labeled with an asterisk in Figure 12f), but in much lower intensity than in the case of oxo-NiMo/P54-i,5 (Figure 12d). These findings evidence that there was the deposition of Ni3Mo7O24 nanoparticles over the support surface during wet co-impregnation, but it was less pronounced than in the case of the incipient wetness co-impregnation. Supposedly, the prolonged impregnation time employed in wet co-impregnation (24 h) permitted, through an equilibrium displacement mechanism, a gradual dissolution of most (but not all) of the initially formed Ni3Mo7O24 nanoparticles into the abundant impregnating solution (Equation (9)) and posterior adsorption of the resulting ions on the support surface (Equation (10)).
Ni3Mo7O24 (s) ⇌ 3Ni2+ (aq) + Mo7O246− (aq)
AC (s) + Mo7O246− (aq) + 3Ni2+ (aq) ⇌ adsorbed ions
Indeed, the images of elemental mapping (Figure 5a,b) and the data reported in Figure 6d,e show that there was a better Ni dispersion in the catalyst oxo-NiMo/P54-w,4 (prepared through wet co-impregnation) than in the case of the catalyst oxo-NiMo/P54-i,4 (prepared through incipient wetness co-impregnation): the measured average Ni particle size was 3.89 and 4.24 nm, respectively. Since Ni is supposed to act as a catalyst promoter, its better dispersion is believed to be the reason why the catalysts prepared through wet co-impregnation (sulf-NiMo/P54-w,4 and sulf-NiMo/P54-w,5) presented considerably higher HDO activity than their counterparts prepared through incipient wetness co-impregnation (sulf-NiMo/P54-i,4 and sulf-NiMo/P54-i,5). When sulf-NiMo/P54-w,4 and sulf-NiMo/P54-w,5 were employed, the products obtained after the 3 h tests of lauric acid HDO presented AIs of only 0.8 and 1.1, respectively (Table 6, Entries 6 and 7).
Sequential Impregnation of Mo and Ni
To avoid the formation of Ni3Mo7O24 nanoparticles, catalysts were prepared employing a sequential impregnation of Mo and Ni using separate solutions of (NH4)6Mo7O24 and Ni(NO3)2. In the same way as for the co-impregnation procedures, both incipient wetness impregnation and wet impregnation methodologies were employed.
Indeed, the DRX profiles of the resulting oxo-catalysts (oxo-Ni,Mo/P54-i,4, oxo-Ni,Mo/P54-i,5, oxo-Ni,Mo/P54-w,4, and oxo-Ni,Mo/P54-w,5) did not present the peaks characteristic of MoO2 at 2θ 36.9 and 53.5° (Figure 12). Furthermore, the catalysts prepared using sequential impregnation presented lower SSA and porosity than their counterparts prepared by co-impregnation. According to the reasoning presented in Section 2.3.3, these findings result from the non-formation of Ni3Mo7O24 nanoparticles during catalyst preparation using sequential impregnation.
The resulting catalysts were tested for lauric acid HDO, and all of them presented high activity. After 3 h tests, the catalysts prepared by sequential incipient wetness impregnation of Mo and Ni (sulf-Ni,Mo/P54-i,4 and sulf-Ni,Mo/P54-i,5) rendered products with AIs of 2.0 and 0.8 (Table 6; Entries 8 and 9), respectively, which were much lower than those verified in the case of the counterpart catalysts prepared without the addition of Ni (Table 6; Entries 2 and 3) and also by co-impregnation of Mo and Ni (Table 6; Entries 4 and 5). These results confirm that Ni effectively acted as a promoter to increase catalyst activity. Although elemental mapping images (Figure 5) evidence the presence of Ni clusters, the promoting effect of this metal is mainly attributed to the fact that part of it replaces some Mo atoms in the metal sulfide structure. According to Byskov et al. [78] and Romero et al. [79], this replacement weakens Mo-S bonds, thus favoring the formation of vacant sites that promote hydrotreating reactions. More than that, Mo4+ and Ni2+ cations have two and eight valence electrons, respectively, so that it is perfectly understandable that the replacement of Mo4+ by Ni2+ creates defects in the MoS2 structure, and these defects could create kinds of sulfur vacancies, thereby increasing the catalyst hydrotreating activity. Furthermore, Ni is widely recognized for its ability to catalyze hydrogenation reactions [80,81], which is certainly contributing to the accomplishment of the reactions involved in Equations (5), (7) and (8).
In the cases of the catalysts prepared by wet impregnation (sulf-Ni,Mo/P54-w,4 and sulf-Ni,Mo/P54-w,5), products with AIs of 0.5 and 0.8 were obtained after 3 h tests of lauric acid HDO (Table 6; Entries 10 and 11). Since these values were very similar to that verified for sulf-Ni,Mo/P54-i,5 and close to zero, new tests with the catalysts sulf-Ni,Mo/P54-i,5 and sulf-Ni,Mo/P54-w,4 were carried out using a shorter reaction time (2 h). The AIs of the resulting products (13.7 and 1.9, respectively; Table 6; Entries 9′ and 10′) confirmed that the catalyst with the highest activity for HDO was sulf-Ni,Mo/P54-w,4, prepared by sucessive wet impregnation of Mo and Ni. Supposedly, the long impregnation time employed in this methodology permits a better metal dispersion throughout the support surface, thus resulting in a higher number of edge sites to promote HDO reactions. Indeed, the elemental mapping images (Figure 5) and the data displayed in Figure 6 confirm that the catalyst oxo-Ni,Mo/P54-w,4 has a higher dispersion of Mo and Ni nanoclusters.
Figure 16 shows the gas chromatography/flame ion detector (GC/FID) chromatogram of the product obtained after a 3 h test of lauric acid HDO with the sulf-Ni,Mo/P54-w,4 catalyst. The chromatogram presents only two peaks, assigned to n-undecane (n-C11) and n-dodecane (n-C12), with relative areas of 55.8 and 44.2%, respectively. n-C11 resulted from decarboxylation and decarbonylation reactions (Equations (1) and (2)), while n-C12 resulted from hydrogenation/dehydration reactions (Equation (3)). These results show that cracking and isomerization reactions were not relevant, which is in accordance with the low acidity of the employed support (see pertinent discussion in the Introduction Section). Therefore, it is possible to infer that these catalysts are more interesting to be used in the production of diesel-like fuels, where long and linear chains are desirable because they present a higher cetane number [82]. In turn, if the aim is to produce sustainable aviation fuel (SAF), an additional isomerization step should be added to the process because branched chains enable the fuel to meet the cold properties required for aviation fuels [4,82]. Alternatively, the obtained product, rich in linear alkane chains, could be mixed with a fuel fraction with appropriate content of branched alkanes, besides aromatics/cycloalkanes (the latter are important because they swell the rubber seals used in high-pressure fuel systems, thus preventing fuel leak [4,83]).
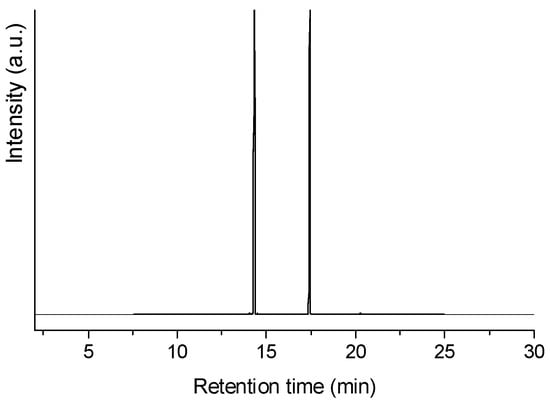
Figure 16.
GC/FID chromatogram of the product obtained after the 3 h test of lauric acid HDO using the sulf-Ni,Mo/P54-w,4 catalyst.
2.3.4. Effect of Calcination Temperature
Figure 12 shows that basically the same peaks were present in the XRD profiles of the oxo-catalysts prepared at 400 or 500 °C, except for the catalysts prepared through co-impregnation because, as already reported in Section 2.3.3, in these cases, partial reduction of Mo6+ to Mo4+ took place between 400 and 500 °C. Therefore, the influence of the calcination temperature on the HDO activity of the resulting catalysts was rather limited.
2.3.5. The Effects of Sulfiding the Catalyst
To evaluate the usefulness of performing the sulfidation of the AC-supported Mo-based catalysts (whose procedure is described in Section 3.4), a lauric acid HDO test was carried out using the catalyst oxo-Ni,Mo/P54-w,4; that is to say, without proceeding with the catalyst sulfidation step. However, the reaction mixture darkened during the test, and its viscosity gradually increased. These findings evidence that pronounced coking took place, which highlights the need to perform the sulfidation step. Indeed, Mukundan et al. [62] reported that Mo oxides can trigger the formation of carbon precursors on the catalyst surface, while Ojagh et al. [84] stated that sulfur phases are less prone to deactivation by coke deposition.
2.4. HDO Tests with Coconut Oil
The catalyst sulf-Ni,Mo/P54-w,4 (the one that presented the highest HDO activity in the tests with lauric acid) was employed in the subsequent tests with coconut oil. The 3 h test resulted in a product with an AI of 1.1 (Table 6, Entry 12), which was somewhat higher than that verified for the product of the test with lauric acid under identical conditions (0.5; Table 6, Entry 10). This result can be attributed to the fact that the triacylglyceride molecules that compose the oil have to be first split into propane and the corresponding fatty chains before the latter are deoxygenated, which slows the process [35].
In turn, a reaction time of 5 h rendered a product with an AI 0.0 (Table 6, Entry 12′). Accordingly, the GC/FID chromatogram of the obtained product (Figure 17a) presented only peaks corresponding to alkanes (practically only n-alkanes). These alkanes cover a large chain length range, mostly from C7 to C18, with emphasis on n-C11 and n-C12 (Table 7), which is in accordance with the coconut oil composition [26] and the occurrence of a nearly balanced distribution between decarboxylation/decarbonylation and hydrogenation/dehydration pathways (see Section 2.3.3).
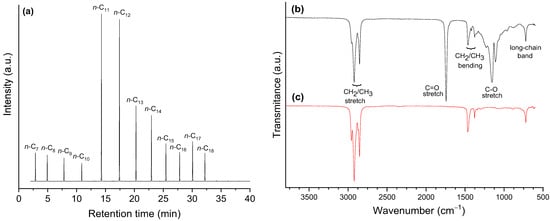
Figure 17.
(a) GC/FID chromatogram and FTIR spectra of (b) coconut oil and (c) the product of coconut oil HDO for 5 h using the sulf-Ni,Mo/P54-w,4 catalyst.

Table 7.
Relative content of n-alkanes in the product of coconut oil HDO for 5 h using the sulf-Ni,Mo/P54-w,4 catalyst. (obtained from the respective GC/FID chromatogram).
In accordance with the measured AI (zero) and the respective chromatogram, the Fourier-transform infrared spectroscopy (FTIR) spectrum of the obtained product (Figure 17c) presents only absorptions characteristics of alkanes: (i) C-H stretch at 2970–2825 cm−1; (ii) C–H bending of CH2 and CH3 at ~1460 and ~1370 cm−1, respectively; (iii) long-chain band at ~720 cm−1. Outstandingly, the bands characteristic of oxygenated groups that were present in the spectrum of coconut oil (Figure 17b) vanished, namely the C=O stretch at ~1740 cm−1 and the C–O stretch at ~1150 cm−1. In addition, there is no evidence of the O–H stretch band in the range of 3500–3200 cm−1 (which would appear if there were free fatty acids in the mixture) or any other absorption characteristic of oxygenated functional groups.
2.5. Catalyst Reusability
The reusability of the catalyst sulf-Ni,Mo/P54-w,4 was investigated by using the same catalyst sample in four consecutive cycles of coconut oil HDO. At the end of each cycle, the spent catalyst was recovered from the liquid product by filtration and dried overnight at 100 °C. The employed reaction time was 5 h. In a first set of cycles, the in situ catalyst sulfidation was carried out only before the first cycle. In these conditions, there was a small but gradual increase in the AI of the products obtained from successive cycles (Figure 18), which reflects a loss of catalyst activity. In a second set of cycles, the in situ catalyst sulfidation was repeated before each new cycle. In this way, the AI of the obtained products remained zero even after the fourth cycle. These results disclose that a slight loss of catalyst activity gradually takes place due to the partial replacement of sulfur by oxygen in the active phase, which is potentialized by the abundant presence of oxygenated molecules in the reaction medium. However, this undesirable effect can be avoided by ensuring that the sulfidation degree of the catalyst is kept high.
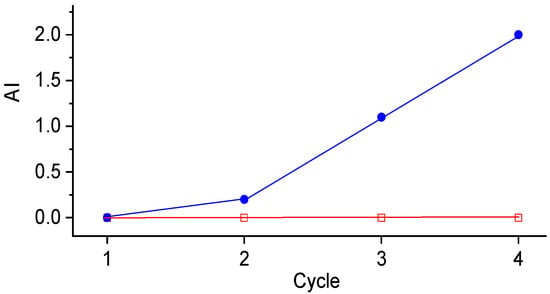
Figure 18.
AI of the products obtained after successive cycles of coconut oil HDO (catalyst: sulf-Ni,Mo/P54-w,4; 5 h reaction time) with catalyst sulfidation carried out only before the first cycle (blue closed circles), or before each new cycle (red open squares).
At this point, it is valid to mention that activity loss due to partial oxidation by H2O has also been reported for Al2O3-supported sulfided Mo-based catalysts [39,85,86,87]. In this sense, some authors have proceeded with the co-feeding of S-containing compounds (e.g., dimethyl disulfide (DMDS) or H2S) to avoid catalyst deactivation during the hydroprocessing of biomass-derived feedstocks [38,39,49,87,88]. Table 8 presents some results, selected from the literature, concerning these issues.

Table 8.
Some results, selected from the literature, concerning (i) the deactivation of sulfided Mo-based catalysts due to the oxidation by water and (ii) the continuous addition of S-containing compounds aiming to prevent this effect.
To the best of our knowledge, the work of Aiamsiri et al. [89] is the only one published in the literature concerning the use of a supported sulfided Mo-based catalyst in successive batch cycles for the hydroprocessing of a lipidic feedstock. The authors reported a gradual and considerable loss of activity for a sulfided NiMo/γ-Al2O3 catalyst along successive cycles of palm oil HDO: the hydrocarbons yield (C14–C18) verified in the first three cycles were 54.1, 43.5, and 39.4%. It is worth mentioning that the catalyst was not resulfided after each cycle, so that it is not possible to specify if the referred weight loss was simply due to the oxidation of the sulfided active phase by the formed water or whether support-related effects (i.e., coke formation and hydrolysis of Al2O3) also contributed to it. Anyway, it is noticeable that the reported activity loss was much more pronounced than that verified in the present work for the cycles carried out using the AC-supported catalyst sulf-Ni,Mo/P54-w,4 without resulfidation.
2.6. Comparison of the HDO Activity
The comparison of the HDO activity of the catalysts prepared in the present work with those of other sulfided NiMo catalysts is highly desirable. However, a comparison with studies reported in the literature is difficult due to the different systems and conditions employed. Therefore, in order to have a reference to evaluate the performance of the prepared catalysts, some HDO tests were carried out under similar conditions employing an industrial sulfided NiMo/Al2O3 catalyst supplied by Petrobras Oil Company (Rio de Janeiro, Brazil). This Al2O3-supported catalyst, which was characterized elsewhere [35], rendered a product with AI 0.3 after a 3 h test of lauric acid HDO, a result that was similar to that achieved with the sulf-Ni,Mo/P54-w,4 catalyst (which rendered a product with AI 0.5; Table 6, Entry 10). On the other hand, after 3 h tests with coconut oil, the sulfided NiMo/Al2O3 catalyst rendered a product with AI 6.3, while sulf-Ni,Mo/P54-w,4 gave rise to a product whose AI was 1.1.
Two important conclusions can be taken from these results: firstly, that AC-supported sulfided NiMo catalysts can be prepared with a capacity to promote HDO as high as those of well-consolidated industrial catalysts having Al2O3 as support; secondly, that in comparison to Al2O3, the employment of the AC P54 as support favored the initial step of triglycerides split to render the respective fatty acids, so that a higher activity for coconut oil HDO was observed for the prepared AC-supported catalyst. Supposedly, the mesopores in the P54 AC provided better access of the bulky triglycerides molecules to the active sites inside the pores network, therefore favoring the initial step of hydrogenolysis [19,90]. For these reasons, the P54-supported catalyst and the Al2O3-supported catalysts presented similar performance in the HDO of lauric acid (a linear molecule with relatively small hydrodynamic dimension), whereas the former presented a much better performance for the HDO of bulky molecules such as those that constitute lipidic feedstocks.
3. Materials and Methods
3.1. Catalysts Preparation
A dried endocarp of coconut shell (Cocos nucifera L.) originally from the region of Federal District, Brazil, was crushed and sieved. The fraction in the range of 40–80 mesh (0.177–0.400 mm) was submitted to chemical activation with H3PO4 (85%, Vetec, Speyer, Germany) following the procedure described in previous papers [66]. Shortly, the shell was impregnated with an aqueous solution containing the desired amount of H3PO4 and carbonized up to 450 °C under an inert atmosphere (N2). Then, the carbonized material was washed with deionized water to remove the chemical, leaving behind a porous structure, and dried overnight at 110 °C. The higher the proportion of impregnated H3PO4, the larger the developed porosity and the higher the pore dimensions. Thus, aiming to obtain a mesoporous-rich AC, a relatively high phosphorus/shell ratio was used in the present work: 0.54 (for this reason, the obtained sample was termed P54).
During catalysts preparation, Mo and Ni were deposited from aqueous solutions of (NH4)6Mo7O24·4H2O (99%; Vetec) and Ni(NO3)2·6H2O (99%; Vetec), respectively, over the surface of the P54 AC. The solutions were always prepared with concentrations corresponding to Mo and Ni proportions of 42 and 1.8 wt% relative to the support, respectively. As shown in Table 5, this procedure resulted in maximum Mo and Ni contents of about 20 and 1.0 wt% in the prepared catalysts (ICP/OES measurements).
The metals depositions were carried out by incipient wetness impregnation or wet impregnation. In the former, a volume of impregnating solution was added dropwise over the support, so that the solution penetrated the pores by capillarity. The solution volume (determined through a preliminary test on aliquot samples) corresponded to that beyond which the catalyst outside began to look wet. Then, the material was dried overnight in an oven at 110 °C. In turn, in the wet technology, an excess solution was used (13 mL per gram of AC). The system was closed and kept under stirring for 48 h at 50 °C. After that, the excess water and volatiles were permitted to evaporate by opening the system. Finally, the material was dried overnight in an oven at 110 °C.
For preparing the catalyst containing both Mo and Ni, two different approaches were evaluated: the metals were co-impregnated from a single solution; the metals were sequentially impregnated with separate solutions (first Mo, then Ni).
The impregnated materials were calcined up to 400 or 500 °C (2 h, 5 °C min−1) in an inert atmosphere (N2, 100 mL min−1). The calcined materials were sulfided in situ as described in Section 3.4.
3.2. Catalysts Labels
The catalysts were systematically labelled accordingly to the procedure employed for their preparation as follows.
- (a)
- The prefixes “oxo” or “sulf” were used to indicate the non-sulfided and sulfided forms, respectively (“oxo” is used because oxides are the predominant form of the metals after calcination and before sulfidation, as depicted in Section 3.2).
- (b)
- The deposited metals were indicated in the sequence. If Mo and Ni were co-impregnated, their elemental symbols were listed together (NiMo). If they were sequentially impregnated, the elemental symbols were separated by a comma. Repair that, despite Mo being impregnated first in the sequential protocol, and even so, the Ni symbol has been listed before Mo in the catalysts labels because this is the more usual format verified in the literature.
- (c)
- In the sequence, the employed support (P54) is indicated after a slash.
- (d)
- Then, separated by a hyphen, “w” or “i” indicate if wet impregnation or incipient wetness impregnation was used, respectively.
- (e)
- Finally, separated by a comma, the calcination temperature is indicated: 4 and 5 represent 400 °C and 500 °C, respectively.
Thus, just as an example, oxo-Ni,Mo/P54-w,4 refers to the non-sulfided form of the catalyst that was prepared with sequential deposition of Mo and Ni over the P54 support by wet impregnation, with a calcination temperature of 400 °C.
3.3. Characterization of P54 and Catalysts
The textural properties of ACs and catalysts were evaluated from the excess adsorption/desorption isotherms of N2 (−196 °C) recorded up to 1 bar on a volumetric automatic system Quantachrome NovaWin 2200e (Boynton Beach, FL, USA). SSA and Vmic were determined from the adsorption branch by applying the Brunauer–Emmett–Teller (BET) and Dubinin–Radushkevich (DR) equations, respectively. The volume of liquid N2 adsorbed at p/p0 0.95 was termed V0.95 and was considered as the sum of Vmic and the Vmes. Therefore, Vmes was calculated as the difference between V0.95 and Vmic.
P54 had its content of C, H, and N determined by EA performed on a Parkin Elmer CHN Elemental Analyzer model EA 2400 Series II equipped with an AD6 ultra-microbalance (Waltham, MA, USA). The ash content was determined on a dry basis by heating the material at 600 °C for 6 h in a furnace oven open to the atmospheric air. The bulk contents of Mo and Ni were determined by ICP/OES on an Agilent 5100 equipment (Santa Clara, CA, USA).
TPD/MS analyses were performed on an automated reaction system model AMI-90R coupled to a Dymaxion quadrupole mass spectrometer (Altamira Instruments, Pittsburgh, PA, USA). The sample (~100 mg) was placed in a U-tube of quartz under an Ar flow of 10 cm3 min−1 at atmospheric pressure. The system was first heated to 110 °C (10 °C min−1) and maintained at this temperature for 30 min to release physisorbed small molecules. Thereafter, the temperature was raised to 950 °C (10 °C min−1). The evolving gases were monitored with the mass spectrometer. CO and CO2-profiles were fitted according to a Gaussian distribution. Peak areas were correlated to the amount of gas through a daily routine calibration that consisted of the injection of a given volume of pure gas using a calibrated loop [91].
XPS measurements were performed on a Physical Electronics 5700 spectrometer using the Mg-Kα line (1253.6 eV) from a PHI model 04-548 Dual Anode X-rays Source (Chanhassen, MN, USA). The X-rays source was run at a power of 300 W (10 keV and 30 mA). The pressure inside the vacuum chamber was 5 × 10−7 Pa. A Perkin Elmer 10-360 hemispherical analyzer (Eden Prairie, MN, USA) with a multi-channel detector was employed. The samples were analyzed at an angle of 45° to the surface plane. BEs were referred to the C 1s line of adventitious carbon at 284.8 eV and determined with the resolution of ±0.1 eV. The spectra were fitted assuming a Gaussian–Lorentzian distribution for each peak.
High-resolution transmission electron microscopy (HRTEM) was carried out with a TALOS F200x instrument operating in scanning transmission electron microscopy (STEM) mode, equipped with a high-angle annular dark-field (HAADF) detector, at 200 kV and 200 nA (Thermo Fisher Scientific, Waltham, MA, USA). The elemental mapping was carried out on an EDX Super-X system provided with four X-ray detectors and an X-FEG (field emission gun) beam (Thermo Fisher Scientific, Waltham, MA, USA). Particle size distributions were achieved by using ImageJ 1.51k software, sampling at least 700 particles for each metal in each catalyst.
XRD diffractograms were obtained using a Ni-filtered Cu-Kα radiation (λ = 0.15406 nm). The analyses were usually carried out on a Rigaku instrument model Miniflex 300 (Akishima-shi, Tokyo, Japan).
The contents of acidic and basic groups of P54 were determined through a procedure adapted from the Boehm titration methodology [92]. To determine the basic groups, ~0.5 g of AC was added to an Erlenmeyer flask containing 50 mL of a standard HCl solution (~0.100 mol L−1). After stirring for 24 h at room temperature (~25 °C), the solid was filtered and aliquots of 10 mL of the filtrate were titrated against a standard NaOH solution (~0.100 mol L−1). In turn, the contents of acidic groups were determined by backtitration: about 0.50 g of AC was added to an Erlenmeyer flask containing 50 mL of a standard solution of NaOH, NaHCO3, or Na2CO3 (~0.100 mol L−1). After magnetic stirring for 24 h and filtration, 10 mL aliquots of the filtrate were added to 15 mL of the standard HCl solution. Finally, the excess acid was titrated against the standard NaOH solution. Blank tests were performed following the same procedure, but without contact with P54. Thus, the contents of acidic or basic groups were determined by considering the difference between the volumes of NaOH solution required to reach the endpoint in the titrations of the sample and the blank. Measurements were taken in triplicate.
To interpret the data, it was assumed that the NaOH solution neutralizes all the acidic groups on the AC surface; Na2CO3 solution neutralizes strong and medium strength acidic groups; NaHCO3 solution neutralizes only strong acids. Therefore, (i) the amount of HCl consumed in the test with NaHCO3 corresponded to the content of strong acids. Furthermore, the difference between the amount of HCl consumed in the tests with (ii) Na2CO3 and NaHCO3 corresponded to the content of medium-strength acidic groups; (iii) NaOH and Na2CO3 corresponded to the content of weak acids.
It is usually assumed in the literature that strong, medium, and weak acids correspond to carboxylic acids, lactones, and phenols, respectively (see, just as a few examples, references [93,94]). However, as stressed by Schönherr et al. [95], pKa values of acidic groups strongly depend on their chemical environment, so that a strict allocation of acidic functional groups to the reaction with specific bases can lead to misunderstandings. Therefore, in the present work, we opted to report the titration results in terms of the more general terms strong, medium strength, and weak acidic groups, without making attributions to specific groups.
Another aspect that deserves to be highlighted is that anhydrides are usually neglected by the authors who employ titration to characterize the surface of ACs. Nevertheless, Schönherr et al. [95] reported that, in basic aqueous solution, anhydrides are hydrolyzed to render two carboxylic acids. Therefore, we infer that, besides carboxylic acids themselves, the content of strong acids reported in Table 4 also encompasses two carboxylic acids resulting from each anhydride group.
The total content of basic groups was determined through a similar procedure as above described for acidic groups, with some obvious modifications: the AC was put in contact with an acidic solution (HCl~0.100 mol L−1); after filtration, the aliquots were titrated against a standard basic solution (NaOH~0.100 mol L−1). It was assumed that HCl neutralizes all the AC surface basic groups. Therefore, the difference between the amount of NaOH spent in the titration of the blank acidic solution and the acidic solution remaining after soaking with P54 corresponded to the total amount of basic groups on the AC surface.
3.4. HDO Tests
The feedstocks employed in the HDO tests were lauric acid (99.0%, Sigma Aldrich, St. Louis, MO, USA) and an extra virgin coconut oil (98%) (dr. Orgânico, Brazil). They were used without further purification. The tests were carried out in a cylindrical stainless-steel reactor with an internal volume of ~100 cm3. A scheme of the reactor was presented elsewhere [35]. Prior to the tests, the catalysts in the oxo form (0.500 g) were sulfided in situ with 0.500 mL of CS2 (PA, Vetec) at 400 °C (1 h) in H2 atmosphere (initial pressure of 30 bar). After sulfidation, the system was cooled and purged with N2. Then, 10.0 mL of feedstock were charged, the reactor was pressurized with H2 at 30 bar, and heated to 340 °C. The heating from room temperature up to 340 °C took nearly 45 min. The time the temperature reached 340 °C was considered the initial reaction time. After the target reaction time, the system was cooled to room temperature, which took around 60 min. Then, the liquid product was dried with Na2SO4 and separated by centrifugation.
3.5. Characterization of the Liquid Reaction Products
The liquid reaction products were qualitatively analyzed by gas chromatography/mass spectrometry (GC/MS) using a GC-17a chromatograph interfaced with a QP5050A spectrometer (Shimadzu, Kyoto, Japan). For quantitative analyses, GC/FID chromatograms were acquired on a GC-2010 equipment (Shimadzu, Kyoto, Japan). A Rtx-5MS polydimethylsiloxane column (30 m, 25 mm) was used in both GC/MS and GC/FID analyses. The AI was determined according to the AOCS method Cd-3d-63-O. FTIR spectra were acquired on an IR Prestige-21 spectrometer using a diamond ATR (attenuated total reflection) accessory and a DLATGS detector (Shimadzu, Kyoto, Japan).
4. Conclusions
In the present work, an AC produced from chemical activation of coconut shell with H3PO4 was employed as the support of sulfided Mo-based catalysts intended for the hydroprocessing of lipidic feedstocks. The effects of preparation conditions on the properties and HDO activity of the resulting catalysts were systematically investigated for the first time.
Catalysts with high HDO activity were achieved. They presented good stability, permitting the accomplishment of several reaction cycles without significant loss of activity, provided that the sulfidation degree was kept high. Ni acted as a promoter, increasing the catalyst activity. Sulfidation proved to be an essential step to prevent coking.
The catalyst with the highest activity was the one prepared through sequential deposition of Mo and Ni by wet impregnation. It presented activity for lauric acid HDO similar to that of an industrial sulfided NiMo/Al2O3 catalyst. However, in the case of the tests with coconut oil, the AC-supported catalyst was even more efficient than the commercial Al2O3-supported catalyst because the presence of mesopores in the synthesized AC provided better access of bulky triglyceride molecules to the metallic active sites inside the pores network, thus favoring the initial step of hydrogenolysis to render the respective fatty acids.
Due to the low acidity of ACs, low degrees of cracking and isomerization were observed in the hydroprocessing tests with the AC-supported catalysts, so that the resulting products were mainly constituted by long-chain n-alkanes. Therefore, it is possible to infer that these catalysts are more interesting to be used in the production of diesel-like fuels with high cetane number. In turn, if the aim is to produce SAF, an additional step of cracking/isomerization should be added to the process, or the obtained product should be mixed with another fuel fraction with an appropriate content of branched alkanes and aromatic/cycloalkanes.
Author Contributions
Conceptualization and methodology, M.J.P. and E.R.-C.; funding acquisition, M.J.P. and E.R.-C.; supervision, M.J.P.; formal analysis, M.J.P., A.M.d.F.J., R.D.B., M.S.T., J.G., W.d.N.M., D.B.-P. and E.R.-C.; investigation, A.M.d.F.J., R.D.B., M.S.T., J.G., W.d.N.M. and D.B.-P.; writing—original draft preparation, M.J.P.; writing—review and editing, A.M.d.F.J., R.D.B., W.d.N.M., E.R.-C. and D.B.-P. All authors have read and agreed to the published version of the manuscript.
Funding
D.B.-P. and E.R.-C. thank the Spanish Ministry of Science and Innovation, project PID2021-126235OB-C32 funded by MCIN/AEI/10.13039/501100011033 and FEDER funds, and project TED2021-130756B-C31 funded by MCIN/AEI/10.13039/501100011033 and by “ERDF A way of making Europe” by the European Union Next Generation EU/PRTR. All authors thank CAPES (Brazilian Federal Agency for Support and Evaluation of Graduate Education, Brazil; Finance Code 001) for the financial support for this research.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AC | Activated carbon |
| ATR | Attenuated total reflection |
| BE | Binding energy |
| BET | Brunauer–Emmett–Teller |
| DR | Dubinin–Radushkevitch |
| EDX | Energy-dispersive X-ray |
| FEG | Field emission gun |
| FID | Flame ionization detector |
| FTIR | Fourier-transform infrared spectroscopy |
| GC | Gas chromatography |
| HAADF | High-angle annular dark-field |
| HDO | Hydrodeoxygenation |
| HEFA | Hydroprocessing of esters and fatty acids |
| HR | High-resolution |
| HRTEM | High-resolution transmission electron microscopy |
| ICP | Inductively coupled plasma |
| IUPAC | International Union of Pure and Applied Chemistry |
| JCPDS | Joint Committee on Powder Diffraction Standards |
| MS | Mass spectrometry |
| OES | Optical emission spectrometry |
| SAF | Sustainable aviation fuel |
| SPK | Synthetic paraffinic kerosene |
| SSA | Specific surface area |
| STEM | Scanning transmission electron microscopy |
| TEM | Transmission electron microscopy |
| TPD | Temperature-programmed desorption |
| Vmic | Micropores volume |
| Vmes | Mesopores volume |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
References
- Fregolente, P.B.L.; Wolf Maciel, W.M.; Oliveira, L.S. Removal of Water Content From Biodiesel and Diesel Fuel Using Hydrogel Adsorbents. Braz. J. Chem. Eng. 2015, 32, 895–901. [Google Scholar] [CrossRef]
- Dwivedi, G.; Sharma, M. Cold Flow Behaviour of Biodiesel-A Review. Int. J. Ren. Energy Res. 2013, 3, 827–836. [Google Scholar]
- Ferrari, R.A.; Oliveira, V.d.S.; Scabio, A. Oxidative Stability of Biodiesel from Soybean Oil Fatty Acid Ethyl Esters. Sci. Agric. 2005, 62, 291–295. [Google Scholar] [CrossRef]
- Prauchner, M.J.; DBrandão, R.; de Freitas Júnior, A.M.; da COliveira, S. Alternative Hydrocarbon Fuels, with Emphasis on Sustainable Jet Fuels. Rev. Virtual Química 2023, 15, 498–518. [Google Scholar] [CrossRef]
- Wei, H.; Liu, W.; Chen, X.; Yang, Q.; Li, J.; Chen, H. Renewable Bio-Jet Fuel Production for Aviation: A Review. Fuel 2019, 254, 115599. [Google Scholar] [CrossRef]
- Yang, J.; Xin, Z.; He, Q.; Corscadden, K.; Niu, H. An Overview on Performance Characteristics of Bio-Jet Fuels. Fuel 2019, 237, 916–936. [Google Scholar] [CrossRef]
- Watson, M.J.; Machado, P.G.; da Silva, A.V.; Saltar, Y.; Ribeiro, C.O.; Nascimento, C.A.O.; Dowling, A.W. Sustainable Aviation Fuel Technologies, Costs, Emissions, Policies, and Markets: A Critical Review. J. Clean. Prod. 2024, 449, 141472. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Pereira, M.F.R. Sustainable Aviation Fuel Production through Catalytic Processing of Lignocellulosic Biomass Residues: A Perspective. Sustainability 2024, 16, 3038. [Google Scholar] [CrossRef]
- Díaz-Pérez, M.A.; Serrano-Ruiz, J.C. Catalytic Production of Jet Fuels from Biomass. Molecules 2020, 25, 802. [Google Scholar] [CrossRef]
- Peters, M.A.; Alves, C.T.; Onwudili, J.A. A Review of Current and Emerging Production Technologies for Biomass-Derived Sustainable Aviation Fuels. Energies 2023, 16, 6100. [Google Scholar] [CrossRef]
- Amhamed, A.I.; Al Assaf, A.H.; Le Page, L.M.; Alrebei, O.F. Alternative Sustainable Aviation Fuel and Energy (SAFE)- A Review with Selected Simulation Cases of Study. Energy Rep. 2024, 11, 3317–3344. [Google Scholar] [CrossRef]
- Song, M.; Zhang, X.; Chen, Y.; Zhang, Q.; Chen, L.; Liu, J.; Ma, L. Hydroprocessing of Lipids: An Effective Production Process for Sustainable Aviation Fuel. Energy 2023, 283, 129107. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; dos Santos, I.A.; Arcanjo, M.R.A.; Cavalcante, C.L.; de Luna, F.M.T.; Fernandez-Lafuente, R.; Vieira, R.S. Production of Jet Biofuels by Catalytic Hydroprocessing of Esters and Fatty Acids: A Review. Catalysts 2022, 12, 237. [Google Scholar] [CrossRef]
- Kim, S.K.; Han, J.Y.; Lee, H.; Yum, T.; Kim, Y.; Kim, J. Production of Renewable Diesel via Catalytic Deoxygenation of Natural Triglycerides: Comprehensive Understanding of Reaction Intermediates and Hydrocarbons. Appl. Energy 2014, 116, 199–205. [Google Scholar] [CrossRef]
- Lam, J.E.; Mohamed, A.R.; Kay Lup, A.N.; Koh, M.K. Palm Fatty Acid Distillate Derived Biofuels via Deoxygenation: Properties, Catalysts and Processes. Fuel Process. Technol. 2022, 236, 107394. [Google Scholar]
- Mohammad, M.; Kandaramath Hari, T.; Yaakob, Z.; Chandra Sharma, Y.; Sopian, K. Overview on the Production of Paraffin Based-Biofuels via Catalytic Hydrodeoxygenation. Renew. Sustain. Energy Rev. 2013, 22, 121–132. [Google Scholar]
- Ding, S.; Parlett, C.M.A.; Fan, X. Recent Developments in Multifunctional Catalysts for Fatty Acid Hydrodeoxygenation as a Route towards Biofuels. Mol. Catal. 2022, 523, 111492. [Google Scholar] [CrossRef]
- Rahmawati, Z.; Santoso, L.; McCue, A.; Azua Jamari, N.L.; Ninglasari, S.Y.; Gunawan, T.; Fansuri, H. Selectivity of Reaction Pathways for Green Diesel Production towards Biojet Fuel Applications. RSC Adv. 2023, 13, 13698–13714. [Google Scholar] [CrossRef]
- Khan, S.; Kay Lup, A.N.; Qureshi, K.M.; Abnisa, F.; Wan Daud, W.M.A.; Patah, M.F.A. A Review on Deoxygenation of Triglycerides for Jet Fuel Range Hydrocarbons. J. Anal. Appl. Pyrolysis 2019, 140, 1–24. [Google Scholar]
- Chen, S.; Zhou, G.; Miao, C. Green and Renewable Bio-Diesel Produce from Oil Hydrodeoxygenation: Strategies for Catalyst Development and Mechanism. Renew. Sustain. Energy Rev. 2019, 101, 568–589. [Google Scholar]
- Mahdi, H.I.; Bazargan, A.; McKay, G.; Azelee, N.I.W.; Meili, L. Catalytic Deoxygenation of Palm Oil and Its Residue in Green Diesel Production: A Current Technological Review. Chem. Eng. Res. Des. 2021, 174, 158–187. [Google Scholar]
- Mussa, N.-S.; Toshtay, K.; Capron, M. Catalytic Applications in the Production of Hydrotreated Vegetable Oil (HVO) as a Renewable Fuel: A Review. Catalysts 2024, 14, 452. [Google Scholar] [CrossRef]
- Pattanaik, B.P.; Misra, R.D. Effect of Reaction Pathway and Operating Parameters on the Deoxygenation of Vegetable Oils to Produce Diesel Range Hydrocarbon Fuels: A Review. Renew. Sustain. Energy Rev. 2017, 73, 545–557. [Google Scholar] [CrossRef]
- Arun, N.; Sharma, R.V.; Dalai, A.K. Green Diesel Synthesis by Hydrodeoxygenation of Bio-Based Feedstocks: Strategies for Catalyst Design and Development. Renew. Sustain. Energy Rev. 2015, 48, 240–255. [Google Scholar]
- Mäki-Arvela, P.; Martínez-Klimov, M.; Murzin, D.Y. Hydroconversion of Fatty Acids and Vegetable Oils for Production of Jet Fuels. Fuel 2021, 306, 121673. [Google Scholar] [CrossRef]
- Cheah, K.W.; Yusup, S.; Loy, A.C.M.; How, B.S.; Skoulou, V.; Taylor, M.J. Recent Advances in the Catalytic Deoxygenation of Plant Oils and Prototypical Fatty Acid Models Compounds: Catalysis, Process, and Kinetics. Mol. Catal. 2022, 523, 111469. [Google Scholar]
- Zhao, X.; Wei, L.; Cheng, S.; Julson, J. Review of Heterogeneous Catalysts for Catalytically Upgrading Vegetable Oils into Hydrocarbon Biofuels. Catalysts 2017, 7, 83. [Google Scholar] [CrossRef]
- Gosselink, R.W.; Hollak, S.A.W.; Chang, S.; van Haveren, J.; de Jong, K.P.; Bitter, J.H.; van Es, D.S. Reaction Pathways for the Deoxygenation of Vegetable Oils and Related Model Compounds. ChemSusChem 2013, 6, 1576–1594. [Google Scholar] [CrossRef]
- Ooi, X.Y.; Gao, W.; Ong, H.C.; Lee, H.V.; Juan, J.C.; Chen, W.H.; Lee, K.T. Overview on Catalytic Deoxygenation for Biofuel Synthesis Using Metal Oxide Supported Catalysts. Renew. Sustain. Energy Rev. 2019, 112, 834–852. [Google Scholar]
- Mishra, R.K.; Jaya Prasanna Kumar, D.; Sankannavar, R.; Binnal, P.; Mohanty, K. Hydro-Deoxygenation of Pyrolytic Oil Derived from Pyrolysis of Lignocellulosic Biomass: A Review. Fuel 2024, 360, 130473. [Google Scholar]
- Yücel, S.B.; Donar, Y.O.; Ergenekon, S.; Özoylumlu, B.; Sinağ, A. Green Catalyst for Clean Fuel Production via Hydrodeoxygenation. Turk. J. Chem. 2023, 47, 968–990. [Google Scholar]
- Sharma, V.; Getahun, T.; Verma, M.; Villa, A.; Gupta, N. Carbon Based Catalysts for the Hydrodeoxygenation of Lignin and Related Molecules: A Powerful Tool for the Generation of Non-Petroleum Chemical Products Including Hydrocarbons. Renew. Sustain. Energy Rev. 2020, 133, 110280. [Google Scholar] [CrossRef]
- Itthibenchapong, V.; Srifa, A.; Kaewmeesri, R.; Kidkhunthod, P.; Faungnawakij, K. Deoxygenation of Palm Kernel Oil to Jet Fuel-like Hydrocarbons Using Ni-MoS2/γ-Al2O3 Catalysts. Energy Convers. Manag. 2017, 134, 188–196. [Google Scholar]
- Wang, H.; Rogers, K.; Zhang, H.; Li, G.; Pu, J.; Zheng, H.; Lin, H.; Zheng, Y.; Ng, S. The Effects of Catalyst Support and Temperature on the Hydrotreating of Waste Cooking Oil (WCO) over CoMo Sulfided Catalysts. Catalysts 2019, 9, 689. [Google Scholar] [CrossRef]
- Brandão, R.D.; de Freitas Júnior, A.M.; Oliveira, S.C.; Suarez, P.A.Z.; Prauchner, M.J. The Conversion of Coconut Oil into Hydrocarbons within the Chain Length Range of Jet Fuel. Biomass Convers. Biorefinery 2021, 11, 837–847. [Google Scholar] [CrossRef]
- Seo, D.-J.; Lee, J.B.; Kim, Y.-J.; Cho, H.-R.; Kim, S.-Y.; Kim, G.-E.; Park, Y.-D.; Kim, G.-H.; An, J.-C.; Oh, K.; et al. The Characteristics of Hydrodeoxygenation of Biomass Pyrolysis Oil over Alumina-Supported NiMo Catalysts. Catalysts 2024, 15, 6. [Google Scholar] [CrossRef]
- Eller, Z.; Varga, Z.; Hancsók, J. Renewable Jet Fuel from Kerosene/Coconut Oil Mixtures with Catalytic Hydrogenation. Energy Fuels 2019, 33, 6444–6453. [Google Scholar] [CrossRef]
- Verma, V.; Mishra, A.; Anand, M.; Farooqui, S.A.; Sinha, A.K. Catalytic Hydroprocessing of Waste Cooking Oil for the Production of Drop-in Aviation Fuel and Optimization for Improving Jet Biofuel Quality in a Fixed Bed Reactor. Fuel 2023, 333, 126348. [Google Scholar]
- Şenol, O.İ.; Viljava, T.-R.; Krause, A.O.I. Hydrodeoxygenation of Aliphatic Esters on Sulphided NiMo/γ-Al2O3 and CoMo/γ-Al2O3 Catalyst: The Effect of Water. Catal. Today 2005, 106, 186–189. [Google Scholar] [CrossRef]
- Kaluža, L.; Soukup, K.; Koštejn, M.; Karban, J.; Palcheva, R.; Laube, M.; Gulková, D. On Stability of High-Surface-Area Al2O3, TiO2, SiO2-Al2O3, and Activated Carbon Supports during Preparation of NiMo Sulfide Catalysts for Parallel Deoxygenation of Octanoic Acid and Hydrodesulfurization of 1-Benzothiophene. Catalysts 2022, 12, 1559. [Google Scholar] [CrossRef]
- Bara, C.; Lamic-Humblot, A.-F.; Fonda, E.; Gay, A.-S.; Taleb, A.-L.; Devers, E.; Digne, M.; Pirngruber, G.D.; Carrier, X. Surface-Dependent Sulfidation and Orientation of MoS2 Slabs on Alumina-Supported Model Hydrodesulfurization Catalysts. J. Catal. 2016, 344, 591–605. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Tuxen, A.; Knudsen, K.G.; Brorson, M.; Topsøe, H.; Lægsgaard, E.; Lauritsen, J.V.; Besenbacher, F. Comparative atomic-scale analysis of promotional effects by late 3d-transition metals in MoS2 hydrotreating catalysts. J. Catal. 2010, 272, 195–203. [Google Scholar] [CrossRef]
- Lauritsen, J.V.; Kibsgaard, J.; Olesen, G.H.; Moses, P.G.; Hinnemann, B.; Helveg, S.; Nørskov, J.K.; Clausen, B.S.; Topsøe, H.; Lægsgaard, E.; et al. Location and coordination of promoter atoms in Co- and Ni-promoted MoS2-based hydrotreating catalyst. J. Catal. 2007, 249, 220–233. [Google Scholar] [CrossRef]
- Laurent, E.; Delmon, B. Influence of Water in the Deactivation of a Sulfided Catalyst during Hydrodeoxygenation. J. Catal. 1994, 146, 281–291. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Zhao, X.; Julson, J. Application, Deactivation, and Regeneration of Heterogeneous Catalysts in Bio-Oil Upgrading. Catalysts 2016, 6, 195. [Google Scholar] [CrossRef]
- He, Z.; Wang, X. Hydrodeoxygenation of Model Compounds and Catalytic Systems for Pyrolysis Bio-Oils Upgrading. Catal. Sustain. Energy 2012, 1, 28–52. [Google Scholar] [CrossRef]
- Kordouli, E.; Kordulis, C.; Lycourghiotis, A.; Cole, R.; Vasudevan, P.T.; Pawelec, B.; Fierro, J.L.G. HDO Activity of Carbon-Supported Rh, Ni and Mo-Ni Catalysts. Mol. Catal. 2017, 441, 209–220. [Google Scholar] [CrossRef]
- Mahene, W.L.; Kivevele, T.; Machunda, R. The Role of Textural Properties and Surface Chemistry of Activated Carbon Support in Catalytic Deoxygenation of Triglycerides into Renewable Diesel. Catal. Commun. 2023, 181, 106737. [Google Scholar] [CrossRef]
- Coumans, A.E.; Hensen, E.J.M. A Real Support Effect on the Hydrodeoxygenation of Methyl Oleate by Sulfided NiMo Catalysts. Catal. Today 2017, 298, 181–189. [Google Scholar] [CrossRef]
- de la Puente, G.; Gil, A.; Pis, J.J.; Grange, P. Effects of Support Surface Chemistry in Hydrodeoxygenation Reactions over CoMo/Activated Carbon Sulfided Catalysts. Langmuir 1999, 15, 5800–5806. [Google Scholar] [CrossRef]
- He, W.; Hu, A.; Qiu, L.; Wang, W.; Xiang, Y.; Han, W.; Xu, G.; Zhang, L.; Zheng, A. Insight into the Microstructure and Deactivation Effects on Commercial NiMo/γ-Al2O3 Catalyst through Aberration-Corrected Scanning Transmission Electron Microscopy. Catalysts 2019, 9, 810. [Google Scholar] [CrossRef]
- Busca, G. Acid Catalysts in Industrial Hydrocarbon Chemistry. Chem. Rev. 2007, 107, 5366–5410. [Google Scholar] [CrossRef] [PubMed]
- Prauchner, M.J.; Sapag, K.; Rodríguez-Reinoso, F. Tailoring Biomass-Based Activated Carbon for CH4 Storage by Combining Chemical Activation with H3PO4 or ZnCl2 and Physical Activation with CO2. Carbon 2016, 110, 138–147. [Google Scholar] [CrossRef]
- Prauchner, M.J.; Oliveira, S.d.C.; Rodríguez-Reinoso, F. Tailoring Low-Cost Granular Activated Carbons Intended for CO2 Adsorption. Front. Chem. 2020, 8, 581133. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.C.; Dutra, R.C.; León, J.J.L.; Martins, G.A.V.; Silva, A.M.A.; Azevedo, D.C.S.; Santiago, R.G.; Ballesteros Plata, D.; Rodríguez-Castellón, E.; Prauchner, M.J. Activated Carbon Ammonization: Effects of the Chemical Composition of the Starting Material and the Treatment Temperature. C 2025, 11, 15. [Google Scholar] [CrossRef]
- van Veen, J.A.R.; Gerkema, E.; van der Kraan, A.M.; Knoester, A. A Real Support Effect on the Activity of Fully Sulphided CoMoS for the Hydrodesulphurization of Thiophene. J. Chem. Soc. Chem. Commun. 1987, 22, 1684. [Google Scholar] [CrossRef]
- Topsøe, H.; Clausen, B.S. Active Sites and Support Effects in Hydrodesulfurization Catalysts. Appl. Catal. 1986, 25, 273–293. [Google Scholar] [CrossRef]
- Dugulan, A.I.; van Veen, J.A.R.; Hensen, E.J.M. On the Structure and Hydrotreating Performance of Carbon-Supported CoMo- and NiMo-Sulfides. Appl. Catal. B 2013, 142–143, 178–186. [Google Scholar] [CrossRef]
- Tan, Z.; Xiao, H.; Zhang, R.; Zhang, Z.-S.; Kaliaguine, S. Potential to Use Mesoporous Carbon as Catalyst Support for Hydrodesulfurization. New Carbon Mater. 2009, 24, 333–343. [Google Scholar] [CrossRef]
- Rambabu, N.; Badoga, S.; Soni, K.K.; Dalai, A.K.; Adjaye, J. Hydrotreating of Light Gas Oil Using a NiMo Catalyst Supported on Activated Carbon Produced from Fluid Petroleum Coke. Front. Chem. Sci. Eng. 2014, 8, 161–170. [Google Scholar] [CrossRef]
- Kouzu, M.; Kuriki, Y.; Hamdy, F.; Sakanishi, K.; Sugimoto, Y.; Saito, I. Catalytic Potential of Carbon-Supported NiMo-Sulfide for Ultra-Deep Hydrodesulfurization of Diesel Fuel. Appl. Catal. A. 2004, 265, 61–67. [Google Scholar] [CrossRef]
- Mukundan, S.; Konarova, M.; Atanda, L.; Ma, Q.; Beltramini, J. Guaiacol Hydrodeoxygenation Reaction Catalyzed by Highly Dispersed, Single Layered MoS2/C. Catal. Sci. Technol. 2015, 5, 4422–4432. [Google Scholar] [CrossRef]
- Ospina, V.; Buitrago, R.; Lopez, D.P. HDO Del Guaiacol Mediante El Uso de Catalizadores NiMo Soportados Sobre Carbón Activado Obtenido a Partir de La Torta de Higuerilla. Ing. Investig. 2015, 35, 49–55. [Google Scholar]
- Tapia, J.; Acelas, N.Y.; López, D.; Moreno, A. NiMo-Sulfide Supported on Activated Carbon to Produce Renewable Diesel. Univ. Sci. 2017, 22, 71. [Google Scholar]
- Ferreira, K.K.; Ribeiro, L.S.; Pereira, M.F.R. Analysis of Reaction Conditions in Palmitic Acid Deoxygenation for Fuel Production. Catalysts 2024, 14, 853. [Google Scholar] [CrossRef]
- Prauchner, M.J.; Rodríguez-Reinoso, F. Chemical versus Physical Activation of Coconut Shell: A Comparative Study. Microporous Mesoporous Mat. 2012, 152, 163–171. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar]
- Biesinger, M.C. Accessing the Robustness of Adventitious Carbon for Charge Referencing (Correction) Purposes in XPS Analysis: Insights from a Multi-User Facility Data Review. Appl. Surf. Sci. 2022, 597, 153681. [Google Scholar] [CrossRef]
- López-Cruz, C.; Guzman, J.; Cao, G.; Martínez, C.; Corma, A. Modifying the catalytic properties of hydrotreating NiMo–S phases by changing the electrodonor capacity of the support. Catal. Today 2021, 382, 130–141. [Google Scholar]
- Afanasiev, P. Calculation of MoS2 slabs morphology descriptors from transmission electron microscopy data revisited. Case study of the influence of citric acid and treatment conditions on the properties of MoS2/Al2O3. Appl. Catal. A Gen. 2017, 529, 10–19. [Google Scholar] [CrossRef]
- Rathnasamy, R.; Thangamuthu, R.; Alagan, V. Sheet-like Orthorhombic MoO3 Nanostructures Prepared via Hydrothermal Approach for Visible-Light-Driven Photocatalytic Application. Res. Chem. Intermed. 2018, 44, 1647–1660. [Google Scholar] [CrossRef]
- Alam, U.; Kumar, S.; Bahnemann, D.; Koch, J.; Tegenkamp, C.; Muneer, M. Harvesting Visible Light with MoO3 Nanorods Modified by Fe(iii) Nanoclusters for Effective Photocatalytic Degradation of Organic Pollutants. Phys. Chem. Chem. Phys. 2018, 20, 4538–4545. [Google Scholar] [CrossRef]
- Liu, H.; Hu, H.; Wang, J.; Niehoff, P.; He, X.; Paillard, E.; Eder, D.; Winter, M.; Li, J. Hierarchical Ternary MoO2/MoS2/Heteroatom-Doped Carbon Hybrid Materials for High-Performance Lithium-Ion Storage. ChemElectroChem 2016, 3, 922–932. [Google Scholar] [CrossRef]
- Samaniego-Benitez, J.E.; Mendoza-Cruz, R.; Bazán-Díaz, L.; Garcia-Garcia, A.; Arellano-Jimenez, M.J.; Perez-Robles, J.F.; Plascencia-Villa, G.; Velázquez-Salazar, J.J.; Ortega, E.; Favela-Camacho, S.E.; et al. Synthesis and Structural Characterization of MoS2 Micropyramids. J. Mater. Sci. 2020, 55, 12203–12213. [Google Scholar] [CrossRef]
- Zhang, X.; Ke, Y.; Wang, T.; Cai, J.; Huang, Q.; Lin, S. Green Synthesis of Flowerball-like MoS2/VC Nanocomposite and Its Efficient Catalytic Performance for Oxygen Reduction Either in Alkaline or Acid Media. Catalysts 2022, 12, 259. [Google Scholar] [CrossRef]
- Basha, I.K.; Abd El-Monaem, E.M.; Khalifa, R.E.; Omer, A.M.; Eltaweil, A.S. Sulfonated Graphene Oxide Impregnated Cellulose Acetate Floated Beads for Adsorption of Methylene Blue Dye: Optimization Using Response Surface Methodology. Sci. Rep. 2022, 12, 9339. [Google Scholar] [CrossRef]
- Chorkendorff, I.; Niemantsverdriet, J.W. Concepts of Modern Catalysis and Kinetics; Wiley: Weinheim, Germany, 2003. [Google Scholar]
- Byskov, L.S.; Hammer, B.; Nørskov, J.K.; Clausen, B.S.; Topsøe, H. Sulfur Bonding in MoS2 and Co-Mo-S Structures. Catal. Lett. 1997, 47, 177–182. [Google Scholar] [CrossRef]
- Romero, Y.; Richard, F.; Brunet, S. Hydrodeoxygenation of 2-Ethylphenol as a Model Compound of Bio-Crude over Sulfided Mo-Based Catalysts: Promoting Effect and Reaction Mechanism. Appl. Catal. B 2010, 98, 213–223. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, M.; Bartling, S.; Lund, H.; Atia, H.; Dyson, P.J.; Beller, M.; Jagadeesh, R.V. A general and robust Ni-based nanocatalyst for selective hydrogenation reactions at low temperature and pressure. Sci. Adv. 2023, 9, eadj8225. [Google Scholar] [CrossRef]
- Stoffels, M.A.; Klauck, F.J.R.; Hamadi, T.; Glorius, F.; Leker, J. Technology Trends of Catalysts in Hydrogenation Reactions: A Patent Landscape Analysis. Adv. Synth. Catal. 2020, 362, 1258–1274. [Google Scholar] [CrossRef]
- Prauchner, M.J.; Brandão, R.D.; de Freitas Júnior, A.M.; da, C. Oliveira, S. Combustíveis Derivados do Petróleo: Obtenção, Propriedades e Usos. Rev. Virtual Química 2023, 15, 43–60. [Google Scholar]
- Lokesh, K.; Sethi, V.; Nikolaidis, T.; Goodger, E.; Nalianda, D. Life Cycle Greenhouse Gas Analysis of Biojet Fuels with a Technical Investigation into Their Impact on Jet Engine Performance. Biomass Bioenergy 2015, 77, 26–44. [Google Scholar] [CrossRef]
- Ojagh, H.; Creaser, D.; Tamm, S.; Arora, P.; Nyström, S.; Lind Grennfelt, E.; Olsson, L. Effect of Dimethyl Disulfide on Activity of NiMo Based Catalysts Used in Hydrodeoxygenation of Oleic Acid. Ind. Eng. Chem. Res. 2017, 56, 5547–5557. [Google Scholar]
- Bykova, E.S.; Nadeina, K.A.; Vatutina, Y.V.; Chesalov, Y.A.; Pakharukova, V.P.; Larina, T.V.; Prosvirin, I.P.; Gerasimov, E.Y.; Klimov, O.V.; Noskov, A.S. The Impact of the Water Phase in the Gasoil Fraction on the CoMo Hydrotreating Catalyst’s Performance. Fuel 2024, 365, 131229. [Google Scholar]
- Gong, S.; Shinozaki, A.; Shi, M.; Qian, E.W. Hydrotreating of Jatropha Oil over Alumina Based Catalysts. Energy Fuels 2012, 26, 2394–2399. [Google Scholar] [CrossRef]
- Kubička, D.; Horáček, J. Deactivation of HDS Catalysts in Deoxygenation of Vegetable Oils. Appl. Catal. A 2011, 394, 9–17. [Google Scholar]
- Bezergianni, S.; Dimitriadis, A.; Kalogianni, A.; Pilavachi, P.A. Hydrotreating of Waste Cooking Oil for Biodiesel Production. Part I: Effect of Temperature on Product Yields and Heteroatom Removal. Bioresour. Technol. 2010, 101, 6651–6656. [Google Scholar] [PubMed]
- Aiamsiri, P.; Tumnantong, D.; Yoosuk, B.; Ngamcharussrivichai, C.; Prasassarakich, P. Biohydrogenated Diesel from Palm Oil Deoxygenation over Unsupported and γ-Al2O3 Supported Ni–Mo Catalysts. Energy Fuels 2021, 35, 14793–14804. [Google Scholar]
- Žula, M.; Grilc, M.; Likozar, B. Hydrocracking, Hydrogenation and Hydro-Deoxygenation of Fatty Acids, Esters and Glycerides: Mechanisms, Kinetics and Transport Phenomena. Chem. Eng. J. 2022, 444, 136564. [Google Scholar]
- Rocha, R.P.; Pereira, M.F.R.; Figueiredo, J.L. Characterisation of the Surface Chemistry of Carbon Materials by Temperature-Programmed Desorption: An Assessment. Catal. Today 2023, 418, 114136. [Google Scholar]
- Boehm, H.P. Surface Oxides on Carbon and Their Analysis: A Critical Assessment. Carbon 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Wu, H.; Lu, W.; Chen, Y.; Zhang, P.; Cheng, X. Application of Boehm Titration for the Quantitative Measurement of Soot Oxygen Functional Groups. Energy Fuels 2020, 34, 7363–7372. [Google Scholar]
- Juan, Z.; Kaixuan, F.; Pingping, W.; Yue, Z.; Yongke, Z. Enhancement of the Adsorption of Bilirubin on Activated Carbon via Modification. Results Mater. 2021, 9, 100172. [Google Scholar] [CrossRef]
- Schönherr, J.; Buchheim, J.R.; Scholz, P.; Adelhelm, P. Boehm Titration Revisited (Part II): A Comparison of Boehm Titration with Other Analytical Techniques on the Quantification of Oxygen-Containing Surface Groups for a Variety of Carbon Materials. C 2018, 4, 22. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).