Hydrogen Responses of Propylene Polymerization with MgCl2-Supported Ziegler–Natta Catalysts in the Presence of Different Silane External Donors
Abstract
1. Introduction
2. Results and Discussion
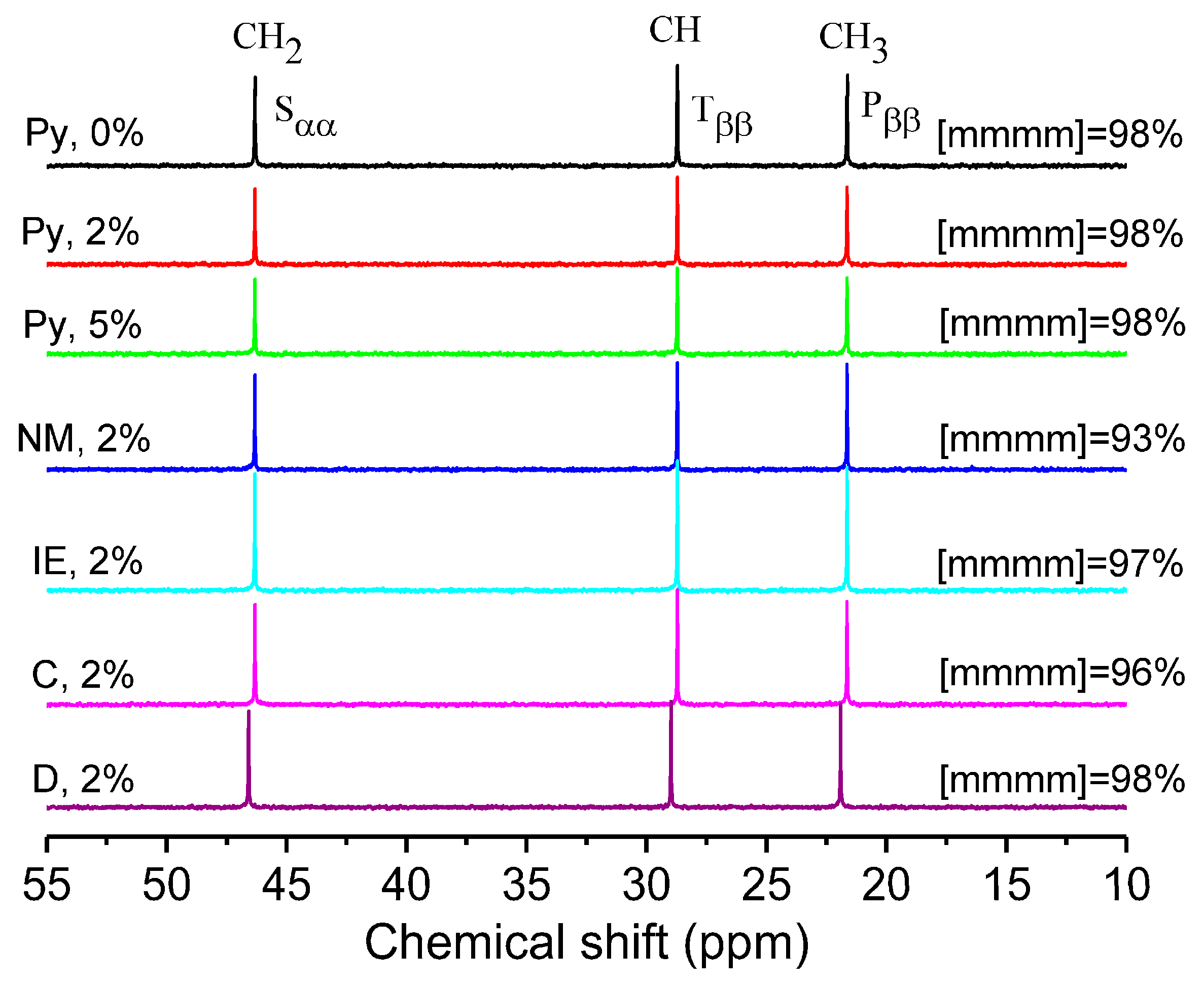
2.1. Polymerization Activity and Chain Structure of the PP Products
2.2. Active Center Distribution and Reactivity of Different Active Centers
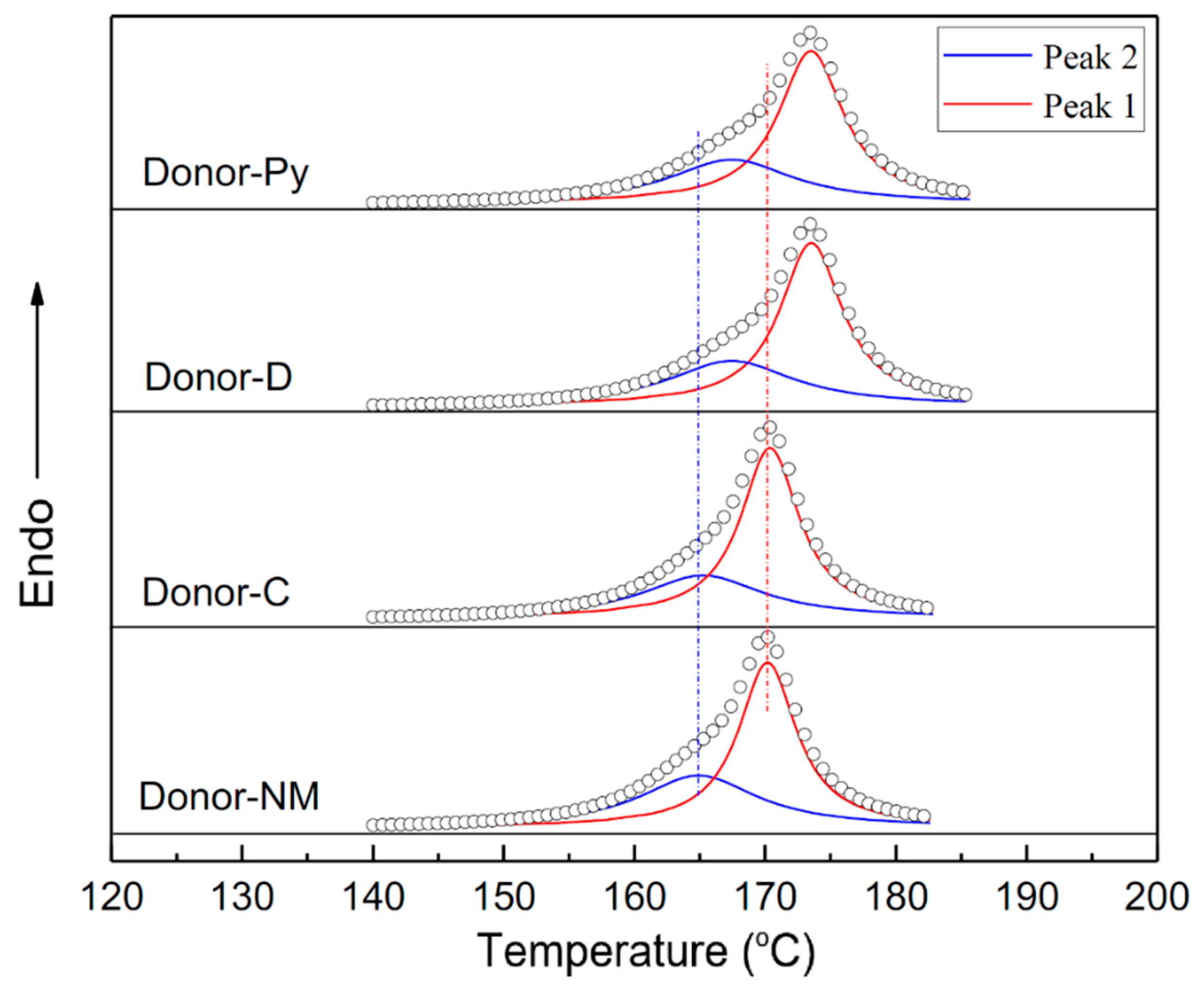
2.3. Further Characterization of the Isotactic Fraction of PP Products
2.4. Discussions on Mechanism of the External Donor and Hydrogen Effects
3. Experimental Section
3.1. Materials
3.2. Synthesis of Dipiperidyldimethoxysilane (Donor-Py)
3.3. Propylene Polymerization
3.4. Polymer Fractionation
3.5. Measurement of the Molecular Weight
3.6. Sulfur Content Determination
3.7. Thermal Analysis
3.7.1. Differential Scanning Calorimetry (DSC)
3.7.2. Successive Self-Nucleation and Annealing (SSA)
3.8. Nuclear Magnetic Resonance (NMR)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiao, J.L.; Guo, M.F.; Wang, L.S.; Liu, D.B.; Zhang, X.F.; Yu, L.Q.; Song, W.B.; Liu, Y.Q. Recent Advances in Polyolefin Technology. Polym. Chem. 2011, 2, 1611. [Google Scholar] [CrossRef]
- Zhang, X.F.; Xie, F.; Pen, Z.L.; Zhang, Y.; Zhang, Y.X.; Zhou, W. Effect of Nucleating Agent on the Structure and Properties of Polypropylene/Poly(ethylene-octene) Blends. Eur. Polym. J. 2002, 38, 1–6. [Google Scholar] [CrossRef]
- Tang, W.H.; Tang, J.; Yuan, H.L.; Jin, R.G. Crystallization Behavior and Mechanical Properties of Polypropylene Random Copolymer/Poly(ethylene-octene) Blends. J. Appl. Polym. Sci. 2011, 122, 461–468. [Google Scholar]
- Song, S.J.; Feng, J.C.; Wu, P.Y.; Yang, Y.L. Shear-Enhanced Crystallization in Impact-Resistant Polypropylene Copolymer: Influence of Compositional Heterogeneity and Phase Structure. Macromolecules 2009, 42, 7067–7078. [Google Scholar]
- Salakhov, I.I.; Bukatov, G.D.; Batyrshin, A.Z.; Matsko, M.A.; Barabanov, A.A.; Temnikova, E.V.; Shaidullin, N.M. Polypropylene Synthesis in Liquid Monomer with Titanium-Magnesium Catalyst: Effect of Different Alkoxysilanes as External Donors. J. Polym. Res. 2019, 26, 126. [Google Scholar]
- Chadwick, J.C. Polyolefins-Catalyst and Process Innovations and Their Impact on Polymer Properties. Macromol. React. Eng. 2009, 3, 428–432. [Google Scholar] [CrossRef]
- Weng, Y.H.; Jiang, B.Y.; Fu, Z.S.; Fan, Z.Q. Mechanism of Internal and External Electron Donor Effects on Propylene Polymerization with MgCl2-supported Ziegler-Natta Catalyst: New Evidences Based on Active Center Counting. J. Appl. Polym. Sci. 2018, 135, 46605. [Google Scholar] [CrossRef]
- Chang, H.F.; Li, H.Y.; Zheng, T.; Zhang, L.Y.; Yuan, W.; Li, L.; Huang, H.; Hu, Y.L. Characterization of the Effects of the C/N Mixed External Donors on the Stereo-Defects Distribution of Polypropylene by Successive Self-Nucleating and Annealing and 13C-NMR Techniques. J. Polym. Res. 2013, 20, 207. [Google Scholar]
- Chadwick, J.C.; Morini, G.; Balbontin, G.; Mingozzi, I.; Albizzati, E. Propene Polymerization with MgCl2-supported Catalysts: Effects of Using a Diether as External Donor. Macromol. Chem. Phys. 1997, 198, 1181–1188. [Google Scholar] [CrossRef]
- Moballegh, L.; Hakim, S.; Morshedian, J.; Nekoomanesh, M. Investigating Effects of Using Mixtures of Two External Electron Donors on Microstructure and Properties of Polypropylene/Poly(ethylene-co-propylene) in-reactor Blends Based on Ziegler-Natta Catalyst. Macromol. React. Eng. 2015, 9, 350–359. [Google Scholar]
- Fan, Z.Q.; Forlini, F.; Tritto, I.; Locatelli, P.; Sacchi, M.C. Effect of Ethoxy- and Methoxysilane Donors in Propene/1-hexene Copolymerization with High-yield Supported Ziegler-Natta Catalysts. Macromol. Chem. Phys. 1994, 195, 3889–3899. [Google Scholar]
- Sacchi, M.C.; Forlini, F.; Tritto, I.; Locatelli, P.; Morini, G.; Noristi, L.; Albizzati, E. Polymerization Stereochemistry with Ziegler-Natta Catalysts Containing Dialkylpropane Diethers: A Tool for Understanding Internal/External Donor Relationships. Macromolecules 1996, 29, 3341–3345. [Google Scholar]
- Kang, K.K.; Shiono, T.; Jeong, Y.T.; Lee, D.H. Polymerization of Propylene by Using Mg(OEt)2-DNBP-TiCl4 Catalyst with Alkoxy Disilanes as External Donor. J. Appl. Polym. Sci. 1999, 71, 293–301. [Google Scholar]
- Wang, Q.; Murayama, N.; Liu, B.P.; Terano, M. Effects of Electron Donors on Active Sites Distribution of MgCl2-Supported Ziegler-Natta Catalysts Investigated by Multiple Active Sites Model. Macromol. Chem. Phys. 2005, 206, 961–966. [Google Scholar]
- Correa, A.; Piemontesi, F.; Morini, G.; Cavallo, L. Key Elements in the Structure and Function Relationship of the MgCl2/TiCl4/Lewis Base Ziegler-Natta Catalytic System. Macromolecules 2007, 40, 9181–9189. [Google Scholar]
- Shen, X.R.; Fu, Z.S.; Hu, J.; Wang, Q.; Fan, Z.Q. Mechanism of Propylene Polymerization with MgCl2-Supported Ziegler-Natta Catalysts Based on Counting of Active Centers: The Role of External Electron Donor. J. Phys. Chem. C 2013, 117, 15174–15182. [Google Scholar]
- Fu, Z.S.; Tu, S.T.; Fan, Z.Q. Effect of the Combined External Electron Donors on the Structure and Properties of Polypropylene/Poly(ethylene-co-propylene) In-Reactor Alloys Prepared by High-Efficiency Industrial Ziegler-Natta Catalyst. Ind. Eng. Chem. Res. 2013, 52, 5887–5894. [Google Scholar]
- Vittoria, A.; Meppelder, A.; Friederichs, N.; Busico, V.; Cipullo, R. Demystifying Ziegler-Natta Catalysts: The Origin of Stereoselectivity. ACS Catal. 2017, 7, 4509. [Google Scholar]
- Sacchi, M.C.; Fan, Z.Q.; Forlini, F.; Tritto, I.; Locatelli, P. Use of Different Alkoxysilanes as External Donors in MgCl2-supported Ziegler-Natta Catalysts to Obtain Propene/l-butene Copolymers with Different Microstructure. Macromol. Chem. Phys. 1994, 195, 2805–2816. [Google Scholar]
- Busico, V.; Cipullo, R.; Monaco, G.; Talarico, G.; Vacatello, M.; Chadwick, J.C.; Segre, A.L.; Sudmeijer, O. High-Resolution 13C NMR Configurational Analysis of Polypropylene Made with MgCl2-supported Ziegler-Natta Catalysts. 1. The “Model” System MgCl2/TiCl4-2,6-Dimethylpyridine/Al(C2H5)3. Macromolecules 1999, 32, 4173–4182. [Google Scholar]
- Chang, H.F.; Li, H.Y.; Zheng, T.; Zhou, Q.; Zhang, L.Y.; Hu, Y.L. The Effects of New Aminosilane Compounds as External Donors on Propylene Polymerization. J. Polym. Res. 2014, 21, 554. [Google Scholar]
- Chadwick, J.C.; Morini, G.; Balbontin, G.; Camurati, I.; Heere, J.J.R.; Mingozzi, I.; Testoni, F. Effects of Internal and External Donors on the Regio-and Stereoselectivity of Active Species in MgCl2-supported Catalysts for Propene Polymerization. Macromol. Chem. Phys. 2001, 202, 1995–2002. [Google Scholar]
- Zhang, H.X.; Lee, Y.J.; Park, J.R.; Lee, D.H.; Yoon, K.B. Control of Molecular Weight Distribution for Polypropylene Obtained by Commercial Ziegler-Natta Catalyst: Effect of Electron Donor. Macromol. Res. 2011, 19, 622–628. [Google Scholar]
- Chang, H.F.; Ren, S.T.; Dang, X.F.; Zhang, L.Y.; Li, H.Y.; Hu, Y.L. The Effect of the Mixed External Donors on the Sequence Length Distribution of Polypropylene. J. Appl. Polym. Sci. 2013, 129, 1026–1035. [Google Scholar]
- Kang, J.; Yang, F.; Wu, T.; Li, H.L.; Cao, Y.; Xiang, M. Polymerization Control and Fast Characterization of the Stereo-Defect Distribution of Heterogeneous Ziegler-Natta Isotactic Polypropylene. Eur. Polym. J. 2012, 48, 425–434. [Google Scholar]
- Gnanakumar, E.S.; Chokkapu, E.R.; Kunjir, S.; Ajithkumar, T.G.; Rajamohanan, P.R.; Chakraborty, D.; Gopinath, C.S. 9-Fluorenemethanol: An internal electron donor to fine tune olefin polymerization activity. Dalton Trans. 2014, 43, 9143–9151. [Google Scholar]
- Ratanasak, M.; Parasuk, V. Roles of Malonate Donor on Activity and Stereoselectivity of Ziegler-Natta Catalyzed Propylene Polymerization. J. Organomet. Chem. 2015, 775, 6–11. [Google Scholar]
- Zhou, Q.; Zheng, T.; Li, H.Y.; Luo, Z.; Wang, A.L.; Zhang, L.Y.; Hu, Y.L. Microstructure of polypropylene and active center in Ziegler-Natta catalyst: Effect of novel salicylate internal donor. RSC Adv. 2016, 6, 75023–75031. [Google Scholar]
- Nissinen, V.H.; Linnolahti, M.; Bazhenov, A.S.; Pakkanen, T.T.; Pakkanen, T.A.; Deni, P.; Leinonen, T.; Jayaratne, K.; Pakkanen, A. Polyethylenimines: Multidentate Electron Donors for Ziegler-Natta Catalysts. J. Phys. Chem. C 2013, 121, 23413–23421. [Google Scholar]
- Zhou, Q.; Xu, H.; Wang, A.L.; Ma, Z.F.; Li, H.Y.; Zhang, L.Y.; Hu, Y.L. Polypropylene with High Melt Flow Rate and High Isotacticity Prepared by Phosphate-Mixed External Donors. J. Appl. Polym. Sci. 2017, 134, 44704. [Google Scholar]
- Shigeru, I.; Hiroyuki, I.; Hiroshi, S.; Tokuji, I.; Hideo, I. Process for Polymerization of Alpha-Olefin, a Poly-Alpha-Olefin Prepared Thereby, and an Aminosilane Compound Usable as the Constituent of Catalyst for the Process. EP0841348 A2, 13 May 1998. [Google Scholar]
- Ikeuchi, H.; Yano, T.; Ikai, S.; Sato, H.; Yamashita, J. Study on Aminosilane Compounds as External Electron Donors in Isospecific Propylene Polymerization. J. Mol. Catal. A Chem. 2003, 193, 207–215. [Google Scholar]
- Chang, H.F.; Ren, S.T.; Zheng, T.; Dang, X.F.; Yang, Y.; Zhang, L.Y.; Li, H.Y. Studies on the Influence of Different Substitued Groups of the External Donors on Propylene Proymerization. Acta Polym. Sin. 2013, 2, 199–207. [Google Scholar]
- Alshaiban, A.; Soares, J.B.P. Effect of Varying Hydrogen Concentration, External Donor Concentration, and Temperature on Propylene Polymerization Kinetics and Microstructure of Polypropylene Made with a 4th Generation Ziegler-Natta Catalyst. Macromol. React. Eng. 2014, 8, 723–735. [Google Scholar]
- Zhang, B.; Fu, Z.; Fan, Z.; Phiriyawirut, P.; Charoenchaidet, S. Preparation and Characterization of High MFR Polypropylene and Polypropylene/Poly(ethylene-co-propylene) In-reactor Alloys. J. Appl. Polym. Sci. 2016, 133, 42984. [Google Scholar]
- Shen, X.R.; Hu, J.; Fu, Z.S.; Lou, J.Q.; Fan, Z.Q. Counting the Number of Active Centers in MgCl2-Supported Ziegler-Natta Catalysts by Quenching with 2-Thiophenecarbonyl Chloride and Study on the Initial Kinetics of Propylene Polymerization. Catal. Commun. 2013, 30, 66–69. [Google Scholar]
- Hu, J.; Shen, X.; Fu, Z.; Wang, Q.; Fan, Z. Mechanistic study on hydrogen effects in propylene polymerization with MgCl2-supported Ziegler-Natta catalysts: New evidences from active center counting and reaction kinetics. Appl. Catal. A Gen. 2023, 667, 119460. [Google Scholar]
- Busico, V.; Cipullo, R.; Corradini, P. Ziegler-Natta Oligomerization of 1-alkenes: A Catalyst’s “Fingerprint”, 1. Hydrooligomerization of Propene in the Presence of a Highly Isospecific MgCl2-supported Catalyst. Makromol. Chem. 1993, 194, 1079–1093. [Google Scholar]
- Busico, V.; Chadwick, J.C.; Cipullo, R.; Ronca, S.; Talarico, G. Propene/Ethene-[1-13C] Copolymerization as a Tool for Investigating Catalyst Regioselectivity. MgCl2/Internal Donor/TiCl4–External Donor/AlR3 Systems. Macromolecules 2004, 37, 7437–7443. [Google Scholar]
- Shen, X.R.; Fu, Z.S.; Fan, Z.Q. Effects of Hydrogen on Active Centers in Propylene Polymerization with Supported Ziegler-Natta Catalyst. Acta Polym. Sin. 2013, 12, 1537–1544. [Google Scholar]
- Kouzai, I.; Wada, T.; Taniike, T.; Terano, M. Hydrogen Effects for Propylene Polymerization with Ultra Low TiCl3 Loading MgCl2-supported Catalyst. Macromol. Symp. 2007, 260, 179–183. [Google Scholar]
- Jiang, B.Y.; He, F.; Yang, P.J.; Zhang, Z.; Weng, Y.H.; Cheng, Z.M.; Fu, Z.S.; Fan, Z.Q. Enhancing Stereoselectivity of Propylene Polymerization with MgCl2-Supported Ziegler-Natta Catalysts by Electron Donor: Strong Effects of Titanium Dispersion State. Catal. Commun. 2019, 121, 38–42. [Google Scholar] [CrossRef]
- Nishiyama, I.; Liu, B.P.; Matsuoka, H.; Nakatani, H.; Terano, M. Kinetic Evaluation of Various Isospecific Active Sites on MgCl2-Supported Ziegler Catalysts. Macromol. Symp. 2003, 193, 71–80. [Google Scholar] [CrossRef]
- Bukatov, G.D.; Zakharov, V.A.; Barabanov, A.A. Mechanism of Olefin Polymerization on Supported Ziegler-Natta Catalysts Based on Data on the Number of Active Centers and Propagation Rate Constants. Kinet. Catal. 2005, 46, 166–176. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, B.Y.; Zhang, B.; Fu, Z.S.; Fan, Z.Q. Deactivation Effect Caused by Catalyst-Cocatalyst Pre-Contact in Propylene Polymerization with MgCl2-Supported Ziegler-Natta Catalyst. Chin. J. Polym. Sci. 2019, 37, 1023–1030. [Google Scholar] [CrossRef]
- Dong, Q.; Fan, Z.Q.; Fu, Z.S.; Xu, J.T. Fractionation and Characterization of an Ethylene-Propylene Copolymer Produced with a MgCl2/SiO2/TiCl4/Diester-type Ziegler-Natta Catalyst. J. Appl. Polym. Sci. 2008, 107, 1301–1309. [Google Scholar] [CrossRef]
- Tu, S.T.; Fu, Z.S.; Fan, Z.Q. Influence of Cocatalyst on the Structure and Properties of Polypropylene/Poly (ethylene-co-propylene) in-reactor Alloys Prepared by MgCl2/TiCl4/Diester Type Ziegler-Natta Catalyst. J. Appl. Polym. Sci. 2011, 124, 5154–5164. [Google Scholar] [CrossRef]
- Biao Zhang, B.; Qingyun Qian, Q.; Pengjia Yang, P.; Baiyu Jiang, B.; Zhisheng Fu, Z.; Fan, Z. Responses of a Supported Ziegler–Natta Catalyst to Comonomer Feed Ratios in Ethylene–Propylene Copolymerization: Differentiation of Active Centers with Different Catalytic Features. Ind. Eng. Chem. Res. 2021, 60, 4575–4588. [Google Scholar] [CrossRef]
- Müller, A.J.; Hernández, Z.H.; Arnal, M.L.; Sánchez, J.J. Successive Self-Nucleation/Annealing (SSA): A Novel Technique to Study Molecular Segregation during Crystallization. Polym. Bull. 1997, 39, 465–472. [Google Scholar] [CrossRef]
- Müller, A.J.; Michell, R.M.; Pérez, R.A.; Lorenzo, A.T. Successive Self-Nucleation and Annealing (SSA): Correct Design of Thermal Protocol and Applications. Eur. Polym. J. 2015, 65, 132–154. [Google Scholar] [CrossRef]
- Chang, H.F.; Zhang, Y.; Ren, S.T.; Dang, X.F.; Zhang, L.Y.; Li, H.Y.; Hu, Y.L. Study on the Sequence Length Distribution of Polypropylene by the Successive Self-Nucleation and Annealing (SSA) Calorimetric Technique. Polym. Chem. 2012, 3, 2909–2919. [Google Scholar] [CrossRef]
- Kang, J.; Yang, F.; Wu, T.; Li, H.L.; Liu, D.M.; Cao, Y.; Xiang, M. Investigation of the Stereodefect Distribution and Conformational Behavior of Isotactic Polypropylene Polymerized with Different Ziegler-Natta Catalysts. J. Appl. Polym. Sci. 2012, 125, 3076–3083. [Google Scholar] [CrossRef]
- Busico, V.; Cipullo, R.; Monaco, G.; Vacatello, M.; Bella, J.; Segre, A.L. Full Assignment of the 13C NMR Spectra of Regioregular Polypropylenes: Methine Region. Macromolecules 1998, 31, 8713–8719. [Google Scholar]
- Chadwick, J.C. Advances in Propene Polymerization Using MgCl2-Supported Catalyst. Fundamental Aspects and the Role of Electron Donors. Macromol. Symp. 2001, 173, 21–36. [Google Scholar]
- Sacchi, M.C.; Tritto, I.; Locatelli, P. Stereochemical Investigation of the Effect of Lewis Bases in Heterogeneous Ziegler-Natta Initiator Systems. Prog. Polym. Sci. 1991, 16, 331–360. [Google Scholar] [CrossRef]
- Fan, Z.; Zhang, L.; Fu, Z. Effects of Ethylene as Comonomer on the Active Center Distribution of 1-Hexene Polymerization with MgCl2-supported Ziegler-Natta Catalysts. J. Mol. Catal. A 2011, 351, 93–99. [Google Scholar] [CrossRef]
- Kissin, Y.V.; Rishina, L.A. Hydrogen effects in propylene polymerization reactions with titanium-based Ziegler-Natta catalysts. I. Chemical mechanism of catalyst activation. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 1353–1365. [Google Scholar]

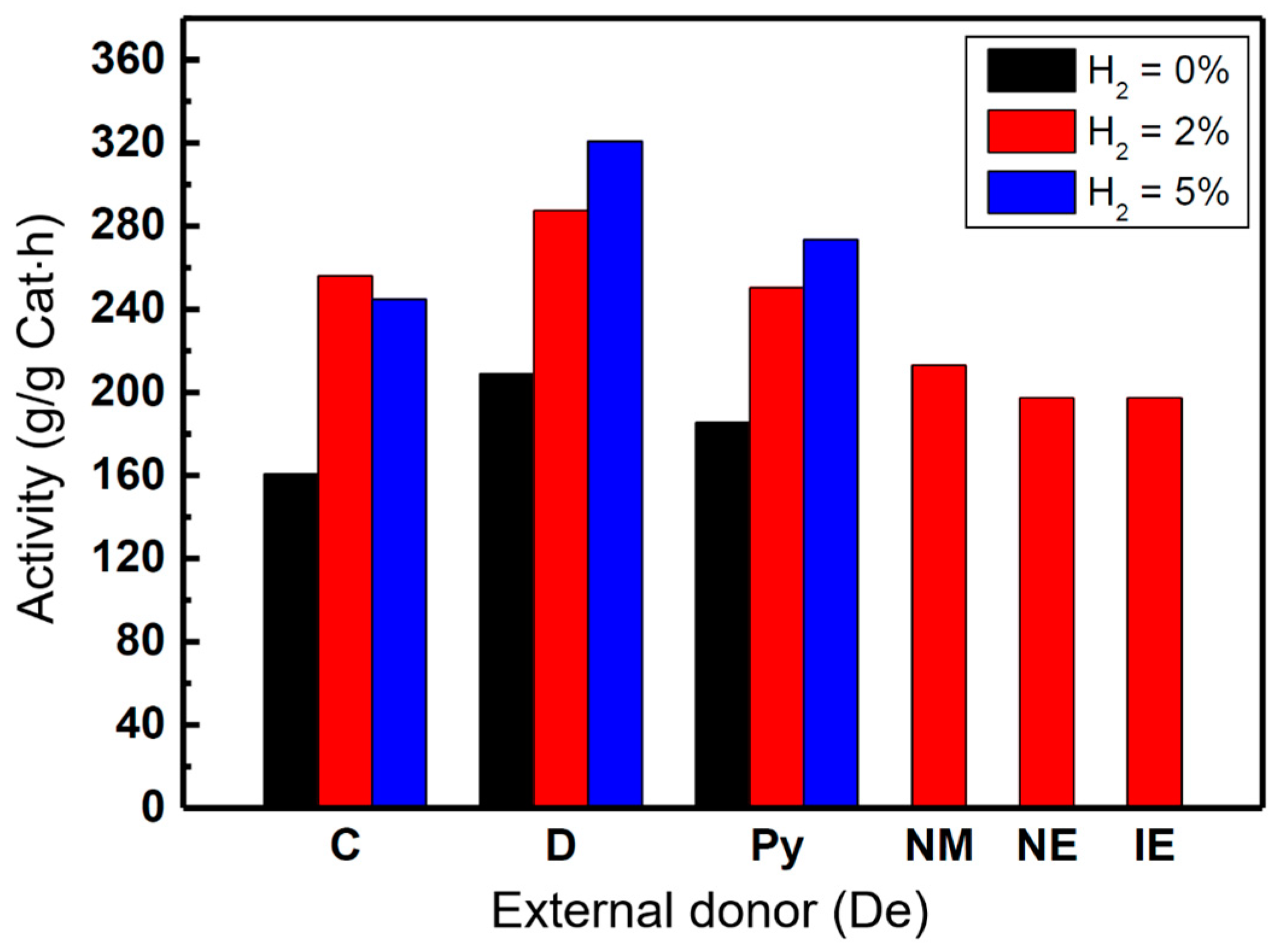
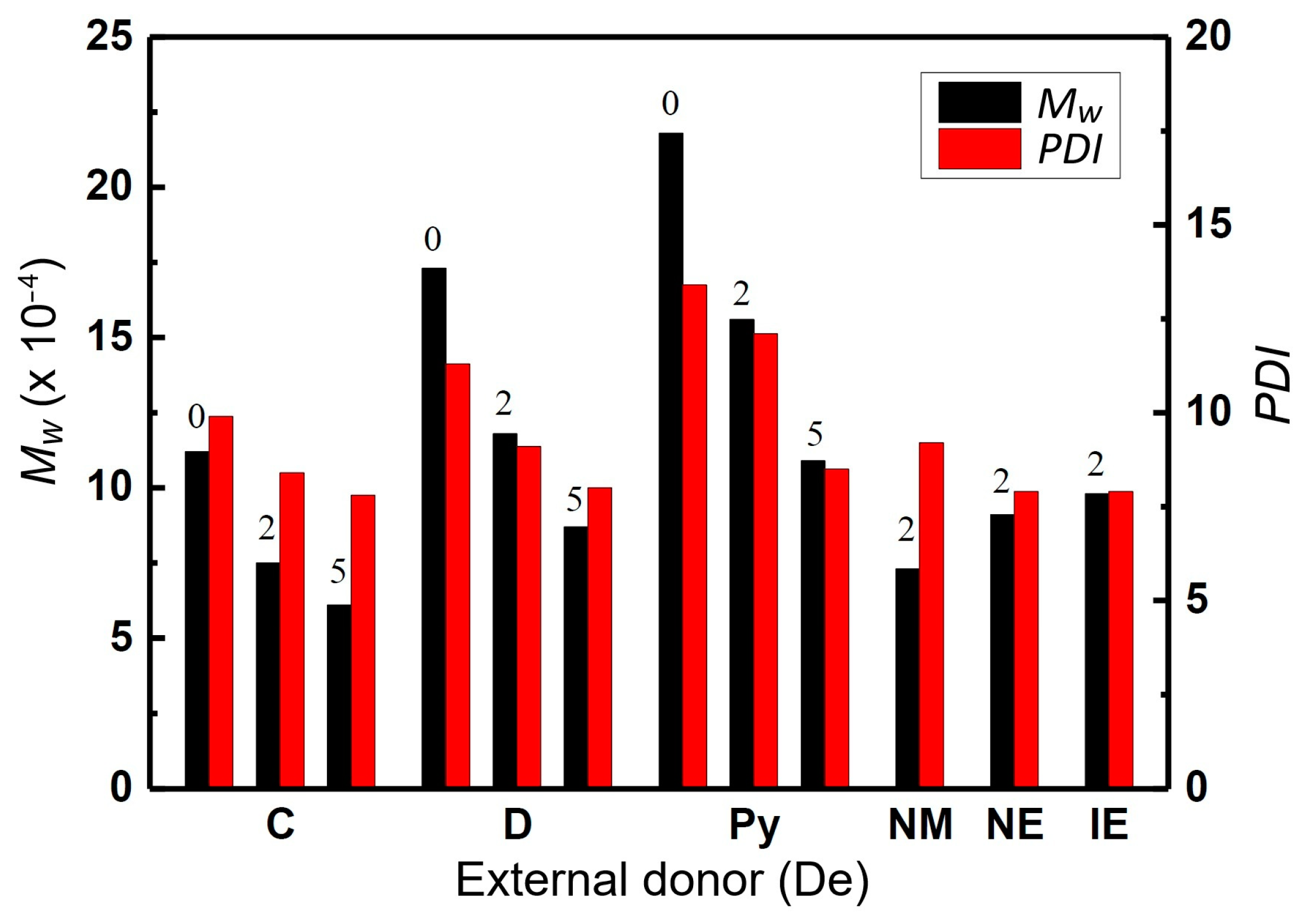
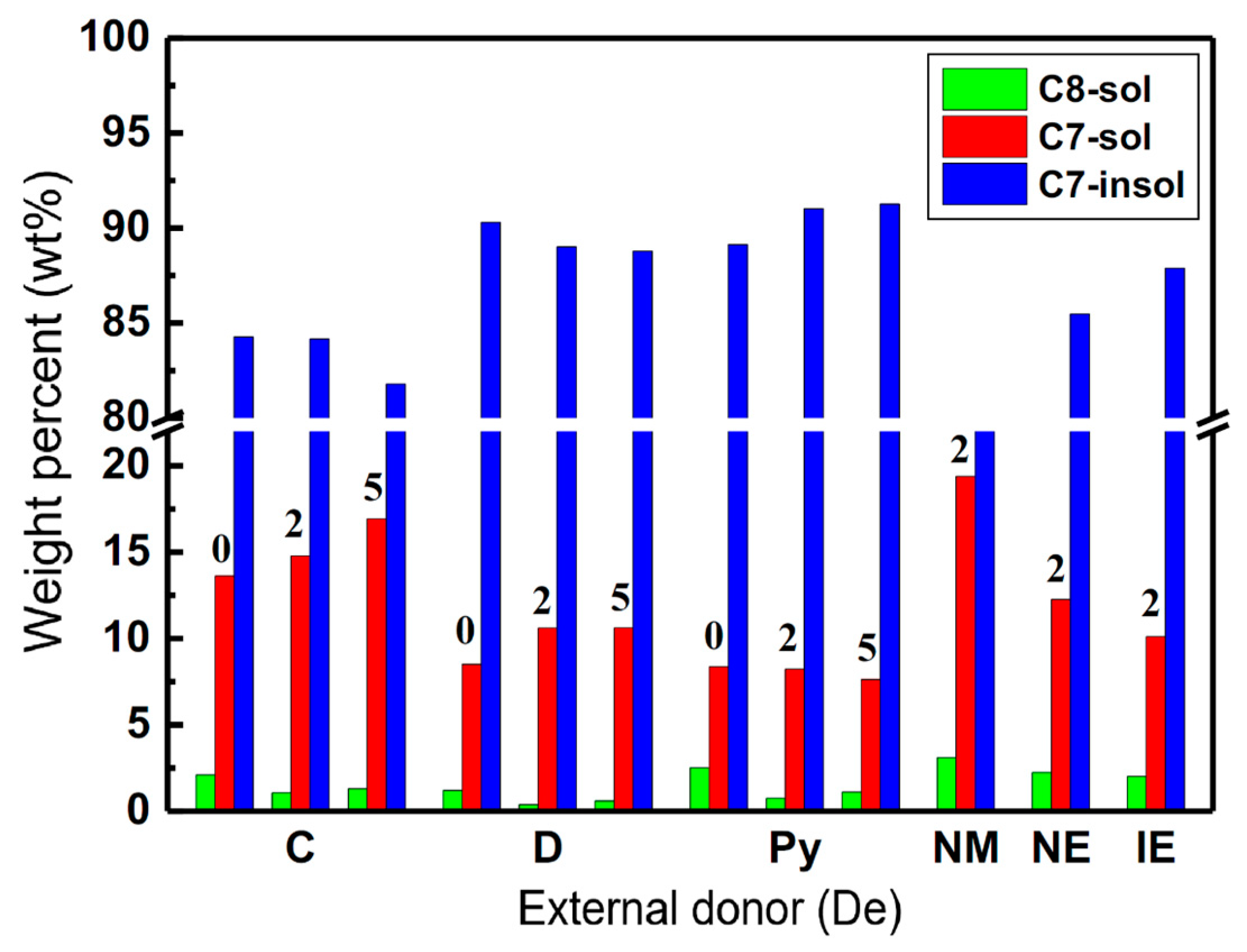
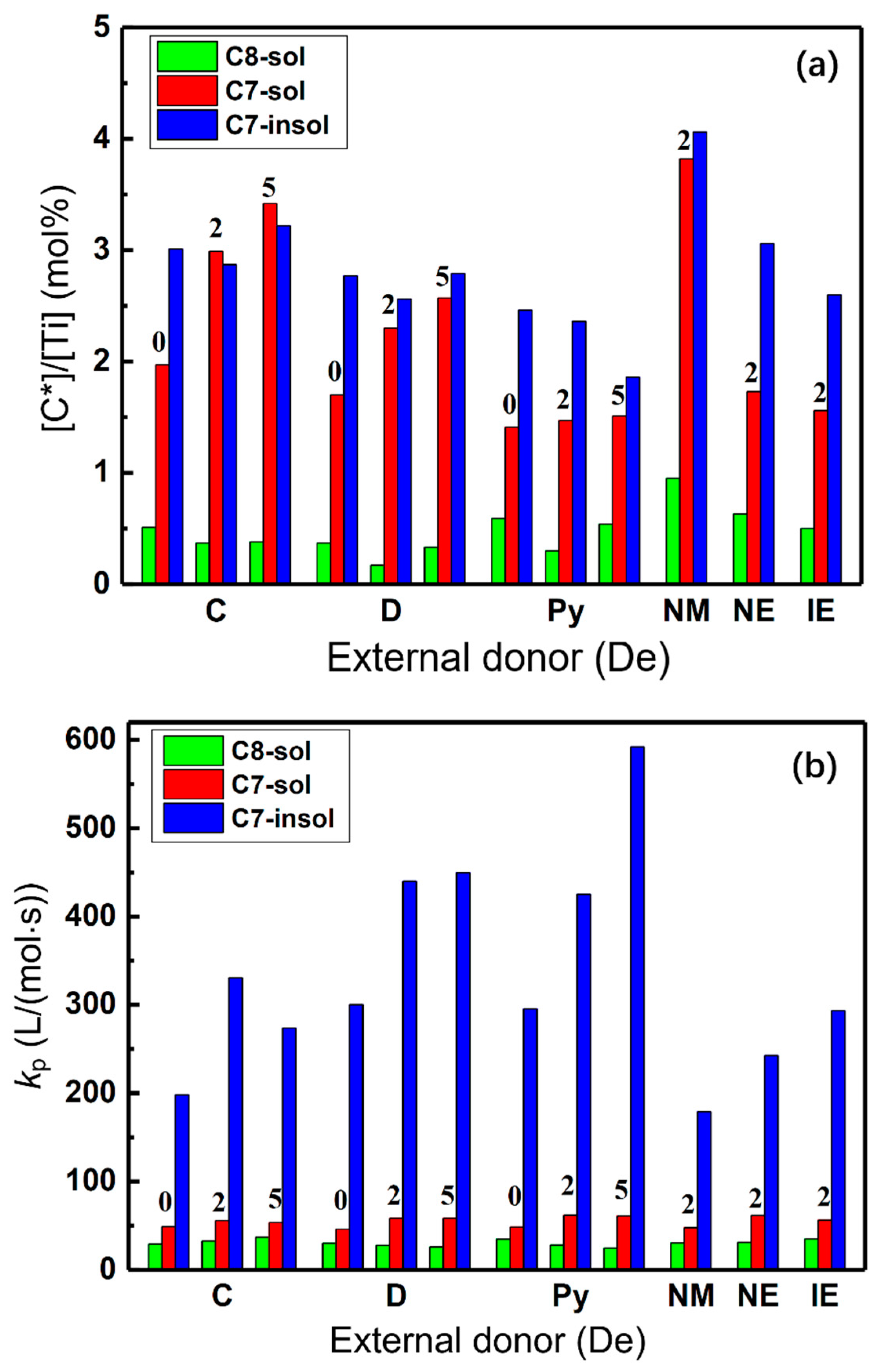

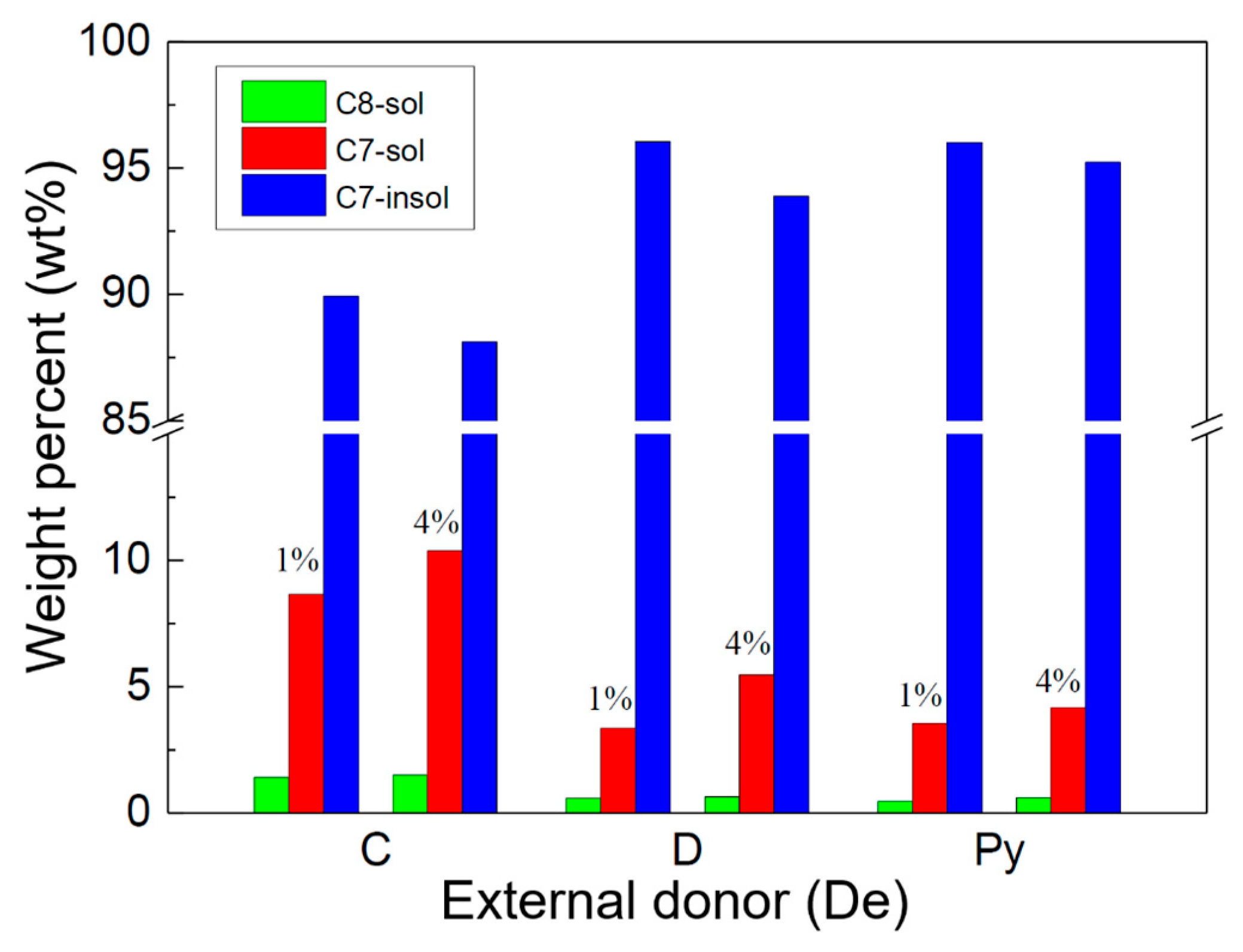
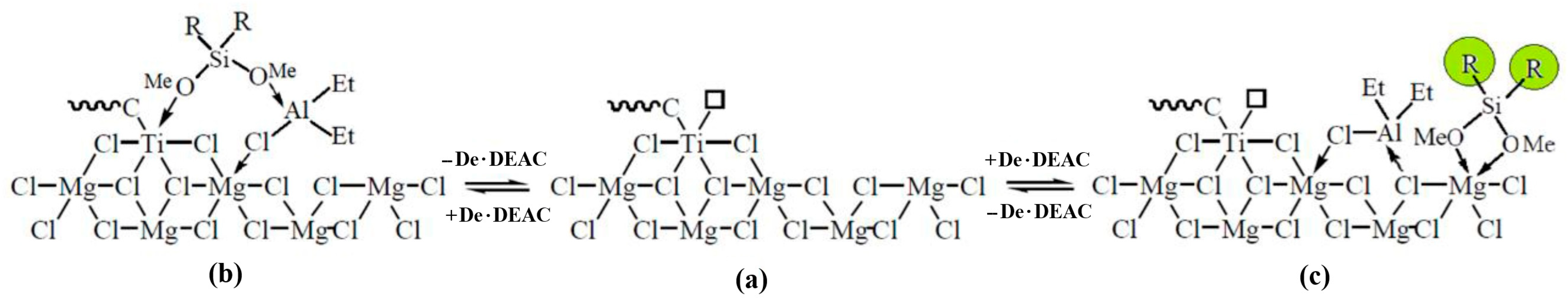
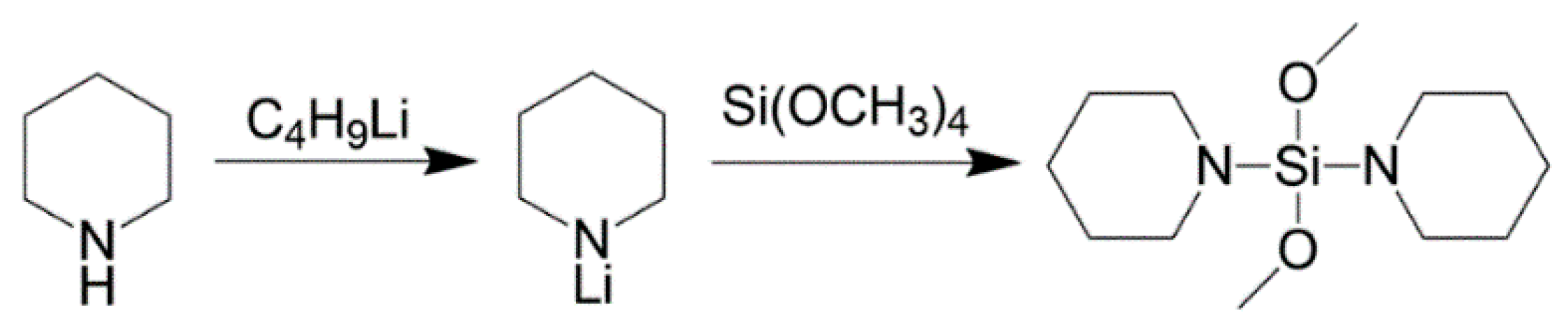
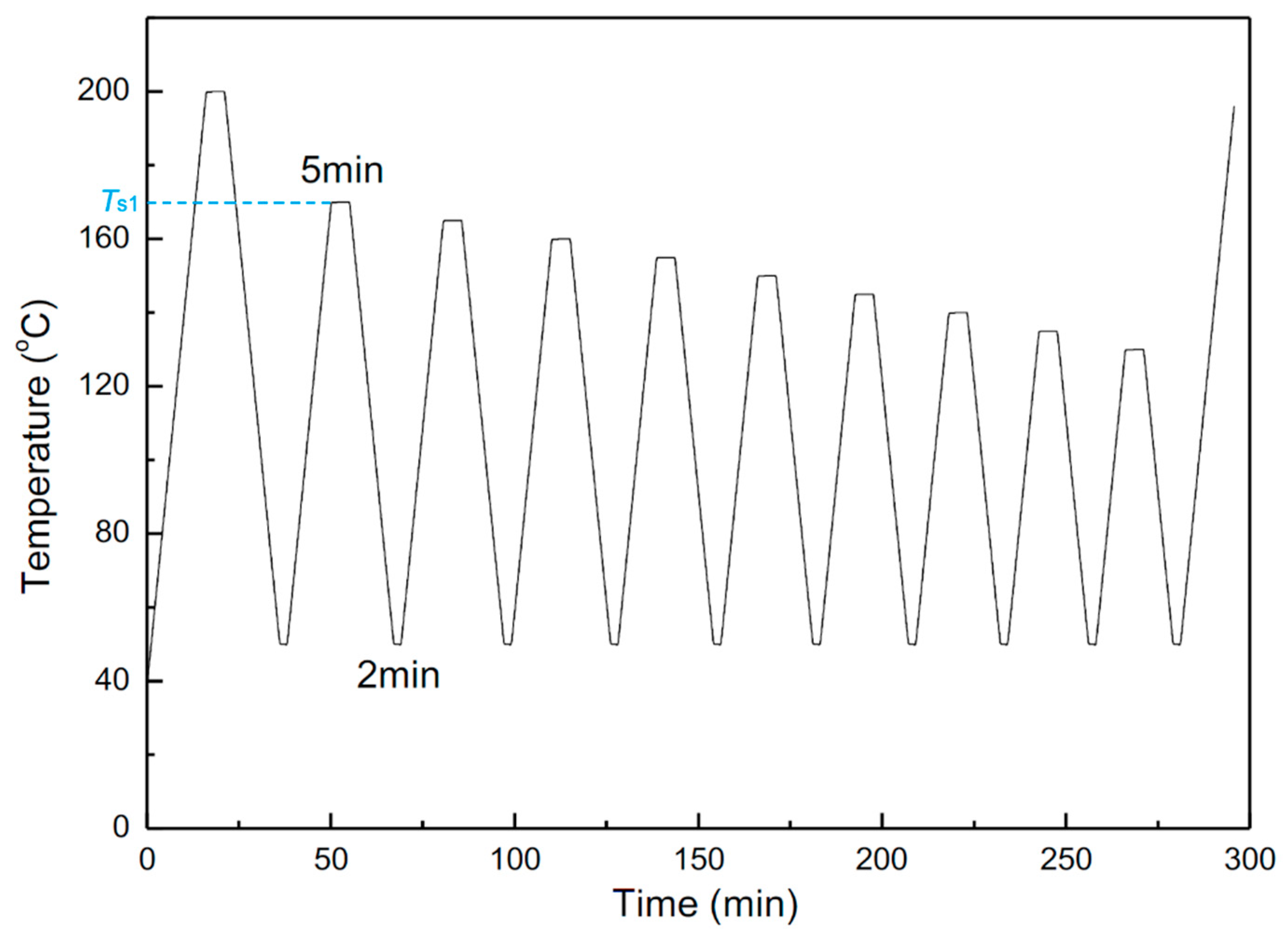
| Samples | De | H2 (mL) | C8-sol | C7-sol | C7-insol | C7-sol + C7-insol |
|---|---|---|---|---|---|---|
| PP-C-0 | Donor-C | 0 | 2.11 | 13.61 | 84.27 | 97.89 |
| PP-C-2 | Donor-C | 2 | 1.06 | 14.78 | 84.16 | 98.94 |
| PP-C-5 | Donor-C | 5 | 1.29 | 16.92 | 81.79 | 98.71 |
| PP-D-0 | Donor-D | 0 | 1.20 | 8.51 | 90.30 | 98.80 |
| PP-D-2 | Donor-D | 2 | 0.38 | 10.60 | 89.02 | 99.62 |
| PP-D-5 | Donor-D | 5 | 0.60 | 10.61 | 88.79 | 99.40 |
| PP-Py-0 | Donor-Py | 0 | 2.51 | 8.36 | 89.13 | 97.49 |
| PP-Py-2 | Donor-Py | 2 | 0.75 | 8.22 | 91.03 | 99.25 |
| PP-Py-5 | Donor-Py | 5 | 1.11 | 7.63 | 91.26 | 98.89 |
| PP-NM-2 | Donor-NM | 2 | 3.10 | 19.39 | 77.50 | 96.90 |
| PP-NE-2 | Donor-NE | 2 | 2.24 | 12.27 | 85.48 | 97.76 |
| PP-IE-2 | Donor-IE | 2 | 2.01 | 10.10 | 87.88 | 97.99 |
| Samples | Tm (°C) | ΔH (J/g) | Peak 1 | Peak 2 | ||||
|---|---|---|---|---|---|---|---|---|
| Tm1 (°C) | PP1 a (%) | ΔHP1 b (J/g) | Tm2 (°C) | PP2 a (%) | ΔHP2 c (J/g) | |||
| PP-NM-2 | 170.02 | 125.7 | 170.18 | 62.8 | 61.1 | 164.91 | 37.2 | 36.3 |
| PP-C-5 | 170.39 | 124.2 | 170.36 | 67.9 | 68.9 | 165.24 | 32.2 | 32.7 |
| PP-D-5 | 173.39 | 125.9 | 173.52 | 66.2 | 74.0 | 167.43 | 33.8 | 37.8 |
| PP-Py-5 | 173.36 | 126.2 | 173.50 | 66.2 | 76.2 | 167.43 | 33.8 | 38.9 |
| External Donor | Sample | Activity (g/g Cat·h) | Tm (°C) | ΔH (J/g) |
|---|---|---|---|---|
| Donor-C | HP-PP-C1 a | 1314 ± 2.37% | 162.58 | 97.74 |
| HP-PP-C4 b | 1343 ± 0.22% | 161.96 | 103.4 | |
| Donor-D | HP-PP-D1 a | 1597 ± 0.08% | 163.71 | 98.92 |
| HP-PP-D4 b | 1553 ± 1.74% | 163.70 | 101.2 | |
| Donor-Py | HP-PP-Py1 a | 1387 ± 0.67% | 165.05 | 100.8 |
| HP-PP-Py4 b | 1376 ± 0.45% | 164.73 | 100.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhang, B.; Guo, W.; Jiang, B.; Du, M.; Fu, Z.; Fan, Z. Hydrogen Responses of Propylene Polymerization with MgCl2-Supported Ziegler–Natta Catalysts in the Presence of Different Silane External Donors. Catalysts 2025, 15, 330. https://doi.org/10.3390/catal15040330
Liu X, Zhang B, Guo W, Jiang B, Du M, Fu Z, Fan Z. Hydrogen Responses of Propylene Polymerization with MgCl2-Supported Ziegler–Natta Catalysts in the Presence of Different Silane External Donors. Catalysts. 2025; 15(4):330. https://doi.org/10.3390/catal15040330
Chicago/Turabian StyleLiu, Xiaoyu, Biao Zhang, Wenqi Guo, Baiyu Jiang, Miao Du, Zhisheng Fu, and Zhiqiang Fan. 2025. "Hydrogen Responses of Propylene Polymerization with MgCl2-Supported Ziegler–Natta Catalysts in the Presence of Different Silane External Donors" Catalysts 15, no. 4: 330. https://doi.org/10.3390/catal15040330
APA StyleLiu, X., Zhang, B., Guo, W., Jiang, B., Du, M., Fu, Z., & Fan, Z. (2025). Hydrogen Responses of Propylene Polymerization with MgCl2-Supported Ziegler–Natta Catalysts in the Presence of Different Silane External Donors. Catalysts, 15(4), 330. https://doi.org/10.3390/catal15040330









