Revisiting the Impact of CO2 on the Activity and Selectivity of Cobalt-Based Catalysts for Fischer–Tropsch Synthesis Under Industrial-Relevant Conditions
Abstract
1. Introduction
2. Results
2.1. Experiment 1: CO Hydrogenation Performance with Increasing CO2 Concentration (12.5% and 25%)
2.2. Experiment 2: Comparative Fischer–Tropsch Synthesis with and Without CO2 Addition in Syngas (25 mol%)
2.3. Experiment 3: CO Hydrogenation with Switching Between Ar and Low CO2 Concentration (6 mol%) and Catalyst Regeneration
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Catalyst Characterization
4.3. Activity and Selectivity Measurements in Fischer–Tropsch Synthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| P-t-L | power-to-liquid |
| P-t-X | power-to-X |
| SOECs | solid oxide electrolysis cells |
| FTS | Fischer–Tropsch synthesis |
| FT-IR | Fourier Transform Infrared Spectroscopy |
| DRIFTS | Diffuse Reflectance Infrared Fourier Transform Spectroscopy |
| 20CoReAl | 20 wt% Co-0.5 wt% Re/γ-Al2O3 |
| 12CoReAl | 12 wt% Co-0.5 wt% Re/γ-Al2O3 |
| ICP | Inductively Coupled Plasma |
| XRD | X-ray diffraction |
| BET | Brunauer–Emmett–Teller method |
| CO-CoRe | CO-treated 20 wt% Co-0.5 wt% Re/γ-Al2O3 |
| TPH | temperature-programmed hydrogenation |
| MS | mass spectrometry |
| DFT | density functional theory |
| AIAT | ab initio atomistic thermodynamics |
| TCD | thermal conductivity detector |
| FID | flame ionization detector |
References
- IEA. Net Zero by 2050. Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 1 May 2021).
- Wulf, C.; Zapp, P.; Schreiber, A. Review of Power-to-X Demonstration Projects in Europe. Front. Energy Res. 2020, 8, 191. [Google Scholar] [CrossRef]
- Andika, R.; Nandiyanto, A.B.D.; Putra, Z.A.; Bilad, M.R.; Kim, Y.; Yun, C.M.; Lee, M. Co-electrolysis for power-to-methanol applications. Renew. Sustain. Energy Rev. 2018, 95, 227–241. [Google Scholar] [CrossRef]
- The Royal Society. Sustainable Synthetic Carbon Based Fuels for Transport. Available online: https://cris.brighton.ac.uk/ws/portalfiles/portal/6852944/Sustainable_synthetic.pdf (accessed on 1 September 2019).
- Kim, S.-M.; Bae, J.W.; Lee, Y.-J.; Jun, K.-W. Effect of CO2 in the feed stream on the deactivation of Co/γ-Al2O3 Fischer–Tropsch catalyst. Catal. Commun. 2008, 9, 2269–2273. [Google Scholar] [CrossRef]
- Park, S.-J.; Bae, J.W.; Lee, Y.-J.; Ha, K.-S.; Jun, K.-W.; Karandikar, P. Deactivation behaviors of Pt or Ru promoted Co/P-Al2O3 catalysts during slurry-phase Fischer–Tropsch synthesis. Catal. Commun. 2011, 12, 539–543. [Google Scholar] [CrossRef]
- Riedel, T.; Schaub, G. Low-Temperature Fischer–Tropsch Synthesis on Cobalt Catalysts—Effects of CO2. Top. Catal. 2003, 26, 145–156. [Google Scholar] [CrossRef]
- Visconti, C.G.; Lietti, L.; Tronconi, E.; Forzatti, P.; Zennaro, R.; Finocchio, E. Fischer–Tropsch synthesis on a Co/Al2O3 catalyst with CO2 containing syngas. Appl. Catal. A Gen. 2009, 355, 61–68. [Google Scholar]
- Bredy, P.; Farrusseng, D.; Schuurman, Y.; Meunier, F.C. On the link between CO surface coverage and selectivity to CH4 during CO2 hydrogenation over supported cobalt catalysts. J. Catal. 2022, 411, 93–96. [Google Scholar] [CrossRef]
- Riedel, T.; Claeys, M.; Schulz, H.; Schaub, G.; Nam, S.-S.; Jun, K.-W.; Choi, M.-J.; Kishan, G.; Lee, K.-W. Comparative study of Fischer–Tropsch synthesis with H2/CO and H2/CO2 syngas using Fe- and Co-based catalysts. Appl. Catal. A Gen. 1999, 186, 201–213. [Google Scholar] [CrossRef]
- Zhang, Y.; Jacobs, G.; Sparks, D.E.; Dry, M.E.; Davis, B.H. CO and CO2 hydrogenation study on supported cobalt Fischer–Tropsch synthesis catalysts. Catal. Today 2002, 71, 411–418. [Google Scholar] [CrossRef]
- Chakrabarti, D.; de Klerk, A.; Prasad, V.; Gnanamani, M.K.; Shafer, W.D.; Jacobs, G.; Sparks, D.E.; Davis, B.H. Conversion of CO2 over a co-based Fischer–Tropsch catalyst. Ind. Eng. Chem. Res. 2015, 54, 1189–1196. [Google Scholar] [CrossRef]
- Claeys, M.; Dry, M.E.; van Steen, E.; du Plessis, E.; van Berge, P.J.; Saib, A.M.; Moodley, D.J. In situ magnetometer study on the formation and stability of cobalt carbide in Fischer–Tropsch synthesis. J. Catal. 2014, 318, 193–202. [Google Scholar] [CrossRef]
- Delannay, F. Characterization of Heterogeneous Catalysts; Marcel Dekker Inc.: New York, NY, USA, 1984. [Google Scholar]
- Niemelä, M.; Krause, A. The long-term performance of Co/SiO2 catalysts in CO hydrogenation. Catal. Lett. 1996, 42, 161–166. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Freide, J.J.F.; Gamlin, T.D.; Hensman, J.R.; Nay, B.; Sharp, C. Development of a CO2 tolerant Fischer-Tropsch catalyst: From laboratory to commercial-scale demonstration in Alaska. J. Energy Chem. 2004, 13, 1. Available online: https://www.jenergychem.com/CN/Y2004/V13/I1/1 (accessed on 8 January 2004).
- Zonnevylle, M.; Geerlings, J.; Van Santen, R. Conversion of surface carbidic to subsurface carbon on cobalt (0001): A theoretical study. Surf. Sci. 1990, 240, 253–262. [Google Scholar] [CrossRef]
- Gholami, Z.; Tišler, Z.; Svobodová, E.; Hradecká, I.; Sharkov, N.; Gholami, F. Catalytic Performance of Alumina-Supported Cobalt Carbide Catalysts for Low-Temperature Fischer–Tropsch Synthesis. Catalysts 2022, 12, 1222. [Google Scholar] [CrossRef]
- Hazemann, P.; Decottignies, D.; Maury, S.; Humbert, S.; Meunier, F.C.; Schuurman, Y. Selectivity loss in Fischer-Tropsch synthesis: The effect of cobalt carbide formation. J. Catal. 2021, 397, 1–12. [Google Scholar] [CrossRef]
- Sattler, J.J.; Beale, A.M.; Weckhuysen, B.M. Operando Raman spectroscopy study on the deactivation of Pt/Al2O3 and Pt–Sn/Al2O3 propane dehydrogenation catalysts. Phys. Chem. Chem. Phys. 2013, 15, 12095–12103. [Google Scholar] [CrossRef]
- Hofer, L.; Peebles, W. Preparation and X-ray diffraction studies of a new cobalt carbide1. J. Am. Chem. Soc. 1947, 69, 893–899. [Google Scholar] [CrossRef]
- Kwak, G.; Woo, M.H.; Kang, S.C.; Park, H.-G.; Lee, Y.-J.; Jun, K.-W.; Ha, K.-S. In situ monitoring during the transition of cobalt carbide to metal state and its application as Fischer–Tropsch catalyst in slurry phase. J. Catal. 2013, 307, 27–36. [Google Scholar] [CrossRef]
- Moodley, D.J.; van de Loosdrecht, J.; Saib, A.M.; Overett, M.J.; Datye, A.K.; Niemantsverdriet, J.W. Carbon deposition as a deactivation mechanism of cobalt-based Fischer–Tropsch synthesis catalysts under realistic conditions. Appl. Catal. A Gen. 2009, 354, 102–110. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, B.; Jiang, F.; Fang, X.; Xu, Y.; Liu, X. Assessing the formation of cobalt carbide and its catalytic performance under realistic reaction conditions and tuning product selectivity in a cobalt-based FTS reaction. Catal. Sci. Technol. 2019, 9, 3238–3258. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, L.; Yu, F.; An, Y.; Dai, Y.; Yang, Y.; Lin, T.; Li, S.; Wang, H.; Gao, P. Effects of sodium on the catalytic performance of CoMn catalysts for Fischer–Tropsch to olefin reactions. ACS Catal. 2017, 7, 3622–3631. [Google Scholar] [CrossRef]
- Choi, Y.I.; Yang, J.H.; Park, S.J.; Sohn, Y. Energy storage and CO2 reduction performances of Co/Co2C/C prepared by an anaerobic ethanol oxidation reaction using sacrificial SnO2. Catalysts 2020, 10, 1116. [Google Scholar] [CrossRef]
- Tsakoumis, N.E.; Rønning, M.; Borg, Ø.; Rytter, E.; Holmen, A. Deactivation of cobalt based Fischer–Tropsch catalysts: A review. Catal. Today 2010, 154, 162–182. [Google Scholar] [CrossRef]
- Pei, Y.-P.; Liu, J.-X.; Zhao, Y.-H.; Ding, Y.-J.; Liu, T.; Dong, W.-D.; Zhu, H.-J.; Su, H.-Y.; Yan, L.; Li, J.-L. High alcohols synthesis via Fischer–Tropsch reaction at cobalt metal/carbide interface. ACS Catal. 2015, 5, 3620–3624. [Google Scholar] [CrossRef]
- Pan, Z.; Parvari, M.; Bukur, D.B. Fischer–Tropsch Synthesis on Co/Al2O3 Catalyst: Effect of Pretreatment Procedure. Top. Catal. 2014, 57, 470–478. [Google Scholar] [CrossRef]
- Pan, Z.; Bukur, D.B. Fischer–Tropsch synthesis on Co/ZnO catalyst—Effect of pretreatment procedure. Appl. Catal. A Gen. 2011, 404, 74–80. [Google Scholar] [CrossRef]
- Jacobs, G.; Patterson, P.M.; Zhang, Y.; Das, T.; Li, J.; Davis, B.H. Fischer–Tropsch synthesis: Deactivation of noble metal-promoted Co/Al2O3 catalysts. Appl. Catal. A Gen. 2002, 233, 215–226. [Google Scholar] [CrossRef]
- Tavasoli, A.; Abbaslou, R.M.M.; Dalai, A.K. Deactivation behavior of ruthenium promoted Co/γ-Al2O3 catalysts in Fischer–Tropsch synthesis. Appl. Catal. A Gen. 2008, 346, 58–64. [Google Scholar] [CrossRef]
- Jacobs, G.; Das, T.K.; Zhang, Y.; Li, J.; Racoillet, G.; Davis, B.H. Fischer–Tropsch synthesis: Support, loading, and promoter effects on the reducibility of cobalt catalysts. Appl. Catal. A Gen. 2002, 233, 263–281. [Google Scholar] [CrossRef]
- Cats, K.H.; Weckhuysen, B.M. Combined Operando X-ray Diffraction/Raman Spectroscopy of Catalytic Solids in the Laboratory: The Co/TiO2 Fischer–Tropsch Synthesis Catalyst Showcase. ChemCatChem 2016, 8, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Ding, Y.; Wang, T.; Yan, L.; Chen, W.; Zhu, H.; Lu, Y. The formation of Co2C species in activated carbon supported cobalt-based catalysts and its impact on Fischer–Tropsch reaction. Catal. Lett. 2005, 102, 265–269. [Google Scholar] [CrossRef]
- Lynch, J. Development of structural characterisation tools for catalysts. Oil Gas Sci. Technol. 2002, 57, 281–305. [Google Scholar] [CrossRef][Green Version]
- Ducreux, O.; Lynch, J.; Rebours, B.; Roy, M.; Chaumette, P. In Situ Characterisation of Cobalt Based Fischer-Tropsch Catalysts: A New Approach to the Active Phase. Stud. Surf. Sci. Catal. 1998, 119, 125–130. [Google Scholar] [CrossRef]
- Fei Tan, K.; Xu, J.; Chang, J.; Borgna, A.; Saeys, M. Carbon deposition on Co catalysts during Fischer–Tropsch synthesis: A computational and experimental study. J. Catal. 2010, 274, 121–129. [Google Scholar] [CrossRef]
- Swart, J.C.W.; Ciobîcǎ, I.M.; van Santen, R.A.; van Steen, E. Intermediates in the Formation of Graphitic Carbon on a Flat FCC-Co(111) Surface. J. Phys. Chem. C 2008, 112, 12899–12904. [Google Scholar] [CrossRef]
- Corral Valero, M.; Raybaud, P. Stability of Carbon on Cobalt Surfaces in Fischer–Tropsch Reaction Conditions: A DFT Study. J. Phys. Chem. C 2014, 118, 22479–22490. [Google Scholar] [CrossRef]
- Moodley, D.; Van De Loosdrecht, J.; Saib, A.; Niemantsverdriet, J. The formation and influence of carbon on cobalt-based Fischer-Tropsch synthesis catalysts: An integrated review. Adv. Fisch.-Tropsch Synth. Catal. Catal. 2010, 128, 49–81. [Google Scholar] [CrossRef]
- Moodley, D.J. On the Deactivation of Cobalt-Based Fischer-Tropsch Synthesis Catalysts. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2008. [Google Scholar]
- ten Have, I.C.; Weckhuysen, B.M. The active phase in cobalt-based Fischer-Tropsch synthesis. Chem Catal. 2021, 1, 339–363. [Google Scholar] [CrossRef]
- Rahmati, M.; Huang, B.; Schofield, L.M.; Fletcher, T.H.; Woodfield, B.F.; Hecker, W.C.; Bartholomew, C.H.; Argyle, M.D. Effects of Ag promotion and preparation method on cobalt Fischer-Tropsch catalysts supported on silica-modified alumina. J. Catal. 2018, 362, 118–128. [Google Scholar] [CrossRef]
- Xu, R.; Hou, C.; Xia, G.; Sun, X.; Li, M.; Nie, H.; Li, D. Effects of Ag promotion for Co/Al2O3 catalyst in Fischer-Tropsch synthesis. Catal. Today 2020, 342, 111–114. [Google Scholar] [CrossRef]
- Mauldin, C.H. Cobalt Catalysts for the Conversion of Methanol to Hydrocarbons and for Fischer-Tropsch Synthesis. U.S. Patent 4,568,663, 4 February 1986. [Google Scholar]
- Mauldin, C.H.; Vamado, D.E. Rhenium as a promoter of titania-supported cobalt Fischer-Tropsch catalysts. In Studies in Surface Science and Catalysis; Iglesia, E., Spivey, J.J., Fleisch, T.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 136, pp. 417–422. [Google Scholar]
- Promotion Effects in Co-based Fischer–Tropsch Catalysis. In Catalysis; Spivey, J.J., Dooley, K.M., Dooley, K.M., Spivey, J.J., Eds.; The Royal Society of Chemistry: London, UK, 2006; Volume 19. [Google Scholar]
- Schanke, D.; Eri, S.; Rytter, E.; Aaserud, C.; Hilmen, A.-M.; Asbjørn Lindvg, O.; Bergene, E.; Holmen, A. Fischer-Tropsch synthesis on cobalt catalysts supported on different aluminas. In Studies in Surface Science and Catalysis; Bao, X., Xu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 147, pp. 301–306. [Google Scholar]
- Storsæter, S.; Borg, Ø.; Blekkan, E.A.; Holmen, A. Study of the effect of water on Fischer–Tropsch synthesis over supported cobalt catalysts. J. Catal. 2005, 231, 405–419. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Blöchl, P.E.; Jepsen, O.; Andersen, O.K. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev. B 1994, 49, 16223–16233. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Caldeweyher, E.; Bannwarth, C.; Grimme, S. Extension of the D3 dispersion coefficient model. J. Chem. Phys. 2017, 147, 034112. [Google Scholar] [CrossRef]
- Caldeweyher, E.; Ehlert, S.; Hansen, A.; Neugebauer, H.; Spicher, S.; Bannwarth, C.; Grimme, S. A generally applicable atomic-charge dependent London dispersion correction. J. Chem. Phys. 2019, 150, 154122. [Google Scholar] [CrossRef]
- Lin, H.; Liu, J.-X.; Fan, H.-J.; Li, W.-X. Morphology Evolution of FCC and HCP Cobalt Induced by a CO Atmosphere from Ab Initio Thermodynamics. J. Phys. Chem. C 2020, 124, 23200–23209. [Google Scholar] [CrossRef]
- Liu, J.-X.; Su, H.-Y.; Sun, D.-P.; Zhang, B.-Y.; Li, W.-X. Crystallographic Dependence of CO Activation on Cobalt Catalysts: HCP versus FCC. J. Am. Chem. Soc. 2013, 135, 16284–16287. [Google Scholar] [CrossRef] [PubMed]
- Saib, A.M.; Moodley, D.J.; Ciobîcă, I.M.; Hauman, M.M.; Sigwebela, B.H.; Weststrate, C.J.; Niemantsverdriet, J.W.; van de Loosdrecht, J. Fundamental understanding of deactivation and regeneration of cobalt Fischer–Tropsch synthesis catalysts. Catal. Today 2010, 154, 271–282. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Su, H.-Y.; Sun, K.; Liu, J.; Li, W.-X. Structural and electronic properties of cobalt carbide Co2C and its surface stability: Density functional theory study. Surf. Sci. 2012, 606, 598–604. [Google Scholar] [CrossRef]
- Chen, P.-P.; Liu, J.-X.; Li, W.-X. Carbon Monoxide Activation on Cobalt Carbide for Fischer–Tropsch Synthesis from First-Principles Theory. ACS Catal. 2019, 9, 8093–8103. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]

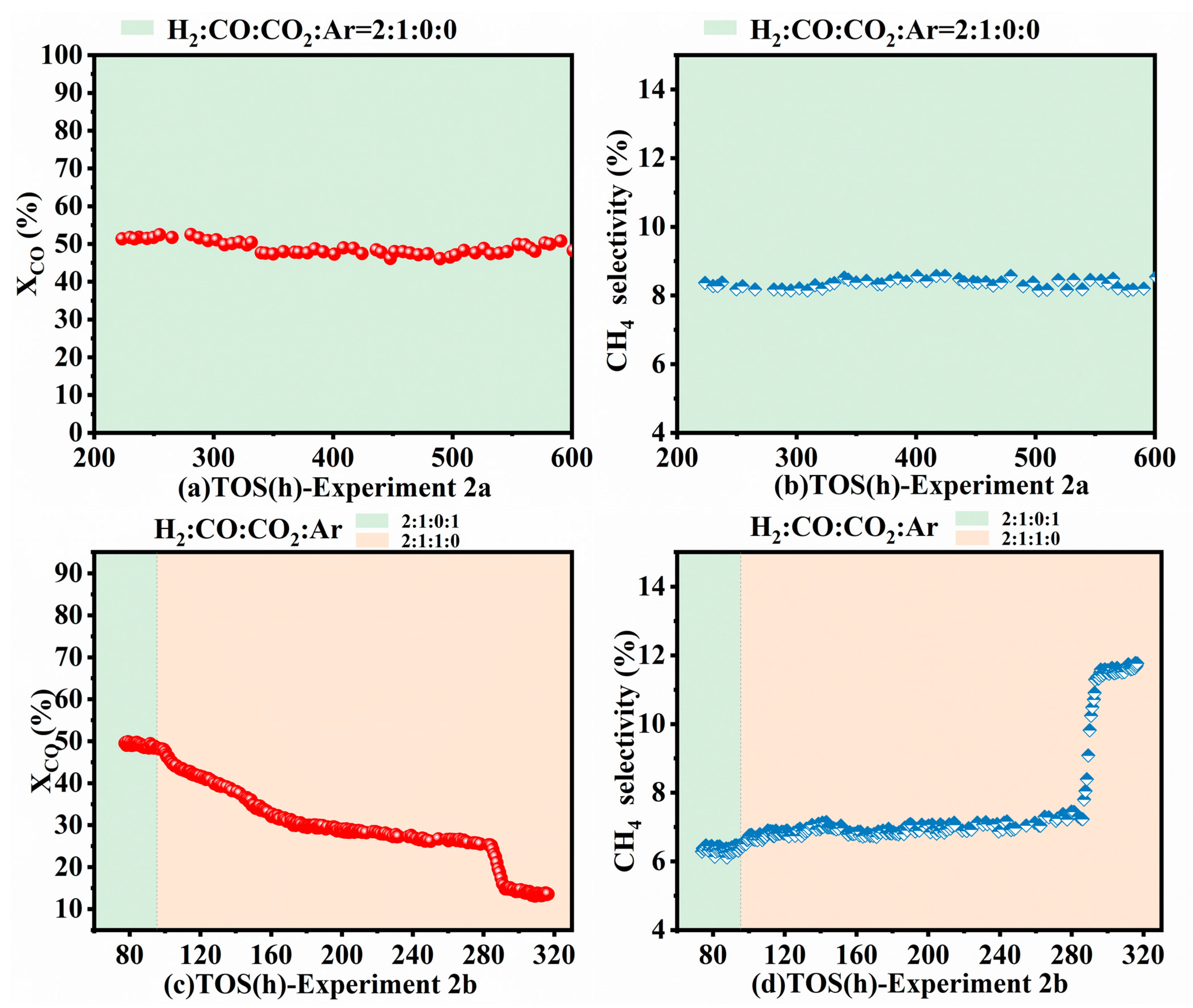
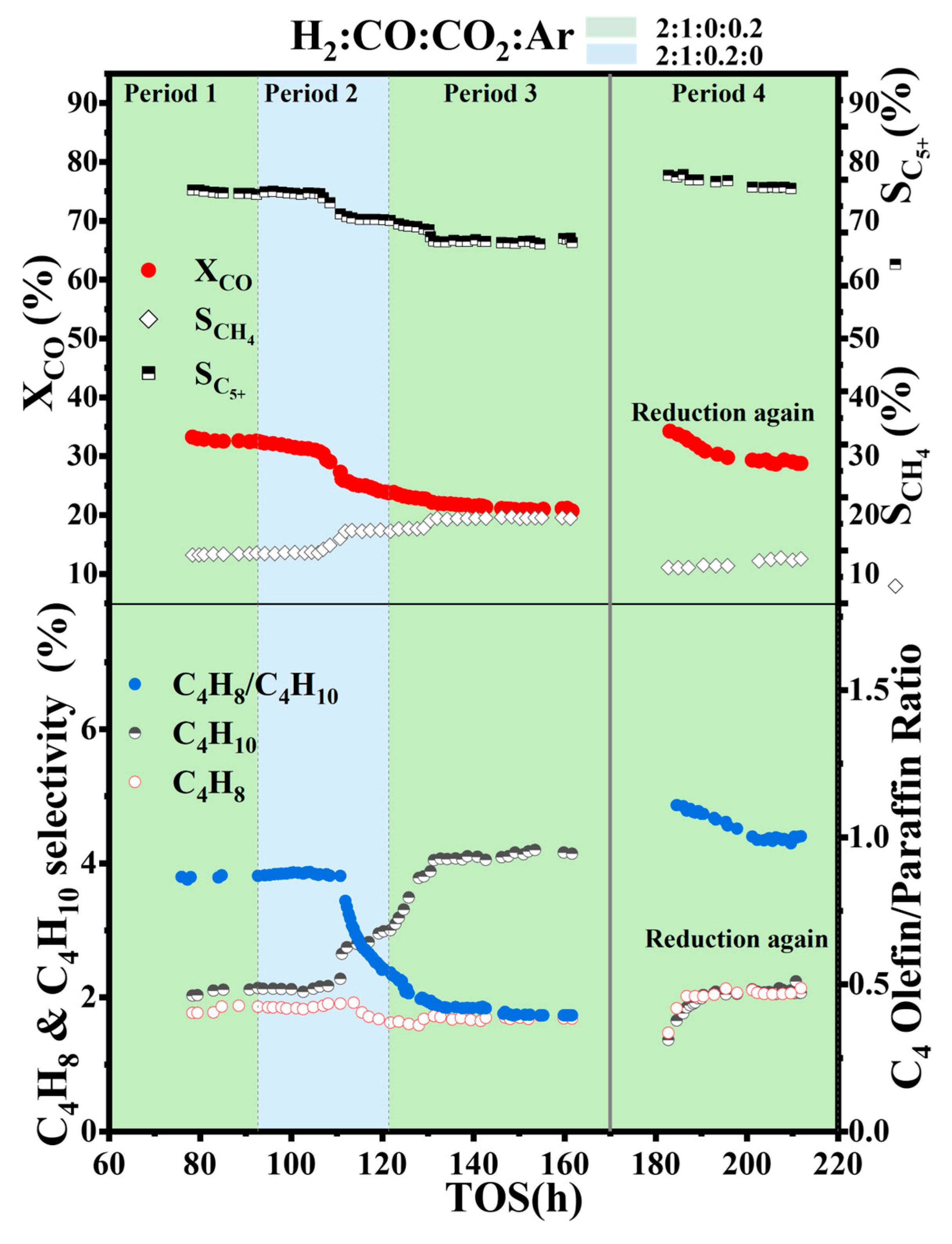
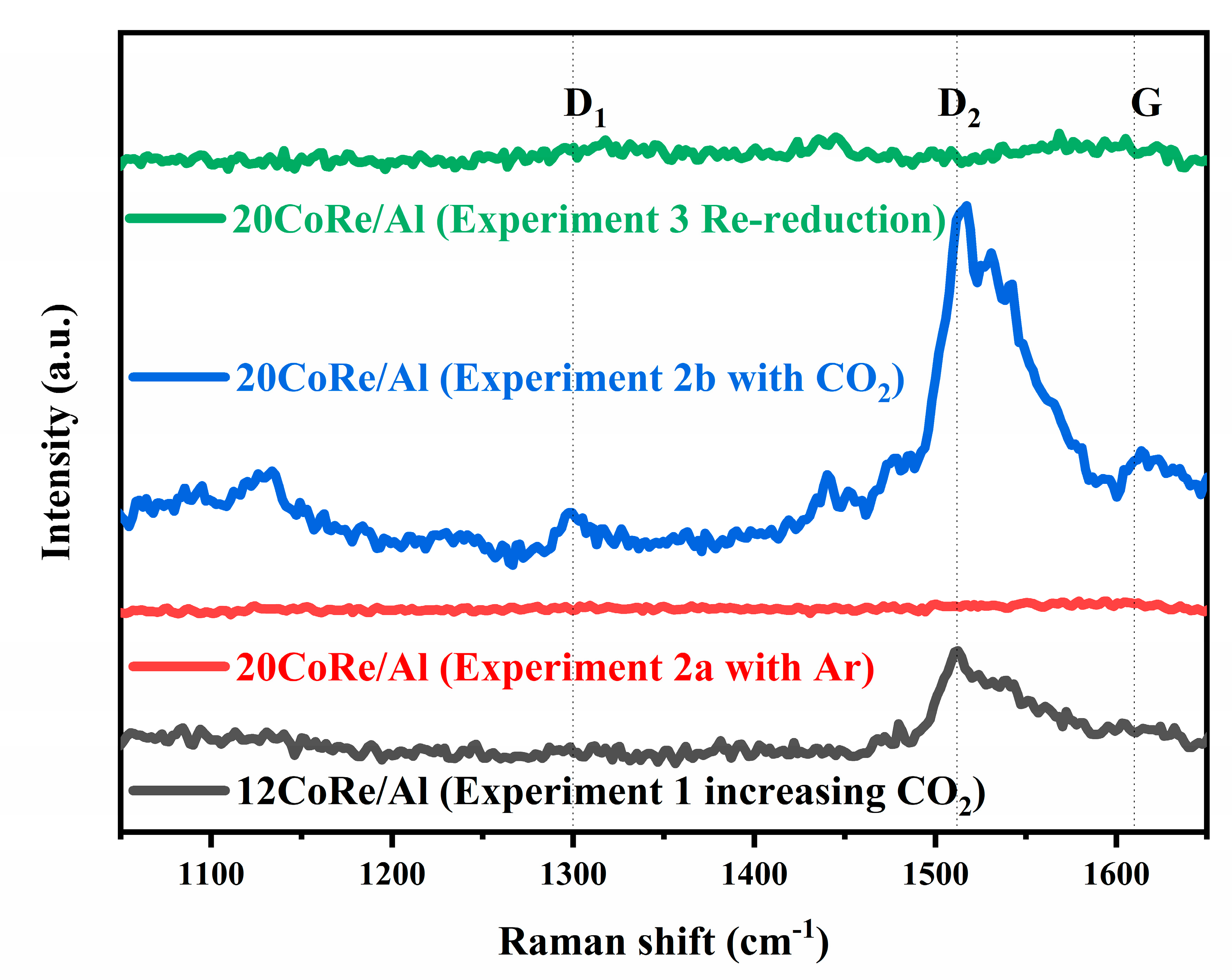
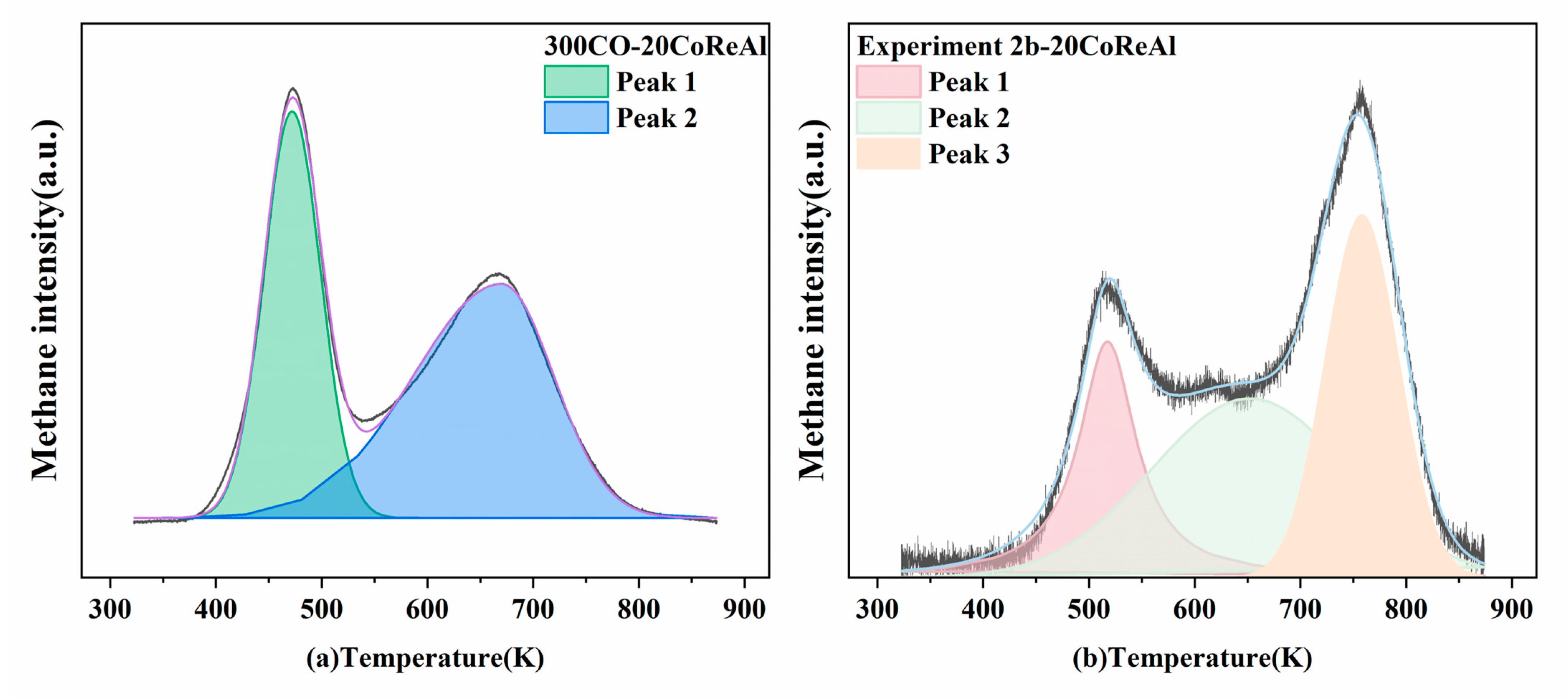


| Catalyst (Impregnated/Precipitation) | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore diameter (nm) | Particle Size (nm) | Type of Reactor/Pressure (Mpa)/Temperate (K)/GHSV (cm3/h/gcat) | CO2 Addition | CO Conversion 1 | Methane Selectivity 1 | C5+ Selectivity 1 | Overall Effect of CO2 on Catalyst Stability and Deactivation | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20%Co/γ-Al2O3(imp) | 218.9 | 0.734 | 10.1 | 9.5 | FBR-2 Mpa-493 K-2000 | H2/CO/CO2/Ar cm3/min = 32/16/0~13.3/5.4~18.7 | Negative | Positive | Negative | CO2 is found to be responsible for the partial oxidation of surface cobalt metal with the coexistence of generated water. | [5] |
| 20%Co/P-Al2O3(imp) | 192 | 0.28 | 4.5 | 14.9 | CSTR-2 Mpa-503 K-2000 | H2/CO/CO2/Ar mol% = 57.3/28.4/9.3/5 | Negative | - | - | Increased the rate of deactivation. | [6] |
| 20%Co-0.2%Ru/P-Al2O3(imp) | 198 | 0.31 | 4.8 | 13.4 | CSTR-2 Mpa-503 K-2000 | H2/CO/CO2/Ar mol% = 57.3/28.4/9.3/5 | Negative | - | -- | Increased the rate of deactivation. Greater deactivation than unprompted catalyst. | [6] |
| 20%Co-0.2%Pt/P-Al2O3(imp) | 194 | 0.26 | 4.3 | 10.3 | CSTR-2 Mpa-503 K-2000 | H2/CO/CO2/Ar mol% = 57.3/28.4/9.3/5 | Negative | - | - | Increased the rate of deactivation. | [6] |
| 31.2%Co-Zr-Ru/SiO2(Prec) | 248 | - | 7.9 | 1.69 | CFSSR-463 K | H2/CO/CO2 Mpa = 0.54/0.27/ 0~0.62 | Negative | Positive | Negative | Increased the rate of deactivation. Irreversible after removal of CO2. | [7] |
| 27.3%Co-Zr-Pt/SiO2(Imp) | 116.2 | - | 12.5 | 6.57 | CFSSR-463 K | H2/CO/CO2 Mpa = 0.35/0.18/ 0~0.7 | no change | no change | no change | CO2 behaves as an inert gas | [7] |
| 25.9%Co-La-Ru/Al2O3(Imp) | 81.6 | - | 13.8 | 2.21 | CFSSR-463 K | H2/CO/CO2 Mpa = 0.33/0.17/ 0~0.68 | no change | no change | no change | CO2 behaves as an inert gas | [7] |
| 19.4%Co-La-Ru/Al2O3(Prec) | 124.1 | - | 12.4 | 3.21 | CFSSR-463 K | H2/CO/CO2 Mpa = 0.54/0.29/ 0~0.52 | no change | no change | no change | CO2 behaves as an inert gas | [7] |
| 15%Co/γ-Al2O3(imp) | 120 | 0.31 | - | - | FBR-2 Mpa-493 K-4800 | H2/CO/CO2/N2 bar = 14.2/2.9/2.9~0/0~2.9 | no change | no change | no change | CO2 behaves as an inert gas. | [8] |
| 15%Co-0.45%Pt/Sirolox (imp) | - | - | - | 18 | Operando DRIFTS-483 K | H2/CO/CO2/He cm3/min = 45/0.25~1/15/15 | - | Positive | Negative | As CO decreases and CO2 increases, the reaction shifts from Fischer–Tropsch synthesis to methanation. | [9] |
| 15%Co/Sirolox (imp) | - | - | - | 12 | Operando DRIFTS-483 K | H2/CO/CO2/He cm3/min = 45/0.25~1/15/15 | - | Positive | Negative | As CO decreases and CO2 increases, the reaction shifts from Fischer–Tropsch synthesis to methanation. | [9] |
| 15%Co-0.56%Pt/TiO2 (imp) | - | - | - | 15 | Operando DRIFTS-483 K | H2/CO/CO2/He cm3/min = 45/0.25~1/15/15 | - | Positive | Negative | As CO decreases and CO2 increases, the reaction shifts from Fischer–Tropsch synthesis to methanation. | [9] |
| 15%Co/TiO2 (imp) | - | - | - | 15 | Operando DRIFTS-483 K | H2/CO/CO2/He cm3/min = 45/0.25~1/15/15 | - | Positive | Negative | As CO decreases and CO2 increases, the reaction shifts from Fischer–Tropsch synthesis to methanation. | [9] |
| 100Co-60MnO-0.15Pt/147SiO2 (Prec) | - | - | - | - | FBR-1 Mpa-463 K-1800 | H2/CO/CO2 mol% = 2/1~0/0~1 | - | Positive | Negative | As CO decreases and CO2 increases, the reaction shifts from Fischer–Tropsch synthesis to methanation. | [10] |
| 15%Co/SiO2(imp) | - | - | - | - | FBR/2.4 Mpa/493 K | H2/CO/CO2 | - | - | - | CO2 participates in the reaction during the FT process, The conversion rate of CO is four times that of CO2. | [11] |
| 25%Co-0.5%Pt/γ-Al2O3(imp) | 130 | 0.28 | 1.91 | FBR-2-493 K-6000 | H2/CO/CO2 mol% = 3/1~0/0~1 | Positive | Negative | 14CO2 was indeed converted, The only products derived from 14CO2 were C1-C3 hydrocarbons. | [12] |
| EXP Number | Catalyst | T(K)/P(bar)/GHSV (Ncm3/h/gcat) | Feed Gas Ratio: H2/CO/CO2/Ar | |||
|---|---|---|---|---|---|---|
| Period 1 | Period 2 | Period 3 | Period 4 | |||
| 1 (increasing CO2) | 12CoRe/Al | 493/21/7200 | 2:1:0:1 | 2:1:0.5:0.5 | 2:1:1:0 | 2:1:0:1 |
| 2a (syngas) | 20CoRe/Al | 493/20/10,000 | 2:1:0:0 | |||
| 2b (CO2) | 20CoRe/Al | 493/20/10,000 | 2:1:0:1 | 2:1:1:0 | ||
| 3 (Re-reduction) | 20CoRe/Al | 493/23/5000 | 2:1:0:0.2 | 2:1:0.2:0 | 2:1:0:0.2 | 2:1:0:0.2 |
| Exp. Number | Catalyst | Co/Re (ICP) [wt%] | Crystallite Size (XRD) [nm] 1 | BET and BJH Methods (Before/After Reaction) | ||
|---|---|---|---|---|---|---|
| BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) | ||||
| 2a (syngas) | 20CoRe/Al | 19.4/0.48 | 12.8 | 130/102 | 0.31/0.27 | 9.6/8.1 |
| 2b (CO2) | 20CoRe/Al | 19.4/0.48 | 12.8 | 130/75 | 0.31/0.2 | 9.6/7.5 |
| Experiment | Catalyst | Feed Gas Ratio H2:CO:CO2:Ar | TOS (h) | XCO (%) | PCO (atm) |
|---|---|---|---|---|---|
| 1 | 12CoRe/Al | 2:1:1:0 | 150 | 49 | 4.15 |
| 2b | 20CoRe/Al | 2:1:1:0 | 282 | 25 | 4.63 |
| 3 | 20CoRe/Al | 2:1:0.2:0 | 105 | 30 | 6.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Du, J.; Chen, D.; Gong, F.; Gao, Y.; Huang, Z.; Chen, D.; Yang, J. Revisiting the Impact of CO2 on the Activity and Selectivity of Cobalt-Based Catalysts for Fischer–Tropsch Synthesis Under Industrial-Relevant Conditions. Catalysts 2025, 15, 329. https://doi.org/10.3390/catal15040329
Chen Z, Du J, Chen D, Gong F, Gao Y, Huang Z, Chen D, Yang J. Revisiting the Impact of CO2 on the Activity and Selectivity of Cobalt-Based Catalysts for Fischer–Tropsch Synthesis Under Industrial-Relevant Conditions. Catalysts. 2025; 15(4):329. https://doi.org/10.3390/catal15040329
Chicago/Turabian StyleChen, Zhiyu, Jinbo Du, Denghui Chen, Fuqing Gong, Yang Gao, Zhen Huang, De Chen, and Jia Yang. 2025. "Revisiting the Impact of CO2 on the Activity and Selectivity of Cobalt-Based Catalysts for Fischer–Tropsch Synthesis Under Industrial-Relevant Conditions" Catalysts 15, no. 4: 329. https://doi.org/10.3390/catal15040329
APA StyleChen, Z., Du, J., Chen, D., Gong, F., Gao, Y., Huang, Z., Chen, D., & Yang, J. (2025). Revisiting the Impact of CO2 on the Activity and Selectivity of Cobalt-Based Catalysts for Fischer–Tropsch Synthesis Under Industrial-Relevant Conditions. Catalysts, 15(4), 329. https://doi.org/10.3390/catal15040329







