Recent Advances in Cu-Based Metal–Organic Framework Electrocatalysts for CO2 Reduction Reactions
Abstract
1. Introduction
2. Recent Advances in Cu-Based MOF Electrocatalysts
2.1. Overview of CO2RR C1 Products
2.1.1. Carbon Monoxide
2.1.2. Methane
2.1.3. Formic Acid/Formates
2.2. Overview of CO2RR C2+ Products
2.2.1. Ethylene
2.2.2. Ethanol
2.2.3. Other C2+ Products
3. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Using Carbon Dioxide as a Building Block in Organic Synthesis. Nat. Commun. 2015, 6, 15. [Google Scholar] [CrossRef]
- Duma, Z.G.; Moma, J.; Langmi, H.W.; Louis, B.; Parkhomenko, K.; Musyoka, N.M. Towards High CO2 Conversions Using Cu/Zn Catalysts Supported on Aluminum Fumarate Metal-Organic Framework for Methanol Synthesis. Catalysts 2022, 12, 1104. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.B.; Norskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Chang, F.; Xiao, M.; Miao, R.; Liu, Y.; Ren, M.; Jia, Z.; Han, D.; Yuan, Y.; Bai, Z.; Yang, L. Copper-Based Catalysts for Electrochemical Carbon Dioxide Reduction to Multicarbon Products. Electrochem. Energy Rev. 2022, 5, 4. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.; Ma, L.; Zhu, G.; Wang, Y.; Xue, X.; Chen, R.; Yang, S.; Jin, Z. Progress and Perspective of Electrocatalytic CO2 Reduction for Renewable Carbonaceous Fuels and Chemicals. Adv. Sci. 2018, 5, 24. [Google Scholar] [CrossRef]
- Zhong, H.; Ghorbani-Asl, M.; Ly, K.H.; Zhang, J.; Ge, J.; Wang, M.; Liao, Z.; Makarov, D.; Zschech, E.; Brunner, E.; et al. Synergistic electroreduction of carbon dioxide to carbon monoxide on bimetallic layered conjugated metal-organic frameworks. Nat. Commun. 2020, 11, 1409. [Google Scholar] [CrossRef]
- Hernandez, S.; Farkhondehfal, M.A.; Sastre, F.; Makkee, M.; Saracco, G.; Russo, N. Syngas production from electrochemical reduction of CO2: Current status and prospective implementation. Green Chem. 2017, 19, 2326–2346. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Maxakato, N.W. Electrocatalytic CO2 conversion on metal-organic frameworks derivative electrocatalysts. J. CO2 Util. 2023, 69, 34. [Google Scholar] [CrossRef]
- Xie, H.; Wang, T.; Liang, J.; Li, Q.; Sun, S. Cu-based nanocatalysts for electrochemical reduction of CO2. Nano Today 2018, 21, 41–54. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Zhou, Y.; Zhao, Y.; Liu, C.J. Recent progresses in the size and structure control of MOF supported noble metal catalysts. Catal. Today 2016, 263, 61–68. [Google Scholar] [CrossRef]
- Kong, C.; Jiang, G.; Sheng, Y.; Liu, Y.; Gao, F.; Liu, F.; Duan, X. Progress on Cu-based metal-organic frameworks for high-efficiency electrochemical CO2 conversion. Chem. Eng. J. 2023, 460, 141803. [Google Scholar] [CrossRef]
- Wang, C.; Lv, Z.; Yang, W.; Feng, X.; Wang, B. A rational design of functional porous frameworks for electrocatalytic CO2 reduction reaction. Chem. Soc. Rev. 2023, 52, 1382–1427. [Google Scholar] [CrossRef] [PubMed]
- Han, G.H.; Bang, J.; Park, G.; Choe, S.; Jang, Y.J.; Jang, H.W.; Kim, S.Y.; Ahn, S.H. Recent Advances in Electrochemical, Photochemical, and Photoelectrochemical Reduction of CO2 to C2+ Products. Small 2023, 19, e2205765. [Google Scholar] [CrossRef]
- Qu, J.; Cao, X.; Gao, L.; Li, J.; Li, L.; Xie, Y.; Zhao, Y.; Zhang, J.; Wu, M.; Liu, H. Electrochemical Carbon Dioxide Reduction to Ethylene: From Mechanistic Understanding to Catalyst Surface Engineering. Nano-Micro Lett. 2023, 15, 178. [Google Scholar] [CrossRef]
- Aran-Ais, R.M.; Gao, D.; Roldan Cuenya, B. Structure- and Electrolyte-Sensitivity in CO2 Electroreduction. Acc. Chem. Res. 2018, 51, 2906–2917. [Google Scholar] [CrossRef]
- Goyal, A.; Marcandalli, G.; Mints, V.A.; Koper, M.T.M. Competition between CO2 Reduction and Hydrogen Evolution on a Gold Electrode under Well-Defined Mass Transport Conditions. J. Am. Chem. Soc. 2020, 142, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Kortlever, R.; Shen, J.; Schouten, K.J.P.; Calle-Vallejo, F.; Koper, M.T.M. Catalysts and Reaction Pathways for the Electrochemical Reduction of Carbon Dioxide. J. Phys. Chem. Lett. 2015, 6, 4073–4082. [Google Scholar] [CrossRef]
- Shao, P.; Zhang, H.-X.; Hong, Q.-L.; Yi, L.; Li, Q.-H.; Zhang, J. Enhancing CO2 Electroreduction to Ethylene via Copper-Silver Tandem Catalyst in Boron-Imidazolate Framework Nanosheet. Adv. Energy Mater. 2023, 13, 2300088. [Google Scholar] [CrossRef]
- Zhong, D.; Zhao, Z.-J.; Zhao, Q.; Cheng, D.; Liu, B.; Zhang, G.; Deng, W.; Dong, H.; Zhang, L.; Li, J.; et al. Coupling of Cu(100) and (110) Facets Promotes Carbon Dioxide Conversion to Hydrocarbons and Alcohols. Angew. Chem. Int. Ed. 2021, 60, 4879–4885. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, Z.; Zhong, L.; Hsu, C.-S.; Xu, X.; Li, Y.; Zhao, S.; Chen, S.; Yu, J.; Chen, S.; et al. Product-Specific Active Site Motifs of Cu for Electrochemical CO2 Reduction. Chem 2021, 7, 406–420. [Google Scholar] [CrossRef]
- Jin, H.; Xiong, L.; Zhang, X.; Lian, Y.; Chen, S.; Lu, Y.; Deng, Z.; Peng, Y. Cu-Based Catalyst Derived from Nitrogen-Containing Metal Organic Frameworks for Electroreduction of CO2. Acta Phys. Chim. Sin. 2021, 37, 11. [Google Scholar] [CrossRef]
- Kang, X.; Fu, G.; Fu, X.-Z.; Luo, J.-L. Copper-based metal-organic frameworks for electrochemical reduction of CO2. Chin. Chem. Lett. 2023, 34, 107757. [Google Scholar] [CrossRef]
- Xiong, W.; Si, D.; Yi, J.; Huang, Y.; Li, H.; Cao, R. Morphology and composition dependence of multicomponent Cu-based nanoreactor for tandem electrocatalysis CO2 reduction. Appl. Catal. B-Environ. 2022, 314, 121498. [Google Scholar] [CrossRef]
- Zheng, T.; Liu, C.; Guo, C.; Zhang, M.; Li, X.; Jiang, Q.; Xue, W.; Li, H.; Li, A.; Pao, C.-W.; et al. Copper-catalysed exclusive CO2 to pure formic acid conversion via single-atom alloying. Nat. Nanotechnol. 2021, 16, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Howarth, A.J.; Peters, A.W.; Vermeulen, N.A.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Best Practices for the Synthesis, Activation, and Characterization of Metal-Organic Frameworks. Chem. Mater. 2017, 29, 26–39. [Google Scholar] [CrossRef]
- Dae-Hyun, N.; Bushuyev, O.S.; Li, J.; De Luna, P.; Seifitokaldani, A.; Cao-Thang, D.; de Arquer, F.P.G.; Wang, Y.; Liang, Z.; Proppe, A.H.; et al. Metal-Organic Frameworks Mediate Cu Coordination for Selective CO2 Electroreduction. J. Am. Chem. Soc. 2018, 140, 11378–11386. [Google Scholar] [CrossRef]
- Xu, X.; Sun, H.; Jiang, S.P.; Shao, Z. Modulating metal-organic frameworks for catalyzing acidic oxygen evolution for proton exchange membrane water electrolysis. Susmat 2021, 1, 460–481. [Google Scholar] [CrossRef]
- Zheng, W.; Lee, L.Y.S. Metal-Organic Frameworks for Electrocatalysis: Catalyst or Precatalyst? Acs Energy Lett. 2021, 6, 2838–2843. [Google Scholar] [CrossRef]
- Shao, P.; Yi, L.; Chen, S.; Zhou, T.; Zhang, J. Metal-organic frameworks for electrochemical reduction of carbon dioxide: The role of metal centers. J. Energy Chem. 2020, 40, 156–170. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Wan, J.; Yu, C. MOF-on-MOF hybrids: Synthesis and applications. Coord. Chem. Rev. 2021, 432, 23. [Google Scholar] [CrossRef]
- Gebremariam, S.K.; Dumee, L.F.; Llewellyn, P.L.; AlWahedi, Y.F.; Karanikolos, G.N. Metal-organic framework hybrid adsorbents for carbon capture-A review. J. Environ. Chem. Eng. 2023, 11, 30. [Google Scholar] [CrossRef]
- Nwosu, U.; Siahrostami, S. Copper-based metal-organic frameworks for CO2 reduction: Selectivity trends, design paradigms, and perspectives. Catal. Sci. Technol. 2023, 13, 3740–3761. [Google Scholar] [CrossRef]
- Chen, R.; Cheng, L.; Liu, J.; Wang, Y.; Ge, W.; Xiao, C.; Jiang, H.; Li, Y.; Li, C. Toward High-Performance CO2-to-C2 Electroreduction via Linker Tuning on MOF-Derived Catalysts. Small 2022, 18, 2200720. [Google Scholar] [CrossRef]
- Jana, A.; Snyder, S.W.; Crumlin, E.J.; Qian, J. Integrated carbon capture and conversion: A review on C2+ product mechanisms and mechanism-guided strategies. Front. Chem. 2023, 11, 11. [Google Scholar] [CrossRef]
- Marcos, F.C.F.; Costa, M.J.F.; Catuzo, G.L.; de Moraes, D.A.; de Oliveira Junior, M.; Mastelaro, V.R.; Assaf, J.M.; Giudici, R.; Assaf, E.M. Supported Cu catalysts on UiO-66 toward enhanced methanol selectivity by CO2 hydrogenation: Effect of Cu loading. J. Catal. 2023, 427, 9. [Google Scholar] [CrossRef]
- Kim, D.; Kim, I.J.; Kwon, H.T.; Paeng, K.; Lee, H. CuBTC Metal-Organic Framework Decorated with FeBTC Nanoparticles with Enhance Water Stability for Environmental Remediation Applications. Acs Omega 2023, 8, 14900–14906. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; Ding, P.; Huang, J.; Dierolf, M.; Kelly, S.D.; Qiu, X.; Chen, Y.; Hussain, M.Z.; Li, W.; et al. Engineering a Cu-Pd Paddle-Wheel Metal-Organic Framework for Selective CO2 Electroreduction. Angew. Chem. Int. Ed. Engl. 2024, 63, e202414600. [Google Scholar] [CrossRef]
- Cho, J.H.; Lee, C.; Hong, S.H.; Jang, H.Y.; Back, S.; Seo, M.-g.; Lee, M.; Min, H.-K.; Choi, Y.; Jang, Y.J.; et al. Transition Metal Ion Doping on ZIF-8 Enhances the Electrochemical CO2 Reduction Reaction. Adv. Mater. 2023, 35, e2208224. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Zhu, H.; Huang, J.; Liao, P.; Chen, X. Ultrathin two-dimensional triptycence-based metal-organic framework for highly selective CO2 electroreduction to CO. Chin. Chem. Lett. 2023, 34, 107134. [Google Scholar] [CrossRef]
- Xin, Z.; Yuan, Z.; Liu, J.; Wang, X.; Shen, K.; Chen, Y.; Lan, Y.-Q. Cu cluster embedded porous nanofibers for high-performance CO2 electroreduction. Chin. Chem. Lett. 2023, 34, 107458. [Google Scholar] [CrossRef]
- Shao, P.; Wan, Y.-M.; Yi, L.; Chen, S.; Zhang, H.-X.; Zhang, J. Enhancing Electroreduction CO2 to Hydrocarbons via Tandem Electrocatalysis by Incorporation Cu NPs in Boron Imidazolate Frameworks. Small 2024, 20, 8. [Google Scholar] [CrossRef]
- Cui, S.; Yu, C.; Tan, X.; Li, W.; Zhang, Y.; Qiu, J. A tandem catalyst with high CO2 capture capability to achieve a promoted CO2-to-CH4 electrochemical conversion. Chem. Eng. J. 2023, 470, 144083. [Google Scholar] [CrossRef]
- Sun, B.; Hu, H.; Liu, H.; Guan, J.; Song, K.; Shi, C.; Cheng, H. Highly-exposed copper and ZIF-8 interface enables synthesis of hydrocarbons by electrocatalytic reduction of CO2. J. Colloid Interface Sci. 2024, 661, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Trinh, Q.T.; Wang, H.; Wu, S.; Arce-Ramos, J.M.; Sullivan, M.B.; Kraft, M.; Ager, J.W.W.; Zhang, J.; Xu, R. Selective and Stable CO2 Electroreduction to CH4 via Electronic Metal-Support Interaction upon Decomposition/Redeposition of MOF. Small 2023, 19, e2301379. [Google Scholar] [CrossRef]
- Lv, J.; Li, W.; Li, J.; Zhu, Z.; Dong, A.; Lv, H.; Li, P.; Wang, B. A Triptycene-Based 2D MOF with Vertically Extended Structure for Improving the Electrocatalytic Performance of CO2 to Methane. Angew. Chem. Int. Ed. 2023, 62, e202217958. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.-Y.; Sun, W.Y. In Situ Carbon-Encapsulated Copper-Doped Cerium Oxide Derived from MOFs for Boosting CO2-to-CH4 Electro-Conversion. Acs Catal. 2023, 13, 1545–1553. [Google Scholar] [CrossRef]
- Xue, H.; Huang, J.-R.; Wang, Z.-S.; Zhao, Z.-H.; Shi, W.; Liao, P.-Q.; Chen, X.-M. Tailoring ligand fields of metal-azolate frameworks for highly selective electroreduction of CO2 to hydrocarbons at industrial current density. Chin. J. Catal. 2023, 53, 102–108. [Google Scholar] [CrossRef]
- Lu, P.; Lv, J.; Chen, Y.; Ma, Y.; Wang, Y.; Lyu, W.; Yu, J.; Zhou, J.; Yin, J.; Xiong, Y.; et al. Steering the Selectivity of Carbon Dioxide Electroreduction from Single-Carbon to Multicarbon Products on Metal-Organic Frameworks via Facet Engineering. Nano Lett. 2024, 24, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, A.; Wei, D.; Huang, F.; Ma, J.; He, H.; Xu, J. Atomic cerium-doped CuOx catalysts for efficient electrocatalytic CO2 reduction to CH4. Chin. Chem. Lett. 2025, 36, 110175. [Google Scholar] [CrossRef]
- Sun, H.; Lin, L.; Hua, W.; Xie, X.; Mu, Q.; Feng, K.; Zhong, J.; Lyu, F.; Deng, Z.; Peng, Y. Atomically dispersed Co-Cu alloy reconstructed from metal-organic framework to promote electrochemical CO2 methanation. Nano Res. 2023, 16, 3680–3686. [Google Scholar] [CrossRef]
- Jing, Z.; Su, W.; Fan, Y. Increasing electrochemical carbon dioxide reduction to methane via a novel copper-based conductive metal organic framework. J. Colloid Interface Sci. 2025, 678, 251–260. [Google Scholar] [CrossRef]
- Sun, M.; Cheng, J.; Anzai, A.; Kobayashi, H.; Yamauchi, M. Modulating Electronic States of Cu in Metal-Organic Frameworks for Emerging Controllable CH4/C2H4 Selectivity in CO2 Electroreduction. Adv. Sci. 2024, 11, 2404931. [Google Scholar] [CrossRef]
- Zou, Y.-H.; Wang, X.; Ning, F.; Yi, J.; Liu, Y. Implanting MWCNTs in BiCu-MOFs to enhance electrocatalytic CO2 reduction to formate. Sep. Purif. Technol. 2023, 317, 11. [Google Scholar] [CrossRef]
- Huang, H.; Yue, K.; Liu, C.; Zhan, K.; Dong, H.; Yan, Y. CuO (111) Microcrystalline Evoked Indium-Organic Framework for Efficient Electroreduction of CO2 to Formate. Small 2024, 20, 2400441. [Google Scholar] [CrossRef]
- Liu, S.; Song, L.; Liu, R.; Li, L.; Yang, D.; Yuan, S.; Dai, X. Controlled One-Step Synthesis of MOF-on-MOF Cocatalysts for Two-Channel Electrocatalytic Conversion of CO2 to Formic Acid. Small 2023, 19, 2304808. [Google Scholar] [CrossRef]
- Lee, J.; Choi, H.; Mun, J.; Jin, E.; Lee, S.; Nam, J.; Umer, M.; Cho, J.; Lee, G.; Kwon, Y.; et al. Nanozyme Based on Porphyrinic Metal-Organic Framework for Electrocatalytic CO2 Reduction. Small Struct. 2023, 4, 2200087. [Google Scholar] [CrossRef]
- He, Q.; Li, H.; Hu, Z.; Lei, L.; Wang, D.; Li, T.-T. Highly Selective CO2 Electroreduction to C2H4 Using a Dual-Sites Cu(II) Porphyrin Framework Coupled with Cu2O Nanoparticles via a Synergetic-Tandem Strategy. Angew. Chem. Int. Ed. 2024, 63, e202407090. [Google Scholar] [CrossRef]
- Jang, J.; Lee, K.; Shin, H.; Lee, H.S.; Lee, B.-H.; Jeong, J.; Kim, J.; Hwang, W.; Park, S.; Bootharaju, M.S.; et al. Distinct reconstruction of aluminum-doped oxide-derived copper enhances the selectivity of C2+ products in CO2 electroreduction. J. Mater. Chem. A 2023, 11, 19066–19073. [Google Scholar] [CrossRef]
- Zhou, Z.; Liang, S.; Xiao, J.; Zhang, T.; Li, M.; Xie, W.; Wang, Q. Surface modification of Cu2O with stabilized Cu+ for highly efficient and stable CO2 electroreduction to C2+ chemicals. J. Energy Chem. 2023, 84, 277–285. [Google Scholar] [CrossRef]
- Zheng, K.; Hu, D.-Y.; Zhang, X.-W.; Xiao, X.-X.; Liang, Z.-J.; Wu, J.-X.; Lin, D.-Y.; Zhuo, L.-L.; Yi, H.; Gong, L.; et al. Bending two-dimensional Cu(i)-based coordination networks to inverse electrocatalytic HER/CO2RR selectivity. J. Mater. Chem. A 2024, 12, 16396–16402. [Google Scholar] [CrossRef]
- Yan, T.; Wang, P.; Sun, W.-Y. Single-Site Metal-Organic Framework and Copper Foil Tandem Catalyst for Highly Selective CO2 Electroreduction to C2H4. Small 2023, 19, e2206070. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Hu, D.-Y.; Wang, C.; Liang, Z.-J.; Zhang, X.-W.; Xiao, X.-X.; Wu, J.-X.; Zhuo, L.-L.; Lin, D.-Y.; Zhou, D.-D.; et al. Isomeric Cu(I) Azolate Frameworks Showing Contrasting Electrocatalytic CO2 Reduction Selectivities and Stabilities. Small 2024, 21, 2408510. [Google Scholar] [CrossRef]
- Ma, M.; Xiong, L.; Dong, Y.; Bai, Q.; Hua, W.; Zheng, Z.; Lyu, F.; Lian, Y.; Wei, Z.; Yuan, H.; et al. Metalloporphyrin Frameworks to Encapsulate Copper Oxides for Boosting Ethylene Production in Neutral Electrolyte. Adv. Funct. Mater. 2024, 34, 2315667. [Google Scholar] [CrossRef]
- Wen, Y.; Cheng, W.-H.; Wang, Y.-R.; Shen, F.-C.; Lan, Y.-Q. Tailoring the Hydrophobic Interface of Core-Shell HKUST-1@Cu2O Nanocomposites for Efficiently Selective CO2 Electroreduction. Small 2024, 20, e2307467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, Y. Enhanced CO2 Electroreduction to ethylene on Cu nanocube coated with nitrogen-doped carbon shell in-situ electro-derived from metal-organic framework. Chem. Eng. J. 2024, 499. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Y. Stabilizing *CO intermediate on nitrogen-doped carbon-coated CuxOy derived from metal-organic framework for enhanced electrochemical CO2-to-ethylene. J. Mater. Chem. A 2024, 13, 2902–2910. [Google Scholar] [CrossRef]
- Heng, J.-M.; Zhu, H.-L.; Zhao, Z.-H.; Liao, P.-Q.; Chen, X.-M. Fabrication of Ultrahigh-Loading Dual Copper Sites in Nitrogen-Doped Porous Carbons Boosting Electroreduction of CO2 to C2H4 Under Neutral Conditions. Adv. Mater. 2024, 37, e2415101. [Google Scholar] [CrossRef]
- Chang, B.; Zhang, F.; Min, Z.; Wang, N.; Xia, Q.; Fan, J.; Fan, M.; Wang, J. Confined CuI sites in partially electro-reduced 2D conductive Cu-MOF film for boosting CO2 electrocatalysis to C2 products. Chem. Eng. J. 2024, 496, 153993. [Google Scholar] [CrossRef]
- Wen, C.F.; Yang, S.; He, J.J.; Niu, Q.; Liu, P.F.; Yang, H.G. Anionic Metal-Organic Framework Derived Cu Catalyst for Selective CO2 Electroreduction to Hydrocarbons. Small 2024, 20, e2405051. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Wajrak, M.; Bhatelia, T.; Jiang, S.P.; Shao, Z. Grain boundary engineering: An emerging pathway toward efficient electrocatalysis. Infomat 2024, 6, e12608. [Google Scholar] [CrossRef]
- Sun, D.; Xu, X.; Qin, Y.; Jiang, S.P.; Shao, Z. Rational Design of Ag-Based Catalysts for the Electrochemical CO2 Reduction to CO: A Review. Chemsuschem 2020, 13, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shin, D.; Park, J.; Jung, J.-Y.; Song, H. Grain Boundary-Rich Copper Nanocatalysts Generated from Metal-Organic Framework Nanoparticles for CO2-to-C2+ Electroconversion. Adv. Sci. 2023, 10, 11. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Song, Y.; Geng, S.; Peng, Z.; Yu, J.; Liu, F.; Wang, Y.; Xi, S.; Zhang, Z.; et al. Simultaneous Defect and Size Control of Metal-Organic Framework Nanostructures for Highly Efficient Carbon Dioxide Electroreduction to Multicarbon Products. Acs Mater. Lett. 2023, 5, 2121–2130. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Z.; Gao, G.; Chen, C.; Xue, Y.; Zhao, J.; Lei, Q.; Jin, M.; Zhu, C.; Han, Y.; et al. Enhanced CO2 Electroreduction Selectivity toward Ethylene on Pyrazolate-Stabilized Asymmetric Ni-Cu Hybrid Sites. J. Am. Chem. Soc. 2023, 145, 26444–26451. [Google Scholar] [CrossRef]
- Deng, J.; Qiu, L.; Xin, M.; He, W.; Zhao, W.; Dong, J.; Xu, G. Boosting Electrochemical CO2 Reduction on Copper-Based Metal-Organic Frameworks via Valence and Coordination Environment Modulation. Small 2024, 20, e2311060. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xiao, J.; Guo, L.; Xie, Z.; Wang, K.; Wang, Y.; Hao, F.; Ma, Y.; Zhou, J.; Lu, P.; et al. In Situ Phase Transformation-Enabled Metal-Organic Frameworks for Efficient CO2 Electroreduction to Multicarbon Products in Strong Acidic Media. ACS Nano 2024, 18, 33602–33613. [Google Scholar] [CrossRef]
- Yin, B.; Wang, C.; Xie, S.; Gu, J.; Sheng, H.; Wang, D.-X.; Yao, J.; Zhang, C. Regulating Spin Density using Tempol Molecules for Enhanced CO2-to-Ethylene Conversion by HKUST-1 Framework Derived Electrocatalysts. Angew. Chem. Int. Ed. 2024, 63, e202405873. [Google Scholar] [CrossRef]
- Bohan, A.; Jin, X.; Wang, M.; Wang, Y.; Chen, W.; Wei, Z.; Du, Z.; Liu, X.; Wang, Y.; Zhang, L. Steering CO2 electroreduction toward C2+ products by defect-engineered coordination microenvironments of Cu-MOFs. Chem. Eng. J. 2024, 500, 157076. [Google Scholar] [CrossRef]
- Xie, G.; Guo, W.; Fang, Z.; Duan, Z.; Lang, X.; Liu, D.; Mei, G.; Zhai, Y.; Sun, X.; Lu, X. Dual-Metal Sites Drive Tandem Electrocatalytic CO2 to C2+ Products. Angew. Chem. Int. Ed. 2024, 63, e202412568. [Google Scholar] [CrossRef]
- Su, W.; Guo, W.; Fan, Y. CuAg bimetallic catalysts derived from an Ag-anchored Cu-based metal-organic framework for CO2 electroreduction to ethanol. Chem. Eng. J. 2023, 477, 147204. [Google Scholar] [CrossRef]
- Zhao, Z.-H.; Huang, J.-R.; Liao, P.-Q.; Chen, X.-M. Highly Efficient Electroreduction of CO2 to Ethanol via Asymmetric C-C Coupling by a Metal-Organic Framework with Heterodimetal Dual Sites. J. Am. Chem. Soc. 2023, 145, 26783–26790. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Huang, D.; Huang, R.-K.; He, X.-L.; Luo, Y.; Xiang, Y.-L.; Jiang, L.-b.; Dong, M.; Li, S.; Zhang, Z.; et al. Metal-Organic Framework Supported Low-Nuclearity Cluster Catalysts for Highly Selective Carbon Dioxide Electroreduction to Ethanol. Angew. Chem. Int. Ed. 2024, 63, e202409270. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Wang, X.; Feng, Y.; Zhang, H.; Zhang, G. Self-Polarization Triggered Multiple Polar Units Toward Electrochemical Reduction of CO2 to Ethanol with High Selectivity. Angew. Chem. Int. Ed. 2023, 62, e202302241. [Google Scholar] [CrossRef]
- Wang, C.; Lv, Z.; Liu, Y.; Dai, L.; Liu, R.; Sun, C.; Liu, W.; Feng, X.; Yang, W.; Wang, B. Asymmetric Cu-N1O3 Sites Coupling Atop-type and Bridge-type Adsorbed *C1 for Electrocatalytic CO2-to-C2 Conversion. Angew. Chem. Int. Ed. 2024, 63, e202411216. [Google Scholar] [CrossRef]
- Liu, W.; Tang, B.; Huang, K.; Zhang, Z.; Wang, Z.; An, G.; Zhang, M.; Wang, K.; Fu, S.; Guo, H.; et al. Radiation-Synthesized Metal-Organic Frameworks with Ligand-Induced Lewis Pairs for Selective CO2 Electroreduction. Small 2024, 20, e2408688. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, M.; Ye, J.; Liu, L.; Li, L.; Li, Y.; Huang, X. Highly Selective CO2 Electroreduction to C2+Products over Cu2O-Decorated 2D Metal-Organic Frameworks with Rich Heterogeneous Interfaces. Nano Lett. 2023, 23, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Li, A.; Ma, C.; Gao, Z.; Zhou, B.; Zhu, L.; Huang, Z. Nitrogen-doped carbon confined Cu-Ag bimetals for efficient electroreduction of CO2 to high-order products. Chem. Eng. J. 2023, 468, 143606. [Google Scholar] [CrossRef]
- Yang, K.; Sun, Y.; Chen, S.; Li, M.; Zheng, M.; Ma, L.; Fan, W.; Zheng, Y.; Li, Q.; Duan, J. Less-Coordinated Atomic Copper-Dimer Boosted Carbon-Carbon Coupling During Electrochemical CO2 Reduction. Small 2023, 19, 9. [Google Scholar] [CrossRef]
- Mao, X.; Gong, W.; Fu, Y.; Li, J.; Wang, X.; O’Mullane, A.P.; Xiong, Y.; Du, A. Computational Design and Experimental Validation of Enzyme Mimicking Cu-Based Metal-Organic Frameworks for the Reduction of CO2 into C2 Products: C-C Coupling Promoted by Ligand Modulation and the Optimal Cu-Cu Distance. J. Am. Chem. Soc. 2023, 145, 21442–21453. [Google Scholar] [CrossRef]


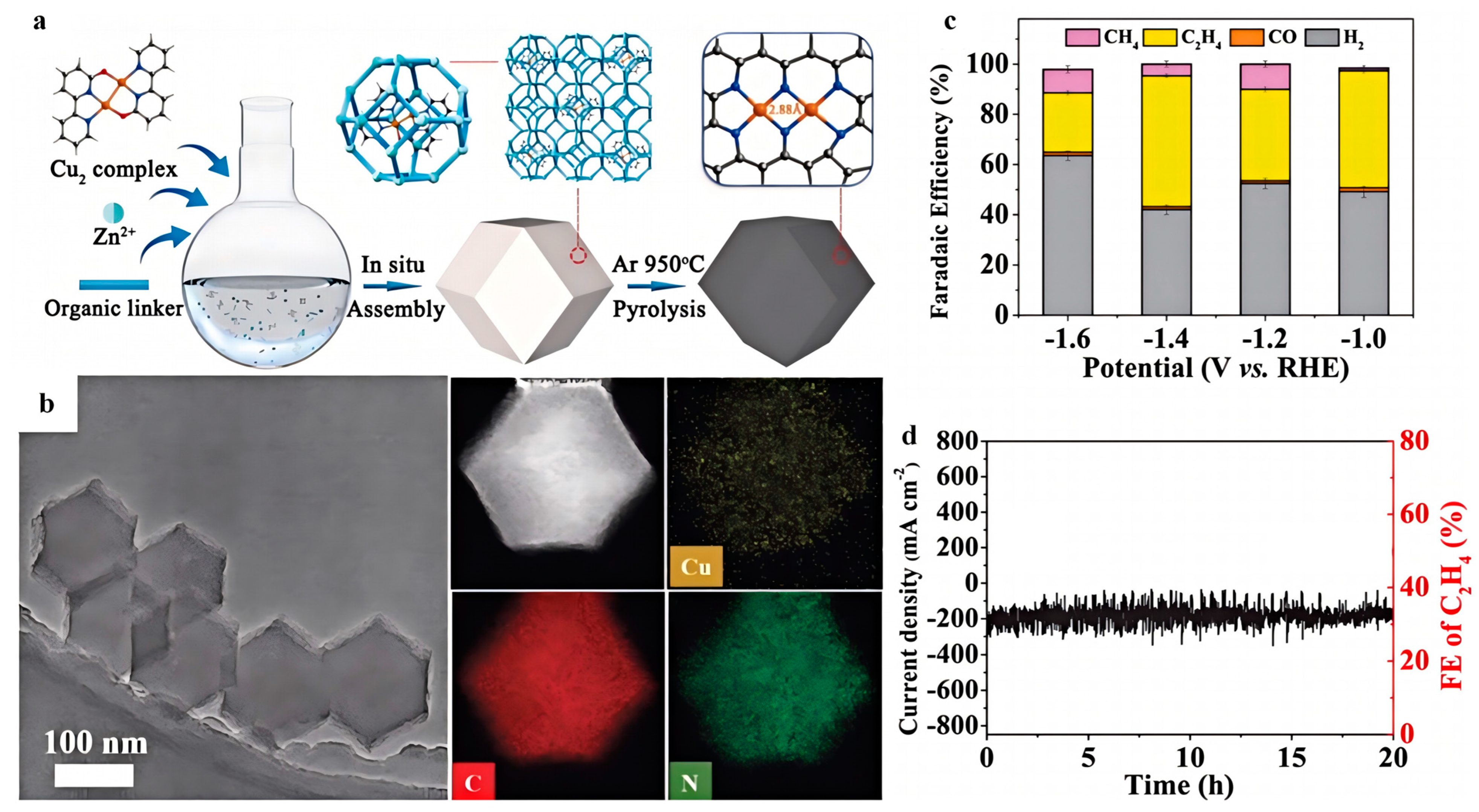
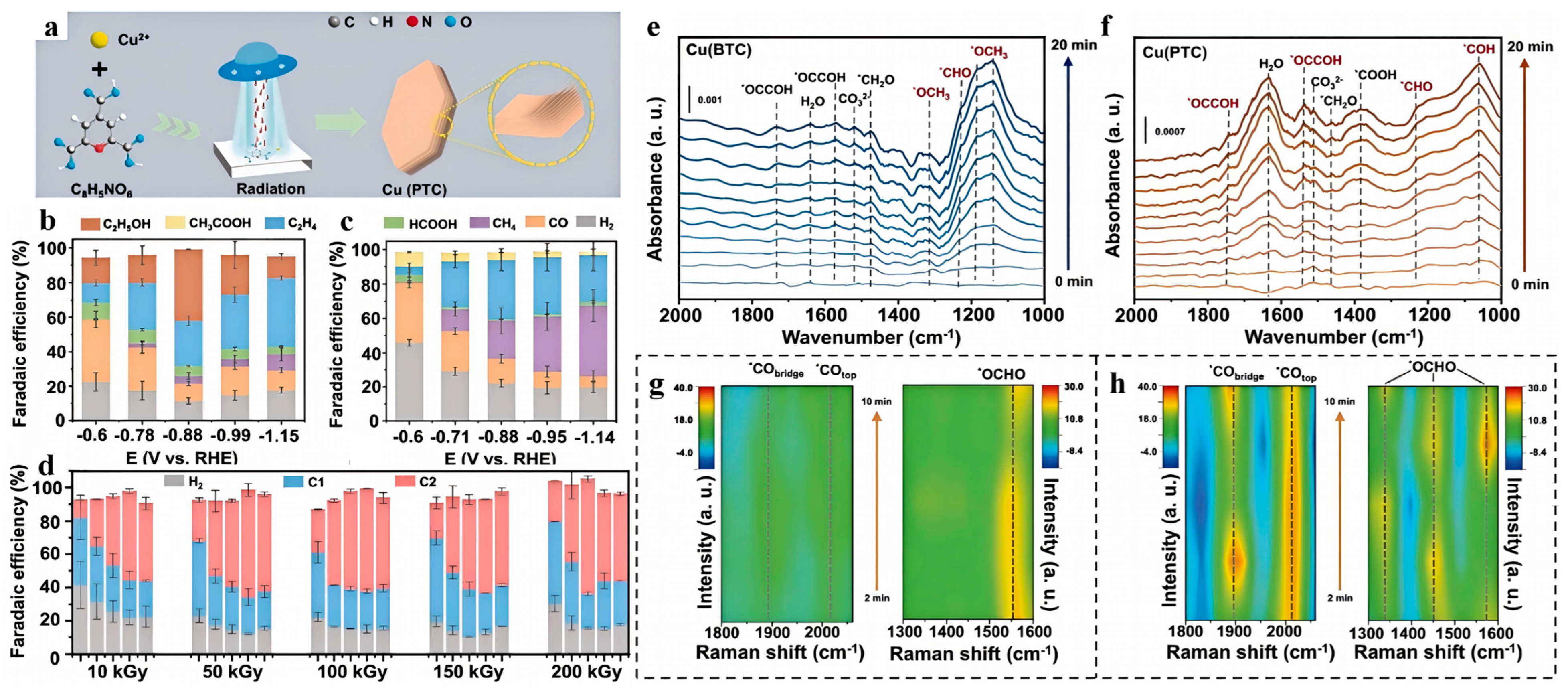
| Electrocatalyst | Electrolyte | Main Product | FE (%) | Potential (V vs. RHE) | Ref. |
|---|---|---|---|---|---|
| Cu3−xPdx(BTC)2 | 0.5 M KHCO3 | CO | 84.8 | −0.77 | [38] |
| Cu0.5Zn0.5/ZIF-8 | 0.5 M KHCO3 | CO | 88.5 | −1.0 | [39] |
| Cu-HHTT | 0.1 M KHCO3 | CO | 96.6 | −0.6 | [40] |
| MCP-500 | 0.1 M KHCO3 | CO | 98 | −0.8 | [41] |
| Cu@BIF-144(Zn) | 0.1 M KHCO3 | CH4 C2H4 | 41.8 12.9 | −1.6 −1.5 | [42] |
| R-Cu-TAl-CNTs1 | 0.1 M KHCO3 | CH4 | 54 | −1.56 | [43] |
| Cu@ZIF-8 NWs | 0.1 M KHCO3 | CH4 C2H4 | 57.5 | −0.7 | [44] |
| Cu-MIL-derived Cu/C | 0.1 M KHCO3 | CH4 | 55 | −1.4 | [45] |
| 2D-vc-MOF(Cu) | 0.1 M KCl | CH4 | 65 | −1.4 | [46] |
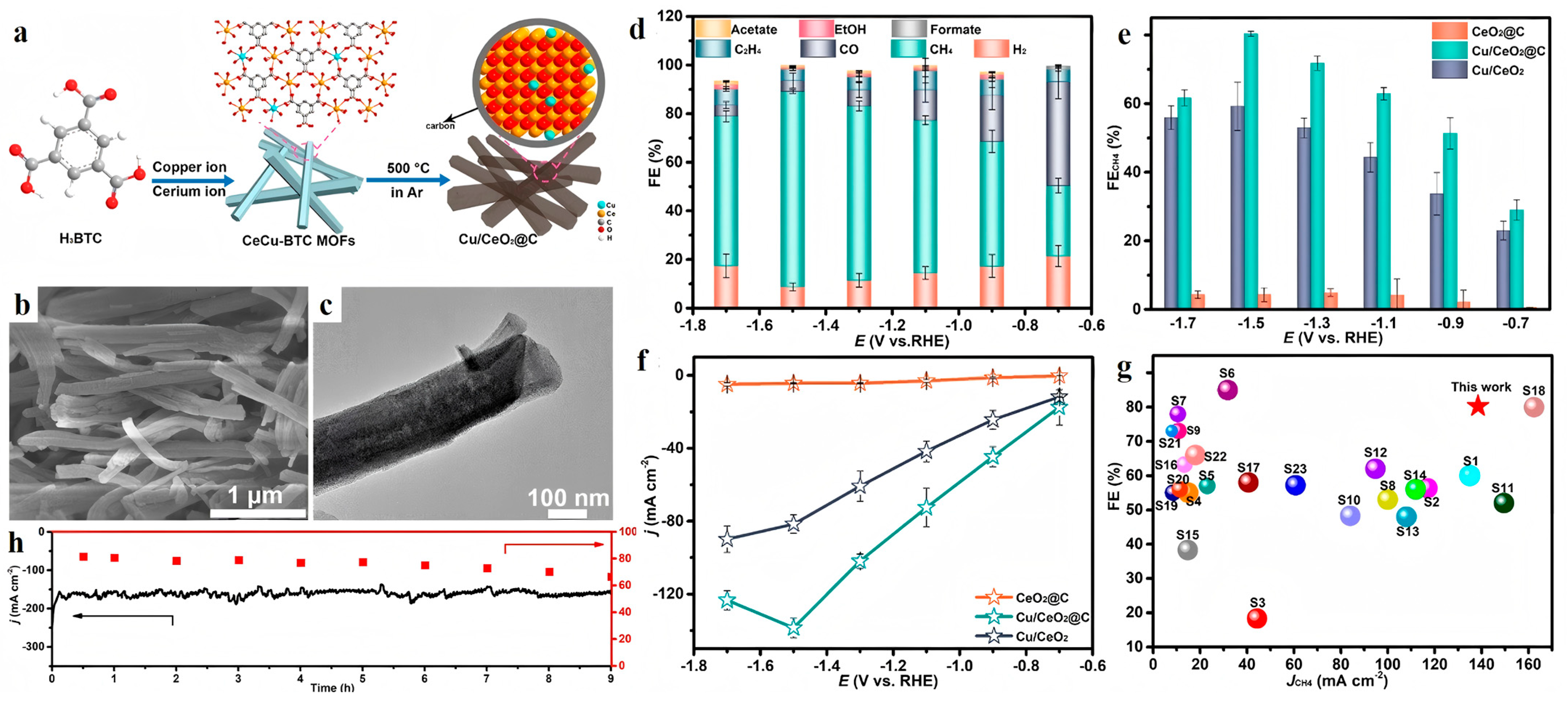
| Cu/CeO2@C | 0.5 M KOH | CH4 | 80.3 | −1.5 | [47] |
| Cu-BTP | 0.1 M KHCO3 | CH4 C2H4 | 60 22 | −1.6 | [48] |
| Cu-MMT-H2O Cu-MMT-IPA | 0.1 M KHCO3 | CH4 C2H4 | 55.22 50.98 | −1.4 −1.15 | [49] |
| Ce/CuOx-NPs | 1 M KOH | CH4 | 67.4 | −1.6 | [50] |
| Co0.02-CuBDC | 1 M KOH | CH4 | 60 | −1.02 | [51] |
| Cu-PD-2-MBI | 1 M KOH | CH4 | 73.7 | −1.3 | [52] |
| Cu/UiO-66-F (Ce) | 1 M KOH | CH4 C2H4 | 58 44 | −1.33 −0.96 | [53] |
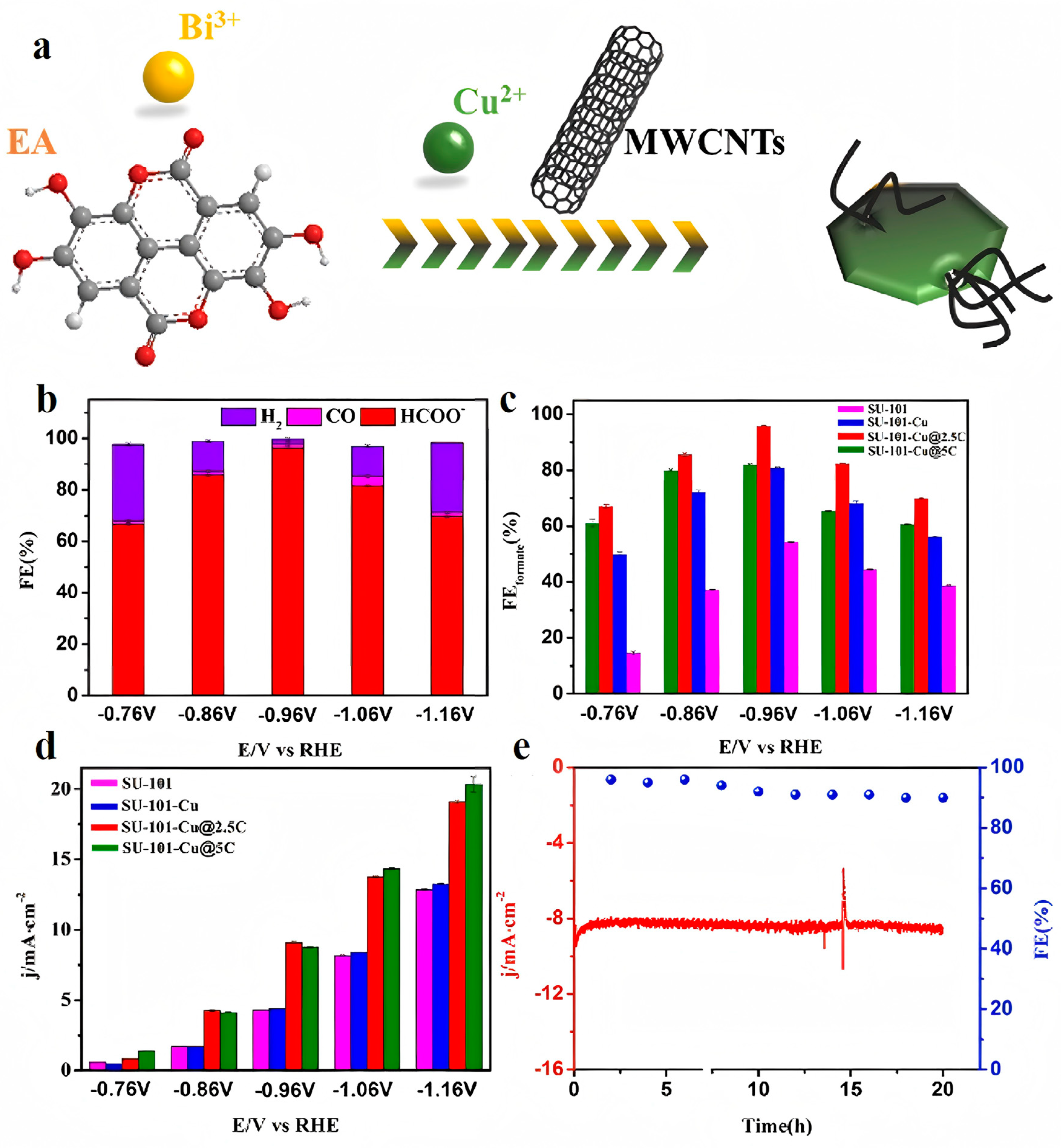
| SU-101-Cu@2.5C | 0.5 M KHCO3 | HCOOH/HCOO− | ≈100 | −0.86 | [54] |
| MIL-68(In)/CuO | 1 M KOH | HCOO− | 89.7 | −0.7 | [55] |
| Cu@Bi1/2 | 0.5 M KHCO3 | HCOOH | 89.26 | −0.95 (V vs. Ag/AgCl) | [56] |
| PPF-100 | 0.5 M BMImBF4 + H2O/DMSO | CO HCOOH | 72.4 24.1 | −3.0 (V vs. Ag/AgNO3) | [57] |
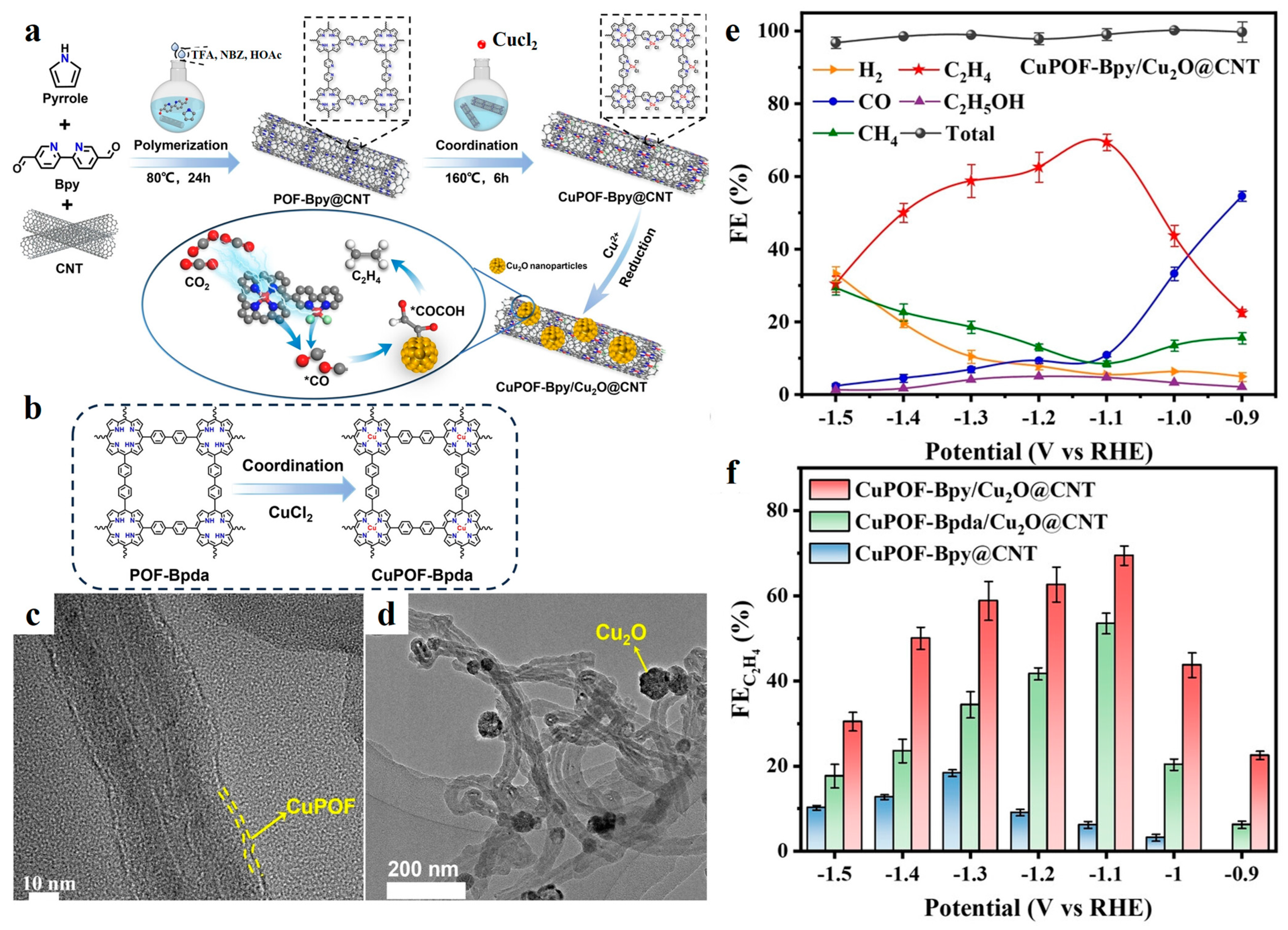
| CuPOF-Bpy/Cu2O@CNT | 0.5 M KHCO3 | C2H4 | 71 | −1.1 | [58] |
| CuO_Al | 1 M KHCO3 | C2H4 C2+ | 43.4 69.3 | −1.08 −0.98 | [59] |
| Cu2O@CuBTC | 1 M KOH | C2H4 C2H5OH | 40 19 | -- | [60] |
| w-A100H0 | 0.1 M KHCO3 | C2H4 | 51.8 | −1.3 | [61] |
| Cu-MOF-CF | 0.1 M KHCO3 | C2H4 | 48.6 | −1.11 | [62] |
| Ag@BIF-104NSs(Cu) | 0.5 M KHCO3 + 0.5 M KCl | C2H4 | 21.43 | −1.2 | [18] |
| MAF-2Fa | 0.1 M KHCO3 | C2H4 | 52.9 | −1.2 | [63] |
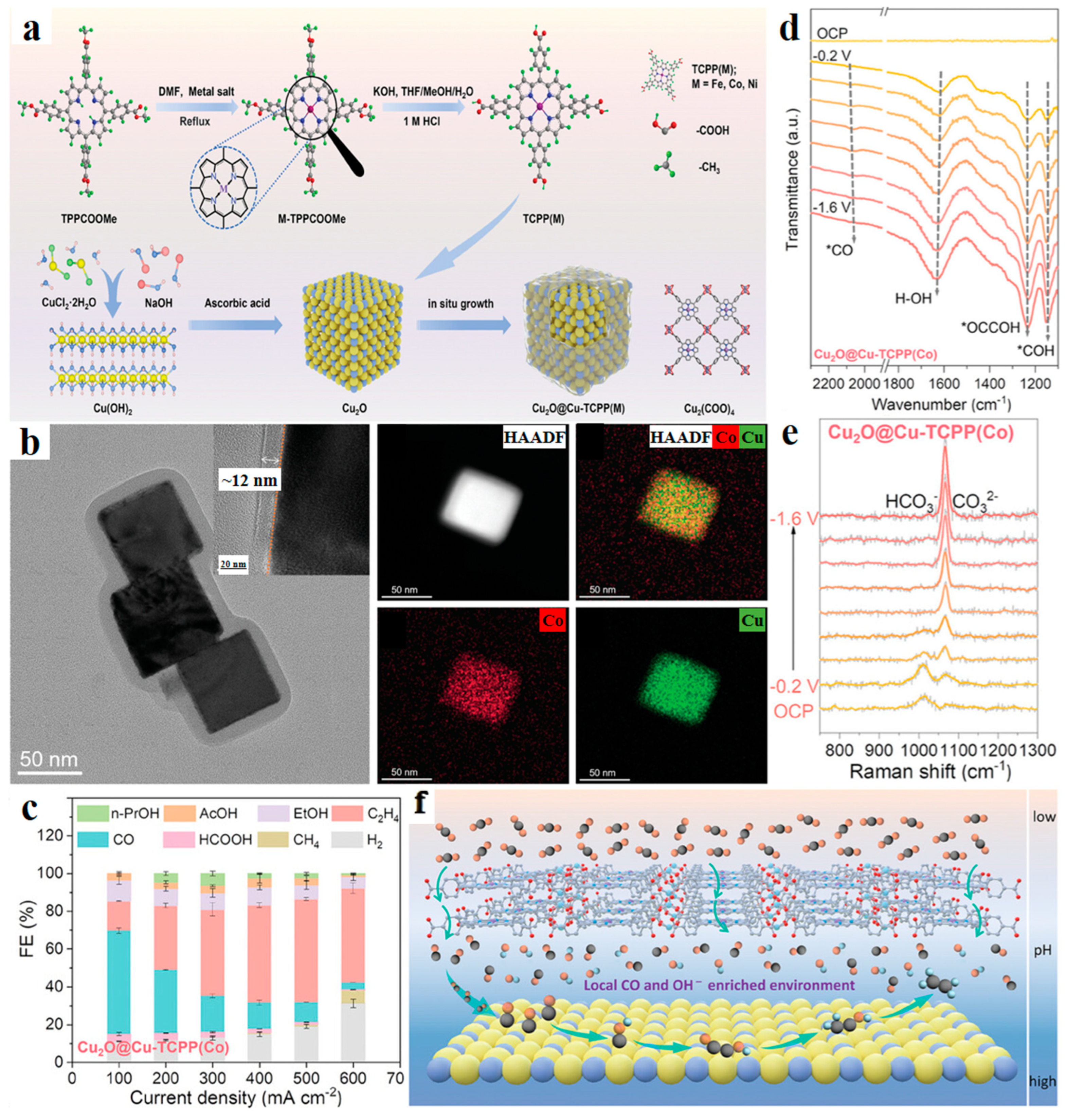
| Cu2O@Cu-TCPP(Co) | 1 M KCl | C2H4 C2+ | 54 69 | -- | [64] |
| HKUST-1@Cu2O/PTFE-1 | -- | C2H4 C2+ | 46.08 67.41 | −1.0 | [65] |
| Cu-cube/CN | 0.1 M KHCO3 | C2H4 | 49.6 | −1.15 | [66] |
| CuxOy/CN | 0.1 M KHCO3 | C2H4 | 44 | −1.1 | [67] |
| NPCMAF-4-Cu2-21 | 0.1 M KHCO3 | C2H4 | 52 | −1.4 | [68] |
| CuI-CuIIHHTP@Cu0 | 0.5 M KHCO3 | C2H4 C2H5OH | 42.3 30.3 | −1.17 | [69] |
| aHD-Cu | 1 M KOH | C2H4 C2+ | 56 ≈80 | −0.7 | [70] |
| n-MDC-250 | 0.1 M KHCO3 | C2H4 C2+ | 63.1 80.9 | −1.01 | [73] |
| CuTrz-109 nm | 0.1 M KHCO3 | C2H4 C2+ | 55.4 81.8 | −1.15 | [74] |
| Cu1Ni-BDP MOF | 1 M KOH | C2H4 | 52.7 | −1.3 | [75] |
| HC-HKUST-3 | 0.1 M KHCO3 | C2H4 | 57 | −1.6 | [76] |
| Cu-btca-s | 3.0 M KCl and 0.05 M H2SO4 | C2H4 C2+ | 51.2 81.9 | −2.5 | [77] |
| TEMPOL@HKUST-1 | 0.1 M KHCO3 | C2H4 | 44.7 | −1.8 (V vs. Ag/AgCl) | [78] |
| UC-Cu-BTEC-2 | 0.1 M KHCO3 | C2H4 C2+ | 50.1 77.2 | −1.7 | [79] |
| 1.4%Pd-Cu@CuPz2 | 0.1 M KCl | C2H5OH C2+ | 47.5 81.9 | −1.09 | [80] |
| CuAg5@NC | 0.5 M KHCO3 | C2H5OH C2+ | 51.8 82.6 | −1.0 −1.2 | [81] |
| CuSn-HAB | 1 M KOH | C2H5OH | 56 | −0.57 | [82] |
| Cu-MOF-20 | 0.5 M KHCO3 | C2H5OH | 82.5 | −1.0 | [83] |
| Cu2O@MOF/CF | 0.5 M KHCO3 | C2H5OH | 44.3 | −0.615 | [84] |
| BIT-119 | 0.1 M KHCO3 | C2H5OH C2+ | 42 75 | −1.8 (V vs. Ag/AgCl) | [85] |
| Cu(PTC) | 1 M KOH | C2+ | 70 | −0.88 | [86] |
| Cu2O@Cu-BDC | 0.1 M KBr | C2+ | 72.1 | −1.19 | [87] |
| Cu-Ag/NC | 0.1 M KHCO3 | C2+ | 55.3 | −1.2 | [88] |
| Cu-TDC | 1 M KOH | C2+ | 71 | -- | [89] |
| MIL-53(Cu) | 0.1 M KHCO3 | C2+ | 55.5 | −1.19 | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Yang, T.; Nie, W.; Gao, Y.; Wang, Z.; Dong, A. Recent Advances in Cu-Based Metal–Organic Framework Electrocatalysts for CO2 Reduction Reactions. Catalysts 2025, 15, 328. https://doi.org/10.3390/catal15040328
Gao H, Yang T, Nie W, Gao Y, Wang Z, Dong A. Recent Advances in Cu-Based Metal–Organic Framework Electrocatalysts for CO2 Reduction Reactions. Catalysts. 2025; 15(4):328. https://doi.org/10.3390/catal15040328
Chicago/Turabian StyleGao, Honglin, Ting Yang, Wen Nie, Yuefeng Gao, Zhen Wang, and Aiyi Dong. 2025. "Recent Advances in Cu-Based Metal–Organic Framework Electrocatalysts for CO2 Reduction Reactions" Catalysts 15, no. 4: 328. https://doi.org/10.3390/catal15040328
APA StyleGao, H., Yang, T., Nie, W., Gao, Y., Wang, Z., & Dong, A. (2025). Recent Advances in Cu-Based Metal–Organic Framework Electrocatalysts for CO2 Reduction Reactions. Catalysts, 15(4), 328. https://doi.org/10.3390/catal15040328






