Titanosilicate ETS-10-Modified Cu2O for Enhanced Visible-Light Photoelectrochemical Activity
Abstract
1. Introduction
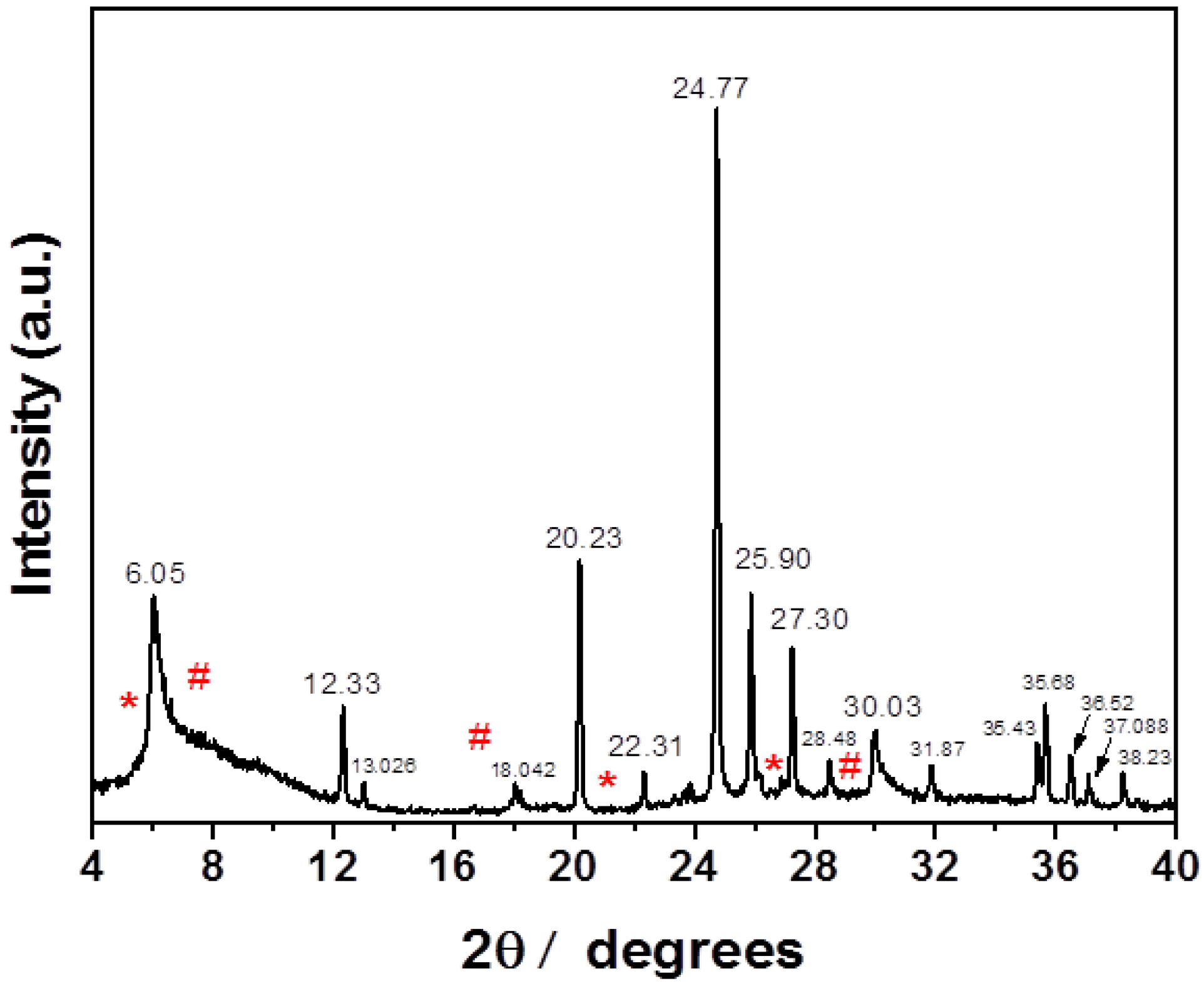
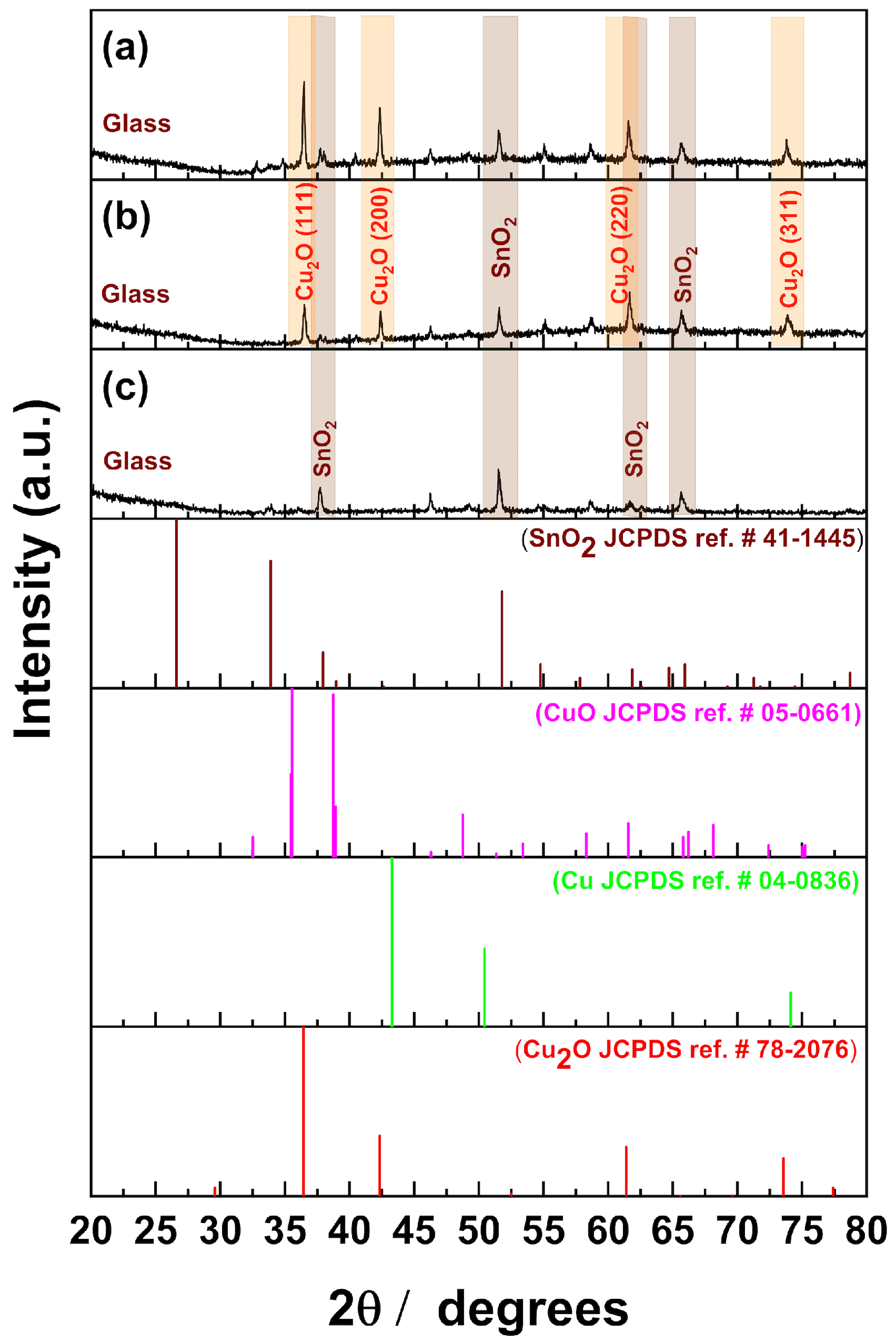
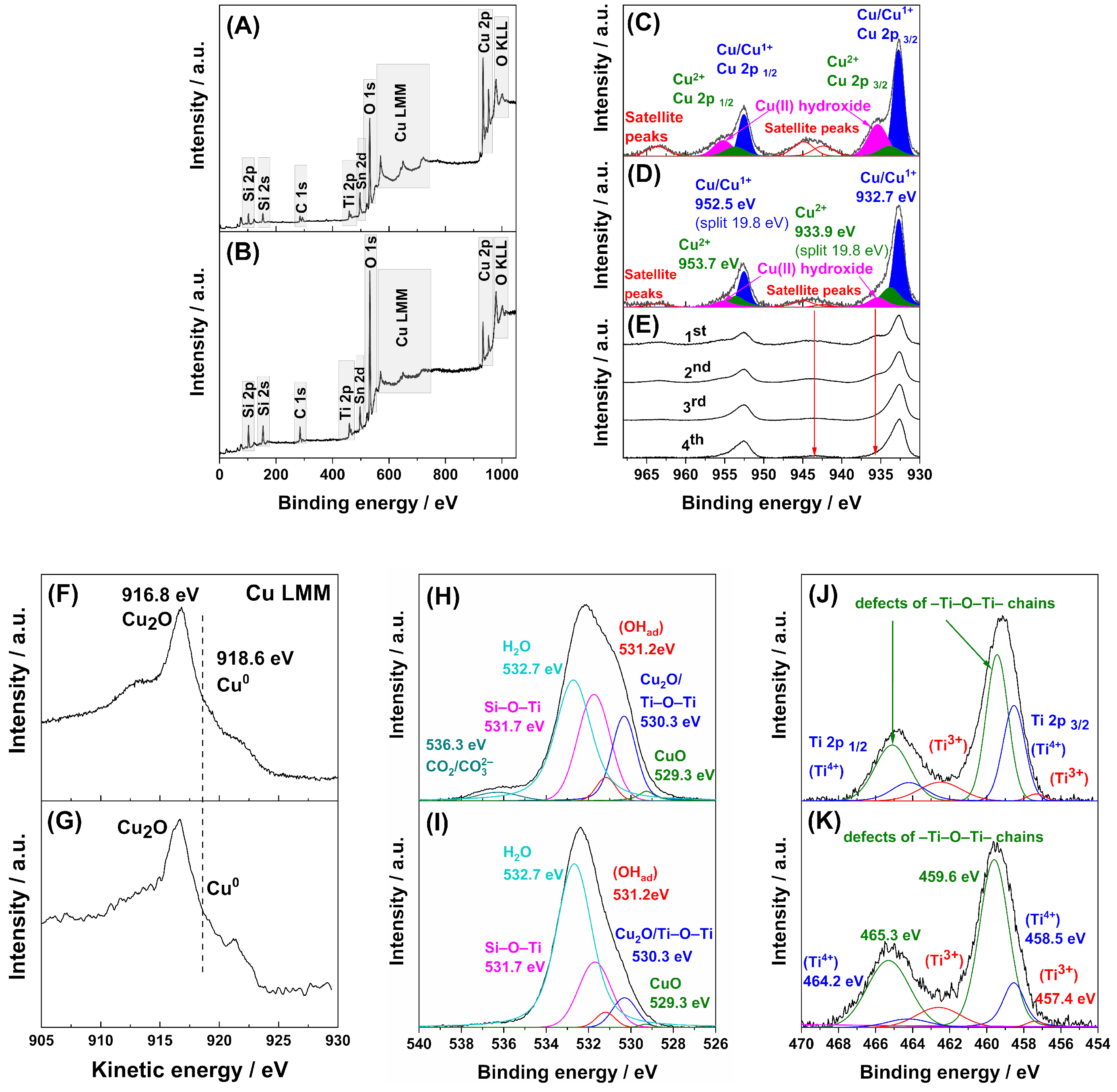
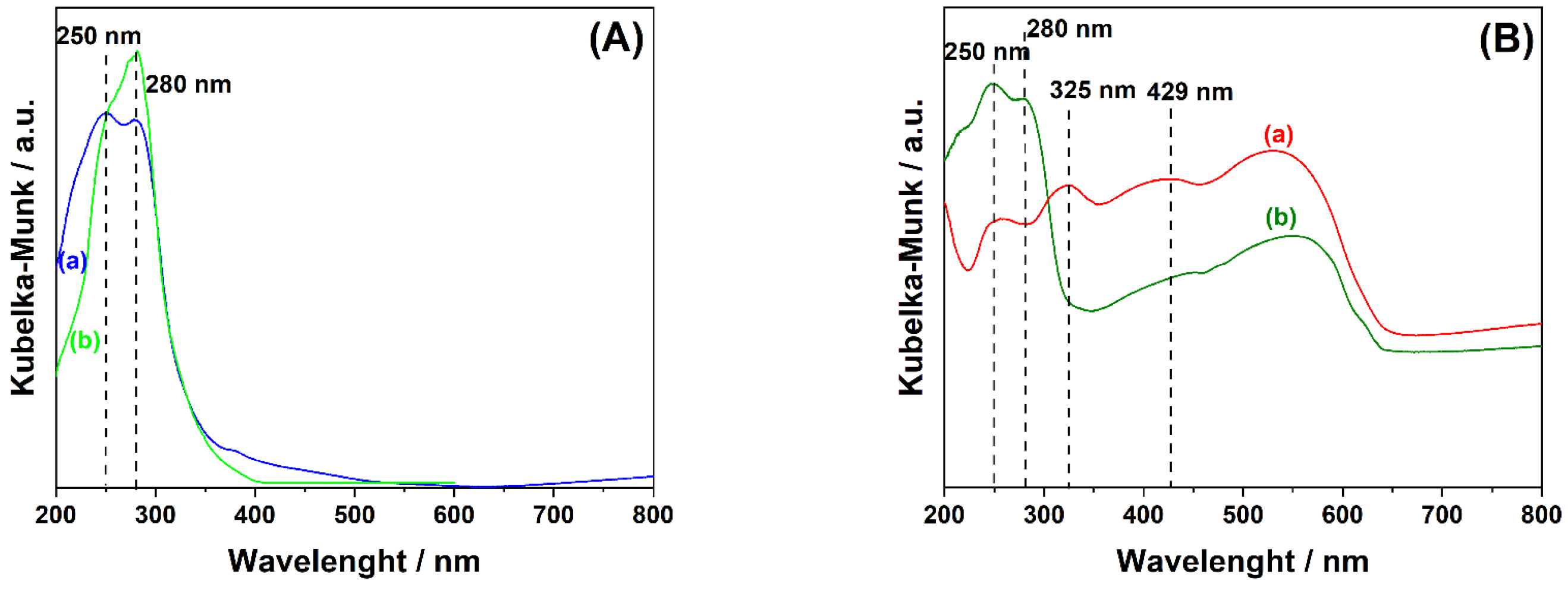
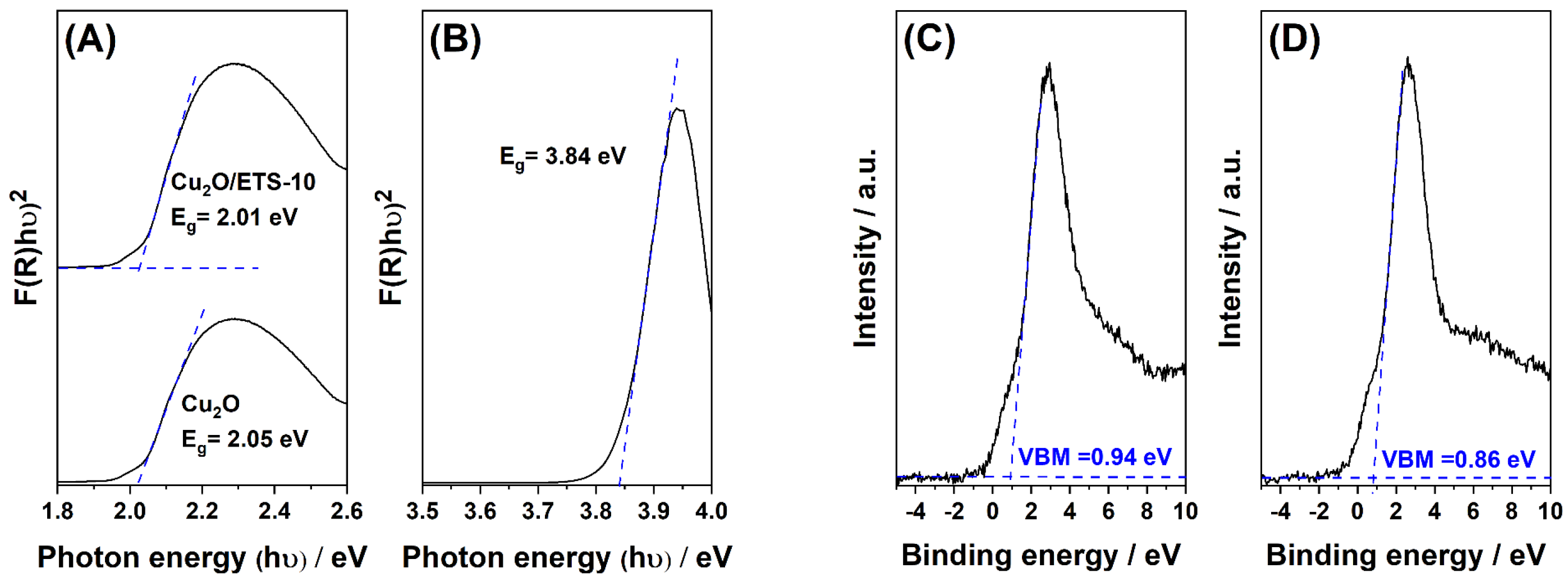
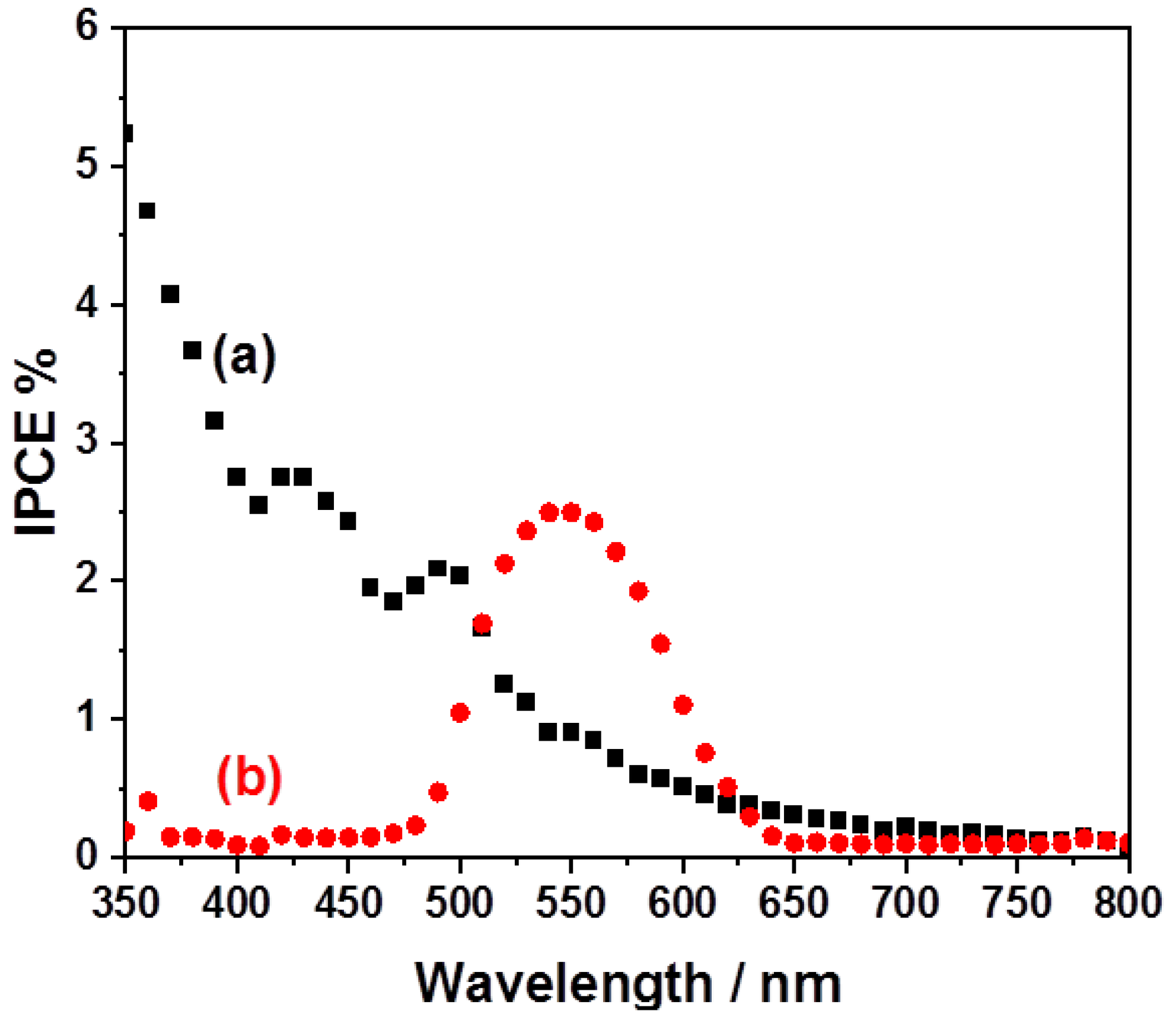
2. Results and Discussion
Product Identification
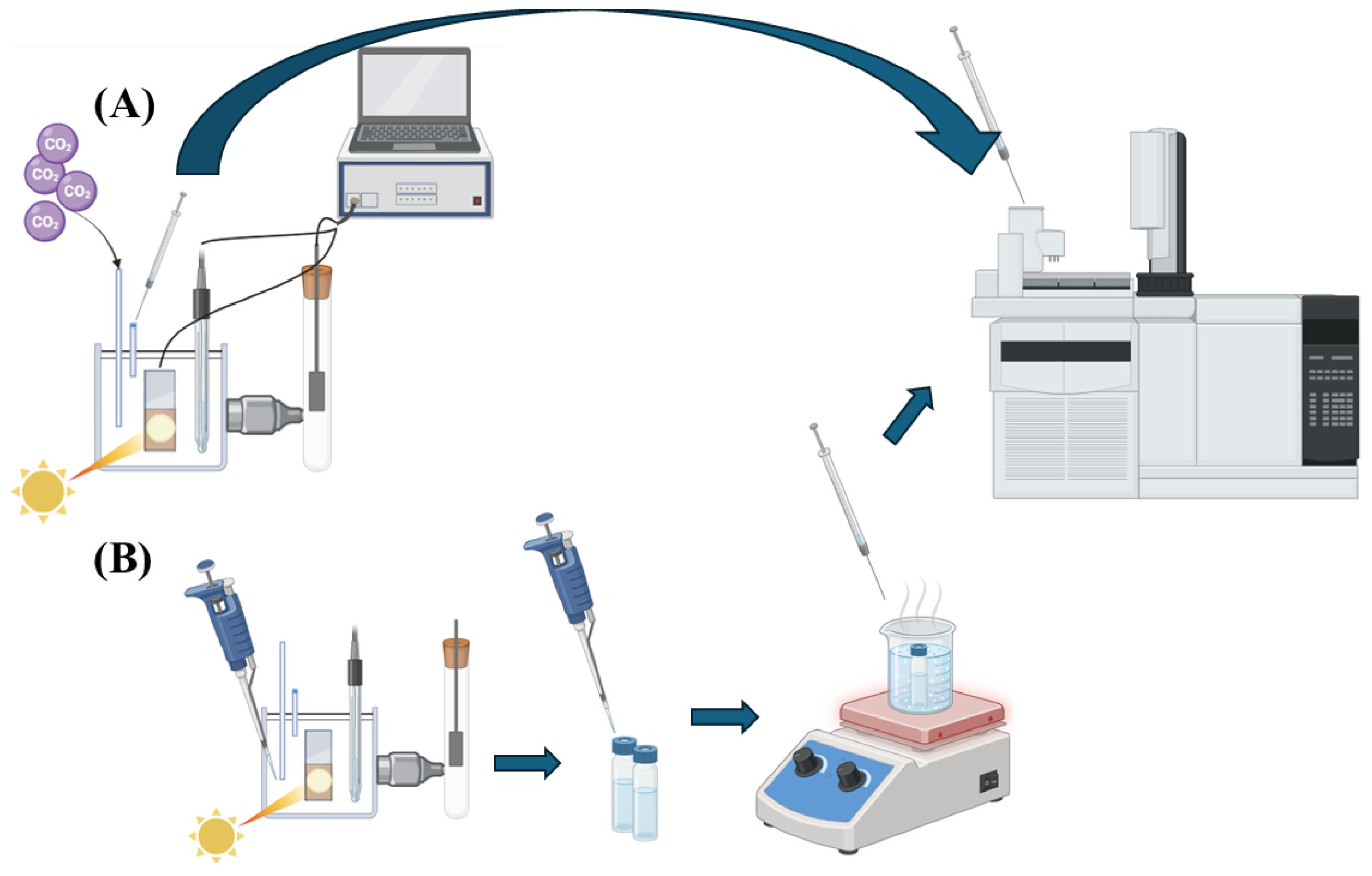
3. Experimental Section
3.1. Materials
3.2. Copper(I) Oxide Electrodeposition
3.3. Synthesis of Titanosilicate (ETS-10) as Protective Layer
3.4. Characterization
3.4.1. XRD
3.4.2. XPS
3.4.3. SEM/EDS
3.4.4. UV–Vis Diffuse Reflectance
3.4.5. Photoelectrochemical Measurements
3.4.6. Product Identification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoshino, S.; Takayama, T.; Yamaguchi, Y.; Iwase, A.; Kudo, A. CO2 Reduction Using Water as an Electron Donor over Heterogeneous Photocatalysts Aiming at Artificial Photosynthesis. Accounts Chem. Res. 2022, 55, 966–977. [Google Scholar] [CrossRef]
- Kumaravel, V.; Bartlett, J.; Pillai, S.C. Photoelectrochemical Conversion of Carbon Dioxide (CO2) into Fuels and Value-Added Products. ACS Energy Lett. 2020, 5, 486–519. [Google Scholar] [CrossRef]
- Tang, B.; Xiao, F.X. An Overview of Solar-Driven Photoelectrochemical CO2 Conversion to Chemical Fuels. ACS Catal. 2022, 12, 9023–9057. [Google Scholar] [CrossRef]
- Kecsenovity, E.; Endrödi, B.; Tóth, P.S.; Zou, Y.; Dryfe, R.A.; Rajeshwar, K.; Janáky, C. Enhanced Photoelectrochemical Performance of Cuprous Oxide/Graphene Nanohybrids. J. Am. Chem. Soc. 2017, 139, 6682–6692. [Google Scholar] [CrossRef]
- Baran, T.; Visibile, A.; Busch, M.; He, X.; Wojtyla, S.; Rondinini, S.; Minguzzi, A.; Vertova, A. Copper Oxide-Based Photocatalysts and Photocathodes: Fundamentals and Recent Advances. Molecules 2021, 26, 7271. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gao, Z.; Cui, Z.; Liang, Y.; Li, Z.; Zhu, S.; Yang, X.; Ma, J. Synthesis of Cu2O Octadecahedron/TiO2 Quantum Dot Heterojunctions with High Visible Light Photocatalytic Activity and High Stability. ACS Appl. Mater. Interfaces 2016, 8, 91–101. [Google Scholar] [CrossRef]
- Janáky, C.; Hursán, D.; Endrödi, B.; Chanmanee, W.; Roy, D.; Liu, D.; De Tacconi, N.R.; Dennis, B.H.; Rajeshwar, K. Electro- and Photoreduction of Carbon Dioxide: The Twain Shall Meet at Copper Oxide/Copper Interfaces. ACS Energy Lett. 2016, 1, 332–338. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, B.; Wang, Z.; Guo, M.; Qin, X.; Zhang, X.; Wang, P.; Dai, Y. Crystal faces of Cu2O and their stabilities in photocatalytic reactions. J. Phys. Chem. C 2009, 113, 14448–14453. [Google Scholar] [CrossRef]
- Siegfried, M.J.; Choi, K.S. Elucidating the effect of additives on the growth and stability of Cu2O surfaces via shape transformation of pre-grown crystals. J. Am. Chem. Soc. 2006, 128, 10356–10357. [Google Scholar] [CrossRef]
- Soon, A.; Todorova, M.; Delley, B.; Stampfl, C. Thermodynamic stability and structure of copper oxide surfaces: A first-principles investigation. Phys. Rev. B Condens. Matter Mater. Phys. 2007, 75, 125420. [Google Scholar] [CrossRef]
- Luo, J.; Steier, L.; Son, M.K.; Schreier, M.; Mayer, M.T.; Grätzel, M. Cu2O Nanowire Photocathodes for Efficient and Durable Solar Water Splitting. Nano Lett. 2016, 16, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Szaniawska, E.; Bienkowski, K.; Rutkowska, I.A.; Kulesza, P.J.; Solarska, R. Enhanced photoelectrochemical CO2-reduction system based on mixed Cu2O–nonstoichiometric TiO2 photocathode. Catal. Today 2018, 300, 145–151. [Google Scholar] [CrossRef]
- Deng, X.; Li, R.; Wu, S.; Wang, L.; Hu, J.; Ma, J.; Jiang, W.; Zhang, N.; Zheng, X.; Gao, C.; et al. Metal-Organic Framework Coating Enhances the Performance of Cu2O in Photoelectrochemical CO2 Reduction. J. Am. Chem. Soc. 2019, 141, 10924–10929. [Google Scholar] [CrossRef] [PubMed]
- Szaniawska, E.; Rutkowska, I.A.; Frik, M.; Wadas, A.; Seta, E.; Krogul-Sobczak, A.; Rajeshwar, K.; Kulesza, P.J. Reduction of carbon dioxide at copper(I) oxide photocathode activated and stabilized by over-coating with oligoaniline. Electrochim. Acta 2018, 265, 400–410. [Google Scholar] [CrossRef]
- Szaniawska, E.; Rutkowska, I.A.; Seta, E.; Tallo, I.; Lust, E.; Kulesza, P.J. Photoelectrochemical reduction of CO2: Stabilization and enhancement of activity of copper(I) oxide semiconductor by over-coating with tungsten carbide and carbide-derived carbons. Electrochim. Acta 2020, 341, 136054. [Google Scholar] [CrossRef]
- Akbar, M.B.; Gong, Y.; Wang, Y.; Woldu, A.R.; Zhang, X.; He, T. Role of TiO2coating layer on the performance of Cu2O photocathode in photoelectrochemical CO2 reduction. Nanotechnology 2021, 32, 395707. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Zhou, R.; Eugene, A.J.; Guzman, M.I.; Grela, M.A. Cu2O/TiO2 heterostructures for CO2 reduction through a direct Z-scheme: Protecting Cu2O from photocorrosion. Appl. Catal. B Environ. 2017, 217, 485–493. [Google Scholar] [CrossRef]
- Suib, S.L.; Přech, J.; Čejka, J.; Kuwahara, Y.; Mori, K.; Yamashita, H. Some novel porous materials for selective catalytic oxidations. Mater. Today 2020, 32, 244–259. [Google Scholar] [CrossRef]
- Barrocas, B.T.; Přech, J.; Filip Edelmannová, M.; Szaniawska, E.; Kočí, K.; Čejka, J. Titanosilicates enhance carbon dioxide photocatalytic reduction. Appl. Mater. Today 2022, 26, 101392. [Google Scholar] [CrossRef]
- Krisnandi, Y.K.; Southon, P.D.; Adesina, A.A.; Howe, R.F. ETS-10 as a photocatalyst. Int. J. Photoenergy 2003, 5, 131–140. [Google Scholar] [CrossRef]
- Anderson, M.W.; Terasaki, O.; Ohsuna, T.; Philippou, A.; MacKay, S.P.; Ferreira, A.; Rocha, J.; Lidin, S. Structure of the microporous titanosilicate ETS-10. Nature 1994, 367, 347–351. [Google Scholar] [CrossRef]
- Melcher, J.; Feroz, S.; Bahnemann, D. Comparing photocatalytic activities of commercially available iron-doped and iron-undoped aeroxide TiO2 P25 powders. J. Mater. Sci. 2017, 52, 6341–6348. [Google Scholar] [CrossRef]
- Krisnandi, Y.K.; Lachowski, E.E.; Howe, R.F. Effects of Ion Exchange on the Structure of ETS-10. ChemInform 2006, 37, 928–933. [Google Scholar] [CrossRef]
- Lv, L.; Su, F.; Zhao, X.S. A reinforced study on the synthesis of microporous titanosilicate ETS-10. Microporous Mesoporous Mater. 2004, 76, 113–122. [Google Scholar] [CrossRef]
- Zaheer, M.A.; Poppitz, D.; Feyzullayeva, K.; Wenzel, M.; Matysik, J.; Ljupkovic, R.; Zarubica, A.; Karavaev, A.A.; Pöppl, A.; Gläser, R.; et al. Synthesis of highly active ETS-10-based titanosilicate for heterogeneously catalyzed transesterification of triglycerides. Beilstein J. Nanotechnol. 2019, 10, 2039–2061. [Google Scholar] [CrossRef]
- Bojestig, E.; Cao, Y.; Nyborg, L. Surface chemical analysis of copper powder used in additive manufacturing. Surf. Interface Anal. 2020, 52, 1104–1110. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Liu, Y.; Wang, K.; Qiu, W.; Chen, L.; Li, W.; Li, J. Photocorrosion behavior of Cu2O nanowires during photoelectrochemical CO2 reduction. J. Electroanal. Chem. 2022, 912, 116252. [Google Scholar] [CrossRef]
- Pauly, N.; Tougaard, S.; Yubero, F. Determination of the Cu 2p primary excitation spectra for Cu, Cu2O and CuO. Surf. Sci. 2014, 620, 17–22. [Google Scholar] [CrossRef]
- Iijima, Y.; Niimura, N.; Hiraoka, K. Prevention of the reduction of CuO during X-ray photoelectron spectroscopy analysis. Surf. Interface Anal. 1996, 24, 193–197. [Google Scholar] [CrossRef]
- Poulston, S.; Parlett, P.M.; Stone, P.; Bowker, M. Surface oxidation and reduction of CuO and Cu2O studied using XPS and XAES. Surf. Interface Anal. 1996, 24, 811–820. [Google Scholar] [CrossRef]
- Wilson, S.S.; Bosco, J.P.; Tolstova, Y.; Scanlon, D.O.; Watson, G.W.; Atwater, H.A. Interface stoichiometry control to improve device voltage and modify band alignment in ZnO/Cu2O heterojunction solar cells. Energy Environ. Sci. 2014, 7, 3606–3610. [Google Scholar] [CrossRef]
- Zhu, C.; Osherov, A.; Panzer, M.J. Surface chemistry of electrodeposited Cu2O films studied by XPS. Electrochim. Acta 2013, 111, 771–778. [Google Scholar] [CrossRef]
- Baklanov, M.R.; Shamiryan, D.G.; Tökei, Z.; Beyer, G.P.; Conard, T.; Vanhaelemeersch, S.; Maex, K. Characterization of Cu surface cleaning by hydrogen plasma. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2001, 19, 1201–1211. [Google Scholar] [CrossRef]
- Lv, L.; Lee, F.Y.; Zhou, J.; Su, F.; Zhao, X.S. XPS study on microporous titanosilicate ETS-10 upon acid treatment. Microporous Mesoporous Mater. 2006, 96, 270–275. [Google Scholar] [CrossRef]
- Trinh, Q.T.; Bhola, K.; Amaniampong, P.N.; Jérôme, F.; Mushrif, S.H. Synergistic Application of XPS and DFT to Investigate Metal Oxide Surface Catalysis. J. Phys. Chem. C 2018, 122, 22397–22406. [Google Scholar] [CrossRef]
- McIntyre, N.S.; Sunder, S.; Shoesmith, D.W.; Stanchell, F.W. Chemical Information From Xps—Applications To the Analysis of Electrode Surfaces. J. Vac. Sci. Technol. 1980, 18, 714–721. [Google Scholar] [CrossRef]
- Manangon-Perugachi, L.E.; Smeets, V.; Vivian, A.; Kainthla, I.; Eloy, P.; Aprile, C.; Debecker, D.P.; Gaigneaux, E.M. Mesoporous methyl-functionalized titanosilicate produced by aerosol process for the catalytic epoxidation of olefins. Catalysts 2021, 11, 196. [Google Scholar] [CrossRef]
- Su, Y.; Balmer, M.L.; Bunker, B.C. Raman spectroscopic studies of silicotitanates. J. Phys. Chem. B 2000, 104, 8160–8169. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W.; Zhu, W. Shape evolution and size-controllable synthesis of Cu2O octahedra and their morphology-dependent photocatalytic properties. J. Phys. Chem. B 2006, 110, 13829–13834. [Google Scholar] [CrossRef]
- Anderson, M.W.; Terasaki, O.; Ohsuna, T.; Malley, P.J.O.; Philippou, A.; Mackay, S.P.; Ferreira, A.; Rocha, J.; Lidin, S. Microporous titanosilicate ETS-10: A structural survey. Philos. Mag. B 1995, 71, 813–841. [Google Scholar] [CrossRef]
- Kuzyaka, D.; Galioglu, S.; Altın, I.; Sokmen, M.; Akata, B. The effect of microporous vanadosilicate AM-6 thin films as photocatalysts for the degradation of methylene blue. J. Photochem. Photobiol. A Chem. 2018, 366, 127–135. [Google Scholar] [CrossRef]
- Ng, Y.C.; Jei, C.Y.; Shamsuddin, M. Titanosilicate ETS-10 derived from rice husk ash. Microporous Mesoporous Mater. 2009, 122, 195–200. [Google Scholar] [CrossRef]
- Galioǧlu, S.; Zahmakiran, M.; Eren Kalay, Y.; Özkar, S.; Akata, B. Effect of silver encapsulation on the local structure of titanosilicate ETS-10. Microporous Mesoporous Mater. 2012, 159, 1–8. [Google Scholar] [CrossRef]
- Das, T.K.; Chandwadkar, A.J.; Budhkar, A.P.; Belhekar, A.A.; Sivasanker, S. Studies on the synthesis of ETS-10 I. Influence of synthesis parameters and seed content. Microporous Mater. 1995, 4, 195–203. [Google Scholar] [CrossRef]
- Suib, S.L.; Přech, J.; Szaniawska, E.; Čejka, J. Recent Advances in Tetra- (Ti, Sn, Zr, Hf) and Pentavalent (Nb, V, Ta) Metal-Substituted Molecular Sieve Catalysis. Chem. Rev. 2023, 123, 877–917. [Google Scholar] [CrossRef]
- Liepold, A.; Roos, K.; Reschetilowski, W.; Lin, Z.; Rocha, J.; Philippou, A.; Anderson, M.W. Characterisation studies on the new microporous aluminium-containing ETS-10 molecular sieve used for processing larger molecules. Microporous Mater. 1997, 10, 211–224. [Google Scholar] [CrossRef]
- Philippou, A.; Anderson, M.W. Aldol-type reactions over basic microporous titanosilicate ETS-10 type catalysts. J. Catal. 2000, 189, 395–400. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Nowak, M.; Kauch, B.; Szperlich, P. Determination of energy band gap of nanocrystalline SbSI using diffuse reflectance spectroscopy. Rev. Sci. Instrum. 2009, 80, 046107. [Google Scholar] [CrossRef] [PubMed]
- Borello, E.; Lamberti, C.; Bordiga, S.; Zecchina, A.; Areán, C.O. Quantum-size effects in the titanosilicate molecular sieve. Appl. Phys. Lett. 1997, 71, 2319–2321. [Google Scholar] [CrossRef]
- Pan, L.; Kim, J.H.; Mayer, M.T.; Son, M.K.; Ummadisingu, A.; Lee, J.S.; Hagfeldt, A.; Luo, J.; Grätzel, M. Boosting the performance of Cu2O photocathodes for unassisted solar water splitting devices. Nat. Catal. 2018, 1, 412–420. [Google Scholar] [CrossRef]
- Liu, X.; Thomas, J.K. Synthesis of microporous titanosilicates ETS-10 and ETS-4 using solid TiO2 as the source of titanium. Chem. Commun. 1996, 1435–1436. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szaniawska-Białas, E.; Parzuch, A.; Trinh, L.; Eliášová, P.; Solarska, R. Titanosilicate ETS-10-Modified Cu2O for Enhanced Visible-Light Photoelectrochemical Activity. Catalysts 2025, 15, 313. https://doi.org/10.3390/catal15040313
Szaniawska-Białas E, Parzuch A, Trinh L, Eliášová P, Solarska R. Titanosilicate ETS-10-Modified Cu2O for Enhanced Visible-Light Photoelectrochemical Activity. Catalysts. 2025; 15(4):313. https://doi.org/10.3390/catal15040313
Chicago/Turabian StyleSzaniawska-Białas, Ewelina, Aleksandra Parzuch, Linh Trinh, Pavla Eliášová, and Renata Solarska. 2025. "Titanosilicate ETS-10-Modified Cu2O for Enhanced Visible-Light Photoelectrochemical Activity" Catalysts 15, no. 4: 313. https://doi.org/10.3390/catal15040313
APA StyleSzaniawska-Białas, E., Parzuch, A., Trinh, L., Eliášová, P., & Solarska, R. (2025). Titanosilicate ETS-10-Modified Cu2O for Enhanced Visible-Light Photoelectrochemical Activity. Catalysts, 15(4), 313. https://doi.org/10.3390/catal15040313






