The Effect of Support and Reduction Methods on Catalyst Performance in the Selective Oxidation of 1,2-Propanediol
Abstract
1. Introduction
2. Results
2.1. XRD Analysis
2.2. CO2-TPD Analysis
2.3. CO-TPD Analysis
2.4. BET Analysis
2.5. SEM Analysis
2.6. TEM and HRTEM Analysis
2.7. XPS Analysis
3. Catalytic Oxidation of 1,2–PDO
3.1. Catalyst Screening Experiments
3.2. Effect of Reaction Conditions on 1,2-PDO Oxidation
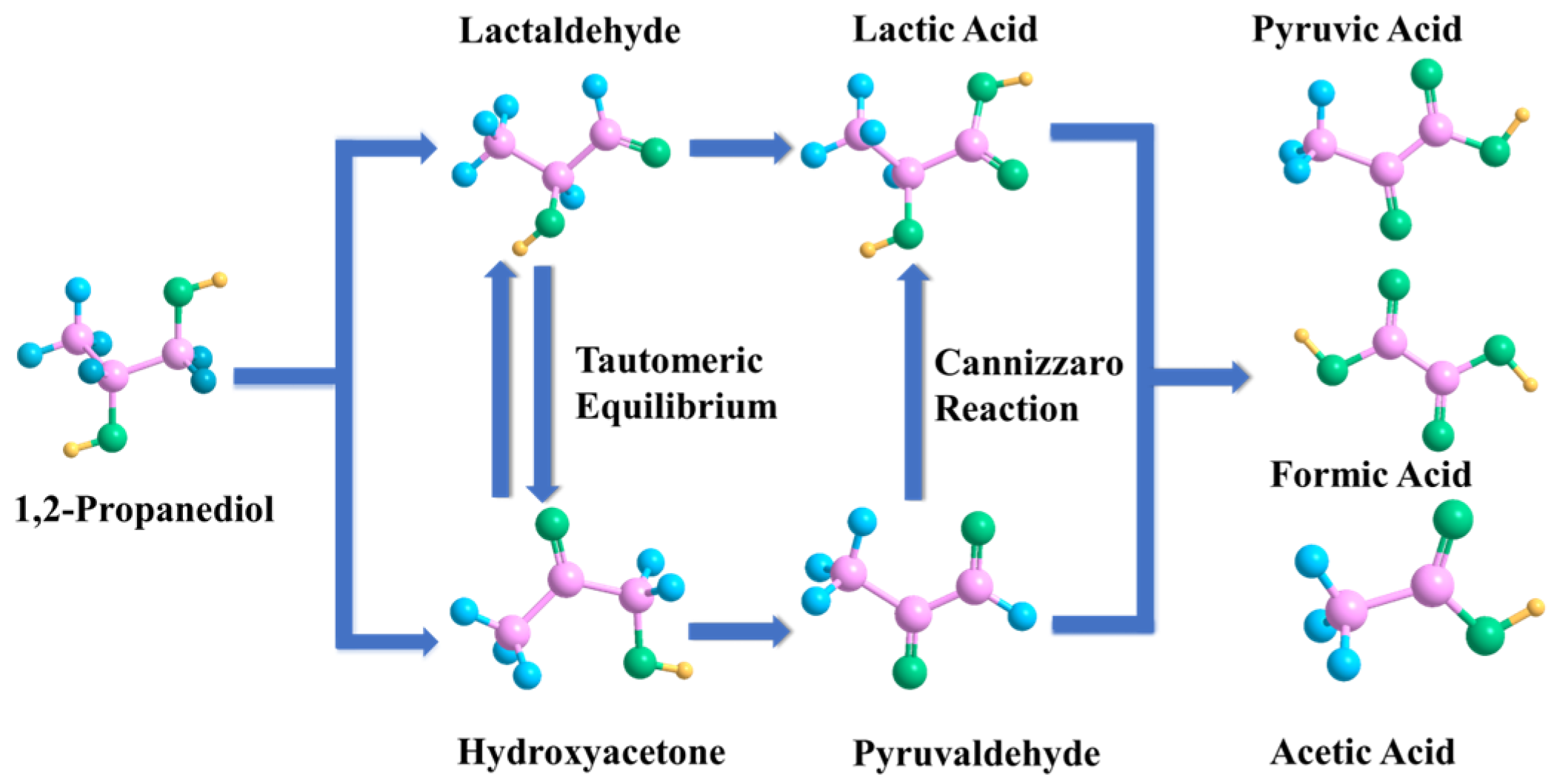
3.3. Possible Reaction Mechanism
4. Materials and Methods
4.1. Materials
4.2. Catalyst Preparation
4.3. Catalyst Characterization
4.4. Product Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, Q.; Ji, Q.; Chen, L.; Zhu, Z.; Tu, S.; Okonkwo, C.E.; Out, P.; Zhou, C. Multimode ultrasound and ternary deep eutectic solvent sequential pretreatments enhanced the enzymatic saccharification of corncob biomass. Ind. Crops Prod. 2022, 188, 115574. [Google Scholar] [CrossRef]
- Okonkwo, C.E.; Hussain, S.Z.; Onyeaka, H.; Adeyanju, A.A.; Nwonuma, C.O.; Bashir, A.A.; Farooq, A.; Zhou, C.; Shittu, T.D. Lignin polyphenol: From biomass to innovative food applications, and influence on gut microflora. Ind. Crops Prod. 2023, 206, 117696. [Google Scholar] [CrossRef]
- Gong, C.; Meng, X.; Jin, C.; Yang, M.; Liu, J.; Sheng, K.; Pu, Y.; Ragauskas, A.; Ji, G.; Zhang, X. Green synthesis of cellulose formate and its efficient conversion into 5-hydroxymethylfurfural. Ind. Crops Prod. 2023, 192, 115985. [Google Scholar] [CrossRef]
- Srinivas, D.; Satyanarayana, C.V.V.; Potdar, H.S.; Ratnasamy, P. Structural studies on NiO-CeO2-ZrO2 catalysts for steam reforming of ethanol. Appl. Catal. A Gen. 2003, 246, 323–334. [Google Scholar] [CrossRef]
- Boasiako, T.A.; Hua, F.; Xiong, Y.; Boateng, I.D.; Ma, Y. Enzymatic catalytic dynamics of lactic-acetic acid co-fermentation: Effect of cellulase on the physicochemical, phytochemicals, volatiles, and antioxidant activity of jujube puree extracts. Ind. Crops Prod. 2024, 222, 119590. [Google Scholar] [CrossRef]
- Chen, M.; Tong, H.; Liu, H.; Lu, J.; Du, J.; Tao, Y.; Cheng, Y.; Wang, H. A cyclic process for enzymatic hydrolysis and fermentation of lactic acid pretreated reed. Ind. Crops Prod. 2022, 181, 114848. [Google Scholar] [CrossRef]
- Gumus, M.; Kasifoglu, S. Performance and emission evaluation of a compression ignition engine using a biodiesel (apricot seed kernel oil methyl ester) and its blends with diesel fuel. Biomass Bioenergy 2010, 34, 134–139. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Ren, X.; Li, W.; Sun, J.; Wang, X.; Huang, Y.; Guo, Y.; Zeng, H. Novel fluorescence immunoassay for the detection of zearalenone using HRP-mediated fluorescence quenching of gold-silver bimetallic nanoclusters. Food Chem. 2021, 355, 129633. [Google Scholar] [CrossRef]
- Shi, Y.; Li, W.; Feng, X.; Lin, L.; Nie, P.; Shi, J.; Zou, X.; He, Y. Sensing of mercury ions in Porphyra by Copper @ Gold nanoclusters based ratiometric fluorescent aptasensor. Food Chem. 2021, 344, 128694. [Google Scholar] [CrossRef]
- Sun, Y.; Zhai, X.; Xu, Y.; Liu, C.; Zou, X.; Li, Z.; Shi, J.; Huang, X. Facile fabrication of three-dimensional gold nanodendrites decorated by silver nanoparticles as hybrid SERS-active substrate for the detection of food contaminants. Food Control 2021, 122, 107772. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, L.; Yin, L.; Arslan, M.; El-Seedi, H.R.; Zou, X. Novel mesoporous silica surface loaded gold nanocomposites SERS aptasensor for sensitive detection of zearalenone. Food Chem. 2023, 403, 134384. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Cai, J.; Jayan, H.; Yosri, N.; Majeed, U.; Guo, Z.; Zou, X. Assembly of stabilized dendritic magnetic ferric-silver nanocomposite with gold nanoparticles for sensitive detection of ochratoxin A using SERS aptasensor. Food Control 2024, 165, 110704. [Google Scholar] [CrossRef]
- Li, H.; Hassan, M.M.; He, Z.; Haruna, S.A.; Chen, Q.; Ding, Z. A sensitive silver nanoflower-based SERS sensor coupled novel chemometric models for simultaneous detection of chlorpyrifos and carbendazim in food. Lwt 2022, 167, 113804. [Google Scholar] [CrossRef]
- Sun, Y.; Zhai, X.; Zou, X.; Shi, J.; Huang, X.; Li, Z. A Ratiometric Fluorescent Sensor Based on Silicon Quantum Dots and Silver Nanoclusters for Beef Freshness Monitoring. Foods 2023, 12, 1464. [Google Scholar] [CrossRef]
- Hao, M.Y.; Li, Z.H.; Huang, X.W.; Wang, Y.; Wei, X.O.; Zou, X.B.; Shi, J.Y.; Huang, Z.Q.; Yin, L.T.; Gao, L.Y.; et al. A cell-based electrochemical taste sensor for detection of Hydroxy-?-sanshool. Food Chem. 2023, 418, 135941. [Google Scholar] [CrossRef]
- Ali, S.; Chen, X.J.; Shah, M.A.; Ali, M.; Zareef, M.; Arslan, M.; Ahmad, S.; Jiao, T.H.; Li, H.H.; Chen, Q.S. The avenue of fruit wastes to worth for synthesis of silver and gold nanoparticles and their antimicrobial application against foodborne pathogens: A review. Food Chem. 2021, 359, 129912. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, Y.; Qian, L.; Yin, Y.H.; Yuan, Z.Y.; Dai, Y.T.; Zhang, T.; Yang, D.Y.; Qiu, F.X. Lamellar Ti3C2 MXene composite decorated with platinum-doped MoS2 nanosheets as electrochemical sensing functional platform for highly sensitive analysis of organophosphorus pesticides. Food Chem. 2024, 459, 140379. [Google Scholar] [CrossRef]
- Han, E.; Pan, Y.Y.; Li, L.; Cai, J.R. Bisphenol A detection based on nano gold-doped molecular imprinting electrochemical sensor with enhanced sensitivity. Food Chem. 2023, 426, 136608. [Google Scholar] [CrossRef]
- Li, H.H.; Geng, W.H.; Sun, X.; Wei, W.Y.; Mu, X.F.; Ahmad, W.; Hassan, M.M.; Ouyang, Q.; Chen, Q.S. Fabricating a nano-bionic sensor for rapid detection of H2S during pork spoilage using Ru NPs modulated catalytic hydrogenation conversion. Meat Sci. 2021, 177, 108507. [Google Scholar] [CrossRef]
- Guo, Z.M.; Chen, P.; Yin, L.M.; Zuo, M.; Chen, Q.S.; El-Seedi, H.R.; Zou, X.B. Determination of lead in food by surface-enhanced Raman spectroscopy with aptamer regulating gold nanoparticles reduction. Food Control 2022, 132, 108498. [Google Scholar] [CrossRef]
- Zhou, X.H.; Pan, W.W.; Li, N.; Salah, M.; Guan, S.N.; Li, X.L.; Wang, Y. Development of a Sensitive Monoclonal Antibody-Based Colloidal Gold Immunochromatographic Strip for Lomefloxacin Detection in Meat Products. Foods 2024, 13, 2550. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Saltsburg, H.; Flytzani-Stephanopoulos, M. Active Nonmetallic Au and Pt Species on Ceria-Based Water-Gas Shift Catalysts. Science 2003, 301, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Rabis, A.; Rodriguez, P.; Schmidt, T.J. Electrocatalysis for Polymer Electrolyte Fuel Cells: Recent Achievements and Future Challenges. ACS Catal. 2012, 2, 864–890. [Google Scholar] [CrossRef]
- de Bruijn, F.A.; Dam, V.A.T.; Janssen, G.J.M. Review: Durability and Degradation Issues of PEM Fuel Cell Components. Fuel Cells 2008, 8, 3–22. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Zeng, J.; Wang, Q.; Li, Z.; Qin, R.; Wu, C.; Xie, Z.; Zheng, L. The synergy between atomically dispersed Pd and cerium oxide for enhanced catalytic properties. Nanoscale 2017, 9, 6643–6648. [Google Scholar] [CrossRef]
- Sadalage, P.S.; Dar, M.A.; Bhor, R.D.; Bhalerao, B.M.; Kamble, P.N.; Paiva-Santos, A.C.; Nimbalkar, M.S.; Sonawane, K.D.; Pai, K.; Patil, P.S.; et al. Optimization of biogenic synthesis of biocompatible platinum nanoparticles with catalytic, enzyme mimetic and antioxidant activities. Food Biosci. 2022, 50, 102024. [Google Scholar] [CrossRef]
- Yang, X.-F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef]
- Dimitratos, N.; Lopez-Sanchez, J.A.; Meenakshisundaram, S.; Anthonykutty, J.M.; Brett, G.; Carley, A.F.; Taylor, S.H.; Knight, D.W.; Hutchings, G.J. Selective formation of lactate by oxidation of 1,2-propanediol using gold palladium alloy supported nanocrystals. Green Chem. 2009, 11, 1209–1216. [Google Scholar] [CrossRef]
- Ma, H.; Nie, X.; Cai, J.; Chen, C.; Gao, J.; Miao, H.; Xu, J. Au/Mg(OH)2: Highly efficient for selective oxidation of 1,2-propanediol to lactic acid with molecular oxygen. Sci. China Chem. 2010, 53, 1497–1501. [Google Scholar] [CrossRef]
- Ryabenkova, Y.; He, Q.; Miedziak, P.J.; Dummer, N.F.; Taylor, S.H.; Carley, A.F.; Morgan, D.J.; Dimitratos, N.; Willock, D.J.; Bethell, D.; et al. The selective oxidation of 1,2-propanediol to lactic acid using mild conditions and gold-based nanoparticulate catalysts. Catal. Today 2013, 203, 139–145. [Google Scholar] [CrossRef]
- Wang, H.; Rao, D.; Meng, M.; Liu, L.; Feng, Y. Modulating depth of 1,2-propanediol oxidation over La(III) doped MCM-41 supported binary Pd and Bi nanoparticles for selective production of C3 carbonyl compounds. Appl. Surf. Sci. 2021, 554, 149528. [Google Scholar] [CrossRef]
- Tang, W.; Hu, Z.; Wang, M.; Stucky, G.D.; Metiu, H.; McFarland, E.W. Methane complete and partial oxidation catalyzed by Pt-doped CeO2. J. Catal. 2010, 273, 125–137. [Google Scholar] [CrossRef]
- Kuang, Q.; Wang, X.; Jiang, Z.; Xie, Z.; Zheng, L. High-Energy-Surface Engineered Metal Oxide Micro- and Nanocrystallites and Their Applications. Acc. Chem. Res. 2014, 47, 308–318. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.-Y.; Jia, X.; Liu, B.; Yang, Y. One-dimensional metal oxide nanostructures for heterogeneous catalysis. Nanoscale 2013, 5, 7175–7183. [Google Scholar] [CrossRef]
- Zhang, Z.; Jung, J.C.; Yan, N. Designed synthesis of MOx(M = Zn, Fe, Sn, Ni, Mn, Co, Ce, Mg, Ag), Pt, and Au nanoparticles supported on hierarchical CuO hollow structures. Nanoscale 2016, 8, 19684–19695. [Google Scholar] [CrossRef]
- Wang, X.; Han, X.; Xie, S.; Kuang, Q.; Jiang, Y.; Zhang, S.; Mu, X.; Chen, G.; Xie, Z.; Zheng, L. Controlled Synthesis and Enhanced Catalytic and Gas-Sensing Properties of Tin Dioxide Nanoparticles with Exposed High-Energy Facets. Chem. Eur. J. 2012, 18, 2283–2289. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; Zheng, B.; Jiang, Y.; Zhang, L.; Xie, Z.; Zheng, L. Controlled synthesis of concave Cu2O microcrystals enclosed by {hhl} high-index facets and enhanced catalytic activity. J. Mater. Chem. A 2013, 1, 282–287. [Google Scholar] [CrossRef]
- Zhou, G.; Li, T.; Chen, J.; Deng, L.; Xie, H. Nano-Pd/CeO2 catalysts for hydrogen storage by reversible benzene hydrogenation/dehydrogenation reactions. Int. J. Hydrogen Energy 2021, 46, 14540–14555. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, L.; Cheng, D.; Chen, F.; Zhan, X.; Gong, J. Synthesis of Pd nanoparticles supported on CeO2 nanotubes for CO oxidation at low temperatures. Chin. J. Catal. 2016, 37, 83–90. [Google Scholar] [CrossRef]
- Yoo, S.; Lee, E.; Jang, G.H.; Kim, D.H. Effect of Pd precursors on the catalytic properties of Pd/CeO2 catalysts for CH4 and CO oxidation. Mol. Catal. 2022, 533, 112791. [Google Scholar] [CrossRef]
- Ahasan, M.R.; Wang, Y.; Wang, R. In situ DRIFTS and CO-TPD studies of CeO2 and SiO2 supported CuOx catalysts for CO oxidation. Mol. Catal. 2022, 518, 112085. [Google Scholar] [CrossRef]
- Cargnello, M.; Doan-Nguyen, V.V.T.; Gordon, T.R.; Diaz, R.E.; Stach, E.A.; Gorte, R.J.; Fornasiero, P.; Murray, C.B. Control of Metal Nanocrystal Size Reveals Metal-Support Interface Role for Ceria Catalysts. Science 2013, 341, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, Z.; An, H.; Xue, W.; Wang, Y. Oxidative carbonylation of phenol with a Pd-O/CeO2-nanotube catalyst. Chin. J. Catal. 2015, 36, 1142–1154. [Google Scholar] [CrossRef]
- Boronin, A.I.; Slavinskaya, E.M.; Danilova, I.G.; Gulyaev, R.V.; Amosov, Y.I.; Kuznetsov, P.A.; Polukhina, I.A.; Koscheev, S.V.; Zaikovskii, V.I.; Noskov, A.S. Investigation of palladium interaction with cerium oxide and its state in catalysts for low-temperature CO oxidation. Catal. Today 2009, 144, 201–211. [Google Scholar] [CrossRef]
- Liu, X.; Ning, P.; Xu, L.; Liu, Q.; Song, Z.; Zhang, Q. Low-temperature catalytic oxidation of CO over highly active mesoporous Pd/CeO2–ZrO2–Al2O3 catalyst. RSC Adv. 2016, 6, 41181–41188. [Google Scholar] [CrossRef]
- Bedrane, S.; Descorme, C.; Duprez, D. Investigation of the oxygen storage process on ceria- and ceria–zirconia-supported catalysts. Catal. Today 2002, 75, 401–405. [Google Scholar] [CrossRef]
- Meng, L.; Lin, J.-J.; Pu, Z.-Y.; Luo, L.-F.; Jia, A.-P.; Huang, W.-X.; Luo, M.-F.; Lu, J.-Q. Identification of active sites for CO and CH4 oxidation over PdO/Ce1−xPdxO2−δ catalysts. Appl. Catal. B Environ. 2012, 119–120, 117–122. [Google Scholar] [CrossRef]
- Ding, D.; Xu, X.; Tian, P.; Liu, X.; Xu, J.; Han, Y.-F. Promotional effects of Sb on Pd-based catalysts for the direct synthesis of hydrogen peroxide at ambient pressure. Chin. J. Catal. 2018, 39, 673–681. [Google Scholar] [CrossRef]
- Shi, J.; Yuan, T.; Zheng, M.; Wang, X. Metal-Free Heterogeneous Semiconductor for Visible-Light Photocatalytic Decarboxylation of Carboxylic Acids. ACS Catal. 2021, 11, 3040–3047. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, W.; Zhang, K.; Zhang, Y.; Wang, X.; Zhang, T.; Wu, X.; Chen, C.; Jiang, L. Facile synthesis of Mn–Fe/CeO2 nanotubes by gradient electrospinning and their excellent catalytic performance for propane and methane oxidation. Dalton Trans. 2017, 46, 16967–16972. [Google Scholar] [CrossRef]
- Ma, J.; Lou, Y.; Cai, Y.; Zhao, Z.; Wang, L.; Zhan, W.; Guo, Y.; Guo, Y. The relationship between the chemical state of Pd species and the catalytic activity for methane combustion on Pd/CeO2. Catal. Sci. Technol. 2018, 8, 2567–2577. [Google Scholar] [CrossRef]
- Shen, Q.; Wu, M.; Wang, H.; He, C.; Hao, Z.; Wei, W.; Sun, Y. Facile synthesis of catalytically active CeO2 for soot combustion. Catal. Sci. Technol. 2015, 5, 1941–1952. [Google Scholar] [CrossRef]
- Hu, Z.; Zhou, G.; Xu, L.; Yang, J.; Zhang, B.; Xiang, X. Preparation of ternary Pd/CeO2-nitrogen doped graphene composites as recyclable catalysts for solvent-free aerobic oxidation of benzyl alcohol. Appl. Surf. Sci. 2019, 471, 852–861. [Google Scholar] [CrossRef]
- Tan, H.; Wang, J.; Yu, S.; Zhou, K. Support Morphology-Dependent Catalytic Activity of Pd/CeO2 for Formaldehyde Oxidation. Environ. Sci. Technol. 2015, 49, 8675–8682. [Google Scholar] [CrossRef]
- Feng, Y.; Li, W.; Meng, M.; Yin, H.; Mi, J. Mesoporous Sn(IV) doping MCM-41 supported Pd nanoparticles for enhanced selective catalytic oxidation of 1,2-propanediol to pyruvic acid. Appl. Catal. B Environ. 2019, 253, 111–120. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Shen, K.; Vohs, J.M.; Gorte, R.J. Characterization of Ceria Films in SBA-15. J. Phys. Chem. C 2024, 128, 3751–3758. [Google Scholar] [CrossRef]
- Xin, P.; Li, J.; Xiong, Y.; Wu, X.; Dong, J.; Chen, W.; Wang, Y.; Gu, L.; Luo, J.; Rong, H.; et al. Revealing the Active Species for Aerobic Alcohol Oxidation by Using Uniform Supported Palladium Catalysts. Angew. Chem. Int. Ed. 2018, 57, 4642–4646. [Google Scholar] [CrossRef]
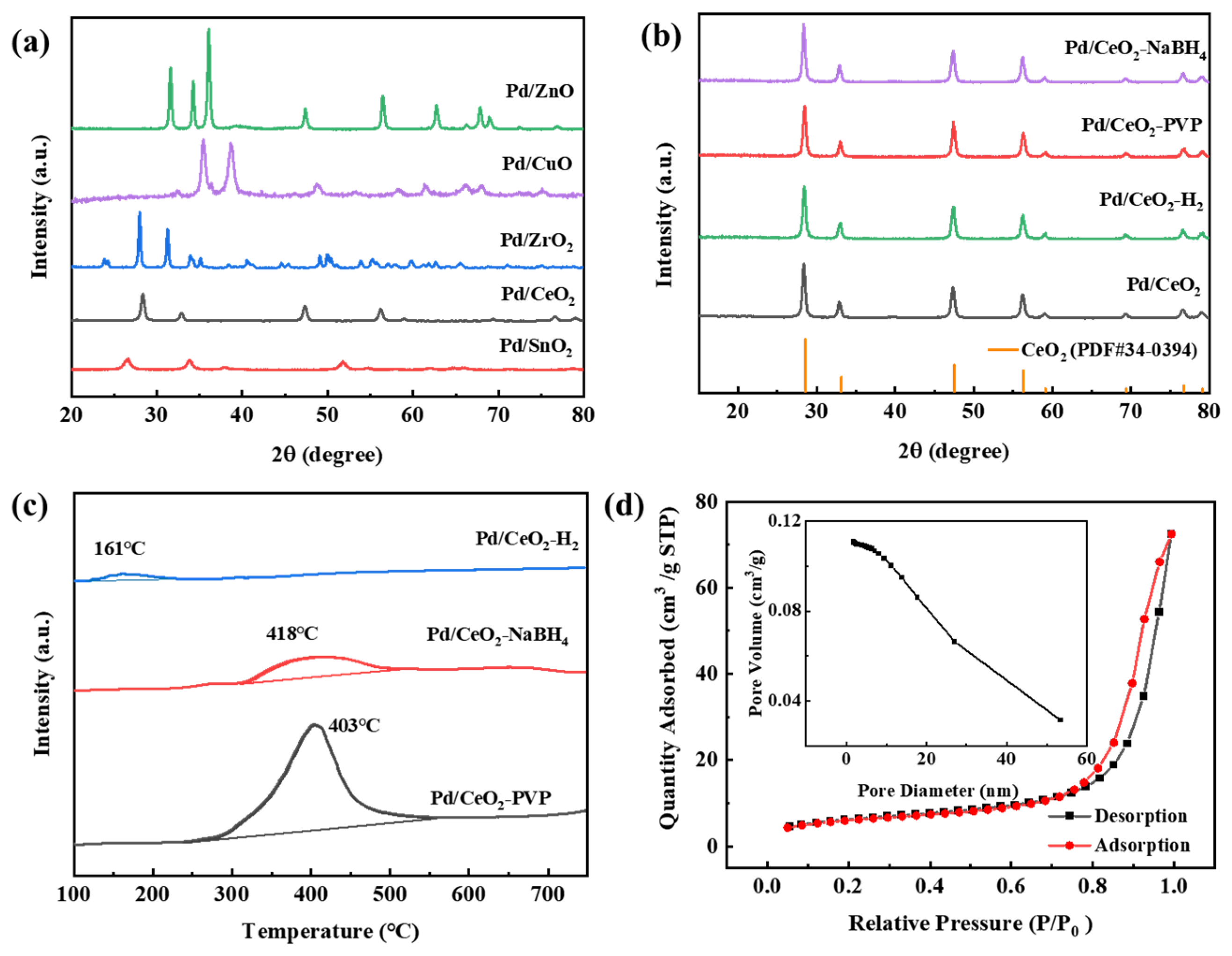


| Catalyst | BET Surface Area a (m2·g−1) | Pore Volume a (cm3·g−1) | Pore Diameter a (nm) | CO Adsorption b (µmol g−1) | Dispersion of Pd b (%) | Pd Crystallite Size b (nm) | Base Quantity c (mmol·g−1) | Pd Loading d (wt, %) |
|---|---|---|---|---|---|---|---|---|
| CeO2 | 42.6 | 0.17 | 15.5 | - | - | - | - | - |
| Pd/CeO2-H2 | 26.3 | 0.13 | 19.8 | 35.4 | 11.8 | 8.5 | 0.28 | 0.53 |
| Pd/CeO2-NaBH4 | 26.7 | 0.14 | 20.3 | 40.9 | 15.6 | 6.4 | 1.84 | 0.61 |
| Pd/CeO2-PVP | 22.0 | 0.11 | 19.7 | 110.4 | 31.1 | 3.2 | 12.61 | 0.58 |
| Catalyst | Binding Energy (eV) | Ratio | ||||
|---|---|---|---|---|---|---|
| Pd 3d5/2 | Pd 3d3/2 | Pd0/Pd0 + Pd2+ | Pd/Ce | Ce3+/Ce4+ | Oads/Olatt | |
| Fresh Pd/CeO2-PVP | 335.29 | 340.58 | 0.18 | 0.048:1 | 0.36 | 0.42 |
| Spent Pd/CeO2-PVP | 335.88 | 341.18 | 0.17 | 0.061:1 | 0.32 | 0.49 |
| Fresh Pd/CeO2-NaBH4 | 335.06 | 340.40 | 0.58 | 0.016:1 | 0.41 | 0.34 |
| Spent Pd/CeO2-NaBH4 | 335.41 | 340.78 | 0.22 | 0.037:1 | 0.16 | 0.43 |
| Fresh Pd/CeO2-H2 | 335.21 | 340.47 | 0.44 | 0.010:1 | 0.22 | 0.31 |
| Spent Pd/CeO2-H2 | 335.85 | 340.96 | 0.22 | 0.052:1 | 0.17 | 0.48 |
| Catalysts | NaOH/1,2-PDO | LA Yield (%) | 1,2-PDO Conversion (%) | LA Selectivity (%) |
|---|---|---|---|---|
| 1.7%Pd/CuO | 2.0 | 48.8 | 73.5 | 66.4 |
| 1.7%Pd/SnO2 | 2.0 | 45.8 | 70.0 | 65.4 |
| 1.7%Pd/ZrO2 | 2.0 | 42.4 | 65.5 | 64.8 |
| 1.7%Pd/ZnO | 2.0 | 38.9 | 65.3 | 59.6 |
| 1.7%Pd/CeO2 | 2.0 | 51.7 | 74.2 | 69.7 |
| 3%Pd/CeO2-NaBH4 | 3.0 | 59.8 | 90.9 | 65.8 |
| 3%Pd/CeO2-H2 | 3.0 | 57.3 | 77.6 | 73.9 |
| 3%Pd/CeO2-PVP | 3.0 | 62.7 | 86.9 | 72.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, Z.; Xiong, X.; Shen, L.; Yin, H. The Effect of Support and Reduction Methods on Catalyst Performance in the Selective Oxidation of 1,2-Propanediol. Catalysts 2025, 15, 304. https://doi.org/10.3390/catal15040304
Li X, Wang Z, Xiong X, Shen L, Yin H. The Effect of Support and Reduction Methods on Catalyst Performance in the Selective Oxidation of 1,2-Propanediol. Catalysts. 2025; 15(4):304. https://doi.org/10.3390/catal15040304
Chicago/Turabian StyleLi, Xin, Zhiqing Wang, Xiong Xiong, Lingqin Shen, and Hengbo Yin. 2025. "The Effect of Support and Reduction Methods on Catalyst Performance in the Selective Oxidation of 1,2-Propanediol" Catalysts 15, no. 4: 304. https://doi.org/10.3390/catal15040304
APA StyleLi, X., Wang, Z., Xiong, X., Shen, L., & Yin, H. (2025). The Effect of Support and Reduction Methods on Catalyst Performance in the Selective Oxidation of 1,2-Propanediol. Catalysts, 15(4), 304. https://doi.org/10.3390/catal15040304







