Modified Resazurin Ink Testing and the Fluorescence Probe Method for Simple and Rapid Photocatalytic Performance Evaluation
Abstract
1. Introduction
2. Results
2.1. Fabrication and Performance Evaluation of Standard TiO2 Films
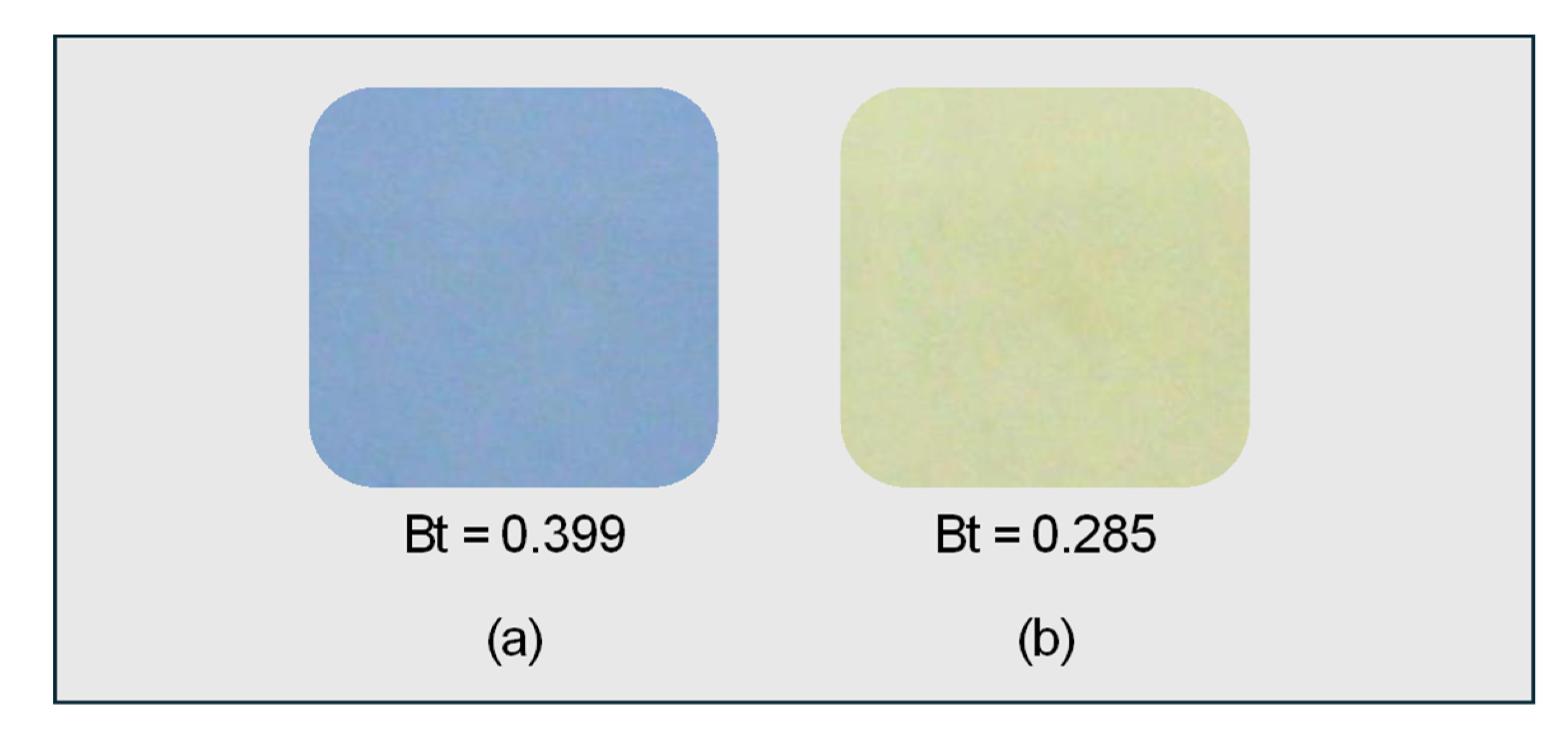
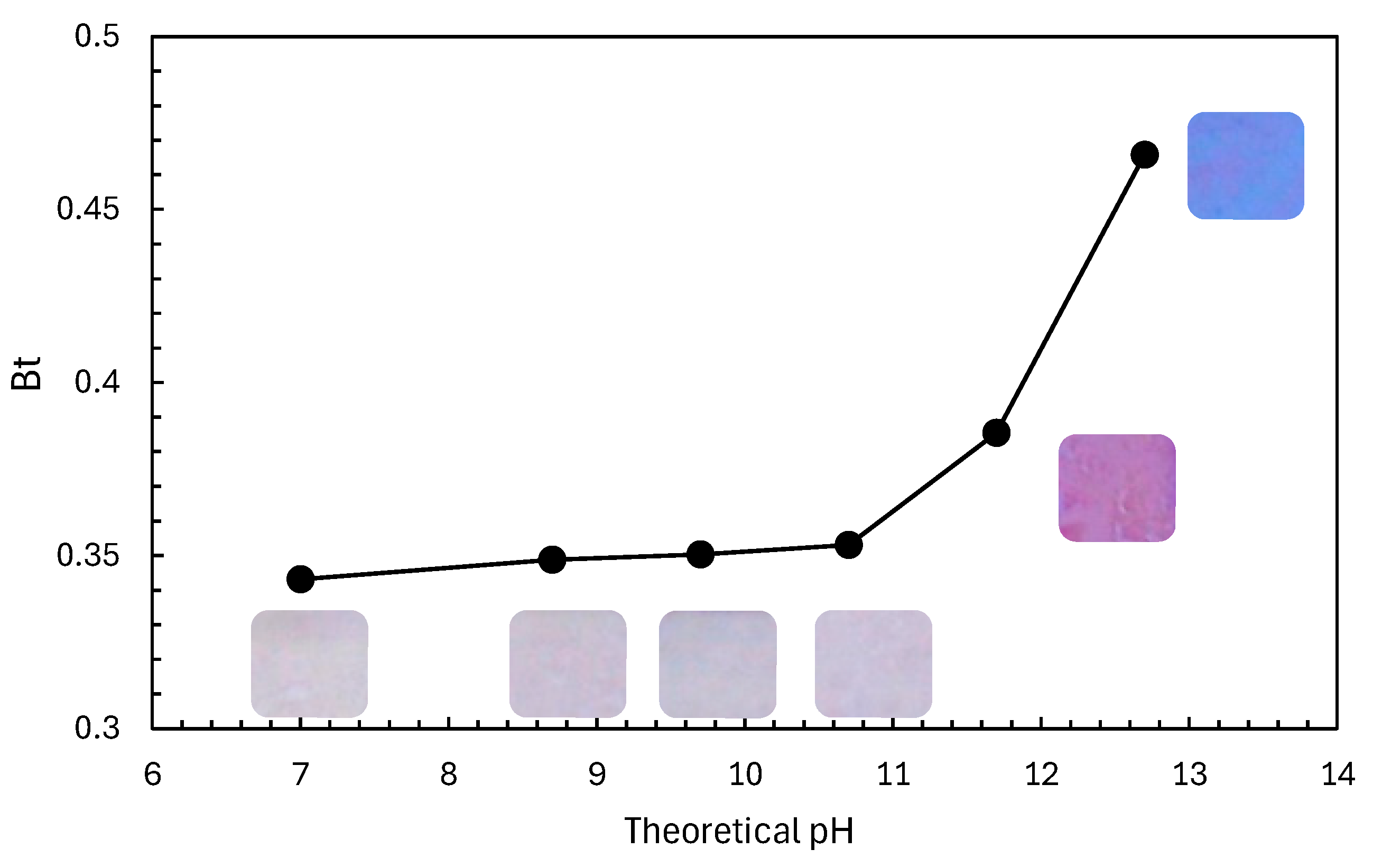
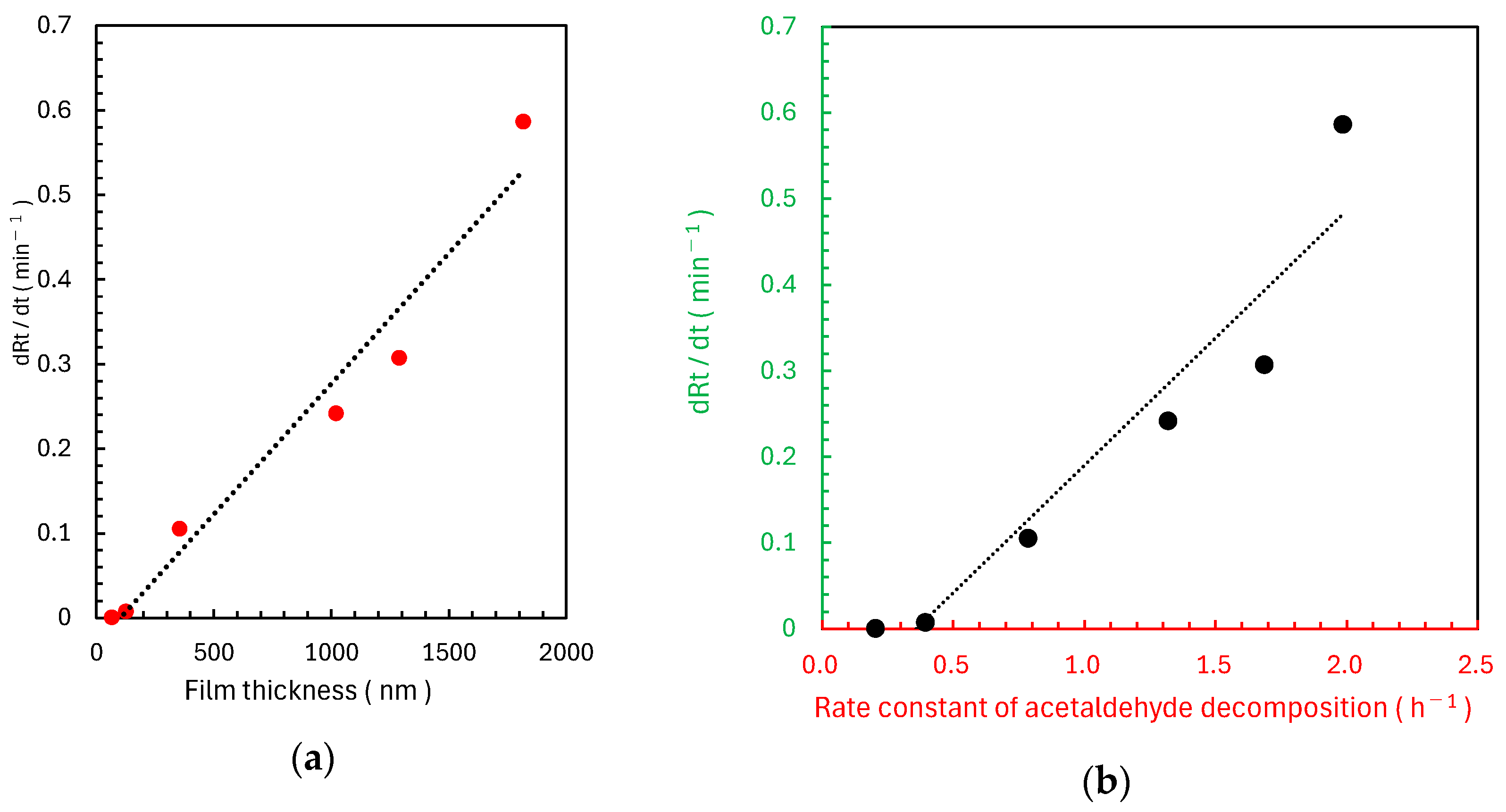
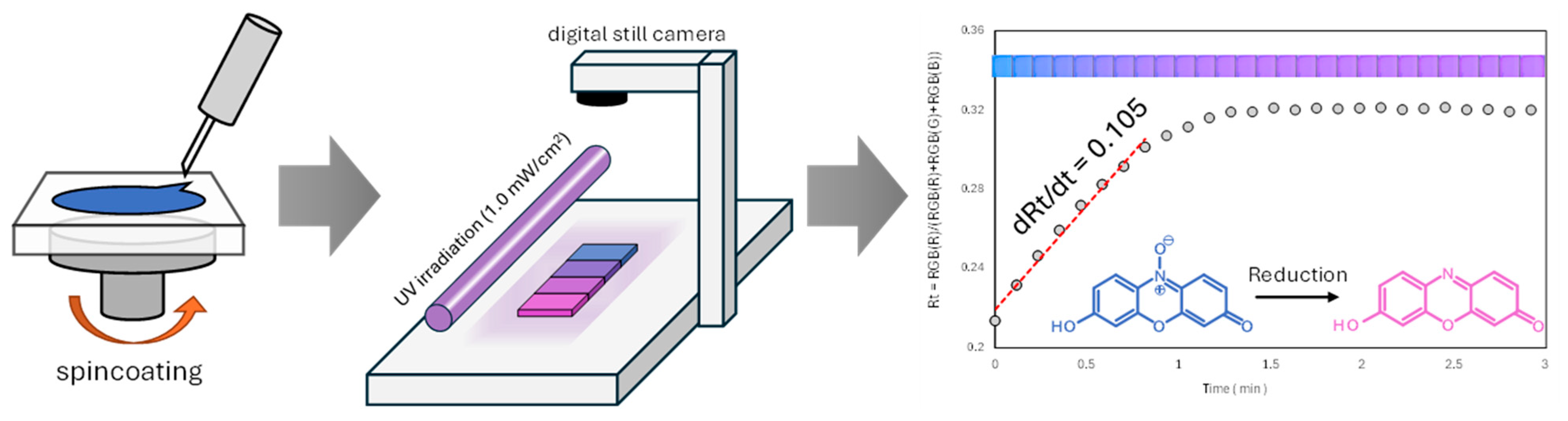
2.2. Evaluation Method of Photocatalytic Performance Using Rz Ink Tests
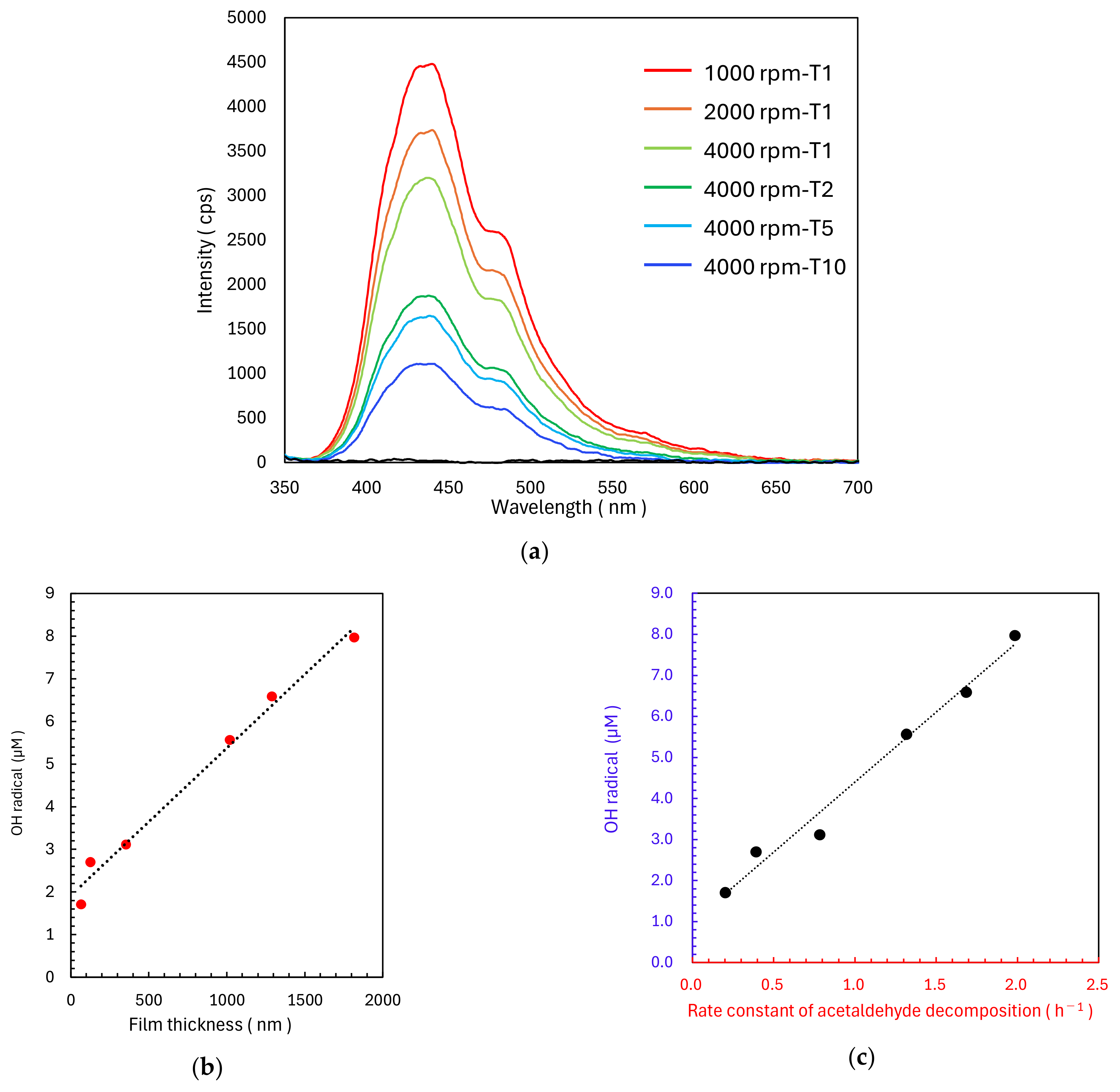
2.3. Evaluation Method of Photocatalytically Generated OH Radicals
3. Discussion
4. Materials and Methods
4.1. Fabrication of TiO2 Films on Glass Substrates
4.2. Structural Analysis of TiO2 Films
4.3. Photocatalytic Performance Test for Acetaldehyde Decomposition
4.4. Evaluation of Photocatalytic Performance Using Rz Ink Tests
4.5. Evaluation of Photocatalytically Generated OH Radicals
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Hamada, K.; Ochiai, T.; Aoki, D.; Akutsu, Y.; Hirabayashi, Y. Decomposition of Gaseous Styrene Using Photocatalyst and Ozone Treatment. Catalysts 2022, 12, 316. [Google Scholar] [CrossRef]
- Mills, A.; Wang, J.; Lee, S.-K.; Simonsen, M. An intelligence ink for photocatalytic films. Chem. Commun. 2005, 21, 2721–2723. [Google Scholar] [CrossRef]
- ISO 21066: 2018; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Qualitative and Semiquantitative Assessment of the Photocatalytic Activities of Surfaces by the Reduction of Resazurin in a Deposited Ink Film. International Organization for Standardization: Geneva, Switzerland, 2018.
- JIS R 1703-1: 2020; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Self-Cleaning Performance of Photocatalytic Materials—Part 1: Measurement of Water Contact Angle. Japanese Standards Association: Tokyo, Japan, 2020.
- JIS R 1703-2: 2014; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Self-Cleaning Performance of Photocatalytic Materials—Part 2: Decomposition of Wet Methylene Blue. Japanese Standards Association: Tokyo, Japan, 2014.
- Mills, A.; Cusick, A.; Hepburn, J. The Kinetics of Semiconductor Photocatalysis in Activity-Indicator Films. J. Adv. Oxid. Technol. 2009, 12, 152–157. [Google Scholar] [CrossRef]
- Mills, A.; Hill, C.; Robertson, P.K.J. Overview of the current ISO tests for photocatalytic materials. J. Photochem. Photobiol. A Chem. 2012, 237, 7–23. [Google Scholar] [CrossRef]
- Mills, A.; Hepburn, J.; Hazafy, D.; O’Rourke, C.; Krysa, J.; Baudys, M.; Zlamal, M.; Bartkova, H.; Hill, C.E.; Winn, K.R.; et al. A simple, inexpensive method for the rapid testing of the photocatalytic activity of self-cleaning surfaces. J. Photochem. Photobiol. A Chem. 2013, 272, 18–20. [Google Scholar] [CrossRef]
- Mills, A.; Hepburn, J.; Hazafy, D.; O’Rourke, C.; Wells, N.; Krysa, J.; Baudys, M.; Zlamal, M.; Bartkova, H.; Hill, C.E.; et al. Photocatalytic activity indicator inks for probing a wide range of surfaces. J. Photochem. Photobiol. A Chem. 2014, 290, 63–71. [Google Scholar] [CrossRef]
- Ishibashi, K.-I.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Detection of active oxidative species in TiO2 photocatalysis using the fluorescence technique. Electrochem. Commun. 2000, 2, 207–210. [Google Scholar] [CrossRef]
- Bubacz, K.; Kusiak-Nejman, E.; Tryba, B.; Morawski, A.W. Investigation of OH radicals formation on the surface of TiO2/N photocatalyst at the presence of terephthalic acid solution. Estimation of optimal conditions. J. Photochem. Photobiol. A Chem. 2013, 261, 7–11. [Google Scholar] [CrossRef]
- Tampieri, F.; Ginebra, M.-P.; Canal, C. Quantification of Plasma-Produced Hydroxyl Radicals in Solution and their Dependence on the pH. Anal. Chem. 2021, 93, 3666–3670. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Hirakawa, T.; Yawata, K.; Nosaka, Y. Photocatalytic reactivity for O2•- and •OH radical formation in anatase and rutile TiO2 suspension as the effect of H2O2 addition. Appl. Catal. A 2007, 325, 105. [Google Scholar] [CrossRef]
- Yan, G.; Chen, J.; Hua, Z. Roles of H2O2 and OHradical dot radical in bactericidal action of immobilized TiO2 thin-film reactor: An ESR study. J. Photochem. Photobiol. A Chem. 2009, 207, 153–159. [Google Scholar] [CrossRef]
- Sawada, T.; Yoshino, F.; Kimoto, K.; Takahashi, Y.; Shibata, T.; Hamada, N.; Sawada, T.; Toyoda, M.; Lee, M.-C. ESR Detection of ROS Generated by TiO2 Coated with Fluoridated Apatite. J. Dent. Res. 2010, 89, 848–853. [Google Scholar] [CrossRef]
- Suzuki, S.; Okazaki, T.; Amano, F. Continuous-Flow Electron Spin Resonance Measurements of Hydroxyl Radicals Produced during Photocatalytic Water Oxidation. J. Phys. Chem. C 2024, 128, 19669–19678. [Google Scholar] [CrossRef]
- Zhang, J.; Nosaka, Y. Photocatalytic oxidation mechanism of methanol and the other reactants in irradiated TiO2 aqueous suspension investigated by OH radical detection. Appl. Catal. B Environ. 2015, 166–167, 32–36. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Understanding Hydroxyl Radical (•OH) Generation Processes in Photocatalysis. ACS Energy Lett. 2016, 1, 356–359. [Google Scholar] [CrossRef]
- Tochikubo, F.; Shimokawa, Y.; Shirai, N.; Uchida, S. Chemical reactions in liquid induced by atmospheric-pressure dc glow discharge in contact with liquid. Jpn. J. Appl. Phys. 2014, 53, 126201. [Google Scholar] [CrossRef]
- Kanazawa, S.; Furuki, T.; Nakaji, T.; Akamine, S.; Ichiki, R. Measurement of OH Radicals in Aqueous Solution Produced by Atmospheric-pressure LF Plasma Jet. Int. J. Plasma Environ. Sci. Technol. 2012, 6, 166–171. [Google Scholar]
- Kanazawa, S.; Furuki, T.; Nakaji, T.; Akamine, S.; Ichiki, R. Application of chemical dosimetry to hydroxyl radical measurement during underwater discharge. J. Phys. Conf. Ser. 2013, 418, 012102. [Google Scholar] [CrossRef]
- Hamada, K.; Ochiai, T.; Tsuchida, Y.; Miyano, K.; Ishikawa, Y.; Nagura, T.; Kimura, N. Eco-Friendly Cotton/Linen Fabric Treatment Using Aqueous Ozone and Ultraviolet Photolysis. Catalysts 2020, 10, 1265. [Google Scholar] [CrossRef]
- Sahni, M.; Locke, B.R. Quantification of Hydroxyl Radicals Produced in Aqueous Phase Pulsed Electrical Discharge Reactors. Ind. Eng. Chem. Res. 2006, 45, 5819–5825. [Google Scholar] [CrossRef]
- Sahni, M.; Locke, B.R. The Effects of Reaction Conditions on Liquid-Phase Hydroxyl Radical Production in Gas-Liquid Pulsed-Electrical-Discharge Reactors. Plasma Process. Polym. 2006, 3, 668–681. [Google Scholar] [CrossRef]
- Kanazawa, S.; Kawano, H.; Watanabe, S.; Furuki, T.; Akamine, S.; Ichiki, R.; Ohkubo, T.; Kocik, M.; Mizeraczyk, J. Observation of OH radicals produced by pulsed discharges on the surface of a liquid. Plasma Sources Sci. Technol. 2011, 20, 034010. [Google Scholar] [CrossRef]
- Hayashi, H.; Akamine, S.; Ichiki, R.; Kanazawa, S. Comparison of OH radical concentration generated by underwater discharge using two methods. Int. J. Plasma Environ. Sci. Technol. 2016, 10, 24–28. [Google Scholar]
- Charbouillot, T.; Brigante, M.; Mailhot, G.; Maddigapu, P.R.; Minero, C.; Vione, D. Performance and selectivity of the terephthalic acid probe for OH as a function of temperature, pH and composition of atmospherically relevant aqueous media. Photochem. Photobiol. A Chem. 2011, 222, 70–76. [Google Scholar] [CrossRef]
- Khan, M.S.I.; Lee, E.-J.; Kim, Y.-J. Roles of individual radicals generated by a submerged dielectric barrier discharge plasma reactor during Escherichia coli O157:H7 inactivation. AIP Adv. 2015, 5, 107111. [Google Scholar] [CrossRef]
- Tochikubo, F.; Uchida, S.; Watanabe, T. Study on Decay Characteristics of OH Radical Density in Pulsed Discharge in Ar/H2O. Jpn. J. Appl. Phys. 2004, 43, 315. [Google Scholar] [CrossRef]
- Aparna, N.; Philip, R.S.; Mathew, M. Tailoring the properties of SnS thin films deposited at room temperature by varying the amount of complexing agent for enhanced photocatalytic activity. Mater. Today Sustain. 2024, 27, 100941. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.; Cao, Q.; Liao, H.; Gao, J.; Zhang, L.; Wei, W.; Li, H.; Lu, J. Structural Regulation of Photocatalyst to Optimize Hydroxyl Radical Production Pathways for Highly Efficient Photocatalytic Oxidation. Adv. Mater. 2024, 36, 2306758. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.-H.; Chuang, H.-Y.; Jiang, Z.-W.; Liu, X.-Z.; Horng, J.-J. Novel quantification of formation trend and reaction efficiency of hydroxyl radicals for investigating photocatalytic mechanism of Fe-doped TiO2 during UV and visible light-induced degradation of acid orange 7. Chemosphere 2020, 251, 126380. [Google Scholar] [CrossRef]
- Shiraki, D.; Ishibashi, N.; Takeuchi, N. Quantitative Estimation of OH Radicals Reacting in Liquid Using a Chemical Probe for Plasma in Contact with Liquid. IEEE Trans. Plasma Sci. 2016, 44, 3158–3163. [Google Scholar] [CrossRef]
- Matthews, R.W. The Radiation Chemistry of the Terephthalate Dosimeter. Radiat. Res. 1980, 83, 27–41. [Google Scholar] [CrossRef]
- Fang, X.; Mark, G.; von Sonntag, C. OH radical formation by ultrasound in aqueous solutions Part I: The chemistry underlying the terephthalate dosimeter. Ultrason. Sonochem. 1996, 3, 57–63. [Google Scholar] [CrossRef]
- Yahagi, T.; Ohji, T.; Yamaguchi, H.; Takahashi, T.; Nakano, H.; Iijima, M.; Tatami, J. Deformation Behavior and Fracture Strength ofSingle-Crystal 4H-SiC Determined by MicrocantileverBending Tests. Adv. Eng. Mater. 2024, 26, 2400095. [Google Scholar] [CrossRef]
- Hanawalt, J.D.; Rinn, W.; Frevel, L.K. Chemical Analysis by X-Ray Diffraction. Ind. Eng. Chem. Anal. Ed. 1938, 10, 457–512. [Google Scholar] [CrossRef]
- Hanawalt, J.D.; Rinn, W. Identification of Crystalline Materials. Ind. Eng. Chem. Anal. Ed. 1936, 8, 244–247. [Google Scholar] [CrossRef]
- Mills, A.; Wells, N. Reductive photocatalysis and smart inks. Chem. Soc. Rev. 2015, 44, 2849–2864. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; Wells, N.; O’Rourke, C. Probing the activities of UV and visible-light absorbing photocatalyst powders using a resazurin-based photocatalyst activity indicator ink (Rz Paii). J. Photochem. Photobiol. A Chem. 2017, 338, 123–133. [Google Scholar] [CrossRef]
- Ochiai, T.; Nagai, T.; Hamada, K.; Tobe, T.; Aoki, D.; Sunada, K.; Ishiguro, H. Estimating the Anti-Viral Performance of Photocatalytic Materials: The Correlation between Air Purification Efficiency and Severe Acute Respiratory Syndrome Coronavirus 2 Inactivation. Catalysts 2024, 14, 163. [Google Scholar] [CrossRef]
- JIS R 1757: 2020; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method of Complete Decomposition by Photocatalytic Materials Under Indoor Lighting Environment-Decomposition of Acetaldehyde. Japanese Standards Association: Tokyo, Japan, 2020.
- ISO 19652: 2018; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics), Test Method for Complete Decomposition Performance of Semiconducting Photocatalytic Materials Under Indoor Lighting Environment, Decomposition of Acetaldehyde. International Organization for Standardization: Geneva, Switzerland, 2018.
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamada, K.; Minami, D.; Nishino, M.; Ochiai, T. Modified Resazurin Ink Testing and the Fluorescence Probe Method for Simple and Rapid Photocatalytic Performance Evaluation. Catalysts 2025, 15, 288. https://doi.org/10.3390/catal15030288
Hamada K, Minami D, Nishino M, Ochiai T. Modified Resazurin Ink Testing and the Fluorescence Probe Method for Simple and Rapid Photocatalytic Performance Evaluation. Catalysts. 2025; 15(3):288. https://doi.org/10.3390/catal15030288
Chicago/Turabian StyleHamada, Kengo, Daichi Minami, Misa Nishino, and Tsuyoshi Ochiai. 2025. "Modified Resazurin Ink Testing and the Fluorescence Probe Method for Simple and Rapid Photocatalytic Performance Evaluation" Catalysts 15, no. 3: 288. https://doi.org/10.3390/catal15030288
APA StyleHamada, K., Minami, D., Nishino, M., & Ochiai, T. (2025). Modified Resazurin Ink Testing and the Fluorescence Probe Method for Simple and Rapid Photocatalytic Performance Evaluation. Catalysts, 15(3), 288. https://doi.org/10.3390/catal15030288








