Abstract
In this article, Pt-SnxOy hybrid nanoparticles encaged in porous silica nanospheres (Pt-SnxOy@PSNs) were prepared by using 1-dodecanethiol (C12-SH) as a coordination agent to confine Pt and Sn ions in a microemulsion system, which is formed by cetyltrimethylammonium bromide (CTAB) and C12-SH as co-surfactants in water. Compared with Pt@PSNs, when different molar ratios of SnxOy were introduced into Pt@PSNs to form Pt-SnxOy@PSNs, the catalytic efficiency of 4-nitrophenol (4-NP) reduction with NaBH4 can be significantly enhanced. At molar ratios of 4-NP/Pt of 150/1, the 4-NP conversion reached 100% over Pt-SnxOy@PSNs with Pt/Sn molar ratios of 1/0.75 in 8 min. This catalytic performance showed a slight decrease after six reaction cycles. This enhanced catalytic efficiency can be ascribed to the synergistic effect between Pt and SnxOy, and the protection of porous silica nanostructures can effectively improve the stability of the catalyst.
1. Introduction
4-aminophenol (4-AP), as an important organic intermediate, is widely used in the pharmaceutical, herbicide, pesticide, and rubber industries [1,2,3]. Traditionally, it is produced through the hydrogenation of 4-nitrophenol (4-NP) by using strong reducing agents, such as NaBH4 [3,4] and ammonia borane [5,6]. Although the thermodynamic conversion of 4-NP to 4-AP is feasible, it has a high kinetic barrier without catalysts due to mutual repelling between 4-nitrophenolate ions and BH4− [4,7,8]. Thus, a large excess of reducing agents is often required for the conversion of 4-NP to 4-AP. However, these remaining reducing agents after the reaction can cause serious environmental pollution problems [9]. Therefore, it is vital to develop an efficient, cheap, and environment-friendly catalytic system for 4-NP reduction [4].
Noble metals, such as Pd [10,11,12], Pt [13,14,15], Au [16,17,18], and Ag [3,19,20], are the main active metals in 4-NP reduction. The catalytic activity of noble metals in 4-NP reduction has a strong correlation with their particle size [21]. However, noble metals’ tendency to aggregate and undergo metal loss during catalytic reactions leads to low catalytic activity and stability in 4-NP reduction. Recently, various strategies, such as alloy effects [22,23], support properties [24,25], and the synergistic effect of metal–metal oxides [26,27,28], have been proposed to enhance the catalytic performance of 4-NP reduction. Among them, the synergistic effect of metal–metal oxides can not only greatly enhance the activity and stability of catalysts for a series of hydrogenation reactions but also reduce the cost of catalysts. Although extensive studies have been conducted on the synergistic effect of metal–metal oxides, the preparation of close-contact metal–metal oxide hybrid nanoparticles remains a challenge.
In this article, we prepared Pt-SnxOy hybrid nanoparticles encaged in porous silica nanospheres (Pt-SnxOy@PSNs) as an efficient hydrogen catalyst for 4-NP reduction with NaBH4. Scheme 1 illustrates the specific preparation process for Pt-SnxOy@PSNs. Due to the coordination of 1-dodecanethiol (C12-SH), Pt4+, and Sn4+, metal ions were confined in a microemulsion system formed by the co-surfactants cetyltrimethylammonium bromide (CTAB) and C12-SH in water [29]. Silicified micelles were obtained through the hydrolysis and polymerization of tetraethyl orthosilicate (TEOS). Pt-SnxOy@PSNs can be obtained by calcination. Compared with Pt@PSNs, SnxOy@PSNs, and Pt-SnxOy@PSNs with different Pt/Sn molar ratios, Pt-SnxOy, with molar ratios of 1/0.75, encaged in porous silica nanospheres (Pt1-(SnxOy)0.75@PSNs) shows the best catalytic efficiency and stability during 4-NP reduction with NaBH4, which can be ascribed to the synergistic effect between Pt and SnxOy and the protection of the silica shell.
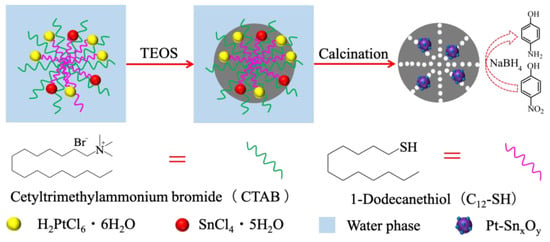
Scheme 1.
Synthetic procedures of Pt-SnxOy hybrid nanoparticles in PSNs.
2. Results and Discussion
2.1. Synthesis and Characterization
In this article, Pt@PSNs, SnxOy@PSNs, and different Pt/Sn molar ratios of Pt-SnxOy@PSNs and Pt2-(SnxOy)1.5@PSNs (two times higher metal ion concentration of Pt1-(SnxOy)0.75@PSNs) were synthesized through the microemulsion method. Their corresponding transmission electron microscopy (TEM) images are presented in Figure 1. As shown in Figure 1a–h, all PSNs show a clear porous structure. Pt and Pt-SnxOy nanoparticles can be observed from the insert image in Figure 1a–g. Moreover, the high-angle annular dark-field image of Pt1-(SnxOy)0.75@PSNs in Figure 1h further confirms the structure of the prepared materials. The analysis diameter of overall Pt@PSNs and different Pt/Sn molar ratios of Pt-SnxOy@PSNs are shown in Figure S1, while the average diameter of Pt and Pt-SnxOy with different Pt/Sn molar ratios are shown in Figure S2. As shown in Figures S1 and S2, the average diameters of overall PSNs ranged from 78.5 to 89.1; for Pt and Pt-SnxOy nanoparticles, it was around 2.1–2.2 nm. The TEM images of Pt1-(SnxOy)0.75@PSNs at different calcination temperatures are shown in Figure S3. As shown in Figure S3b,c, with the calcination temperature increasing to 400 °C and 500 °C, a partial aggregation of Pt-SnxOy hybrid nanoparticles occurred. The analysis diameter of Pt-SnxOy nanoparticles, with the calcination temperature at 400 °C and 500 °C, are presented in Figure S4. When the calcination was increased from 300 °C to 400 °C and 500 °C, the average diameter of Pt-SnxOy nanoparticles also increased from 2.1 nm to 3.8 nm and 4.6 nm, respectively.
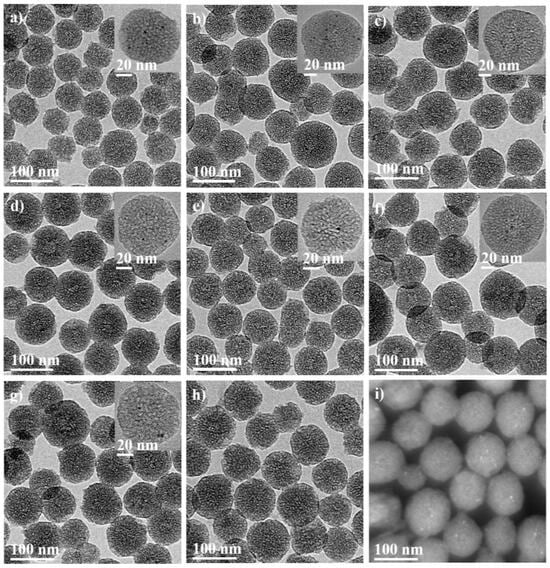
Figure 1.
TEM images showing (a) Pt@PSNs, (b) Pt1-(SnxOy)0.25@PSNs, (c) Pt1-(SnxOy)0.5@PSNs, (d) Pt1-(SnxOy)0.75@PSNs, (e) Pt1-(SnxOy)1@PSNs, (f) Pt1-(SnxOy)1.25@PSNs, (g) Pt2-(SnxOy)1.5@PSNs, (h) SnxOy@PSNs, (i) high-angle annular dark-field image of Pt1-(SnxOy)0.75@PSNs. The inserted images in (a–f) show the enlarged Pt@PSNs and Pt-SnxOy@PSNs with different Pt/Sn molar ratios. Scale bars in (a–i) are 100 nm, and scale bars in the inserts in (a–g) are 20 nm, respectively. All materials were calcined at 300 °C.
X-ray diffraction (XRD) patterns of Pt@PSNs and different Pt/Sn molar ratios of Pt-SnxOy@PSNs are shown in Figure 2, while SnxOy@PSNs and Pt2-(SnxOy)1.5@PSNs XRD patterns are presented in Figure S5. As shown in Figure 2, XRD curves of Pt@PSNs and different Pt/Sn molar ratios of Pt-SnxOy@PSNs only present the SiO2 (~22°) and Pt diffractions (~39.8°, 46.2°, 67.5°). There are no obvious SnxOy diffractions. However, the energy-dispersive spectrometry (EDS) measurement of Pt1-(SnxOy)0.75@PSNs in Figure S6a shows small peaks of Pt and Sn. Moreover, when the concentrations of Pt4+ and Sn4+ were doubled, there were clear Pt and Sn peaks in the EDS measurement of Pt2-(SnxOy)1.5 in Figure S6b. The absence of distinct Sn peaks may be related to the formation of amorphous Sn-related phases for SnxOy [29,30].

Figure 2.
(a) X-ray diffraction (XRD) patterns of Pt@PSNs and (b) different Pt/Sn molar ratios of Pt-SnxOy@PSNs.
To characterize the specific distribution of Pt and Sn in catalysts, the EDS line scans in high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and phase mapping of Pt2-(SnxOy)1.5@PSNs were measured in Figure 3. Figure 3a,b presents the dark field image of selected Pt2-(SnxOy)1.5@PSNs and the corresponding Pt/Sn EDS counts. As shown in Figure 3b, the highest concentration of Pt and Sn occur in the same place, suggesting the close contact of Pt and SnxOy. Moreover, the phase mapping of Pt and Sn in Figure 3c,d further confirm the consistency of Pt and SnxOy.

Figure 3.
(a) High-angle annular dark-field image of Pt2-(SnxOy)1.5@PSNs, and the yellow arrows indicate the selected nanospheres for EDS line scans; (b) the corresponding EDS line scans of selected nanospheres; (c) the corresponding Pt phase mapping; (d) the corresponding Sn phase mapping.
The X-ray photoelectron spectroscopy (XPS) measurement of Pt2@PSNs, Pt2-(SnxOy)0.5@PSNs, Pt2-(SnxOy)1@PSNs, Pt2-(SnxOy)1.5@PSNs, Pt2-(SnxOy)2@PSNs, and Pt2-(SnxOy)2.5@PSNs (all materials with two times higher metal ion concentration of normal Pt-SnxOy@PSNs with calcination at 300 °C) further characterizes the valence states of Pt and Sn elements in PSNs. As shown in Figure S7a, the binding energies at 74.5/71.1(eV/eV), 75.4/72(eV /eV) and 77.4/74(eV/eV) can be assigned to 4f7/2/4f5/2 of the Pt0 species, 4f7/2/4f5/2 of the Pt2+ species, and 4f7/2/4f5/2 of the Pt4+ species, respectively [31,32,33,34,35]. For Pt2-(SnxOy)0.5@PSNs, Pt2-(SnxOy)1@PSNs, Pt2-(SnxOy)1.5@PSNs, Pt2-(SnxOy)2@PSNs, and Pt2-(SnxOy)2.5@PSNs, the peaks of Pt0 species in Figure S7b,d,f,h,j shift by 0.2 eV to lower binding energies. From the results of XPS analysis, the Pt species in Pt2-(SnxOy)0.5@PSNs, Pt2-(SnxOy)1@PSNs, Pt2-(SnxOy)1.5@PSNs, Pt2-(SnxOy)2@PSNs, and Pt2-(SnxOy)2.5@PSNs, which calcined at 300 °C, is electron-rich, similar to the reported Pt/tin oxide heterointerfaces [36,37]. Sn XPS spectra, with binding energies at 487.5/496.0 (eV/eV), can be attributed to 3d5/2/3d3/2 of Sn4+ species, as shown in Figure S7g [38,39]. However, there are no obvious Sn XPS spectra peaks in Figure S7c,e,i,k, which may be ascribed to the core–shell structure and low loading of Sn. The Sn species of Pt2-(SnxOy)1.5@PSNs was mainly in the Sn4+ state, indicating the oxidized state of Sn with the calcination at 300 °C.
To characterize the effect of the calcination temperature, N2 adsorption–desorption isotherms and pore size distributions at various calcination temperatures of Pt1-(SnxOy)0.75@PSNs are shown in Figure 4, while textural properties of Pt1-(SnxOy)0.75@PSNs at different calcination temperatures are presented in Table 1. As shown in Figure 4a, all the prepared Pt1-(SnxOy)0.75@PSNs at different calcination temperatures exhibited type IV isotherms with hysteresis loops, indicating a mesoporous structure. The sharp adsorption at P/P0 around 0.90 is attributed to the aggregation void of PSNs. From the pore size distribution in Figure 4b, there are mainly 1–2 nm micropores and 2–6 nm mesopores in Pt1-(SnxOy)0.75@PSNs at different calcination temperatures. Moreover, when the calcination temperature is 200 °C, there is low pore volume and Brunauer–Emmett–Teller (BET) surface areas in Figure 4 and Table 1, which can be ascribed to the residual of organic agents in Pt1-(SnxOy)0.75@PSNs after 200 °C calcination.

Figure 4.
(a) N2 adsorption–desorption isotherms of Pt1-(SnxOy)0.75@PSNs calcined at various temperatures; (b) their corresponding pore size distributions by density functional theory method.

Table 1.
Textural properties of Pt1-(SnxOy)0.75@PSNs calcined at different temperatures.
Figure 5 illustrates the Fourier transform infrared (FT-IR) spectra and thermogravimetric (TG)/differential thermogravimetric (DTG) curves of Pt1-(SnxOy)0.75@PSNs to study the effect of the calcination temperature on organic removal. For Pt1-(SnxOy)0.75@PSNs before calcination in Figure 5a, signals at 2854 and 2923 cm−1 are assigned to the C-H stretching vibrations [40], signals at 1550cm−1 are attributed to the bending vibration of the C-H bond [41], and signals at 720–570 cm−1 are indexed to peaks of C-S [42]. These signals are extremely small after the calcination of Pt1-(SnxOy)0.75@PSNs at 200 °C, indicating that most of the organic agents in Pt1-(SnxOy)0.75@PSNs were removed. By further increasing the calcination temperature to 300 °C and 400 °C, the corresponding signals completely disappeared, suggesting the complete removal of organic agents in Pt1-(SnxOy)0.75@PSNs. Moreover, the TG/DTA results of Pt1-(SnxOy)0.75@PSNs before calcination showed similar results to FT-IR.
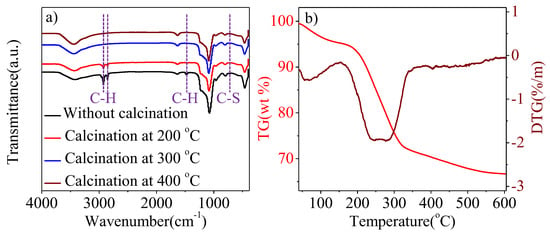
Figure 5.
(a) FT-IR spectra of Pt1-(SnxOy)0.75@PSNs before and after calcination at different temperatures; (b) TG/DTG analysis of Pt1-(SnxOy)0.75@PSNs before calcination.
2.2. Catalytic Reduction of 4-NP
The catalytic reduction of 4-NP with NaBH4 over Pt@PSNs, SnxOy@PSNs, and various Pt-SnxOy@PSNs in water was studied at room temperature and atmospheric pressure. Through inductively coupled plasma–optical emission spectroscopy (ICP-OES), Table S1 illustrates the real Pt and Sn loading of Pt-SnxOy@PSNs with different Pt/Sn molar ratios. As shown in Table S1, the real Pt/Sn molar ratios were close to the theoretical value, indicating the nearly total metal ions into PSNs. UV-vis absorption spectra were used to measure the 4-NP concentration change over time due to a strong adsorption (around 400 nm) of 4-nitrophenolate ions in the UV-vis spectra [26,27].
Figure 6 shows the effect of different PSNs on the catalytic reduction of 4-NP. As shown in Figure 6b, the individual Pt@PSNs, SnxOy@PSNs, and those without a catalyst can undergo 60.5%, 26.6%, and 27.1%4-NP conversion in 10 min, suggesting that NaBH4 can slowly react with 4-NP, and Pt@PSNs can accelerate the rate of catalytic reduction of 4-NP with NaBH4. In contrast, the conversion of 4-NP over different Pt/Sn molar ratios of Pt-SnxOy@PSNs in Figure 6a can achieve over 95% within 10 min. Moreover, when the Pt/Sn molar ratios of Pt-SnxOy@PSNs increased from 1/0.25 to 1/0.75, the time to achieve 95% 4-NP conversion decreased from 10 min to 5 min, which can be attributed to the synergistic effect of Pt and SnxOy. However, further increasing the molar ratios of Pt/Sn to 1/1 and 1/1.25, the time to achieve 95% 4-NP conversion increased from 5 min to 9 min, suggesting the less exposed Pt interface with the converge of too much SnxOy [26,27]. Therefore, the best catalytic efficiency of Pt1-(SnxOy)0.75@PSNs was chosen for further study.
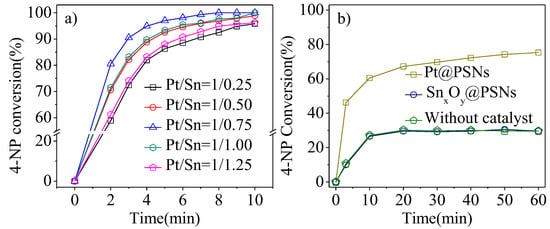
Figure 6.
Catalytic reduction of 4-NP with NaBH4 of (a) using various Pt/Sn molar ratios of PSNs and (b) using SnxOy@PSNs and Pt@PSNs. Reaction conditions: 0.025 mmol of 4-NP, 4-NP/Pt molar ratio-150/1 for Pt-containing PSNs, 4-NP/Sn molar ratio-248/1 for SnxOy@PSNs, 1.50 mmol of NaBH4, 20.0 mL of H2O, room temperature, reaction time-10 min, stirring speed-750 rpm. All materials were calcined at 300 °C.
Figure S8 presents the effect of the calcination temperature of Pt1-(SnxOy)0.75@PSNs on the catalytic reduction of 4-NP with NaBH4. When the calcination temperature was increased from 200 °C to 300 °C, the conversion of 4-NP enhanced from 39.4% to 97% in 5 min, indicating the complete removal of organic agents in Pt1-(SnxOy)0.75@PSNs, as shown in Figure 5. However, when the calcination temperature was further increased to 400 °C and 500 °C, the conversion of 4-NP decreased to 94.5% and 86%, respectively, in 5 min. As shown in Figures S3 and S4, this conversion decline can be related to the aggregation of Pt-SnxOy hybrid nanoparticles. In summary, the best calcination temperature for catalytic efficiency was 300 °C.
According to the literature, the rate constants (k) of 4-NP catalytic reductions can be calculated by the slopes of the fitted lines [43,44]. Figure 7a illustrates the effect of the reaction temperature on the catalytic reduction of 4-NP over Pt1-(SnxOy)0.75@PSNs. For reaction temperatures of 10 °C, 25 °C, and 45 °C, the k of Pt1-(SnxOy)0.75@PSNs is 0.0068 s−1, 0.012 s−1, and 0.028 s−1, respectively. Based on the above calculated k and the Arrhenius theory [43,44], the activation energy of Pt1-(SnxOy)0.75@PSNs in Figure 7b is 30.1 kJ/mol.
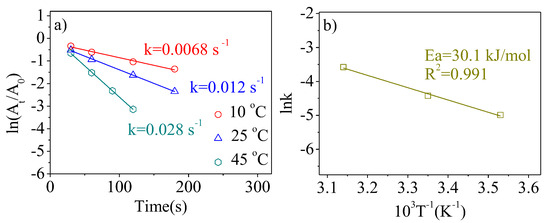
Figure 7.
(a) The effect of reaction temperature on rate constants (k) over Pt1-(SnxOy)0.75@PSNs calcined at 300 °C; (b) the corresponding relationship between lnk and T−1.
Figure 8a shows the influence of the 4-NP concentration on catalytic reduction at a constant molar ratio of NaBH4/4-NP. When the concentration of 4-NP was 5 mM, the catalytic activity was slower than other concentrations of 4-NP, which can be ascribed to the lower probability of collision between 4-NP and the catalyst particles [18,26,27]. When the concentration of 4-NP increased to 50 mM, the catalytic activity obviously improved (4-NP achieved 100% conversion within 8 min). As the concentration of 4-NP further increased to 100 and 150 mM, the reaction activity only slightly decreased; complete conversion was obtained in 10 min, indicating good catalytic performance of Pt1-(SnxOy)0.75@PSNs. The influence of NaBH4 concentration on catalytic reduction of 4-NP at a constant amount of 4-NP is shown in Figure 8b. With the increase of NaBH4 concentration, the catalytic efficiency of 4-NP reduction rapidly improves. But when the concentration of NaBH4 is over 300 mM, the catalytic reaction activity slightly increases, suggesting a sufficient concentration of NaBH4 [18,26,27]. In this work, the best catalytic efficiency with a turnover frequency of 7322.2 h−1 (defined as the moles of 4-NP converted per total molar Pt atoms per hour) was obtained when the concentration of NaBH4 was 300 mM. Compared with the existing reported catalytic system in Table S2, Pt1-(SnxOy)0.75@PSNs show good catalytic efficiency.
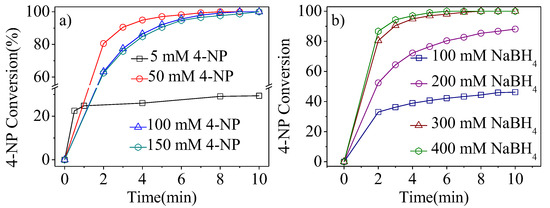
Figure 8.
(a) Effect of 4-NP concentration on catalytic reduction over Pt1-(SnxOy)0.75@PSNs calcined at 300 °C; (b) effect of NaBH4 concentration on catalytic reduction over Pt1-(SnxOy)0.75@PSNs calcined at 300 °C. Reaction conditions: 5.0 mg Pt1-(SnxOy)0.75@PSNs, 20.0 mL of H2O, room temperature, stirring speed-750 rpm, NaBH4/4-NP molar ratio of 60/1 for (a), 0.025 mmol of 4-NP for(b).
Compared with a typical 4-NP catalytic reduction, the concentration of 4-NP was 5 times higher with static 4-NP/NaBH4 to test the catalytic stability of Pt1-(SnxOy)0.75@PSNs. Figure 9a illustrates the catalytic performance of Pt1-(SnxOy)0.75@PSNs for 4-NP reduction at a 750/1 molar ratio of 4-NP/Pt. In 9 min, 100.0% conversion of 4-NP was reached. The catalytic performance of 4-NP reduction under normal conditions over Pt1-(SnxOy)0.75@PSNs after having undergone five reaction cycles is shown in Figure 9b. Compared with the fresh catalyst in Figure 9b, the catalytic performance of the recycled catalyst showed a slight decrease, but the conversion of 4-NP still could reach 99% in 10 min, suggesting the good catalytic stability of Pt1-(SnxOy)0.75@PSNs. Figure S9 presents the TEM image and size distribution of Pt1-(SnxOy)0.75@PSNs after six cycles of catalytic reaction. The structure and size of Pt1-(SnxOy)0.75@PSNs did not show obvious changes, and Pt and Sn ions were not detected in the solution after five cycles of 4-NP reduction using ICP-OES, further demonstrating the good reusability of Pt1-(SnxOy)0.75@PSNs, which can be attributed to the protection of porous silica nanostructures.
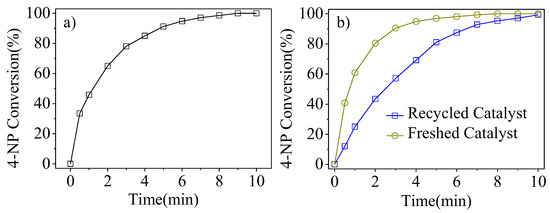
Figure 9.
(a) Influence of reaction time on 4-NP conversion over Pt1-(SnxOy)0.75@PSNs at a 4-NP/Pt ratio of 750 (five times higher); (b) effect of reaction time on 4-NP conversion over recycled Pt1-(SnxOy)0.75@PSNs after being treated by 4-NP five times. Reaction conditions: 4-NP, 0.25 mmol for (a) and 0.025 mmol for (b); 4-NP/Pt ratio of 750 for (a) and 150 for (b); H2O, 40.0 mL for (a) and 20.0 mL for (b); NaBH4/4-NP ratio of 60/1; room temperature; speed of agitation, 750 rpm.
3. Materials and Methods
3.1. Materials
Sodium borohydride (NaBH4, ≥96.0%), Ethanol (AR, ≥99.7%), ammonia (AR, 25–28%), Sodium bicarbonate (NaHCO3, ≥99.5%) and Sodium hydroxide (NaOH, ≥96.0%) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). 4-nitrophenol (≥98.0%), Chloroplatinic acid hexahydrate (H2PtCl6·6H2O, Pt ≥ 37.5%), Cetyltrimethylammonium bromide (CTAB, 99.0%) and 1-Dodecanethiol (C12-SH, 98.0%) were purchased from Aladdin Chemical (Shanghai, China). Tetraethyl orthosilicate (TEOS, 98.0%) and Tin(IV) chloride pentahydrate (SnCl4·5H2O, 99.0%) were purchased from Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China). All reagents were used as received without purification.
3.2. Synthesis of Pt-SnxOy@PSNs, Pt@PSNs, and SnxOy@PSNs
For the synthesis of Pt1-(SnxOy)0.75@PSNs, 0.1 g of CTAB, and 10 μL C12-SH were added in a bottle glass with 46.0 g of deionized water under magnetic stirring to achieve a clear solution at 80 °C. Subsequently, 900 μL of 10 mM H2PtCl6 aqueous solution, 675 μL of 10 mM SnCl4 aqueous solution, and 350 μL of 2 M NaOH aqueous solution were charged into the bottle. After the mixture was magnetically stirred at 500 rpm at 80 °C for 1.0 h, TEOS (750 μL) was added. After reaction 2 h, the solids were obtained by centrifugation, washed with ethanol several times, dried in an oven at 60 °C overnight, and then calcined at 200–500 °C for 3.0 h in a muffle furnace.
The procedures for Pt1-(SnxOy)0.25@PSNs, Pt1-(SnxOy)0.5@PSNs, Pt1-(SnxOy)1@PSNs, and Pt1-(SnxOy)1.25@PSNs were the same as those of Pt1-(SnxOy)0.75@PSNs except for changing the concentrations of SnCl4 aqueous solutions to 3.3, 6.7, 13.3, and 16.7 mM, respectively. As for the synthesis of Pt@PSNs, Pt2@PSNs (with Pt concentrations twice of Pt@PSNs), and SnxOy@PSNs, the procedures were the same as those of Pt1-(SnxOy)0.75@PSNs, except 900 μL of 10.0 mM H2PtCl6, 900 μL of 20.0 mM H2PtCl6, and 675 μL of 10.0 mM SnCl4 aqueous solutions were used, respectively.
The procedures for Pt2-(SnxOy)0.5@PSNs, Pt2-(SnxOy)1@PSNs, Pt2-(SnxOy)1.5@PSNs, Pt2-(SnxOy)2@PSNs, and Pt2-(SnxOy)2.5@PSNs were the same as those of Pt1-(SnxOy)0.75@PSNs, except 900 μL of 20.0 mM H2PtCl6 and 675 μL of 6.6 mM SnCl4; 900 μL of 20.0 mM H2PtCl6 and 675 μL of 13.4 mM SnCl4; 900 μL of 20.0 mM H2PtCl6 and 675 μL of 20.0 mM SnCl4; 900 μL of 20.0 mM H2PtCl6 and 675 μL of 26.6 mM SnCl4; and 900 μL of 20.0 mM H2PtCl6 and 675 μL of 33.4 mM SnCl4 aqueous solutions were used, respectively.
3.3. Catalytic Reduction of 4-NP with NaBH4
In a typical run, 0.025 mmol of 4-NP (0.5 mL of 50 mM 4-NP aqueous solution), 5.0 mg of Pt1-(SnxOy)0.75@PSNs (Pt, 0.65 wt%; Sn, 0.3 wt%; Pt/4-NP molar ratio, 1/150), 1.50 mmol of NaBH4 (5.0 mL of 300 mM NaBH4 aqueous solution), and 20.0 mL of water were mixed in a 30 mL glass bottle and vigorously stirred at room temperature. At the defined time intervals, a small fraction of the mixtures was sampled for UV-vis spectroscopy measurement to determine 4-NP conversion.
3.4. Catalytic Stability of 4-NP Reduction
In total, 0.25 mmol of 4-NP and 10.0 mg of catalyst, 15.0 mmol of NaBH4, and 40.0 mL of water were added in 65 mL glass bottle and vigorously stirred at room temperature. The reaction was sampled for UV-vis spectroscopy measurement to determine 4-NP conversion. After 15 min of reaction, the catalysts were collected and washed with deionized water and ethanol several times and dried at 60 °C. The recovered catalyst underwent another cycle at the same reaction conditions as those in Section 3.3.
4. Conclusions
In this study, a microemulsion method was used to prepare Pt-SnxOy hybrid nanoparticles encaged in porous silica nanospheres. In this microemulsion system, CTAB and C12-SH served as co-surfactants to form micelles in water. C12-SH was coordinated with Pt and Sn ions to confine metal ions in the microemulsion system. After calcination, Pt-SnxOy@PSNs were formed. Due to the synergistic effect of Pt and SnxOy, the catalytic efficiency of the reduction of 4-NP was greatly enhanced. Moreover, the protection of the silica shell enabled Pt-SnxOy@PSNs to show good stability in 4-NP reduction. We believe this microemulsion method can be extended to preparing other bimetal porous silica nanospheres, and these materials can have more applications in various fields.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal15030263/s1, Figure S1: Size distributions of whole Pt@PSNs and different molar ratios of Pt-SnxOy@PSNs; Figure S2: Size distributions of inner nanoparticles of Pt@PSNs and different molar ratios of Pt-SnxOy@PSNs; Figure S3: TEM images of Pt1-(SnxOy)0.75@PSNs with different calcination temperatures; Figure S4: Size distributions of inner nanoparticles of Pt1-(SnxOy)0.75@PSNs with different calcination temperatures; Figure S5: XRD patterns of SnxOy@PSNs and Pt2-(SnxOy)1.5@PSNs; Figure S6: The EDS measurement of Pt1-(SnxOy)0.75@PSNs and Pt2-(SnxOy)1.5@PSNs. Figure S7: XPS spectra of Pt2@PSNs and Pt2-(SnxOy)1.5@PSNs. Figure S8: The effect of calcination temperature on 4-NP conversion over Pt1-(SnxOy)0.75@PSNs calcined at various temperatures. Figure S9: TEM images and size distribution of recycled Pt1-(SnxOy) 0.75@PSNs; Table S1: The real Pt and Sn loading of Pt-SnxOy@PSNs with different Pt/Sn ratios; Table S2: Comparisons of catalytic performance between Pt1-(SnxOy)0.75@PSNs and various reported catalysts for 4-NP reduction [45,46,47,48,49,50].
Author Contributions
The project was conceived and designed by K.L. K.L. and Q.Z. carried out the materials syntheses/characterization and the catalytic experiments. K.L., Q.W., H.Y. (Hongbo Yu) and H.Y. (Hongfeng Yin) supervised this project. These authors (K.L. and Q.W.) are equal contributors. All authors contributed to the discussion on the project. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financial supported by National Key Research and Development Program (2023YFB4005902), Ningbo Yongjiang Talent Introduction Program (2022A-008-C, 2023D-002-02), the National Natural Science Foundation of China (Grant Nos. 22072171), Ningbo Youth Science and Technology Leading Talents Project (2024QL017), Ningbo Natural Science Foundation (2023J274).
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no competing interests.
References
- Huang, C.; Ye, W.; Liu, Q.; Qiu, X. Dispersed Cu2O Octahedrons on h-BN Nanosheets for p-Nitrophenol Reduction. ACS Appl. Mater. Interfaces 2014, 6, 14469–14476. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Luo, M.; Lan, J.; Xie, F.; Cai, L.; Chan, T.-S.; Peng, M.; Tan, Y. Pd Atomic Engineering of Nanoporous Ni/NiO for Efficient Nitrophenol Hydrogenation Reaction. ACS Appl. Mater. Interfaces 2023, 15, 26746–26754. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Jian, M.; Qiao, L.; Zhao, Z.; Yang, Y.; Jiao, T.; Zhang, Q. Efficient Removal and Recovery of Ag from Wastewater Using Charged Polystyrene-Polydopamine Nanocoatings and Their Sustainable Catalytic Application in 4-Nitrophenol Reduction. ACS Appl. Mater. Interfaces 2024, 16, 5834–5846. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Liang, X.; Zhang, L.; Tang, Z.; Al-Mamun, M.; Zhao, H.; Su, X. Fabrication of Highly Stable Metal Oxide Hollow Nanospheres and Their Catalytic Activity toward 4-Nitrophenol Reduction. ACS Appl. Mater. Interfaces 2017, 9, 18207–18214. [Google Scholar] [CrossRef]
- Wang, X.; Liu, D.; Song, S.; Zhang, H. Pt@CeO2 Multicore@Shell Self-Assembled Nanospheres: Clean Synthesis, Structure Optimization, and Catalytic Applications. J. Am. Chem. Soc. 2013, 135, 15864–15872. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Chen, L.; Miao, S.; Wu, S.; Hao, X.; Zhang, W.; Jia, M. Interfacial Synergy of PtPd Nanoparticles Dispersed on Amine-Modified ZrSBA-15 in Catalytic Dehydrogenation of Ammonia Borane and Reduction of p-Nitrophenol. J. Phys. Chem. C 2018, 122, 12975–12983. [Google Scholar] [CrossRef]
- Strachan, J.; Barnett, C.F.; Masters, A.; Maschmeyer, T. 4-Nitrophenol Reduction: Probing the Putative Mechanism of the Model Reaction. ACS Catal. 2020, 10, 5516–5521. [Google Scholar] [CrossRef]
- Li, M.; Chen, G. Revisiting Catalytic Model Reaction p-nitrophenol/NaBH4 Using Metallic Nanoparticles Coated on Polymeric Spheres. Nanoscale 2013, 5, 11919–11927. [Google Scholar] [CrossRef]
- Yu, H.; Wu, C.; Wang, S.; Li, T.; Yin, H. Transition Metal Oxide-Modified Ir Nanoparticles Supported on SBA-15 Silica for Selective Hydrogenation of Substituted Nitroaromatics. ACS Appl. Nano Mater. 2021, 4, 7213–7220. [Google Scholar] [CrossRef]
- Cui, K.; Zhong, W.; Li, L.; Zhuang, Z.; Li, L.; Bi, J.; Yu, Y. Well-Defined Metal Nanoparticles@Covalent Organic Framework Yolk-Shell Nanocages by ZIF-8 Template as Catalytic Nanoreactors. Small 2019, 15, 1804419. [Google Scholar] [CrossRef]
- Liu, T.; Sun, Y.; Jiang, B.; Guo, W.; Qin, W.; Xie, Y.; Zhao, B.; Zhao, L.; Liang, Z.; Jiang, L. Pd Nanoparticle-Decorated 3D-Printed Hierarchically Porous TiO2 Scaffolds for the Efficient Reduction of a Highly Concentrated 4-Nitrophenol Solution. ACS Appl. Mater. Interfaces 2020, 12, 28100–28109. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Yang, Q.; Chen, W. One-Step Rapid and Facile Synthesis of Subnanometer-Sized Pd6 (C12H25S) 11 Clusters with Ultra-High Catalytic Activity for 4-Nitrophenol Reduction. ACS Sustainable Chem. Eng. 2019, 7, 2916–2923. [Google Scholar] [CrossRef]
- Li, W.; Xian, L.; Zhao, Y.; Sun, C. Stepwise Preparation of Uniform-Size Ultrafine Pt Nanoparticles for High-Performance Catalysis of Methanol Oxidation and Nitrophenol Reduction. ACS Appl. Nano Mater. 2023, 6, 19176–19188. [Google Scholar] [CrossRef]
- Chairunnisa, R.; Noorenza Biutty, M.; Yoo, S.; Il Yoo, S. Porous Polydopamine-Coated Polydimethylsiloxane/Pt Composites for Solar-Light-Mediated Catalytic Reactions for Water Treatment. ACS Appl. Polym. Mater. 2024, 6, 12049–12058. [Google Scholar] [CrossRef]
- Qi, B.; Wu, C.; Liu, Y.; Liu, J.; Zhang, H. Self-Assembled Magnetic Pt Nanocomposites for the Catalytic Reduction of Nitrophenol. ACS Appl. Nano Mater. 2019, 2, 4377–4385. [Google Scholar] [CrossRef]
- Neal, R.D.; Hughes, R.A.; Sapkota, P.; Ptasinska, S.; Neretina, S. Effect of Nanoparticle Ligands on 4-Nitrophenol Reduction: Reaction Rate, Induction Time, and Ligand Desorption. ACS Catal. 2020, 10, 10040–10050. [Google Scholar] [CrossRef]
- Gamraoui, H.; Khojastehnezhad, A.; Bélanger-Bouliga, M.; Nazemi, A.; Siaj, M. Covalent Organic Framework-Templated N-Heterocyclic Carbene-Functionalized Gold Nanoparticles for the Catalytic Reduction of Nitrophenol. ACS Appl. Nano Mater. 2024, 7, 22570–22580. [Google Scholar] [CrossRef]
- Hu, R.; Xu, R.; Wang, Z.; Wang, J.; Zhou, S. Enhanced Catalytic Nitrophenol Reduction via Highly Porous Hollow Silica Nanospheres Stabilized Au Nanoclusters. Chem. Eng. J. 2023, 471, 144780. [Google Scholar] [CrossRef]
- Yeh, Y.-J.; Chiang, W.-H. Ag Microplasma-Engineered Nanoassemblies on Cellulose Papers for Surface-Enhanced Raman Scattering and Catalytic Nitrophenol Reduction. ACS Appl. Nano Mater. 2021, 4, 6364–6375. [Google Scholar] [CrossRef]
- Chen, T.; Liu, Z.; Zhang, K.; Su, B.; Hu, Z.; Wan, H.; Chen, Y.; Fu, X.; Gao, Z. Mussel-Inspired Ag NPs Immobilized on Melamine Sponge for Reduction of 4-Nitrophenol, Antibacterial Applications and Its Superhydrophobic Derivative for Oil-Water Separation. ACS Appl. Mater. Interfaces 2021, 13, 50539–50551. [Google Scholar] [CrossRef]
- Zaarour, M.; Cazemier, J.; Navarro de Miguel, J.C.; Almukhtar, F.; Komaty, S.; Ruiz-Martinez, J. Influence of Support Polarity on the Selective Hydrogenation of Nitrostyrene over Pt-based Heterogenous Catalysts. Micropor. Mesopor. Mat. 2024, 379, 113272. [Google Scholar] [CrossRef]
- Li, Z.; He, M.; Wen, Y.; Zhang, X.; Hu, M.; Li, R.; Liu, J.; Chu, J.; Ma, Z.; Xing, X.; et al. Highly Monodisperse Cu-Sn Alloy Nanoplates for Efficient Nitrophenol Reduction Reaction via Promotion Effect of Tin. Inorg. Chem. 2020, 59, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Fageria, P.; Uppala, S.; Nazir, R.; Gangopadhyay, S.; Chang, C.-H.; Basu, M.; Pande, S. Synthesis of Monometallic (Au and Pd) and Bimetallic (AuPd) Nanoparticles Using Carbon Nitride (C3N4) Quantum Dots via the Photochemical Route for Nitrophenol Reduction. Langmuir 2016, 32, 10054–10064. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-Q.; Zhao, J.; Wu, Y.-P.; Dong, W.-W.; Li, D.-S.; Li, J.-R.; Zhang, Q. Ultrafine Pt Nanoparticles and Amorphous Nickel Supported on 3D Mesoporous Carbon Derived from Cu-Metal-Organic Framework for Efficient Methanol Oxidation and Nitrophenol Reduction. ACS Appl. Mater. Interfaces 2018, 10, 12740–12749. [Google Scholar] [CrossRef]
- Feng, D.; Wei, Z.; Wang, Q.; Feng, A.; Zhang, H. Controllable Synthesis of Cobalt-Containing Nanosheet Array-Like Ternary CuCoAl-LDH/rGO Hybrids to Boost the Catalytic Efficiency for 4-Nitrophenol Reduction. ACS Appl. Mater. Interfaces 2022, 14, 24265–24280. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Z.; Ma, Y.; Xu, C.; Zhou, S. Au-CuxOy Nanoparticles Encapsulated in Hollow Porous Silica Nanospheres as Efficient Catalysts for Nitrophenol Reduction. ACS Appl. Nano Mater. 2023, 6, 461–468. [Google Scholar] [CrossRef]
- Ye, H.; Hu, R.; Wang, Z.; Wang, J.; Zhou, S. Confinement Effect of Hollow Nanoreactors Enforcing the Formation of Pd-NixOy Hybrid Nanoparticles Inside for 4-Nitrophenol Catalytic Reduction. Ind. Eng. Chem. Res. 2024, 63, 288–295. [Google Scholar] [CrossRef]
- Long, Y.; Song, S.; Li, J.; Wu, L.; Wang, Q.; Liu, Y.; Jin, R.; Zhang, H. Pt/CeO2@MOF Core@Shell Nanoreactor for Selective Hydrogenation of Furfural via the Channel Screening Effect. ACS Catal. 2018, 8, 8506–8512. [Google Scholar] [CrossRef]
- Li, K.; Duan, C.; Mao, W.; Wang, D.; Liang, Y.; Ye, W.; He, G.; Liu, X. SnOx-Decorated Pt Catalysts for the Reductive N-Methylation of Quinoline with Methanol. ACS Sustain. Chem. Eng. 2024, 12, 7644–7654. [Google Scholar] [CrossRef]
- Wu, P.; He, Z.; Liu, Y.; Song, L.; Wang, C.; Muhumuza, E.; Bai, P.; Zhao, L.; Mintova, S.; Yan, Z. Compatibility between Activity and Selectivity in Catalytic Oxidation of Benzyl Alcohol with Au-Pd Nanoparticles through Redox Switching of SnOx. ACS Appl. Mater. Interfaces 2021, 13, 49780–49792. [Google Scholar] [CrossRef]
- Liu, M.; Tang, W.; Xie, Z.; Yu, H.; Yin, H.; Xu, Y.; Zhao, S.; Zhou, S. Design of Highly Efficient Pt-SnO2 Hydrogenation Nanocatalysts Using Pt@Sn Core-Shell Nanoparticles. ACS Catal. 2017, 7, 1583–1591. [Google Scholar] [CrossRef]
- Tang, J.; Liu, P.; Liu, X.; Chen, L.; Wen, H.; Zhou, Y.; Wang, J. In Situ Encapsulation of Pt Nanoparticles within Pure Silica TON Zeolites for Space-Confined Selective Hydrogenation. ACS Appl. Mater. Interfaces 2020, 12, 11522–11532. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yao, S.; Zhang, M.; Huang, F.; Fan, Q.; Zhang, S.; Liu, H.; Ma, D.; Gao, C. Ultra-Small Platinum Nanoparticles Encapsulated in Sub-50 nm Hollow Titania Nanospheres for Low-Temperature Water-Gas Shift Reaction. ACS Appl. Mater. Interfaces 2018, 10, 36954–36960. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Xu, C.; Zhou, S. Synthesis of Alumina Supported Pt-SnO2 Hybrid Nanostructures by In Situ Transformation of PtSn Alloy Nanoparticles and Their Application as Highly Efficient Catalysts for Selective Hydrogenation of Furfural. J. Phys. Chem. C 2023, 127, 4033–4041. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, R.; Wang, L.; Zhou, S. Enhanced Selective Hydrogenation of Cinnamaldehyde to Cinnamyl Alcohol over Silica-Coated Pt-CoxOy Hybrid Nanoparticles. ACS Appl. Mater. Interfaces 2024, 16, 924–932. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, J.; Wu, C.; Guan, L. Pt Nanoparticles Densely Coated on SnO2-Covered Multiwalled Carbon Nanotubes with Excellent Electrocatalytic Activity and Stability for Methanol Oxidation. ACS Appl. Mater. Interfaces 2017, 9, 26921–26927. [Google Scholar] [CrossRef]
- Li, W.; Li, Z.; Yang, F.; Fang, X.; Tang, B. Synthesis and Electrochemical Performance of SnOx Quantum Dots@ UiO-66 Hybrid for Lithium Ion Battery Applications. ACS Appl. Mater. Interfaces 2017, 9, 35030–35039. [Google Scholar] [CrossRef]
- Yan, P.; Ma, L.; Wang, S.; Fang, L.; Meng, X.; Kong, X.; Zhou, S. SnO2 Modified Intermetallic PdSn for Selective Hydrogenation of Phenol to Cyclohexanone with Hydrogen Donor. Chem. Eng. J. 2024, 479, 147794. [Google Scholar] [CrossRef]
- Ye, B.; Xu, L.; Wu, W.; Ye, Y.; Yang, Z.; Qiu, Y.; Gong, Z.; Zhou, Y.; Huang, Q.; Shen, Z.; et al. Construction of Hierarchical SnO2@NC@MoS2/C Nanotubes for Ultrastable Lithium- and Sodium-Ion Batteries. ACS Sustainable Chem. Eng. 2022, 10, 3166–3179. [Google Scholar] [CrossRef]
- Yan, Y.; An, Q.; Xiao, Z.; Zheng, W.; Zhai, S. Flexible Core-Shell/Bead-Like Alginate@PEI with Exceptional Adsorption Capacity, Recycling Performance toward Batch and Column Sorption of Cr (VI). Chem. Eng. J. 2017, 313, 475–486. [Google Scholar] [CrossRef]
- Do, Q.C.; Kim, D.-G.; Ko, S.-O. Nonsacrificial Template Synthesis of Magnetic-Based Yolk-Shell Nanostructures for the Removal of Acetaminophen in Fenton-like Systems. ACS Appl. Mater. Interfaces 2017, 9, 28508–28518. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, T.; Zhang, X.; Huo, K.; Chu, P.K.; He, J. One-Step Synthesis of Monodisperse and Hierarchically Mesostructured Silica Particles with a Thin Shell. Langmuir 2010, 26, 13556–13563. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Han, B.; Jiang, X.; Zhou, C.; Xia, K.; Gao, Q.; Wu, J. Toward High Activity and Durability: An Oxygen-Rich Boron NitrideSupported Au Nanoparticles for 4-Nitrophenol Hydrogenation. J. Phys. Chem. C 2019, 123, 10389–10397. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, H.; Huang, H.; Zong, W.; Miao, Y.-E.; He, G.; Parkin, I.P.; Lai, F.; Liu, T. Electron-Deficient Au Nanoparticles Confined in Organic Molecular Cages for Catalytic Reduction of 4-Nitrophenol. ACS Appl. Nano Mater. 2022, 5, 1276–1283. [Google Scholar] [CrossRef]
- Ye, J.; Li, C.; Yao, X.; Jin, M.; Wan, D. Customizing a Hyperbranched Ligand Confers Supported Platinum Nanoclusters with Unexpected Catalytic Activity toward the Reduction of 4-Nitrophenol. Langmuir 2023, 39, 18093–18100. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Qi, S.-D.; Niu, X.-Y.; Hu, J.; Ren, C.-L.; Chen, H.-L.; Chen, X.-G. Metallic Nanoparticles Immobilized in Magnetic Metal-Organic Frameworks: Preparation and Application as Highly Active, Magnetically Isolable and Reusable Catalysts. Catal. Sci. Technol. 2014, 4, 3013–3024. [Google Scholar] [CrossRef]
- Dai, Y.; Chai, Y.; Sun, Y.; Fu, W.; Wang, X.; Qing Gu, Q.; Ying, T.; Zeng, H.; Sun, Y. New Versatile Pt Supports Composed of Graphene Sheets Decorated by Fe2O3 Nanorods and N-dopants with High Activity based on Improved Metal/Support Interactions. J. Mater. Chem. A 2015, 3, 125–130. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, Z.; Li, J.; Feng, D.; Feng, A.; Zhang, H. Hierarchical-Structured Pd Nanoclusters Catalysts x-PdNCs/CoAl(O)/rGO-T by the Captopril-Capped Pd Cluster Precursor Method for the Highly Efficient 4-Nitrophenol Reduction. ACS Appl. Mater. Interfaces 2022, 14, 27775–27790. [Google Scholar] [CrossRef]
- Ye, W.; Yu, J.; Zhou, Y.; Gao, D.; Wang, D.; Wang, C.; Xue, D. Green synthesis of Pt-Au Dendrimer-Like Nanoparticles Supported on Polydopamine-Functionalized Graphene and Their High Performance toward 4-Nitrophenol Reduction. Appl. Catal. B Environ. 2016, 181, 371. [Google Scholar] [CrossRef]
- Long, Y.; Li, J.; Wu, L.; Li, J.; Wang, X.; Yao, S.; Song, S.; Zhang, H. One-Pot Synthesis of Cobalt-Doped Pt-Au Alloy Nanoparticles Supported on Ultrathin α-Co(OH)2 Nanosheets and Their Enhanced Performance in the Reduction of p-Nitrophenol. Eru. J. Inorg. Chem. 2017, 2017, 146–152. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).